Abstract
Harmonious interactions between radiation, medical, interventional and surgical oncologists, as well as other members of multidisciplinary teams, are essential for the optimization of patient care in oncology. This multidisciplinary approach is particularly important in the current landscape, in which standard-of-care approaches to cancer treatment are evolving towards highly targeted treatments, precise image guidance and personalized cancer therapy. Herein, we highlight the importance of multidisciplinarity and interdisciplinarity at all levels of clinical oncology training. Potential deficits in the current career development pathways and suggested strategies to broaden clinical training and research are presented, with specific emphasis on the merits of trainee involvement in functional multidisciplinary teams. Finally, the importance of training in multidisciplinary research is discussed, with the expectation that this awareness will yield the most fertile ground for future discoveries. Our key message is for cancer professionals to fulfil their duty in ensuring that trainees appreciate the importance of multidisciplinary research and practice.
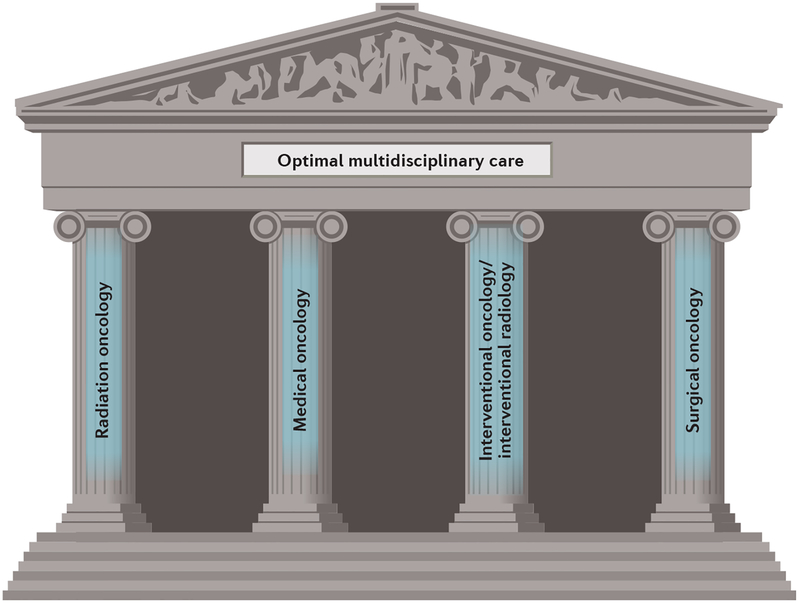
Communication across different specialties remains a core element of cancer care. Communication skills are important in establishing a good relationship with patients, but in the increasingly complex field of cancer treatment, oncologists from each specialty need to be equally skilled at communicating with, and learning the art of, those focused on other specialties. One current view is that four ‘pillars’ exist in oncology: radiation oncology, medical oncology, interventional oncology and surgical oncology, with some degree of interdependence between all four disciplines (FIG. 1). Nearly all patients will come into contact with clinicians practising one or more of these specialties during their cancer care continuum. To achieve the best outcomes for patients, expertise relating to these four pillars needs to be integrated and combined sensibly, and all treatment options need to be considered in order to provide an optimal care pathway for each patient. This approach should also drive innovation and efficient use of health-care resources across populations.
Figure 1 |. The four pillars of oncology.
The importance of all four principal oncology specialties is depicted.
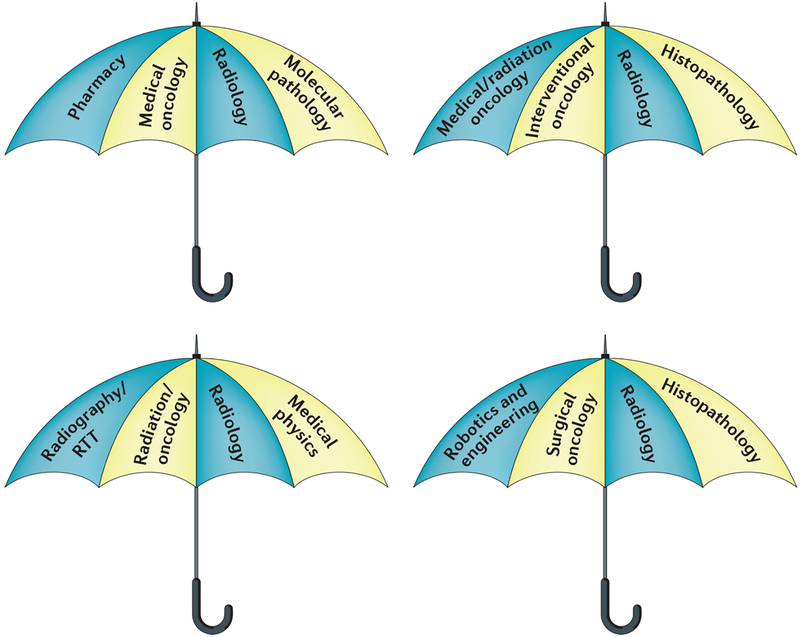
The length and scope of oncology training varies between different health-care systems, but typically specialization in radiation and medical oncology requires 4–5 years of clinical training to obtain accreditation from a national regulatory body, such as the Royal College of Radiologists (RCR) in the UK, or the American Board of Internal Medicine (ABIM) in the USA. Surgical oncology and interventional oncology training can vary in duration between 4–8 years. These timescales for clinical training do not include time taken out of specialist training to undertake research or a higher degree. Trainees usually follow a systems-based or organ-based syllabus to study different tumour sites and practical procedures (particularly in the specialties of radiation, surgical and interventional oncology). The proportion of oncology trainees undertaking full-time research varies widely between countries and regions. This research can be either laboratory-based or clinical, and can lead to the award of a higher research degree or to securing medium-term placements to learn specific skills, such as stereotactic radiotherapy or radiofrequency ablation (FIG. 2).
Figure 2 |. Examples of functional teams that can benefit from strong interdisciplinary collaboration.
Each umbrella shows an example of a functional team required to deliver the optimal package of care. All four specialties are equally important, although one speciality should be tasked with coordinating multidisciplinary work for each of the teams.
Similar to the patterns observed for many medical specialties, the proportion of female trainees and specialists in oncology has increased over the years. The ASCO State of Cancer Care in America report1 notes that the proportion of women in all oncology specialties continues to rise and, in 2015, 46% of trainees in oncology fellowship programmes were women. In the UK, the RCR 2014 workforce census2 recorded that 65% of clinical oncology trainees were women, compared with 46%of consultants at that time. Of note, 25% of consultants included in the census worked less than full time, with this figure rising to 40% for female consultants. As well as having implications for workforce planning, this disparity highlights the need to ensure training, academic work and family circumstances are balanced, to enable each individual to achieve a work–life balance that maximizes satisfaction and productivity, while meeting patients’ care needs. General surgery is attracting a growing proportion of female trainees around the world, although they remain a minority compared with male surgical trainees; this gender gap is even more pronounced for non-white female trainees3. In the UK, the proportion of female surgical trainees increased from 15% in 2009 (REF. 4) to 28% in 2013 (REF. 5).
Cancer services are encountering substantial challenges in the current health-care climate, which relate to rapid advances in the development of novel therapies, escalating costs of interventions and ageing populations6. Current financial constraints limit access to cancer therapies, and shortfalls in the required numbers of trained oncologists are expected in several countries owing to poor workforce planning7. In order to achieve the best possible care for all patients, the current deficits inthe training of cancer specialists must be recognized, and strategies for optimized multidisciplinary training should be defined.
