Abstract
Hematopoietic stem cells (HSCs) can stably regenerate all lineages of the adult blood and immune systems following transplantation via a combination of self-renewal and multipotent differentiation. HSCs are therefore an important cell type in both basic research and in the clinic, where HSC transplantation is a curative therapy for a range of diseases. However, as a rare bone marrow cell population, the characterization and collection of HSCs can often be challenging. This has led to a large search for in vitro culture conditions that support the growth of functional HSCs and the in vitro stabilization of the HSC state represents a major challenge in the field. Here, we review recent progress towards stabilizing HSCs in vitro.
Keywords: hematopoietic stem cell, hematopoiesis, ex vivo, in vitro, stem cell culture
Introduction
The hematopoietic system is made up of a number of specialized cell types including oxygen-carrying red blood cells, megakaryocyte-producing platelets, and pathogen-fighting innate and adaptive immune cells (including monocytes, neutrophils, T lymphocytes, and B lymphocytes). Hematopoiesis (blood formation) from hematopoietic stem and progenitor cells (HSPCs) within the bone marrow (and other hematopoietic organs e.g. spleen) is essential for sustaining blood and immune functions1-3. The most primitive hematopoietic cell type, the hematopoietic stem cell (HSC), retains throughout life the unique capacity for both self-renewal and multipotent differentiation4. Self-renewal allows HSCs to repopulate the HSC pool while multipotent differentiation allows HSCs to differentiate into any mature adult hematopoietic cell type. HSCs initially differentiate into hematopoietic progenitor cells (HPCs), which lack self-renewal capacity but can retain multipotent differentiation potential2,4. As HPCs differentiate further, they become progressively more lineage restricted before forming a mature hematopoietic cell type. Loss of homeostasis within the hematopoietic system by either over- or under-production of certain lineages, blocked differentiation, or loss of hematopoietic cell function, results in morbidity and mortality. Understanding the mechanism of hematopoiesis therefore represents a major goal in biomedical research.
Besides their biological importance, HSCs are also significant from a therapeutic standpoint. HSC transplantation (HSCT) represents a curative therapy for a number of malignant and non-malignant diseases5,6. The aim of HSCT is the reconstitution of a healthy hematopoietic system within the patient. This is usually achieved through collecting healthy allogeneic HSCs from a suitable immune-matched donor and transplanting them into the recipient who has received bone marrow conditioning (usually radiation and/or chemotherapy), which destroys their hematopoietic system. Successful engraftment of donor HSCs leads to long-term hematopoietic system reconstitution, and is key to the curative ability of HSCT. Autologous HSCT involves using the patient’s own HSCs and is also used clinically, for example in high-dose radiation/chemotherapy solid cancer treatments, where such a therapy would otherwise cause bone marrow failure. Autologous HSCT has been used in the context of gene therapy to treat certain congenital blood diseases such as primary immunodeficiencies, where patient HSCs are collected, “corrected” by transferring missing genes by retrovirus or lentivirus, and then returned to the patient7.
Given the large interest in HSC biology and clinical use of HSCT therapies for over half a century, it is perhaps surprising that HSCs are still poorly maintained outside the body8. For example, donor HSCs for HSCT are transplanted fresh or after only minimal in vitro culture to avoid loss of activity. Improvements in expansion of HSCs could dramatically improve engraftment success rates and also open up the wider use of umbilical cord blood HSCs in HSCT; cord blood is an accessible source of HSCs but often do not contain sufficient numbers of HSCs for transplantation9. Even improvements in the maintenance of HSCs in vitro during gene correction (e.g. lentiviral or retroviral transduction, or more recent CRISPR/Cas9 gene editing10,11) could have a major impact on access to therapy. The in vitro stabilization of HSCs therefore represents a major challenge in the stem cell field. Efforts to overcome this challenge have stimulated a range of studies into the mechanism of HSC self-renewal1,12,13 and the in vivo HSC microenvironment (niche)3,14. Here, we review the recent progress towards stabilizing adult HSC in vitro. To provide context for non-HSC experts, we start with a summary of HSC assays and nomenclature before focusing our discussion on recent progress (in the last 1-2 years) and landmark papers in this field.
