Abstract
Aims
Rodent cystometry has provided valuable insights into the impact of disease, injury, and aging on the cellular and molecular pathways, neurologic processes, and biomechanics of lower urinary tract function. The purpose of this white paper is to highlight the benefits and shortcomings of different experimental methods and strategies, and to provide guidance on the proper interpretation of results.
Methods
Literature search, selection of articles, and conclusions based on discussions among a panel of workers in the field
Results
A range of cystometric tests and techniques used to explore biological phenomena relevant to the lower urinary tract are described, the advantages and disadvantages of various experimental conditions are discussed, and guidance on the practical aspects of experimental execution and proper interpretation of results are provided.
Conclusions
Cystometric evaluation of rodents comprises an extensive collection of functional tests that can be performed under a variety of experimental conditions. Decisions regarding which approaches to choose should be determined by the specific questions to be addressed and implementation of the test should follow standardized procedures.
Introductory Statements
Cystometry broadly describes a variety of procedures used to measure bladder pressure during filling and voiding. Cystometry is a central procedure of human clinical urodynamic testing and is used to interrogate the systemic regulation of lower urinary tract (LUT) function with the overall goal of clinical diagnosis. While the testing methods can be precise in human cystometry (as defined by formal recommendations)1, it generally requires active patient participation and interpretation of the output, and, therefore, data collected includes subjective components2,3. Moreover, the impacts of disease, injury, and aging on specific mechanisms underlying LUT function often cannot be directly tested in humans, and we therefore must turn to non-human experimental models for these purposes.
The most commonly utilized experimental models for understanding LUT function are muriform rodents (rats and mice). Cystometry in rodents can provide precise objective information derived from mechanistically designed experiments, as described here and elsewhere 4,5. The validity of rodent cystometric testing depends on the choice of methods and technology as applied to the hypothesis, the rigor in application of the methods, accurate interpretation of results and an understanding of fluid dynamics and the underlying physiology. Decisions regarding which experimental approaches to choose should be determined by the particular questions to be addressed. Rodent cystometry has provided valuable insights into the impact of disease, injury, and aging on the cellular and molecular pathways, neurologic processes, and biomechanics of lower urinary tract function. The purpose of this white paper is to highlight the benefits and shortcomings of different experimental methods and strategies, and to provide guidance on the proper interpretation of results.
Function of the Lower Urinary Tract
The function of the LUT is to collect and store urine internally, and to periodically release urine externally when environmentally appropriate. Simply described, the bladder is the anatomical reservoir for urine storage and also generates the pressure to evacuate urine, and the urethra functions as the outlet, and actively participates both in storage and release functions. The actions of these structures are coordinated through reflexes involving the spinal cord and brainstem, with inhibitory influences exerted from higher cortical centers6. This concept of LUT reflex organization is not new: In the early 1900s, Barrington described several spinal and supraspinal reflexes originating from the bladder or urethra via afferent pathways through each of the three peripheral nervous system branches that innervate the LUT7,8.
Muriform Rodent-Specific Considerations
Muriform rodents are habitually quadrupedal, although they may spend some amounts of time in a vertically-oriented “seated” position or transiently reach vertically during exploration or item acquisition. In quadrupedal mammals, gravity tends to act on the urinary bladder drawing it toward the ground through the ventral abdominal wall, rather than through the pelvic floor, as in the case of the habitually bipedal human. This can be altered somewhat by positioning during voiding in quadrupeds, such as in the readily observable case of squatting during urination in female cats and dogs.
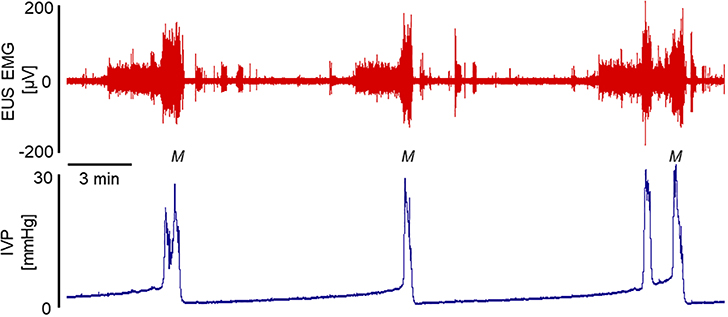
As is the case for almost all other non-human animals studied to date9, muriform rodents exhibit phasic external urethral sphincter (EUS) activity during micturition10–14. This phasic EUS activity directly results in the high frequency pressure oscillations (HFPO) seen in urethra pressure traces (see Figure 2 for example)15–20, and can be detected in bladder pressure traces under the proper conditions (see Figure 3 for example). Micturition-associated EUS phasic activity contributes to fully efficient voiding in the rat10,21. Thus, synergistic EUS behavior during micturition in these animals is one of phasic activity rather than either lack of activity (which defines a synergistic EUS in the healthy human) or tonic contractile activity (which would constitute detrusor-sphincter dyssynergia in any species).
Figure 2.
Pressure traces obtained by the isolated bladder-urethra preparation in urethane anesthetized female rats. Top trace is anterograde urethral perfusion pressure, bottom trace is isovolumetric bladder pressure. Left panel is control period, right panel is following 333 ug/kg of a-bungarotoxin. Note the drop in baseline urethral pressure, absence of HFPO, and smooth muscle relaxation of the urethra, with no change in bladder pressure characteristics, following neuromuscular blockade (right panel). [Fraser MO, unpublished observation]
Figure 3.
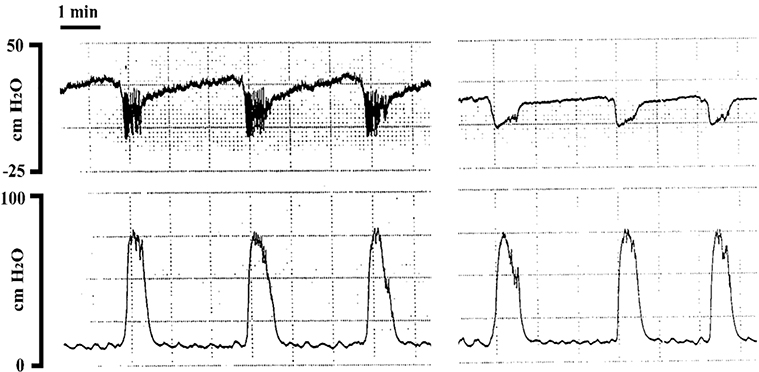
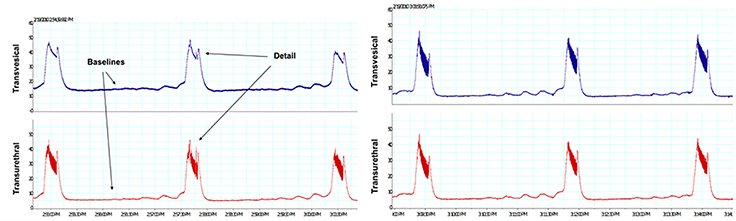
Left panel: Simultaneous pressure recordings from a transvesical filling catheter (PE50; top trace) and a transurethral static catheter (PE50; bottom trace). Right panel: Simultaneous pressure recordings from a transvesical static catheter (top trace) and a transurethral static catheter (bottom trace), demonstrating that fidelity was not influenced by catheter tip location. Filling was achieved via a transureteral catheter (PE10) at the same rate as used by transvesical route in the left panel. Note that when recording through the infusion catheter, baseline pressures are higher and HFPO details are lost. [Fraser MO, unpublished observation]
Utilization of Rodents for Neurourological Research
Rats and mice are the animals most widely used for performance of preclinical cystometry, due to their well-characterized anatomy, widespread availability, and ease of handling and housing, and are therefore the focus of this white paper. Relevant models of human disease can be readily developed in rodents using chemical, pharmacological, immunological, surgical, behavioral, or genetic approaches. While no animal model can be expected to fully recapitulate human physiology or disease, these models are nonetheless invaluable for both understanding LUT physiology and elucidating potential mechanisms that contribute to or are responsible for LUT pathologies. Further, different animal models may more closely recapitulate different specific sub-phenotypes of syndromic clinical conditions than others. It is important in this regard to understand the phenotype produced by the manipulations leading to a particular animal model in order to assign relevance to pathological process for which it will be used to study. Additionally, these animals provide an essential means of evaluating the safety and efficacy of new drugs, medical devices, and surgical treatments. Perhaps most importantly, benefits from animal research can only be attained by comprehensive functional testing that is properly designed, carefully executed, and correctly interpreted.
