Abstract
Purpose –
To determine associations between beta-peripapillary atrophy (B-PPA) and incidence and growth of geographic atrophy (GA) in eyes treated with anti-VEGF agents in the Comparison of Age-related Macular Degeneration (AMD) Treatments Trials (CATT).
Methods –
We included 245 cases with incident GA and 245 controls matched by baseline demographics and characteristics associated with development of GA in CATT. Baseline color images were graded for type of B-PPA, defined as presence of hypopigmentation with visible choroidal vessels and sclera that is adjacent to the optic disc. B-PPA was further classified as scleral ring, sclera, sclera/choroidal blood vessels, or combination. Areas of each type of B-PPA as well as the circumferential extent of B-PPA were measured.
Results –
B-PPA was present in 58% of eyes developing GA and in 52% without GA (p=0.17). Greater circumferential extent of sclera/choroidal blood vessels B-PPA in relation to the optic disc was associated with incident GA (p=0.02) and GA size at first observation (p=0.047). B-PPA was not associated with GA growth rate (p>0.05). Patients without B-PPA had a higher number of GA-associated risk alleles of ARMS2 (p=0.0003) and HTRA1 (p=0.001).
Conclusions –
The extent of sclera/choroidal blood vessel B-PPA was associated with GA incidence and size, but not growth rate in eyes treated for neovascular AMD. B-PPA and GA may share some common pathophysiologic pathways unrelated to the GA-associated risk alleles evaluated.
Keywords: Age-related macular degeneration, geographic atrophy, beta peripapillary atrophy, Comparison of Age-related Macular Degeneration Treatments Trials (CATT)
Summary Statement
Our study identified a significant association between the extent of sclera/choroidal blood vessel beta peripapillary atrophy (B-PPA) and incidence and size of geographic atrophy (GA) in Comparison of Age-related Macular Degeneration Treatments Trials (CATT). Presence of B-PPA was also significantly associated with several risk alleles for GA.
Introduction
Peripapillary atrophy (PPA) can be detected on clinical examination, has been defined using several imaging modalities, and is associated with several clinical conditions, most commonly myopia and glaucoma.1, 2 Several subtypes of PPA have been described. Alpha-PPA (A-PPA) is an irregular retinal pigment epithelium (RPE) hyper- or hypo-pigmentation that is farther away from the optic nerve than beta-PPA (B-PPA), which is itself characterized by hypopigmentation with visible choroidal vessels and sclera that is adjacent to the disc.3 Gamma-PPA (G-PPA) is B-PPA but without the presence of RPE or Bruch’s membrane (BM)4 as assessed via optical coherence tomography (OCT).5 Furthermore, “delta zone” has been defined as part of G-PPA when blood vessels are >50 μm in diameter and <300 μm in length.5
Depending on the patient population and ocular pathology, A-PPA may be present in up to 100% of eyes and B-PPA in up to 75–80% of eyes.6, 7 Several studies have focused on changes in PPA area and morphology over time.8 For example, B-PPA has been found to expand on the order of 10 μm2/year9 and is associated with increasing age.10 Typical OCT-defined characteristics in the area subtending B-PPA include a preserved retinal nerve fiber layer (RNFL), and RPE and photoreceptor loss, RPE disruption, and inner/outer retinal thinning.11, 12 Other findings may include an RNFL plaque, intraretinal cystoid changes, and abnormal retinal sloping. Progressive outer retinal/RPE/BM changes are characteristic of geographic atrophy (GA) as well.13
Several prior studies explored the relationship between B-PPA, choroidal thickness, pseudodrusen, and age-related macular degeneration (AMD).14–17 For example, presence of broad B-PPA was associated with AMD; however, age may have been a confounding variable.17 In another study, B-PPA was associated with reticular pseudodrusen in early AMD; although, the relationship was attenuated when subfoveal choroidal thickness was taken into account.14 Using data from the Geographic Atrophy Progression (GAP) study, Chang et al showed that PPA (type not defined) was identified at presentation in 86.4% of eyes with macular GA.18 However, we are unaware of any studies analyzing the relationship between B-PPA and GA, in particular, in eyes with neovascular AMD treated with anti-VEGF agents. Based on the above, we surmised that there might be an association between B-PPA and GA. To test this hypothesis, we investigated the relationship between B-PPA at baseline and development of GA over five years in the study eyes with anti-VEGF treatment for neovascular AMD in the Comparison of AMD Treatments Trials (CATT).
Methods
CATT study population
This is a secondary analysis of the data from the CATT Study. Design, methods, study population, treatment, and outcomes for CATT over five years of follow-up have been published previously.19–21 Eligibility criteria for the trial included presence of neovascular AMD as evidenced by leakage on fluorescein angiography and fluid on OCT, visual acuity (VA) between 20/25 and 20/320, and no prior treatment for the neovascularization. Study eyes were randomly assigned to treatment with either intravitreal ranibizumab or bevacizumab and to one of three treatment regimens: monthly treatment for 2 years, pro re nata (PRN) treatment for 2 years, or monthly treatment for 1 year and PRN treatment for the second year. The study was approved by institutional review boards at each center and was Health Insurance Portability and Accountability Act compliant. The study is registered on http://www.clinicaltrials.gov (no. NCT00593450, accessed July 24, 2018).
Selected patient population
To assess the association between B-PPA at baseline with incident GA over 5 years in the study eye, we conducted a matched case-control study. The cases were from 266 patients who developed GA in the study eye at any point over 5 years of CATT follow-up.22 After excluding those with GA at baseline, poor photo quality, and no follow-up, 245 (92.1%) study eyes with incident GA were included. 245 controls without incident GA were 1:1 matched with cases based on demographics and characteristics shown to be associated with development of GA in CATT, which included GA in the fellow eye, subretinal tissue complex thickness at foveal center, and subretinal and intraretinal fluid.23, 24 Two additional case-control matched pairs were excluded because of inability to reliably determine area of B-PPA in one of the eyes, leaving 243 (91.4%) case-control pairs for analysis. The baseline characteristics of this sub-set of CATT cases and matched controls are shown in Table 1. In addition, we characterized the association between B-PPA at baseline and GA size at year-1, −3, and −5, as well as GA growth over 5 years of CATT.22
Table 1.
