Abstract
After fertilization, mouse embryos go through preimplantation development to give rise to blastocyst. Two key molecular events, zygotic genome activation (ZGA) and the first cell lineage specification, are essential for the process. Recent advances in low-input epigenomics profiling techniques allow the analysis of these events at a molecular level, which revealed a critical role of epigenetic and chromatin reprogramming in ZGA and the first cell lineage specification. Additionally, the establishment of an in vitro embryonic stem cell (ESC) to two-cell embryo-like conversion system have also contributed to the molecular understanding of preimplantation development. In this review, we summarize recent advances in epigenetic regulation of mouse preimplantation development, point out the remaining questions, and propose strategies to tackle these questions.
Keywords: Epigenetics, Chromatin, ZGA, 2-cell embryo like cell, Preimplantation
Introduction
Mammalian life starts with the fertilization of an egg by a sperm. After fertilization, the totipotent zygotes go through preimplantation development before implanting into the mother’s uterus to generate an entire organism that includes both embryonic and extra-embryonic tissues. Preimplantation development includes two key events, zygotic genome activation (ZGA) and the first cell lineage specification [1]. Mouse ZGA includes minor and major waves. While the minor ZGA occurs in zygotes and early two-cell embryos where about one hundred of zygotic genes are transcribed [2], the major ZGA mainly takes place in late two-cell embryos with thousands of genes are actively transcribed [3]. After ZGA, the embryos go through a few cell cleavages before the first cell lineage specification to generate trophectoderm (TE) and inner cell mass (ICM). During the past several years, great progress has been made in understanding the molecular events, particularly in ZGA and the first cell lineage specification, thanks to the development of low-input epigenomic profiling techniques as well as the establishment of an in vitro cell fate conversion model for early embryos. In this review, we summarize the progress with an emphasis on epigenetic regulation during mouse preimplantation development.
Epigenetic dynamics during mouse preimplantation development
Since the sperm and egg genomes are organized very differently, it is anticipated that they go through dramatic changes during preimplantation development to become epigenetically equalized, with the exception of imprinted loci. However, the limited availability of mammalian oocytes and embryos impeded global analyses of this dynamic process. Now, this challenge has been largely resolved with the development of low-input techniques for epigenomic profiling.
Immunostaining and whole-genome bisulfite sequencing (WGBS) analysis revealed global DNA demethylation soon after fertilization (Figure 1). While both paternal and maternal genomes undergo demethylation, the paternal genome demethylation is at a faster rate. This demethylation process is driven by a combination of Tet3-mediated 5-methylcytosine (5mC) oxidation and DNA-replication-dependent dilution [4–6]. However, Tet3-dependent DNA demethylation is not required for ZGA and preimplantation development [5, 7].
Figure 1. Overview of the mRNA level, DNA methylation, and chromatin landscapes during mouse preimplantation development.
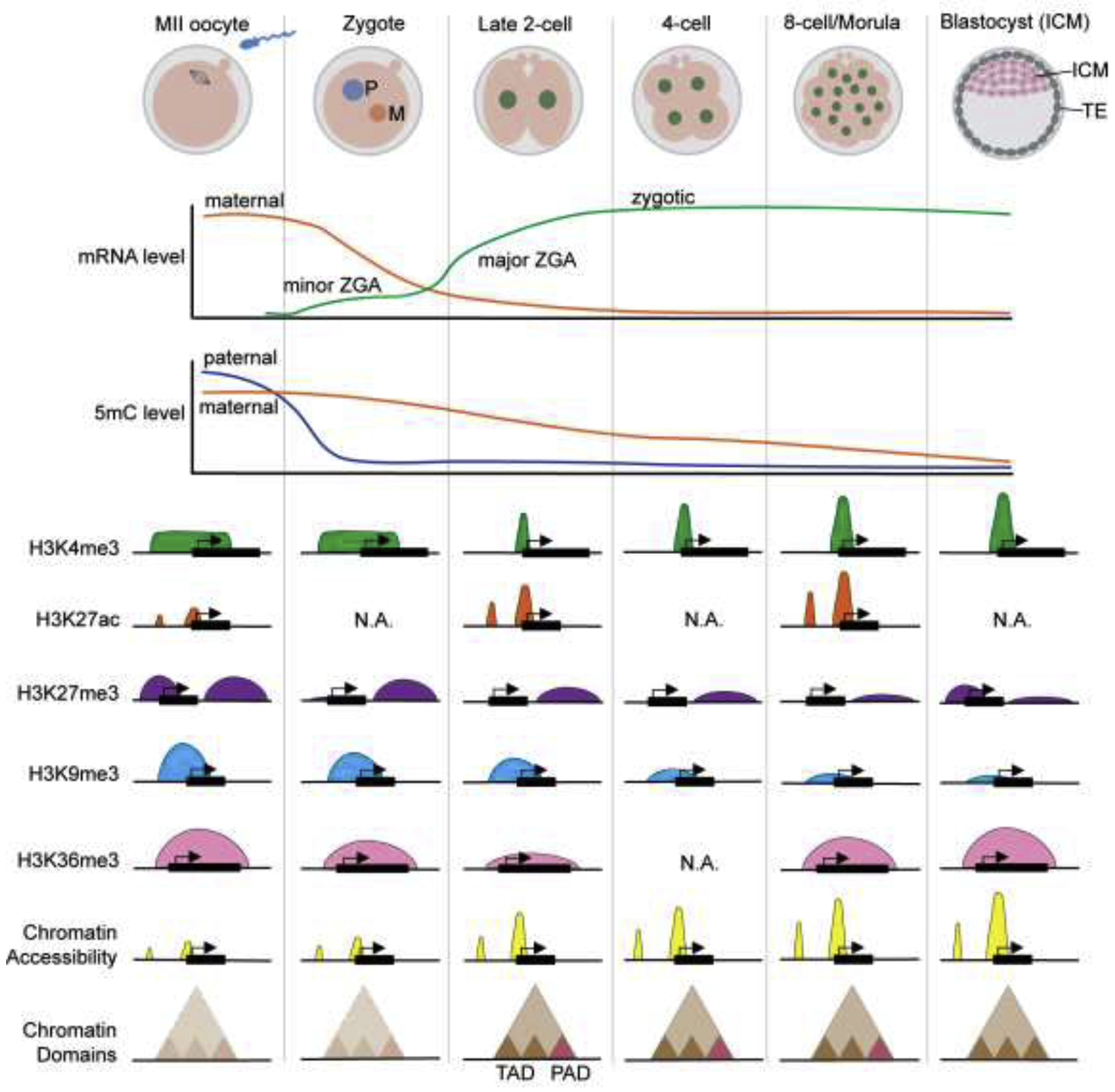
