Abstract
Educational attainment is widely used as a surrogate for socioeconomic status (SES). Low SES is a risk factor for hypertension and high blood pressure (BP). To identify novel BP loci, we performed multi-ancestry meta-analyses accounting for gene-educational attainment interactions using two variables, “Some College” (yes/no) and “Graduated College” (yes/no). Interactions were evaluated using both a 1 degree of freedom (DF) interaction term and a 2DF joint test of genetic and interaction effects. Analyses were performed for systolic BP, diastolic BP, mean arterial pressure, and pulse pressure. We pursued genome-wide interrogation in Stage 1 studies (N=117 438) and follow-up on promising variants in Stage 2 studies (N=293 787) in five ancestry groups. Through combined meta-analyses of Stages 1 and 2, we identified 84 known and 18 novel BP loci at genome-wide significance level (P<5×10−8). Two novel loci were identified based on the 1DF test of interaction with educational attainment, while the remaining 16 loci were identified through the 2DF joint test of genetic and interaction effects. Ten novel loci were identified in individuals of African ancestry. Several novel loci show strong biological plausibility since they involve physiologic systems implicated in BP regulation. They include genes involved in the central nervous system-adrenal signaling axis (ZDHHC17, CADPS, PIK3C2G), vascular structure and function (GNB3, CDON), and renal function (HAS2 and HAS2-AS1, SLIT3). Collectively, these findings suggest a role of educational attainment or SES in further dissection of the genetic architecture of BP.
Introduction
Educational attainment is among the most widely used indices of socioeconomic status (SES) in epidemiologic studies.1, 2 Multiple studies have demonstrated a step-wise decline in all-cause mortality with increasing levels of education.1 Compared with other measures of SES, such as income and occupation, the use of educational attainment has several advantages: it is stable after young adulthood, simple to capture, has a low non-response rate, and is not affected by poor health in adulthood.1, 3 Furthermore, the relationship between educational attainment and cardiovascular disease traits tend to be more consistent and stronger.4 Higher educational attainment is related to improved health efficacy (such as preventive health behaviors and problem-solving capacity), improved access to health care, and more favorable socio-psychological conditions (such as personal control and social support).2, 3
Several variables of educational attainment investigated in epidemiologic studies in relation to cardiovascular risk traits include continuous variables (such as years of education) and various partitions (such as completing high school or completing college degree).5–11 Low educational attainment is related to high blood pressure (BP) and increased hypertension (HTN) risk as evidenced in a meta-analysis of 51 studies across 20 countries.3 Educational attainment is also related to coronary artery disease,12 coronary calcification,13 and other cardiovascular risk traits including metabolic syndrome,10 lipid levels,9, 10, 14 smoking behavior,12, 15 salt intake,16, 17 and leisure-time physical activity.18 Furthermore, the genetic effects on HTN may vary as a function of educational attainment. For example, a heritability study of European-ancestry male twins showed higher heritability of HTN at higher education levels (h2 = 0.63 with >14 years of education compared to h2 = 0.46 with ≤ 14 years of education),19 suggesting interactions between genes and educational attainment.
While genome-wide association studies have investigated the genetic contributions to educational attainment,6 there has been no comprehensive effort to examine the role played by gene-environment interactions in BP using educational attainment as the environmental exposure. Within the CHARGE Gene-Lifestyle Interactions Working Group,20 we performed genome-wide meta-analysis of systolic BP (SBP), diastolic BP (DBP), mean arterial pressure (MAP), and pulse pressure (PP), accounting for gene-educational attainment interactions. Based on the availability of data across participating studies, we considered two educational attainment variables, “Some College” (yes/no, for any education beyond high school) and “Graduated College” (yes/no, for completing a 4-year college degree). Herein we report our findings based on up to 411 225 individuals from five ancestry groups.
Subjects and Methods
Participating studies
We performed our analysis in two stages (Figure 1). A total of 42 cohorts including 117 438 men and women (aged 18–80 years) from European (EUR), African (AFR), Asian (ASN), Hispanic (HIS), and Brazilian (BRZ) ancestries contributed to Stage 1 genome-wide interaction analyses (Table S1). An additional 49 cohorts including 293 787 individuals contributed to Stage 2 analyses of top single nucleotide variants (SNVs, also including a small number of insertion and deletion [indels] variants) selected from Stage 1 (Table S2). Participating studies are described in the Supplementary Material. Since discoveries to date are largely from EUR populations, considerable effort was made to recruit most of the available non-EUR cohorts into Stage 1. Each study obtained informed consent from participants and approval from the appropriate institutional review boards.
Figure 1.
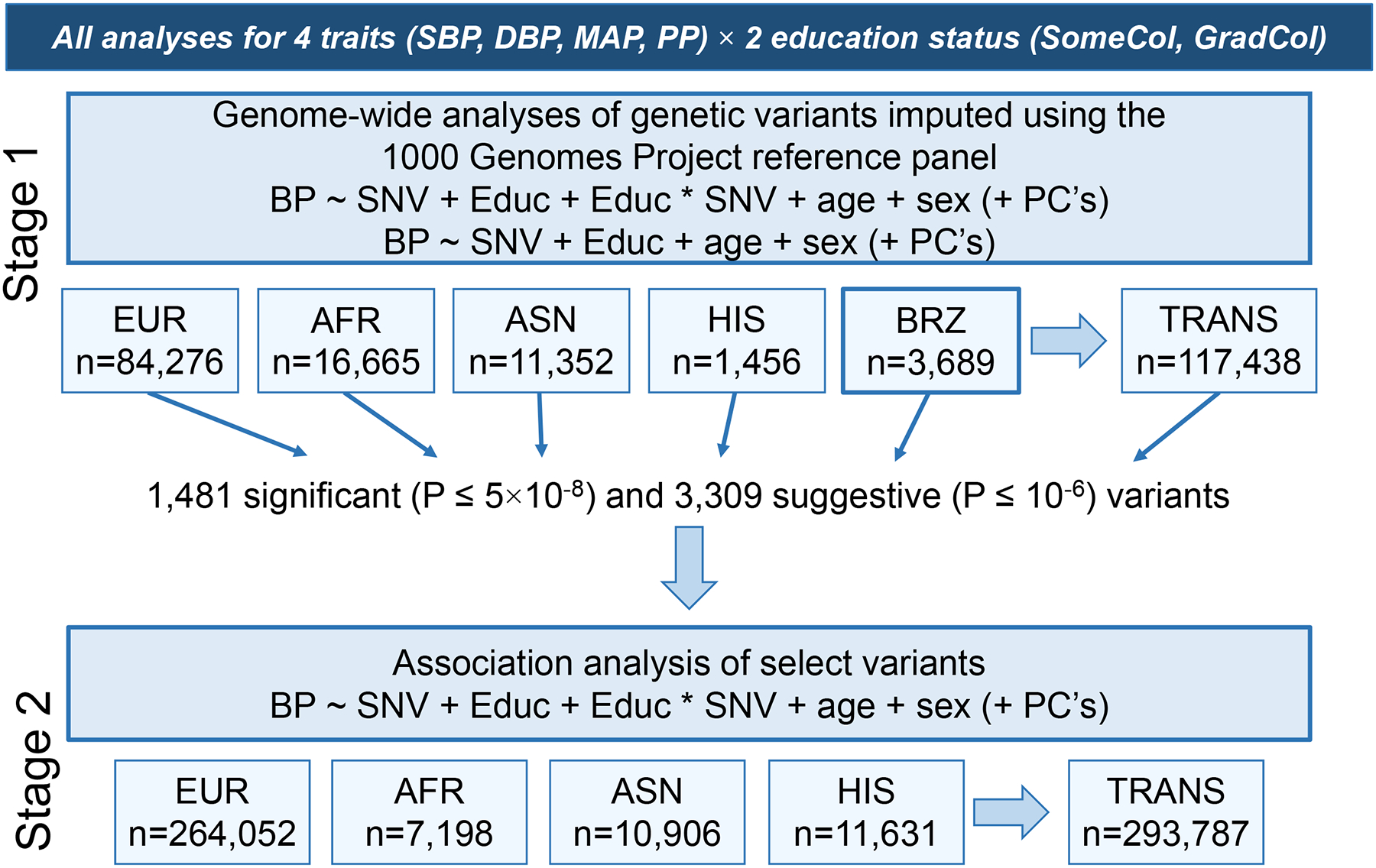
Study design with summary of data included in this study. Educ: education status (considering either SomeCol or GradCol status separately); PC: principal component; EUR: European; AFR: African; ASN: Asian; HIS: Hispanic; BRZ: Brazilian; SNV: single nucleotide variant; TRANS; trans-ancestry (i.e., combining all ancestry groups through meta-analysis).