Clinical training
Clinical training should provide oncologists with a standard toolkit with which to approach the care of all patients with cancer; this toolkit should be tailored to each discipline’s common and local practice. Data-driven clinical trials should be a driving force for progress in clinical oncology; training in regulatory and clinical trial science and administration will facilitate the incorporation of clinical trials into oncology care in the future. Academic translational efforts in conducting phase I–III oncology drug trials should be combined with innovations in medical devices, and an increasing proportion of clinical trials should be focused on a rational methodology for combining drugs, devices, radiotherapy and imaging guidance for local therapy. An example is the phase I–III clinical trial advancement of 90Y microspheres combined with chemotherapy, which has been achieved by radiation oncologists, medical oncologists and interventional oncologists working collaboratively8,9. Such demonstrations of academic multidisciplinarity in practice send a clear message to trainees on how collaboration can strengthen both research and clinical practice.
The current reality is that clinical research is often conducted within independent silos of research, clinical collaborations and conferences, with different specialties having varying levels of appreciation of emerging therapies in other disciplines. A basic understanding of clinical and translational research should be mandated as a part of all training programmes — for example, as part of the ‘core competencies’ governed by the Accreditation Council for Graduate Medical Education (ACGME) in the USA10. By making this aspect of training mandatory, more oncologists will become familiar with the ethics and regulatory science of clinical trials.
Several professional organizations have a multidisciplinary teaching role (BOX 1). These initiatives are breaking new ground by developing cross-speciality courses, but further developments are required. Most innovation in cancer care continues to originate from specialist organizations, such as the European Organisation for Research and Treatment of Cancer or the Radiation Therapy Oncology Group, which generally remain predominantly oncology-focused and specialty-specific. Because innovation in academia has run along speciality-specific tracks, innovation in industry has tended to proceed in parallel rather than in synergy and hence, academic training, detached from from commercial partners, has generally not benefited from the expertise of industry. Improvements in cross-specialty integration with industry partners could potentially offer the ability to steer innovation and development of new products, and to better integrate them into multidisciplinary care pathways.
Box 1 |. Professional organizations supporting multidisciplinary oncology training.
Multidisciplinary meetings
The British Uro-Oncology Group organizes annual meetings (predominantly for medical and clinical oncologists) and regional training sessions, and is involved in guideline development
The British Thoracic Oncology Group organizes an annual meeting, and assumes a teaching and advocacy role across lung cancer disciplines
Multidisciplinary learning
The ECCO-AACR-EORTC-ESMO Workshop on Methods in Clinical Cancer Research (formerly known as Flims) includes European and US experts from all oncological specialties as wells as clinical trial experts, statisticians and radiologists. The workshop provides a fertile environment in which clinical trial ideas can be considered from multiple expert perspectives
The ESMO school runs international multidisciplinary courses to enable trainees to learn effectively from and alongside other professionals
International organizations
UK national organizations
The Clinical and Translational Radiotherapy Research Working Group (CTRad) is split into different workstreams aiming to deliver a cohesive response to the challenges of implementing both new technologies57,58 and new trials relating to radiotherapy. In addition, they provide a forum for trainees to confidentially discuss a new clinical trial idea with a panel of experts
In the Radiotherapy–Drug Combinations Consortium (RaDCom), leading UK laboratory researchers collaborate with the aim of delivering high-quality preclinical projects, which should inform subsequent clinical research on radiotherapy–drug combinations
Industry collaborations
Combined research agenda
Collaborations between academic centres supported by an industry partner
Synergistic research and development work
Financial, organizational and academic support for meetings and learning opportunities
AACR, American Association for Cancer Research; ECCO, European CanCer Organization; EORTC, European Organisation for the Research and Treatment of Cancer.
Radiation oncology
Radiation oncology is a specialty focused on the assessment of patients receiving radiotherapy, and the technical design, delivery and overall optimization of these therapeutic approaches. In the UK and other countries, many oncologists are dually trained in radiation oncology and medical oncology, in a discipline termed ‘clinical oncology’. Specialization in radiation or clinical oncology in the UK, USA, Australia and New Zealand typically requires 5 years of training designed to impart knowledge of the physics, anatomical and pharmacological aspects that are integral to these disciplines. Proficiency in these areas is tested through formal examinations, such as those conducted by the RCR in the UK or by the Royal Australian and New Zealand College of Radiologists. Research is not mandatory in most training schemes, but is increasingly encouraged to foster a better understanding of the changing therapeutic landscape in oncology.
In all countries, radiation oncology trainees benefit from a multidisciplinary approach involving close liaison with radiographers and radiation therapists (allied health professionals who deliver the radiotherapy), and physicists and dosimetrists (who plan the technical aspects of treatment). However, because current treatments are commonly guided by daily imaging scans, radiation oncology trainees are increasingly required to receive advanced training in radiology. The most frequently used imaging modality is CT, but magnetic resonance (MR)-based approaches are also being developed11. In the absence of a radiation oncologist with the necessary advanced skill levels, including those relating to novel imaging techniques12, 13, the recurrence rates of patients with cancer might increase, owing to inadequate tumour delineation or ‘geographical misses’. The faculty at Duke University Medical Centre have therefore designed a specialist radiology training programme for its radiation oncology residents14. An unmet need currently exists for this type of training in many oncology subspecialties: in the 2013 Annual Survey of UK Clinical Oncology trainees, only 2.6% reported they had received formal radiology training from a radiologist, and 35% had ‘self-taught’ the radiology skills they needed in clinical practice15. Faculty in charge of future training of radiation oncologists should identify potential synergies with radiology teaching programmes, in order to improve the training in both specialties.
Trainees in radiation oncology also need to learn how to respect and work with interventional radiologists and medical oncologists. New paradigms in radiation treatment, such as the radical treatment of oligometastases with stereotactic body radiotherapy16,17, require close integration between oncologists from a range of disciplines who also have the ability to deliver ablative treatments, to ensure that the patient receives the most appropriate treatment. For example, radiofrequency ablation18, which can be delivered by interventional or surgical oncologists, is an alternative to stereotactic body radiotherapy for some patients. Thus, all oncologists need to develop a good understanding of alternative ablative treatments (such as microwave, laser, cryoablation, or focused ultrasonography for thermal ablation) in order to optimize care for patients. Likewise, combinations of targeted agents (such as sunitinib) with radiotherapy might result in improvements in cancer control. Hence, close collaboration between radiation oncologists and medical oncologists is required to maximise the benefits of radiotherapy in combination with other therapeutic modalities, and to minimize the risk of adverse events associated with treatment modalities that might potentiate the toxic effects of radiotherapy19.
Research within clinical training programmes.
Access to full-time research fellowships during training is limited and highly competitive. In most health-care systems, dedicated research time is not contemplated as a priority in training programmes. Indeed, data from a small survey indicate that trainees who do not conduct full-time research publish an average of less than one peer-reviewed article before becoming a consultant20, thus supporting our view that, for academic outputs to flourish, the allocation of dedicated research time within training is required. Since 2005, trainees in radiation oncology in Australia and New Zealand have been mandated to complete at least one piece of independent research of ‘publishable quality’ as part of their training, although this requirement is not supported by allocation of dedicated research time. A survey of 116 trainees in these countries published in 2014 revealed that 53% had published research in a peer-reviewed journal, and 59% had presented their work at an international meeting21. For some of these trainees, encouragement to conduct academic work could foster career-long enthusiasm for research; thus, the effectiveness of other training schemes might be improved using this approach.
