Abstract
Purpose
Men diagnosed with atypical small acinar proliferation (ASAP) are counseled to undergo early re-biopsy, as their risk of prostate cancer (PCa) is high. However, random re-biopsies may not re-sample areas of concern. Magnetic resonance imaging/transrectal ultrasound (MRI/TRUS) fusion-guided biopsy offers an opportunity to accurately target, and later re-target, specific areas within the prostate. The purpose of this study is to describe the ability of MRI/TRUS fusion-guided prostate biopsy to detect PCa in areas with an initial diagnosis of ASAP.
Materials and Methods
From March 2007 to February 2014, 1,028 patients underwent MP-MRI of the prostate and MRI/TRUS fusion-guided biopsy. Twenty patients met the following stringent inclusion criteria: no history of PCa; index biopsy demonstrating at least one core of ASAP and benign glands in all remaining cores; fusion targeted re-biopsy with at least one targeted core directly re-sampling an area of the prostate which previously contained ASAP.
Results
At index biopsy, the 20 patients had a median age of 60 years (IQR 57–64) and median PSA of 5.92ng/ml (IQR 3.34–7.48). At fusion targeted re-biopsy in a median 11.6 months, 5/20 (25%, 95% CI 6.02–43.98) patients were diagnosed with PCa, all of which was primary Gleason grade 3, low-volume disease. On fusion re-biopsy, cores which directly re-targeted areas of previous ASAP detected the highest tumor burden.
Conclusions
When MRI/TRUS fusion-guided biopsy detects isolated ASAP on index biopsy, early re-biopsy is unlikely to detect clinically significant PCa. Cores which re-target areas of previous ASAP are more effective than random re-biopsy cores.
Keywords: atypia, magnetic resonance imaging, transrectal ultrasound, cancer detection, image-guided biopsy
INTRODUCTION
In the current era of prostate-specific antigen (PSA) testing, 5% of men who undergo prostate biopsy are diagnosed with atypical small acinar proliferation (ASAP).1 This diagnosis presents a frustrating challenge to physicians and patients alike. Contrary to the well-defined, pre-malignant finding of high-grade prostatic intraepithelial neoplasia (HGPIN), ASAP is not a discrete entity. Rather, it is a broad term that is used to describe features that are suspicious for, but not diagnostic of, prostate cancer (PCa). Cores containing a small number of visible acini, or distorted acini, for example, may all be labeled as ASAP.2 Nevertheless, ASAP is clinically relevant because as many as 40% of men with ASAP are diagnosed with PCa on their first re-biopsy.1 No clinical variables reliably predict which men with ASAP are at the highest risk; therefore, current guidelines suggest that all patients with a diagnosis of ASAP undergo re-biopsy in 3–6 months.1, 3
The mechanism by which ASAP predisposes to PCa is unknown. It is theorized that many cases of ASAP are simply poorly sampled foci of cancer. In this setting, the biopsy under-sampled the PCa and therefore an immediate re-biopsy may demonstrate PCa. This concept is supported by the spatial distribution of PCa that is found on re-biopsy. Malignant cells are found in the same sextant as the original ASAP in 48–57% of cases, as opposed to the contralateral lobe of the prostate in only 17–27%.1 This skewed distribution has led some to advocate for increased sampling within the sextant previously harboring ASAP.1 However, because the exact biopsy location can only be estimated with conventional TRUS guidance, it is subject to operator dependency and inaccuracy.
Recent advances in imaging and biopsy technology have made possible the precise re-sampling of areas within the prostate.4 Magnetic resonance imaging/ultrasound (MRI/TRUS) fusion-guided biopsy is a particularly promising technique for PCa detection.5–8 It uses software-based fusion to combine high quality diagnostic MRI and real time transrectal ultrasound TRUS-guided biopsy and tracks movement of the ultrasound probe in relation to the MRI. Such a system not only allows for targeted biopsy of lesions that are suspicious on MRI, but can also record the location of every core, including random extended-sextant samples. Sites that yield suspicious glands on pathology, such as those seen with ASAP, can be then be more accurately re-biopsied. The purpose of the present study is to describe the ability of MRI/TRUS fusion-guided re-biopsy to detect PCa after an initial diagnosis of ASAP.
MATERIALS AND METHODS
Patient Selection
Patients were enrolled as a part of an Institutional Review Board (IRB) approved, prospective trial evaluating prostate multiparametric MRI (MP-MRI) and MRI/TRUS fusion-guided biopsy at the National Cancer Institute of the National Institutes of Health (NIH). From March 2007 to February 2014, 1,028 men underwent MRI/TRUS fusion guided biopsy of the prostate. Of these, 94 patients underwent multiple biopsy sessions. Inclusion criteria for the present analysis were: no history of PCa; diagnostic 3 Tesla (3T) MP-MRI of the prostate at presentation; index MRI/TRUS fusion-guided and 12-core biopsies; index biopsy pathology showing no prostate cancer, at least one core of ASAP, and benign glands in all remaining cores; re-biopsy with MRI/TRUS fusion-guided re-biopsy and 12-core biopsies, with at least one targeted core directly re-sampling an area of the prostate which on index biopsy session demonstrated ASAP. All patients provided written informed consent.
