Abstract
Objectives
Idiopathic subglottic stenosis (iSGS) is an inflammatory process leading to fibrosis and narrowing of the laryngotracheal airway. There is variability in patient response to surgical intervention, but the mechanisms underlying this variability are unknown. In this pilot study, we measure expression of candidate targets at the mucosal surface of the subglottis in iSGS patients. We aim to identify putative biomarkers for iSGS that provide insights into the molecular basis of disease progression, yield a gene signature for the disease, and/or predict a response to therapy.
Study Design
In vitro comparative study of human cells.
Methods
Levels of candidate transcripts and proteins were measured in healthy and stenotic laryngotracheal tissue specimens taken from the mucosal surface in 16 iSGS patients undergoing endoscopic balloon dilation. Pre- and post-operative pulmonary function test and patient reported voice and breathing outcomes were also assessed. Unsupervised clustering was used to define patient subgroups based on expression profile.
Results
Pulmonary function and voice and breathing outcome metrics demonstrated significant post-operative improvement. Transcript levels of αSMA, CCL2, COL1A1, COL3A1, FN1, IFNG, and TGFB1 and protein levels of CCL2, IFNG, and IL-6 were significantly upregulated in stenotic as compared to healthy tissues. Marked heterogeneity was observed in the patterns of expression of candidate markers across individuals and tissue types. Patient subgroups defined by expression profile did not show a statistically significant difference in dilation interval.
Conclusion
Pro-inflammatory and pro-fibrotic pathways are significantly upregulated along the mucosal surface of stenotic laryngotracheal tissues, and CCL2 and IFNG merit further investigation as potential iSGS biomarkers.
Level of Evidence
4
Keywords: Idiopathic subglottic stenosis, dilation, gene expression, protein expression
INTRODUCTION
Idiopathic subglottic stenosis (iSGS) is an inflammatory process that leads to fibrosis and narrowing of the airway in the distal larynx and proximal trachea. Several factors have been implicated in the pathogenesis of this condition, including a hormonal component given the female predominance seen in iSGS populations, mycobacterial colonization, and extra-esophageal reflux; however, the etiology may be multifactorial and remains unknown.1–3 The preferred treatments for iSGS are surgical and include endoscopic dilation, laryngotracheal reconstruction, endoscopic resection, endoscopic laryngotracheoplasty, or cricotracheal resection.4–6 More recently, adjuvant medical therapies, including serial steroid injections and a combination of trimethoprim-sulfamethoxazole, high dose inhaled corticosteroid, and proton pump inhibitors have been investigated.5,7 Endoscopic balloon dilation remains the most commonly employed surgical therapy for iSGS.8 There are, however, certain subpopulations that respond less favorably, experiencing a more rapid return in airway symptoms and shorter post-dilation intervals.9 Clinical characteristics, including a vertical length of stenosis greater than 1 cm, the presence of circumferential stenosis, and involvement of the interarytenoid area at the posterior commissure, have been previously identified as poor prognostic factors.10 However, the mechanistic factors underlying an individual’s disease trajectory or predisposition to treatment response remain unknown.
Canonical fibrosis signaling mechanisms, including growth factors, angiogenic factors, and inflammatory cytokines, are known to lead to myofibroblast activation and extracellular matrix deposition.11,12 These mechanisms likely contribute, at least in part, to the pathogenesis of iSGS, and gene expression studies have been performed to better understand the transcriptomic alterations that occur in this setting. Extracellular matrix components, including type I collagen, type III collagen, and fibronectin, and inflammatory responses, including activation of the IL-17A/IL-23 pathway, have been shown to be upregulated in iSGS.13 Evidence suggests that γδ T cells and interactions between Mycobacterium and the host immune system may be driving IL-17A overactivity.1,13 Polymorphisms in the ligand and receptor upstream of IL-17A activation have been documented in the literature and may be a potential mechanistic pathway for varied susceptibility to Mycobacterium infection and iSGS pathology.1
In this pilot study, we investigate expression of candidate genes and proteins in laryngotracheal surface tissues collected from iSGS patients undergoing endoscopic balloon dilation. We also evaluate pre- and post-operative pulmonary function test (PFT) results and patient reported voice and breathing outcomes and correlate these outcome measures with candidate biomarker expression. We hypothesize that there is transcriptional and translational heterogeneity in the laryngotracheal tissues of iSGS patients, and we predict that this heterogeneity is related, at least in part, to clinically observed variability in disease susceptibility and treatment response. We aim to identify putative biomarkers for iSGS that provide insights into the molecular basis of disease progression, yield a gene signature for the disease, and/or predict a response to therapy.
