Abstract
Bilateral internal mammary artery (BIMA) in coronary artery bypass grafting (CABG) has traditionally been limited. This review looks at the recent outcome data on BIMA in CABG focusing on the management of risk factors for mediastinitis, one of the potential barriers for more extensive BIMA utilization. A combination of pre-, intra- and postoperative strategies are essential to reduce mediastinitis. Limited data indicate that the incidence of mediastinitis can be reduced using closed incision negative-pressure wound therapy as a part of these strategies with the possibility of offering patients best treatment options by extending BIMA to those with a higher risk of mediastinitis. Recent economic data imply that the technology may challenge the current low uptake of BIMA by reducing the short-term cost differentials between single internal mammary artery and BIMA. Given that most published randomized controlled trials and meta-analyses of observational long-term outcome data favor BIMA, if short-term complications of BIMA including mediastinitis can be controlled adequately, there may be opportunities for more extensive use of BIMA leading to improved long-term outcomes. An ongoing study looking at BIMA in high-risk patients may provide evidence to support the hypothesis that mediastinitis should not be a factor in limiting the use of BIMA in CABG.
Keywords: CABG, mediastinitis, bilateral, outcomes, costs
Background
Coronary artery bypass grafting (CABG) is the most commonly performed cardiac surgery procedure worldwide1,2) with annual volumes of approximately 160000 isolated cases in the US.3) There is a significant inter-country variation with recent incidence rates ranging from 17 to 73 per 100000 inhabitants in western European countries.4)
The absolute number of CABG has fallen during the last decade due to an increase in percutaneous coronary intervention (PCI) procedures. For example, in Germany, between 2008 and 2018, isolated CABG surgery decreased from 47337 to 33999 cases (-28%). According to current international guidelines, in single-vessel disease, low-risk multi-vessel coronary artery disease or isolated left main disease PCI is generally preferred; on the other hand, CABG is usually recommended in patients with complex two-vessel disease, three-vessel disease, and/or non-isolated left main disease.5)
Clinical Evidence
Internal mammary artery (IMA) or internal thoracic artery has been established as the “gold standard” graft in CABG through large observational studies.6) Even though many studies are not randomized, more than 90% of CABG patients in the United Kingdom and more than 95% of CABG patients in the United States currently receive single internal mammary artery (SIMA) graft.7,8)
Based on the most recent 2018 European Society of Cardiology (ESC)/European Association for Cardio-Thoracic Surgery (EACTS) guidelines on myocardial revascularization,9) an additional arterial graft should be considered in appropriate patients where the use of the radial artery is recommended over the saphenous vein in patients with high-grade coronary artery stenosis,9) and as an alternative, the ESC/EACTS guidelines also state that the use of two IMAs, referred to as “bilateral internal mammary artery (BIMA) grafting,” should be considered in patients who do not have a high risk of sternal wound infection.9) In fact, several studies have reported that the use of BIMA grafting is associated with a significant long-term survival benefit over SIMA graft irrespective of age, left ventricular function, and diabetes.10,11) The debate on the difference in clinical outcomes associated with the use of single versus multiple arterial grafts has been going on for more than 40 years.12) Over the last 20 years, this comparison has been strengthened through the availability of several observational analyses. For the comparison between SIMA and BIMA, six meta-analyses have been published. Although there were different inclusion criteria and variations in the statistical methods, BIMA has been shown to be associated with significantly better long-term survival with a 20% mean reduction in relative risk in all the reviews13,14) (Table 1).
Table 1. Aggregate meta-analyses comparing the use of one versus two arterial grafts.
| Author, year | Studies/patients | Conduits compared | Relative risk reduction |
|---|---|---|---|
| Taggart, 2001 | 7/15962 | SIMA vs. BIMA | 19% in favor of BIMA |
| Rizzoli, 2002 | 7/15299 | SIMA vs. BIMA | 21% in favor of BIMA |
| Weiss, 2013 | 27/79063 | SIMA vs. BIMA | 22% in favor of BIMA |
| Takagi, 2014 | 20/70897 | SIMA vs. BIMA | 20% in favor of BIMA |
| Yi, 2014 | 9/15583 | SIMA vs. BIMA | 21% in favor of BIMA |
| Buttar et al., 2017 | 29/89399 | SIMA vs. BIMA | 22% in favor of BIMA |
Adapted from Gaudino et al.14) SIMA: single internal mammary artery; BIMA: bilateral internal mammary artery
In one of the most recent studies,15) data on almost 90000 patients from 29 observational studies were pooled. After over 8 years of surgery, the BIMA cohort had a superior long-term, myocardial infarction-free, and angina-free survival and a reduced operative mortality, need for repeat revascularization, and risk of cerebrovascular accident compared to the SIMA group.14) The principal additional clinical risk associated with BIMA was a significantly higher incidence of deep sterna wound infection,14) and this has been reported elsewhere.16)
Although the number of observational studies is substantial, the number of randomized controlled trials (RCTs) comparing the use of single and multiple arterial grafts is relatively limited. Results from four RCTs comparing BIMA with SIMA (summarized in Table 2) have been published.14)
Table 2. Randomized trials comparing bilateral and single internal thoracic arteries.
| Author, year | Number of patients | Country | Follow-up |
|---|---|---|---|
| Myers et al., 2000 | 162 | United States | Median 90 months |
| Gaudino et al., 2005 | 60 | Italy | Mean 52 month |
| Nasso et al., 2009 | 850 | Italy | Mean 2 years |
| Head and Kappetein, 2019 | 3102 | International | Mean 10 years |
Adapted from Gaudino et al.14)
Some studies17,18) had a very limited sample size (<100 patients). An Italian study randomized 850 patients to four different strategies.19) No difference in survival between the groups after 2 years of mean follow-up was observed, although event-free survival was better in patients who received two arterial grafts.
The Arterial Revascularization Trial (ART) is the largest RCT comparing BIMA and SIMA over 10 years. The ART included over 3000 patients randomized to a BIMA (n = 1548) or an SIMA (n = 1554).20) The primary end point of the ART was survival.