Imaging and Biopsy Protocols
The initial diagnostic 3T MP-MRI of the prostate (Achieva, Philips Healthcare, Best, The Netherlands) incorporated both an endorectal coil (BPX-30, Medrad, Pittsburgh, PA) and a 16-channel cardiac surface coil (SENSE, Philips Healthcare) positioned over the pelvis, as previously described.9 Two urologic radiologists (P.L.C., B.T.) prospectively evaluated each MP-MRI, which included T2-weighted (T2W), diffusion weighted imaging (DWI), dynamic contrast enhanced (DCE), and MR spectroscopy (MRS) sequences (Figure 1). The results of these sequences were used to generate prostate cancer suspicion scores of low, moderate, or high suspicion. This system is based on the number of MP-MRI sequences which are positive for any intraprostatic lesion, with low suspicion lesions typically positive on any one sequence, moderate suspicion on two, and high suspicion on all sequences, as previously described and validated.10
Figure 1.
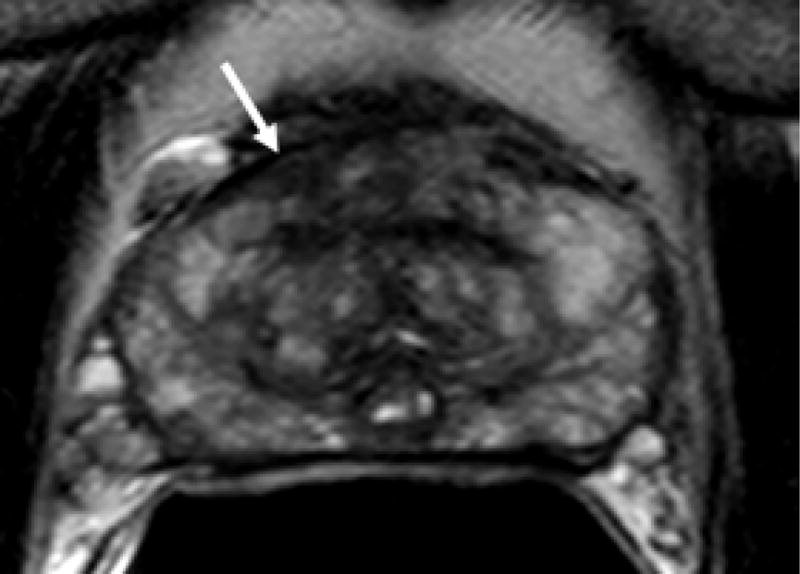
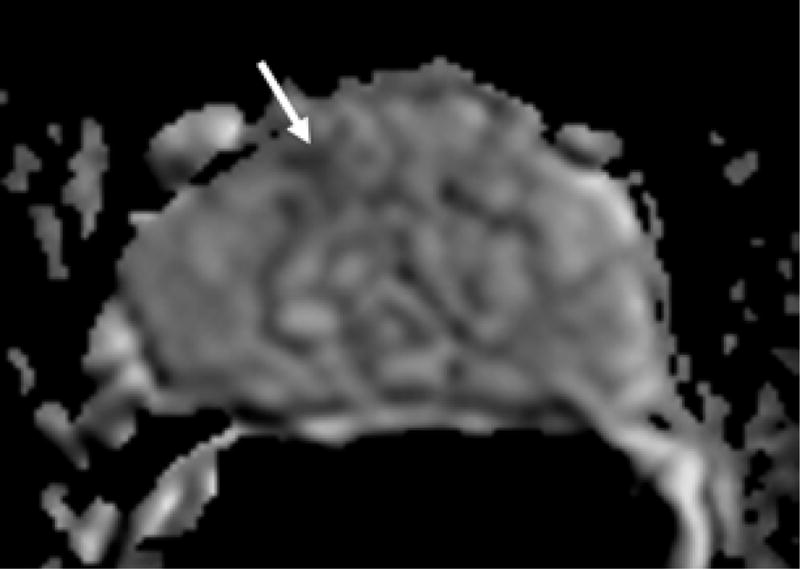
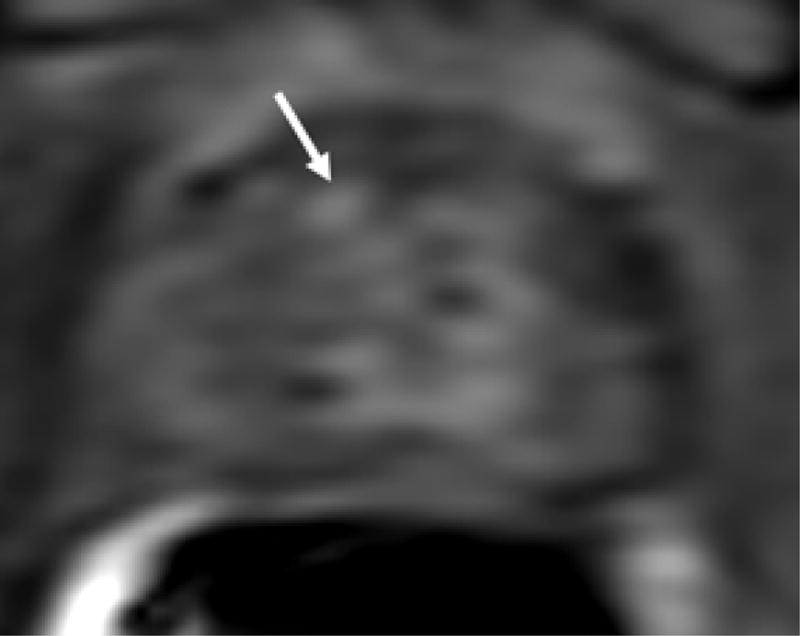
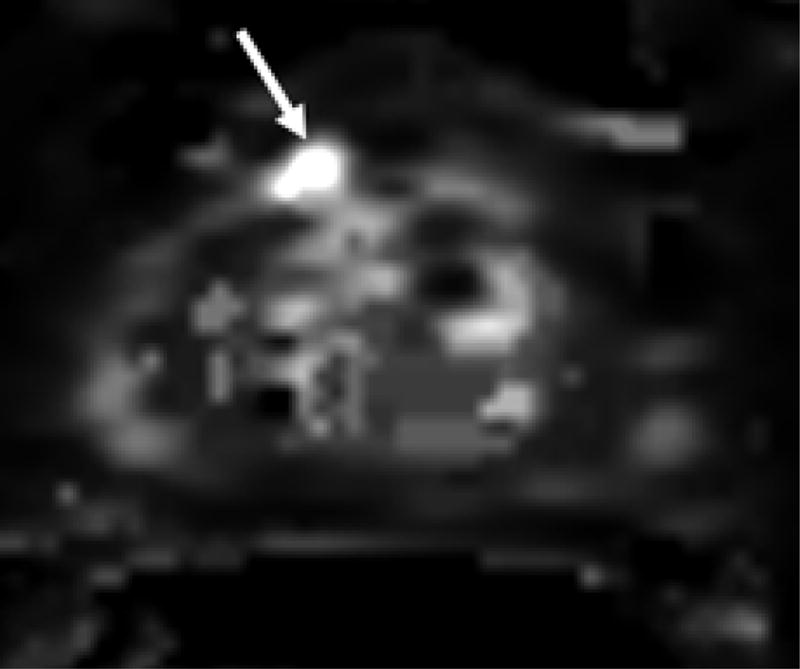
