Abstract
Purpose
Magnetic resonance imaging detects extracapsular extension by prostate cancer with excellent specificity but low sensitivity. This limits surgical planning, which could be modified to account for focal extracapsular extension with image directed guidance for wider excision. In this study we evaluate the performance of multiparametric magnetic resonance imaging in extracapsular extension detection and determine which preoperative variables predict extracapsular extension on final pathology when multiparametric magnetic resonance imaging predicts organ confined disease.
Materials and Methods
From May 2007 to March 2014, 169 patients underwent pre-biopsy multiparametric magnetic resonance imaging, magnetic resonance imaging/transrectal ultrasound fusion guided biopsy, extended sextant 12-core biopsy and radical prostatectomy at our institution. A subset of 116 men had multiparametric magnetic resonance imaging negative for extracapsular extension and were included in the final analysis.
Results
The 116 men with multiparametric magnetic resonance imaging negative for extracapsular extension had a median age of 61 years (IQR 57–66) and a median prostate specific antigen of 5.51 ng/ml (IQR 3.91–9.07). The prevalence of extracapsular extension was 23.1% in the overall population. Sensitivity, specificity, and positive and negative predictive values of multiparametric magnetic resonance imaging for extracapsular extension were 48.7%, 73.9%, 35.9% and 82.8%, respectively. On multivariate regression analysis only patient age (p=0.002) and magnetic resonance imaging/transrectal ultrasound fusion guided biopsy Gleason score (p=0.032) were independent predictors of extracapsular extension on final radical prostatectomy pathology.
Conclusions
Because of the low sensitivity of multiparametric magnetic resonance imaging for extracapsular extension, further tools are necessary to stratify men at risk for occult extracapsular extension that would otherwise only become apparent on final pathology. Magnetic resonance imaging/transrectal ultrasound fusion guided biopsy Gleason score can help identify which men with prostate cancer have extracapsular extension that may not be detectable by imaging.
Keywords: prostate, adenocarcinoma, neoplasm staging, risk, prognosis
In the era of serum PSA testing the majority of prostate cancer diagnoses represent organ confined disease, for which radical prostatectomy is curative.1 However, certain adverse pathological features on RP pathology are associated with worse postoperative oncologic outcomes. The presence of extracapsular extension is of particular importance as it is associated with higher rates of positive surgical margins and early biochemical recurrence.2
Preoperative knowledge of the presence and location of potential ECE allows for a modified surgical technique, which minimizes the risk of positive margins and optimizes the likelihood of complete extirpation via wide resection.3 However, no preoperative tool reliably detects and localizes ECE in all cases. Traditional anatomical sequences of MRI reliably detect gross ECE. However, MRI is limited by a low sensitivity for microfocal tumor growth invading the capsule, with a reported sensitivity for ECE of 33% to 64%.4–6 Thus, as prostatic imaging has evolved, attention has turned toward multiparametric prostate MRI.
This technique has a high accuracy for the identification of clinically relevant intraprostatic PCa lesions, leading to hopes of improved staging accuracy as well.7–9 Somford et al recently published their initial experience with MP-MRI for the detection of ECE.10 Despite the use of a 3 Tesla (3T) scanner with an endorectal coil and MP-MRI sequences interpreted by experienced radiologists, the sensitivity of MP-MRI for ECE as confirmed by final pathology was only 58.2%. PSA was an independent predictor of ECE while random biopsy Gleason score was not.10 These findings suggest that even with optimal imaging protocols, MRI under stages disease in men who ultimately choose to undergo RP.
Further adjunct tools are necessary to risk stratify men who are likely to harbor ECE that is not detectable on the highest level imaging.11 At our institution patients with suspected prostate cancer undergo 3T MP-MRI of the prostate with an endorectal coil. This is followed by MRI/TRUS fusion guided biopsy, whereby MRI suspicious lesions can be targeted and directly sampled. Because this biopsy technique is thought to more accurately represent true prostate tumor burden,12–14 we hypothesized that targeted biopsy Gleason score could better predict the presence of ECE. Thus, in this study we evaluate the performance of MP-MRI and fusion biopsy for the detection of ECE, and determine which preoperative variables can predict ECE on final RP pathology in cases in which MP-MRI estimates organ confined disease.