Comparison of baseline characteristics between eyes with versus without development of geographic atrophy during follow-up*
| Baseline Characteristics | Incident geographic atrophy | P-value | |
|---|---|---|---|
| No (n=243) | Yes (n=243) | ||
| Age (years), mean (standard deviation) | 79.4 (7.9) | 79.8 (7.0) | 0.57 |
| Smoking status | 0.66 | ||
| Never | 101 (41.6%) | 110 (45.3%) | |
| Quit | 127 (52.3%) | 117 (48.1%) | |
| Current | 15 (6.2%) | 16 (6.6%) | |
| Hypercholesterolemia, yes | 129 (53.1%) | 154 (63.4%) | 0.02 |
| Drug group, Avastin | 127 (52.3%) | 111 (45.7%) | 0.15 |
| Regimen group | 0.18 | ||
| Pro re nata | 123 (50.6%) | 112 (46.1%) | |
| Switched | 64 (26.3%) | 57 (23.5%) | |
| Monthly | 56 (23.0%) | 74 (30.5%) | |
| Baseline visual acuity in study eye | 0.55 | ||
| 20/200–320 | 12 (4.9%) | 18 (7.4%) | |
| 20/100–160 | 64 (26.3%) | 57 (23.5%) | |
| 20/50–80 | 89 (36.6%) | 96 (39.5%) | |
| 20/25–40 | 78 (32.1%) | 72 (29.6%) | |
| Baseline choroidal neovascularization area (disc areas) | 0.42 | ||
| <=1 | 109 (44.9%) | 106 (43.6%) | |
| >1 to <=2 | 50 (20.6%) | 38 (15.6%) | |
| >2 to <=4 | 42 (17.3%) | 48 (19.8%) | |
| >4 | 18 (7.4%) | 27 (11.1%) | |
| Unknown | 24 (9.9%) | 24 (9.9%) | |
| Retinal angiomatous proliferation, yes | 24 (9.9%) | 49 (20.2%) | 0.002 |
| Glaucoma in study eye, yes | 32 (13.2%) | 27 (11.1%) | 0.49 |
| Cataract status in study eye | 0.42 | ||
| No history | 14 (5.8%) | 11 (4.5%) | |
| Pseudophakic/aphakic | 125 (51.4%) | 139 (57.2%) | |
| Ongoing | 104 (42.8%) | 93 (38.3%) | |
| Spherical equivalent in study eye† | 0.56 | ||
| Pseudophakic/aphakic | 125 | 139 | |
| Myopia −5 to −0.5 diopters | 19 (16.1%) | 12 (11.5%) | |
| Emmetropia −0.49 to 0.49 diopters | 11 (9.3%) | 15 (14.4%) | |
| Hyperopia 0.5 to 5 diopters | 85 (72.0%) | 74 (71.2%) | |
| Hyperopia >= 5 diopters | 3 (2.5%) | 3 (2.9%) | |
The eyes were matched by geographic atrophy in the fellow eye, subretinal tissue complex thickness at foveal center, and subretinal and intraretinal fluid in the fovea
Excluding pseudophakic/aphakic eyes
Grader training and initial grading
Graders (AMK and ES) were trained by ED from the Reading Center of the Center for Preventative Ophthalmology and Biostatistics to recognize B-PPA on multiple disc-centered color fundus images (Figure 1). We did not use OCT images in this study since baseline OCT images in CATT were all time-domain rather than spectral-domain OCT (SD-OCT), which would make it difficult to delineate the BM. Furthermore, these OCT images were fovea centered scans so the area adjacent to the disc was not visualized typically. Guided by definitions used in previous studies,3, 5 B-PPA was defined as a hypopigmented area with absence of RPE/BM resulting in visible choroidal vessels and/or sclera that was adjacent to the scleral ring or disc margin.3 Representative images were selected and agreed upon by all graders (AMK and ES) and an adjudicator (ED) and were used in the study as reference during the adjudication process.
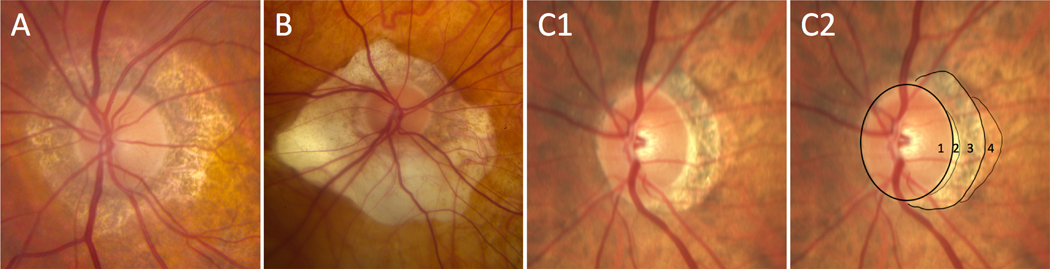
Figure 1. Different examples of peripapillary atrophy.
A and B. Examples of 360-degree circumferential beta-peripapillary atrophy with normal thickness scleral ring and no obvious alpha-peripapillary atrophy. C1 and C2. Original image (C1) and image with tracings (C2) outlining optic disc (1), thick scleral ring (2), beta-peripapillary atrophy (3), and alpha-peripapillary atrophy (4).