MATERIALS AND METHODS
This investigation was approved by the Johns Hopkins University School of Medicine Institutional Review Boards, adhered to the tenets of the Declaration of Helsinki, and complied with the Health Insurance Portability and Accountability Act. All subjects provided written informed consent prior to study participation and were recruited prospectively between December 2015 and May 2018. Eligible participants were patients with iSGS treated in a faculty clinic (ATH) in the Department of Otolaryngology-Head and Neck Surgery at the Johns Hopkins University School of Medicine, 18 years of age or older, and not currently pregnant. Patients with subglottic stenosis secondary to traumatic or autoimmune etiologies, including prolonged intubation and granulomatosis with polyangiitis, were excluded. All study procedures were performed in addition to and not in place of standard clinical care.
Dilation of Subglottic Stenosis and Tissue Collection
After informed consent, the patient was brought to the operating room and maintained under general anesthesia. Tooth guards were placed. A laryngoscope was used to expose the airway, and the patient was placed into suspension. High flow apneic oxygenation was used with an endotracheal tube introduced through the laryngoscope for ventilation if needed to maintain oxygen saturation above 95%.14 Brush biopsies (ConMed Disposable Bronchial Cytology Brush, ConMed Corporation, Utica, NY) and Gelfoam swabs (Surgifoam Absorbable Gelatin Sponge, U.S.P., Ethicon, Inc., Somerville, NJ) were collected from the area of stenosis and adjacent healthy tissue.15 The brushes and swabs were immediately placed in microcentrifuge tubes on dry ice and subsequently stored at ‒80° C prior to further processing. Excision, destruction, and balloon dilation (CRE Pulmonary Balloon Dilators, Boston Scientific, Marlborough, MA) of the stenosis was performed in the usual fashion. The location of the stenosis relative to the superior aspect of the vocal folds, the length of the stenosis, the percent stenosis before dilation, and the percent stenosis after dilation were noted. Triamcinolone was injected submucosally. Lidocaine was applied topically for anesthesia to the larynx and trachea.
Data Collection
Patient demographic and clinical characteristics, including age, race, gender, body mass index (BMI), and prior surgical history, including total number of endoscopic dilations, were extracted from the medical record. The dilation interval was calculated by determining the median lengths of time between serial dilations. Individuals with only one dilation and no defined dilation interval were excluded from analyses of dilation interval. The location of the stenosis relative to the superior aspect of the vocal folds, the length of the stenosis, and the percent stenosis pre- and post-dilation were recorded.
Pulmonary function testing (PFT) was performed using the MicroLoop Spirometer (Vyaire Medical, Mettawa, IL) in the office pre- and postoperatively, and PFT results were processed using the Spirometry PC Software (Vyaire Medical, Mettawa, IL). Individual PFT test results were exported. PFT parameters, including AUCTOTAL (total area under the curve), FEF50 (forced expiratory flow at 50% FVC), FIF50 (forced inspiratory flow at 50% FVC), FEV1 (forced expiratory volume in 1 second), FVC (forced vital capacity), PEF (peak expiratory flow), and PIF (peak inspiratory flow), were extracted. Additional PFT parameters of interest, including AUCTOTAL/FVC, FEF50/FIF50, FEV1/FVC, FEV1/PEF, and PEF/PIF, were calculated.
Patient reported outcomes were also measured pre- and post-operatively. During routinely scheduled clinic visits, patients were provided with and asked to complete the Voice-Related Quality of Life Survey (V-RQOL), Voice Handicap Index-10 (VHI-10), and Clinical COPD Questionnaire (CCQ). Raw V-RQOL scores were converted to calculated V-RQOL scores prior to analysis.16
RNA Isolation and Quantitative PCR
Gene expression was measured using previously described methods.15 Briefly, RNA was extracted from brush biopsies using the RNeasy Mini Kit (Qiagen, Germantown, MD). cDNA synthesis was performed using the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). Quantitative polymerase chain reaction (qPCR) was performed with technical duplicates using Power SYBR Green PCR Master Mix (Thermo Fisher Scientific, Waltham, MA) in 20 μL reactions with 0.25 μM forward and reverse primer on the StepOnePlus Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA). The following thermal cycling protocol was used: 95° C 10 minutes followed by 40 cycles of 95° C 15 seconds and 60° C 60 seconds. Levels of gene expression were calculated using the AACt method and normalized to GAPDH. Sequences for the gene specific primers used in this study are provided in Table S1.