MATERIALS AND METHODS
Patient Selection
Patients were enrolled in an institutional review board approved, prospective study of prostate MP-MRI and MRI/TRUS fusion guided biopsy at the National Cancer Institute of the National Institutes of Health. From May 2007 to March 2014, 370 patients underwent RP at our institution (fig. 1). Of these patients 201 were excluded from the study because of an incomplete preoperative evaluation, which in all other cases included MP-MRI, MRI/TRUS fusion guided biopsy and extended sextant 12-core biopsy. The remaining 169 patients were included in the analysis of MP-MRI diagnostic performance for the detection of ECE on final RP pathology.
Figure 1.
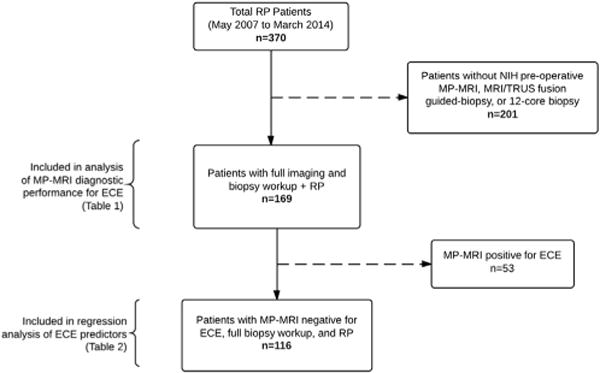
Flowchart illustrating patient selection
Of the 169 patients comprising the overall study population 116 had MP-MRI negative for ECE (fig. 1). These patients were included in the analysis of preoperative predictors of pathological ECE in the setting of MP-MRI that is negative for this finding. The 53 patients with MP-MRI positive for ECE were excluded from analysis because of their heterogeneity. Some of these 53 patients had MRI with frank ECE but many reports instead described probable ECE or extracapsular bulge. By excluding all such patients the analysis of preoperative predictors of pathological ECE may be performed on our study’s specific target population, that is patients with PCa and no MRI suspicion of ECE.
Imaging and Biopsy Protocols
Diagnostic MP-MRI of the prostate was performed with a 3T scanner (Achieva, Philips Healthcare, Best, The Netherlands) using an endorectal coil (BPX-30, Medrad, Pittsburgh, Pennsylvania) and a 16-channel cardiac surface coil (SENSE, Philips Healthcare) as previously described.15 The prostate MP-MRI was evaluated by 2 radiologists (BT, PLC) with extensive prostate MRI experience (7 and 15 years, respectively). MP-MRI incorporated triplanar T2-weighted, diffusion weighted, DCE and magnetic resonance spectroscopy sequences in most cases (fig. 2). These sequences were combined to produce a PCa suspicion score of low, moderate or high as previously validated.16 MP-MRI evidence of ECE included gross violation of the planes between the prostate gland and adjacent tissue, the presence of a visible capsular bulge, capsular retraction or irregularity altering the expected contour of the prostatic capsule perimeter, or obliteration of the rectoprostatic angle on MRI.
Figure 2.
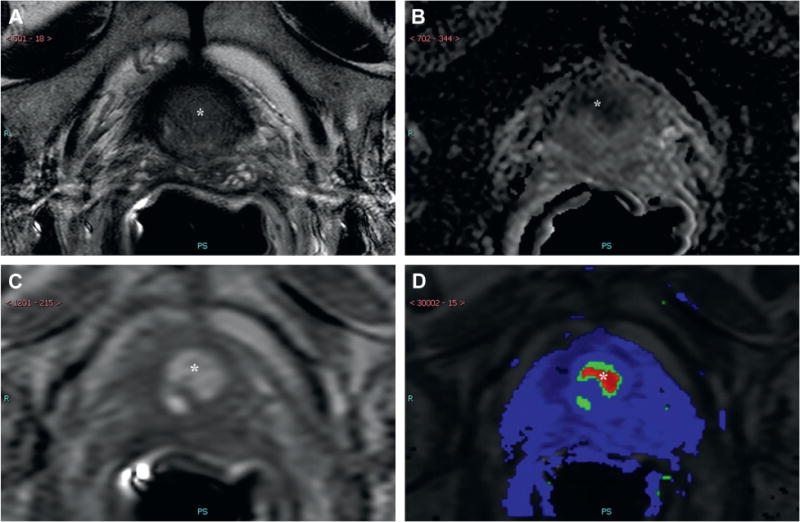
MP-MRI demonstrates lesion in right midline mid-base anterior central gland, which is positive (asterisks) on axial T2-weighted imaging (A), apparent diffusion coefficient maps of diffusion weighted imaging (B), raw DCE MRI (C) and ktrans map derived from DCE (D). There is no MP-MRI evidence of ECE. Targeted biopsy revealed Gleason 8 PCa in 85% of 3 cores. Final pathology was positive for ECE.