The apparent contradiction between the ART with no difference in survival and event-free survival between the BIMA and SIMA groups at 10 years20) and the large amount of observational evidence that does support the superiority of multiple arterial grafts is part of an ongoing debate.14) There are a number of important methodological limitations of the ART that have been proposed to explain the neutral findings. These limitations include the sample size calculation, the primary outcome used in the study21) and the high crossover rate in the BIMA group (16.4%), which may reflect on the lack of experience of the surgeons with systematic BIMA use.14,21,22) As a consequence, an additional as-treated analysis involving patients who received a single arterial graft against those who received multiple arterial grafts and the primary analysis using the intention-to-treat principle were carried out.20) This as-treated analysis did show that there was a benefit with BIMA for the primary and secondary outcomes.14)
Additionally, the use of the radial-artery graft rather than a saphenous-vein graft in 23% of the patients of the SIMA arm may have improved outcomes in the SIMA group since radial-artery graft results in improved graft patency and fewer clinical events.23-25) The high observance with optimal medical therapy may also have served to reduce the differences in the clinical outcome rates between the two groups,20) and the high proportion of patients older than 70 years (the benefit of BIMA grafts seems to remain to an approximate age of 69 years at surgery26)) with a treatment–age interaction close to significance have all been proposed.
However, the results of ART are interpreted; there is likely to be considerable discussion concerning the study methodology. Similarly, the observational evidence may suffer from limitations such as treatment allocation bias and the problem of hidden confounders.14) Given the uncertainty of a definitive interpretation of the results, a further trial (the Randomized comparison of the clinical Outcome of single versus Multiple Arterial grafts [ROMA] trial) has been proposed that potentially addresses many of the identified limitations of the ART.14)
Despite the observational evidence and ESC/EACTS guidelines, uptake of BIMA grafts in CABG remains low worldwide: 4.1% of all CABG procedure in the US, 12% in Europe, 12.6% in Australia, and 30% in Japan use BIMA.8,27,28)
Reasons for the limited use of BIMA grafts, despite the superior long-term observational evidence, include technical challenges, longer operating times, the possible conduit-coronary perfusion mismatch, the lack of clear guidance on when and how to use multi-arterial grafting,29,30) and morbidity including the increased risk of mediastinitis.31-33)
Mediastinitis is defined as a deep infection of the surgical wound of heart surgery, with involvement of the retrosternal space, associated or not with sternal instability/osteomyelitis.34) The incidence ranges from 0.6% to 5.6%, with mortality rates between 14% and 32%, leading to high rates of morbidity, longer in-patient hospital stays, an increased postoperative recovery, and a likely increase in hospital costs.34) According to the National Society of Thoracic Surgeons (STS) database, the incidence of mediastinitis was 0.4% among 140000 isolated coronary bypass procedures performed in 2002 irrespective of coronary bypass conduits used, while other studies have reported the incidence of mediastinitis between 0.4% and 2.7%.35-37) The incidence of mediastinitis following BIMA harvest ranges from 0.6% to 4.2%.38,39) It should also be noted that many infections occur after hospital discharge leading to a probable underestimation of the true mediastinitis incidence rate. Although published data are limited, a recent prospective study by Perrault et al.40) showed that 65% of mediastinal infections occurred after index hospitalization discharge.
In a multivariate analysis, Risnes et al.41) identified six preoperative variables as highly significant independent predictors for the development of mediastinitis: diabetes, obesity, BMI >30 kg/m2, chronic obstructive pulmonary disease (COPD), age, and male gender. Postoperative hyperglycemia is associated with an increased risk of mediastinal infection in non-diabetics but not in diabetics.40)
Given the association between certain risk factors and the likelihood of mediastinitis, several scoring systems have been developed to assist in the prediction of mediastinitis occurrence. Fowler et al.42) developed a simple bedside risk score using the STS National Cardiac Database, including 331429 patients undergoing CABG surgery. The STS score estimates the patient risk for major infection (mediastinitis, thoracotomy or vein harvest site infection, or septicemia) after CABG. Other scoring systems include the EuroSCORE, the MagedanzSCORE, the Gatti score (specifically designed for BIMA), and the Med-Score 24, a bedside risk score for poststernotomy mediastinitis, which according to the authors showed excellent predictive power 24 hours after admission to the intensive care unit (ICU) for mediastinitis risk. It should be noted that in a recent validation study, current predictive models for surgical site infections (SSIs) after CABG showed low accuracy of prediction despite satisfactory calibration and moderate predictive power.43)
Resource Use and Costs Associated with Mediastinitis
Patients with mediastinitis experience higher mortality and morbidity, including longer length of in-patient stay, the need for re-operation, ICU admissions, and hospital readmissions.44,45) Hospital-acquired conditions (HACs) also have a significant financial impact on healthcare systems.46) The estimated additional costs associated with mediastinitis range from $19000 to $56000 per case44,47) in the US. Another study showed that of all HACs, mediastinitis after CABG had the highest marginal impact for both length of stay (LOS) and total costs.46) In a German study,48) mediastinitis represented an important economic factor for the hospital as it almost tripled the costs for patients undergoing CABG. Additionally, there was a financial loss of 9154 euro per patient given that the median reimbursement from health-care insurance companies was 27107 euro per case.48) Hence, reducing the incidence and the subsequent management of mediastinitis is of interest to all involved: patients, hospitals, and payers.
The impact of mediastinitis for hospitals should not be underestimated. Extended hospital LOS, costs associated with treating mediastinitis and readmissions, and the loss of reimbursement due to approaches such as the Centers for Medicare & Medicaid Services (CMS) value-based purchasing initiative49) and payments by results in the UK National Health Service (NHS),50) have major financial implications.
Cardiac surgical programmes need to review their outcomes, be active in identifying opportunities for improvement, and implement practices that are known to reduce mediastinitis.51)
Strategies to Reduce Mediastinitis
A number of risk factors for the development of mediastinitis have been cited in the literature: advanced age, active smoking, coronary artery disease, chronic infections, chronic lung disease, obesity, diabetes, end-stage renal disease, low ejection fraction, osteoporosis, and steroid treatment.52)
Likewise, several risk factors associated with the development of mediastinitis following CABG using single or two internal mammary arteries are modifiable.35,53,54) There are several strategies (broadly divided into preoperative, intraoperative, and postoperative strategies [Table 3]) that can be adopted to reduce mediastinitis following the harvest of BIMAs in order to reduce the difference in mediastinitis rates between SIMA and BIMA.