In order to pursue high-quality research, however, individuals need dedicated research time, both during training and after certification as a specialist. For future oncologists to run practice-changing trials, research training needs to be provided. In the USA, the need for a formal clinical trial training programme has been identified in response to data showing that many trainees lack confidence in their ability to design a clinical trial22. In the UK, the Academic Clinical Fellow (ACF) clinical oncology training programme incorporates dedicated time for research, enabling trainees to spend time in the laboratory or on a full-time clinical research project23. In the Netherlands, all trainees spend at least 10% of their residency time engaged in full-time research, and many already have completed a PhD in a related discipline before starting their radiation oncology training.
Medical oncology
When ESMO was first established in1975, its founders defined seven core principles24 (BOX 2), many of which focused on the importance of multidisciplinary care. These principles should remain a driving force in medical oncology training, because they reflect the current key areas of training: clinical and translational research, acquisition of clinical skills, and the ability to establish effective and fit-for-purpose clinical and research networks.
Box 2 |. Principles of ESMO22.
To improve the quality of prevention, diagnosis, treatment, supportive and palliative care, as well as the follow-up monitoring of patients with malignant disorders
To advance the art, science, recognition and practice of oncology
To disseminate knowledge in oncology to patients with cancer and the public
To educate and train persons involved in clinical cancer care and research
To promote education in oncology in order to ensure a high standard of qualification of medical oncologists within the multidisciplinary team
To facilitate equal access to optimal cancer care to all patients with cancer
To maintain liaisons with other oncology specialties, cancer leagues, universities, patient groups and, if appropriate, the pharmaceutical industry
Clinical training programmes.
Medical students considering a career in medical oncology often gain only limited experience in this discipline at medical school. In Europe, both ESMO and the European School of Oncology (ESO) have recognized this problem and currently run a joint 5-day residential course that is available to medical students who wish to gain more insight into medical oncology25. By offering this intensive educational programme, ESMO and ESO aim to motivate medical students to commit to working for the benefit of patients with cancer in the fast-evolving field of medical oncology.
In most countries, including the UK, training of medical oncologists is structured as a 4-year specialist programme, with trainees rotating through a variety of supervised posts, in which they are involved in caring for patients with common tumour types (such as gastrointestinal, breast or lung cancers), to cover the core syllabus set out by the training boards. The introduction of postgraduate structured exams by the UK Royal College of Physicians (RCP), ESMO and the ABIM has enabled the core syllabi to gain international recognition, and guarantees that accredited medical trainees obtain a uniform level of clinical knowledge before completing their training.
The future needs of medical services are difficult to predict, but undoubtedly the demands placed on cancer services as a whole will increase as a result of demographic changes, such as ageing, worldwide. This demographic trend will also necessitate a broader understanding of the additional challenges relating to cancer care specifically in elderly patients. Joint training programmes in geriatrics and oncology do exist worldwide, but their numbers and availability remain limited. The need for such training was first identified in the USA in the 1990s26, and the introduction of similar programmes in some European countries (for example, France) followed shortly after27. Both ASCO28 and ESMO29 have now published specific guidelines on cancer therapies for the elderly, an international society has been established30, and pilot schemes exist to address the unmet training needs related to this previously under-represented area. Certain countries, including the UK, might have fallen behind by taking less proactive approaches than those adopted in the USA and in several European countries (among others)31; this need could be addressed by including tools for assessment of geriatric patients in all training schemes32.
Although not considered traditional pillars of cancer care, clinical genetics, immunology and molecular pathology are specialties that have become critical to delivering patient-centric cancer care. Over the past decade, the proliferation of tissue-based and blood-based biomarkers of prognosis or a response to treatment has increased dramatically, for example, in non-small-cell lung cancer33. Patient stratification on the basis of mutations in cancer-related genes is now a reality in clinical trials, and molecular criteria determine patients’ access to treatments outside of clinical trials. The expanding use of immunotherapies, particularly immune-checkpoint inhibitors, in clinical practice means that knowledge of immunology is becoming increasingly important for all oncologists to evaluate the full range of treatment options available and to manage the adverse events associated with immunotherapies34,35.
Palliative care is another important discipline, for which the approach to training varies widely between countries. In many respects, training in palliative care gives oncologists a broader perspective on patient care than any of the other disciplines because they have to learn about managing pain and other symptoms, as well as the importance of considering the patient’s physical, psychosocial and spiritual wellbeing. Palliative care is delivered in a variety of settings, including hospices, hospitals and the community, which creates challenges in training oncologists adequately in this discipline. Despite this potential barrier, the management of symptoms in patients with cancer is acknowledged as a key aspect of training; thus, the Joint RCP Training Board in the UK has prioritized training in palliative care within the medical oncology curriculum36.
Improving trainee access to clinical and translational research.
Traditionally, medical oncologists have tended to have more opportunities to perform clinical and translational research than other oncologists, and approximately 75% of medical oncology trainees will undertake a higher research degree37. In the current global financial climate, however, securing funding for such endeavours has become increasingly challenging for clinicians. In the UK, most funding for junior clinical fellowships comes from cancer charities, such as Cancer Research UK (CRUK), or from large medical research charities, such as the Medical Research Council and the Wellcome Trust. In the USA, early career clinicians are eligible for prestigious government-sponsored funding opportunities, such as the NIH Mentored Clinical Scientist Research Career Development Award (K08)38, Early Investigator Award, and Patient-Oriented Research Career Development Award (K23)39, which enable clinicians to dedicate a 3–5 year period of their career to intense, supervised research as a pathway to becoming an autonomous researcher, and with the ultimate aim of educating a future generation of NIH researchers.
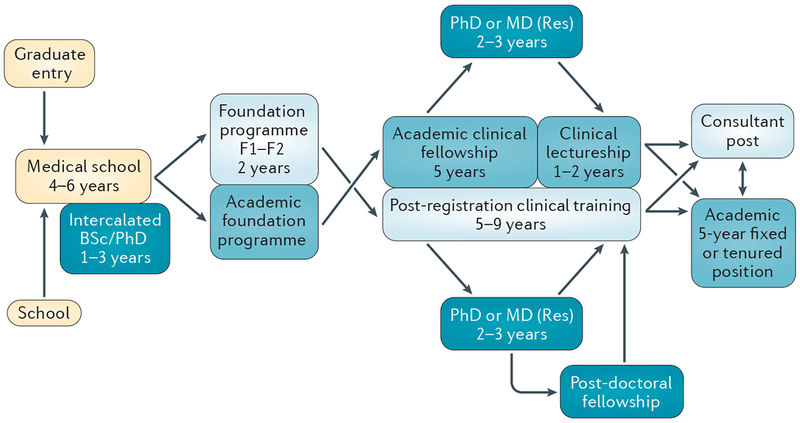
Competition for obtaining these early career grants is fierce and, somewhat paradoxically, previous experience in laboratory research considerably improves the chances of a successful application. Without funding, trainees often cannot leave clinical training positions to gain experience in the laboratory. As mentioned previously, the UK ACF programme (FIG. 3) might better prepare trainees for the competitive world of academic medicine, and should, therefore, be made available to an increasing number of trainees.
Figure 3 |. Academic career pathways for oncology.