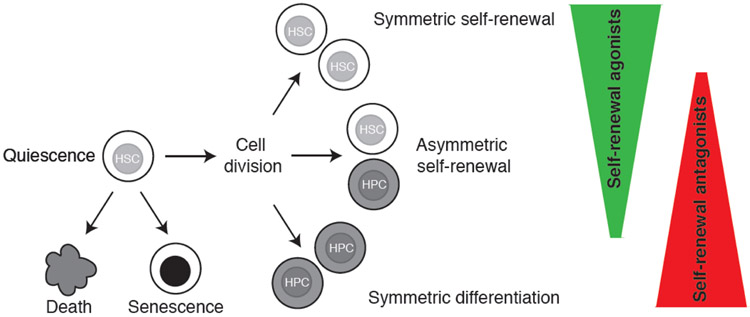
Defining HSC fate decisions
In adults, HSCs are a rare cell population and largely remain in a quiescent G0 (dormant) state throughout their life in the bone marrow, entering cell cycle infrequently unless hematopoietic stress occurs1. The current dogma is that once in culture, an HSC may retain quiescence, undergo self-renewal via symmetric or asymmetric cell division, differentiate (thereby losing self-renewal), undergo senescence, or die (through apoptosis or other cell death mechanisms) (Figure 1). While cell death and senescence are relatively easy to assess within in vitro cultures, tracking the retention and expansion of HSCs (vs. HPCs) is more challenging because the only functional assay for an HSC is transplantation. It is worth noting that phenotypic identity and functional identity of HSCs are not necessarily the same4,15. Even the best phenotypic markers fail to identify functional HSCs at 100% purity and the HSC population displays significant heterogeneity16,17. This also means that any starting bulk cell population in an HSC culture is unlikely to contain functional pure HSCs and instead represents a mixture of HSCs with self-renewal potential and HPCs without.
Figure 1: In vitro cell fates for hematopoietic stem cells.
Hematopoietic stem cells (HSCs) can be either maintained in quiescence, or stimulated into cell cycle where symmetric self-renewal, asymmetric self-renewal, or symmetric differentiation is possible. HSCs differentiate into hematopoietic progenitor cells (HPCs). Alternatively, HSCs may undergo cell death or senescence. Recent improvements in the in vitro expansion of HSCs have been made through identifying and modulating both agonists and antagonists of HSC self-renewal.
In mice, functional HSCs reconstitute multilineage hematopoiesis long-term (usually defined as 16-24 weeks) in an irradiated recipient, while HPCs only reconstitute hematopoiesis partially or transiently15. Similar reconstitution potential can be assessed for human HSCs by transplantation into sub-lethally irradiated immunodeficient mice, although the xenogeneic nature of this limit the full assessment of HSC function18. HSCs can be subdivided into short-term (ST) and long-term (LT)-HSCs, with LT-HSCs being able to reconstitute hematopoiesis serially in secondary recipient mice, a demonstration of potent self-renewal potential15. By contrast, ST-HSCs can only reconstitute primary recipients and therefore lack potent self-renewal potential (and likely are more similar to a progenitor than a stem cell). An ultimate goal of the field is the stable maintenance and expansion of functional LT-HSCs.
Maintaining HSC function in vitro through quiescence
To expand the absolute number of HSCs in a culture, symmetric self-renewal cell division is needed. Unfortunately, most traditional in vitro HSC cultures appear to induce HSC differentiation during cell proliferation, or at best asymmetric self-renewal (generating one HSC and one non-HSC) to maintain HSCs. Instead, stably maintaining HSCs in vitro was first achieved through sustaining the HSC quiescent state in vitro. One of the first demonstrations of in vitro maintenance of quiescent HSCs was through the use of transforming growth factor-beta (TGFb)19, with recombinant TGFb able to maintain HSCs in a dormant state in vitro for several weeks without cell division. Following transplantation into irradiated recipients, the cultured HSCs functioned normally, reconstituting multi-lineage hematopoiesis long-term. More recently, a combination of low cytokine concentrations, lipids, and hypoxia have been identified to support in vitro HSC quiescence20. Through screening a number of media conditions, Takubo and colleagues generated in vitro culture conditions that maintained functional HSCs with minimal cell division for over a month20. Interestingly, they found that HSC quiescence required high fatty acids and lipid carrying bovine serum albumin (BSA).