Phenotype and lifestyle variables
We performed our analysis for SBP, DBP, MAP, and PP. After computing SBP and DBP when multiple measurements were available, we adjusted for antihypertensive medication use by adding 15 mmHg and 10 mmHg to SBP and DBP, respectively.21 After medication adjustment, MAP was computed as (SBP + 2DBP)/3, and PP was computed as SBP minus DBP (SBP - DBP). To reduce the influence of possible outliers, Winsorizing was performed for each BP value that was more than 6 standard deviations away from the mean. Descriptive statistics for these 4 BP traits are presented in Tables S3 and S4. For educational attainment, two dichotomous variables were created. The first variable, ‘Some College’ (SomeCol), was coded as 1 if the subject received any education beyond high school, including vocational school (and as 0 if no education beyond high school). The second variable, ‘Graduated College’ (GradCol), was coded as 1 if the subject completed at least a 4-year college degree (and as 0 for any education less than a 4-year degree). Subjects with missing data for BP, education attainment, or any covariates were excluded from analysis.
Genotype data
Genotyping was performed by each participating study using Illumina (San Diego, CA, USA) or Affymetrix (Santa Clara, CA, USA) genotyping arrays. Imputation was performed using the 1000 Genomes Project22 Phase I Integrated Release Version 3 Haplotypes (2010–11 data freeze, 2012–03–14 haplotypes) as a reference panel, in most cohorts. Information on genotype and imputation for each study is presented in Tables S5 and S6.
Analysis methods
Each study performed association analyses using the following model:
where Y is the BP variable (SBP, DBP, MAP, or PP value), Educ is the educational variable (SomeCol or GradCol), and G is the dosage of the imputed genetic variant coded additively from 0 to 2. C is the vector of covariates, including age, sex, field center (for multi-center studies), and principal components. In addition, studies in Stage 1 performed association analysis using the following genetic main effect model with education attainment:
Each study provided the estimated SNV effect (βG), estimated SNV-educational attainment interaction effect (βGE), their robust standard errors, and a robust estimate of the covariance between βG and βGE. We performed meta-analysis using the 1 degree of freedom (DF) test of the interaction effect (βGE) and 2DF test of both SNV (βG) and interaction effects (βGE). Inverse-variance weighted meta-analysis was performed for the 1DF test and the joint meta-analysis of Manning et al23 for the 2DF test, both using METAL.24 In Stage 1 EUR, AFR, ASN meta-analyses, variants were included if they were available in more than 5 000 samples or at least 3 cohorts (these filters were not applied to BRZ or HIS because of the fewness of cohorts included in these meta-analyses). We applied genomic control correction25 twice in Stage 1, first for study-specific GWAS results and again for meta-analysis results. Genome-wide significant (P < 5×10−8) and suggestive (P < 1×10−6) variants in Stage 1 were taken forward into Stage 2 analysis. Genomic control correction was not applied to the Stage 2 results as association test was performed for select variants. Results presented reflect meta-analyses combining Stages 1 and 2. Loci were defined by physical distance (± 1Mb around the index SNV of the respective locus).
Quality control (QC)
Each participating cohort in Stage 1 excluded variants with minor allele frequency (MAF) < 1%. We performed extensive QC using the R package EasyQC26 for all cohort-specific and meta-analysis results. For Stages 1 and 2, we excluded all variants with imputation quality measure < 0.5. In addition, to remove unstable study-specific results that reflected small sample size, low minor allele count (MAC), or low imputation quality, we excluded variants for which the minimum of (MAC0, MAC1) x imputation quality < 20, where MAC0 and MAC1 are the MAC in the two education strata (Educ = 0 and Educ = 1). The allele frequencies provided by each cohort were compared against those from the relevant ancestry-specific 1000 Genomes reference panel. Marker names were harmonized to ensure consistency across cohorts. In addition, we visually compared summary statistics (e.g., mean, median, standard deviation, inter-quartile range) of all effect estimates, standard errors (SEs), and p-values. We examined SE-N plots26 and quantile-quantile (QQ) plots to reveal issues with trait transformation or other analytical problems. Any problems encountered during QC steps were resolved through communication with the analysts from the participating studies. More detailed information about the QC steps, including major QC problems encountered and how they were resolved, are described elsewhere.20, 27
Characterization of functional roles
A suite of tools implemented in FUMA GWAS28 were used to identify functional roles for the index variants and nearby variants in linkage disequilibrium (LD; r2 ≥ 0.2) in each of the novel BP loci. LD information was obtained from the 1000 Genomes Project reference genome for the ancestry with the most significant ancestry-specific association. If the most significant association was in trans-ancestry analyses, the reference genome for the ancestry with the next most significant association was used instead.29 One index insertion/deletion locus was not identified in any of the reference genomes by FUMA and therefore not detailed. Nearest gene annotations were limited to protein coding, long non-coding RNAs (lncRNAs), and non-coding RNAs (ncRNAs) within 10kb of index variants and variants in LD (r2 ≥ 0.2) with the index variant.30
For the index and LD variants, we used the RegulomeDB score,31 which reflects a summary of annotations with known and predicted regulatory elements such as DNAase hypersensitivity, binding sites of transcription factors, and promoter regions, and Combined Annotation Dependent Depletion (CADD)32 scores, which predict deleteriousness of variants. The 15-core chromatin state (ChromHMM)33, 34 was assessed for 129 epigenomes (labeled E001-E129) to identify histone modifications consistent with epigenetic regulation of gene expression. Expression quantitative trait loci (eQTLs) were determined using the GTEx_v7 database35 for index and LD variants. Using nearest-gene annotations, FUMA GWAS was used to generate tissue-specific gene expression data (GTEx V7 dataset, 53 tissue types); significance was determined as a Benjamini-Hochberg false discovery rate (FDR) < 0.05.