69 year old male with PSA 7.18 ng/ml and one prior negative biopsy. (a) axial T2W MRi demonstrates a lesion in the right anterior prostate (arrow), with (b) ADC maps of DW MRI showing restricted diffusion corresponding to a hypointense signal intensity within the lesion. (c) Raw DCE MRI and (d) ktrans maps obtained from DCE MRI confirm hypervascularity in the corresponding area. MRI/TRUS fusion-guided biopsy revealed ASAP. On image-guided targeted re-biopsy, Gleason 6 PCa was detected in a core which had directly resampled an area of previous ASAP.
All patients then underwent MRI/TRUS fusion-guided biopsy using iterations of the UroNav platform (Philips/InVivo, Gainesville, FL, USA) and an end-fire TRUS (Philips Healthcare, Bothell, WA) as previously described.6 The initial biopsy session included a systematic extended-sextant 12-core biopsy in addition to targeted biopsies of all MRI-suspicious lesions. At least two cores were obtained from every MRI-visible lesion, one each in the axial and sagittal planes.11 The intraprostatic location that was sampled by each biopsy core was recorded by the fusion biopsy software, with semi-automatic detection of needle tip and offset core biopsy location (Figure 2). Biopsy pathology was reviewed by a single genitourinary pathologist (M.J.M.). In all patients, this index biopsy revealed at least one core of ASAP and benign prostatic tissue in the remaining cores (Figure 3).
Figure 2.