Identification of self-renewal agonists
While maintenance of quiescent HSCs may be useful, to achieve in vitro expansion of HSCs, self-renewal through cell division is required. To survive and grow in vitro, HSCs require exogenous stimulation and in vitro HSC cultures are commonly supplemented with a cocktail of hematopoietic cytokines that are aimed at promoting the survival, proliferation, and self-renewal of HSCs. A number of different cytokines have been reported, but HSC cultures typically include stem cell factor (SCF), thrombopoietin (TPO), interleukin-11 (IL-11), Flt3 ligand (Flt3l), and/or IL-6 (and are reviewed elsewhere8). Alongside these cytokines, fetal bovine serum or some form of serum albumin supplement (such as BSA or human serum albumin) plus insulin are also usually added. Besides providing the buffering protein serum albumin, these supplements also contain other bioactive molecules, including fatty acids and proteins, which can support HSC self-renewal. However, depending on the batch of serum albumin, differentiation-inducing factors can also be found in these supplements21, and in general these types of cultures only support modest expansion of HSCs.
Over the years, a number of attempts have been made to identify molecules and culture conditions that support symmetric self-renewal of HSCs. Probably the most successful small molecule self-renewal agonists to date are UM171 and StemRegin-1 (SR1), which together in combination with cytokines can support ~30-fold expansion of human LT-HSCs from umbilical cord blood during a two-week culture22. Recent mechanistic studies of UM171 suggest that it drives HSC self-renewal through modulating inflammatory signaling to limit the accumulation of cell-damaging reactive oxygen species (ROS)23. Both UM171 and SR1 have now been evaluated in clinical trials, both demonstrating feasibility and safety for cord blood transplantation24,25. More recently, other small molecules, including histone de-acetylase inhibitors have also shown efficacy in expanding human HSCs in vitro26. Transgene overexpression has also recently been used to induce HSC self-renewal in vitro, with Mikkola and colleagues using expression of a key self-renewal factor, MLLT3, to induce human HSC expansion in vitro27.
Exciting improvements in human HSC expansion have recently been demonstrated through the use of zwitterionic hydrogels in 3-dimensional (3D) HSC cultures28. These 3D cultures afforded an impressive 73-fold expansion of LT-HSCs during a 24-day culture. Interestingly, the expansion efficient in 3D cultures was limited to use of zwitterionic hydrogels, with polyethylene glycol-based 3D cultures offering only modest support for HSCs. Mechanistic studies suggested that these 3D hydrogel cultures at least in part supported HSC self-renewal through inhibiting aerobic metabolism and suppressing of DNA-damaging ROS.
In vitro HSC stabilization has also been recently been attempted through mimicking the in vivo HSC microenvironment. Frenette and colleagues achieved this by developing a novel stromal co-culture system using genetically revitalized primary bone marrow mesenchymal stromal cells (MSCs)29. In this system, overexpression of five transcription factors; Klf4, Ostf1, Xbp1, Irf3, and Irf7, in MSCs was used to preserve their HSC-supportive activities in vitro and could maintain and modestly expand HSCs (~7-fold). Interestingly, the revitalized MSCs also appeared to support HSCs at least in part through reducing accumulation of DNA damage29.
Removal of self-renewal antagonists
An alternative approach to those described above has been the identification and removal of self-renewal antagonists from HSC cultures. For example, HSCs are often cultured in commercially available liquid media, which were not specifically developed for HSC growth and can include inhibitory factors. Even simple changes to these media such as removing the amino acid tryptophan can improve HSC expansion in vitro30. More recently, the high concentration of calcium in standard medias were found to reduce HSC maintenance in vitro31 through regulating mitochondrial metabolism31,32. However, it was suggested that low concentrations of calcium inhibit calpain proteases, which play a role in reducing the known HSC self-renewal antagonists, Ten-eleven translocated (Tet) enzymes such as Tet233. Notably, a Tet2 co-factor, ascorbic acid (vitamin C), was also recently identified as an HSC self-renewal antagonist34,35.