Acute Preparations
a. Anesthesia
Anesthetics are widely used for animal studies because of convenience to the experimenter and for ethical reasons associated with conducting in vivo experiments (e.g. diminution/elimination of anxiety, pain, and discomfort). Generally, anesthetics exert their effects by either enhancing inhibitory pathways, suppressing excitatory pathways, or both, within the central nervous system (CNS). It is assumed that by using anesthetics that spare LUT function, thereby allowing cystometric evaluation, spinal and supraspinal/subcortical reflexes are relatively intact without the influence of cortical control, and experimental evidence supports this as a conclusion22,23. A caveat to the use of anesthetics is that, as biologically active chemical compounds, they may alter physiological responses and may interact or interfere with the effects of any drug that is to be examined in pharmacological experiments. Whether the anesthesia of choice is actually an anesthetic (produces a state of unconsciousness) or a hypnotic (dissociative anesthetic which uncouples sensory, motor, integrative, memory and emotional activities in the brain; a state similar to catalepsy) should also be considered. The former leads to CNS depression, while the latter does not. Not all anesthetics are equal in terms of usefulness for cystometric study in rats and mice. Many anesthetics, including inhalation agents, can suppress physiologic responses to bladder filling, including complete suppression of the voiding reflex at doses required to effect surgical anesthesia.
The use of urethane (ethyl carbamate) in rodent lower urinary tract research has been well described in the literature22–24. This anesthetic provides a wide margin of safety and has minimal impact on hemodynamic parameters. Bladder activity is well preserved under urethane anesthesia (1.2 g/kg dose s.c., i.p., or divided between the two routes; occasionally with small additional doses to achieve a suitable anesthetic plane) 22,23,25. Despite these advantages, urethane is known to interfere with urethral activity, resulting in increased post-void residual volume26,27. Best results may be achieved by s.c. only administration22,28,29, with divided-dose injection sites lateral to the spinal cord (Fraser, unpublished observations). The i.p. approach may ensure faster knock down but may exacerbate the undesirable effects on LUT function22,28–30. Regardless, urethane remains a main choice for the study of reflex mechanisms of LUT function and dysfunction, as other anesthetics have been shown to exert greater adverse effects on the micturition reflex31.
Urethane produces cystometric pressure profiles most similar to those observed in conscious and decerebrate preparations when compared to other anesthetics31 and has the additional benefit of lasting several hours. This long duration of anesthetic plane precludes having to re-dose the animals, as would be required with a shorter acting agent, and the variability of anesthetic plane that such repeat dosing inherently creates. However, the use of urethane anesthesia is generally limited to non-survival procedures due to its adverse post-operative health effects in rodents and suspected carcinogenic and mutagenic risks32,33.
b. Conscious Approaches
Possible interactions of anesthetics with test substances can be avoided when conducting pharmacological experiments by utilizing unanesthetized, conscious preparations. In addition, the contribution of the conscious forebrain to lower urinary tract activity can be examined. Unlike anesthetized preparations, in which environmental cues and diurnal variations are presumably suppressed, conscious approaches are necessarily sensitive to such effects, and these influences must be considered when utilizing conscious preparations. While such influences may have a greater effect on results obtained using infusion rates that approach physiological filling, these may be, in part, over-ridden by more rapid infusion rates that may engage reflex pathways without/despite cortical influences. Humoral responses (i.e., stress hormone release), rather than behavioral responses, may still affect results despite, and in response to, increased flow rates. Other potentially important environmental factors that may influence conscious preparations include, noise, odors, temperature, lighting, sex and behavior of experimenters, presence of other conspecifics, etc. Of particular physiological concern, but at odds with practicality, is that muriform rodents are nocturnal, but most laboratories perform experiments during the animal’s subjective “night”. These potential environmental influences require careful consideration when performing conscious cystometry.
Urodynamic monitoring of spontaneous voiding can be performed with a conscious rodent placed in a restraining cage (such as a Ballman cage)31,34–36. It is easier to manage the position of the catheter placed in the apex of the bladder dome during the urodynamic experiment in a restrained animal rather than an unrestrained (i.e., freely-moving) one. However, the restraint is likely to cause stress to an animal, which would increase sympathetic activity, thereby favoring storage mechanisms, and potentially prolonging the time of bladder filling until micturition34. Such stress may be alleviated by subjecting the animals to restraint training. Typically, the animal is placed in the restraint cage for a period of time (e.g., one hour) over several days (3–5) prior to performing the cystometric evaluation. Animals subsequently tested appear to be less agitated, as they are more likely to attend to food and water, rather than try to escape37. Commercially available single animal micturition units are advertised to provide stress-free enclosures for this purpose, but, even if true, such a system still requires restraint training due to the restraint mechanism itself, the novel environment of the enclosure, and the sensory isolation of a social animal that is accustomed to the day to day activities of other animals and humans in its home cage environment. It is therefore unclear whether such devices provide any real advantage.
In addition to avoiding potential effects of anesthetics, the use of conscious, unrestrained (i.e., freely-moving) approaches reduces the stress that might be caused by restraint34,38,39. The novel environment factor is still an issue, as well as the nature of the environment (as discussed above for restrained cystometry). Moreover, this approach necessitates a catheter connected to the bladder and exiting through the muscle and the skin in such a way as to be inaccessible to the animal during the post-surgical period, whether using an acute or a chronic catheter preparation. The presence of the catheter and its connection to the recording apparatus would also be expected to affect the experimental animal’s behavior. Moreover, during urodynamic monitoring, the bladder may be tonically or episodically twisted or be unevenly compressed by the catheter placed in the apex of the bladder dome, as the animal moves in a monitoring cage, resulting in artifactual pressure fluctuations and changes in bladder filling geometry during cystometry. While a twisted bladder may still fill and void, such a configuration may be expected to decrease compliance, bladder capacity and voiding efficiency.
c. Decerebration
The precollicular forebrain receives peripheral inputs and is responsible for decision making regarding the timing of micturition (conscious control). Precollicular decerebration disconnects the forebrain from the brainstem, thereby preventing this conscious control and is therefore a valuable alternative for in vivo urological research by allowing reflex micturition without anesthetic effects27,40,41. The involuntary reflex activities of the bladder and urethra are maintained as the neural circuits in the brain stem (including periaqueductal gray and pons), spinal cord (including thoracic sympathetic neurons and lumbosacral parasympathetic neurons), and peripheral nerves that are responsible for reflex voiding and storage remain intact.
In decerebrate, unanesthetized rodents, efficient voiding is preserved and post-void residuals are low40–42 because activity of urethra and bladder is not disturbed by anesthetics. However, decerebration reduces bladder capacity by approximately 40 % (from 0.5 ml to 0.3 ml), as compared to conscious restrained (neurally-intact) rats36. As is the case in the use of general anesthetics, spinal and supraspinal reflexes can be evaluated, but unlike anesthetized rodents, there are no confounding drugs on board. This model also necessarily excludes intracerebroventricular delivery of drugs and prohibits evaluation of forebrain involvement in the micturition process. Additionally, heightened somatic reflexes and decerebrate spasticity are to be expected and need to be addressed to reduce/eliminate movement artifact. However, decerebration allows for the study of reflex micturition without the potential confounding influences of anesthesia in anesthetized preparations and the numerous environmental factors in conscious preparations.
Chronic Preparations
Clearly, acute surgical stress and pain can influence the outcomes of any in vivo experiment. Activation of the CNS pain and stress pathways and the release of stress hormones, corticosterone and catecholamines, in response to pain may influence lower urinary tract activity 43,44. Thus, anesthetized or decerebrate preparations may be preferred to avoid pain in spinal cord intact animals. In spinal cord injured animals, surgical pain may not be an issue at dermatomes distal to the lesion and depending on extent of surgical injury. Nonetheless, acute surgical trauma is expected to result in acute nociception and inflammation that may affect traumatized tissues either directly or indirectly (via cross-sensitization mechanisms11) regardless of whether the animal is conscious, anesthetized, or decerebrate. To minimize such concerns, chronic catheter implantation techniques, in which much of the surgical preparation is performed days or weeks prior to experimentation, have been developed.