The progression of oncology trainees and the opportunities to gain academic experience during training is shown from left to right. The terminology shown is specific to the UK, but principles of training are common across Europe and North America. Approximate timescales are shown as examples, but exact timescales vary between institutions.
Clinical research is a valuable component of training that, unfortunately, is often incorporated into clinical training programmes in an ad hoc manner. Few trainees have the opportunity to work in large early clinical trials units, but such experience is an important aspect of the professional development of future oncologists. Access to local research networks and first-hand experience in trial units will assist oncologists in the referral of well-selected patients for enrolment in clinical trials. If fellowships in such units are not available, trainees should have access to local research networks, and the opportunity to participate in multidisciplinary forums for local research, such as a the pan-UK Cancer Research Network40.
Interventional oncology
Despite the current global challenges to health care capacity, vascular and interventional radiology (VIR) with specific application to cancer care has flourished as an emerging specialty that is closely connected to the other three pillars of oncology. Previously, these types of interventional procedures were mainly associated with symptomatic control and palliative or supportive care but, at present, interventional oncology is increasingly used with the goals of increasing the survival and/or cure rates of patients with certain types of cancer41, such as liver cancer or renal cell carcinoma42, which can be achieved using cryoablation or thermal ablation with radiofrequency, microwave or laser electromagnetic radiation. Interventional oncology has become a vibrant and dynamic component of most interventional radiology practices, aimed at standardizing the use of multidisciplinary personalized therapies.
The Society of Interventional Radiology (SIR) in the USA43, and the Cardiovascular and Interventional Radiology Society of Europe44 have promoted the adoption and harmonization of clinical practice guidelines and reporting standards, and organized training symposiums and workshops to discuss paradigms in interventional oncology. In addition, education and cross-disciplinary training in minimally invasive, image-guided, local and regional delivery of cancer therapies has been the main goal of numerous large meetings and multidisciplinary conferences, such as the World Conference on Interventional Oncology45, European Conference on Interventional Oncology46, Synergy47, Symposium on Clinical Interventional Oncology48, and Interventional Oncology Sans Frontieres. These meetings promote multidisciplinary attendance, often by offering free or discounted registration to partners from the host institution.
Practitioners of the three other oncology subspecialities are increasingly recognizing the importance of interventional oncology. The RCR Sub-Faculty Board, for example, have stated that such training should be included in the clinical oncology curricula49. New techniques, such as selective internal radiotherapy treatment, require the skills of both oncologists and interventional radiologists for optimal patient selection, treatment and follow-up assessment50. Acquiring such skills can be difficult because a limited number of centres offer these services, and not every oncologist needs to be proficient in the techniques themselves. Nevertheless, a familiarity with interventional techniques will enable the appropriate selection of patients from all centres, thus improving access to these pioneering techniques.
Postgraduate training needs to evolve constantly in order to adapt to rapidly changing treatment paradigms. In previous decades, minimally invasive, image-guided therapies (for example, ablation and chemoembolization) have been increasingly integrated into the treatment algorithms for many cancers, with prominent roles for these modalities promulgated in National Comprehensive Cancer Network guidelines and various treatment algorithms for neoplasms of the liver, kidney, bone, and lung. These locally and regionally delivered interventional oncology therapies are most-commonly administered by physicians trained in VIR or interventional body imaging (a specialty within diagnostic radiology), who often lack formal independent dedicated or structured training in interventional oncology. The interventional radiology fellowship has traditionally been a 1-year fellowship completed after a radiology residency; however, this short-term fellowship alone is clearly not sufficient for a trainee to acquire the requisite clinical and technical skills, owing to the rapid expansion of this specialty, with enormous technological and scientific advances in areas including interventional oncology51. Traditionally, VIR specialists receive fellowship training encompassing interventional oncology after a diagnostic radiology residency, but the extent of the oncology training experience varies widely across VIR fellowships. In 2013, the US ACGME approved an independent residency pathway for VIR training and primary certification43. Future US trainees will be able to receive VIR and/or interventional oncology training via a fellowship, or through an integrated or independent VIR residency pathway. Thus, medical students can enter VIR residency directly, or via a VIR fellowship or residency after completing a diagnostic radiology residency (6–7 years of postgraduate training). The ‘Residents, Fellows and Student’ section of the SIR promotes training opportunities in interventional oncology at multiple levels52, and the Medical Student Council of the SIR serves future students interested in interventional oncology within interventional radiology25.
In the USA, clinical office-based care reimbursement coding for interventional radiology physicians increased by 1,200% between 1998–2008 (REF. 53). Interventional radiology is currently in transition to acquiring independent residency status, which will result in improved procedural and clinical training of interventional radiologists, without affecting the number of postgraduate years they spend in training (total 6–7 years). This transition will also enable medical students to enter directly into the interventional radiology specialty through multiple pathways. The most important aspect of this transition will be the increase in clinical training, because interventional radiology trainees will rotate with their surgical and medical colleagues in oncology and intensive care, among other subspecialties. Nevertheless, imaging, procedures, and nonprocedural clinical care remain the three basic facets of the interventional radiology training pathway53.
Interventional radiology has never been a more popular specialty among medical trainees than it is today. Between 2009–2013, the uptake of available fellowship positions increased from 54% to >90% in the USA54. Unfortunately, interventional radiology is not one of the six basic clinical rotations that all medical students must undertake in US medical schools, and only ≤25% of schools require a rotation in diagnostic radiology55. Surveys of medical students indicate that <1% of students require a VIR rotation. The results of studies performed in the USA, in several European countries and in Canada have demonstrated a general lack of knowledge of interventional radiology among medical students54. As interventional oncology becomes a larger discipline within interventional radiology, the exposure of students earlier in their careers to the existence and uniqueness of multidisciplinary interventional oncology is critical for this emerging discipline to assume a role in multidisciplinary team training. With the designation of specialty status for VIR by the American Board of Medical Specialties in the USA43 and the recognition of VIR as an emerging subspecialty of radiology in the UK41, further integration of interventional oncology into multidisciplinary care will maintain and even increase the strength of the fourth and newest pillar of cancer care.
Surgical oncology
One of the demands for contemporary training in surgery is to produce fully qualified surgeons who are aware of the particular needs associated with clinical oncology and who are capable of functioning within complex multidisciplinary teams. The expertise of surgeons should include a blend of technical ability (with subspecialty skills), knowledge of oncology treatments, and the capability of enrolling patients into randomized clinical trials in high volumes. For example, three quarters of the 41,000 newly diagnosed patients with colorectal cancer each year in the UK undergo surgical treatment for their cancer, but <10% of these enrol in a clinical trial56. Poor trial enrolment rates illustrate the need to formally improve provision and awareness of research within current surgical training programmes.
Technical skills in surgical oncology.
Surgical oncologists are trained to a high standard in the prevention, diagnosis, treatment and rehabilitation of patients with cancer. These principles are championed by two pan-specialty umbrella organizations, the Society of Surgical Oncology in North America and the British Association of Surgical Oncology in the UK. Trainee-led groups exist within these organizations, including the Surgical Oncological Trainee Association57, which promotes the needs and oncology-focused education of future surgeons.