As mentioned above, serum albumin supplements such as FBS are one of the most variable components of HSC media. Even purified bovine serum albumin-fraction V display large batch-to-batch variability in HSC maintenance and expansion21. It is therefore perhaps unsurprising that most success to date in expanding functional HSCs has been achieved through the use of albumin-free conditions36,37. We recently found that serum albumin can be replaced with polyvinyl alcohol (PVA) in HSC media. In combination with optimized concentrations of SCF and TPO, and the use a fibronectin matrix, this albumin-free approach afforded long-term expansion of mouse HSCs, for over 1-2 months. Limiting dilution assays estimated ex vivo expansion at between 236-899-fold, and symmetric self-renewal was also demonstrated by clone-splitting transplantation experiments. While human HSCs can also be maintained in PVA-based media, further work is needed to demonstrate long-term expansion of human HSCs in these conditions.
Use of PVA appears to minimize the introduction of differentiation-inducing factors associated with serum albumin supplements36, while similarly stabilizing the activity of recombinant cytokines in liquid media38. However, the exact function of PVA in HSC cultures is still incompletely understood. Interestingly, the functions of PVA in supporting HSC self-renewal and proliferation could be dissociated through modulating the chemical structure of PVA36. PVA is formed from hydrolysis of polyvinyl acetate, which can be incompletely hydrolyzed (generating a polymer containing a mixture of alcohol and acetate domains) or completely hydrolyzed (generating a polymer containing only alcohol domains). Incompletely (~87%) hydrolyzed PVA supported rapid proliferation and expansion of HSCs while completely (>99%) hydrolyzed PVA could support expansion of HSCs but with significantly reduced cell proliferation. These results that further optimization of PVA structure may be possible to further improve HSC expansion.
Besides the use of PVA to reduce the introduction of exogenous self-renewal antagonists, limiting the buildup of differentiation-inducing factors secreted by HSCs and HPCs within the culture was also important to expand HSCs more than a week in vitro. Long-term in vitro HSC cultures required regular complete media changes, which were essential for the maintenance and expansion of mouse HSCs in long-term in vitro cultures36,37. A similar build of secreted differentiation-inducing factors in human HSC cultures was previously been observed by Zandstra and colleagues39, who suggested that this could be overcome using an automated batch-fed method, where HSC cultures are constantly provided with fresh media to limit a concentration buildup of negative factors. Taken together, observations highlight that self-renewal antagonists can be both found with common HSC medias and build up within the HSC cultures, for example by cell secretion of differentiation-inducing factors or metabolic activity (e.g. in the case of ROS).
Conclusions
In conclusion, exciting progress has been made over the last couple of years in stabilizing HSCs in vitro through adding self-renewal agonists and removing self-renewal antagonists. In particular, the application of new reagents has also offered improvement, such as the use of zwitterionic hydrogels for 3D culture28 and use of PVA36. It will be in interesting to understand whether these different approaches are synergistic and can further improve HSC stability and expansion in vitro in combination. These new systems provide important tools for experimental hematology, to study the mechanism of hematopoiesis and to develop new therapeutic strategies for clinical application.