After fertilization, maternal mRNA (orange line) are rapidly degraded while two waves of ZGA (both minor and major ZGA, green line) take place. Paternal genome 5-methylcytosine (5mC, blue line) is rapidly decreased in zygotes, while maternal 5mC level (orange line) is gradually decreased during preimplantation development. The broad H3K4me3 (green) domain in oocytes is removed at two-cell stage and replaced by the promoter H3K4me3 peaks. H3K27ac (orange) level is increased at two-cell stage. The H3K27me3 (purple) at promoter region is rapidly erased after fertilization, while the H3K27me3 at distal region is gradually decreased from two-cell stage. The H3K9me3 (blue) at promoter region is gradually decreased during preimplantation development. Maternal H3K36me3 (pink) across the entire gene body is attenuated after fertilization and lost at eight-cell stage, zygotic H3K36me3 starts to establish from two-cell stage and becomes stronger at eight-cell stage. Chromatin accessibility (yellow) is increased from two-cell stage and further enhanced during preimplantation development. Higher order chromatin structure, indicated by compartments and topologically associating domains (TADs), is disordered in MII oocytes and zygotes (light brown triangles with dashed lines) and established from late two-cell stage (brown triangles with solid lines). Polycomb-associating domains (PADs, red triangles with solid lines) are present transiently during two- to eight-cell stages.
In addition to DNA methylation, histone modifications are also reprogrammed during mouse preimplantation development (Figure 1). The most notable change on histone modifications is the trimethylation of histone 3 lysine 4 (H3K4me3), a mark of active transcription when located at gene promoter. However, broad non-canonical H3K4me3 domains have also been identified in transcriptionally silent oocytes, which may function as a repressive mark to prevent transcription before ZGA [8]. The broad H3K4me3 domains are replaced by the promoter-associated canonical H3K4me3 peaks at the late two-cell stage [8, 9]. Interestingly, removal of the broad H3K4me3 domains appears to be essential for ZGA as knockdown of the H3K4me3 demethylases Kdm5a/b results in preimplantation developmental arrest with defects in activation of a subset of ZGA genes [9]. The establishment of promoter H3K4me3 peaks coincides with the appearance of histone 3 lysine 27 acetylation (H3K27ac) [9], another mark for gene activation, indicating that these active marks may function together to mediate ZGA. It has been proposed that genomic elements close to the broad H3K4me3 that harbor H3K27ac may serve as putative active cis-regulatory elements (cREs) for ZGA [9]. Removal of the H3K4me3 broad domains after fertilization may allow binding of transcription factors (TFs) at cREs to deposit active H3K27ac marks, resulting in activation of the ZGA genes [9]. Whether this is indeed the case await to be shown.
In addition to active histone marks, inactive marks such as trimethyl-histone 3 lysine 27 (H3K27me3) and lysine 9 (H3K9me3) also exhibit dynamic changes during preimplantation development. While promoter H3K27me3 and H3K9me3 are quickly erased or decreased after fertilization, the broad H3K27me3 domains in distal regions can be maintained until the blastocyst stage (Figure 1) [10–12]. Removal of H3K9me3 is essential for preimplantation development as failure to do so in somatic cell nuclear transfer (SCNT) embryos leads to developmental arrest due to ZGA defects [13]. Whether removal of H3K27me3 is required for preimplantation development still await to be shown. Yet, maternal H3K27me3-mediated non-canonical imprinting in preimplantation embryos is largely lost in the embryonic lineage after implantation, but maintained in the extraembryonic lineage at certain loci that include the maternal Xist and certain genes important for placenta development [14, 15].
In oocytes, the establishment of the aforementioned DNA methylation, non-canonical H3K4me3, and H3K27me3 are largely modulated by another histone modification, trimethylation of histone 3 lysine 36 (H3K36me3) [16]▪. The depletion of maternal H3K36me3 in oocytes results in the disruption of the maternal epigenome, thus causing defects in ZGA and embryonic development [16]▪. After fertilization, maternal H3K36me3 is attenuated from the late two-cell stage and disappears at the eight-cell stage, while zygotic H3K36me3 is established gradually during preimplantation development (Figure 1) [16]▪.