Qualitative and quantitative characterization of B-PPA
Disc-centered images for all 243 case-control matched pairs were independently double-graded for presence of B-PPA (AMK and ES). The graders were masked to all demographics, clinical characteristics, and status of case or control. For each image, confidence of B-PPA presence (0 – not confident, 1 – probable, and 2 – definite) and image quality (0 – poor, 1 – fair, and 2 – good) were graded. Images with poor quality were excluded from analysis. Among eyes with presence of B-PPA, we determined different subtypes of B-PPA: 1) thick scleral ring, 2) sclera only, 3) sclera/choroidal blood vessels, and 4) combination (Figure 2),3, 25 and performed the following area measurements: 1) optic disc, 2) optic disc plus scleral ring, and 3) optic disc plus scleral ring and total B-PPA using the drawing tool in Image J software (available for download from https://imagej.nih.gov/ij/download.html). Because the images were obtained with different cameras and magnifications, we measured the areas in pixels rather than mm2. To account for the differences in image magnification, we reported PPA area in pixels as a percent of optic disc area or optic disc plus scleral ring area.25 For “scleral ring” and “sclera/choroidal blood vessel” subtype of B-PPA, we also graded “extent” of PPA with “extent” being defined as percent of circumference of the optic disc encompassed by the specific subtype of B-PPA (e.g., 360 degrees would represent 100%). These were graded as <25%, 26–74%, and >75%. All quantitative measurements were adjudicated if the difference was >20% of the mean of the two graders and all qualitative differences were adjudicated. There is no consensus in the literature regarding inclusion of scleral ring as part of total B-PPA area.3, 25 To account for this, we performed two separate statistical analyses with and without inclusion of scleral ring as part of B-PPA area.
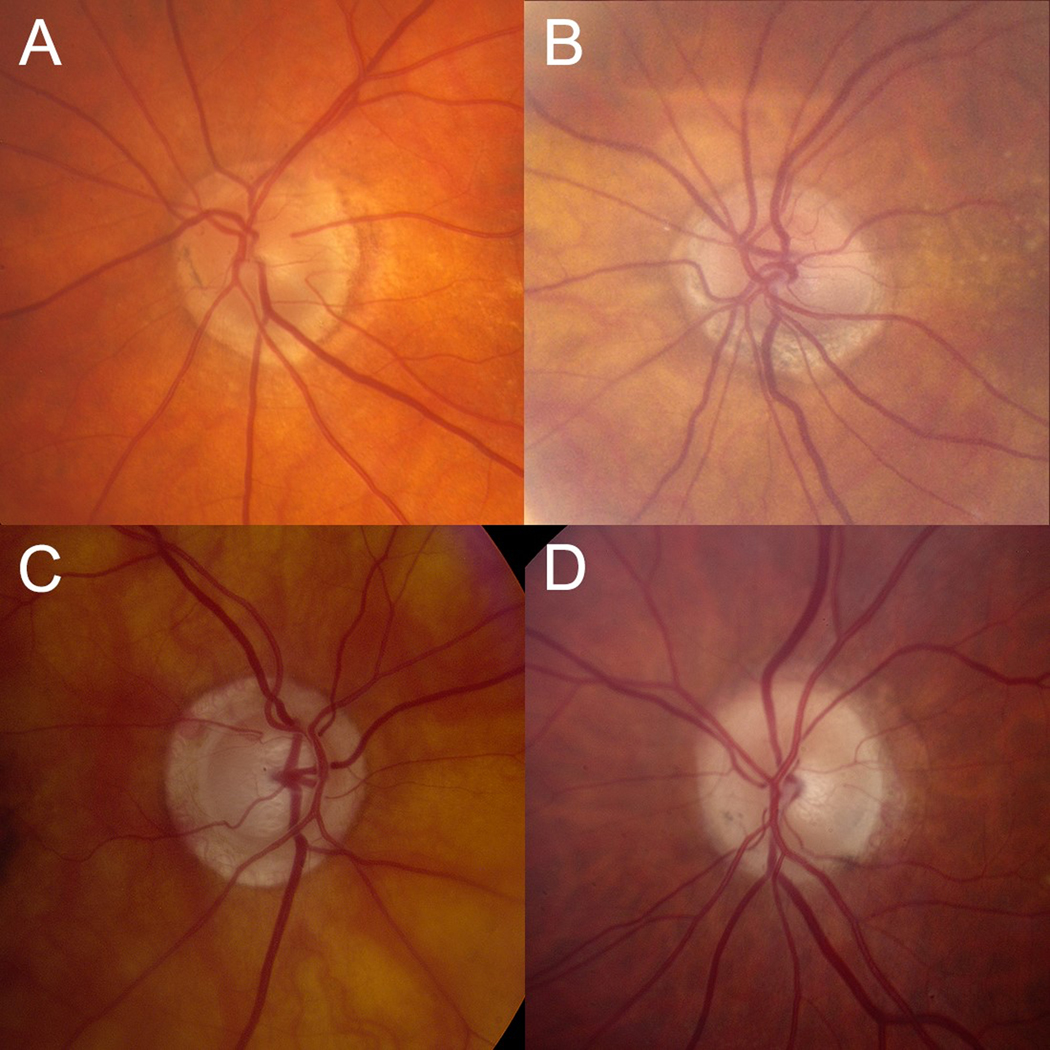
Figure 2. Different sub-types of beta peripapillary atrophy.
A. Thick scleral ring. B. Sclera only. C. Sclera/choroidal blood vessels. D. Thick scleral ring and sclera/choroidal blood vessels. Note that alpha-peripapillary atrophy is present in A, B, and D.
Pseudodrusen and genetic analysis
The presence of pseudodrusen in the study/fellow eye, as well as detailed genetic analysis were previously assessed.26 In CATT, only 835/1185 (70.5%) of participants consented to genetic testing.27 GA-associated small nucleotide polymorphism (SNP) alleles included CFH rs1061170, ARMS2 rs1049092, HTRA1 rs1120063, C3 rs2230199, and TLR3 rs3775291.28, 29 The risk alleles are C for CFH, T for ARMS2, A for HTRA1, and G for C3, while the protective allele is T for TLR3.