Results
Overview
We performed a meta-analysis of gene-education interactions on SBP, DBP, MAP, and PP in two stages (Figure 1). In Stage 1, we pursued genome-wide interrogation in 117 438 individuals of European (EUR), African (AFR), Asian (ASN), Hispanic (HIS), and Brazilian (BRZ) ancestries (summary information, Table 1). After extensive quality control (QC), we performed genome-wide meta-analyses at approximately 18.8 million single nucleotide variants (SNVs) and a small number of insertion and deletion (indels) variants imputed using the 1000 Genomes Project reference panel (QQ plots, Figure S1). Through the 1DF test of the interaction effect and the 2DF joint test of the SNV and interaction effects, we identified 1 481 genome-wide significant (P < 5×10−8) and 3 309 suggestive (P < 1×10−6) variants associated with any BP trait in any ancestry and/or in trans-ancestry analysis. All significant and suggestive variants were tested for association in 293 787 additional individuals of EUR, AFR, ASN, and HISP ancestries in Stage 2.
Table 1.
Basic characteristics of cohorts in Stages 1 and 2 in each ancestry
| Stage 1 | Max. N | Some College? (Yes/No) | Grad. College? (Yes/No) | % Male | % HT | % HT Meds | Age | SBP | DBP | MAP | PP | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % Yes | N | % Yes | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||||
| EUR | 84 276 | 47 870 | 57.7 | 79 040 | 34.6 | 32.9 | 28.7 | 17.0 | 54.7 | 7.8 | 130.3 | 19.1 | 78.3 | 11.2 | 95.6 | 12.6 | 52.0 | 13.1 |
| AFR | 16 665 | 16 665 | 48.8 | 16 665 | 21.6 | 40.6 | 52.4 | 40.3 | 51.5 | 9.2 | 134.4 | 23.2 | 81.2 | 13.4 | 98.9 | 15.5 | 53.2 | 16.1 |
| ASN | 11 352 | 11 352 | 10.0 | 748 | 38.9 | 53.0 | 49.6 | 29.5 | 56.8 | 9.4 | 139.3 | 16.1 | 80.2 | 11.1 | 99.9 | 13.5 | 59.1 | 16.2 |
| BRZ | 3 689 | 3 689 | 36.8 | 3 689 | 17.5 | 45.6 | 29.0 | 7.6 | 34.5 | 3.8 | 123.4 | 15.3 | 76.6 | 10.0 | 92.2 | 11.1 | 46.8 | 10.2 |
| HIS | 1 456 | 1 456 | 35.2 | 1 456 | 9.8 | 48.4 | 41.4 | 33.3 | 60.8 | 9.73 | 131.2 | 24.8 | 74.9 | 11.7 | 93.6 | 14.9 | 56.4 | 18.5 |
| Stage 1 Total | 117 438 | 81 032 | 47.8 | 101 598 | 31.5 | 36.5 | 34.2 | 21.4 | 53.9 | 8.1 | 131.6 | 19.4 | 78.8 | 11.5 | 96.4 | 13.1 | 52.8 | 13.8 |
| Stage 2 | ||||||||||||||||||
| EUR | 264 052 | 242 524 | 54.3 | 257 037 | 26.3 | 47.6 | 48.5 | 21.7 | 54.9 | 8.9 | 138.5 | 20.2 | 83.3 | 11.4 | 101.7 | 13.4 | 55.2 | 13.6 |
| AFR | 7 198 | 7 198 | 20.8 | 7 198 | 10.9 | 40.4 | 56.8 | 44.1 | 54.7 | 9.5 | 137.6 | 21.8 | 83.8 | 12.9 | 101.7 | 14.8 | 53.8 | 14.9 |
| ASN | 10 906 | 10 906 | 21.4 | 5 947 | 10.9 | 40.1 | 37.4 | 24.9 | 55.8 | 9.0 | 134.2 | 23.1 | 80.7 | 12.8 | 98.6 | 15.4 | 53.5 | 14.8 |
| HIS | 11 631 | 11 631 | 35.5 | 11 631 | 14.6 | 41.1 | 25.4 | 15.0 | 45.3 | 13.6 | 123.6 | 19.7 | 75.0 | 11.8 | 91.2 | 13.6 | 48.6 | 13.1 |
| Stage 2 Total | 293 787 | 272 259 | 51.3 | 281 813 | 25.1 | 46.6 | 47.4 | 22.1 | 54.6 | 9.1 | 137.7 | 20.3 | 82.9 | 11.5 | 101.2 | 13.6 | 54.8 | 13.7 |
| TOTAL | 411 225 | 353 291 | 50.5 | 383 411 | 26.8 | 43.7 | 43.6 | 21.9 | 54.4 | 8.8 | 136.0 | 20.1 | 81.7 | 11.5 | 99.8 | 13.4 | 54.2 | 13.7 |
The cell entries for the covariates and BP traits corresponds to sample-size weighted averages across all cohorts in each category.
We performed meta-analyses combining Stages 1 and 2 (Manhattan Plots, Figure S2). We identified 84 known BP loci. This includes 82 loci identified through main-effect only analyses,36–41 including 18 recently reported by Hoffmann et al,42 Evangelou et al,43 and Giri et al;44 and two loci (TFAP2A and PCDH9) recently reported by our consortium through gene-smoking and gene-alcohol interaction analyses,27, 45 which suggest the inter-correlated nature of the various lifestyle traits.
We identified 18 novel genome-wide significant loci (P < 5×10−8) located at least 1Mb away from any known BP loci (Table 2). Nine loci were identified through the combined analyses of Stage 1 and 2; the remaining nine loci were identified in Stage 1 but not available in Stage 2 for combined analyses (Table S7). The LocusZoom plots of these novel loci are presented in Figure S3. Two loci (SLIT3 and HRH4) were identified through the 1DF test of interaction effects. At both loci, the genetic effect on DBP was stronger and beneficial in higher education and weaker and detrimental in lower education. For example, at SLIT3, the minor allele A was associated with a 4.82 mmHg lower DBP in higher education (GradCol=1), whereas it was associated with a 2.25 mmHg higher DBP in GradCol=0. The remaining 16 loci were identified through the 2DF joint test of the SNV and interaction effects; twelve loci were identified considering ‘Some College’ (SomeCol) and four loci were identified considering ‘Graduated College’ (GradCol).
Table 2.