Specialty-specific oncology training programmes are integrated into current surgical training programmes, which are supplemented by fellowships bridging the gap between senior trainees and established consultants58. Fellowships in laparoscopic surgery are provided for senior trainees with gynaecological, urological and colorectal expertise; these fellowships have international scope (for example, the European Society of Coloproctology (ESCP) offers pan-European fellowships in colorectal surgical and procedure skills). Similar high-quality training fellowships are available across all surgical oncology subspecialties, on aspects including oncoplastic breast surgery, international travelling fellowships for gastroesophageal resection, minimally invasive urological training and ocular oncology.
Surgical training needs to keep pace with the rapid evolution of new technologies and the introduction of multidisciplinary teams. For example, the British Association of Plastic, Reconstructive and Aesthetic Surgeons endorse courses in sentinel-lymph-node biopsy in patients with malignant melanoma, and simulator models have been developed for teaching sentinel-node biopsy of patients with breast cancer. Similar courses are delivered by the Cleveland Clinic and other large institutions specializing in oncology in the USA. Robot-assisted surgery for urological, neurosurgical, neck, gynaecological and colorectal cancers is evolving, and training courses and fellowships are already available. Current challenges in robot-assisted surgery include the achievement of cost-effectiveness and real patient benefits over use of conventional approaches. Assessment of the effects of these novel technologies on patient outcomes includes both registry-based commissioning and randomized clinical trials involving new technologies59, in concordance with the IDEAL Collaboration’s recommendations for evaluation of surgical innovation60. Currently, a limited number of patients access such programmes and, therefore, deficits in the tutelage of trainees in the importance of clinical and economic benefit assessment must be addressed.
Translational research skills.
All surgical trials face recruitment challenges59, thus illustrating a need to improve surgical oncology training not only in the UK, but also worldwide. Surgical training currently supports the development of skills required to conduct clinical trials among both dedicated academic and non-academic clinical trainees; hopefully, newly trained consultant surgeons emerging from these programmes will recruit patients into randomized trials as part of routine clinical practice, with the aim that every patient undergoing surgery should be offered the opportunity to partake in a trial. Indeed, this skillset is starting to be incorporated into training programmes, with Good Clinical Practice certification61 and the definition of milestones, such as a minimum number of recruited patients62. Trainee surgeons are supported within national cancer networks — for example, trainee members are part of the surgical subgroups of the UK National Cancer Research Institute. These members actively contribute to designing and conducting new trials. For example, the management groups of both the STAR-TREC and FOXTROT trials include surgical and oncological trainees who are shadowing their senior counterparts and are working together to contribute to funding applications63.
In the UK, surgical trainees have pioneered trainee-led research collaboratives64, which consist of regional and national networks of trainees connected by their annual rotations, and have been involved in planning and delivering multisite research65, with numerous benefits (BOX 3). General surgical networks have complete national coverage and now encompass all surgical subspecialties66. These networks have the ability to deliver both multicentre observational research and randomized clinical trials. For example, the randomized controlled ROSSINI trial42 was conducted across 21 hospitals to test the efficacy of a wound guard in preventing infection of the surgical site after major abdominal surgery. As a national portfolio trial in the UK, this trial was designed and conducted by surgical trainees, who recruited 760 patients ahead of schedule67. The MAStectomy Decisions Audit (MASDA) study is an ongoing multicentre observational study that is being led by breast surgery trainees; the aim of this study is to describe the current UK practice in multidisciplinary team decision-making for patients undergoing mastectomy68. Trainee networks can also expand into Europe, as exemplified by the cohort studies delivered by the ESCP, in which trainees participate in study design and conduct at each level.
Box 3 |. Benefits of participation in trainee-led research collaborations.
For trainees
An opportunity to acquire experience in research methodology and data analysis
‘Improved CV’ owing to inclusion of publications, presentations and posters
Acquisition of transferable skills, such as teamwork, leadership, management and/or public speaking
Experience in research administration
Helps trainees achieve excellence in training
For patients
Improved quality of research
Increased awareness of patients’ needs when trainees frame the research question
For research
Improved quality of research
Improved recruitment through trainees
Increased number of surgical studies
Increased multicentre collaboration
For region or training scheme
Improved reputation of scheme
Helps trainees achieve excellence in training
Increased research infrastructure in region
CV, curriculum vitae.
Medical students are the next generation of potential surgical researchers and, therefore, starting their collaborative and research training at an early stage of their careers would be advantageous. Such a strategy would immerse the prospective surgeons in the culture of multidisciplinary trials, thereby facilitating the identification of future surgical oncology leaders. In the UK, a national network involving students interested in surgical research has been granted funding by the Bowel Disease Research Foundation to train 40 senior medical students per year in the practical recruitment of patients into randomized trials69.This initiative will deliver a research-ready cohort of junior doctors across multiple specialties, who will subsequently transition into regional surgical research collaborations70.
Multidisciplinary working
The multidisciplinary team meeting
In many countries, multidisciplinary team meetings and tumour boards have become embedded in day-to-day clinical practice as a way of improving and standardizing treatment decision-making. In one international survey of practice in the care of patients with breast cancer, excluding those in the USA, 92% of respondents worked in a centre with a multidisciplinary team and, in more than half of the 39 countries surveyed in Eastern and Western Europe, multidisciplinary-team-led decision-making was mandatory71 and trainee attendance was encouraged. In these meetings, all newly diagnosed patients with cancer, and specifically the management plans for these patients, are typically discussed by teams comprising medical oncologists, radiation oncologists, surgical oncologists, radiologists, interventional radiologists, nuclear medicine physicians, histopathologists and, importantly, trainees in these disciplines. Representatives of all four pillars of oncology are present and, therefore, local standardization can be achieved while promoting a community-wide and culture-wide approach to treatment, and instilling in trainees the importance of contributions from experts in other disciplines. Indeed, mutual respect and cross-disciplinary understanding of the different treatment options offered by each specialty should be incentivised and highly valued, and facilitated by an exchange of data, ideas and new approaches to therapy.
Multidisciplinary teams provide an important setting for multidisciplinary learning to take place, but no evidence exists on the extent to which trainees benefit from this learning opportunity, or whether dissemination of knowledge relating to various specialties occurs broadly and optimally among trainees. The financial costs associated with these meetings are high72, but the meetings are credited with improving the outcomes of patients with cancer in the UK by reducing variations in clinical practice. Multidisciplinary team members believe that these meetings improve patient care and increase efficiency73. In addition, evidence exists supporting the hypothesis that conducting such team meetings leads to improved patient survival74. Involving patients in multidisciplinary team meetings has also been shown to be of benefit in terms of their quality of life75. Thus, the adoption of multidisciplinary teams can improve both the consistency and quality of patient care, and generate opportunities for multidisciplinary learning and exchange of knowledge.
Team working
Involvement of a multidisciplinary team is now an expected feature of publicly funded health research. Development of personal skills is a key feature in the training of capable professionals within the team; however, a shortfall in formal training of budding oncologists in communication skills is well documented76. In addition, the high rate of ‘burnout’ among health-care professionals involved in the treatment of patients with cancer is recognized77, and the constant development of new anticancer therapies requires a lifelong commitment to continued professional learning and development.
Multidisciplinary teams are at the heart of public–private partnerships, collaborations and think-tanks. Gone are the days when specialists in a single discipline (such as medical oncology) would develop a new drug without first collaborating with other health professionals. Funding bodies expect the integration of combination therapies, medical devices, radiotherapy and systemic therapy in order to understand the context of the treatment being proposed78.