Statistical analysis
We used two-sample t-tests for comparison of means and chi-square tests for comparison of proportions between two groups. We used univariate and multivariate logistic regression models to assess the associations between presence of B-PPA and characteristics of B-PPA with incidence of GA during 5 years of follow-up. Correlation from pairings between GA cases and controls were accounted for by using generalized estimating equation. In multivariate analyses, the models were adjusted by age, smoking status, treatment drug, regimen, hypercholesterolemia, retinal angiomatous proliferation (RAP) lesion, glaucoma, cataract status, and refractive error. The association of ordered categorical variables with GA was assessed using linear trend test. Analysis of variance was used to evaluate the association of B-PPA with GA size when first observed. Linear mixed effects models were used to evaluate the association of B-PPA with rate of GA growth. In the mixed effects model, the square root of GA area (in mm) was modeled as a function of time (relative to the first observation of GA). Linear trend tests were used to determine the association of area of sclera/choroidal blood vessels B-PPA as percent of optic disc or optic disc plus scleral ring area with incidence, initial size, and growth rate of GA. For the above, area of B-PPA was categorized into three equally sized groups, while those without B-PPA was considered as another separate group. All statistical analyses were performed in SAS v9.4 (SAS Institute Inc., Cary, NC) and two-sided p-value<0.05 (without correction for multiple comparisons) was considered to be statistically significant.
Results
Demographics and baseline characteristics
Demographic and baseline study eye characteristics for the 243 incident GA cases during 5-year CATT follow-up and their matched controls are shown in Table 1. GA cases and controls were similar in age, smoking status, treatment drug group and regimen, baseline VA and choroidal neovascularization (CNV) size, history of glaucoma, lens status, and refractive error in the study eye. However, participants with GA in the study eye were more likely to have hypercholesterolemia (p=0.02) and a RAP lesion (p=0.002).
Presence of B-PPA on disc centered color fundus photos was identified in 267 (54.9%) patients. Patients with B-PPA were significantly older (p=0.0003) than those without B-PPA, but did not differ significantly in gender, smoking status, and presence of pseudodrusen at baseline in study/fellow eye (Table 2).
Table 2.
Comparison of demographics and pseudodrusen between eyes with versus without beta peripapillary atrophy at baseline
| Characteristics | Beta peripapillary atrophy | P-value | |
|---|---|---|---|
| No (n=219) | Yes (n=267) | ||
| Age (years), mean (standard deviation) | 78.2 (7.6) | 80.7 (7.1) | 0.0003 |
| Gender, female | 142 (64.8%) | 163 (61.1%) | 0.39 |
| Smoking status | 0.42 | ||
| Never | 102 (46.6%) | 109 (40.8%) | |
| Former | 103 (47.0%) | 141 (52.8%) | |
| Current | 14 (6.4%) | 17 (6.4%) | |
| Pseudodrusen in fellow eye*, yes | 52 (26.0%) (n=201) | 81 (32.1%) (n=260) | 0.18 |
| Pseudodrusen in either eye†, yes | 61 (31.0%) (n=197) | 94 (37.6%) (n=250) | 0.16 |
Pseudodrusen could not be determined in 25 fellow eyes due to poor image quality or presence of fluid, which prevented pseudodrusen assessment
Pseudodrusen could not be determined in either study or fellow eye of 39 patients due to poor image quality or presence of fluid, which prevented pseudodrusen assessment
B-PPA grading agreement and data adjudication
Pre-adjudication inter-grader agreement for categorical variables – B-PPA type, extent of scleral ring, and extent of B-PPA sclera/choroidal blood vessels – was 61.2% (kappa, 0.27), 58.6% (kappa, 0.21), and 52.2% (kappa, 0.32), respectively. Intraclass correlation (ICC) coefficients for continuous variables – area of optic disc and optic disc plus scleral ring and B-PPA – was 0.97 (ICC 95% CI, 0.97–0.98) and 0.57 (ICC 95% CI, 0.48–0.64), respectively. Adjudication was performed on 3.6–49.4% of measurements depending on previously outlined criteria (see “Qualitative and quantitative characterization of B-PPA” in the Methods section).
Association of B-PPA with development of GA
B-PPA was present in 58.0% of eyes with GA and in 52.0% of eyes without GA (adjusted OR=1.35, p=0.12; Table 3). Greater area of B-PPA as percent of disc size was weakly associated with development of GA (p=0.08). Type of B-PPA was not associated with risk of GA (p=0.30). Greater circumferential extent of sclera/choroidal blood vessels B-PPA was significantly associated with higher incidence of GA over the CATT 5-year follow-up period (p=0.02). In relation to GA development, a trend towards statistical significance was detected for increasing area of sclera/choroidal blood vessels B-PPA (including scleral ring) as percent of disc area (p=0.10) and increasing area of sclera/choroidal blood vessels B-PPA as percent of optic disc plus scleral ring area (p=0.08).
Table 3.