Table 3. Strategies to reduce mediastinitis.
| Preoperative strategies | Intraoperative strategies | Postoperative strategies |
|---|---|---|
| Optimization of glycemic control in diabetics (HbA1c <8.0%)56,57) Reduction of weight in obese (BMI <30 kg/m2)57) Cessation of cigarette smoking57) Optimization of pulmonary function in COPD patients (FEV1/FVC <0.70)32,57) Systematic preoperative decolonization measures (e.g. mupirocin nasal ointment)55) |
Skin disinfection with remnant active agents, e.g. chlorhexidine58) Iodine-impregnated skin drapes67) Repetitive surgical glove exchange58) Avoidance of (excessive) bone wax67) Meticulous skeletonized IMA harvesting67) Cautious use of electrocautery67) Avoidance of long operative period (<7 hours)57) Avoidance of long cardiopulmonary bypass time (<180 minutes)57) Avoidance of the need for IABP support57) Use of antibiotic sponge or paste below/ on sternal marrow before closure66) Use >8 sternal wires for closure72) |
Avoidance of low cardiac output status57) Avoid the usage of sympathomimetic agents and vasopressors57) Reduction of ventilator support time <48 hours57) Avoiding transfusion of packed red blood cell, single donor platelets57) Use of chest stabilizing vests (e.g. posthorax)77) Use of NPWT (e.g. PICO)79) |
HbA1c: hemoglobin A1c; BMI: body mass index; COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in the first second; FVC: forced vital capacity; IMA: internal mammary artery; NPWT: negative pressure wound therapy
There are a number of basic process improvement opportunities for cardiac surgery populations including pre-operative showering and nasal decolonization programs,55) antibiotic prophylaxis, hair removal, glucose control,56,57) surgical skin antisepsis, instrument flashing, aseptic technique, surgical technique, and postoperative dressings.51)
Cardiac surgery is considered as clean surgery where the majority of pathogens responsible for infections come from the patient’s skin. Thus, careful preoperative skin decolonization and use of disinfectants based on chlorhexidine-isopropanol rather than povidon-iodine ethanol can reduce surgical-site infections.58) After numerous studies favoring chlorhexidine, the CLEAN2 trial is the first RCT comparing povidon-iodine with chlorhexidine in a cardiac surgery cohort.59) Preoperative showering with chlorhexidine is widely accepted but clear evidence of its effectiveness is still lacking,60) whereas prophylactic perioperative antibiotic therapy and intra-nasal prophylaxis with mupirocin seem to be effective in reducing the incidence of postoperative sternal wound infection.61)
Poor perioperative glycemic control deteriorates the patient’s outcome after cardiac surgery. Postoperative glucose levels of >250 mg/dl increase the risk of postoperative complications by a factor of 10. Based on the increased cardiovascular risk of diabetes patients and the detrimental effects of perioperative hyperglycemia especially in BIMA patients, strict glucose level control is essential in avoiding mediastinitis.56)
In a recent review by Schiraldi et al.,52) additional risk factors that worsen wound healing were identified. These include poor skin preparation, emergency operation, transfusion with high volumes of red blood cells, transfusion with platelets, extended operative and perfusion time, bleeding after surgery, sternal rewiring, employment of an intra-aortic balloon pump, prolonged use of electrocautery, and maladjusted prophylactic antibiotic administration (>60 minutes prior to incision).52)
In a large meta-analysis by Dai et al.,62) the use of BIMA was shown to increase the relative risk of deep sternal wound infection (DSWI) by 62% when compared with LIMA. This increased risk was most prominent in patients with diabetes and in the elderly. However, skeletonization in BIMA harvesting was not associated with an increased risk of DSWI, proving special operative techniques to maintain sternal perfusion and hence reduce wound infections.63)
Various aspects of perioperative primary surgical care were reported to have a significant impact on mediastinitis risk in the literature.64) In addition to meticulous disinfection and compliance with sterility principles, the topical use of antimicrobials applied to the sternum during cardiac procedures combined with standard intravenous agents may yield satisfactory results for mediastinitis prevention.52) Osawa et al.65) showed that spraying a solution of gentamicin and cefazolin on the surgical site multiple times during cardiac surgery had beneficial effects in terms of protecting high-risk patients.
There is evidence that prophylactic implantation of gentamicin–collagen sponges reduces the incidence of sternal wound infections after cardiac surgery. The analysis of 22135 patients revealed a significant reduction, but especially in high-risk patients (e.g. after BIMA harvest), only a combination of different measures might be beneficial in prevention of SWI.66)
Topical vancomycin paste applied to the sternal cutting edges instead of bone wax67) and before sternal closure seems to reduce sternal wound infections and is recommended by the expert consensus review.68) However, literature results are inconclusive. Whereas a meta-analysis of mainly observational studies shows a reduction of wound infections in 2017,69) a retrospective review of 14492 patients failed to show that vancomycin reduced the risk of DSWI.70)
Strong coughing has a considerable effect on sternal stability, since it induces powerful shearing forces that may easily untwist the wires.52,71) Sternal fixation techniques were found to significantly affect the infection rate.52,72) Unsatisfactory treatment results have resulted in the search for alternative surgical concepts. Moving beyond traditional sternal closure with sternal wires, hybrid techniques have been proposed to achieve better sternal stability. Novel approaches combine stainless steel monofilament wire sutures with peristernal cable-tie devices (ZipFix; DePuy Synthes GmbH, Oberdorf, Switzerland), which appears to be an effective way of reducing the mediastinitis rate.52)
Strong coughing, raising the intra-thoracic pressure up to 300 mmHg, was considered to be a factor with a considerable effect on sternal stability, as it induces strong shearing forces that may easily untwist the wires.
Dehiscence and infection of the sternum may preclude rewiring, especially in patients with multiple morbidities. Titanium plate sternal fixation, despite making it more difficult to quickly reaccess the mediastinum, reduced the need for multiple rounds of debridement, offering good sternal stability and reducing mortality (11.1%) when compared to traditional wire refixation (19.2%). This strategy was suggested for primary surgery in high-risk patients or in patients undergoing sternal wound debridement.73) Nonetheless, reinfection and postoperative sternal pain limit the application of sternal titanium plates to high-risk patients who are unsuitable for standard rewiring.74)
Vogt et al.64) have also recently reported the results from a multinational study on the use of an innovative closing protocol using defined measures preoperatively, intraoperatively, and postoperatively. These results led to a markedly reduced number of postoperative sternal wound infections.64)
Autologous platelet-rich plasma (PRP) use for the prevention of mediastinitis has been suggested, with positive clinical results.52,75,76) PRP wound irrigation was found to improve healing and led to a reduced incidence of sternal infections.52,76) Additionally, PRP was also able to restrain the proliferation of Staphylococcus aureus, one of the most prevalent bacteria responsible for mediastinitis.52,76) However, BIMA were used only in 5 % of the patients included in this study. At the time of the publication of this article, there was no study comparing PRP in a BIMA patient collective.