Acknowledgements:
ACW is supported by the Leukemia & Lymphoma Society (3385-19) and the NIH (K99HL150218). HN is supported by CIRM (LA1_C12-06917; DISC1-10555); the NIH (R01DK116944; R01HL147124; R21AG061487); and the Ludwig Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Competing interests:
H.N. is a co-founder and shareholder of ReproCELL Inc, Megakaryon Corp., and Century Therapeutics.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eaves CJ. Hematopoietic stem cells: concepts, definitions, and the new reality. Blood. 2015;125(17):2605–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laurenti E, Göttgens B. From haematopoietic stem cells to complex differentiation landscapes. Nature. 2018;553(7689):418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinho S, Frenette PS. Haematopoietic stem cell activity and interactions with the niche. Nat Rev Mol Cell Biol. 2019;20(5):303–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamamoto R, Wilkinson AC, Nakauchi H. Changing concepts in hematopoietic stem cells. Science. 2018;362(6417):895–896. [DOI] [PubMed] [Google Scholar]
- 5.Chabannon C, Kuball J, Bondanza A, et al. Hematopoietic stem cell transplantation in its 60s: A platform for cellular therapies. Sci Transl Med. 2018;10(436). [DOI] [PubMed] [Google Scholar]
- 6.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354(17):1813–1826. [DOI] [PubMed] [Google Scholar]
- 7.Morgan RA, Gray D, Lomova A, Kohn DB. Hematopoietic Stem Cell Gene Therapy: Progress and Lessons Learned. Cell Stem Cell. 2017;21(5):574–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar S, Geiger H. HSC Niche Biology and HSC Expansion Ex Vivo. Trends Mol Med. 2017;23(9):799–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broxmeyer HE. Enhancing the efficacy of engraftment of cord blood for hematopoietic cell transplantation. Transfus Apher Sci. 2016;54(3):364–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gundry MC, Dever DP, Yudovich D, et al. Technical considerations for the use of CRISPR/Cas9 in hematology research. Exp Hematol. 2017;54:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dever DP, Porteus MH. The changing landscape of gene editing in hematopoietic stem cells: a step towards Cas9 clinical translation. Curr Opin Hematol. 2017;24(6):481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orkin SH, Zon LI. Hematopoiesis: An evolving paradigm for stem cell biology. Cell. 2008;132(4):631–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilkinson AC, Gottgens B. Transcriptional regulation of haematopoietic stem cells. Adv Exp Med Biol. 2013;786:187–212. [DOI] [PubMed] [Google Scholar]
- 14.Crane GM, Jeffery E, Morrison SJ. Adult haematopoietic stem cell niches. Nat Rev Immunol. 2017;17(9):573–590. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto R, Morita Y, Ooehara J, et al. Clonal analysis unveils self-renewing lineage-restricted progenitors generated directly from hematopoietic stem cells. Cell. 2013;154(5):1112–1126. [DOI] [PubMed] [Google Scholar]
- 16.Wilson NK, Kent DG, Buettner F, et al. Combined Single-Cell Functional and Gene Expression Analysis Resolves Heterogeneity within Stem Cell Populations. Cell Stem Cell. 2015;16(6):712–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haas S, Trumpp A, Milsom MD. Causes and Consequences of Hematopoietic Stem Cell Heterogeneity. Cell Stem Cell. 2018;22(5):627–638. [DOI] [PubMed] [Google Scholar]
- 18.Goyama S, Wunderlich M, Mulloy JC. Xenograft models for normal and malignant stem cells. Blood. 2015;125(17):2630–2640. [DOI] [PubMed] [Google Scholar]
- 19.Yamazaki S, Iwama A, Takayanagi S, Eto K, Ema H, Nakauchi H. TGF-beta as a candidate bone marrow niche signal to induce hematopoietic stem cell hibernation. Blood. 2009;113(6):1250–1256. [DOI] [PubMed] [Google Scholar]
- 20.*.Kobayashi H, Morikawa T, Okinaga A, et al. Environmental Optimization Enables Maintenance of Quiescent Hematopoietic Stem Cells Ex Vivo. Cell Rep. 2019;28(1):145–158.e149.This paper describes the identification of conditions to maintain quiescent HSCs for over 1 month in vitro.