Univariate and multivariate analysis for the association between baseline beta peripapillary atrophy and incident geographic atrophy
| Beta peripapillary atrophy | Geographic atrophy | Univariate analysis | Multivariate analysis* | |||
|---|---|---|---|---|---|---|
| No (n=243) | Yes (n=243) |
Odds ratio (95% CI) |
P-value | Odds ratio (95% CI) |
P-value | |
| Presence of B-PPA | 0.17 | 0.12 | ||||
| No | 117 (48.1%) | 102 (42.0%) | 1.00 (Ref) | 1.00 (Ref) | ||
| Yes | 126 (51.9%) | 141 (58.0%) | 1.28 (0.90, 1.83) | 1.35 (0.92, 1.99) | ||
| B-PPA area as % of disc area | 0.08† | 0.08† | ||||
| No B-PPA | 117 (48.1%) | 102 (42.0) | 1.00 (Ref) | 1.00 (Ref) | ||
| ≤50% | 49 (20.2%) | 44 (18.1%) | 1.03 (0.65, 1.64) | 1.10 (0.66, 1.81) | ||
| 51–75% | 31 (12.8%) | 41 (16.9%) | 1.52 (0.89, 2.59) | 1.65 (0.92, 2.95) | ||
| 76–100% | 16 (6.6%) | 18 (7.4%) | 1.29 (0.65, 2.58) | 1.27 (0.60, 2.69) | ||
| >100% | 30 (12.3%) | 38 (15.6%) | 1.45 (0.84, 2.52) | 1.56 (0.86, 2.82) | ||
| B-PPA type | 0.36 | 0.30 | ||||
| No B-PPA | 117 (48.1%) | 102 (42.0%) | 1.00 (Ref) | 1.00 (Ref) | ||
| Scleral ring only | 4 (1.6%) | 8 (3.3%) | 2.29 (0.68, 7.79) | 2.40 (0.71, 8.16) | ||
| Scleral ring + sclera/ChBV | 35 (14.4%) | 35 (14.4%) | 1.15 (0.68, 1.93) | 1.19 (0.68, 2.10) | ||
| Sclera/ChBV only | 87 (35.8%) | 98 (40.3%) | 1.29 (0.87, 1.92) | 1.36 (0.88, 2.09) | ||
| B-PPA sclera/ChBV circumferential extent | 0.03† | 0.02† | ||||
| None | 117 (48.1%) | 102 (42.0%) | 1.00 (Ref) | 1.00 (Ref) | ||
| ≤25% | 37 (15.2%) | 32 (13.2%) | 0.99 (0.60, 1.64) | 1.06 (0.63, 1.79) | ||
| 26–74% | 67 (27.6%) | 68 (28.0%) | 1.16 (0.75, 1.80) | 1.25 (0.77, 2.01) | ||
| >75% | 22 (9.1%) | 41 (16.9%) | 2.14 (1.18, 3.88) | 2.39 (1.26, 4.53) | ||
| B-PPA sclera/ChBV area as % of disc area‡** | 0.07† | 0.10† | ||||
| No B-PPA | 117 (49.0%) | 102 (43.4%) | 1.00 (Ref) | 1.00 (Ref) | ||
| <30% | 44 (18.4%) | 42 (17.9%) | 1.09 (0.69, 1.75) | 1.13 (0.68, 1.88) | ||
| 30–70% | 40 (16.7%) | 46 (19.6%) | 1.32 (0.80, 2.16) | 1.38 (0.80, 2.37) | ||
| >70% | 38 (15.9%) | 45 (19.1%) | 1.36 (0.81, 2.27) | 1.39 (0.79, 2.43) | ||
| B-PPA sclera/choroidal blood vessel as % of disc plus scleral ring area‡** | 0.06† | 0.08† | ||||
| No B-PPA | 117 (49.0%) | 102 (43.4%) | 1.00 (Ref) | 1.00 (Ref) | ||
| <25% | 42 (17.6%) | 43 (18.3%) | 1.17 (0.74, 1.86) | 1.27 (0.77, 2.09) | ||
| 25–55% | 42 (17.6%) | 42 (17.9%) | 1.15 (0.71, 1.86) | 1.13 (0.67, 1.91) | ||
| >55% | 38 (15.9%) | 48 (20.4%) | 1.45 (0.86, 2.43) | 1.52 (0.86, 2.68) | ||
Adjusted by age, smoking status, treatment drug, regimen, hypercholesterolemia, retinal angiomatous proliferation lesion, glaucoma, cataract, and spherical equivalent
From test of linear trend
Area of B-PPA could not be determined in 12 patients with B-PPA (4 in no GA group and 8 in GA group)
The subjects were divided into 3 equal groups
Abbreviations: CI, confidence interval; B-PPA, beta-peripapillary atrophy; ChBV, choroidal blood vessels
Association of B-PPA with GA size and rate of growth
The extent of sclera/choroidal blood vessels B-PPA in relation to the optic disc was significantly (p=0.047) associated with GA size at first observation during follow-up (Table 4). Presence and type of B-PPA and area of sclera/choroidal blood vessels were not significantly associated with initial size or growth rate of GA (p>0.08 for all comparisons, Table 4).
Table 4.
Univariate analysis for the association between baseline beta peripapillary atrophy and size of geographic atrophy area and growth rate of geographic atrophy
| Geographic atrophy size at first observation during follow-up* | Geographic atrophy growth† | |||||
|---|---|---|---|---|---|---|
| n | Mean (SE) in mm2 | P-value | n | mm/year (SE) | P-value | |
| Presence of B-PPA | 0.32 | 0.46 | ||||
| No | 102 | 3.47 (0.41) | 44 | 0.277 (0.037) | ||
| Yes | 141 | 4.21 (0.56) | 77 | 0.312 (0.030) | ||
| B-PPA area as % of disc area | 0.16‡ | 0.28‡ | ||||
| No B-PPA | 102 | 3.47 (0.41) | 44 | 0.291 (0.036) | ||
| ≤50% | 44 | 3.87 (1.00) | 26 | 0.318 (0.047) | ||
| 51–75% | 41 | 4.07 (1.02) | 21 | 0.309 (0.060) | ||
| 76–100% | 18 | 2.89 (0.98) | 6 | 0.164 (0.107) | ||
| >100% | 38 | 5.39 (1.26) | 24 | 0.390 (0.052) | ||
| B-PPA type | 0.56 | 0.71 | ||||
| No B-PPA | 102 | 3.47 (0.41) | 44 | 0.291 (0.037) | ||
| Scleral ring only | 8 | 6.05 (3.72) | 5 | 0.210 (0.116) | ||
| Scleral ring + sclera/ChBV | 35 | 4.47 (1.03) | 20 | 0.346 (0.055) | ||
| Sclera/ChBV only | 98 | 3.97 (0.66) | 52 | 0.330 (0.037) | ||
| B-PPA sclera/ChBV circumferential extent | 0.047‡ | 0.29‡ | ||||
| None | 102 | 3.47 (0.41) | 44 | 0.258 (0.036) | ||
| ≤25% | 32 | 3.93 (1.11) | 21 | 0.304 (0.061) | ||
| 26–74% | 68 | 2.80 (0.55) | 34 | 0.245 (0.043) | ||
| >75% | 41 | 6.78 (1.41) | 22 | 0.370 (0.057) | ||
| B-PPA sclera/ChBV area as % of disc area** | 0.16‡ | 0.22‡ | ||||
| No B-PPA | 102 | 3.47 (0.41) | 44 | 0.265 (0.036) | ||
| ≤23% | 42 | 3.32 (0.80) | 24 | 0.312 (0.050) | ||
| 23–79% | 46 | 4.07 (0.97) | 21 | 0.223 (0.057) | ||
| >79% | 45 | 4.86 (1.09) | 27 | 0.377 (0.052) | ||
| B-PPA sclera/ChBV as % of the disk plus scleral ring area** | 0.08‡ | 0.31‡ | ||||
| No B-PPA | 102 | 3.47 (0.41) | 44 | 0.267 (0.036) | ||
| <25% | 43 | 2.86 (0.70) | 24 | 0.322 (0.052) | ||
| 25–55% | 42 | 4.27 (1.05) | 20 | 0.237 (0.058) | ||
| >55% | 48 | 5.07 (1.06) | 28 | 0.355 (0.051) | ||
GA was first observed at year 1 in 111 eyes, at year 2 in 37 eyes, and at year 5 in 95 eyes
Among those with two or more measurements of GA size during follow-up (those with baseline GA were excluded from this study)
From test of linear trend
The subjects were divided into 3 equal groups
Abbreviations: SE, standard error; B-PPA, beta-peripapillary atrophy; ChBV, choroidal blood vessels
Genetic analysis of GA-associated alleles
Genetic data was available on 155/219 (70.8%) patients without B-PPA and on 179/267 (67.0%) patients with B-PPA (Table 5). Patients without B-PPA had a significantly higher likelihood of the T risk allele in ARMS2 rs1049092 (p=0.0003) and the A risk allele in HTRA1 rs1120063 (p=0.001). There were no statistically significant differences in the C risk allele for CFH rs1061170, the G risk allele for C3 rs2230199, or the protective T allele for TLR3 rs3775291.