Local and high order chromatin dynamics during mouse preimplantation development
In eukaryotic cells, histones and DNA are organized in the form of chromatin. As mentioned earlier, the chromatin of sperm cells and eggs are organized differently. Upon fertilization, chromatin from both sperm cells and eggs undergo reprogramming to achieve similar chromatin accessibility so that gene expression on both parental alleles can be equalized. Using the assay for transposase-accessible chromatin sequencing (ATAC-seq) and low-input DNase I sequencing (liDNase-seq), the dynamics of chromatin accessibility during mouse preimplantation development have been profiled [17–19]. The analysis revealed a very rapid reprogramming process with the two parental genomes exhibit similar chromatin accessibility within 12 hours of fertilization and this reprogramming process is DNA replication-independent [19]. However, the protein factors that mediate the reprogramming process are largely unknown. During preimplantation development, chromatin accessibility gradually increases with zygotes and two-cell embryos exhibiting less chromatin accessibility. However, a rapid increase in chromatin accessibility takes place during and after ZGA and mainly occurs in the distal regions, which correlates with the usage of enhancer elements (Figure 1) [17, 18]. Although the dynamics of chromatin accessibility in human preimplantation embryos are largely similar [20], species-specific differences have been observed, including the establishment of open chromatin at the OCT4 binding motifs at the time of ZGA [20], which does not occur in mouse until morula stage [18].
In addition to the local chromatin accessibility revealed by ATAC-seq and liDNase-seq, chromatin structural changes can also be assessed by their folding patterns, including the self-interacting topologically associating domains (TADs), and the non-DNA-structure-interacting domains, such as the lamina-associated domains (LADs) [21, 22]. Dynamic changes in TADs and LADs have been reported during preimplantation development (Figure 1). Specifically, sperm cells show a structured organization, while the MII oocytes lack TADs and compartments [23, 24]. After fertilization, TADs undergo reorganization, from weak TADs in zygotes and early two-cell embryos to newly established TADs at late two-cell embryos, and this reorganization is ZGA-independent [23, 24]. A recent study has reported a new self-interacting compartmental domain, the Polycomb-associating domains (PADs), marked by H3K27me3 over large genomic blocks [25]▪. PADs are established during oocyte growth and disassembled upon meiotic resumption, but briefly reappeared in the maternal genome after fertilization. Polycomb repressive complex 1 (PRC1) and PRC2 are responsible for the establishment of PADs in oocytes and after fertilization, respectively. Another study also reported the existance of Polycomb-marked compartments in early embryos [26]. Besides TADs and PADs, LADs were reported to be established de novo after fertilization [27]. LADs formation, which may be H3K4me3-dependent, occurs before TAD consolidation and may help to prime the repressive compartments for certain genomic regions. Whether these higher-order chromatin dynamics play any role in ZGA and preimplantation development, or they are the outcome of development cue, remains to be determined.
Regulators of ZGA in mouse
ZGA is one of the key molecular events during preimplantation development yet the major regulators of this event still remains elusive. Recent studies using low-input genomic profiling approaches and the two-cell-embryo-like cell (2CLC) system, several candidate regulators for ZGA have been identified.
An integrative analysis of transcriptome and chromatin accessibility of zygotes and two-cell embryos revealed Nfya as one of the candidate TFs regulating ZGA because the Nfya-binding motif is the most enriched motifs in two-cell embryo activated gene promoters with newly gained chromatin accessibility [18]. The knockdown of maternal Nfya resulted in a decrease in chromatin accessibility and defects in the activation of a subset of ZGA genes [18]. A second candidate TF for ZGA is Dux as it is specifically expressed right before major ZGA and its enforced expression in embryonic stem cells (ESC) resulted in activation of a subset of ZGA genes [28–30]. However, Dux knockout mice (both zygotic KO and maternal/zygotic KO) only exhibit minor defects on ZGA and developmental potential, and homozygous knockout mice can survive to adulthood [31–33]▪, indicating that Dux does not play a major role in regulating ZGA. A third TF that might contribute to ZGA is Yap1 because maternal-Yap1 knockouts exhibit defects in the activation of a subset of ZGA genes [34]. However, the major defects of the knockout embryos are in cell compaction and TE lineage specification instead of two- or four-cell arrest [34].
In addition to TFs, chromatin remodeling is known to be essential for preparing the ZGA genes to be poised for activation. In this regard, it is interesting to note that maternal depletion of the chromatin remodeling factor Brg1 results in a two-cell arrest and causes defects in ZGA [35]. Another chromatin remodeler Snf2h has been shown to affect ZGA gene expression in an in vitro 2CLCs system [36]. However, whether it performs a similar function in vivo in developing embryos remains to be shown. As discussed above, ZGA is also affected by several histone modifications [8, 9, 16]▪.
In summary, although several candidate factors for mammalian ZGA have been proposed, their role in ZGA gene activation appears to be minor, suggesting major mammalian ZGA regulators remain to be identified. In contrast, master ZGA regulators in other organisms have been identified, including Zelda in Drosophila [37], Pou5f1, SoxB1, and Nanog in zebrafish [38]. These factors are unlikely to be responsible for mammalian ZGA as no mammalian Zelda homology has been identified, and the pluripotent factors involved in zebrafish ZGA either do not express in mammalian embryos until after ZGA, or has no function in mice ZGA [39]. It is possible that the mammalian master ZGA regulators might be maternal proteins or newly translated proteins activated by fertilization trigged signaling. New approaches focusing on the maternal RNAs or proteins may reveal their identities.