Patients then underwent a single prostate biopsy session which included MRI/TRUS fusion guided biopsy and extended sextant 12-core biopsy using the now commercially available UroNav platform (Philips/InVivo, Gainesville, Florida) or earlier research iterations of the current platform. All MRI suspicious lesions were targeted with a minimum of 2 cores, which were sampled in the axial and sagittal planes using an end-fire TRUS probe.17 All biopsy and RP pathology was reviewed by a single genitourinary pathologist (MJM). Robot-assisted RP was performed by a single urologist (PAP).
Data Analysis
Univariate and multivariate logistic regression analysis as well as descriptive statistics were calculated using JMP® Pro 10.0. Potential predictors of ECE were included in the multivariate regression model if significantly associated on univariate analysis, with statistical significance predefined as p <0.05. ROC curves were calculated, with AUC compared using DeLong’s test.
RESULTS
Of the 370 patients who underwent RP 169 met the inclusion criteria for analysis of the diagnostic performance of MP-MRI for ECE (fig. 1). The prevalence of ECE on final RP pathology in this population was 39 of 169 (23.1%). Sensitivity, specificity, and positive and negative predictive values of MP-MRI for correctly detecting the presence of pathological ECE were 48.7%, 73.9%, 35.9% and 82.8%, respectively.
The subset of 116 patients with MP-MRI negative for ECE was then analyzed to determine the preoperative predictors of ECE in this particular population (fig. 1). Clinical variables for this cohort are presented in table 1. The 116 patients had a median age of 61 years (IQR 57–66) and a median PSA of 5.51 ng/ml (IQR 3.91–9.07). On univariate regression analysis patient age, PSA, and highest biopsy Gleason score on random biopsy and MRI/TRUS fusion guided biopsy were associated with ECE on final RP pathology (p <0.05, table 2 and fig. 3). On multivariate regression analysis built on these parameters only patient age and MRI/TRUS fusion guided biopsy Gleason score remained statistically significant (p <0.05). In the cohort of 116 patients targeted biopsy Gleason score was higher than random biopsy Gleason score in 7 of 20 (35%) patients who ultimately had ECE on final pathology. The same finding was true for 18 of 96 (19%) patients without ECE on final pathology.
Table 1.
Preoperative clinical parameters
| No. (%) | |
|---|---|
| MP-MRI lesions: | |
| None | 0 |
| 1–2 | 62 (53.5) |
| 3–4 | 47 (40.5) |
| 5+ | 7 (6.3) |
| MP-MRI suspicion level: | |
| Low | 10 (8.6) |
| Moderate | 88 (75.9) |
| High | 18 (15.5) |
| Highest Gleason score: | |
| Extended sextant 12-core: | |
| Benign | 14 (12.1) |
| Gleason 6 | 37 (31.9) |
| Gleason 7 | 46 (39.7) |
| Gleason 8+ | 19 (16.4) |
| MRI/TRUS fusion guided: | |
| Benign | 23 (19.8) |
| Gleason 6 | 35 (30.1) |
| Gleason 7 | 35 (30.1) |
| Gleason 8+ | 23 (19.8) |
Table 2.
Univariate and multivariate regression analysis of predictors of ECE on final pathology
| Univariate Analysis
|
Multivariate Analysis
|
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Age, per yr | 1.2 | 1.1–1.3 | <0.001* | 1.2 | 1.1–1.3 | 0.002* |
| PSA, per ng/ml | 1.1 | 1.0–1.2 | 0.008* | 1.0 | 1.0–1.1 | 0.259 |
| MP-MRI: | ||||||
| No. lesions, per lesion | 0.9 | 0.5–1.3 | 0.484 | – | – | – |
| Suspicion level | 0.9 | 0.3–2.4 | 0.562 | – | – | – |
| Biopsy Gleason score: | ||||||
| Random 12-core | 2.5 | 1.4–5.0 | <0.001* | 1.5 | 0.8–3.0 | 0.219 |
| MRI/TRUS fusion guided | 3.0 | 1.7–5.9 | <0.001* | 2.0 | 1.1–4.2 | 0.032* |
Statistically significant.