Protein Isolation and Quantification
Protein quantification was performed as previously described.17 Briefly, protein was extracted from Gelfoam swabs by vortexing in cold saline. Total protein concentration was measured using absorbance at 280 nm on the NanoDrop Spectrophotometer (Thermo Fisher Scientific, Waltham, MA). The concentrations of IL-4, IL-6, IL-17, CCL2, and IFNG were measured using BD CBA Flex Set bead-based immunoassays (BD Biosciences, San Jose, CA) and the Accuri C6 Plus Flow Cytometer (BD Biosciences, San Jose, CA). Target protein concentration was normalized to the total protein concentration.
Statistical Analysis
Unless otherwise indicated, all analyses were performed using R (v3.2.2). For paired variables, the non-parametric Wilcoxon signed rank test was used for statistical testing. For unpaired variables, the non-parametric Wilcoxon rank sum test was used for statistical testing. The Hommel correction was used to calculate adjusted P-values in the setting of multiple testing.18 High and low gene expression subgroups were defined by k-means clustering of stenotic tissue gene expression profiles.
RESULTS
A total of 16 patients were enrolled, and the demographic and clinical characteristics for these individuals are summarized in Table I. All were female and Caucasian, and the mean age at time of surgery was 55.3 ± 12.0 years. The median number of dilations was 2.5 [interquartile range (IQR): 1.0, 7.8], and the median dilation interval was 10.7 months [IQR: 8.0, 16.3]. The median interval between the pre-operative clinic visit and the day of surgery and between the day of surgery and the post-operative clinic visit were 33.0 days [IQR: 15.5, 67.8] and 21.0 days [IQR: 17.0, 38.0], respectively. The mean distance between the start of the stenotic region and the superior aspect of the vocal folds was 14.0 ± 5.9 mm, and the mean length of the stenotic region was 15.4 ± 6.2 mm. The mean percent stenosis prior to dilation was 70.0% ± 12.7% (Cotton-Myer Grade 2), and the mean percent stenosis after dilation was 12.8% ± 6.4% (Cotton-Myer Grade 1).19
TABLE I.
Demographic and Clinical Characteristics.
| Age (years), mean ± SD | 55.3 ± 12.0 |
| Female, n (%) | 16 (100%) |
| Caucasian, n (%) | 16 (100%) |
| BMI, mean ± SD | 30.5 ± 7.6 |
| Total number of dilations, median [IQR] | 2.5 [1, 7.8] |
| Dilation interval (months), median [IQR] | 10.7 [8.0, 16.3] (n = 13) |
| Pre-operative interval (days), median [IQR] | 33.0 [15.5, 67.8] |
| Post-operative interval (days), median [IQR] | 21.0 [17.0, 38.0] |
| Stenosis distance from VF (mm), mean ± SD | 14.0 ± 5.9 |
| Stenosis length (mm), mean ± SD | 15.4 ± 6.2 |
| Stenosis pre-dilation (%), mean ± SD | 70.0 ± 12.7 |
| Stenosis post-dilation (%), mean ± SD | 12.8 ± 6.4 |
BMI = body mass index; IQR = interquartile range; SD = standard deviation; VF = superior aspect of vocal folds.
Pre-dilation and post-dilation PFTs were obtained for all 16 study participants, and summary statistics are shown in Table II. Significant improvements in pulmonary function were evident after dilation, including changes in total area under the curve (AUCTOTAL), forced expiratory flow at 50% forced vital capacity (FEF50), the ratio between forced expiratory volume in 1 second and peak expiratory flow (FEV1/PEF), forced inspiratory flow at 50% forced vital capacity (FIF50), peak expiratory flow (PEF), and peak inspiratory flow (PIF). Patients also demonstrated significant improvements in health-related quality of life, based on changes in self-reported outcome parameters measured using the Voice-Related Quality of Life (V-RQOL), Voice Handicap Index-10 (VHI-10), and Clinical CoPD Questionnaire (CCQ) assessment tools pre- and post-dilation. Patient reported outcome parameters are summarized in Table III.
TABLE II.