Epigenetic regulation of ICM and TE lineage specification
After ZGA, embryos go through several cell divisions before the first cell lineage separation to generate TE and ICM cells. The TE cell lineage, with the specific expression of Cdx2 and Gata3, contributes to the placenta; while the ICM cells, marked by the expression of pluripotent factors such as Oct4, Nanog, and Sox2, differentiate into epiblast and primitive endoderm, which eventually give rise to all embryonic tissues and some extraembryonic membranes, respectively [40, 41]. During the first cell lineage differentiation, the Hippo pathway controls the TE lineage specification [42–44, 45], while epigenetic modifications are involved in the consolidation of the ICM cell lineage [46, 47]. A recent study suggested that asymmetric distribution of Carm1-deposited dimethyl-arginine 26 of histone H3 (H3R26me2) in the blastomeres is observed as early as four-cell stage embryos [48]. Blastomeres with higher levels of Carm1 and H3R26me2 have a higher tendency to contribute to the ICM [48]. In addition, a higher level of H3R26me2 increases the expression of pluripotent genes and facilitates Sox2 binding to its targets for ICM specification [49, 50]. Furthermore, overexpression of Carm1 can increase the frequency of asymmetric divisions and lead cells biased to take inner position of the embryo [51]. In addition to Carm1, another chromatin modifier Prdm14 is also asymmetrically expressed in four-cell embryos to modulate H3R26me2 level for ICM contribution [52].
Two recent studies extended the above observations by demonstrating that long non-coding RNAs (lncRNAs) are involved in Carm1-mediated H3R26me2 heterogeneity in four-cell embryos [53, 54]▪. Wang et al. showed that a lncRNA, LincGET, is asymmetrically expressed during two- to four-cell stages [53]▪. LincGET interacts with Carm1 and promotes its nuclear localization, leading to an increased H3R26me2 level. Importantly, injection of LincGET into one of the blastomeres of two-cell embryos can bias the injected blastomere toward ICM specification [53]▪. Another lncRNA Neat1 is also required for Carm1-dependent H3R36me2 deposition and ICM specification [54]▪. Neat1 is asymmetrically expressed between blastomeres in 4-cell embryo and recruits Carm1 to nuclear foci paraspeckles [54]▪. Disruption of Neat1 results in reduced H3R26me2, increased Cdx2 expression, and biased TE specification [54]▪. Whether LincGET and Neat1 coordinately regulate Carm1 on lineage specification merits further investigation.
Taken together, the asymmetric distribution of H3R26me2 serves as one of the first signals for the first lineage specification. The heterogeneity of H3R26me2 is modulated by the asymmetric expression of Carm1, Prdm14, LincGET, and Neat1 in early blastomeres. What causes the initial biased expression of Carm1/Prdm14/LincGET/Neat1 in two- and four-cell embryos is currently unknown.
Understanding ZGA and totipotency to pluripotency transition using the 2CLC system
During preimplantation development, embryos go through ZGA and transit from totipotency to pluripotency [55]. Understanding the molecular mechanism of this process is impeded by the scarcity of mammalian early embryos. The observation that cultured mouse ESCs have a rare totipotent cell population, 2CLCs, that expresses two-cell-embryo-specific genes, provides an in vitro system for understanding this process [56].
The rare cell population in cultured ESCs gained their name 2CLCs because they share several key features of two-cell embryos, including expression of a group of two-cell-embryo-specific transcripts, loss expression of pluripotent genes, and have the capacity to contribute to both TE and ICM cell lineages [55–57]▪. Further studies show that the transition from pluripotent mESC to totipotent 2CLC establishes multiple two-cell-embryo-like molecular features, including chromatin decondensation [58], increased histone mobility [59], loss of chromocenters [58], decreased chromatin accessibility [29], and formation of MERVL-mediated insulating domain boundaries [60]. Since 2CLC has many features of the totipotent two-cell embryos, they provide a unique model for studying ZGA and totipotency to pluripotency transition.
As part of the ZGA genes, two-cell-embryo-specific genes are transiently activated in two-cell embryos and quickly shutdown in four-cell embryos [55]. Factors that regulate this transcriptional dynamic were largely unknown. Since the same group of genes, including MERVL repeats, are activated during ESC to 2CLC transition, this transition provides a unique system for understanding how the two-cell embryo-specific genes are activated during ZGA (Figure 2). Recent studies in the 2CLC system have revealed a transcriptional axis that includes Dppa2/4-mediated Dux activation, which in turn activates two-cell-embryo-specific genes [28–30, 61–63]. Importantly, the loss function of either Dux or Dppa2/4 partly affected activation of some two-cell-embryo-specific genes during ZGA [62–65], indicating that Dppa2/4 and Dux may have some role in ZGA, although Dux is not essential for ZGA in mouse models [31–33]▪. Whether Dppa2/4 has an essential role in ZGA remains to be determined. Additional factors, including Patz1, Nelfa, and Gata3, have also been shown to activate two-cell-embryo-specific genes in mESCs [36, 61, 66], their role in ZGA remains to be shown.
Figure 2. Relationship of 2CLCs with mouse preimplantation development.