Health-care systems globally must incorporate measures to reinforce mechanisms that reward multidisciplinary approaches to treating patients with cancer. In the era of precision medicine, the multispecialty approach can have numerous effects, from enabling patients to receive the most-appropriate treatment to delivering such treatments in a timely fashion. The best example of multidisciplinarity would be a combined specialty clinic, in which patients would be jointly examined by professionals from two or more specialties79. Decreased time to diagnosis and decreased patient anxiety levels, and increased patient satisfaction are achieved in multidisciplinary clinics compared with other settings75. Collaborative care might also inspire multidisciplinary research and quality assurance41. An example of such multidisciplinary research is provided by the PACE trial, in which participants with early-stage prostate cancer were randomly assigned to receive either surgery or stereotactic radiotherapy; recruitment has been shown to be possible despite considerable differences between these two modalities within a multidisciplinary clinical context, and quality assurance is an important consideration throughout the clinical trial80.
Conclusions
Cancer care has undergone a technological revolution over the past 10 years, evolving from general specialties using techniques that had not changed substantially for several decades into highly specialized fields, in which the pace of innovation threatens to outstrip the ability of medical professionals to educate their trainees in an integrated fashion. How these trainees are expected to keep up with advances relating to their own specialty, let alone the other three pillars of cancer care, is a pressing question that will only be addressed with the introduction of an integrative training system (BOX 4; TABLE 1).
Box 4 |. Proposed solutions to improve global oncology training.
Formal training in complementary oncology disciplines
Adoption of competency-based training programmes to allow time for multispecialty training and research exposure
Embed research training into all oncology training schemes
Leverage efficiencies of e-learning programmes (such as ASCO’s Education Essentials for Oncology Fellows programme), online modules and simulation strategies
Reimbursement of health-care costs should favour multidisciplinary clinics and multidisciplinary decision-making mechanisms
Conferences and training days should prioritize contents related to multidisciplinary learning
Table 1 |.
Unmet training needs and proposed solutions
| Unmet need | Proposed solution |
|---|---|
| Poor trainee knowledge of other pillars of oncology |
|
| Decision-making made in subspecialty silos |
|
| Inadequate exposure to research methodology and administration |
|
| Few opportunities to gain laboratory experience before PhD |
|
This is an exciting time for all cancer-related medical specialties, and great strides have been made towards improving the outcomes of patients. This progress must be matched by a drive to train the next generation of oncologists in an integrated way that prepares them for the challenges ahead. Improving the training in all four pillars of cancer care is achievable, and many of the most-striking opportunities require comprehensive knowledge of the other three pillars. For example, in radiation oncology, advanced technologies such as MR Linac11, proton therapy81 and molecular radiotherapy (for example, selective internal radiation therapy (SIRT)8 and 223Ra therapy82) must be paired with state-of-the-art imaging techniques, which require cross-disciplinary input from many professional groups. With the increased precision of cancer-targeting, a greater certainty of the location of the tumour and the area most at risk of recurrence is required. Functional imaging (such as multiparametric or whole-body diffusion-weighted MRI and PET83), including novel tracers, will be crucial to this effort. The extent to which clinical oncology training can evolve to rapidly accommodate new advances and techniques will be integral to the success of efforts to nurture future oncology leaders — these efforts must start at medical school via provision of students with opportunities to explore the oncological specialties.
To continue these fast-paced improvements in cancer treatment, academically minded oncologists in all four specialties of cancer care must drive forward innovation and research, and the integration of research and training needs to be promoted. The broad range of skills acquired during dedicated research time will not only enhance the future academic output of the trainee oncologists, but the analytical and logical ways of thinking that such schemes promote will also augment the ability of health-care systems to manage and implement changes.
Cancer care requires mutual interdependence between a wide range of multidisciplinary colleagues, and the adoption of multidisciplinary team meetings is key to delivering the best possible care. Research, training, and patient care benefit from a multidisciplinary approach, and adoption of this approach will ensure that trainees of all four specialties in clinical oncology are ready to face the new challenges ahead together.
Acknowledgements
A.C.T. receives support from the NIHR RMH/ICR Biomedical Research Centre. V.K. and B.J.W. are supported by the NIH Center for Interventional Oncology, The National Cancer Institute, and the Intramural Research Program of the National Institutes of Health. R.A.S. is supported by Cancer Research UK, the CRUK-EPSRC UCL Cancer Imaging Centre and the NIHR University College London Hospitals Biomedical Research Centre. The opinions expressed herein are those of the authors in their personal capacity and do not necessarily represent those of any institution, the National Institutes of Health, nor any government entity.
Competing interests statement
A.C.T. receives research funding from Accuray, Elekta and MSD, and has received travel support or honoraria from Astellas, Bayer and Janssen. R.A.S. has received research funding from Sirtex Technology and consultation fees from Affidea, Astra Zeneca, BTG, Cancer Research Technology, Eisai, Sirtex Medical, Varian and Vertex. The other authors declare no competing interests.
Contributor Information
Alison C. Tree, Royal Marsden NHS Foundation Trust and the Institute of Cancer Research, Downs Road, Sutton, Surrey SM2 5PT, UK.
Victoria Harding, Division of Cancer, ICTEM Hammersmith Campus, Du Cane Road, London W12 0NN, UK..
Aneel Bhangu, Academic Department of Surgery, Room 29, 4th Floor, Queen Elizabeth Hospital, Edgbaston, Birmingham B15 2TH, UK..
Venkatesh Krishnasamy, Center for Interventional Oncology, National Cancer Institute and NIH Clinical Center, National Institutes of Health, 10 Center Drive, Bethesda, Maryland 20814, USA..
Dion Morton, Academic Department of Surgery, Room 29, 4th Floor, Queen Elizabeth Hospital, Edgbaston, Birmingham B15 2TH, UK..
Justin Stebbing, Imperial College/Imperial Healthcare NHS Trust, Charing Cross Hospital, 1st Floor, E Wing, Fulham Palace Road, London, W6 8RF, UK;; Division of Cancer, ICTEM Hammersmith Campus, Du Cane Road London W12 0NN, UK.
Bradford J. Wood, Center for Interventional Oncology, National Cancer Institute and NIH Clinical Center, National Institutes of Health, 10 Center Drive, Bethesda, Maryland 20814, USA.
Ricky A. Sharma, NIHR University College London Hospitals Biomedical Research Centre, UCL Cancer Institute, University College London, London WC1E 6DD, UK.