- 21.Ieyasu A, Ishida R, Kimura T, et al. An All-Recombinant Protein-Based Culture System Specifically Identifies Hematopoietic Stem Cell Maintenance Factors. Stem Cell Reports. 2017;8(3):500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fares I, Chagraoui J, Gareau Y, et al. Cord blood expansion. Pyrimidoindole derivatives are agonists of human hematopoietic stem cell self-renewal. Science. 2014;345(6203):1509–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chagraoui J, Lehnertz B, Girard S, et al. UM171 induces a homeostatic inflammatory-detoxification response supporting human HSC self-renewal. PLoS One. 2019;14(11):e0224900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagner JE, Brunstein CG, Boitano AE, et al. Phase I/II Trial of StemRegenin-1 Expanded Umbilical Cord Blood Hematopoietic Stem Cells Supports Testing as a Stand-Alone Graft. Cell Stem Cell. 2016;18(1):144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen S, Roy J, Lachance S, et al. Hematopoietic stem cell transplantation using single UM171-expanded cord blood: a single-arm, phase 1-2 safety and feasibility study. Lancet Haematol. 2020;7(2):e134–e145. [DOI] [PubMed] [Google Scholar]
- 26.Zimran E, Papa L, Djedaini M, Patel A, Iancu-Rubin C, Hoffman R. Expansion and preservation of the functional activity of adult hematopoietic stem cells cultured ex vivo with a histone deacetylase inhibitor. Stem Cells Transl Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.*.Calvanese V, Nguyen AT, Bolan TJ, et al. MLLT3 governs human haematopoietic stem-cell self-renewal and engraftment. Nature. 2019;576(7786):281–286.This paper describes MLLT3 as an important human HSC self-renewal factor and that overexpression of MLLT3 during in vitro culture can improve HSC expansion.
- 28.**.Bai T, Li J, Sinclair A, et al. Expansion of primitive human hematopoietic stem cells by culture in a zwitterionic hydrogel. Nat Med. 2019.This paper described the use of zwitterionic hydrogels to expand human HSCs in 3D culture conditions, which support ~73-fold expansion.
- 29.*.Nakahara F, Borger DK, Wei Q, et al. Engineering a haematopoietic stem cell niche by revitalizing mesenchymal stromal cells. Nat Cell Biol. 2019;21(5):560–567.This paper described the development of revitalized bone marrow MSCs as an approach to maintain HSCs within an in vitro co-culture system.
- 30.Taya Y, Ota Y, Wilkinson AC, et al. Depleting dietary valine permits nonmyeloablative mouse hematopoietic stem cell transplantation. Science. 2016;354(6316):1152–1155. [DOI] [PubMed] [Google Scholar]
- 31.*.Luchsinger LL, Strikoudis A, Danzl NM, et al. Harnessing Hematopoietic Stem Cell Low Intracellular Calcium Improves Their Maintenance In Vitro. Cell Stem Cell. 2019.This paper described to negative consequences of calcium on HSC maintenance in vitro.
- 32.Umemoto T, Hashimoto M, Matsumura T, Nakamura-Ishizu A, Suda T. Ca. J Exp Med. 2018;215(8):2097–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moran-Crusio K, Reavie L, Shih A, et al. Tet2 loss leads to increased hematopoietic stem cell self-renewal and myeloid transformation. Cancer Cell. 2011;20(1):11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agathocleous M, Meacham CE, Burgess RJ, et al. Ascorbate regulates haematopoietic stem cell function and leukaemogenesis. Nature. 2017;549(7673):476–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cimmino L, Dolgalev I, Wang Y, et al. Restoration of TET2 Function Blocks Aberrant Self-Renewal and Leukemia Progression. Cell. 2017;170(6):1079–1095.e1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.**.Wilkinson AC, Ishida R, Kikuchi M, et al. Long-term ex vivo haematopoietic-stem-cell expansion allows nonconditioned transplantation. Nature. 2019;571(7763):117–121.This paper describes the optimization of albumin-free PVA-based culture conditions that can support HSCs in vitro for at least 1–2 months and can offer up to ~900-fold expansion of functional HSCs.
- 37.Wilkinson AC, Ishida R, Nakauchi H, Yamazaki S. Long-term ex vivo expansion of mouse hematopoietic stem cells. Nat Protoc. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishimura T, Hsu I, Martinez-Krams DC, et al. Use of polyvinyl alcohol for chimeric antigen receptor T-cell expansion. Exp Hematol. 2019;80:16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Csaszar E, Kirouac DC, Yu M, et al. Rapid expansion of human hematopoietic stem cells by automated control of inhibitory feedback signaling. Cell Stem Cell. 2012;10(2):218–229. [DOI] [PubMed] [Google Scholar]