In 1986, Yaksh et al. published results of the first chronic bladder catheter methodology in rats31. This methodological advance allowed cystometric approaches in awake animals without acute surgical trauma. This technique, or modifications of it, has been extensively utilized in both rats and mice since the original publication. Briefly, the catheter is inserted in the dome of the bladder, the abdominal wall musculature is closed, and the catheter is tunneled subcutaneously to exit from the high mid-scapular region. The overlying skin of the ventral laparotomy and the exit wound of the free end of the catheter are closed, and the animal is allowed to recover prior to cystometric evaluation. While bladder activity was evident at 2 days post-implantation, it was not normal until 6 days post-implantation of the bladder catheter. These results have been replicated by others23,45–47, demonstrating that the timing of experiments following chronic catheter implantation must be considered.
Telemetric recording of bladder pressure can also be made with placement of a chronic bladder catheter and repetitive assessment of bladder pressure at different time-points in the same animal without externalization of tubing. The pressure catheter of a radiotelemetry device is inserted into the bladder, and the body of the transmitter is placed intra-abdominally. This method was compared to traditional cystometry before and after traumatic brain injury in rats and was found to provide similar results in bladder pressure recording and intermicturition intervals48. In small animals, such as muriform rodents, the relative size of the telemetric transmitting units compared to body size may be an important consideration, as it may impact normal rodent behavior.
Catheter Placement
a. Transurethral
Transurethral placement of the catheter into the bladder can be achieved in female rodents by inserting it through the urethral meatus and entering the bladder lumen in a retrograde fashion. In male rodents, because the penile urethra ends in a sigmoid flexure, transurethral catheterization via the external meatus is not possible. Rather, the catheter can be inserted through an incision into the urethra made caudal to the prostate gland and inserting the catheter into the bladder through this opening16. Thus, transurethral placement requires anesthesia or decerebration, because neither can be performed in a freely moving or loosely restrained animal.
Transurethral catheterization results in a significant increase in peak bladder pressures and areas under the curve of bladder pressure during voiding compared to transvesical catheterization, suggesting obstruction of flow through the outlet. Transurethral catheterization also causes a stronger abdominal wall visceromotor response to voiding compared to both transvesical catheterization and voiding in response to physiologic diuresis (uncatheterized animals), but does not appear to affect flow rates49. These results are unresponsive to α-adrenergic receptor blockade and therefore suggestive of direct physical obstruction. Indirect perturbation of voiding may result from urethral irritation and/or activation of urethral afferents. For example, mechanical irritation was shown to induce neurogenic inflammation in the rat urethra that was capsaicin-sensitive50, suggesting that transurethral catheterization may alter outlet afferent sensitivity. This view is also supported by observations in humans in which transient transurethral catheterization resulted in deviation from a bell-shaped uroflow curve, suggesting that mechanical urethral stimulation may influence LUT function51.
b. Transvesical
The transvesical approach is most commonly performed by inserting the catheter tip through the apex of the bladder dome and securing it with a purse string suture or circular external ligature. A flare is created in the tip of the catheter with heat to prevent tube displacement. When flaring the tip, one must strike a balance between maximal radius of the flared edges and occlusion of the internal diameter, as the latter will affect flow resistance and therefore adversely influence the system contribution to baseline pressure. If the urothelium is not sufficiently cut to allow easy insertion of the flared catheter tip, one may unintentionally tear the urothelium from the smooth muscle with catheter insertion. The tube either exits the abdominal cavity and overlying skin through the abdominal incision, or, following exit from abdominal cavity, is tunneled subcutaneously and externalized through the skin in the mid-scapular region/back of the neck where the animal cannot reach it (for conscious preparations). The abdominal wall and overlying skin are then closed in layers, unless one is using an anesthetized or decerebrate unanesthetized preparation and wishes to observe the bladder and measure lower urinary tract activity in the absence of abdominal wall contraction.
c. Combination Approaches
i. Simultaneous Bladder and Urethral Pressure Measurements
A method that combines isovolumetric bladder pressure and anterograde urethral pressure methods without interrupting periurethral nerve pathways was described previously17–20. A special double-lumen anterograde infusion and static internal recording catheter system with a conical tip is inserted into the dome of the bladder and fitted into the bladder neck. An additional transvesical catheter is inserted for bladder filling/emptying and pressure recording (Figure 1.). This preparation allows determination of effects of systemic treatments on each compartment independently, as well localized delivery of test substances to each compartment individually18. This method does, however, necessarily remove the bladder neck as a contributor to measured activity and likely stimulates proximal urethral afferent innervation.
Figure 1.
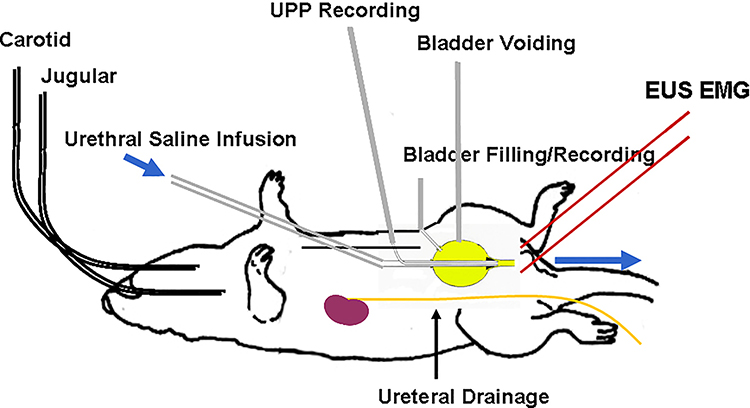
The isolated bladder-urethra preparation. The double-lumen urethral catheter consisted of external PE-160 tubing for urethral perfusion and internal PE-50 tubing for recording anterograde urethral perfusion pressure (UPP). A cone-shaped plug fashioned from a 200pl Eppendorf pipette tip and serving as the tip of the double-lumen urethral catheter was seated in the bladder neck to functionally separate the urethra from the bladder. A separate PE-50 catheter was used for bladder filling and pressure recording. PE10 catheters are used for externalized ureteral diversion. An additional PE-50 catheter may be inserted as a vent/artificial outlet to allow for continuous “open” cystometry. [Fraser MO].
Additional methods that have been used to measure isolated bladder and urethral function (anterograde perfusion pressure) include physical separation of the urethra and bladder by dissection15,16. The relatively low yield of this technique due to nerve damage associated with surgical isolation in the bladder neck region led to the development of the double-lumen catheter preparation described above (Figures 1 and 2).
ii. Dual Bladder Catheters
Using a single catheter for both fluid infusion and pressure measurement is often employed due to simplicity; however, this practice risks signal contamination from pump-induced artifacts and substantial damping of the physiologic signal by the pump and additional connections required. High quality, well-maintained syringe pumps driving small-diameter syringes, short lengths of stiffer tubing, very tight connections, and meticulous technique with flushing out all air from the system are needed to optimize signal fidelity. Pump/syringe and multiple connection artifacts may be avoided by separating the fluid lines for infusion and pressure measurement. A double-lumen catheter can be inserted transvesically or a combination of catheters inserted separately for infusion and recording may be utilized. Figure 3 illustrates the potential for improved signal fidelity of a dual catheter system.
Cystometric Approaches
The fundamental measurement in rodent cystometry is intravesical pressure in response to changing fluid volumes. Gradual bladder filling can be accomplished by renal output, either at typical physiologic rates by making water available to the animal during testing, or enhanced renal output rates/polyuria induced by increasing voluntary water intake by offering sweetened water52,53, gavage, intravascular infusion of a balanced salt solution, and/or administration of diuretic agents54. While arguably “more physiologic”, these techniques cannot assure a known steady volume delivery to the bladder, complicating interpretation of filling curves.