Pre- and Post-Dilation PFT Parameters.
| Pre-dilation (mean ± SD) | Post-dilation (mean ± SD) | P-value* | |
|---|---|---|---|
| AUCTOTAL | 9.00 ± 3.71 | 15.28 ± 6.82 | .00006 |
| AUCTOTAL/FVC | 3.49 ± 0.84 | 5.40 ± 1.68 | .00031 |
| FEF50 (L/s) | 2.43 ± 1.05 | 3.73 ± 1.30 | .00015 |
| FEF50/FIF50 | 1.28 ± 0.49 | 1.21 ± 0.41 | .35190 |
| FEV1 (L) | 2.04 ± 0.69 | 2.42 ± 0.53 | .00214 |
| FEV1/FVC | 0.81 ± 0.15 | 0.89 ± 0.09 | .14386 |
| FEV1/PEF (s) | 0.66 ± 0.20 | 0.43 ± 0.09 | .00006 |
| FIF50 (L/s) | 1.88 ± 0.36 | 3.18 ± 1.06 | .00003 |
| FVC (L) | 2.52 ± 0.65 | 2.72 ± 0.47 | .00919 |
| PEF (L/s) | 3.32 ± 1.30 | 5.83 ± 1.58 | .00006 |
| PEF/PIF | 1.47 ± 0.41 | 1.67 ± 0.24 | .00629 |
| PIF (L/s) | 2.23 ± 0.51 | 3.51 ± 0.91 | .00015 |
AUCTOTAL = total area under the curve; FEF50 = forced expiratory flow at 50% FVC; FIF50 = forced inspiratory flow at 50% FVC; FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; PEF = peak expiratory flow; PIF = peak inspiratory flow.
Wilcoxon signed rank test.
TABLE III.
Pre- and Post-Dilation Patient Reported Outcome Parameters.
| Pre-dilation (mean ± SD) | Post-dilation (mean ± SD) | P-value* | |
|---|---|---|---|
| V-RQOL | 82.19 ± 12.68 | 93.75 ± 10.08 | .00205 |
| VHI-10 | 11.57 ± 6.73 | 5.07 ± 5.44 | .00249 |
| CCQTOTAL | 3.09 ± 1.48 | 1.17 ± 0.69 | .00072 |
CCQTOTAL = Clinical COPD Questionnaire Total Score (n = 16); VHI-10 = Voice Handicap Index-10 (n = 14); V-RQOL = Voice-Related Quality of Life Calculated Measure (n = 16).
Wilcoxon signed rank test.
Expression levels of candidate genes in areas of stenosis (Fig. 1A) and adjacent healthy tissue (Fig. 1B) are shown for each individual. There is marked heterogeneity in the patterns of expression across individuals and across tissue types. Certain inflammatory markers, including IL4, IL6, IL13, IL17A, IFNG, TGFB1, TNF, and CCL2, and fibrotic markers, including COL3A1, and αSMA (ACTA2), are relatively upregulated in both stenotic and healthy tissues from a subset of patients. Unsupervised k-means clustering analysis was performed to segregate patients into subgroups based on stenotic tissue gene expression profile. The group of 8 patients including ID_03, ID_05, ID_07, ID_09, ID_12, ID_14, ID_15, and ID_16 is hereafter referred to as the High Gene Expression Subgroup, and the group of the remaining 8 patients including ID_01, ID_02, ID_04, ID_06, ID_08, ID_10, ID_11, and ID_13 is hereafter referred to as the Low Gene Expression Subgroup.
Fig. 1.
Expression of candidate genes in healthy and stenotic tissues in iSGS. Levels of expression of candidate genes were measured by qPCR in intra-operatively collected brush biopsies and presented as a heatmap centered and scaled by column and false colored by column Z-score for (A) stenotic tissue and (B) healthy tissue. ID: numeric identifier for study participant; H: healthy tissue; S: stenotic tissue.