Table 5.
Comparison of genetic characteristics between eyes with versus without beta peripapillary atrophy at baseline
| Gene alleles* | Beta peripapillary atrophy | P-value | |
|---|---|---|---|
| No (n=155) | Yes (n=179) | ||
| CFH rs1061170 | 0.29 | ||
| CC | 47 (30.3%) | 48 (26.8%) | |
| TC | 78 (50.3%) | 88 (49.2%) | |
| TT | 30 (19.4%) | 43 (24.0%) | |
| ARMS2 rs1049092 | 0.0003 | ||
| GG | 37 (23.9%) | 61 (34.1%) | |
| GT | 68 (43.9%) | 93 (52.0%) | |
| TT | 50 (32.3%) | 25 (14.0%) | |
| HTRA1 rs1120063 | 0.001 | ||
| AA | 47 (30.3%) | 25 (14.0%) | |
| AG | 70 (45.2%) | 94 (52.5%) | |
| GG | 38 (24.5%) | 60 (33.5%) | |
| C3 rs2230199 | 0.41 | ||
| CC | 86 (55.5%) | 114 (63.7%) | |
| CG | 61 (39.4%) | 51 (28.5%) | |
| GG | 8 (5.2%) | 14 (7.8%) | |
| TLR3 rs3775291 | 0.31 | ||
| CC | 86 (55.5%) | 88 (49.2%) | |
| CT | 58 (37.4%) | 77 (43.0%) | |
| TT | 11 (7.1%) | 14 (7.8%) | |
The risk alleles are C for CFH, T for ARMS2, A for HTRA1, and G for C3. The protective allele is T for TLR3.
Discussion
In the current study, we examined the relationship between B-PPA at baseline and incident GA in the study eye over 5 years of CATT follow-up. We found that B-PPA was present at baseline in 58% of study eyes that developed GA throughout 5 years of follow-up versus 52% that did not. This difference was not statistically significant. Interestingly, the 58% prevalence of B-PPA was considerably lower than the 86% reported in the GAP study among eyes with GA; however, Chang and colleagues did not specify the type of PPA in the GAP study.18 Although presence of B-PPA was not associated with development of GA, there was a weak trend (p=0.08, Table 3) for an association between larger B-PPA area and higher incidence of GA. Presence of B-PPA was also significantly associated with older age, which has been shown previously.10
We separated B-PPA into different morphologic categories – thick scleral ring, sclera, sclera/choroidal blood vessels, or combination (Figure 2). In prior studies, the scleral ring has not been considered as a separate subcategory of B-PPA and was usually incorporated into the overall area of B-PPA as it was assumed that it was so thin that it would not significantly affect the overall area of B-PPA measurement.1, 25 However, we felt that in certain patients the ring was “too thick” to be included and wanted to separate it out (Figure 1 C1 and C2). In addition, to be consistent with the literature, we also performed data analysis with the area of the scleral ring incorporated into the area of sclera/choroidal blood vessels B-PPA. We found that the scleral ring (scleral ring only versus scleral ring/sclera/choroidal blood vessels) did not significantly affect any of the comparisons performed in the study, and it was not independently associated with development of GA (Table 3).
In addition to the type and area of PPA, circumferential extent of PPA has been evaluated in several prior studies. For example, eccentric (<270 degrees) versus concentric (>270 degrees) PPA in myopic glaucomatous eyes was shown to associate with glaucoma worsening and PPA progression.8 We found that circumferential extent (>75% or >270 degrees) of sclera/choroidal blood vessels B-PPA was significantly associated with development of GA over 5 years of CATT follow-up (Table 3) as well as size of GA at first observation (Table 4). The sclera/choroidal blood vessels B-PPA subtype was not significantly associated with GA development probably because the smaller areas/lesions of this type of B-PPA do not seem to be associated with GA development. Overall, these data suggest that enlarging area and extent of sclera/choroidal blood vessels B-PPA may indicate wide spread outer retina/RPE/BM susceptibility to degeneration, including the macula. In addition, a relationship between peripapillary strain, mechanical stress, and changes in the lamina cribrosa as related to age and development of glaucoma has been explored.30, 31 These findings imply that the peripapillary area may have a pre-existing susceptibility to degeneration due to mechanical stress.