Rodent cystometry is usually performed by infusing fluid into the bladder via either a transurethral or transvesical catheter to reduce the time required to complete studies and avoid systemic effects induced by polyuric models. These experiments allow determination of useful measures of bladder physiology, such as true bladder capacities (TBC; volume required to elicit a voiding contraction during a single fill from an initially verified empty bladder), functional bladder capacities (FBC; volume required to elicit a voiding contraction during continuous cystometry), filling compliance, voiding contraction pressures (opening, micturition, and closing pressures), non-voiding contractions (when present; number, frequency and amplitudes), and voiding contraction duration and voiding durations (Phase II; expulsive phase10). Measurement of voided volumes (VV) and post-void residual volumes (PVR) allows determination of voiding efficiency (VE; % of bladder volume voided during micturition, estimated as VV/(VV+PVR) x 100). VE may also be estimated by simply performing single fill cystometry followed by continuous fill cystometry and using the equation VE=average FBC)/TBC x 100. EMG measures (EUS, lower abdominal musculature), blood pressure, respiratory rate, and other physiological measures provide additional data that may be of importance depending on the experimental questions asked. It is always prudent to recognize that one cannot reliably assess data generated by a system without becoming part of it, and as such is the case, more measurement techniques (e.g. cystometric, cardiovascular, EMG) may not be better than fewer. Such decisions regarding experimental design depend on the questions being asked. Voided volume can be determined without interfering with the animal or other systems of interest.
Types of Cystometry
a. Open Outlet
Open outlet cystometry refers to a preparation in which the bladder is filled and can void through the urethra to the external environment. As previously described with catheter placement, both transurethral and transvesical approaches may be utilized to perform open outlet cystometry.
i. Single Fill
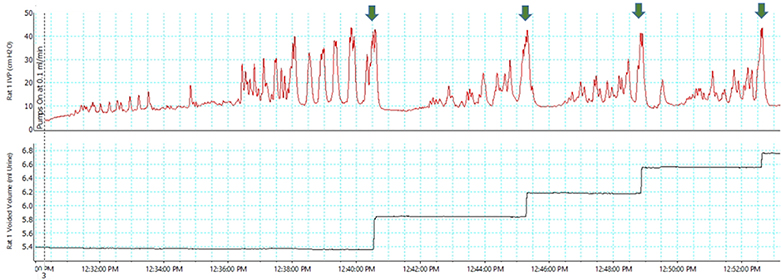
Single filling cystometry in rodents is similar to human cystometry (Figures 4 and 6), and thus may be considered more translatable. It is performed by infusing saline (or other liquid) into the empty bladder to determine TBC, VV, RV, and volume threshold (VT) for inducing micturition (VT = TBC for single fill cystometrogram) 27,34,55–58. The infusion is stopped at the beginning of a micturition contraction, and the fluid voided from the bladder is collected and measured to determine the voided volume (VV). The bladder is then emptied to measure the RV. Voiding efficiency (VE; % of bladder volume voided during micturition) is estimated as (VV/VT) x 100. This procedure is usually repeated multiple times under basal conditions and following interventions such as drug administration.
Figure 4.
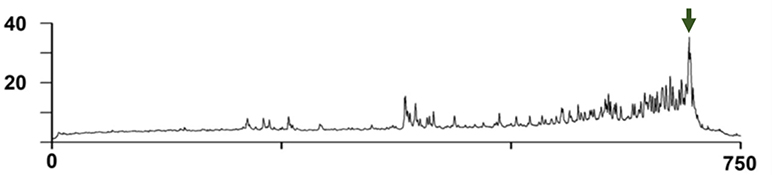
A pressure trace from a complete voiding cycle in an unanesthetized mouse using a chronic catheter. The Y axis is pressure in mmHg and the X axis is time in seconds. The pump is turned on at a constant rate at T=0 to an empty bladder and filling ensues until the voiding contraction (green arrow). [Modified from Dolber et al., 2015]37
Figure 6.
Bladder (top trace) and voided volume (bottom trace) in a female Sprague-Dawley SCI rat under conscious, restrained conditions during combined single fill and continuous cystometry [Fraser MO. Unpublished observation]. Green arrows indicate spinal micturition center-mediated ME.9 Note that TBC is >2× FBC. [Fraser MO, unpublished observation]
ii. Continuous Fill
Continuous cystometry (Figure 5) is performed using a constant infusion of fluid into the bladder to elicit repetitive micturitions, which allows collection of data for a number of micturition cycles 10. This is useful to determine if a drug has effects on intraluminal pressure (e.g., opening, voiding, or closing pressures during bladder contraction) and voided volume (i.e., functional bladder capacity). Continuous cystometry may also be advantageous when investigating a short-acting drug. A faster infusion rate is often chosen for continuous filling cystometry than for single filling cystometry, especially when multiple doses of drugs are being tested in cumulative dose-response studies.
Figure 5.
Bladder (bottom trace) and external urethral sphincter EMG activity (top trace) in a female C57BL/6N mouse under decerebrate, unanesthetized conditions during continuous cystometry [Yoshiyama M, unpublished observation].
iii. Combined Single Fill and Continuous Fill
Continuous cystometry may be used immediately following single cystometry to provide estimates of both TBC and FBC in the same preparation (Figure 6 for example) and as an alternate method for estimating VE (VE=Average FBC/TBC). For this approach, the pump is not stopped at the end of the single fill micturition event (ME).
iv. Important Measurement Considerations
When a filling /void cycle begins from an empty bladder (either as a single fill cystometrogram or during continuous open cystometry with a theoretical 100% voiding efficiency), the volumes that are infused to trigger the void reflect true bladder capacity (TBC) under the conditions of the measurement (e.g., conscious, decerebrate, anesthetized). If the bladder is not emptied, as is the case for continuous cystometry with or without a preceding single fill, then there is no guarantee that the bladder is empty at the start of any filling/voiding cycle within a series of ME during continuous cystometry. Therefore, the volumes required to trigger a ME during continuous cystometry are considered to reflect functional bladder capacity (FBC), and are equivalent in this regard to measures such as those achieved by metabolism cage and voided spots on paper (VSOP) studies. It is important to recognize that the necessary use of catheters for cystometric evaluation can influence voiding efficiency (e.g., surgical trauma and bladder deformation by transvesical catheters or outlet obstruction by transurethral catheters), and it is therefore not a good assumption that voiding efficiency is 100% for any cystometric preparations.
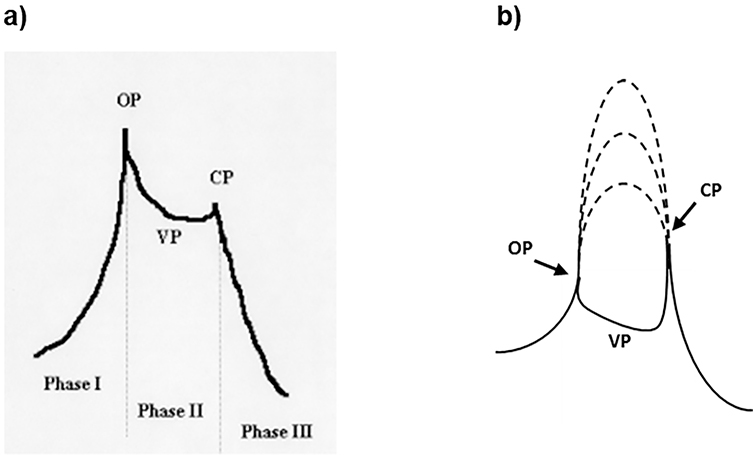
The pattern of the voiding contraction pressure trace was described in the rat10 and mouse42,59 as having three phases (Figure 7a). Phase I is the isovolumetric initial phase of the voiding contraction. The transition between Phases I and II is the opening of the outlet and may appear as a distinct peak called opening pressure (OP). Phase II is the period during the void when the bladder and urethral lumens communicate with the external environment through the urethral meatus, and bladder pressure during this phase represents voiding pressure (VP). The transition between Phase II and Phase III represents the closing of the outlet and may also appear as a distinct peak called closing pressure (CP). Phase III is the isovolumetric final phase, as the bladder contraction decays. In an open system, maximal bladder contraction pressure is either OP or CP, or sometimes, if there is outlet obstruction, VP. These pressures bear no direct relationship to maximal in vivo bladder contractile force generation40,56, as may be seen by superimposing hypothetical isovolumetric contractions on an open outlet voiding pressure trace (Figure 7b). Additionally, conditions and treatments may affect which of the 3 pressures is maximum, and this can change over the course of a given experiment (fast flow rates favor high OP, irritation favors high CP; Fraser, unpublished observations).