Figure 3.
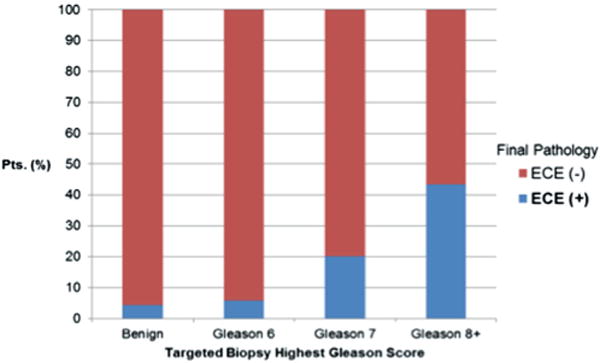
Likelihood of ECE on final pathology vs targeted biopsy Gleason score in patients with MP-MRI negative for ECE (116).
ROC curves were generated for 2 models using known predictors of ECE as determined on univariate analysis. The first model incorporated age, PSA and random biopsy Gleason score. The second model incorporated age, PSA and MRI/US fusion guided biopsy Gleason score. AUCs for the 2 curves were 0.83 and 0.86 (p=0.4).
Due to the association of ECE with positive surgical margins, a subgroup analysis was performed for surgical margin status and the presence of ECE. Of the 20 patients with MP-MRI falsenegative for ECE, surgical margins were involved by PCa in 7 (35%). Among the remaining patients with MRI true negative for ECE, the rate was 9 of 96 (9.4%). This difference in the rate of positive surgical margins was statistically significant (p=0.003).
DISCUSSION
In this study we demonstrated that MRI/TRUS fusion guided biopsy Gleason score can predict which men with PCa harbor ECE, even when MP-MRI fails to do so. In a cohort of 116 patients with MP-MRI findings negative for ECE who then underwent MRI/TRUS fusion guided biopsy, extended sextant 12-core biopsy and RP, only age and targeted biopsy Gleason score were independent predictors of ECE on final RP pathology. This knowledge may help improve surgical planning, which is currently hindered by the low sensitivity of even the most advanced imaging techniques.
Other existing methods of detecting ECE involve substantial limitations. Predictive nomograms such as the Partin tables are appealing because they require no additional imaging or cost and can be implemented quickly.18 However, nomograms rely on population based statistics whose value is limited for individualized counseling and surgical planning. Digital rectal examination not only has poor interobserver reliability but also has low accuracy.19 This is of particular importance for anteriorly located PCa, which represents a substantial proportion of all PCa, and yet is not amenable to digital rectal examination and, thus, may escape detection.20–22 Analogous problems face TRUS when it is used for the local staging of PCa.19 TRUS has poor soft tissue resolution, making it a poor tool for the detection of focal ECE.
Thus, conventional MRI has proven to be the most useful tool for detecting ECE. However, it too fails to detect ECE in a large number of patients. While the specificity of MRI for this purpose is high (71% to 100%), the sensitivity is low (33% to 64%).4–6 This inconsistency has 3 possible explanations. 1) Radiologists’ experience with prostate MRI varies substantially. The detection of ECE in particular is subject to high interobserver variability between more and less experienced readers.5,6 2) Not all pathologically detectable ECE may be visible on imaging. While gross violation of adjacent structures by PCa may be obvious on TRUS, cases of microfocal tumor extension into the capsule may be invisible even with the millimeter level soft tissue resolution of MRI. 3) The definition of ECE may differ among radiologists as well as among institutions. Thus, MP-MRI has been welcomed as an opportunity to improve the diagnosis and local staging of PCa.