Numerous AMD and GA-associated alleles have been identified.32 Genetic testing is not yet recommended as part of routine patient management since allele-specific treatments are not currently available, but may in the future influence choice of therapy.33, 34 While select alleles are associated with higher or lower incidence of GA,22 they were not associated with GA growth in CATT.27 In the current study, patients without B-PPA had significantly higher likelihoods of the risk alleles in ARMS2 and HTRA1 genes (Table 5). While development of B-PPA is strongly genetically inherited,35 these data imply that any associations between B-PPA and GA may not be mechanistically related to GA-specific risk alleles evaluated in the current study. In addition, the results indicate that the known association of the T risk allele for ARMS2 and the A risk allele for HTRA1 with advanced AMD may be specifically related to the neovascular type. This finding may warrant further validation in future studies with a much more robust sample size.
The strengths of this study include: 1) our matched case-control approach using a well-characterized clinical trial cohort with 5 years of follow-up, 2) rigorous double grading of images with adjudication, and 3) thorough analysis of different aspects of B-PPA as they relate to incident GA. Our data regarding GA development is most applicable to eyes with neovascular AMD treated with anti-VEGF agents. Therefore, the results may be different if GA of non-exudative AMD eyes were to be studied. The use of anti-VEGF agents, which themselves have been implicated in development/progression of atrophy,23 may have affected our results. Differences in the vascular supply and structural/biochemical characteristics between the peripapillary and macular areas may have also had an effect.30, 36
Our study also has several limitations. These include subjective assessment of B-PPA as well as outlining the margins on color photographs for B-PPA area quantification. This may explain low inter-grader agreement for type and extent of B-PPA. Use of OCT technology may aid in more reliable determination of B-PPA presence and delineation for area measurement.12 However, disc centered spectral-domain OCT images were not available, as they were not routinely done under CATT methodology. In addition, SD-OCT has recently been used to differentiate B-PPA from G-PPA,37 which cannot be done on color photographs, and so the area of B-PPA represented in the current study could have included G-PPA. Using flattened photographs representing a curved fundus may result in measurement errors, especially if the patients have significant refractive errors. However, we employed previously reported approaches for area measurements on color photographs.25 Assessment of pseudodrusen and genetic testing data were not available for all patients. B-PPA area was represented as percent of disc (or disc plus scleral ring) area rather than mm2 due to differences in cameras and image acquisition methods. Since we did not measure areas of optic disc or B-PPA in mm2, potential comparisons to future studies may be difficult. We did not perform any corrections for multiple comparisons in this secondary data analysis study. Finally, these data are exploratory and hypothesis generating; additional studies would be needed to confirm the association of sclera/choroidal blood vessel B-PPA with incident GA.
In summary, we identified a statistically significant association between circumferential extent of sclera/choroidal blood vessels B-PPA and incidence as well as size of GA over 5 years of CATT follow-up. Although there remains an urgent need to investigate biomarkers for incident GA, the exact mechanisms for development of GA and B-PPA still remain unclear. Our results suggest that further investigation of B-PPA could be meaningful, especially as a biomarker in clinical trials focusing on GA. With the advent and application of artificial intelligence and deep-learning algorithms in medicine, and especially ophthalmology, this technology might be adapted to aid in identification of such biomarkers in future endeavors.
Supplementary Material
Supplemental Digital Content 1. A full listing of the CATT Research Group (PDF)
Acknowledgments
Financial support: Supported by cooperative agreements U10 EY017823, U10 EY017825, U10 EY017826, and U10 EY017828 from the National Eye Institute, National Institutes of Health, Department of Health and Human Services. Supported by the Heed Ophthalmic Foundation. ClinicalTrials.gov number, NCT00593450.
Footnotes
Conflict of interest: No conflicting relationships or proprietary interests exist for any author as it pertains to the current manuscript. The following commercial relationships include: Dr. Kolomeyer is a consultant to Alimera Sciences and Allergen. Dr. Kim is a consultant to Allergan, Synergy Research, Inc. and Apellis Pharamaceuticals. Dr. Ying is a biostatistical consultant for Chengdu Kanghong Biotechnology Co. Ltd; Ziemer Ophthalmic Systems AG; Synergy Research, Inc. and Apellis Pharamaceuticals. Dr. Maguire serves on data and safety monitoring committees for Genentech-Roche.
References
- 1.Uchida H, Ugurlu S, Caprioli J. Increasing peripapillary atrophy is associated with progressive glaucoma. Ophthalmology 1998;105:1541–1545. [DOI] [PubMed] [Google Scholar]
- 2.Xu L, Li Y, Wang S, Wang Y, Wang Y, Jonas JB. Characteristics of highly myopic eyes: the Beijing Eye Study. Ophthalmology 2007;114:121–126. [DOI] [PubMed] [Google Scholar]
- 3.Jonas JB, Nguyen XN, Gusek GC, Naumann GO. Parapapillary chorioretinal atrophy in normal and glaucoma eyes. I. Morphometric data. Investigative ophthalmology & visual science 1989;30:908–918. [PubMed] [Google Scholar]
- 4.Banitt MR, Sidoti PA, Gentile RC, et al. Pars plana Baerveldt implantation for refractory childhood glaucomas. Journal of glaucoma 2009;18:412–417. [DOI] [PubMed] [Google Scholar]
- 5.Jonas JB, Jonas SB, Jonas RA, et al. Parapapillary atrophy: histological gamma zone and delta zone. PloS one 2012;7:e47237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mataki N, Tomidokoro A, Araie M, Iwase A. Beta-peripapillary atrophy of the optic disc and its determinants in Japanese eyes: a population-based study. Acta ophthalmologica 2018;96:e701–e706. [DOI] [PubMed] [Google Scholar]
- 7.Lee KY, Tomidokoro A, Sakata R, et al. Cross-sectional anatomic configurations of peripapillary atrophy evaluated with spectral domain-optical coherence tomography. Investigative ophthalmology & visual science 2010;51:666–671. [DOI] [PubMed] [Google Scholar]