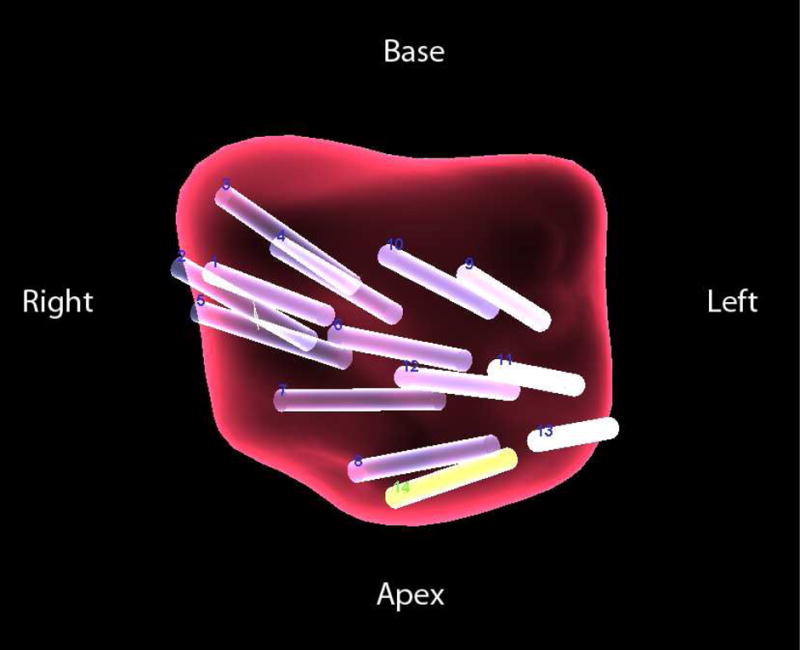
Post-procedural 3-D mapping of extended sextant 12-core (cores 1–2) and MRI/TRUS fusion-guided (cores 3–14) biopsy. 64 year old man with a PSA of 7.79 ng/ml. Index biopsy revealed ASAP in 2/16 cores, with benign glands in the remaining cores. Biopsy cores which produced ASAP were then assigned as targets for MRI/TRUS fusion-guided re-biopsy. Approximately one year later, targeted re-biopsy of areas of previous ASAP produced Gleason 6 PCa in 30% or less of 3 cores.
Figure 3.
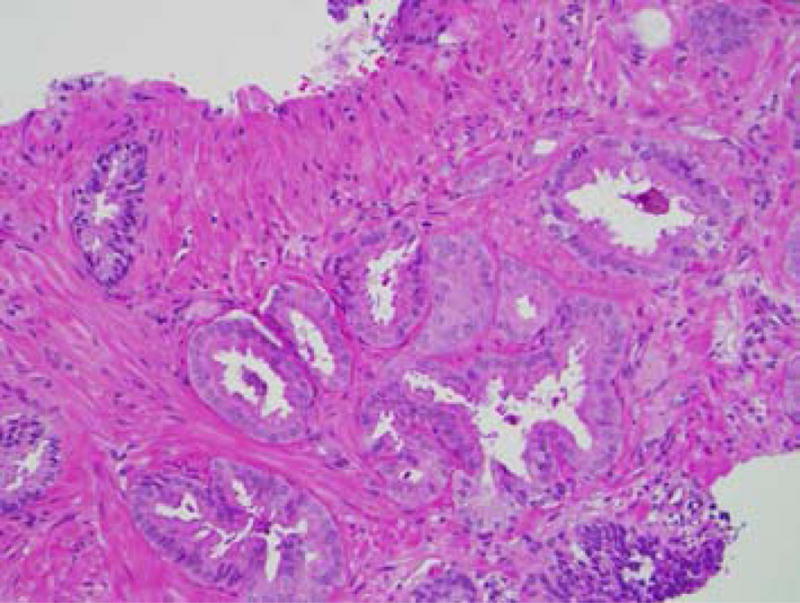
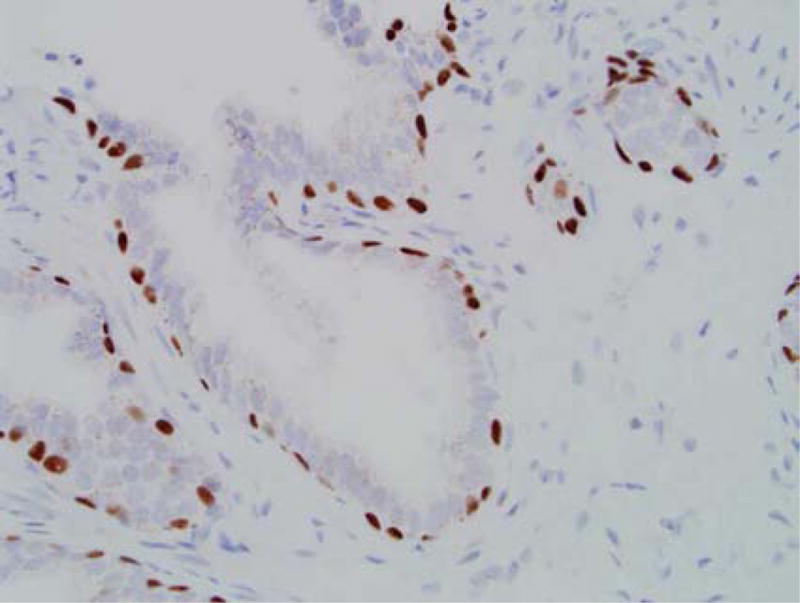
(a) Atypical small acinar proliferation, H&E stain (150×). (b) Immunohistochemical stain for p63 showing preservation of the basal cell layer (200×).
Because of the presence of ASAP, patients were counseled to return for re-biopsy. At this second visit, patients were re-assessed with PSA, DRE, and a repeat 3T MP-MRI of the prostate. Before undergoing repeat targeted biopsy, each patient’s index biopsy pathology and imaging were reviewed. Sites of ASAP on initial biopsy, whether on an index targeted biopsy or as part of the index extended-sextant 12-core biopsy, were assigned as new targets for re-biopsy using the fusion software, with point targets assigned and displayed at the midpoint of the prior core biopsy. All patients then underwent repeat MRI/TRUS fusion-guided biopsy, which again included both extended-sextant 12-core biopsy and targeted biopsy of MRI-visible lesions, as well as targeted biopsy of sites of prior ASAP. Target needle placement was tracked throughout the biopsy session to ensure accuracy. Biopsy pathology was reviewed by the same genitourinary pathologist (M.J.M.).