Besides careful sternal closure techniques, extracorporal stabilization vests (Posthorax; Epple Inc., Vienna, Austria) can help to avoid sternal dehiscence when worn 24 hours for 6 weeks, but there is no beneficial effect on DSWI when patients deviate from this protocol. Thus, sternum stabilization vests can be a helpful preventive tool for compliant and well-informed patients.77)
Negative pressure wound therapy (NPWT) is a treatment concept in acute and chronic wound therapy. The negative wound pressure continually drains bacteria, debris, and exudates, enhancing microcirculation, and accelerates tissue granulation. Owing to its increasing use, a number of studies have found the clinical effect of NWPT to be similar to traditional closed drainage or open packing, with the added benefit of an improvement in sternal wound healing, length of ICU stay, reinfection rates, and possibly mortality.78) Closed incision NPWT adopts the principles of NPWT to promote healing in closed surgical wounds and to reduce surgical site complications (SSCs) such as mediastinitis (Fig. 1).79,80)
Fig. 1. Application of PICO single-use NPWT.80) (A) Immediately postoperative. Drain lines need to be more than 5 cm away from incision. (B) Application of PICO in the operating room. Dressing is better too long than too short. (C) Application of PICO in the operating room. Port is positioned proximally. Side strips are used to secure tubing. Dressing is rigid to touch, and pump vibration is less than 5 seconds per minute. NPWT: negative pressure wound therapy.
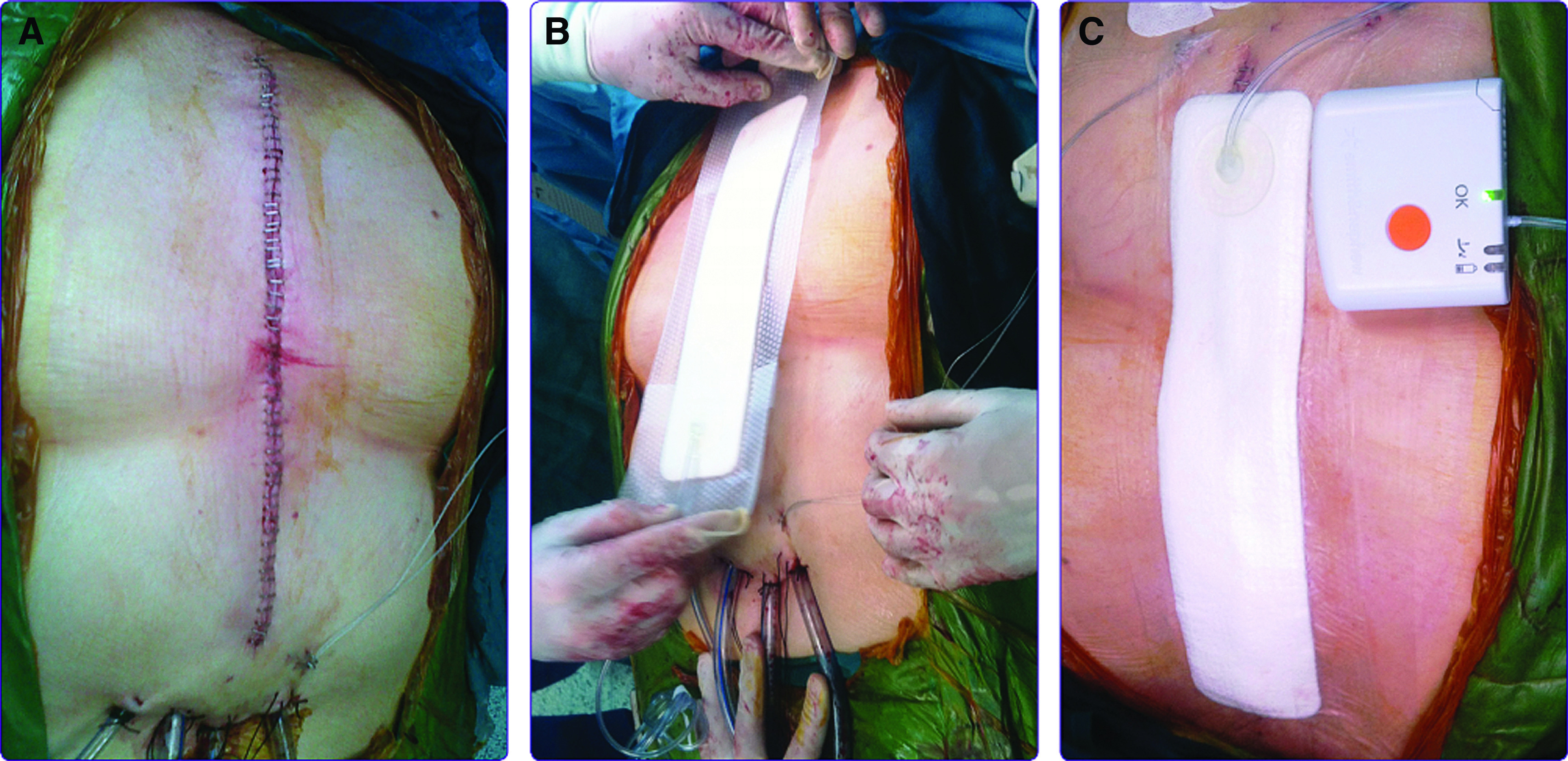
The specific dressings create a negative-pressure environment at the wound site. This helps to hold the incision edges together, reduces lateral tension and edema, stimulates perfusion, enhances the development of granulation tissue, reduces bacterial colonization of wound tissues, and protects the surgical site from external infectious sources.81)
A medical technology briefing from NICE (last updated: August 2019) has reviewed the evidence for the PICO NPWT for closed surgical incisions.79) A meta-analysis of 16 comparative studies with a total of 1895 people showed lower rates of SSIs in patients treated with PICO.79) Besides observational studies, one RCT showed promising results in reducing sternal infections after cardiac surgery.60) University Hospitals Bristol, UK, started implementing the PICO pathway in high-risk patients based on results with 153 non-CABG and 148 CABG procedures. There was a 50% reduction in the SSI rate of the CABG procedure after the implementation of the pathway.82) In the absence of guidelines on specific indications for the use of PICO in CABG surgery, the cardiac centre in Hamburg uses PICO in prophylaxis against mediastinitis for all BIMA CABG procedures.