Eighteen new loci associated with BP traits that are at least 1Mb away from any known BP locus.
| Locus | Nearest Gene | rsID | Chr:Pos | EA | EAF (AFR/ASN/EUR/HIS) | Genetic Effect | GxE Interaction | P-value | Trait | Educ. | Anc. | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect | SE | Effect | SE | Interaction | 2DF Joint | |||||||||
| 1 | CDC14A | rs114558965 | 1:100829685 | a | 0.97/. /. /1.00 | −0.23 | 1.02 | 5.02 | 1.27 | 2.54E-03 | 2.86E-09 | SBP | SC | AFR |
| 2 | LIN01249 | rs9308788 | 2:4711095 | a | 0.02/0.22/0.06/0.06 | 2.97 | 2.13 | −11.93 | 2.64 | 5.04E-05 | 4.47E-08 | SBP | GC | AFR |
| 3 | ARL4C | rs145586115 | 2:235604646 | t | 0.97/. /. /0.99 | 4.80 | 0.87 | −2.57 | 1.29 | 1.16E-01 | 1.92E-08 | SBP | SC | AFR |
| 4 | CADPS | rs141962517 | 3:62514061 | t | 0.98/. /. /. | −2.46 | 1.54 | 12.85 | 2.23 | 1.99E-07 | 3.07E-10 | SBP | GC | AFR |
| 5 | PCDH7 | rs74458816 | 4:31363388 | a | 0.03/. /. /0.00 | −0.18 | 0.70 | −3.46 | 0.91 | 2.26E-03 | 4.90E-09 | MAP | SC | AFR |
| 6 | EIF4E | rs2141284 | 4:99704167 | a | . /. /0.01/0.01 | −1.35 | 0.22 | 1.01 | 0.38 | 2.16E-02 | 6.73E-09 | MAP | GC | TRANS |
| 7 | SPEF2 | rs115523707 | 5:35756623 | t | 0.03/. /0.00/0.00 | −0.83 | 0.64 | −2.73 | 0.88 | 5.91E-02 | 7.22E-09 | PP | SC | AFR |
| 8 | ANKRD34B | rs66907226 | 5:79863455 | d | 0.46/0.81/0.58/0.69 | 0.48 | 0.14 | 0.04 | 0.18 | 9.86E-01 | 4.43E-08 | SBP | SC | EUR |
| 9 | SLCO4C1 | rs114175587 | 5:100994204 | t | 0.02/. /. /0.00 | −4.84 | 0.83 | 3.84 | 1.29 | 3.55E-03 | 2.40E-08 | PP | SC | TRANS |
| 10 | SLIT3 | rs142385399 | 5:168166731 | a | 0.04/. /0.00/0.01 | 2.25 | 0.74 | −7.07 | 1.22 | 2.79E-08 | 4.76E-08 | DBP | GC | AFR |
| 11 | THSD7A | rs200612978 | 7:11493906 | d | 0.03/0.03/. /0.01 | −0.40 | 1.27 | −4.94 | 1.57 | 5.71E-02 | 4.81E-08 | SBP | SC | AFR |
| 12 | HAS2-AS1 | rs112332671 | 8:122673983 | a | 0.02/. /. /0.00 | −1.37 | 1.18 | −4.55 | 1.52 | 6.03E-02 | 1.89E-09 | PP | SC | AFR |
| 13 | CDON | rs12295584 | 11:125841078 | a | 0.95/. /0.99/0.99 | 2.87 | 0.57 | −1.07 | 0.84 | 2.20E-01 | 4.71E-08 | SBP | SC | TRANS |
| 14 | DSTNP2 | rs75535814 | 12:6996683 | t | 0.02/. /. /0.00 | −8.78 | 1.45 | 7.24 | 1.95 | 3.17E-04 | 5.79E-09 | SBP | SC | AFR |
| 15 | PIK3C2G | rs189555401 | 12:18443065 | t | 0.03/. /. /0.01 | −3.23 | 0.57 | 1.82 | 1.15 | 8.92E-02 | 4.10E-08 | DBP | GC | TRANS |
| 16 | E2F7; ZDHHC17 | rs17043233 | 12:77648216 | t | 0.02/. /. /0.00 | −4.07 | 0.58 | 4.26 | 0.90 | 1.72E-05 | 1.39E-11 | MAP | SC | TRANS |
| 17 | HRH4 | rs8099516 | 18:22108763 | t | 0.04/. /. /0.01 | 1.71 | 0.61 | −5.39 | 0.95 | 7.83E-09 | 5.04E-08 | DBP | GC | AFR |
| 18 | LOC100289473 | rs113809930 | 20:1711768 | a | 0.98/0.88/0.97/0.94 | −0.69 | 0.79 | 3.67 | 0.94 | 1.70E-03 | 3.48E-08 | DBP | SC | AFR |
Positions are based on build 37. Effect is in mmHg unit. BP: blood pressure; DBP: diastolic BP; EA: effect allele; EAF: effect allele frequency observed in our cohorts; MAP: mean arterial pressure; P: P-value of the joint test with 2 degrees of freedom of genetic main and interaction effects; PP: pulse pressure; SBP: systolic BP; SE: standard error; 2DF Joint 1DF Interaction P: P-value of the interaction test with 1 degree of freedom. The smallest P-values between 1DF interaction test and the joint 2DF test are in boldface
SC: SomeCol; GC: GradCol; EUR: European; AFR: African; TRANS; trans-ancestry (i.e., combining all ancestry groups through meta-analysis).
Ancestry-specific and trans-ancestry analyses
Novel loci were identified through separate analyses of AFR (12 loci), EUR (1 locus), trans-ancestry (4 loci), and in both AFR and trans-ancestry (1 locus). This highlights the importance of including non-EUR populations to identify novel BP loci. By nature, AFR populations carry more rare and low-frequency variants that may be very rare or monomorphic in other ancestral groups;22 the MAF for the novel index SNVs range from 0.02–0.04 in AFR. The enhanced discovery of novel loci in AFR ancestry may be attributable to the relatively higher MAF in this population versus in EUR. For example, rs141962517 (CAPDS) with a MAF = 0.02 in AFR was significantly associated with SBP (2DF P = 3.07×10−10; 1DF Interaction P = 1.99×10−7); this variant was not present in other ancestries after filtering.
Among the 18 novel loci, three loci were identified only through trans-ancestry analyses, as none of the ancestry-specific analyses reached genome-wide significance. For example, the index SNV (rs189555401) representing the four-variant locus within PIK3C2G was suggestively associated with DBP (P = 1.31×10−7) in AFR and not even nominally associated in HIS (P = 9.67×10−2). However, in trans-ancestry analysis combining these two ancestral groups, the association reached genome-wide significance (P = 4.10×10−8).
Functional annotation and eQTL evidence
To obtain functional annotations for the index variants and nearby variants in LD (r2 ≥ 0.2), we used FUMA GWAS.28 Among the 18 index variants representing our novel loci, two variants were intronic to a non-coding RNA (ncRNA), six variants were intronic, nine variants were intergenic, and the remaining variant (rs66907226) was an indel without available annotation. Among the 499 variants that include both the index variants and nearby variants in LD, there were four exonic, four exonic-ncRNA, 119 intronic, 67 intronic-ncRNA, two 3’ untranslated region (UTR), seven up/downstream flanking, and 296 intergenic variants (Table S8). Of the 499 variants, 13 had RegulomeDB31 scores better than or equal to 3a, suggesting at least moderate evidence for involvement in transcription regulation (Table S9). Thirty-two SNVs have CADD32 scores ≥10, representing the top 10% of predicted deleteriousness for SNVs genome-wide. A single SNV (rs112332671) ~20kb upstream of HAS2 and 16 kb downstream of the ncRNA HAS2-AS1 had a CADD score of 20.1, placing it in the top 1% of predicted deleteriousness.
The 15-core chromatin state (ChromHMM)33 was assessed for 127 epigenomes in the 17 index variants (Table S9). Two had histone chromatin markers in regions flanking the transcription start site and one in a region associated with strong transcription in relevant tissues including brain. Among all 499 LD variants, 45 had histone chromatin markers characteristic of a transcription start site, 64 had markers consistent with strong transcription, and 25 were in enhancer regions. One LD variant (rs555713705) was identified as cis-acting expression trait loci (eQTLs)46, 47 for heart tissue in the GTEx_v7 database (FDR p-value ranging from 3.90×10−3).