In a recent study Somford et al reported the first large series of MP-MRI for the local staging of PCa.10 The authors retrospectively evaluated 183 patients who had undergone 3T endorectal coil MP-MRI that incorporated T2-weighted, diffusion weighted and DCE sequences. These images were interpreted by radiologists with extensive prostate MRI experience. After imaging, all study patients underwent extended sextant 12-core biopsy and RP, which allowed for review of final pathology similar to patients in the current study. The prevalence, sensitivity, specificity, and positive and negative predictive values of MP-MRI for detecting ECE were 49.7%, 58.2%, 89.1%, 84.1% and 68.3%, respectively. Our study supports these findings. Both data sets offer further evidence that MP-MRI has relatively high specificity but low sensitivity for ECE. However, the most striking difference between the 2 cohorts is the prevalence of ECE on final RP pathology in the populations of patients investigated (23.1% in our study vs 49.7% in the study by Somford et al).10 Somford et al suggest that their population may be enriched for patients with ECE as PSA screening was not widespread and downward stage migration was not as prevalent during their study period.10
Importantly, random biopsy Gleason score was not an independent predictor of ECE in the study by Somford et al10 and our data corroborate this finding (table 2). Because the presence of high grade PCa predisposes to ECE, a possible explanation for this lack of association is that random biopsy fails to correctly classify the aggressiveness of the prostate tumor driving the pathology of the process. This is consistent with existing data, which have demonstrated a high degree of Gleason score upgrading from random biopsy to final RP pathology.23,24 MRI/TRUS fusion guided biopsy is thought to aid in overcoming this limitation and, thus, offers an opportunity to better gauge PCa aggressiveness preoperatively.14,16,25 MP-MRI and targeted biopsy particularly outperform random biopsy in detecting high grade dominant lesions, which are the most likely to be clinically significant and lead to ECE.9,25–28 In the present study Gleason score upgrading was more common among patients with MP-MRI that was false-negative for ECE (7 of 20, 35%) than among those with MP-MRI that was true negative (18 of 96, 19%). This finding supports the notion that targeted biopsy predicts ECE by identifying aggressive PCa that random biopsy may miss.
In our cohort the rate of involvement of surgical margins by PCa was significantly different between patients with MP-MRI findings that were falsenegative for ECE (35% of cases had surgical margins involved) and those that were true negative (9%, p <0.05). This finding is not surprising as urologic oncologists cannot adequately plan surgical dissections around areas of ECE if they are not suspicious that it exists based on preoperative imaging. Until the sensitivity of MP-MRI for detecting ECE improves, targeted biopsy Gleason score may help identify cases in which preoperative planning and surgical dissections may warrant added caution.
This study has certain limitations. The final cohort of 116 patients is relatively limited. However, this group was obtained from a larger series of more than 1,000 patients who underwent MRI/TRUS fusion guided biopsy at our institution. We chose to include only those patients who underwent MP-MRI, MRI/TRUS fusion guided and extended sextant 12-core biopsies, and RP, a gold standard for pathological diagnosis, that dramatically decreased the number of subjects in our study. The extent of this reduction is further explained by the confines of the clinical trial in which all of these patients were enrolled. While MP-MRI and MRI/TRUS fusion guided biopsy remains the subject of ongoing investigation, the robot-assisted RP which we perform is standard of care. Thus, patients often undergo imaging and fusion guided biopsy at our research institution but then return to their referring urologist for definitive treatment including RP in an appreciable number of cases.
In addition, patients undergoing RP likely represent a cohort that is enriched for high risk disease, as these patients are most likely to benefit from surgery. Thus, the prevalence of ECE in our study is likely higher than the prevalence of ECE in all patients undergoing MP-MRI for suspected PCa. Therefore, patients with low risk disease in whom MP-MRI is likely to have accurately predicted the absence of ECE, ie low risk patients who elected active surveillance, were excluded from final analysis. The present study population’s enrichment for high risk disease may also explain why the 2 models in our study, with and without MRI findings, performed similarly.
Finally, the interpretation of prostate MP-MRI remains to be standardized. Although the NIH (National Institutes of Health) suspicion level scoring system has been validated,16 further work is necessary to construct and validate a structured reporting system that will be widely adopted across many institutions as the use of MP-MRI expands. The PI-RADS (Prostate Imaging Reporting and Data System) has been introduced in European cohorts for this purpose and may be adopted at other institutions as well.29
CONCLUSIONS
Despite advances in prostate imaging, MP-MRI continues to have low sensitivity for the detection of microfocal ECE by PCa. This suggests that some men may undergo RP after MRI that is a falsenegative for ECE, putting them at increased risk for positive surgical margins. Thus, further tools are necessary to stratify men at risk for occult ECE. In this study we demonstrated that MRI/TRUS fusion guided biopsy Gleason score can help identify which men with PCa have ECE that may not be detectable by imaging. Such knowledge may improve urologists’ preoperative planning, possibly reducing rates of surgical margin involvement.