- 8.Song MK, Sung KR, Shin JW, Kwon J, Lee JY, Park JM. Progressive change in peripapillary atrophy in myopic glaucomatous eyes. The British journal of ophthalmology 2018;102:1527–1532. [DOI] [PubMed] [Google Scholar]
- 9.See JL, Nicolela MT, Chauhan BC. Rates of neuroretinal rim and peripapillary atrophy area change: a comparative study of glaucoma patients and normal controls. Ophthalmology 2009;116:840–847. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Xu L, Zhang L, Yang H, Ma Y, Jonas JB. Peripapillary atrophy in elderly Chinese in rural and urban Beijing. Eye 2008;22:261–266. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi K, Tomidokoro A, Lee KY, et al. Spectral-domain optical coherence tomography of beta-zone peripapillary atrophy: influence of myopia and glaucoma. Investigative ophthalmology & visual science 2012;53:1499–1505. [DOI] [PubMed] [Google Scholar]
- 12.Manjunath V, Shah H, Fujimoto JG, Duker JS. Analysis of peripapillary atrophy using spectral domain optical coherence tomography. Ophthalmology 2011;118:531–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schatz H, McDonald HR. Atrophic macular degeneration. Rate of spread of geographic atrophy and visual loss. Ophthalmology 1989;96:1541–1551. [DOI] [PubMed] [Google Scholar]
- 14.Garg A, Blumberg DM, Al-Aswad LA, et al. Associations Between beta-Peripapillary Atrophy and Reticular Pseudodrusen in Early Age-Related Macular Degeneration. Investigative ophthalmology & visual science 2017;58:2810–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Switzer DW Jr., Mendonca LS, Saito M, Zweifel SA, Spaide RF. Segregation of ophthalmoscopic characteristics according to choroidal thickness in patients with early age-related macular degeneration. Retina 2012;32:1265–1271. [DOI] [PubMed] [Google Scholar]
- 16.Xu L, Li Y, Zheng Y, Jonas JB. Associated factors for age related maculopathy in the adult population in China: the Beijing eye study. The British journal of ophthalmology 2006;90:1087–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirvela H, Luukinen H, Laara E, Sc L, Laatikainen L. Risk factors of age-related maculopathy in a population 70 years of age or older. Ophthalmology 1996;103:871–877. [DOI] [PubMed] [Google Scholar]
- 18.Chang P, Tan A, Jaffe GJ, et al. Analysis of Peripapillary Atrophy in Relation to Macular Geographic Atrophy in Age-Related Macular Degeneration. Investigative ophthalmology & visual science 2016;57:2277–2282. [DOI] [PubMed] [Google Scholar]
- 19.Group CR, Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. The New England journal of medicine 2011;364:1897–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Comparison of Age-related Macular Degeneration Treatments Trials Research G, Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology 2012;119:1388–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Comparison of Age-related Macular Degeneration Treatments Trials Research G, Maguire MG, Martin DF, et al. Five-Year Outcomes with Anti-Vascular Endothelial Growth Factor Treatment of Neovascular Age-Related Macular Degeneration: The Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology 2016;123:1751–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grunwald JE, Pistilli M, Daniel E, et al. Incidence and Growth of Geographic Atrophy during 5 Years of Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology 2017;124:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grunwald JE, Daniel E, Huang J, et al. Risk of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology 2014;121:150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daniel E, Ying GS, Kim BJ, et al. Follow up at 5 Years of Non-Fibrotic Scars in the Comparison of Age-related Macular Degeneration Treatments Trials (CATT). Ophthalmology 2019. May;126(5):743–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Savatovsky E, Mwanza JC, Budenz DL, et al. Longitudinal changes in peripapillary atrophy in the ocular hypertension treatment study: a case-control assessment. Ophthalmology 2015;122:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou Q, Daniel E, Maguire MG, et al. Pseudodrusen and Incidence of Late Age-Related Macular Degeneration in Fellow Eyes in the Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology 2016;123:1530–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grunwald JE, Pistilli M, Ying GS, et al. Growth of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology 2015;122:809–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hagstrom SA, Ying GS, Pauer GJT, et al. Pharmacogenetics for genes associated with age-related macular degeneration in the Comparison of AMD Treatments Trials (CATT). Ophthalmology 2013;120:593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Z, Stratton C, Francis PJ, et al. Toll-like receptor 3 and geographic atrophy in age-related macular degeneration. The New England journal of medicine 2008;359:1456–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burgoyne CF, Downs JC, Bellezza AJ, Suh JK, Hart RT. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Progress in retinal and eye research 2005;24:39–73. [DOI] [PubMed] [Google Scholar]
- 31.Fazio MA, Grytz R, Morris JS, et al. Age-related changes in human peripapillary scleral strain. Biomechanics and modeling in mechanobiology 2014;13:551–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warwick A, Lotery A. Genetics and genetic testing for age-related macular degeneration. Eye 2018;32:849–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rojas-Fernandez CH, Tyber K. Benefits, Potential Harms, and Optimal Use of Nutritional Supplementation for Preventing Progression of Age-Related Macular Degeneration. The Annals of pharmacotherapy 2017;51:264–270. [DOI] [PubMed] [Google Scholar]
- 34.Yaspan BL, Williams DF, Holz FG, et al. Targeting factor D of the alternative complement pathway reduces geographic atrophy progression secondary to age-related macular degeneration. Science translational medicine 2017;9. [DOI] [PubMed] [Google Scholar]
- 35.Healey PR, Mitchell P, Gilbert CE, et al. The inheritance of peripapillary atrophy. Investigative ophthalmology & visual science 2007;48:2529–2534. [DOI] [PubMed] [Google Scholar]
- 36.Lim CW, Cheng J, Tay ELT, et al. Optical coherence tomography angiography of the macula and optic nerve head: microvascular density and test-retest repeatability in normal subjects. BMC ophthalmology 2018;18:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dai Y, Jonas JB, Huang H, Wang M, Sun X. Microstructure of parapapillary atrophy: beta zone and gamma zone. Investigative ophthalmology & visual science 2013;54:2013–2018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1. A full listing of the CATT Research Group (PDF)