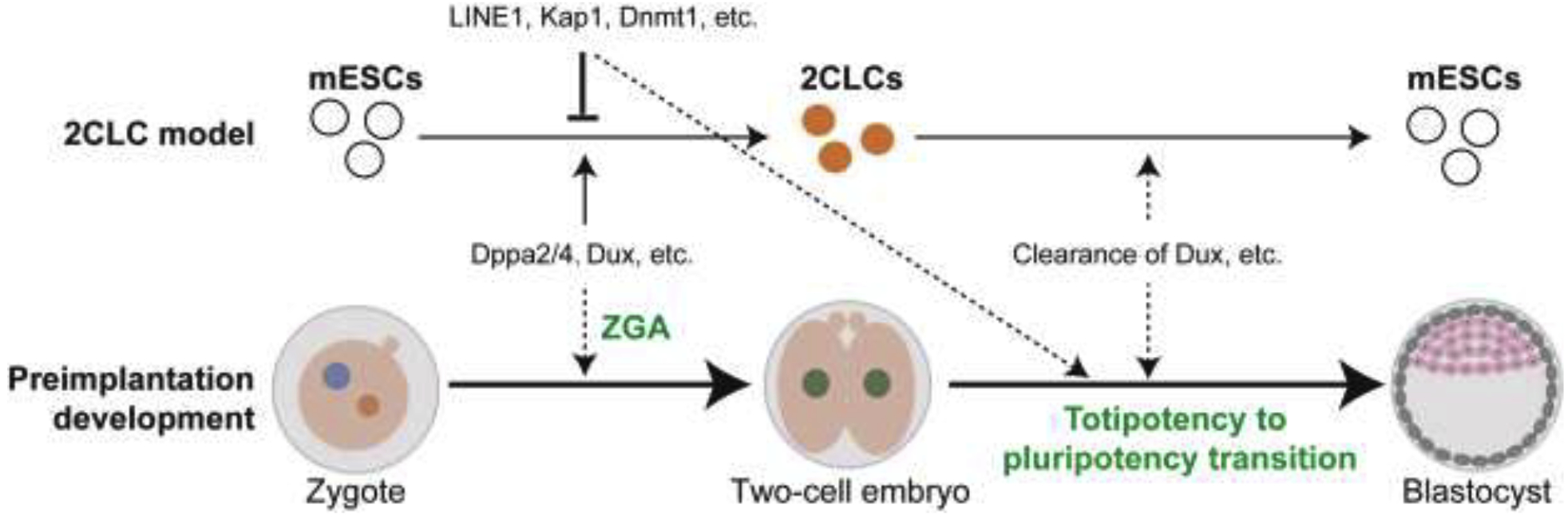
2CLC are a rare two-cell-embryo-like cell population in cultured mESCs, which can serve as an in vitro model for studying preimplantation development. Factors, such as Dppa2/4 and Dux, that drive 2CLC formation may contribute to ZGA; while factors that impede 2CLC formation, such as LINE1, may drive totipotency to pluripotency transition in preimplantation embryos. 2CLCs spontaneously transit back to the pluripotent state and this reversal transition can serve as a model for understanding totipotency to pluripotency transition in embryos. For instance, the rapid clearance of Dux may contribute to both the reversal of the 2C-like state as well as the totipotency to pluripotency transition in preimplantation embryos. Dashed lines indicate the interactions merit further investigation.
In addition to ZGA, the 2CLC system can also be used for understanding cell fate reprogramming between totipotent and pluripotent states. The barriers that prevent ESC to 2CLC transition in vitro might play an important role in preimplantation development to drive totipotency to pluripotency transition (Figure 2). One example is the retrotransposon LINE1 [67]▪. LINE1 has been shown to corporate with Kap1 and nucleolin to suppress Dux expression and the generation of 2CLCs in mESCs. Importantly, LINE1 also suppresses Dux in embryos and promotes totipotency to pluripotency transition [67]▪. Similarly, histone variant H3.3 and Zmym2 prevent 2CLC generation in mESCs and plays a role in the totipotency to pluripotency transition during preimplantation development [68–70]. Several other factors, including H3K4/H3K9 methylation‚ histone acetylation, Tet proteins, DNA methylation, Myc, the PRC1.6 and EP400-TIP60 complexes have also been shown to inhibit 2CLC generation in the mESC culture system [56, 57, 71–74]▪. The role of these factors in totipotency to pluripotency transition in vivo merits further investigation.
It is known that the totipotent state is transient and that the 2CLCs spontaneously transit back into pluripotent mESCs. This reversal transition can also be used for understanding totipotency to pluripotency transition during preimplantation development (Figure 2). For instance, the transcripts of Dux and many two-cell-embryo-specific transcripts are quickly disappeared in four-cell embryos and this clearance appears to be crucial for totipotent to pluripotent state transition in embryos [63, 65, 67]▪. The reversal of 2C-like state is accompanied by a similar clearance of Dux and two-cell-embryo-specific transcripts [57, 75]▪, which can serve as a model for understanding how these transcripts are quickly removed.
Although 2CLC can be used for understanding some of the molecular events during preimplantation development, this system has its limitation [33, 64, 65]. For example, while Dux is necessary and sufficient in inducing 2C-like transition in mESCs, Dux depletion only has a minimum effect on ZGA and embryonic development [33, 64, 65]. This discrepancy is not too surprising as two-cell embryos still contain many maternal transcripts and proteins that are absent in the 2CLCs. Thus, cautions should be taken when extrapolating observations in the 2CLC system to preimplantation embryos.