Economics of Mediastinitis Prevention
Novel approaches for the reduction of mediastinitis are likely to initially cost more than standard care (SC) because of the additional cost of the new intervention. However, there is literature calculating extra costs of novel treatment options – e.g. closed incision NPWT – against secondary economic benefits. Fewer dressing changes, a reduced length of hospital stay, and fewer readmissions can save healthcare resources. Hence, the initial additional cost may be offset. According to NICE support for commissioning for SSI (2013), the cost of an SSI could be as high as £20000 for complex surgeries and £14000 for general surgeries.79)
Nherera et al.83) reviewed the economic implications from the perspective of the NHS of single-use NPWT (sNPWT) compared with conventional postsurgical dressings, in reducing SSC in people having primary hip and knee replacements. The analysis used data from a non-blinded RCT by Karlakki et al.84) comparing PICO to conventional dressings. Results from the economic model showed that patients who had sNPWT had a quality-adjusted life year (QALY) gain of 0.116 and 0.98 complications avoided compared with 0.115 QALY gained and 0.92 complications avoided for conventional dressings.83) The per-patient costs saving was estimated at £1132 in favor of sNPWT.83) In the higher risk subgroups, more cost savings were realized: in people with a body mass index (BMI) of 35 or above, this was £7955, and in people with an American Society of Anaesthesiologists physical status classification of greater than 3, this was £7248.83)
A further study by Nherera et al.85) calculated the cost-effectiveness of sNPWT compared to standard of care in patients following CABG procedure to reduce SSCs defined as dehiscence and sternotomy infections. A decision tree was developed from the Germany Statutory Health Insurance payer’s perspective over a 3-month time horizon. Baseline data on SSC and resources were obtained from a prospective observational study of 2621 CABG patients in Germany. Effectiveness data for sNPWT were taken from a Polish open-label trial that randomized 80 patients to either sNPWT or SC treatment. Cost data (in euros) were taken from the relevant diagnostic-related groups and published literature.85)
The clinical data showed an increase in wounds that healed without complications in 37/40 (92.5%) patients in the sNPWT group compared to 30/40 (75%) patients in the SC group (p = 0.03).85) The estimated mean cost per patient resulted in a cost-saving of €586 in the sNPWT group.85) Sensitivity analyses showed that the findings were robust for a realistic range of values of the key variables.85)
Given the above findings, an observational research database study is currently being carried out in Germany (n = 1200) using PICO as part of an infection prevention strategy for high-risk patients after BIMA. It is expected that the study will show a reduction in SSI incidence rates compared to risk estimates based on standardized risk scores. Additionally, it will be possible to evaluate the cost impact of PICO prophylaxis in different patient subgroups.
Given the lack of clinical evidence to support PICO NPWT in CABG,85) results from the above study will be welcome. Results from the study could be used to calculate program intervention benefits expressed in terms of the following:
Attributable cost : This would consider the difference in LOS for mediastinitis compared with the mean LOS for non-mediastinitis after CABG for different risk scores.
Budget impact: Using standard International Society for Pharmacoeconomics and Outcomes Research (ISPOR) budget impact methodological guidelines and a similar approach used by Gray et al.86) in their 1 year cost study based on ART– different sub-groups and scenarios could be considered to measure the economic impact of a prophylactic approach with PICO NPWT (reduced mediastinitis in CABG but higher initial costs versus SC), reduction in other healthcare-associated infections, and an expected net benefit in avoidable costs (as demonstrated in the limited published economic analyses).
Hence, given that BIMA is associated with a higher incidence of mediastinitis (compared with SIMA), a reduction in the incidence of mediastinitis especially in high-risk subgroups with the associated lower resource utilization and costs combined with superior long-term outcomes (observational data compared with SIMA) may remove one of the barriers to more extensive BIMA implementation in CABG.
Discussion
Many different strategies can be used to reduce the risk of mediastinitis following CABG using BIMA grafts, and it is essential that patient management is continually reviewed in the light of the most recent knowledge and clinical experience. Mediastinitis continues to adversely impact CABG procedures, particularly in the case of BIMA grafts. Strategies can be used to reduce the rate of mediastinitis and minimize preoperative, intraoperative, and postoperative risk factors.57) These include encouraging patients to stop smoking, optimizing perioperative control of hyperglycemia, the administration of prophylactic antibiotics, the maintenance of sterile operative conditions, as well as the selection of the most appropriate surgical techniques in order to lower the rate of mediastinitis following BIMA harvest.57) Other recommendations to prevent mediastinitis include avoiding the use of BIMA grafting in patients with BMI >35 kg/m2, severe COPD, and uncontrolled diabetes.57) The availability of innovative postoperative wound management systems (e.g. PICO NPWT) is likely to reduce the absolute difference in incidence rates of mediastinitis between BIMA and SIMA, especially in those patients classified as high risk. Given that BIMA has been shown to be superior in all meta-analyses of observational studies in terms of long-term outcomes, by reducing the absolute differences in incidence rates of mediastinitis between BIMA and SIMA, it is likely to make BIMA a more appealing option in those regions where currently the use of BIMA in CABG is low. The results of the ongoing observational mediastinitis study in Germany may help inform guidelines on which patients undergoing CABG are most suitable for PICO.
Disclosure Statement
NB and MS are employees of Asklepios Klinik St Georg where the ongoing observational mediastinitis study, supported by Smith &Nephew, is being carried out; WMH serves as a consultant for Genesyze; and TA and CH are directors of Genesyze.
References
- 1). Melly L, Torregrossa G, Lee T, et al. Fifty years of coronary artery bypass grafting. J Thorac Dis 2018; 10: 1960- 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Squiers JJ, Mack MJ. Coronary artery bypass grafting-fifty years of quality initiatives since Favaloro. Ann Cardiothorac Surg 2018; 7: 516- 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). D’Agostino R, Jacobs J, Badhwar V, et al. The society of thoracic surgeons adult cardiac surgery database: 2019 update on outcomes and quality. Ann Thorac Surg 2019; 107: 24- 32. [DOI] [PubMed] [Google Scholar]
- 4). OECD/European Union “Cardiac procedures”, in Health at a Glance: Europe 2016: State of Health in the EU Cycle; 2016.