References
- 1.American Society of Clinical Oncology. The state of cancer care in America 2015. ASCO http://www.asco.org/sites/www.asco.org/files/2015ascostateofcancercare.pdf (2015). [DOI] [PubMed] [Google Scholar]
- 2.The Royal College of Radiologists. Clinical Oncology UK workforce census 2014 report. RCR https://www.rcr.ac.uk/sites/default/files/publication/bfco152_census.pdf (2014). [Google Scholar]
- 3.Frohman HA et al. The nonwhite woman surgeon: a rare species. J. Surg. Educ 72, 1266–1271 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Royal College of Surgeons. Women in surgery, submission to House of Commons Science and Technology Committee, August 2013. RCS https://www.rcseng.ac.uk/about-the-rcs/government-relations-and-consultation/position-statements-and-reports/surgical-profession/ (2013). [Google Scholar]
- 5.Royal College of Surgeons. Women in surgery — statistics. RCS https://www.rcseng.ac.uk/careers-in-surgery/women-in-surgery/mission-and-goals/women-surgeon-statistics/ (2015). [Google Scholar]
- 6.International Agency for Research on Cancer. GLOBOCAN 2012. Estimated cancer incidence, mortality and prevalence worldwide in 2012. IARC http://globocan.iarc.fr/old/burden.asp?selection_pop=207840&Text-p=United+States+of+America&selection_cancer=290&Text-c=All+cancers+excl.+non-melanoma+skin+cancer&pYear=8&type=0&window=1&submit=%C2%A0Execute%C2%A0 (2013). [Google Scholar]
- 7.Datta NR, Samiei M & Bodis S Radiotherapy infrastructure and human resources in Europe — present status and its implications for 2020. Eur. J. Cancer 50, 2735–2743 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Sharma RA et al. Radioembolization of liver metastases from colorectal cancer using yttrium-90 microspheres with concomitant systemic oxaliplatin, fluorouracil, and leucovorin chemotherapy. J. Clin. Oncol 25, 1099–1106 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Dutton SJ et al. FOXFIRE protocol: an open-label, randomised, phase III trial of 5-fluorouracil, oxaliplatin and folinic acid (OxMdG) with or without interventional Selective Internal Radiation Therapy (SIRT) as first-line treatment for patients with unresectable liver-only or liver-dominant metastatic colorectal cancer. BMC Cancer 14, 497 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Accreditation Council for Graduate Medical Education & The American Board of Radiology. The Radiation Oncology Milestone Project. ACGME https://www.acgme.org/acgmeweb/Portals/0/PDFs/Milestones/RadiationOncologyMilestones.pdf (2015). [Google Scholar]
- 11.Lagendijk JJ, Raaymakers BW & van Vulpen M The magnetic resonance imaging-linac system. Semin. Radiat. Oncol 24, 207–209 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Thorwarth D Functional imaging for radiotherapy treatment planning: current status and future directions — a review. Br. J. Radiol 88, 20150056 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bird D et al. Multimodality imaging with CT, MR and FDG-PET for radiotherapy target volume delineation in oropharyngeal squamous cell carcinoma. BMC Cancer 15, 844 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chino J, Doyle S & Marks LB The anatomy of radiation oncology residency training. Int. J. Radiat. Oncol. Biol. Phys 88, 3–4 (2014). [DOI] [PubMed] [Google Scholar]
- 15.The Royal College of Radiologists. The timely delivery of radical radiotherapy: standards and guidelines for the management of unscheduled treatment interruptions. RCR https://www.rcr.ac.uk/system/files/publication/field_publication_files/BFCO(08)6_Interruptions.pdf (2008). [Google Scholar]
- 16.Tree AC et al. Stereotactic body radiotherapy for oligometastases. Lancet Oncol. 14, e28–e37 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Aitken K et al. Initial UK experience of stereotactic body radiotherapy for extracranial oligometastases: can we change the therapeutic paradigm? Clin. Oncol. (R. Coll. Radiol.) 27, 411–419 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Shah DR et al. Current oncologic applications of radiofrequency ablation therapies. World J. Gastrointest. Oncol 5, 71–80 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma RA et al. Clinical development of new drug–radiotherapy combinations. Nat. Rev. Clin. Oncol 13, 627–642 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Goranov BB et al. Academic opportunities within clinical oncology training. Clin. Oncol. (R. Coll. Radiol.) 25, 446 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Thiruthaneeswaran N et al. Promoting a research culture among junior radiation oncologists: outcomes from the introduction of the Australian and New Zealand research requirement in training. Clin. Oncol. (R. Coll. Radiol.) 26, 162–173 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Berman AT et al. Is there a need for resident training in clinical trial design? Int. J. Radiat. Oncol. Biol. Phys 88, 969–970 (2014). [DOI] [PubMed] [Google Scholar]
- 23.National Institute for Health Research. NIHR integrated academic training programme for doctors and dentists. NIHR http://www.nihr.ac.uk/funding-and-support/funding-for-training-and-career-development/training-programmes/integrated-academic-training-programme/ (2015). [Google Scholar]
- 24.European Society for Medical Oncology. Homepage. http://www.esmo.org. [Google Scholar]
- 25.Society of Interventional Radiology. Medical Student Council. RFS http://rfs.sirweb.org/wordpressnstall/medical-student-section/ (2016). [Google Scholar]
- 26.Bennett JM, Sahasrabudhe DM & Hall WJ Medical oncology and geriatric medicine: is it time for fellowship integration? Cancer 80, 1351–1353 (1997). [DOI] [PubMed] [Google Scholar]
- 27.Terret C Management and geriatric assessment of cancer in the elderly. Expert. Rev. Anticancer Ther. 4, 469–475 (2004). [DOI] [PubMed] [Google Scholar]
- 28.American Society of Clinical Oncology. Geriatric oncology resources. ASCO https://www.asco.org/practice-guidelines/cancer-care-initiatives/geriatric-oncology/geriatric-oncology-resources. [Google Scholar]
- 29.European Society for Medical Oncology . ESMO Handbook of Cancer in the Senior Patient (eds Aapro MS & Schrijvers D) (ESMO; 2015). [Google Scholar]
- 30.International Society of Geriatric Oncology . Homepage. http://siog.org/. [Google Scholar]
- 31.Kalsi T et al. Are the UK oncology trainees adequately informed about the needs of older people with cancer? Br. J. Cancer 108, 1936–1941 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horgan AM et al. Impact and feasibility of a comprehensive geriatric assessment in the oncology setting: a pilot study. Am. J. Clin. Oncol 35, 322–328 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Hiley CT et al. Challenges in molecular testing in non-small-cell lung cancer patients with advanced disease. Lancet 388, 1002–1011 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Atkins MB & Larkin J Immunotherapy combined or sequenced with targeted therapy in the treatment of solid tumors: current perspectives. J. Natl Cancer Inst. 108, djv414 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Zhang T et al. The efficacy and safety of anti-PD-1/PD-L1 antibodies for treatment of advanced or refractory cancers: a meta-analysis. Oncotarget 10.18632/oncotarget.12230 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joint Royal College of Physicians Training Board. Speciality training curriculum for medical oncology. GMC http://www.gmc-uk.org/2010__AUC__Medical_Oncology_curriculum_AUC.pdf_56436828.pdf (2010). [Google Scholar]
- 37.The Royal College of Radiologists. Clinical oncology — the future shape of the specialty. RCR https://www.rcr.ac.uk/sites/default/files/bfco144_co_shape_of_specialty.pdf (2014). [Google Scholar]
- 38.National Institutes of Health. Mentored Clinical Scientist Research Career Development Award. NIH https://researchtraining.nih.gov/programs/career-development/K08 (2016). [Google Scholar]