Concluding remarks
Recent advances in low-input epigenomic profiling techniques have greatly facilitated our understanding of the molecular mechanism of mouse preimplantation development, particularly on ZGA and the first cell lineage specification. In addition, the development of the 2CLC model system further facilitated dissecting molecular features of preimplantation embryos. Importantly, the majority of the new findings, including the dynamics of DNA methylation, histone modifications, and chromatin accessibility, are largely conserved between human and mouse, highlighting mouse as a useful model in understanding human preimplantation development [20, 76–78]. Despite the great progress, many important questions still remain unaddressed, including the identity of the master transcription factors responsible for ZGA and the mechanisms of rapid clearance of the two-cell-embryo-specific transcripts. New techniques and approaches, such as low-input profiling of RNA modifications and polyA-tails, as well as an understanding of translational and post-translational regulations during the maternal-to-zygotic transition should shed light on these questions.
Acknowledgements
We thank Drs. Zhiyuan Chen and Wenhao Zhang for critical reading of the manuscript. This work was supported by HHMI and NIH (R01HD092465). Y.Z. is an Investigator of the Howard Hughes Medical Institute. The authors declare no conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest have been highlighted as:
• of special interest
- 1.Chazaud C and Yamanaka Y, Lineage specification in the mouse preimplantation embryo. Development, 2016. 143(7): p. 1063–74. [DOI] [PubMed] [Google Scholar]
- 2.Hamatani T, et al. , Dynamics of global gene expression changes during mouse preimplantation development. Dev Cell, 2004. 6(1): p. 117–31. [DOI] [PubMed] [Google Scholar]
- 3.Xue Z, et al. , Genetic programs in human and mouse early embryos revealed by single-cell RNA sequencing. Nature, 2013. 500(7464): p. 593–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang L, et al. , Programming and inheritance of parental DNA methylomes in mammals. Cell, 2014. 157(4): p. 979–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen L, et al. , Tet3 and DNA replication mediate demethylation of both the maternal and paternal genomes in mouse zygotes. Cell Stem Cell, 2014. 15(4): p. 459–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inoue A and Zhang Y, Replication-dependent loss of 5-hydroxymethylcytosine in mouse preimplantation embryos. Science, 2011. 334(6053): p. 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inoue A, Matoba S, and Zhang Y, Transcriptional activation of transposable elements in mouse zygotes is independent of Tet3-mediated 5-methylcytosine oxidation. Cell Res, 2012. 22(12): p. 1640–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang B, et al. , Allelic reprogramming of the histone modification H3K4me3 in early mammalian development. Nature, 2016. 537(7621): p. 553–557. [DOI] [PubMed] [Google Scholar]
- 9.Dahl JA, et al. , Broad histone H3K4me3 domains in mouse oocytes modulate maternal-to-zygotic transition. Nature, 2016. 537(7621): p. 548–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng H, et al. , Resetting Epigenetic Memory by Reprogramming of Histone Modifications in Mammals. Mol Cell, 2016. 63(6): p. 1066–79. [DOI] [PubMed] [Google Scholar]
- 11.Liu X, et al. , Distinct features of H3K4me3 and H3K27me3 chromatin domains in pre-implantation embryos. Nature, 2016. 537(7621): p. 558–562. [DOI] [PubMed] [Google Scholar]
- 12.Wang C, et al. , Reprogramming of H3K9me3-dependent heterochromatin during mammalian embryo development. Nat Cell Biol, 2018. 20(5): p. 620–631. [DOI] [PubMed] [Google Scholar]
- 13.Matoba S, et al. , Embryonic development following somatic cell nuclear transfer impeded by persisting histone methylation. Cell, 2014. 159(4): p. 884–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inoue A, et al. , Maternal H3K27me3 controls DNA methylation-independent imprinting. Nature, 2017. 547(7664): p. 419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inoue A, et al. , Genomic imprinting of Xist by maternal H3K27me3. Genes Dev, 2017. 31(19): p. 1927–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Q, et al. , SETD2 regulates the maternal epigenome, genomic imprinting and embryonic development. Nat Genet, 2019. 51(5): p. 844–856. [DOI] [PubMed] [Google Scholar]; • The authors report that H3K36me3 is a central epigenetic regulator mediating the deposition of DNA methylation, H3K4me3, and H3K27me3 in oocytes, and plays an essential role during preimplantation development.
- 17.Wu J, et al. , The landscape of accessible chromatin in mammalian preimplantation embryos. Nature, 2016. 534(7609): p. 652–7. [DOI] [PubMed] [Google Scholar]
- 18.Lu F, et al. , Establishing Chromatin Regulatory Landscape during Mouse Preimplantation Development. Cell, 2016. 165(6): p. 1375–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Djekidel MN, et al. , Reprogramming of Chromatin Accessibility in Somatic Cell Nuclear Transfer Is DNA Replication Independent. Cell Rep, 2018. 23(7): p. 1939–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao L, et al. , Chromatin Accessibility Landscape in Human Early Embryos and Its Association with Evolution. Cell, 2018. 173(1): p. 248–259 e15. [DOI] [PubMed] [Google Scholar]
- 21.Zheng H and Xie W, The role of 3D genome organization in development and cell differentiation. Nat Rev Mol Cell Biol, 2019. 20(9): p. 535–550. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez-Sandoval A and Gasser SM, On TADs and LADs: Spatial Control Over Gene Expression. Trends Genet, 2016. 32(8): p. 485–495. [DOI] [PubMed] [Google Scholar]
- 23.Du Z, et al. , Allelic reprogramming of 3D chromatin architecture during early mammalian development. Nature, 2017. 547(7662): p. 232–235. [DOI] [PubMed] [Google Scholar]
- 24.Ke Y, et al. , 3D Chromatin Structures of Mature Gametes and Structural Reprogramming during Mammalian Embryogenesis. Cell, 2017. 170(2): p. 367–381.e20. [DOI] [PubMed] [Google Scholar]
- 25.Du Z, et al. , Polycomb Group Proteins Regulate Chromatin Architecture in Mouse Oocytes and Early Embryos. Mol Cell, 2019. [DOI] [PubMed] [Google Scholar]; • This paper is the first study reporting the formation of Polycomb-associating domains (PADs) during oocyte growth and their dynamics after fertilization, as well as how they are regulated by Polycomb repressive complexes.