- 5). De Innocentiis C, Zimarino M, De Caterina R. Is complete revascularisation mandated for all patients with multivessel coronary artery disease? Interv Cardiol 2018; 13: 45- 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Cameron A, Davis KB, Green G, et al. Coronary bypass surgery with internal-thoracic-artery grafts–effects on survival over a 15-year period. N Engl J Med 1996; 334: 216- 9. [DOI] [PubMed] [Google Scholar]
- 7). Society of Cardiothoracic Surgeons of Great Britain and Ireland , National Adult Cardiac Surgical Database Report 1999-2000. 2001
- 8). ElBardissi AW, Aranki SF, Sheng S, et al. Trends in isolated coronary artery bypass grafting: an analysis of the society of thoracic surgeons adult cardiac surgery database. J Thorac Cardiovasc Surg 2012; 143: 273- 81. [DOI] [PubMed] [Google Scholar]
- 9). Neumann F, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J 2019; 40: 87- 165. [DOI] [PubMed] [Google Scholar]
- 10). Taggart DP. Bilateral internal mammary artery grafting: are BIMA better? Heart 2002; 88: 7- 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Lytle BW, Blackstone EH, Loop FD, et al. Two internal thoracic artery grafts are better than one. J Thorac Cardiovasc Surg 1999; 117: 855- 72. [DOI] [PubMed] [Google Scholar]
- 12). Barner HB. Double internal mammary-coronary artery bypass. Arch Surg 1974; 109: 627- 30. [DOI] [PubMed] [Google Scholar]
- 13). Gaudino M, Di Franco A, Rahouma M, et al. Unmeasured confounders in observational studies comparing bilateral versus single internal thoracic artery for coronary artery bypass grafting: a meta-analysis. J Am Heart Assoc 2018; 7: 1- 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Gaudino MFL, Taggart DP, Fremes SE. The ROMA trial: why it is needed. Curr Opin Cardiol 2018; 33: 622- 6. [DOI] [PubMed] [Google Scholar]
- 15). Buttar SN, Yan TD, Taggart DP, et al. Long-term and short-term outcomes of using bilateral internal mammary artery grafting versus left internal mammary artery grafting: a meta-analysis. Heart 2017; 103: 1419- 26. [DOI] [PubMed] [Google Scholar]
- 16). Davierwala PM, Mohr FW. Bilateral internal mammary artery grafting: rationale and evidence. Int J Surg 2015; 16: 133- 9. [DOI] [PubMed] [Google Scholar]
- 17). Myers WO, Berg R, Ray JF, et al. All-artery multigraft coronary artery bypass grafting with only internal thoracic arteries possible and safe: a randomized trial. Surgery 2000; 128: 650- 9. [DOI] [PubMed] [Google Scholar]
- 18). Gaudino M, Cellini C, Pragliola C, et al. Arterial versus venous bypass grafts in patients with in-stent restenosis. Circulation 2005; 112: 1265- 9. [DOI] [PubMed] [Google Scholar]
- 19). Nasso G, Coppola R, Bonifazi R, et al. Arterial revascularization in primary coronary artery bypass grafting: direct comparison of 4 strategies–results of the Stand-in-Y Mammary Study. J Thorac Cardiovasc Surg 2009; 137: 1093- 100. [DOI] [PubMed] [Google Scholar]
- 20). Taggart DP, Benedetto U, Gerry S, et al. Bilateral versus single internal-thoracic-artery grafts at 10 Years. N Engl J Med 2019; 380: 437- 46. [DOI] [PubMed] [Google Scholar]
- 21). Wood S. Ten-year follow-up proves disappointing for bilateral arterial grafts in ART. https://wwwtctmdcom/news/ten-year-follow-proves-disappointing-bilateral-arterial-grafts-art 2018.
- 22). Benedetto U, Altman DG, Flather M, et al. Incidence and clinical implications of intraoperative bilateral internal thoracic artery graft conversion. Insights from the Arterial Revascularization Trial. J Thorac Cardiovasc Surg 2018; 155: 2346- 55.e6. [DOI] [PubMed] [Google Scholar]
- 23). Head SJ, Kappetein AP. Coronary bypass surgery - an ART for dedicated surgeons. N Engl J Med 2019; 380: 489- 91. [DOI] [PubMed] [Google Scholar]
- 24). Gaudino M, Benedetto U, Fremes S, et al. Radial-artery or saphenous-vein grafts in coronary-artery bypass surgery. N Engl J Med 2018; 378: 2069- 77. [DOI] [PubMed] [Google Scholar]
- 25). Taggart DP, Altman DG, Flather M, et al. Associations between adding a radial artery graft to single and bilateral internal thoracic artery grafts and outcomes: insights from the arterial revascularization trial. Circulation 2017; 136: 454- 63. [DOI] [PubMed] [Google Scholar]
- 26). Persson M, Sartipy U. Bilateral versus single internal thoracic artery grafts. Curr Cardiol Rep 2018; 20: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Yan BP, Clark DJ, Buxton B, et al. Clinical characteristics and early mortality of patients undergoing coronary artery bypass grafting compared to percutaneous coronary intervention: insights from the Australasian Society of Cardiac and Thoracic Surgeons (ASCTS) and the Melbourne Interventional Group (MIG) Registries. Heart Lung Circ 2009; 18: 184- 90. [DOI] [PubMed] [Google Scholar]
- 28). Sezai Y, Orime Y, Tsukamoto S. Coronary artery surgery results 2015 in Japan. Ann Thorac Cardiovasc Surg 2007; 13: 220- 3. [PubMed] [Google Scholar]
- 29). Emmert MY. CABG in the era of modern PCI. Eur Heart J 2017; 38: 2029- 32. [DOI] [PubMed] [Google Scholar]
- 30). Aldea GS, Bakaeen FG, Pal J, et al. The society of thoracic surgeons clinical practice guidelines on arterial conduits for coronary artery bypass grafting. Ann Thorac Surg 2016; 101: 801- 9. [DOI] [PubMed] [Google Scholar]
- 31). Abu-Omar Y, Kocher GJ, Bosco P, et al. European Association for Cardio-Thoracic Surgery expert consensus statement on the prevention and management of mediastinitis. Eur J Cardiothorac Surg 2017; 51: 10- 29. [DOI] [PubMed] [Google Scholar]
- 32). Diez C, Koch D, Kuss O, et al. Risk factors for mediastinitis after cardiac surgery - a retrospective analysis of 1700 patients. J Cardiothorac Surg 2007, 2- 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). Shaikhrezai K, Robertson FL, Anderson SE, et al. Does the number of wires used to close a sternotomy have an impact on deep sternal wound infection? Interact Cardiovasc Thorac Surg 2012; 15: 219- 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34). Oliveira FDS, Freitas LDO, Rabelo-Silva ER, et al. Predictors of mediastinitis risk after coronary artery bypass surgery: applicability of score in 1.322 cases. Arq Bras Cardiol 2017; 109: 207- 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Borger MA, Rao V, Weisel RD, et al. Deep sternal wound infection: risk factors and outcomes. Ann Thorac Surg 1998; 65: 1050- 6. [DOI] [PubMed] [Google Scholar]