- 39.National Institutes of Health. Mentored Patient-Orientated Research Career Development Award. NIH https://researchtraining.nih.gov/programs/career-development/K23 (2016). [Google Scholar]
- 40.National Institute for Health Research. NIHR Clinical Research Network Cancer. NIHR https://www.crn.nihr.ac.uk/cancer/ (2016). [Google Scholar]
- 41.Adam A & Kenny LM Interventional oncology in multidisciplinary cancer treatment in the 21(st) century. Nat. Rev. Clin. Oncol 12, 105–113 (2015). [DOI] [PubMed] [Google Scholar]
- 42.Breen DJ & Lencioni R Image-guided ablation of primary liver and renal tumours. Nat. Rev. Clin. Oncol 12, 175–186 (2015). [DOI] [PubMed] [Google Scholar]
- 43.Society of Interventional Radiology. New IR residency and IR/DR certificate: future direction of the specialty. SIRWEB http://www.sirweb.org/clinical/IR_DR_cert.shtml (2016). [Google Scholar]
- 44.Cardiovascular and Interventional Radiological Society of Europe . Homepage. http://www.cirse.org. [Google Scholar]
- 45.World Conference on Interventional Oncology Events . Homepage. http://www.wcioevents.org. [Google Scholar]
- 46.European Conference on Interventional Oncology. Congresses. ECIO http://www.ecio.org/index.php?pid=9. [Google Scholar]
- 47.Synergy. Homepage. http://synergymiami.org. [Google Scholar]
- 48.The Annual Symposium on Cinical Interventional Oncology. Homepage http://www.iset.org/oncology/. [Google Scholar]
- 49.The Royal College of Radiologists. Interventional oncology: guidance for service delivery. RCR https://www.rcr.ac.uk/system/files/publication/field_publication_files/RCR(13)_IO_0.pdf (2013). [Google Scholar]
- 50.Sharma RA, Cummins C & Crellin A Selective internal radiotherapy of the liver: at the crossroads of interventional oncology research and national health service commissioning. Clin. Oncol. (R. Coll. Radiol.) 26, 733–735 (2014). [DOI] [PubMed] [Google Scholar]
- 51.LaBerge JM & Anderson JC & Radiology Review Committee. A guide to the Interventional Radiology residency program requirements. J. Am. Coll. Radiol 12, 848–853 (2015). [DOI] [PubMed] [Google Scholar]
- 52.Society of Interventional Radiology. Residents, Fellows and Student section. RFS http://rfs.sirweb.org/wordpressnstall/ (2016). [Google Scholar]
- 53.Kaufman JA The interventional radiology/diagnostic radiology certificate and interventional radiology residency. Radiology 273, 318–321 (2014). [DOI] [PubMed] [Google Scholar]
- 54.Commander CW et al. Assessing medical students’ knowledge of IR at two American medical schools. J. Vasc. Interv. Radiol 25, 1801–1806 (2014). [DOI] [PubMed] [Google Scholar]
- 55.Benedetti NJ, Naeger DM & Webb EM Radiology primer: a novel radiology course for undecided medical students. J. Am. Coll. Radiol 11, 1182–1185 (2014). [DOI] [PubMed] [Google Scholar]
- 56.Treasure T & Morton D GRIST: growing recruitment in interventional and surgical trials. J. R. Soc. Med 105, 140–141 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.The Association for Cancer Surgery. BASO trainees. BASO http://www.baso.org.uk/baso-trainees.aspx. [Google Scholar]
- 58.Mackenzie H et al. Design, delivery, and validation of a trainer curriculum for the national laparoscopic colorectal training program in England. Ann. Surg 261, 149–156 (2015). [DOI] [PubMed] [Google Scholar]
- 59.Franklin JM et al. Clinical trials of interventional oncology — moving from efficacy to outcomes. Nat. Rev. Clin. Oncol 12, 93–104 (2015). [DOI] [PubMed] [Google Scholar]
- 60.McCulloch P et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet 374, 1105–1112 (2009). [DOI] [PubMed] [Google Scholar]
- 61.Department of Health. Good clinical practice for clinical trials. gov.uk https://www.gov.uk/guidance/good-clinical-practice-for-clinical-trials (2016). [Google Scholar]
- 62.Lee MJ et al. Academic requirements for Certificate of Completion of Training in surgical training: consensus recommendations from the Association of Surgeons in Training/National Research Collaborative Consensus Group. Int. J. Surg 10.1016/j.ijsu.2016.08.236 (2016). [DOI] [PubMed] [Google Scholar]
- 63.Foxtrot Collaborative Group. Feasibility of preoperative chemotherapy for locally advanced, operable colon cancer: the pilot phase of a randomised controlled trial. Lancet Oncol. 13, 1152–1160 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bhangu A, Fitzgerald JE & Kolias AG Trainee-led research collaboratives: a novel model for delivering multi-centre studies. ANZ J. Surg 84, 902–903 (2014). [DOI] [PubMed] [Google Scholar]
- 65.Bhangu A Safety of short, in-hospital delays before surgery for acute appendicitis: multicentre cohort study, systematic review, and meta-analysis. Ann. Surg 259, 894–903 (2014). [DOI] [PubMed] [Google Scholar]
- 66.National Research Collaborative . Homepage. http://www.nationalresearch.org.uk/. [Google Scholar]
- 67.Pinkney TD et al. Impact of wound edge protection devices on surgical site infection after laparotomy: multicentre randomised controlled trial (ROSSINI Trial). BMJ 347, f4305 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.West Midlands Research Collaborative . MASDA. WM Research http://wmresearch.org.uk/?page_id=477 (2015). [Google Scholar]
- 69.Bowel Disease Research Foundation. Bowel disease research foundation funded projects. BDRF http://www.bdrf.org.uk/research/projects/ (2016). [Google Scholar]
- 70.Collaborative STARSurg. Impact of postoperative non-steroidal anti-inflammatory drugs on adverse events after gastrointestinal surgery. Br. J. Surg 101, 1413–1423 (2014). [DOI] [PubMed] [Google Scholar]
- 71.Saini KS et al. Role of the multidisciplinary team in breast cancer management: results from a large international survey involving 39 countries. Ann. Oncol 23, 853–859 (2012). [DOI] [PubMed] [Google Scholar]
- 72.De Ieso PB et al. A study of the decision outcomes and financial costs of multidisciplinary team meetings (MDMs) in oncology. Br. J. Cancer 109, 2295–2300 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lamb BW et al. Strategies to improve the efficiency and utility of multidisciplinary team meetings in urology cancer care: a survey study. BMC Health Serv. Res 14, 377 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kesson EM et al. Effects of multidisciplinary team working on breast cancer survival: retrospective, comparative, interventional cohort study of 13 722 women. BMJ 344, e2718 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ellis PM The importance of multidisciplinary team management of patients with non-small-cell lung cancer. Curr. Oncol 19 (Suppl. 1), S7–S15 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kissane DW et al. Communication skills training for oncology professionals. J. Clin. Oncol 30, 1242–1247 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kash KM et al. Stress and burnout in oncology. Oncology (Williston Park) 14, 1621–1633 (2000). [PubMed] [Google Scholar]
- 78.Sackett DL Rules of evidence and clinical recommendations on the use of antithrombotic agents. Chest 95(2 Suppl), 2S–4S (1989). [PubMed] [Google Scholar]
- 79.Howe M & Burkes RL Collaborative care in NSCLC; the role of early palliative care. Front. Oncol 4, 192 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tree A et al. Successful patient acceptance of randomization within the PACE study (Prostate Advances in Comparative Evidence). Int. J. Radiat. Oncol. Biol. Phys 87(2 Suppl.), S365 (2013). [Google Scholar]
- 81.Plastaras JP, Berman AT & Freedman GM Special cases for proton beam radiotherapy: re-irradiation, lymphoma, and breast cancer. Semin. Oncol 41, 807–819 (2014). [DOI] [PubMed] [Google Scholar]
- 82.Parker C et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med 369, 213–223 (2013). [DOI] [PubMed] [Google Scholar]
- 83.Weber WA PET/MR Imaging: a critical appraisal. J. Nucl. Med 55(Suppl. 2), 56S–58S (2014). [DOI] [PubMed] [Google Scholar]