Biologic plausibility of the new BP loci
Three novel BP loci are related to the central nervous system (CNS)-adrenal signaling axis that is critical for BP regulation. A locus (Figure 2A) adjacent to ZDHHC17, identified in AFR and in trans-ancestry analyses, encodes a membrane protein that mediates fusion of synaptic vesicles to the plasma membrane. CADPS (Figure 2B), identified in AFR, is expressed in CNS tissue. Three variants in LD have CADD scores >10, and four SNVs have ChromHMM state signals consistent with strong evidence of transcription regulation. PIK3C2G, identified in trans-ancestry analyses, also shows roles in CNS-adrenal signaling. Three variants in LD in this locus have CADD scores >10, including one with a CADD score of 18 that is predicted to reside in an enhancer region in fetal adrenal cells.
Figure 2: LocusZoom plots for 2 BP loci related to CNS-adrenal signaling.
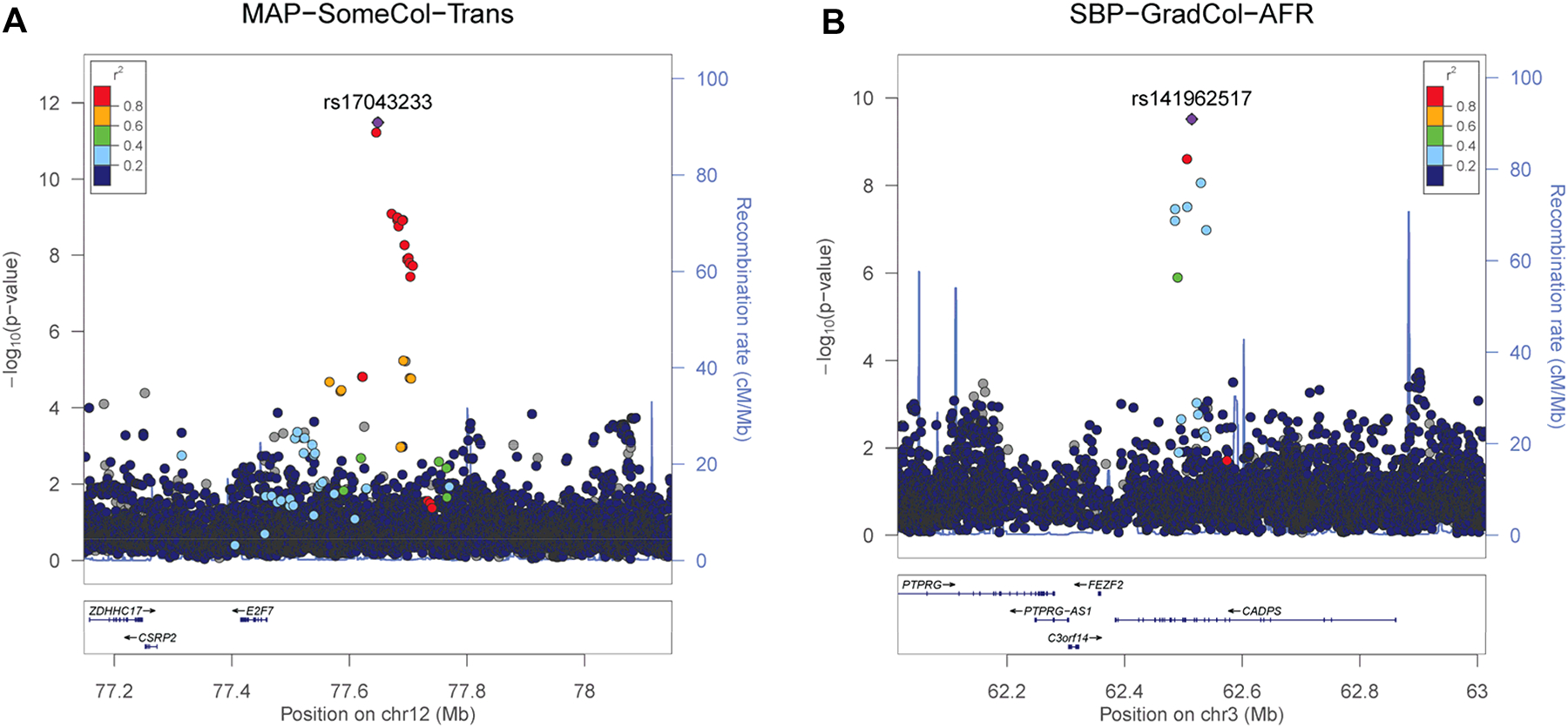
(A) MAP-associated locus adjacent to ZDHHC17, identified in AFR and in trans-ancestry, shows roles in CNS-adrenal signaling. In neurons, ZDHHC17 encodes a membrane protein that mediates fusion of synaptic vesicles to the plasma membrane, enabling the release of neurotransmitters. Murine zdhhc17 knockout models show impaired hippocampal memory and reduced synaptic plasticity, providing potential biological links to working memory and subsequent educational attainment.
(B)A locus intragenic to CADPS, identified in AFR, is of potential biologic relevance given this gene’s expression in CNS tissue and role in regulating the fusion of neuroendocrine vesicles and release of vasoactive catecholamines from both adrenal and neural tissue. Three LD SNVs have CADD scores >10, and four LD SNVs have ChromHMM state signals consistent with strong evidence of transcription regulation.
SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; PP, pulse pressure. The plots were created using LocusZoom (http://locuszoom.sph.umich.edu/).
Two novel BP loci are related to renal fibrosis and cation exchange. A locus, which includes a variant intragenic to SLIT3, showed interaction evidence with educational attainment in AFR (rs142385399, P = 2.79×10−8). A locus also identified in AFR includes HAS2 and HAS2-AS1, which play roles in renal fibrosis. In addition, we identified two novel BP loci related to pathways involved in vascular smooth muscle cell structure and function. A locus identified by trans-ancestry analyses included a variant intragenic to CDON, which is expressed in vascular smooth muscle cells. A locus identified in AFR includes GNB3, which encodes a subunit critical for signal transduction of several vasoactive peptide G protein-coupled receptors involved in BP regulation. A SNV in this locus shows ChromHMM chromatin states consistent with strong transcription regulation in multiple tissues, and three SNVs have strong cis-eQTL association with GNB3 expression in nerve, artery and skeletal muscle tissue (minimum FDR p-value 1.20×10−43).
Discussion
A relationship between educational attainment and BP has been well demonstrated.48–51 Furthermore, African-ancestry individuals have been shown to have a higher burden of HTN than European-ancestry.52 However, higher-educated African-ancestry individuals bear approximately twice the burden of HTN as compared to their European-ancestry counterparts,48, 51 demonstrating that educational differences did not fully account for this observed racial disparity. Herein, we reported genome-wide meta-analyses for SBP, DBP, MAP, and PP accounting for gene-educational attainment interactions across five ancestry groups. We pursued a genome-wide interrogation in 117 438 individuals (in Stage 1) and follow-up analysis at selected variants in 293 787 additional individuals (in Stage 2). Through the combined meta-analysis of stages 1 and 2, we identified 84 known and 18 novel loci at genome-wide significance. As known loci have been discussed elsewhere, this report highlights several novel loci show biologic plausibility by involving physiologic systems implicated in BP regulation.