Figure 7.
a. (left) Phases of the voiding contraction adapted from Maggi et al.9,22 (see text for additional details). b. (right) Maximal isovolumetric bladder contraction amplitudes (dashed lines) cannot be determined from open cystometry, but can be identified from closed outlet systems.
b. Closed outlet
Closed outlet cystometry may be utilized to measure bladder pressure during filling or at constant volumes without allowing expulsion of infused fluid. Generally, filling is continued until the first ME, after which the infusion pump is shut off to measure isovolumetric activity. A transurethral bladder catheter connected to a pressure transducer is typically used to record bladder pressure with the urethral outlet ligated and to infuse fluid into the bladder 55,56,60–62. Closing the outlet can be achieved by many means, including ligation16 or blockage at the bladder neck63, ligation of the external urethral meatus64 or any method that prevents passage of fluid contents from the bladder to the external environment via the external urethral meatus. If the outlet is closed at the external urethral meatus, the system is not isovolumetric for the bladder during a voiding attempt but rather is isovolumetric for the lower urinary tract. In most cases, the objective of closed outlet cystometry is to determine in vivo bladder contractility, which cannot be measured during open cystometry. As previously discussed, some techniques include simultaneous urethra perfusion pressure measurements15–20,63.
Because the outlet is closed, there is no void. This, however, does not indicate the lack of intent to void. Attempted ME can be discerned by the presence of phasic EUS EMG firing and/or the resultant urethral pressure HFPO. Detection of the former requires placement of periurethral or urethral wall EMG electrodes, while the latter may be determined from urethral pressure, or, if the outlet is closed at the external urethra, HFPO may also detected in bladder pressure tracings.
Cystometric Measurements and Approaches Summarized
Tables 1 and 2 list and describe a number of appropriate cystometric measurements and their meanings. Table 3 lists the pros and cons of different states of consciousness (and mobility of conscious animals) of the experimental animal at the time of cystometric measurements. Table 4 lists the pros and cons of different open outlet cystometric approaches (transurethral vs. transvesical catheter placement and single vs. continuous fill cystometry, respectively).
Table 1 -.
Open Cystometric Measures
| Closing Pressure (Outlet) | The peak pressure of transition from Phase II to Phase III of the voiding contraction. |
| Contraction Duration | Includes Phases I-III of voiding contraction or entirety of a nonvoiding contraction, beginning at >45° sustained rise of voiding contraction and ending at <45° return to baseline. |
| Filling Compliance | Compliance= ΔV/ ΔP - May be multiphase and, as such, is best estimated from single fill cystometrogram using bladder pressure at start of infusion (i.e. when empty) and pressure at volume threshold to calculate ΔP, and volume infused to calculate ΔV. |
| Filling Pressure | Refers to the baseline pressure during bladder filling, which is positively related to volume infused. If nonvoiding contractions are present, the nadir values between them are used for this measure. |
| Functional Bladder Capacity | Volume infused during the intermicturition interval (see below) during continuous cystometry. |
| High Frequency Pressure Oscillations | Occur during Phase II of a voiding contraction, these reflect the phasic contractions of the EUS, as the bladder and urethral lumen are in communication. |
| Intercontraction Interval | Surrogate for IMI if no reliable annotation of void start and finish, generally made from <45° inflection point of relaxation of first voiding contraction and sustained rise >45° of the next voiding contraction from baseline. |
| Intermicturition Interval | Time between the end of one void and the start of another during continuous cystometry. Requires some measure of micturition-associated voiding activity. |
| Nonvoiding Contractions | Contractions of the bladder during bladder filling phase that are not part of a true micturition event (i.e. void accompanied by high frequency pressure oscillations or phasic EUS activity). May result in leakage if pressure generated exceeds outlet leak point. |
| Opening Pressure (Outlet) | The peak pressure of transition from Phase I to Phase II of the voiding contraction. Depending on the preparation used, may be obscured by a higher initial voiding pressures. In such cases, may estimate using first appearance of HFPO or voided fluid (although the latter is delayed by a minimal transit time). |
| Pressure at Volume Threshold | The pressure at volume threshold (the beginning of the voiding contraction) used to calculate filling compliance. Often labeled as “Pressure Threshold”. |
| True Bladder Capacity | Volume infused during a single fill of an empty bladder that initiates a void (i.e., volume infused reaches Volume Threshold). |
| True Micturition Pressure | The average nadir pressure between HFO during Phase II of a voiding contraction or the average pressure during Phase II if HFPO are not detectable. |
| Voided Volume | Volume released during a voiding event. |
| Voiding Contraction | A bladder contraction that results in a micturition event. Distinguished from a high pressure leak by the presence of high frequency pressure oscillations or phasic EUS activity. May be described in terms of non-voiding and voiding components (e.g. Phases I-III; Maggi 1986) |
| Voiding Duration | Duration of Phase II (between opening or closing pressure is most sensitive measure, can also estimate based on collection of urine released. |
| Voiding Efficiency | The efficiency of bladder emptying during a micturition event. May be calculated directly from a single micturition cycle by measuring the voided volume (VV) and post-void residual volume (RV) within the bladder. Thus, %VE=(VV/(RV+VV)) × 100. |
| Voiding Frequency | The number of filling/voiding cycles over time during continuous cystometry. |
| Volume Threshold | Generally, the bladder volume at which a voiding contraction is initiated during open- outlet cystometry, but may also be used to refer to the first nonvoiding contraction. |
Table 2 -.
Closed Cystometric Measures
| Bladder Capacity | Starting with an empty bladder, the closed outlet bladder is filled as if performing a single cystometrogram in an open outlet preparation. The onset of the first micturition event marks the Bladder Capacity to Micturition Event. If measures of outlet function are not used, then volume to first stable bladder contraction amplitude may be used. |
| Baseline Pressure | The nadir pressure between stable isovolumetric bladder contractions. May be used to estimate changes in compliance during testing (i.e. baseline pressure will be inversely related to bladder compliance). |
| Bladder Contraction Area Under the Curve | Measured as an index of bladder contraction power. Most data acquisition programs allow measurement of AUC of bladder contractions using the boundaries of bladder contraction duration to delimit data used to calculate AUC. |
| Bladder Contraction Duration | Bladder contraction duration can be measured using the >45° sustained rise of voiding contraction and ending at <45° post-maximal contraction. |
| Bladder Contraction Frequency | The number of bladder contractions over time during rhythmic isovolumetric contractions. |
| Maximal Bladder Contraction Amplitude | Maximal bladder contraction amplitude is an accurate in vivo reflection of bladder contractility in a closed-outlet system. |
Table 3 -.
Effects of States of Consciousness
| Unanesthetized | Anesthetized | |||
| Conscious | Decerebrate | |||
| Voluntary voiding | Reflex micturition | |||
| Restrained | Freely-moving | |||
| Pros | • No influence of anesthesia during experiment • Better model of physiologic conditions |
• Little influence from environmental distractions • Ease of animal handling and catheter management during experiment • No influence of emotional or social behaviors • Allows for greater complexity in experimental design (e.g., measurement of sphincter EMG, urethral pressure, blood pressure) • No effects from surgically-induced injury responses (e.g. wound healing, chronic pain) • Less prone to artifacts |
||
| • Less noisy data acquisition compared to freely moving | • Less stressful compared to restrained | • Eliminates effect of anesthesia during experiment | • Convenience in usage | |
| Cons | • Hard to handle animal and catheters during experiment (e.g. recording artifact) • Influence from environmental factors • Possible influence of animals' emotive responses on cystometric outcomes • Potential chronic catheter implant complications (e.g. infection, catheter blockage/extrusion) 4 |
• No active intervention of the forebrain | ||
| • Affected by acute restraint stress response • Ethical issues |
• Twisting of catheter and bladder | • Challenging surgical procedure requiring extra training and experience in technique • Longer time for surgery |
• Interactions with pharmacological and physiological interventions | |
Table 4 -.