- 36). Dorman MJ, Kurlansky PA, Traad EA, et al. Bilateral internal mammary artery grafting enhances survival in diabetic patients: a 30-year follow-up of propensity score-matched cohorts. Circulation 2012; 126: 2935- 42. [DOI] [PubMed] [Google Scholar]
- 37). Tatoulis J, Buxton BF, Fuller JA, J Maxwell Chamberlain Memorial paper The right internal thoracic artery: the forgotten conduit - 5766 patients and 991 angiograms. Ann Thorac Surg 2011; 92: 9- 15. [DOI] [PubMed] [Google Scholar]
- 38). Sajja LR, Mannam G, Dandu SB, et al. Reduction of sternal wound infections in diabetic patients undergoing off-pump coronary artery bypass surgery and using modified pedicle bilateral internal thoracic artery harvest technique. J Thorac Cardiovasc Surg 2012; 144: 480- 5. [DOI] [PubMed] [Google Scholar]
- 39). De Paulis R, de Notaris S, Scaffa R, et al. The effect of bilateral internal thoracic artery harvesting on superficial and deep sternal infection: the role of skeletonization. J Thorac Cardiovasc Surg 2005; 129: 536- 43. [DOI] [PubMed] [Google Scholar]
- 40). Perrault LP, Kirkwood KA, Chang HL, et al. A prospective multi-institutional cohort study of mediastinal infections after cardiac operations. Ann Thorac Surg 2018; 105: 461- 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41). Risnes I, Abdelnoor M, Almdahl SM, et al. Mediastinitis after coronary artery bypass grafting risk factors and long-term survival. Ann Thorac Surg 2010; 89: 1502- 9. [DOI] [PubMed] [Google Scholar]
- 42). Fowler VG, O'Brien SM, Muhlbaier LH, et al. Clinical predictors of major infections after cardiac surgery. Circulation 2005; 112: I358- 65. [DOI] [PubMed] [Google Scholar]
- 43). Gatti G, Rochon M, Raja SG, et al. Predictive models of surgical site infections after coronary surgery: insights from a validation study on 7090 consecutive patients. J Hosp Infect 2019; 102: 277- 86. [DOI] [PubMed] [Google Scholar]
- 44). Osnabrugge RL, Speir AM, Head SJ, et al. Prediction of costs and length of stay in coronary artery bypass grafting. Ann Thorac Surg 2014; 98: 1286- 93. [DOI] [PubMed] [Google Scholar]
- 45). Hollenbeak CS, Murphy DM, Koenig S, et al. The clinical and economic impact of deep chest surgical site infections following coronary artery bypass graft surgery. Chest 2000; 118: 397- 402. [DOI] [PubMed] [Google Scholar]
- 46). Nero DC, Lipp MJ, Callahan MA. The financial impact of hospital-acquired conditions. J Health Care Finance 2012; 38: 40- 9. [PubMed] [Google Scholar]
- 47). LaPar DJ, Crosby IK, Rich JB, et al. A contemporary cost analysis of postoperative morbidity after coronary artery bypass grafting with and without concomitant aortic valve replacement to improve patient quality and cost-effective care. Ann Thorac Surg 2013; 96: 1621- 7. [DOI] [PubMed] [Google Scholar]
- 48). Graf K, Ott E, Vonberg RP, et al. Economic aspects of deep sternal wound infections. Eur J Cardiothorac Surg 2010; 37: 893- 6. [DOI] [PubMed] [Google Scholar]
- 49). Amin AN, Hofmann H, Owen MM, et al. Reduce readmissions with service-based care management. Prof Case Manag 2014; 19: 255- 62. [DOI] [PubMed] [Google Scholar]
- 50). Department of Health Payment by Results Guidance for 2012-13, London: 2012. [Google Scholar]
- 51). APIC Guide for the prevention of mediastinitis surgical site infections following cardiac surgery; 2008.
- 52). Schiraldi L, Jabbour G, Centofanti P, et al. Deep sternal wound infections: Evidence for prevention, treatment, and reconstructive surgery. Arch Plast Surg 2019; 46: 291- 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53). Lu JC, Grayson AD, Jha P, et al. Risk factors for sternal wound infection and mid-term survival following coronary artery bypass surgery. Eur J Cadiothorac Surg 2003; 23: 943- 9. [DOI] [PubMed] [Google Scholar]
- 54). Pevni D, Mohr R, Lev-Rum O, et al. Influence of bilateral skeletonized harvesting on occurrence of deep sternal wound infection in 1,000 consecutive patients undergoing bilateral thoracic artery grafting. Ann Surj 2003; 237: 277- 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55). Beckmann A, Doebler K, Schaefer E, et al. Sternal surgical site infection prevention - is there any room for improvement? Eur J Cardiothorac Surg 2011; 40: 347- 51. [DOI] [PubMed] [Google Scholar]
- 56). Lazar HL, McDonnell M, Chipkin SR, et al. The society of thoracic surgeons practice guideline series: blood glucose management during adult cardiac surgery. Ann Thorac Surg 2009; 87: 663- 9. [DOI] [PubMed] [Google Scholar]
- 57). Sajja LR. Strategies to reduce deep sternal wound infection after bilateral internal mammary artery grafting. Int J Surg 2015; 16: 171- 8. [DOI] [PubMed] [Google Scholar]
- 58). Macias JH, Arreguin V, Munoz JM, et al. Chlorhexidine is a better antiseptic than povidone iodine and sodium hypochlorite because of its substantive effect. Am J Infect Control 2013; 41: 634- 7. [DOI] [PubMed] [Google Scholar]
- 59). Boisson M, Corbi P, Kerforne T, et al. Multicentre, open-label, randomised, controlled clinical trial comparing 2% chlorhexidine–70% isopropanol and 5% povidone iodine–69% ethanol for skin antisepsis in reducing surgical-site infection after cardiac surgery: the CLEAN 2 study protocol. BMJ Open 2019; 9: e026929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60). Webster J, Osborne S. Preoperative bathing or showering with skin antiseptics to prevent surgical site infection. Cochrane Database Syst Rev 2015, CD004985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61). Ennker IC, Pietrowski D, Vöhringer L, et al. Surgical debridement, vacuum therapy and pectoralis plasty in poststernotomymediastinitis. J Plast Reconstr Aesthet Surg 2009; 62: 1479- 83. [DOI] [PubMed] [Google Scholar]