The central nervous system (CNS)-adrenal signaling axis is critical for BP regulation. Three novel BP loci (ZDHHC17, CADPS, and PIK3C2G) are related to these pathways. In neurons, ZDHHC17 encodes a membrane protein that mediates fusion of synaptic vesicles to the plasma membrane, enabling the release of neurotransmitters.53 Murine zdhhc17 knockout models show impaired hippocampal memory and reduced synaptic plasticity, providing potential biological links to working memory and subsequent educational attainment.54 Although a biological connection between ZDHHC17 and BP traits is not well established, zdhhc17 expression induces neurite outgrowth in a rodent adrenal-derived cell line.55 Cadps plays a role in regulating the fusion of neuroendocrine vesicles and release of vasoactive catecholamines in calf adrenal and neural tissue.56 Pik3c2g encodes a phosphoinositide kinases that is expressed in a sexually dimorphic pattern specifically in a zone of the mouse adrenal cortex believed to play a role in steroid sex hormone production.57 Furthermore, PIK3C2G is under-expressed in human hypertensive kidneys, providing a potential biological link between the expression of adrenal mineralocorticoid hormones and their target organ.58 Among alcohol-preferring rats, pik3c2g expression is also increased in the cerebral periaqueductal gray, a region involved in pain, fear, and anxiety responses,59 possibly providing a link to drivers of socioeconomic status in humans.60 Notably, the loci including ZDHHC17 and CADPS demonstrated some evidence of interaction with educational attainment (P = 1.72×10−5 and 1.99×10−7, respectively).
Two new BP loci (HAS2 and HAS2-AS1, SLIT3) show potential roles in renal function. A locus which includes a variant intragenic to SLIT3 had a significant interaction term with educational attainment. SLIT3 encodes a cell-cell adhesion molecule that binds its receptor, ROBO4, in human-derived endothelial stem cells directing the formation of vascular networks.61 SLIT3 also plays a role in directing neuronal growth in the brain,62, 63 and in renal and cardiac development.64 A locus including HAS2 and HAS2-AS1 is also of interest for roles played in renal fibrosis. HAS2-AS1 is an antisense ncRNA simultaneously expressed and thought to stabilize the HAS2 transcript.65
Two new BP loci (GNB3, CDON) have been shown to regulate pathways involved in vascular smooth muscle cell structure and function. We identified a locus in GNB3, which encodes a G protein-coupled receptor subunit involved in BP regulation. Several candidate gene association studies have identified the synonymous GNB3 variant C825T (rs5443), resulting in a splice variant of the β3 subunit, as significantly associated with essential HTN,66, 67 with BP response to diuretic68 and β-adrenergic receptor blockade,69 and other cardiovascular traits.70 Another locus identified by trans-ancestry analyses included a variant intragenic to CDON; this gene is expressed in vascular smooth muscle cells,71 and encodes a cell-surface receptor complex that regulates myocyte differentiation in rodents.72
This large-scale multi-ancestry study has several limitations. First, the practice of adjusting SBP and DBP by adding 15 and 10 mm Hg for antihypertensive use is based on a method derived from a European-ancestry cohort.21 While this approach is common among GWAS of BP traits,36 we acknowledge that this practice may not be equally appropriate and/or justified in all ancestry groups. Second, while the sample sizes in diverse ancestries are a strength, resulting in the identification of several novel BP loci particularly in African ancestry, several identified loci included low-frequency variants that require further validation. Third, main effect only analysis without educational attainment was not performed, and this limits our ability to resolve if novel loci identified through the 2DF joint test could be found without considering educational attainment. Fourth, the use of educational attainment as a proxy for SES can present some challenges. The socioeconomic impact of education has changed over time and may differ according to birth cohort, as well as in other subgroups defined by gender, ancestry, region, and/or country.1, 49 Even with similar levels of educational attainment, social and environmental experiences were different between AFR and EUR individuals in United States, especially those educated in the 1960s and 1970s, resulting in residual confounding inequities between the ancestral groups.9, 73 This additional source of heterogeneity may have reduced power for trans-ancestry analyses.
In summary, this multi-ancestry study that used gene-education interactions on BP traits identified 18 novel loci and validated 84 known BP loci. Ten novel loci were identified in individuals of African ancestry, demonstrating the need for pursuing genetic studies in diverse populations. Several novel loci involve physiologic systems implicated in BP regulation including genes involved in CNS-adrenal signaling, vascular structure and function, and renal function. Two loci showed interaction evidence with educational attainment. These findings may identify a role for educational attainment and SES in further dissection of the genetic architecture of BP.
Supplementary Material
Acknowledgments
This project was largely supported by a grant from the U.S. National Heart, Lung, and Blood Institute (NHLBI), the National Institutes of Health, R01HL118305. A Career Development Award (K25HL121091), also from the NHLBI, enabled Dr. Sung to play a major role on this project. Dr. Kilpeläinen was supported by the Novo Nordisk Foundation (NNF18CC0034900 and NNF17OC0026848). Full set of study-specific funding sources and acknowledgments appear in the Supplementary Information.
Footnotes
Supplementary Data
Supplementary Data include Supplementary Study Descriptions, Supplementary Acknowledgments, three figures, and nine tables.
Conflict of Interest
The authors declare no conflict of interests.