- 26.Collombet S, et al. , Parental-to-embryo switch of chromosome organization in early embryogenesis. Nature, 2020. 580(7801): p. 142–146. [DOI] [PubMed] [Google Scholar]
- 27.Borsos M, et al. , Genome-lamina interactions are established de novo in the early mouse embryo. Nature, 2019. 569(7758): p. 729–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Iaco A, et al. , DUX-family transcription factors regulate zygotic genome activation in placental mammals. Nat Genet, 2017. 49(6): p. 941–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hendrickson PG, et al. , Conserved roles of mouse DUX and human DUX4 in activating cleavage-stage genes and MERVL/HERVL retrotransposons. Nat Genet, 2017. 49(6): p. 925–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whiddon JL, et al. , Conservation and innovation in the DUX4-family gene network. Nat Genet, 2017. 49(6): p. 935–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Z and Zhang Y, Loss of DUX causes minor defects in zygotic genome activation and is compatible with mouse development. Nat Genet, 2019. 51(6): p. 947–951. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This is the first study of the in vivo function of Dux in mice which demonstrate that Dux plays a minor non-essential role in ZGA, highlighting the difference between 2CLC and 2-cell embryos.
- 32.Guo M, et al. , Precise temporal regulation of Dux is important for embryo development. Cell Res, 2019. 29(11): p. 956–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Iaco A, et al. , DUX is a non-essential synchronizer of zygotic genome activation. Development, 2019: p. dev.177725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu C, et al. , Oocyte-expressed yes-associated protein is a key activator of the early zygotic genome in mouse. Cell Res, 2016. 26(3): p. 275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bultman SJ, et al. , Maternal BRG1 regulates zygotic genome activation in the mouse. Genes Dev, 2006. 20(13): p. 1744–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alda-Catalinas C, et al. , A single-cell transcriptomics CRISPR-activation screen identifies new epigenetic regulators of zygotic genome activation. bioRxiv, 2019: p. 741371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liang HL, et al. , The zinc-finger protein Zelda is a key activator of the early zygotic genome in Drosophila. Nature, 2008. 456(7220): p. 400–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee MT, et al. , Nanog, Pou5f1 and SoxB1 activate zygotic gene expression during the maternal-to-zygotic transition. Nature, 2013. 503(7476): p. 360–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jukam D, Shariati SAM, and Skotheim JM, Zygotic Genome Activation in Vertebrates. Developmental Cell, 2017. 42(4): p. 316–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.White MD, et al. , Instructions for Assembling the Early Mammalian Embryo. Dev Cell, 2018. 45(6): p. 667–679. [DOI] [PubMed] [Google Scholar]
- 41.Cockburn K and Rossant J, Making the blastocyst: lessons from the mouse. J Clin Invest, 2010. 120(4): p. 995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishioka N, et al. , The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev Cell, 2009. 16(3): p. 398–410. [DOI] [PubMed] [Google Scholar]
- 43.Ralston A, et al. , Gata3 regulates trophoblast development downstream of Tead4 and in parallel to Cdx2. Development, 2010. 137(3): p. 395–403. [DOI] [PubMed] [Google Scholar]
- 44.Huang D, et al. , The role of Cdx2 as a lineage specific transcriptional repressor for pluripotent network during the first developmental cell lineage segregation. Sci Rep, 2017. 7(1): p. 17156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strumpf D, et al. , Cdx2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst. Development, 2005. 132(9): p. 2093–2102. [DOI] [PubMed] [Google Scholar]
- 46.White MD and Plachta N, Specification of the First Mammalian Cell Lineages In Vivo and In Vitro. Cold Spring Harb Perspect Biol, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saini D and Yamanaka Y, Cell Polarity-Dependent Regulation of Cell Allocation and the First Lineage Specification in the Preimplantation Mouse Embryo. Curr Top Dev Biol, 2018. 128: p. 11–35. [DOI] [PubMed] [Google Scholar]
- 48.Torres-Padilla ME, et al. , Histone arginine methylation regulates pluripotency in the early mouse embryo. Nature, 2007. 445(7124): p. 214–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.White MD, et al. , Long-Lived Binding of Sox2 to DNA Predicts Cell Fate in the Four-Cell Mouse Embryo. Cell, 2016. 165(1): p. 75–87. [DOI] [PubMed] [Google Scholar]
- 50.Goolam M, et al. , Heterogeneity in Oct4 and Sox2 Targets Biases Cell Fate in 4-Cell Mouse Embryos. Cell, 2016. 165(1): p. 61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parfitt DE and Zernicka-Goetz M, Epigenetic modification affecting expression of cell polarity and cell fate genes to regulate lineage specification in the early mouse embryo. Mol Biol Cell, 2010. 21(15): p. 2649–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burton A, et al. , Single-cell profiling of epigenetic modifiers identifies PRDM14 as an inducer of cell fate in the mammalian embryo. Cell Rep, 2013. 5(3): p. 687–701. [DOI] [PubMed] [Google Scholar]
- 53.Wang J, et al. , Asymmetric Expression of LincGET Biases Cell Fate in Two-Cell Mouse Embryos. Cell, 2018. 175(7): p. 1887–1901 e18. [DOI] [PubMed] [Google Scholar]; • This paper together with reference [54] reveal that lincRNAs are involved in the regulation of lineage specification by Carm1 and H3R26me2.