- 62). Dai C, Lu Z, Zhu H, et al. Bilateral internal mammary artery grafting and risk of sternal wound infection: evidence from observational studies. Ann Thorac Surg 2013; 95: 1938- 45. [DOI] [PubMed] [Google Scholar]
- 63). Sa MP, Ferraz PE, Escobar RR, et al. Skeletonized versus pedicled internal thoracic artery and risk of sternal wound infection after coronary bypass surgery: meta-analysis and meta-regression of 4817 patients. Interact Cardiovasc Thorac Surg 2013; 16: 849- 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64). Vogt P, Berdat P, Santoro G, et al. Significant reduction of sternal wound infection in cardiac surgical patients. American Journal of Clinical Microbiology and Antimicrobials 2019; 2: 1- 7. [Google Scholar]
- 65). Osawa H, Yoshii S, Abraham SJ, et al. Topical spraying of cefazolin and gentamicin reduces deep sternal wound infections after heart surgery: a multicenter, large volume, retrospective study. Gen Thorac Cardiovasc Surg 2016; 64: 197- 202. [DOI] [PubMed] [Google Scholar]
- 66). Kowalewski M, Pawliszak W, Zaborowska K, et al. Gentamicin-collagen sponge reduces the risk of sternal wound infections after heart surgery: meta-analysis. J Thorac Cardiovasc Surg 2015; 149: 1631- 40.e1-6. [DOI] [PubMed] [Google Scholar]
- 67). Meszaros K, Fuehrer U, Grogg S, et al. Risk factors for sternal wound infection after open heart operations vary according to type of operation. Ann Thorac Surg 2016; 101: 1418- 25. [DOI] [PubMed] [Google Scholar]
- 68). Lazar HL, Salm TV, Engelman R, et al. Prevention and management of sternal wound infections. J Thorac Cardiovasc Surg 2016; 152: 962- 72. [DOI] [PubMed] [Google Scholar]
- 69). Kowalewski M, Raffa GM, Szwed KA, et al. Meta-analysis to assess the effectiveness of topically used vancomycin in reducing sternal wound infections after cardiac surgery. J Thorac Cardiovasc Surg 2017; 154: 1320- 3.e3. [DOI] [PubMed] [Google Scholar]
- 70). Lander HL, Ejiofor JI, McGurk S, et al. Vancomycin paste does not reduce the incidence of deep sternal wound infection after cardiac operations. Ann Thorac Surg 2017; 103: 497- 503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71). Irwin RS. Complications of cough: ACCP evidence-based clinical practice guidelines. Chest 2006; 129: 54S- 8S. [DOI] [PubMed] [Google Scholar]
- 72). Kamiya H, Al-maisary SS, Akhyari P, et al. The number of wires for sternal closure has a significant influence on sternal complications in high-risk patients. Interact Cardiovasc Thorac Surg 2012; 15: 665- 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73). Wang W, Wang S. Titanium plate fixation versus conventional approach in the treatment of deep sternal wound infection. J Cardiothorac Surg 2016; 11: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74). Voss S, Will A, Lange R, et al. Mid-term results after sternal reconstruction using titanium plates: is it worth it to plate? Ann Thorac Surg 2018; 105: 1640- 7. [DOI] [PubMed] [Google Scholar]
- 75). Kirmani BH, Jones SG, Datta S, et al. A meta-analysis of platelet gel for prevention of sternal wound infections following cardiac surgery. Blood Transfus 2017; 15: 57- 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76). Patel AN, Selzman CH, Kumpati GS, et al. Evaluation of autologous platelet rich plasma for cardiac surgery: outcome analysis of 2000 patients. J Cardiothorac Surg 2016; 11: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77). Gorlitzer M, Wagner F, Pfeiffer S, et al. Prevention of sternal wound complications after sternotomy: results of a large prospective randomized multicentre trial. Interact Cardiovasc Thorac Surg 2013; 17: 515- 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78). Yusuf E, Chan M, Renz N, et al. Current perspectives on diagnosis and management of sternal wound infections. Infect Drug Resist 2018; 11: 961- 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79). NICE PICO negative pressure wound dressings for closed surgical incisions; 2019.
- 80). Monteagudo Vela M, Sartor L, Sanchez Perez R, et al. Application of PICO single use negative pressure wound therapy as a preventive measure for surgical wound infections (SWIs) and dehiscences in at-risk patients undergoing heart surgery. Smith & Nephew case study 45923 Smith & Nephew Healthcare, 2013. [Google Scholar]
- 81). Dohmen PM, Markou T, Ingemansson R, et al. Use of incisional negative pressure wound therapy on closed median sternal incisions after cardiothoracic surgery: clinical evidence and consensus recommendations. Med Sci Monit 2014; 20: 1814- 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82). University Hospitals Bristol Implementation of PICO Incision Management Negative Pressure Wound therapy in the high-risk Cardiac Surgery Patient Group , Available at: https://www.nice.org.uk/sharedlearning/university-hospitals-bristol-implementation-of-pico-incision-management-negative-pressure-wound-therapy-in-the-high-risk-cardiac-surgery-patient-group.
- 83). Nherera LM, Trueman P, Karlakki SL. Cost-effectiveness analysis of single-use negative pressure wound therapy dressings (sNPWT) to reduce surgical site complications (SSC) in routine primary hip and knee replacements. Wound Repair Regen 2017; 25: 474- 82. [DOI] [PubMed] [Google Scholar]
- 84). Karlakki SL, Hamad AK, Whittall C, et al. Incisional negative pressure wound therapy dressings (iNPWTd) in routine primary hip and knee arthroplasties: a randomised controlled trial. Bone Joint Res 2016; 5: 328- 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85). Nherera LM, Trueman P, Schmoeckel M, et al. Cost-effectiveness analysis of single use negative pressure wound therapy dressings (sNPWT) compared to standard of care in reducing surgical site complications (SSC) in patients undergoing coronary artery bypass grafting surgery. J Cardiothorac Surg 2018; 13: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86). Gray AM, Murphy J, Altman DG, et al. One-year costs of bilateral or single internal mammary grafts in the. Arterial Revascularisation Trial. Heart 2017; 103: 1719- 26. [DOI] [PubMed] [Google Scholar]