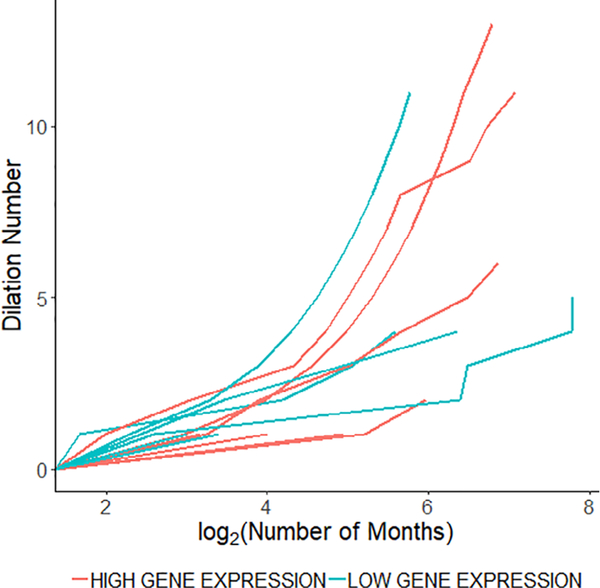
Selected pre- and post-dilation PFT and patient reported outcome parameters are stratified by Gene Expression Subgroup in Figure 2. When comparing pre-dilation to post-dilation within the Low Gene Expression Subgroup, AUCTOTAL, PEF, PIF, CCQTOTAL, VHI-10, and V-RQOL demonstrated statistically significant changes (adjusted P < .05). When comparing pre-dilation to post-dilation within the High Gene Expression Subgroup, AUCTOTAL, PEF, and PIF showed statistically significant differences (adjusted P < .05), whereas CCQTOTAL, VHI-10, and V-RQOL trended towards statistical significance (adjusted P = .06). There was no significant difference between Low and High Gene Expression Subgroups when comparing change in pre- and post-dilation PFT or patient reported outcome parameters (adjusted P > .05). There was also no significant difference in median dilation interval between the two subgroups. After excluding individuals with only one dilation, the median dilation intervals for the High and Low Gene Expression Subgroups were 16.3 [IQR 8.6, 24.7] (n = 7) and 9.4 [IQR 6.6, 12.3] (n = 6), respectively (P = .18). Dilations over time for each individual stratified by gene expression subgroup are shown in Figure 3.
Fig. 2.
PFT and patient reported outcome parameters stratified by gene expression subgroup. Pre- and post-dilation outcomes for High Gene Expression (n = 8) and Low Gene Expression (n = 8) Subgroups. AUCTOTAL: total area under the curve; PEF: peak expiratory flow; PIF: peak inspiratory flow; CCQTOTAL: Clinical COPD Questionnaire Total Score; VHI-10: Voice Handicap Index-10 (High Gene Expression Subgroup n = 7, Low Gene Expression Subgroup n = 7); V-RQOL: Voice-Related Quality of Life Calculated Measure.
Fig. 3.
Dilations over time stratified by gene expression subgroup. Dilation number as a function of time for High Gene Expression Subgroup and Low Gene Expression Subgroup.
At the level of the study population in aggregate, αSMA, CCL2, COL1A1, COL3A1, FN1, IFNG, and TGFB1 were increased in stenotic tissue as compared to healthy tissue (adjusted P < .05), as shown in Figure 4. Sufficient total protein was isolated from 13 patients, and levels of CCL2, IFNG, IL-4, IL-6, and IL-17A protein were measured. As shown in Figure 5, levels of CCL2, IFNG, and IL-6 were elevated in stenotic as compared to healthy tissue (adjusted P < .05). The correlation between gene and protein expression is indistinct, as shown in Figure S1. There are no significant differences between protein expression levels in healthy as compared to stenotic tissue in the High Gene Expression Subgroup (n = 5). In the Low Gene Expression Subgroup (n = 8), however, IL-6 is significantly upregulated (adjusted P < .05), and CCL2 trend towards upregulation (adjusted P = .06), in stenotic tissue as compared to healthy tissue, as shown in Figure S2.
Fig. 4.
Gene expression of candidate biomarkers in iSGS. AU: arbitrary units. Error bars represent standard error. *Adjusted P-value < .05.
Fig. 5.
Protein expression of candidate biomarkers in iSGS. Level of protein expression of candidate targets was measured in intra-operatively collected Gelfoam swabs of stenotic tissue and adjacent healthy tissue (n = 13). Error bars represent standard error. *Adjusted P-value < .05.
DISCUSSION
Consistent with published literature, our study shows that endoscopic balloon dilation leads to significant improvements in pulmonary function. PFTs have been used previously to evaluate postoperative outcomes in patients with iSGS undergoing balloon dilation, and AUCTOTAL/FVC, PEF, PIF, FEV1/PEF, and FIF50 have been reported to demonstrate improvement.20–22 In our cohort, these are among the parameters with the most statistically significant changes pre- and post-dilation. Of note, no statistically significant difference was observed for FEV1/FVC, which is primarily utilized in the evaluation of lower as opposed to upper airway obstruction.23 Patient reported outcomes, as measured by V-RQOL,24 VHI-10,25 CCQ,26 have also been studied in the setting of endoscopic treatment for laryngotracheal stenosis. Using these measures, our iSGS cohort also shows significant postoperative improvement.