References
- 1.Kaplan GA, Keil JE. Socioeconomic factors and cardiovascular disease: a review of the literature. Circulation 1993; 88(4 Pt 1): 1973–1998. [DOI] [PubMed] [Google Scholar]
- 2.Liberatos P, Link BG, Kelsey JL. The measurement of social class in epidemiology. Epidemiol Rev 1988; 10: 87–121. [DOI] [PubMed] [Google Scholar]
- 3.Leng B, Jin Y, Li G, Chen L, Jin N. Socioeconomic status and hypertension: a meta-analysis. J Hypertens 2015; 33(2): 221–229. [DOI] [PubMed] [Google Scholar]
- 4.Winkleby MA, Jatulis DE, Frank E, Fortmann SP. Socioeconomic status and health: how education, income, and occupation contribute to risk factors for cardiovascular disease. Am J Public Health 1992; 82(6): 816–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basson J, Sung YJ, Schwander K, Kume R, Simino J, de las Fuentes L et al. Gene-education interactions identify novel blood pressure loci in the Framingham Heart Study. Am J Hypertens 2014; 27(3): 431–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee JJ, Wedow R, Okbay A, Kong E, Maghzian O, Zacher M et al. Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat Genet 2018; 50(8): 1112–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hertz RP, Unger AN, Cornell JA, Saunders E. Racial disparities in hypertension prevalence, awareness, and management. Arch Intern Med 2005; 165(18): 2098–2104. [DOI] [PubMed] [Google Scholar]
- 8.Steptoe A, Hamer M, Butcher L, Lin J, Brydon L, Kivimaki M et al. Educational attainment but not measures of current socioeconomic circumstances are associated with leukocyte telomere length in healthy older men and women. Brain Behav Immun 2011; 25(7): 1292–1298. [DOI] [PubMed] [Google Scholar]
- 9.Metcalf PA, Sharrett AR, Folsom AR, Duncan BB, Patsch W, Hutchinson RG et al. African American-white differences in lipids, lipoproteins, and apolipoproteins, by educational attainment, among middle-aged adults: the Atherosclerosis Risk in Communities Study. Am J Epidemiol 1998; 148(8): 750–760. [DOI] [PubMed] [Google Scholar]
- 10.Matthews KA, Kelsey SF, Meilahn EN, Kuller LH, Wing RR. Educational attainment and behavioral and biologic risk factors for coronary heart disease in middle-aged women. Am J Epidemiol 1989; 129(6): 1132–1144. [DOI] [PubMed] [Google Scholar]
- 11.Rietveld CA, Medland SE, Derringer J, Yang J, Esko T, Martin NW et al. GWAS of 126,559 individuals identifies genetic variants associated with educational attainment. Science 2013; 340(6139): 1467–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith GD, Hart C, Watt G, Hole D, Hawthorne V. Individual social class, area-based deprivation, cardiovascular disease risk factors, and mortality: the Renfrew and Paisley Study. J Epidemiol Community Health 1998; 52(6): 399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallo LC, Matthews KA, Kuller LH, Sutton-Tyrrell K, Edmundowicz D. Educational attainment and coronary and aortic calcification in postmenopausal women. Psychosom Med 2001; 63(6): 925–935. [DOI] [PubMed] [Google Scholar]
- 14.Jacobsen BK, Thelle DS. Risk factors for coronary heart disease and level of education. The Tromso Heart Study. Am J Epidemiol 1988; 127(5): 923–932. [DOI] [PubMed] [Google Scholar]
- 15.Pierce JP, Fiore MC, Novotny TE, Hatziandreu EJ, Davis RM. Trends in cigarette smoking in the United States. Educational differences are increasing. JAMA 1989; 261(1): 56–60. [PubMed] [Google Scholar]
- 16.Stamler J, Elliott P, Appel L, Chan Q, Buzzard M, Dennis B et al. Higher blood pressure in middle-aged American adults with less education-role of multiple dietary factors: the INTERMAP study. J Hum Hypertens 2003; 17(9): 655–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian HG, Hu G, Dong QN, Yang XL, Nan Y, Pietinen P et al. Dietary sodium and potassium, socioeconomic status and blood pressure in a Chinese population. Appetite 1996; 26(3): 235–246. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan GA, Lazarus NB, Cohen RD, Leu DJ. Psychosocial factors in the natural history of physical activity. Am J Prev Med 1991; 7(1): 12–17. [PubMed] [Google Scholar]
- 19.McCaffery JM, Papandonatos GD, Lyons MJ, Niaura R. Educational attainment and the heritability of self-reported hypertension among male Vietnam-era twins. Psychosom Med 2008; 70(7): 781–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rao DC, Sung YJ, Winkler TW, Schwander K, Borecki I, Cupples LA et al. Multiancestry Study of Gene-Lifestyle Interactions for Cardiovascular Traits in 610 475 Individuals From 124 Cohorts: Design and Rationale. Circ Cardiovasc Genet 2017; 10(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tobin MD, Sheehan NA, Scurrah KJ, Burton PR. Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat Med 2005; 24(19): 2911–2935. [DOI] [PubMed] [Google Scholar]
- 22.1000 Genomes Project Consortium, Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM et al. An integrated map of genetic variation from 1,092 human genomes. Nature 2012; 491(7422): 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manning AK, LaValley M, Liu CT, Rice K, An P, Liu Y et al. Meta-analysis of gene-environment interaction: joint estimation of SNP and SNP x environment regression coefficients. Genet Epidemiol 2011; 35(1): 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 2010; 26(17): 2190–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Devlin B, Roeder K. Genomic control for association studies. Biometrics 1999; 55(4): 997–1004. [DOI] [PubMed] [Google Scholar]
- 26.Winkler TW, Day FR, Croteau-Chonka DC, Wood AR, Locke AE, Magi R et al. Quality control and conduct of genome-wide association meta-analyses. Nat Protoc 2014; 9(5): 1192–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sung YJ, Winkler TW, de Las Fuentes L, Bentley AR, Brown MR, Kraja AT et al. A Large-Scale Multi-ancestry Genome-wide Study Accounting for Smoking Behavior Identifies Multiple Significant Loci for Blood Pressure. Am J Hum Genet 2018; 102(3): 375–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watanabe K, Taskesen E, van Bochoven A, Posthuma D. Functional mapping and annotation of genetic associations with FUMA. Nat Commun 2017; 8(1): 1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ward LD, Kellis M. HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res 2012; 40(Database issue): D930–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res 2010; 38(16): e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boyle AP, Hong EL, Hariharan M, Cheng Y, Schaub MA, Kasowski M et al. Annotation of functional variation in personal genomes using RegulomeDB. Genome Res 2012; 22(9): 1790–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kircher M, Witten DM, Jain P, O’Roak BJ, Cooper GM, Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet 2014; 46(3): 310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roadmap Epigenomics Consortium, Kundaje A, Meuleman W, Ernst J, Bilenky M, Yen A et al. Integrative analysis of 111 reference human epigenomes. Nature 2015; 518(7539): 317–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ernst J, Kellis M. ChromHMM: automating chromatin-state discovery and characterization. Nat Methods 2012; 9(3): 215–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.GTEx Consortium. Genetic effects on gene expression across human tissues. Nature 2017; 550(7675): 204–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newton-Cheh C, Johnson T, Gateva V, Tobin MD, Bochud M, Coin L et al. Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet 2009; 41(6): 666–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD, Chasman DI et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 2011; 478(7367): 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ehret GB, Ferreira T, Chasman DI, Jackson AU, Schmidt EM, Johnson T et al. The genetics of blood pressure regulation and its target organs from association studies in 342,415 individuals. Nat Genet 2016; 48(10): 1171–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu C, Kraja AT, Smith JA, Brody JA, Franceschini N, Bis JC et al. Meta-analysis identifies common and rare variants influencing blood pressure and overlapping with metabolic trait loci. Nat Genet 2016; 48(10): 1162–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Surendran P, Drenos F, Young R, Warren H, Cook JP, Manning AK et al. Trans-ancestry meta-analyses identify rare and common variants associated with blood pressure and hypertension. Nat Genet 2016; 48(10): 1151–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A et al. Genome-wide association study of blood pressure and hypertension. Nat Genet 2009; 41(6): 677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoffmann TJ, Ehret GB, Nandakumar P, Ranatunga D, Schaefer C, Kwok PY et al. Genome-wide association analyses using electronic health records identify new loci influencing blood pressure variation. Nat Genet 2017; 49(1): 54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Evangelou E, Warren HR, Mosen-Ansorena D, Mifsud B, Pazoki R, Gao H et al. Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat Genet 2018; 50(10): 1412–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giri A, Hellwege JN, Keaton JM, Park J, Qiu C, Warren HR et al. Trans-ethnic association study of blood pressure determinants in over 750,000 individuals. Nat Genet 2019; 51(1): 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feitosa MF, Kraja AT, Chasman DI, Sung YJ, Winkler TW, Ntalla I et al. Novel genetic associations for blood pressure identified via gene-alcohol interaction in up to 570K individuals across multiple ancestries. PLoS One 2018; 13(6): e0198166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.The GTEx Consortium. Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science 2015; 348(6235): 648–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Westra HJ, Peters MJ, Esko T, Yaghootkar H, Schurmann C, Kettunen J et al. Systematic identification of trans eQTLs as putative drivers of known disease associations. Nat Genet 2013; 45(10): 1238–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hypertension Detection and Follow-up Program Cooperative Group. Race, education and prevalence of hypertension. Am J Epidemiol 1977; 106(5): 351–361. [PubMed] [Google Scholar]
- 49.Sorel JE, Ragland DR, Syme SL, Davis WB. Educational status and blood pressure: the Second National Health and Nutrition Examination Survey, 1976–1980, and the Hispanic Health and Nutrition Examination Survey, 1982–1984. Am J Epidemiol 1992; 135(12): 1339–1348. [DOI] [PubMed] [Google Scholar]
- 50.Steffen PR. The cultural gradient: culture moderates the relationship between socioeconomic status (SES) and ambulatory blood pressure. J Behav Med 2006; 29(6): 501–510. [DOI] [PubMed] [Google Scholar]
- 51.Vargas CM, Ingram DD, Gillum RF. Incidence of hypertension and educational attainment: the NHANES I epidemiologic followup study. First National Health and Nutrition Examination Survey. Am J Epidemiol 2000; 152(3): 272–278. [DOI] [PubMed] [Google Scholar]
- 52.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018; 137(12): e67–e492. [DOI] [PubMed] [Google Scholar]
- 53.Ohyama T, Verstreken P, Ly CV, Rosenmund T, Rajan A, Tien AC et al. Huntingtin-interacting protein 14, a palmitoyl transferase required for exocytosis and targeting of CSP to synaptic vesicles. J Cell Biol 2007; 179(7): 1481–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Milnerwood AJ, Parsons MP, Young FB, Singaraja RR, Franciosi S, Volta M et al. Memory and synaptic deficits in Hip14/DHHC17 knockout mice. Proc Natl Acad Sci U S A 2013; 110(50): 20296–20301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi W, Wang F, Gao M, Yang Y, Du Z, Wang C et al. ZDHHC17 promotes axon outgrowth by regulating TrkA-tubulin complex formation. Mol Cell Neurosci 2015; 68: 194–202. [DOI] [PubMed] [Google Scholar]
- 56.Elhamdani A, Martin TF, Kowalchyk JA, Artalejo CR. Ca(2+)-dependent activator protein for secretion is critical for the fusion of dense-core vesicles with the membrane in calf adrenal chromaffin cells. J Neurosci 1999; 19(17): 7375–7383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.El Wakil A, Mari B, Barhanin J, Lalli E. Genomic analysis of sexual dimorphism of gene expression in the mouse adrenal gland. Horm Metab Res 2013; 45(12): 870–873. [DOI] [PubMed] [Google Scholar]
- 58.Marques FZ, Campain AE, Tomaszewski M, Zukowska-Szczechowska E, Yang YH, Charchar FJ et al. Gene expression profiling reveals renin mRNA overexpression in human hypertensive kidneys and a role for microRNAs. Hypertension 2011; 58(6): 1093–1098. [DOI] [PubMed] [Google Scholar]
- 59.McClintick JN, McBride WJ, Bell RL, Ding ZM, Liu Y, Xuei X et al. Gene Expression Changes in Glutamate and GABA-A Receptors, Neuropeptides, Ion Channels, and Cholesterol Synthesis in the Periaqueductal Gray Following Binge-Like Alcohol Drinking by Adolescent Alcohol-Preferring (P) Rats. Alcohol Clin Exp Res 2016; 40(5): 955–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann N Y Acad Sci 2010; 1186: 190–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paul JD, Coulombe KLK, Toth PT, Zhang Y, Marsboom G, Bindokas VP et al. SLIT3-ROBO4 activation promotes vascular network formation in human engineered tissue and angiogenesis in vivo. J Mol Cell Cardiol 2013; 64: 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ypsilanti AR, Zagar Y, Chedotal A. Moving away from the midline: new developments for Slit and Robo. Development 2010; 137(12): 1939–1952. [DOI] [PubMed] [Google Scholar]
- 63.Blockus H, Chedotal A. Slit-Robo signaling. Development 2016; 143(17): 3037–3044. [DOI] [PubMed] [Google Scholar]
- 64.Liu J, Zhang L, Wang D, Shen H, Jiang M, Mei P et al. Congenital diaphragmatic hernia, kidney agenesis and cardiac defects associated with Slit3-deficiency in mice. Mech Dev 2003; 120(9): 1059–1070. [DOI] [PubMed] [Google Scholar]
- 65.Michael DR, Phillips AO, Krupa A, Martin J, Redman JE, Altaher A et al. The human hyaluronan synthase 2 (HAS2) gene and its natural antisense RNA exhibit coordinated expression in the renal proximal tubular epithelial cell. J Biol Chem 2011; 286(22): 19523–19532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Siffert W, Rosskopf D, Siffert G, Busch S, Moritz A, Erbel R et al. Association of a human G-protein beta3 subunit variant with hypertension. Nat Genet 1998; 18(1): 45–48. [DOI] [PubMed] [Google Scholar]
- 67.Bagos PG, Elefsinioti AL, Nikolopoulos GK, Hamodrakas SJ. The GNB3 C825T polymorphism and essential hypertension: a meta-analysis of 34 studies including 14,094 cases and 17,760 controls. J Hypertens 2007; 25(3): 487–500. [DOI] [PubMed] [Google Scholar]
- 68.Turner ST, Schwartz GL, Chapman AB, Boerwinkle E. C825T polymorphism of the G protein beta(3)-subunit and antihypertensive response to a thiazide diuretic. Hypertension 2001; 37(2 Pt 2): 739–743. [DOI] [PubMed] [Google Scholar]
- 69.Filigheddu F, Reid JE, Troffa C, PinnaParpaglia P, Argiolas G, Testa A et al. Genetic polymorphisms of the beta-adrenergic system: association with essential hypertension and response to beta-blockade. Pharmacogenomics J 2004; 4(3): 154–160. [DOI] [PubMed] [Google Scholar]
- 70.Bojic T, Milovanovic B, Cupic SJ. Genetic Polymorphisms of Neurocardiovascular Disorders. Archives of Medicine 2015; 7(2:5): 1–22. [Google Scholar]
- 71.Aoki T, Kataoka H, Ishibashi R, Nozaki K, Hashimoto N. Gene expression profile of the intima and media of experimentally induced cerebral aneurysms in rats by laser-microdissection and microarray techniques. Int J Mol Med 2008; 22(5): 595–603. [PubMed] [Google Scholar]
- 72.Takaesu G, Kang JS, Bae GU, Yi MJ, Lee CM, Reddy EP et al. Activation of p38alpha/beta MAPK in myogenesis via binding of the scaffold protein JLP to the cell surface protein Cdo. J Cell Biol 2006; 175(3): 383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tyroler HA. Socioeconomic status in the epidemiology and treatment of hypertension. Hypertension 1989; 13(5 Suppl): I94–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