Pros and Cons of Different Experimental Approaches
| Transurethral | Transvesical | |
| Pros | • Less damage to bladder wall • No requirement of abdominal surgery |
• Can be performed on both females and males • Avoidance of influence on bladder neck/urethral closure • Feasibility of chronic catheter implantation • No necessity of anesthetic use during experiment |
| Cons | • Only possible to perform in females • Possible influence on bladder neck/urethral closure and resistance to flow • Requires anesthesia during experiment |
• Damage to bladder wall • Requirement of abdominal surgery • Potential restriction of bladder contraction in longitudinal direction |
| Single Fill | Continuous Fill | |
| Pros | • Feasibility of analysis with greater accuracy of a variety of cystometry variables (e.g. true bladder capacity, post-void residual volume, and filling compliance) | • Convenient for quickly screening drug effects |
| Cons | • Unsuitability for screening short-acting drugs | • Inability to measure post-void residual volumes • Inability to measure filling parameters from a known empty bladder (e.g. true bladder capacity, filling compliance and baseline pressures) |
Rodent cystometry – Best Practices
a. Appropriate Controls for Experimental manipulations
i. Appropriate Control Period
An appropriate control period may be selected based on the animal model’s anticipated bladder capacity and the selected infusion rate. As a rule of thumb, a longer control period is required for a faster infusion rate to allow time for accommodation. Otherwise, false positive or negative responses may be seen following treatments (for drugs that are thought to increase and decrease bladder capacity, respectively).
ii. Parallel Time- and Volume-Matched Vehicle Only Control Groups for Drug/Device Studies
It is also important to utilize a parallel time/dose repeated vehicle controls or time/test stimulation sham controls as a study arm to account for duration of experimental procedures and influences of environmental factors (interactions between laboratory personnel, etc.). This is in addition to pre-dosing vehicle/pre-testing sham controls as within animal controls. In this way there are within animal and between animal controls.
b. Measurement of Voiding Efficiency during Continuous Cystometry
Muriform rodents utilize phasic external urethral sphincter firing to aid in voiding. Voiding efficiency can be dramatically altered by agents or manipulations that affect this mechanism. Without a measure of voiding efficiency, one can come to improper interpretations regarding comparisons between or within treatment groups. For example, decreased FBC may be due to decreased voiding efficiency or increased afferent sensitivity, while increased FBC may be due to increased voiding efficiency or decreased afferent sensitivity.
It is not possible to measure voiding efficiency with FBC alone and VV alone, as by definition, VV = FBC in a stable preparation.
c. Acclimatization in Conscious Preparations
Conscious control of voiding is very susceptible to stress, especially in animals that “toilette” (i.e., are particular about where and when they void). Thus, the more physiological the infusion rate, the more important the consideration for acclimatizing the animal to the experimental environment prior to the day of experimentation. One may predict that the more rapid the flow rate, the less important acclimatization may be as far as cortical control is concerned, because spinobulbospinal reflex activity is approximated (i.e. any cortical control is likely overwhelmed). Regardless, for the comfort of the animal and to eliminate undue stress-induced sympathetic influences, acclimatization is strongly suggested.
d. Sufficient Recovery Time for Healing in Chronic Catheter/Instrumentation Preparations
As previously discussed, but certainly worth repeating, the original Yaksh paper that first described chronic bladder catheter implantation reported that the bladder began working at day 2 post-implantation31. This has given rise to the frequent use of a 1–4 day recovery time for chronic catheter cystometry and citation of the Yaksh paper for this approach. However, the Yaksh paper also reported that bladder function is not normal at this time, and that the bladder takes at least 6 days to recover, with ≥7 days appearing optimal. Thus, we suggest that at least 1-week time elapse for healing prior to utilization of chronic bladder catheters for cystometric experiments.
e. Bladder Filling Rate as a Variable
Filling of the bladder during cystometry is conventionally achieved by continuous fluid infusion at a constant rapid rate, in contrast to natural filling which occurs intermittently at a variable, slow rate via bilateral bolus delivery of urine from the ureters. The simplicity and convenience of conventional filling presently outweigh any advantages of non-continuous or variable rate infusion patterns.
A wide range of infusion rates during cystometry has been reported in rodents (15–50 μl/min for mice, 40–180 μl/min for rats). Up to some multiple of physiologic filling rates65, active brainstem-mediated detrusor relaxation allows bladder wall tensions to increase minimally as bladder volume increases to capacity66,67. With more rapid filling rates that are sometimes employed in cystometry (clinical and animal), viscoelasticity of the bladder wall, both inherent to the extracellular elements and detrusor reactivity to stretch, results in supraphysiologic increasing tensions68. Due to the inverse relationship of the square of the radius to intravesical pressure, pressure evidence of increasing tension with increasing volume, which is normally not detected at typical cystometric rates (25–100 ml/min) in humans69, is, however, a frequent observation in the small radius rodent bladder. In the same vein, while risking over-interpretation of small drug-induced tension effects, this effect does allow intravesical pressure in rodent cystometry to provide a useful window into LUT physiology that is unattainable in human urodynamics.
Because the infusion rate can have a considerable impact on measurements obtained during the filling phase, the optimal infusion rate should be determined based on the experimental goals. In most cases, the selected infusion rate represents a compromise between the rate of natural diuresis and a rate that is practical for completion of the experiment.
Conclusions
Cystometric evaluation of rodents comprises an extensive collection of functional tests that can be performed under a variety of experimental conditions. Decisions regarding which approaches to choose should be determined by the specific questions to be addressed and implementation of the test should follow standardized procedures. This white paper describes a range of cystometric tests and techniques used to explore biological phenomena relevant to the lower urinary tract, discusses the advantages and disadvantages of various experimental conditions, and provides guidance on the practical aspects of experimental execution and proper interpretation of results. It is our hope that this work provides useful insights for both conducting and interpreting rodent cystometry, and critically evaluating the literature.
ACKNOWLEDGMENTS
We also thank the following participants of the Establishing the Parameters of Void Spot Assays and Cystometrograms for Data Sharing Consensus Meeting, held January 9, 2015 at the American Urological Association Headquarters in Linthicum, MD, who contributed their thoughtful discussion and insights: Rosalyn Adam, Uzoma Anele, Richard Baldock, Carolyn Best, Toby Chai, Warren Hill, Maryann Martone, Michelle Southard-Smith, and Chad Vezina. Special thanks also to the NIH NIDDK program officers Deborah Hoshizaki, Ziya Kirkali, and Tamara Bavendam for organizing and convening the meeting.
GRANTS
The preparation of this white paper was supported by NIH National Institute of Diabetes and Digestive and Kidney Diseases Grant U54-DK-104310 (DEB) and National Institutes on Aging Grant K76-AG-054777 (PPS), Department of Defense Congressionally Directed Medical Research Programs’ Spinal Cord Injury Research Program Grant SC110031 (MOF), Department of Veterans Affairs Biomedical Laboratory Research and Development Service Award I01-BX001790 (MPS) and Rehabilitation Research and Development Service Awards R21-RX001749 (MOF) and I21-RX001398 (MOF), the Japan Society for Promotion of Science KAKENHI Grant 16K11042 (MY), the Canadian Urological Association Scholarship Foundation Career Development Award (LC), and the American Urological Association’s Urology Care Foundation Rising Star in Urology Award (LC) and North Eastern Section Young Investigator Grant (LC).