- 54.Hupalowska A, et al. , CARM1 and Paraspeckles Regulate Pre-implantation Mouse Embryo Development. Cell, 2018. 175(7): p. 1902–1916 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper together with reference [53] reveal that lincRNAs are involved in the regulation of lineage specification by Carm1 and H3R26me2.
- 55.Lu F and Zhang Y, Cell totipotency: molecular features, induction, and maintenance. Natl Sci Rev, 2015. 2(2): p. 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Macfarlan TS, et al. , Embryonic stem cell potency fluctuates with endogenous retrovirus activity. Nature, 2012. 487(7405): p. 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fu X, et al. , Myc and Dnmt1 impede the pluripotent to totipotent state transition in embryonic stem cells. Nat Cell Biol, 2019. 21(7): p. 835–844. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper reveals the transcriptomic dynamics and candidate regulators for the transition between mESCs and 2CLCs, which provides a valuable resource for using 2CLC as a model to study preimplantation development.
- 58.Ishiuchi T, et al. , Early embryonic-like cells are induced by downregulating replication-dependent chromatin assembly. Nat Struct Mol Biol, 2015. 22(9): p. 662–71. [DOI] [PubMed] [Google Scholar]
- 59.Boskovic A, et al. , Higher chromatin mobility supports totipotency and precedes pluripotency in vivo. Genes Dev, 2014. 28(10): p. 1042–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kruse K, et al. , Transposable elements drive reorganisation of 3D chromatin during early embryogenesis. bioRxiv, 2019: p. 523712. [Google Scholar]
- 61.Eckersley-Maslin M, et al. , Dppa2 and Dppa4 directly regulate the Dux-driven zygotic transcriptional program. Genes & development, 2019. 33(3–4): p. 194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.De Iaco A, et al. , DPPA2 and DPPA4 are necessary to establish a 2C‐like state in mouse embryonic stem cells. EMBO reports, 2019: p. e47382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yan YL, et al. , DPPA2/4 and SUMO E3 ligase PIAS4 opposingly regulate zygotic transcriptional program. PLoS Biol, 2019. 17(6): p. e3000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen Z and Zhang Y, Loss of DUX causes minor defects in zygotic genome activation and is compatible with mouse development. Nature Genetics, 2019. 51(6): p. 947–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guo M, et al. , Precise temporal regulation of Dux is important for embryo development. Cell Research, 2019. 29(11): p. 956–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hu Z, et al. , Maternal factor NELFA drives a 2C-like state in mouse embryonic stem cells. Nature Cell Biology, 2020. 22(2): p. 175–186. [DOI] [PubMed] [Google Scholar]
- 67.Percharde M, et al. , A LINE1-Nucleolin Partnership Regulates Early Development and ESC Identity. Cell, 2018. 174(2): p. 391–405 e19. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper reveals a novel function of LINE1 in suppressing Dux both in 2CLCs and early embryos, and suggests that the clearance of Dux may be criticle for totipotency to pluripotency transition.
- 68.Tian Q, et al. , H3.3 impedes zygotic transcriptional program activated by Dux. Biochemical and Biophysical Research Communications, 2020. 522(2): p. 422–427. [DOI] [PubMed] [Google Scholar]
- 69.Kong Q, et al. , Histone variant H3.3–mediated chromatin remodeling is essential for paternal genome activation in mouse preimplantation embryos. Journal of Biological Chemistry, 2018. 293(10): p. 3829–3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang F, et al. , DUX-miR-344-ZMYM2-Mediated Activation of MERVL LTRs Induces a Totipotent 2C-like State. Cell Stem Cell, 2020. 26(2): p. 234–250.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang H, et al. , MLL1 Inhibition and Vitamin D Signaling Cooperate to Facilitate the Expanded Pluripotency State. Cell Reports, 2019. 29(9): p. 2659–2671.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rodriguez-Terrones D, et al. , A molecular roadmap for the emergence of early-embryonic-like cells in culture. Nat Genet, 2018. 50(1): p. 106–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lu F, et al. , Role of Tet proteins in enhancer activity and telomere elongation. Genes Dev, 2014. 28(19): p. 2103–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu K, et al. , SETDB1-Mediated Cell Fate Transition between 2C-Like and Pluripotent States. Cell Reports, 2020. 30(1): p. 25–36.e6. [DOI] [PubMed] [Google Scholar]
- 75.Fu X, Djekidel MN, and Zhang Y, A transcriptional roadmap for 2C-like to pluripotent state transition. Science Advances, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhu P, et al. , Single-cell DNA methylome sequencing of human preimplantation embryos. Nat Genet, 2018. 50(1): p. 12–19. [DOI] [PubMed] [Google Scholar]
- 77.Xia W, et al. , Resetting histone modifications during human parental-to-zygotic transition. Science, 2019. 365(6451): p. 353–360. [DOI] [PubMed] [Google Scholar]
- 78.Wu J, et al. , Chromatin analysis in human early development reveals epigenetic transition during ZGA. Nature, 2018. 557(7704): p. 256–260. [DOI] [PubMed] [Google Scholar]