We next evaluated expression of several candidate genes. Previous studies have shown that extracellular matrix components, including type I collagen, type III collagen, and fibronectin,13 and inflammatory components, including y-interferon and TGF-p,15 are increased in iSGS. In this investigation, inflammatory cytokines and chemoattractants (CCL2, IFNG, and TGFB1) and markers of myofibroblast activity and extracellular matrix deposition (αSMA, COL1A1, COL3A1, and FN1) were transcriptionally upregulated in stenotic tissues. At the translational level, CCL2, IFNG, and IL-6 were upregulated in the stenotic tissues. We note that gene and protein expression are not strongly correlated in our cohort, though evidence suggests that a correlation may not be expected due in part to a temporal delay between mRNA and protein production and the dynamic nature of the biological process.27
We also showed that there was heterogeneity in expression profile across individual iSGS patients. In our cohort, there appeared to be two transcriptionally distinct subgroups of patients, with relatively higher or lower expression of inflammatory and fibrotic markers. The set of upregulated targets suggests the involvement of known pro-fibrotic and inflammatory pathways. Fibrocytes and other pro-fibrotic inflammatory cells may be chemotactically recruited by CCL2, and subsequently, activated myofibroblasts may upregulate alpha-smooth muscle actin and secrete extracellular matrix components, leading to tissue fibrosis and remodeling in regions of stenosis.12 IFN-y, generated as a component of the Th1 immune response, is an antifibrotic agent that may be produced secondary to the disease process and play a modulatory role, attenuating scar formation, in iSGS.12 Our findings indicate that pro-fibrotic and inflammatory pathways are disproportionately active, suggesting a potential mechanistic basis for iSGS pathogenesis and implicating components within these pathways, specifically CCL2 and IFNG, as candidate biomarkers. Investigation into whether gene expression profile could predict clinical response, as represented by dilation interval, revealed a 16.3 month interval for the High Gene Expression Subgroup and a 9.4 month interval for the Low Gene Expression Subgroup; however, the difference between the groups was not significant.
The identification of biomarkers that may be serially monitored is an issue of clinical significance for patients with iSGS, who have varied trajectories of disease progression and degrees of response to endoscopic balloon dilation. Identifying and validating a biomarker in this setting would facilitate the identification of patients who are at high risk for poor response to initial surgical management and for whom more aggressive disease modifying procedures, such as cricotracheal resection, endoscopic resection, or laryngotracheoplasty, may play a role earlier in a patient’s course. Biomarkers in stenotic laryngotracheal tissues are most likely to reflect pathophysiology. Our study made a preliminary attempt to identify biomarkers in surface level laryngotracheal tissues. Surface level tissues have the benefit of ease of collection. However, they may not fully recapitulate the molecular changes that occur in deeper tissues. Previous studies using molecular and immunohistochemical techniques interrogated IL-17A expression in tissue biopsies from mucosal scar (rather than surface sampling) and showed iSGS scar IL-17A expression was increased compared with healthy subjects and iatrogenic post-intubation subglottic scar.13 This difference in methodology with depth of tissue sampling may account for the difference in IL-17A findings between our study and the published literature. The §y T cells are the primary producers of IL-17A, and as they reside in deeper tissues,13 they may not have been robustly sampled in our study. Serum biomarkers, though distant from the tissues of interest, offer advantages in ease of sampling on a routine basis in the clinic and also merit investigation. Potential markers in peripheral blood mononuclear cells, for example, have been previously reported in iSGS.1
Our study has certain limitations. By design, this was a pilot study with a small sample size conducted with a single surgeon at a single institution. Additionally, bias is potentially introduced by identifying biomarkers using a candidate target strategy. Unbiased transcriptome- and proteome-wide approaches would afford additional insights. Our study does suggest, however, that iSGS patients may be subgrouped in an unbiased manner based on transcriptional profile. A dedicated investigation to define the clinical prognostic value of these transcriptionally defined subgroups may be of future interest. In this pilot, we validated the gene and protein quantification methods that could be utilized in such a study. We propose that AUCTOTAL, PEF, and PIF are metrics that may be used to define the degree of functional response and that CCQTOTAL, VHI-10, and V-RQOL may be used to assess patient reported outcomes. To minimize measurement bias, the intervals of time between the day of surgery and the pre- and post-operative clinic visits must be prespecified. Our pilot study was limited by variability in the lengths of these intervals, as clinic visits were scheduled based on patient convenience. Regular surveillance of functional and subjective markers of response to surgery may also be considered at multiple post-operative intervals. Furthermore, there are no published comparisons of the global transcriptional profile for the subglottis vs the trachea, and a future study of expression in the healthy subglottis and proximal trachea would help to provide normative data. Finally, there is potential to apply the methods used herein to study iatrogenic subglottic stenosis.