Footnotes
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.Rosier P, Schaefer W, Lose G, et al. International Continence Society Good Urodynamic Practices and Terms 2016: Urodynamics, uroflowmetry, cystometry, and pressure-flow study. Neurourology and urodynamics. 2017;36(5):1243–1260. [DOI] [PubMed] [Google Scholar]
- 2.Smith PP, Hurtado EA, Appell RA. Post hoc interpretation of urodynamic evaluation is qualitatively different than interpretation at the time of urodynamic study. Neurourology and urodynamics. 2009;28(8):998–1002. [DOI] [PubMed] [Google Scholar]
- 3.Leitner L, Walter M, Sammer U, Knupfer SC, Mehnert U, Kessler TM. Urodynamic Investigation: A Valid Tool to Define Normal Lower Urinary Tract Function? PLoS One. 2016;11(10):e0163847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ito H, Pickering AE, Igawa Y, Kanai AJ, Fry CH, Drake MJ. Muro-Neuro-Urodynamics; a Review of the Functional Assessment of Mouse Lower Urinary Tract Function. Frontiers in physiology. 2017;8:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersson KE, Soler R, Fullhase C. Rodent models for urodynamic investigation. Neurourology and urodynamics. 2011;30(5):636–646. [DOI] [PubMed] [Google Scholar]
- 6.Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nature reviews Neuroscience. 2008;9(6):453–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.BARRINGTON FJF. THE COMPONENT REFLEXES OF MICTURITION IN THE CAT: PARTS I AND II. Brain. 1931;54(2):177–188. [Google Scholar]
- 8.BARRINGTON FJF. THE COMPONENT REFLEXES OF MICTURITION IN THE CAT. PART III1. Brain. 1941;64(4):239–243. [Google Scholar]
- 9.Fraser MO. New Insights into the Pathophysiology of Detrusor-Sphincter Dyssynergia. Current Bladder Dysfunction Reports. 2011;6(2):93–99. [Google Scholar]
- 10.Maggi CA, Giuliani S, Santicioli P, Meli A. Analysis of factors involved in determining urinary bladder voiding cycle in urethan-anesthetized rats. The American journal of physiology. 1986,’251(2 Pt 2):R250–257. [DOI] [PubMed] [Google Scholar]
- 11.Pezzone MA, Liang R, Fraser MO. A model of neural cross-talk and irritation in the pelvis: implications for the overlap of chronic pelvic pain disorders. Gastroenterology. 2005;128(7):1953–1964. [DOI] [PubMed] [Google Scholar]
- 12.Dolber PC, Gu B, Zhang X, Fraser MO, Thor KB, Reiter JP. Activation of the external urethral sphincter central pattern generator by a 5-HT(1A) receptor agonist in rats with chronic spinal cord injury. American journal of physiology Regulatory, integrative and comparative physiology. 2007;292(4):R1699–1706. [DOI] [PubMed] [Google Scholar]
- 13.Gu B, Fraser MO, Thor KB, Dolber PC. Induction of bladder sphincter dyssynergia by kappa-2 opioid receptor agonists in the female rat. J Urol. 2004;171(1):472–477. [DOI] [PubMed] [Google Scholar]
- 14.Chang HY, Cheng CL, Chen JJ, de Groat WC. Roles of glutamatergic and serotonergic mechanisms in reflex control of the external urethral sphincter in urethane-anesthetized female rats. American journal of physiology Regulatory, integrative and comparative physiology. 2006;291(1):R224–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett BC, Kruse MN, Roppolo JR, Flood HD, Fraser M, de Groat WC. Neural control of urethral outlet activity in vivo: role of nitric oxide. J Urol. 1995;153(6):2004–2009. [PubMed] [Google Scholar]
- 16.Conte B, Maggi CA, Parlani M, Lopez G, Manzini S, Giachetti A. Simultaneous recording of vesical and urethral pressure in urethane-anesthetized rats: effect of neuromuscular blocking agents on the activity of the external urethral sphincter. Journal of pharmacological methods. 1991;26(3):161–171. [DOI] [PubMed] [Google Scholar]
- 17.Kakizaki H, Fraser MO, De Groat WC. Reflex pathways controlling urethral striated and smooth muscle function in the male rat. The American journal of physiology. 1997; 272(5 Pt 2):R1647–1656. [DOI] [PubMed] [Google Scholar]
- 18.Jung SY, Fraser MO, Ozawa H, et al. Urethral afferent nerve activity affects the micturition reflex; implication for the relationship between stress incontinence and detrusor instability. J Urol. 1999;162(1):204–212. [DOI] [PubMed] [Google Scholar]
- 19.Yang Z, Dolber PC, Fraser MO. Diabetic urethropathy compounds the effects of diabetic cystopathy. J Urol. 2007;178(5):2213–2219. [DOI] [PubMed] [Google Scholar]
- 20.Yang Z, Dolber PC, Fraser MO. Differential vulnerabilities of urethral afferents in diabetes and discovery of a novel urethra-to-urethra reflex. Am J Physiol Renal Physiol. 2010;298(1):F118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshiyama M, deGroat WC, Fraser MO. Influences of external urethral sphincter relaxation induced by alpha-bungarotoxin, a neuromuscular junction blocking agent, on voiding dysfunction in the rat with spinal cord injury. Urology. 2000;55(6):956–960. [DOI] [PubMed] [Google Scholar]
- 22.Maggi CA, Meli A. Suitability of urethane anesthesia for physiopharmacological investigations in various systems. Part 1: General considerations. Experientia. 1986;42(2):109–114. [DOI] [PubMed] [Google Scholar]
- 23.Matsuura S, Downie JW. Effect of anesthetics on reflex micturition in the chronic cannula-implanted rat. Neurourology and urodynamics. 2000;19(1):87–99. [DOI] [PubMed] [Google Scholar]
- 24.Cannon TW, Damaser MS. Effects of anesthesia on cystometry and leak point pressure of the female rat. Life Sci. 2001;69(10):1193–1202. [DOI] [PubMed] [Google Scholar]
- 25.Thor KB, Muhlhauser MA. Vesicoanal, urethroanal, and urethrovesical reflexes initiated by lower urinary tract irritation in the rat. The American journal of physiology. 1999; 277(4 Pt 2):R1002–1012. [DOI] [PubMed] [Google Scholar]
- 26.Chang HY, Havton LA. Differential effects of urethane and isoflurane on external urethral sphincter electromyography and cystometry in rats. Am J Physiol Renal Physiol. 2008;295(4):F1248–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshiyama M, Roppolo JR, Takeda M, de Groat WC. Effects of urethane on reflex activity of lower urinary tract in decerebrate unanesthetized rats. Am J Physiol Renal Physiol. 2013;304(4):F390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maggi CA, Meli A. Suitability of urethane anesthesia for physiopharmacological investigations. Part 3: Other systems and conclusions. Experientia. 1986;42(5):531–537. [DOI] [PubMed] [Google Scholar]
- 29.Maggi CA, Meli A. Suitability of urethane anesthesia for physiopharmacological investigations in various systems. Part 2: Cardiovascular system. Experientia. 1986;42(3):292–297. [DOI] [PubMed] [Google Scholar]
- 30.Field KJ, White WJ, Lang CM. Anaesthetic effects of chloral hydrate, pentobarbitone and urethane in adult male rats. Lab Anim. 1993;27(3):258–269. [DOI] [PubMed] [Google Scholar]
- 31.Yaksh TL, Durant PA, Brent CR. Micturition in rats: a chronic model for study of bladder function and effect of anesthetics. The American journal of physiology. 1986; 251(6 Pt 2):R1177–1185. [DOI] [PubMed] [Google Scholar]
- 32.Tannenbaum A, Vesselinovitch SD, Maltoni C, Mitchell DS. Multipotential carcinogenicity of urethan in the Sprague-Dawley rat. Cancer research. 1962;22:1362–1371. [PubMed] [Google Scholar]
- 33.Cowen PN. Strain differences in mice to the carcinogenic action of urethane and its non-carcinogenicity in chicks and guinea-pigs. British journal of cancer. 1950;4(2):245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morikawa K, Kakiuchi M, Fukuoka M, Kato H, Ito Y, Gomi Y. Effects of various drugs on bladder function in conscious restrained-denervated rats placed in a restraining cage and produced by transection of the hypogastric nerve. Japanese journal of pharmacology. 1990;52(3):405–411. [DOI] [PubMed] [Google Scholar]
- 35.Yoshiyama M, Nezu FM, Yokoyama O, de Groat WC, Chancellor MB. Changes in micturition after spinal cord injury in conscious rats. Urology. 1999;54(5):929–933. [DOI] [PubMed] [Google Scholar]