Data Analysis
Univariate logistic regression analysis and descriptive statistics were calculated using JMP Pro 10.0 (SAS Institute, Cary, North Carolina).
RESULTS
Twenty patients met this study’s inclusion criteria. At initial presentation, the overall cohort had a median age of 60 years (interquartile range (IQR) 57–64) and median PSA of 5.92 ng/ml (IQR 3.34–7.48) (Table 1). Thirteen of 20 (65%) patients were biopsy naïve and were referred due to elevated PSA or DRE findings alone. All patients exhibited ASAP on at least one core and otherwise benign tissue.
Table 1.
Patient demographics at initial presentation.
| Total # Patients (n), (%) | 20 (100%) |
|
| |
| Age [years] median, (IQR) | 60 (57–64) |
|
| |
| PSA [ng/ml] (median), (IQR) | 5.92 (3.34–7.48) |
|
| |
| Race (n), (%) | |
|
|
|
| White | 16 (80%)
|
| Black | 3 (15%)
|
| Hispanic | 1 (5%) |
|
| |
| Prior Biopsies Per Patient (n patients), (%) | |
|
|
|
| 0 biopsies | 13 (65%)
|
| 1 biopsy | 3 (15%)
|
| 2 or more biopsies | 4 (20%) |
|
| |
| Prior Positive Biopsies Per Patient (n patients), (%) | |
|
|
|
| 0 biopsies | 20 (100%)
|
| 1 or more biopsies | 0 |
At fusion targeted re-biopsy, 5/20 (25%, 95% CI 6.02–43.98) patients were diagnosed with PCa (Table 2). The median time to re-biopsy was 11.6 months (IQR 5.6–15.5). Univariate regression analysis revealed that none of the following factors were predictive of PCa on re-biopsy: patient age, initial PSA, number of MRI lesions, MRI suspicion score, number of cores per patient, biopsy modality which initially detected ASAP, concomitant presence of HGPIN, time to re-biopsy, PSA at re-biopsy, and MRI findings on re-biopsy. In a separate analysis, PSA at re-biopsy was also not a statistically significant predictor of PCa at re-biopsy when censoring the data for the 1 patient with a decreased PSA in the context of 5-alpha reductase inhibitor (5-ARI) use (p=0.052) (Table 3).
Table 2.
Clinical, imaging, and pathologic variables for the 20 patients stratified by biopsy result at fusion targeted re-biopsy. MP-MRI was characterized as stable at the second visit if there was no increase in number of visible lesions, lesion size, or PCa suspicion score.
| Benign | Malignant | ||
|---|---|---|---|
|
| |||
| Patients (n) | 15 | 5 | |
|
| |||
| Biopsy 1 | Age [years] (median), (IQR) | 60 (57 – 62) | 64 (61 – 65) |
|
| |||
| PSA [ng/ml] (median), (IQR) | 4.06 (3.26–6.70) | 7.38 (7.18–7.79) | |
|
| |||
| MP - MRI #1 | |||
| # Lesions (n), (%) | |||
|
|
|||
| 1 | 9 (60%) | 3 (60%) | |
|
|
|||
| 2 or more | 6 (40%) | 2 (40%) | |
|
|
|||
| Suspicion Score (n), (%) | |||
|
|
|||
| Low | 6 (40%) | 1 (20%) | |
|
|
|||
| Moderate | 8 (53%) | 4 (80%) | |
|
|
|||
| High | 1 (7%) | 0 | |
|
| |||
| Total Cores Per Patient (median), (IQR) | 17 (16 – 18) | 16 (16 – 17) | |
|
|
|||
| Source of ASAP on Index Biopsy | |||
|
|
|||
| ASAP cores, total (median), (IQR) | 1 (1 – 2 cores) | 3 (2 – 4 cores) | |
|
|
|||
| ASAP cores, found on 12 -core (% of total) | 70% | 90% | |
|
|
|||
| ASAP cores, found on target cores (% of total) | 30% | 10% | |
|
| |||
| Concurrent HGPIN (cases), (%) | |||
|
|
|||
| ASAP + no HGPIN | 14/15 (95%) | 4/5 (80%) | |
|
|
|||
| ASAP + unifocal HGPIN | 0% | 1/5 (20%) | |
|
|
|||
| ASAP + multifocal HGPIN | 1/15 (5%) | 0% | |
|
| |||
| Biopsy 2 (Fusion targeted Re-Biopsy) | PSA [ng/ml] | ||
|
|
|||
| PSA (median), (IQR) | 5.58 (4.82–7.42) | 8.63 (8.13–11.30) | |
|
|
|||
| PSA change (mean) | +0.65 | +1.58 | |
|
| |||
| MP - MRI #2 (n), (%) | |||
|
|
|||
| No repeat MRI | 3 (20%) | 3 (60%) | |
|
|
|||
| Stable | 10 (67%) | 2 (40 %) | |
|
|
|||
| More Suspicious | 2 (13%) | 0 | |
|
| |||
| Total Cores Per Patient (median), (IQR) | 18 (16 – 20) | 18 (18 – 20) | |
Table 3.