CONCLUSION
Patients with iSGS undergoing endoscopic balloon dilation demonstrate distinct gene and protein expression profiles in stenotic and healthy laryngotracheal tissues. Pro-inflammatory and pro-fibrotic pathways are significantly upregulated in areas of stenosis. Molecular components in these pathways, specifically CCL2 and IFNG, may serve as disease biomarkers.
Supplementary Material
Acknowledgments
Funding and Conflicts of Interest: Research in this publication was supported by National Institute on Deafness and Other Communication Disorders (NIDCD) of the National Institutes of Health (NIH) under grants 1K23DC014082 (a.t.h.) and 1R21DC01722501 (a.t.h.). The North American Airway Collaborative is supported through the Patient Centered Outcomes Research Institute (PCORI) grant number 1409-22214 (a.g.). This study was also supported by the Triological Society and American College of Surgeons (ACS) (a.t.h.). The content is solely the responsibility of the authors and does not represent the official views of the NIH, TRIO, theACS, or PCORI. The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Footnotes
Additional supporting information may be found in the online version of this article.
Contributor Information
Melissa M. Liu, Department of Otolaryngology-Head and Neck Surgery, Johns Hopkins University School of Medicine, Baltimore, Maryland, U.S.A..
Kevin M. Motz, Department of Otolaryngology-Head and Neck Surgery, Johns Hopkins University School of Medicine, Baltimore, Maryland, U.S.A..
Michael K. Murphy, Department of Otolaryngology & Communication,State University of New York Upstate Medical University, Syracuse, New York, U.S.A..
Linda X. Yin, Department of Otorhinolaryngology-Head and Neck Surgery, Mayo Clinic, Rochester, Minnesota, U.S.A..
Dacheng Ding, Department of Otolaryngology-Head and Neck Surgery, Johns Hopkins University School of Medicine, Baltimore, Maryland, U.S.A..
Alexander Gelbard, Department of Otolaryngology, Vanderbilt University School of Medicine, Nashville, Tennessee, U.S.A.; The North American Airway Collaborative, U.S.A..
Alexander T. Hillel, Department of Otolaryngology-Head and Neck Surgery, Johns Hopkins University School of Medicine, Baltimore, Maryland, U.S.A.; The North American Airway Collaborative, U.S.A.
BIBLIOGRAPHY
- 1.Gelbard A, Katsantonis NG, Mizuta M, et al. Molecular analysis of idiopathic subglottic stenosis for mycobacterium species. Laryngoscope 2017; 127:179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blumin JH, Johnston N. Evidence of extraesophageal reflux in idiopathic subglottic stenosis. Laryngoscope 2011;121:1266–1273. [DOI] [PubMed] [Google Scholar]
- 3.Valdez TA, Shapshay SM. Idiopathic subglottic stenosis revisited. Ann Otol Rhinol Laryngol 2002;111:690–695. [DOI] [PubMed] [Google Scholar]
- 4.Menapace DC, Modest MC, Ekbom DC, Moore EJ, Edell ES, Kasperbauer JL. Idiopathic subglottic stenosis: long-term outcomes of open surgical techniques. Otolaryngol Head Neck Surg 2017;156:906–911. [DOI] [PubMed] [Google Scholar]
- 5.Maldonado F, Loiselle A, Depew ZS, et al. Idiopathic subglottic stenosis: an evolving therapeutic algorithm. Laryngoscope 2014;124:498–503. [DOI] [PubMed] [Google Scholar]
- 6.Sandhu GS, Nouraei SAR. Idiopathic subglottic stenosis In: Sandhu GS, SAR N, eds. Laryngeal and Tracheobronchial Stenosis: San Diego, CA: Plural Publishing; 2016:311–326. [Google Scholar]
- 7.Franco RA, Husain I, Reder L, Paddle P. Awake serial intralesional steroid injections without surgery as a novel targeted treatment for idiopathic subglottic stenosis. Laryngoscope 2018;128:610–617. [DOI] [PubMed] [Google Scholar]
- 8.Lee KH, Rutter MJ. Role of balloon dilation in the management of adult idiopathic subglottic stenosis. Ann Otol Rhinol Laryngol 2008;117:81–84. [DOI] [PubMed] [Google Scholar]