Acknowledgments
Supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research and Center for Interventional Oncology. The NIH and Philips Healthcare have a cooperative research and development agreement, and share intellectual property in the field. Also supported by the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and contributions to the Foundation for the NIH from Pfizer Inc., the Doris Duke Charitable Foundation, Alexandria Real Estate Equities, Inc. and Mr. and Mrs. Joel S. Marcus, and the Howard Hughes Medical Institute, as well as other private donors. For a complete list visit http://fnih.org/work/education-training-0/medicalresearch-scholars-program.
Abbreviations and Acronyms
- DCE
dynamic contrast enhanced
- ECE
extracapsular extension
- MP
multiparametric
- MRI
magnetic resonance imaging
- PCa
prostate cancer
- PSA
prostate specific antigen
- RP
radical prostatectomy
- TRUS
transrectal ultrasound
References
- 1.Ung JO, Richie JP, Chen MH, et al. Evolution of the presentation and pathologic and biochemical outcomes after radical prostatectomy for patients with clinically localized prostate cancer diagnosed during the PSA era. Urology. 2002;60:458. doi: 10.1016/s0090-4295(02)01814-9. [DOI] [PubMed] [Google Scholar]
- 2.Hull GW, Rabbani F, Abbas F, et al. Cancer control with radical prostatectomy alone in 1,000 consecutive patients. J Urol. 2002;167:528. doi: 10.1016/S0022-5347(01)69079-7. [DOI] [PubMed] [Google Scholar]
- 3.Hricak H, Wang L, Wei DC, et al. The role of preoperative endorectal magnetic resonance imaging in the decision regarding whether to preserve or resect neurovascular bundles during radical retropubic prostatectomy. Cancer. 2004;100:2655. doi: 10.1002/cncr.20319. [DOI] [PubMed] [Google Scholar]
- 4.D’Amico AV, Schnall M, Whittington R, et al. Endorectal coil magnetic resonance imaging identifies locally advanced prostate cancer in select patients with clinically localized disease. Urology. 1998;51:449. doi: 10.1016/s0090-4295(97)00630-4. [DOI] [PubMed] [Google Scholar]
- 5.Fütterer JJ, Engelbrecht MR, Jager GJ, et al. Prostate cancer: comparison of local staging accuracy of pelvic phased-array coil alone versus integrated endorectal-pelvic phased-array coils. Local staging accuracy of prostate cancer using endorectal coil MR imaging. Eur Radiol. 2007;17:1055. doi: 10.1007/s00330-006-0418-8. [DOI] [PubMed] [Google Scholar]
- 6.Ruprecht O, Weisser P, Bodelle B, et al. MRI of the prostate: interobserver agreement compared with histopathologic outcome after radical prostatectomy. Eur J Radiol. 2012;81:456. doi: 10.1016/j.ejrad.2010.12.076. [DOI] [PubMed] [Google Scholar]
- 7.Rais-Bahrami S, Turkbey B, Grant KB, et al. Role of multiparametric magnetic resonance imaging in the diagnosis of prostate cancer. Curr Urol Rep. 2014;15:387. doi: 10.1007/s11934-013-0387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raskolnikov D, George AK, Rais-Bahrami S, et al. Multiparametric magnetic resonance imaging and image-guided biopsy to detect seminal vesicle invasion by prostate cancer. J Endourol. 2014;28:1283. doi: 10.1089/end.2014.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turkbey B, Mani H, Shah V, et al. Multiparametric 3T prostate magnetic resonance imaging to detect cancer: histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. J Urol. 2011;186:1818. doi: 10.1016/j.juro.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Somford DM, Hamoen EH, Futterer JJ, et al. The predictive value of endorectal 3 Tesla multiparametric magnetic resonance imaging for extraprostatic extension in patients with low, intermediate and high risk prostate cancer. J Urol. 2013;190:1728. doi: 10.1016/j.juro.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 11.Shakir NA, George AK, Siddiqui MM, et al. Identification of threshold prostate specific antigen levels to optimize the detection of clinically significant prostate cancer by magnetic resonance imaging/ultrasound fusion guided biopsy. J Urol. 2014;192:1642. doi: 10.1016/j.juro.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinto PA, Chung PH, Rastinehad AR, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol. 2011;186:1281. doi: 10.1016/j.juro.2011.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raskolnikov D, Rais-Bahrami S, George AK, et al. The role of image guided biopsy targeting in patients with atypical small acinar proliferation. J Urol. 2015;193:473. doi: 10.1016/j.juro.2014.08.