Biopsy pathology for the 5 patients who were diagnosed with PCa at fusion targeted re-biopsy. Right side of the table (shaded) demonstrates which biopsy modality detected (+) the core with the highest Gleason sum.
| Re-Biopsy Results | Targeted Biopsies | Random 12-core Biopsies | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pt | Stage | PSA [ng/ml] | Cores Sampled (n) | Cores Positive (n) | Highest Gleason Sum | Percent of Core(s) Involved (%) | Old ASAP, Re-Targeted | Target, Other | Same Sextant as Old ASAP | Random, Other |
| 1 | cT1c | 8.13 | 18 | 3 | 3+3=6 | 30, 5, 5 | + | − | + | − |
| 2 | cT1c | 8.63 | 16 | 3 | 3+3=6 | 30, 10, 10 | + | + | + | − |
| 3 | cT1c | 11.30 | 21 | 1 | 3+3=6 | 20 | − | − | − | + |
| 4 | cT1c | 0.83† | 20 | 2 | 3+3=6 | 8, 5 | + | − | − | − |
| 5 | cT1c | 11.49 | 20 | 4 | 3+4=7* | 8, 5, 5, 5 | + | − | − | − |
| Detection Rate | 4/5 (80%) | 1/5 (20%) | 2/5 (40%) | 1/5 (20%) | ||||||
On 5-ARI.
This patient had Gleason 3+4=7 PCa in 8% of one targeted core, with the remaining 3 cores as Gleason 3+3=6 in 5% of each core.
Detailed biopsy results for the 5 patients who were diagnosed with PCa on re-biopsy are presented in Table 3. In 4/5 (80%) of cases, the highest tumor burden detected was Gleason 3+3=6 PCa in 30% of the core, with 3 or fewer cores involved in each case. In the remaining patient, Gleason 3+4=7 PCa was detected in 8% of a core that re-targeted a previous area of ASAP. The remaining positive cores in this patient were all Gleason 3+3=6 in 5% of each of 3 cores.
Biopsies were also classified with respect to the source of each core (Table 3, shaded). Targeted re-biopsy cores were sub-classified in two ways: re-targets of areas that had produced ASAP at the index biopsy session (detecting 4/5 cancers), and targets of any other MRI-visible lesions (detecting 1/5 cancers). Extended-sextant 12-core re-biopsies were also sub-classified in two ways: those that sampled the same sextant as that which previously produced ASAP (detecting 2/5 cancers), and those that sampled all other sextants (detecting 1/5 cancers).
DISCUSSION
This study shows that MRI/TRUS fusion biopsy with “ASAP-mapped targets” may be a superior method of following-up ASAP than repeat random biopsies. Tracking where the initial biopsy was positive for ASAP (and then returning to the same location, using the same coordinates as were initially used), enables accurate resampling of the ASAP region. In the present study, 80% of ASAP to PCa transitions or diagnoses were detected using resampling of the same region. In approximately 20% of cases, PCa was identified elsewhere in the gland on repeat biopsy and may or may not be geometrically related to the ASAP. In contrast, random biopsies detected only 40% of the ASAP to PCa transitions in the same location. Thus, MRI/TRUS fusion biopsy may be helpful in following patients initially diagnosed with ASAP and otherwise benign glands.
The term ASAP applies to a broad array of pathologic features. Despite the relatively ill-defined nature of ASAP variants, there is a 40% risk of typically low-grade PCa on first re-biopsy.1 In our series there was a 25% ASAP to PCa transition. Because it is currently impossible to risk stratify men with ASAP, patients are counseled to undergo re-biopsy in 3–6 months.1, 3 This is based in part on a recommendation in 2006 by Epstein et al. who published a comprehensive review of the literature on ASAP and proposed that ASAP be re-biopsied at intervals of 3–6 months. However, the authors acknowledged that this recommendation was based exclusively on studies which had used sextant techniques for the index biopsies.1
Because of the known diagnostic limitations of digital rectal exam (DRE) and PSA, enthusiasm has shifted towards an approach that utilizes advanced prostatic imaging and image guided biopsy. High-quality MP-MRI is an emerging method for imaging prostatic lesions.12 Of particular importance for men with low grade disease, MP-MRI has been specifically validated in the setting of active surveillance (AS).13, 14 MRI/TRUS fusion-guided biopsy allows for an office-based platform that combines the diagnostic accuracy of MRI, the real time capabilities of TRUS and the tissue diagnosis provided by biopsy.6 Of particular interest, many fusion platforms have the ability to automatically record and track biopsy locations. Although the accuracy of these tracking systems has been validated, little if any attention has been paid towards the ability of advanced image-guided biopsy to re-target suspicious findings such as ASAP.15
Our cohort consisted of 20 patients who met stringent inclusion criteria. At approximately 1 year after initial biopsy, only 5/20 (25%) patients were diagnosed with PCa on fusion targeted re-biopsy. This low PCa risk on re-biopsy is noteworthy for three reasons. First, it is not only substantially lower than the 40% risk quoted in other studies after a diagnosis of ASAP, but it in fact approaches the rate of PCa detection after a benign index biopsy.1, 3 Second, all patients in the present study underwent re-biopsy with a minimum of 16 cores (range 16–20). A low PCa detection rate in this context is surprising, as one might expect increased detection with a high number of cores.16 We believe that the initial MRI-TRUS guided biopsy likely discovered more significant lesions that removed patients with PCa from follow-up, thereby lowering the risk of cancer on re-biopsy. Finally, fusion re-biopsy included cores which precisely targeted areas that had specifically been found to harbor ASAP on index biopsy; such cores are among the most likely to detect PCa.