- 9.Gelbard A, Donovan DT, Ongkasuwan J, et al. Disease homogeneity and treatment heterogeneity in idiopathic subglottic stenosis. Laryngoscope 2016;126:1390–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simpson GT, Stuart Strong MM, Healy GB, Shapshay SM, Vaughan CW. Predictive factors of success or failure in the endoscopic management of laryngeal and tracheal stenosis. Ann Otol Rhinol Laryngol 1982;91: 384–388. [DOI] [PubMed] [Google Scholar]
- 11.Rockey DC, Bell PD, Hill JA. Fibrosis—a common pathway to organ injury and failure. N Engl J Med 2015;372:1138–1149. [DOI] [PubMed] [Google Scholar]
- 12.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med 2012;18:1028–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gelbard A, Katsantonis NG, Mizuta M, et al. Idiopathic subglottic stenosis is associated with activation of the inflammatory IL-17A/IL-23 axis. Laryngoscope 2016;126:E356–E361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samad I, Phelps M, Pandian V, et al. High-flow oxygen, a primary oxygenation technique for endolaryngeal airway surgery: our experience with 10 patients. Clin Otolaryngol 2016;41:286–289. [DOI] [PubMed] [Google Scholar]
- 15.Motz KM, Yin LX, Samad I, et al. Quantification of inflammatory markers in laryngotracheal stenosis. Otolaryngol Head Neck Surg 2017;157: 466–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hogikyan ND, Sethuraman G. Validation of an instrument to measure voice-related quality of life (V-RQOL). J Voice 1999;13:557–569. [DOI] [PubMed] [Google Scholar]
- 17.Branski RC, Sandulache VC, Dohar JE, Hebda PA. Mucosal wound healing in a rabbit model of subglottic stenosis: biochemical analysis of secretions. Arch Otolaryngol Head Neck Surg 2005;131:153–157. [DOI] [PubMed] [Google Scholar]
- 18.Hommel G A stagewise rejective multiple test procedure based on a modified bonferroni test. Biometrika 1988;75:383–386. [Google Scholar]
- 19.Myer CM, O’Connor DM, Cotton RT. Proposed grading system for subglottic stenosis based on endotracheal tube sizes. Ann Otol Rhinol Laryngol 1994;103:319–323. [DOI] [PubMed] [Google Scholar]
- 20.Kraft SM, Sykes K, Palmer A, Schindler J. Using pulmonary function data to assess outcomes in the endoscopic management of subglottic stenosis. Ann Otol Rhinol Laryngol 2015;124:137–142. [DOI] [PubMed] [Google Scholar]
- 21.Nouraei SAR, Nouraei SM, Patel A, et al. Diagnosis of laryngotracheal stenosis from routine pulmonary physiology using the expiratory disproportion index. Laryngoscope 2013;123:3099–3104. [DOI] [PubMed] [Google Scholar]
- 22.Nouraei SM, Franco RA, Dowdall JR, et al. Physiology-based minimum clinically important difference thresholds in adult Laryngotracheal stenosis. Laryngoscope 2014;124:2313–2320. [DOI] [PubMed] [Google Scholar]
- 23.Hansen JE, Sun XG, Wasserman K. Spirometric criteria for airway obstruction: use percentage of FEV1/FVC ratio below the fifth percentile, not < 70%. Chest 2007;131:349–355. [DOI] [PubMed] [Google Scholar]
- 24.Hillel AT, Karatayli-Ozgursoy S, Benke JR, et al. Voice quality in laryngotracheal stenosis. Ann Otol Rhinol Laryngol 2015;124: 413–418. [DOI] [PubMed] [Google Scholar]
- 25.Hatcher JL, Dao AM, Simpson CB. Voice outcomes after endoscopic treatment of laryngotracheal stenosis. Ann Otol Rhinol Laryngol 2015;124: 235–239. [DOI] [PubMed] [Google Scholar]
- 26.Samad I, Akst L, Karatayli-Ôzgürsoy S, et al. Evaluation of dyspnea outcomes after endoscopic airway surgery for laryngotracheal stenosis. JAMA Otolaryngol Head Neck Surg 2016;142:1075–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Beyer A, Aebersold R. On the dependency of cellular protein levels on mRNA abundance. Cell 2016;165:535–550. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.