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raskolnikov D, Rais-Bahrami S, Turkbey B, et al. Current ability of multiparametric prostate magnetic resonance imaging and targeted biopsy to improve the detection of prostate cancer. Urol Pract. 2014;1:13. doi: 10.1016/j.urpr.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turkbey B, Pinto PA, Mani H, et al. Prostate cancer: value of multiparametric MR imaging at 3 T for detection–histopathologic correlation. Radiology. 2010;255:89. doi: 10.1148/radiol.09090475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rais-Bahrami S, Siddiqui MM, Turkbey B, et al. Utility of multiparametric magnetic resonance imaging suspicion levels for detecting prostate cancer. J Urol. 2013;190:1721. doi: 10.1016/j.juro.2013.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong CW, Rais-Bahrami S, Walton-Diaz A, et al. Comparison of magnetic resonance imaging and ultrasound (MRI-US) fusion-guided prostate biopsies obtained from axial and sagittal approaches. BJU Int. :2014. doi: 10.1111/bju.12871. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Partin AW, Mangold LA, Lamm DM, et al. Contemporary update of prostate cancer staging nomograms (Partin tables) for the new millennium. Urology. 2001;58:843. doi: 10.1016/s0090-4295(01)01441-8. [DOI] [PubMed] [Google Scholar]
- 19.Mullerad M, Hricak H, Kuroiwa K, et al. Comparison of endorectal magnetic resonance imaging, guided prostate biopsy and digital rectal examination in the preoperative anatomical localization of prostate cancer. J Urol. 2005;174:2158. doi: 10.1097/01.ju.0000181224.95276.82. [DOI] [PubMed] [Google Scholar]
- 20.Ouzzane A, Puech P, Lemaitre L, et al. Combined multiparametric MRI and targeted biopsies improve anterior prostate cancer detection, staging, and grading. Urology. 2011;78:1356. doi: 10.1016/j.urology.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 21.Sundi D, Kryvenko ON, Carter HB, et al. Pathological examination of radical prostatectomy specimens in men with very low risk disease at biopsy reveals distinct zonal distribution of cancer in black American men. J Urol. 2014;191:60. doi: 10.1016/j.juro.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Volkin D, Turkbey B, Hoang AN, et al. Multiparametric magnetic resonance imaging (MRI) and subsequent MRI/ultrasonography fusionguided biopsy increase the detection of anteriorly located prostate cancers. BJU Int. 2014;114:E43. doi: 10.1111/bju.12670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinthus JH, Witkos M, Fleshner NE, et al. Prostate cancers scored as Gleason 6 on prostate biopsy are frequently Gleason 7 tumors at radical prostatectomy: implication on outcome. J Urol. 2006;176:979. doi: 10.1016/j.juro.2006.04.102. [DOI] [PubMed] [Google Scholar]
- 24.Walker R, Lindner U, Louis A, et al. Concordance between transrectal ultrasound guided biopsy results and radical prostatectomy final pathology: are we getting better at predicting final pathology? Can Urol Assoc J. 2014;8:47. doi: 10.5489/cuaj.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siddiqui MM, Rais-Bahrami S, Truong H, et al. Magnetic resonance imaging/ultrasound-fusion biopsy significantly upgrades prostate cancer versus systematic 12-core transrectal ultrasound biopsy. Eur Urol. 2013;64:713. doi: 10.1016/j.eururo.2013.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rastinehad AR, Turkbey B, Salami SS, et al. Improving detection of clinically significant prostate cancer: magnetic resonance imaging/transrectal ultrasound fusion guided prostate biopsy. J Urol. 2014;191:1749. doi: 10.1016/j.juro.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sonn GA, Natarajan S, Margolis DJ, et al. Targeted biopsy in the detection of prostate cancer using an office based magnetic resonance ultrasound fusion device. J Urol. 2013;189:86. doi: 10.1016/j.juro.2012.08.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wysock JS, Rosenkrantz AB, Huang WC, et al. A prospective, blinded comparison of magnetic resonance (MR) imaging-ultrasound fusion and visual estimation in the performance of MR-targeted prostate biopsy: the PROFUS trial. Eur Urol. 2014;66:343. doi: 10.1016/j.eururo.2013.10.048. [DOI] [PubMed] [Google Scholar]
- 29.Barentsz JO, Richenberg J, Clements R, et al. ESUR prostate MR guidelines 2012. Eur Radiol. 2012;22:746. doi: 10.1007/s00330-011-2377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