Our data suggests that patients who are diagnosed with ASAP after MRI/TRUS fusion-guided biopsy may not need to return for early re-biopsy owing to a reduced rate of ASAP to PCa transition and the detection of only low grade (primary Gleason grade 3, low-volume) tumors on re-biopsy. This approach could minimize exposure to biopsy-related complications, such as urosepsis.17 The 5 patients in our study who developed PCa could have all met AS criteria, particularly as these criteria are expanded to include men with features like small amounts of Gleason pattern 4 disease.18, 19 Instead of early re-biopsy, patients can potentially be counseled to return for PSA monitoring and repeat MP-MRI. Given that MP-MRI has a high negative predictive value for clinically significant disease, it may offer an appealing non-invasive tool for monitoring men with ASAP.10, 20 MP-MRI may also offer an opportunity to prolong the currently recommended 3–6 month follow-up period after a diagnosis of ASAP. In the present study, MP-MRI findings did not significantly change over a period of approximately 1 year. This is supported by other studies, which have shown that small index lesions often remain stable on MP-MRI for 2 years or more.21 Delaying re-biopsy could thus reduce the over-detection of clinically-insignificant PCa, the incidence of biopsy-associated morbidity, the psychological burden to patients, and possibly overall healthcare costs.
When re-biopsy is performed, our data indicate that re-targeting prior areas of ASAP with MRI/TRUS fusion-guided biopsy is an effective strategy. In 4/5 (80%) of cases, the cores that re-targeted previous ASAP were the ones that produced the highest grade pathology. Although it may still be premature to advocate replacing extended-sextant 12-core biopsy with targeted biopsies entirely, the present study adds to the growing body of evidence supporting the role of directed sampling.
This study has certain limitations. First, the cohort of 20 patients is small. However, ASAP is a relatively rare entity, and our inclusion criteria were applied to an initial cohort of over 1,000 men who underwent MRI/TRUS fusion-guided biopsy. The resulting sample size is largely a reflection of the referral pattern to institutions like ours which currently have MRI/TRUS fusion-guided biopsy technology. Such patients have often undergone multiple biopsies before presentation, and many have a prior diagnosis of PCa, thus excluding them from this study. Second, patients in the present study were not re-biopsied at a uniform follow-up period. In some cases, this was because patients routinely refused early re-biopsy if their PSA and MRI findings remained stable. The data supporting longer intervals between biopsies for AS or ASAP patients who undergo MRI is not level 1. This is a known limitation of retrospective analysis and should be ideally addressed in a prospective clinical trial. Such a prospective study could also incorporate other recent advances in PCa risk stratification, such as genetic and expression level biomarkers.
CONCLUSION
This study demonstrates that isolated ASAP in the setting of MRI/TRUS fusion-guided biopsy is a rare event, occurring in approximately 2% of biopsies in this series. When such patients undergo re-biopsy, those cores that directly re-target areas of the prostate which produced ASAP on index biopsy are the most likely to detect PCa. More importantly, re-biopsy at a median follow-up of 1 year yields a low detection rate of clinically significant PCa. This suggests that re-biopsy in 3–6 months may not be warranted after a diagnosis of ASAP on MRI/TRUS fusion-guided biopsy. As MP-MRI becomes more widely adopted, validation of these initial findings may give patients and clinicians increased confidence that deferred re-biopsy after ASAP is safe and effective.