- 36.Yokoyama O, Yoshiyama M, Namiki M, de Groat WC. Role of the forebrain in bladder overactivity following cerebral infarction in the rat. Experimental neurology. 2000;163(2):469–476. [DOI] [PubMed] [Google Scholar]
- 37.Dolber PC, Jin H, Nassar R, Coffman TM, Gurley SB, Fraser MO. The effects of Ins2(Akita) diabetes and chronic angiotensin II infusion on cystometric properties in mice. Neurourology and urodynamics. 2015;34(1):72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Conte B, D’Aranno V, Santicioli P, et al. New method for recording cystometrograms in conscious, freely moving rats. Journal of pharmacological methods. 1988;19(1):57–61. [DOI] [PubMed] [Google Scholar]
- 39.Igawa Y, Zhang X, Nishizawa O, et al. Cystometric findings in mice lacking muscarinic M2 or M3 receptors. J Urol. 2004;172(6 Pt 1):2460–2464. [DOI] [PubMed] [Google Scholar]
- 40.Yoshiyama M, Roppolo JR, de Groat WC. Effects of LY215490, a competitive alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor antagonist, on the micturition reflex in the rat. The Journal of pharmacology and experimental therapeutics. 1997;280(2):894–904. [PubMed] [Google Scholar]
- 41.Yoshiyama M, Mochizuki T, Nakagomi H, et al. Functional roles of TRPV1 and TRPV4 in control of lower urinary tract activity: dual analysis of behavior and reflex during the micturition cycle. Am J Physiol Renal Physiol. 2015;308(10):F1128–1134. [DOI] [PubMed] [Google Scholar]
- 42.Kira S, Yoshiyama M, Tsuchiya S, et al. P2Y6-deficiency increases micturition frequency and attenuates sustained contractility of the urinary bladder in mice. Scientific reports. 2017;7(1):771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamazaki Y, Takeda H, Akahane M, Igawa Y, Nishizawa O, Ajisawa Y. Species differences in the distribution of beta-adrenoceptor subtypes in bladder smooth muscle. British journal of pharmacology. 1998;124(3):593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qin C, Greenwood-Van Meerveld B, Foreman RD. Spinal neuronal responses to urinary bladder stimulation in rats with corticosterone or aldosterone onto the amygdala. Journal of neurophysiology. 2003;90(4):2180–2189. [DOI] [PubMed] [Google Scholar]
- 45.Mann-Gow TK, Larson TR, Woien CT, Andersen TM, Andersson KE, Zvara P. Evaluating the Procedure for Performing Awake Cystometry in a Mouse Model. J Vis Exp. 2017(123). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yokoyama O, Ishiura Y, Komatsu K, et al. Effects of MK-801 on bladder overactivity in rats with cerebral infarction. J Urol. 1998;159(2):571–576. [DOI] [PubMed] [Google Scholar]
- 47.Morikawa K, Ichihashi M, Kakiuchi M, et al. Effects of various drugs on bladder function in conscious rats. Japanese journal of pharmacology. 1989;50(4):369–376. [DOI] [PubMed] [Google Scholar]
- 48.Moody BJ, Liberman C, Zvara P, Smith PP, Freeman K, Zvarova K. Acute lower urinary tract dysfunction (LUTD) following traumatic brain injury (TBI) in rats. Neurourology and urodynamics. 2014;33(7):1159–1164. [DOI] [PubMed] [Google Scholar]
- 49.Smith PP, Hurtado E, Smith CP, Boone TB, Somogyi GT. Comparison of cystometric methods in female rats. Neurourology and urodynamics. 2008;27(4):324–329. [DOI] [PubMed] [Google Scholar]
- 50.Abelli L, Conte B, Somma V, Parlani M, Geppetti P, Maggi CA. Mechanical irritation induces neurogenic inflammation in the rat urethra. J Urol. 1991;146(6):1624–1626. [DOI] [PubMed] [Google Scholar]
- 51.Suskind AM, Smith PP. Evidence of a functional effect of transient transurethral catheterization on micturition in women. International urogynecology journal. 2012;23(9):1245–1248. [DOI] [PubMed] [Google Scholar]
- 52.Wood R, Eichel L, Messing EM, Schwarz E. Automated noninvasive measurement of cyclophosphamide-induced changes in murine micturition frequency and volume and demonstration of pharmacologic sensitivity. Urology. 2001;57(6 Suppl 1):115–116. [DOI] [PubMed] [Google Scholar]
- 53.Levin RM, Wein AJ, Eika B, Tammela TL, Longhurst PA. Effects of diuresis on micturition. Neurourology and urodynamics. 1995;14(2):169–176. [DOI] [PubMed] [Google Scholar]
- 54.Tammela TL, Longhurst PA, Wein AJ, Levin RM. The effect of furosemide-induced diuresis on rabbit micturition and bladder contractile function. J Urol. 1993;150(1):204–208. [DOI] [PubMed] [Google Scholar]
- 55.Yoshiyama M, Roppolo JR, Thor KB, de Groat WC. Effects of LY274614, a competitive NMDA receptor antagonist, on the micturition reflex in the urethane-anaesthetized rat. British journal of pharmacology. 1993;110(1):77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoshiyama M, Roppolo JR, de Groat WC. Effects of MK-801 on the micturition reflex in the rat--possible sites of action. The Journal of pharmacology and experimental therapeutics. 1993;265(2):844–850. [PubMed] [Google Scholar]
- 57.Yoshiyama M, Roppolo JR, De Groat WC. Alteration by urethane of glutamatergic control of micturition. European journal of pharmacology. 1994;264(3):417–425. [DOI] [PubMed] [Google Scholar]
- 58.Yoshiyama M, de Groat WC. Supraspinal and spinal alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid and N-methyl-D-aspartate glutamatergic control of the micturition reflex in the urethane-anesthetized rat. Neuroscience. 2005;132(4):1017–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yoshiyama M, Kobayashi H, Araki I, Du S, Zakoji H, Takeda M. Sex-related differences in activity of lower urinary tract in response to intravesical acid irritation in decerebrate unanesthetized mice. American journal of physiology Regulatory, integrative and comparative physiology. 2008;295(3):R954–960. [DOI] [PubMed] [Google Scholar]
- 60.Yoshiyama M, Roppolo JR, Rihmland J, Blastos B, de Groat WC. The effects of MK-801, an NMDA receptor antagonist, on the micturition reflex in the rat. Neuroscience letters. 1991;126(2):141–144. [DOI] [PubMed] [Google Scholar]
- 61.Yoshiyama M, Kakizaki H, de Groat WC. Suppression of the micturition reflex in urethane-anesthetized rats by intracerebroventricular injection of WAY100635, a 5-HT(1A) receptor antagonist. Brain research. 2003;980(2):281–287. [DOI] [PubMed] [Google Scholar]
- 62.Chuang Y-C, Fraser MO, Yu Y, Chancellor MB, de Groat WC, Yoshimura N. THE ROLE OF BLADDER AFFERENT PATHWAYS IN BLADDER HYPERACTIVITY INDUCED BY THE INTRAVESICAL ADMINISTRATION OF NERVE GROWTH FACTOR. The Journal of Urology. 2001;165(3):975–979. [PubMed] [Google Scholar]
- 63.Miyazato M, Sugaya K, Nishijima S, Kadekawa K, Ashimine S, Ogawa Y. Intrathecal or dietary glycine inhibits bladder and urethral activity in rats with spinal cord injury. J Urol. 2005;174(6):2397–2400. [DOI] [PubMed] [Google Scholar]
- 64.Yoshiyama M, De Groat WC. Role of spinal alpha1-adrenoceptor subtypes in the bladder reflex in anesthetized rats. American journal of physiology Regulatory, integrative and comparative physiology. 2001;280(5):R1414–1419. [DOI] [PubMed] [Google Scholar]
- 65.Klevmark B Volume threshold for micturition. Influence of filling rate on sensory and motor bladder function. Scand J Urol NephrolSuppl. 2002(210):6–10. [DOI] [PubMed] [Google Scholar]
- 66.Andersson KE. Changes in bladder tone during filling: pharmacological aspects. Scand J Urol Nephrol Suppl. 1999;201:67–72; discussion 76–99. [DOI] [PubMed] [Google Scholar]
- 67.Andersson KE. Pathways for relaxation of detrusor smooth muscle. Adv Exp Med Biol. 1999;462:241–252. [DOI] [PubMed] [Google Scholar]
- 68.Coolsaet BL, van Duyl WA, van Mastrigt R, van der Zwart A. Visco-elastic properties of the bladder wall. Urol Int. 1975;30(1):16–26. [DOI] [PubMed] [Google Scholar]
- 69.Griffiths DJ. Urodynamics. The Mechanics and Hydrodynamics of the Lower Urinary Tract. Bristol: Adam Hilger Ltd; 1980. [Google Scholar]