Abbreviations and Acronyms
- ASAP
Atypical small acinar proliferation
- IQR
Interquartile range
- MP-MRI
Multiparametric magnetic resonance imaging
- MRI
Magnetic resonance imaging
- MRI/TRUS
Magnetic resonance imaging/transrectal ultrasound
- PCa
Prostate cancer
- PSA
Prostate-specific antigen
- TRUS
Trans-rectal ultrasound
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Epstein JI, Herawi M. Prostate needle biopsies containing prostatic intraepithelial neoplasia or atypical foci suspicious for carcinoma: implications for patient care. J Urol. 2006;175:820. doi: 10.1016/S0022-5347(05)00337-X. [DOI] [PubMed] [Google Scholar]
- 2.Schlesinger C, Bostwick DG, Iczkowski KA. High-grade prostatic intraepithelial neoplasia and atypical small acinar proliferation: predictive value for cancer in current practice. Am J Surg Pathol. 2005;29:1201. doi: 10.1097/01.pas.0000168178.48535.0d. [DOI] [PubMed] [Google Scholar]
- 3.Merrimen JL, Jones G, Hussein SA, et al. A model to predict prostate cancer after atypical findings in initial prostate needle biopsy. J Urol. 2011;185:1240. doi: 10.1016/j.juro.2010.11.063. [DOI] [PubMed] [Google Scholar]
- 4.Raskolnikov D, Rais-Bahrami S, Turkbey B, et al. Current Ability of Multiparametric Prostate Magnetic Resonance Imaging and Targeted Biopsy to Improve the Detection of Prostate Cancer. Urology Practice. 2014;1:13. doi: 10.1016/j.urpr.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delongchamps NB, Peyromaure M, Schull A, et al. Prebiopsy magnetic resonance imaging and prostate cancer detection: comparison of random and targeted biopsies. J Urol. 2013;189:493. doi: 10.1016/j.juro.2012.08.195. [DOI] [PubMed] [Google Scholar]
- 6.Pinto PA, Chung PH, Rastinehad AR, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol. 2011;186:1281. doi: 10.1016/j.juro.2011.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rastinehad AR, Turkbey B, Salami SS, et al. Improving Detection of Clinically Significant Prostate Cancer: Magnetic Resonance Imaging/Transrectal Ultrasound Fusion Guided Prostate Biopsy. J Urol. 2013 doi: 10.1016/j.juro.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rais-Bahrami S, Siddiqui MM, Vourganti S, et al. Diagnostic Value of Biparametric MRI as an Adjunct to PSA-based Detection of Prostate Cancer in Men without Prior Biopsies. BJU Int. 2014 doi: 10.1111/bju.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turkbey B, Pinto PA, Mani H, et al. Prostate cancer: value of multiparametric MR imaging at 3 T for detection–histopathologic correlation. Radiology. 2010;255:89. doi: 10.1148/radiol.09090475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rais-Bahrami S, Siddiqui MM, Turkbey B, et al. Utility of multiparametric magnetic resonance imaging suspicion levels for detecting prostate cancer. J Urol. 2013;190:1721. doi: 10.1016/j.juro.2013.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong CW, Rais-Bahrami S, Walton-Diaz A, et al. Comparison of MR-US fusion-guided prostate biopsies obtained from axial and sagittal approaches. BJU Int. 2014 doi: 10.1111/bju.12871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rais-Bahrami S, Turkbey B, Grant KB, et al. Role of multiparametric magnetic resonance imaging in the diagnosis of prostate cancer. Curr Urol Rep. 2014;15:387. doi: 10.1007/s11934-013-0387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stamatakis L, Siddiqui MM, Nix JW, et al. Accuracy of multiparametric magnetic resonance imaging in confirming eligibility for active surveillance for men with prostate cancer. Cancer. 2013;119:3359. doi: 10.1002/cncr.28216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turkbey B, Mani H, Aras O, et al. Prostate cancer: can multiparametric MR imaging help identify patients who are candidates for active surveillance? Radiology. 2013;268:144. doi: 10.1148/radiol.13121325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu S, Kruecker J, Turkbey B, et al. Real-time MRI-TRUS fusion for guidance of targeted prostate biopsies. Comput Aided Surg. 2008;13:255. doi: 10.1080/10929080802364645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lughezzani G, Sun M, Budaus L, et al. Effect of the number of biopsy cores on prostate cancer detection and staging. Future Oncol. 2010;6:381. doi: 10.2217/fon.10.4. [DOI] [PubMed] [Google Scholar]
- 17.Loeb S, Carter HB, Berndt SI, et al. Complications after prostate biopsy: data from SEER-Medicare. J Urol. 2011;186:1830. doi: 10.1016/j.juro.2011.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooperberg MR, Carroll PR, Klotz L. Active surveillance for prostate cancer: progress and promise. J Clin Oncol. 2011;29:3669. doi: 10.1200/JCO.2011.34.9738. [DOI] [PubMed] [Google Scholar]
- 19.Klotz L, Zhang L, Lam A, et al. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010;28:126. doi: 10.1200/JCO.2009.24.2180. [DOI] [PubMed] [Google Scholar]
- 20.Siddiqui MM, Rais-Bahrami S, Truong H, et al. Magnetic resonance imaging/ultrasound-fusion biopsy significantly upgrades prostate cancer versus systematic 12-core transrectal ultrasound biopsy. Eur Urol. 2013;64:713. doi: 10.1016/j.eururo.2013.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rais-Bahrami S, Turkbey B, Rastinehad AR, et al. Natural history of small index lesions suspicious for prostate cancer on multiparametric MRI: recommendations for interval imaging follow-up. Diagn Interv Radiol. 2014;20:293. doi: 10.5152/dir.2014.13319. [DOI] [PMC free article] [PubMed] [Google Scholar]


