Abstract
Background:
Higher total 25-hydroxyvitamin D [25(OH)D] levels are associated with improved survival among colorectal cancer (CRC) patients, but the relationships between circulating vitamin D binding protein (VDBP), bioavailable or free 25(OH)D, and CRC survival remain unknown.
Methods:
We examined the associations between prediagnostic plasma levels of vitamin D-related markers and survival among 603 white participants diagnosed with CRC from 2 prospective US cohorts. Plasma VDBP and total 25(OH)D were directly measured, while bioavailable and free 25(OH)D were calculated using a validated formula based on total 25(OH)D, VDBP, and albumin levels. Cox proportional hazards regression was used to estimate hazard ratios (HRs) for overall and CRC-specific mortality, adjusted for other prognostic markers and potential confounders.
Results:
Higher VDBP levels were associated with improved overall (Ptrend = 0.001) and CRC-specific survival (Ptrend = 0.02). Compared to patients in the lowest quartile, those in the highest quartile of VDBP had a multivariable HR of 0.58 (95% confidence interval [CI], 0.41–0.80) for overall mortality and 0.58 (95% CI, 0.37–0.91) for CRC-specific mortality. The results remained similar after further adjustment for total 25(OH)D levels. In contrast, neither bioavailable nor free 25(OH)D levels were associated with overall or CRC-specific mortality (all Ptrend > 0.15).
Conclusion:
Prediagnostic circulating concentrations of VDBP were positively associated with survival among CRC patients.
Impact:
The clinical utility of VDBP as a prognostic marker warrants further exploration, as well as research into underlying mechanisms of action.
Introduction
Vitamin D is hypothesized to play a role in the development and progression of colorectal cancer (CRC). Colon cancer cells express vitamin D receptor (VDR) (1, 2) and 1-α-hydroxylase (3), which converts the main circulating form of vitamin D, 25-hydroxyvitamin D [25(OH)D], into the active metabolite, calcitriol [1,25(OH)2D]. Binding of 1,25(OH)2D to VDR leads to several anti-cancer effects, including increased cell differentiation and apoptosis (4, 5) and decreased proliferation (6), angiogenesis (7, 8), and metastasis (9, 10).
Vitamin D binding protein (VDBP), also known as the group-specific component, is the major vitamin D carrier protein. Approximately 88% of circulating 25(OH)D is bound to VDBP, while 12% of 25(OH)D is loosely bound to albumin, leaving very little in the free form (11, 12). Experimental studies demonstrate that VDBP has important biological functions that may inhibit tumor growth, such as actin scavenging, macrophage activation, and chemotaxis (13). A meta-analysis of 28 studies examined VDBP levels in relation to the overall risk of multiple cancers including CRC and found borderline decreased risk in individuals with higher VDBP levels (odds ratio, 0.75; 95% confidence interval [CI], 0.56–1.00) (14). Previous studies did not find an association between VDBP levels and CRC risk (15–17). However, it is unknown whether prediagnostic VDBP levels influence survival outcomes among CRC patients.
The “free hormone hypothesis” postulates that the bound fraction of a hormone is not available to target cells for signaling and gene regulation (18), suggesting that free 25(OH)D and albumin-bound 25(OH)D, which can dissociate during tissue perfusion, may be more biologically active than VDBP-bound 25(OH)D. However, more recent studies found that the 25(OH)D-VDBP complex can also be internalized into cells by transportation of megalin, an endocytic receptor that is expressed in epithelial cells of several organs including colon (19, 20). Although the link between higher total 25(OH)D levels and better CRC survival has been well documented (21–27), the association between bioavailable or free 25(OH)D levels and CRC survival is unknown.
Building upon our prior analyses of total 25(OH)D levels and CRC survival (21), we further investigated the associations of prediagnostic plasma levels of VDBP, bioavailable 25(OH)D, and free 25(OH)D with survival among participants diagnosed with CRC from 2 prospective US cohorts, the Nurses’ Health Study (NHS) and the Health Professionals Follow-Up Study (HPFS).
Materials and Methods
Study population
In 1976, NHS was initiated when 121,700 US female registered nurses aged 30 to 55 years responded to a mailed questionnaire on risk factors for cancer and cardiovascular disease (28). Blood samples were collected from 32,826 NHS participants between 1989 and 1990. In 1986, HPFS was established when 51,529 US male dentists, optometrists, osteopaths, podiatrists, pharmacists, and veterinarians aged 40 to 75 years completed a mailed questionnaire on health-related behaviors and medical history (29). Blood samples were collected from 18,225 HPFS participants between 1993 and 1995. In both cohorts, participants received biennial questionnaires to update information on lifestyle factors and medical diagnoses. A high follow-up rate of more than 90% was achieved in both cohorts.
When an incident case of CRC was identified rom self-report or during follow-up of participant deaths, we asked permission to obtain hospital records and pathology reports. Physicians who were blinded to exposure data reviewed medical records, death certificates, or cancer registry data to ascertain the diagnosis of CRC and record information on important tumor characteristics. We have estimated that 96–97% of patients were captured by using these methods (30, 31).
Patients diagnosed with CRC after the date of blood collection through December 2011 were eligible for the current study. Patients were excluded if they were non-white (due to inability of the monoclonal assay to accurately measure VDBP in non-whites) or had reported any cancer (other than nonmelanoma skin cancer) prior to CRC diagnosis. Patients who were diagnosed with CRC within 2 years after blood collection were also excluded to minimize bias associated with presence of occult cancer. Among 627 eligible patients with total 25(OH)D levels, 603 had available VDBP levels, from which bioavailable and free 25(OH)D were calculated.
The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and the Harvard T.H. Chan School of Public Health, and those of participating registries as required. All participants provided written informed consent for the researchers to access their medical records. The study was conducted in concordance with the International Ethical Guidelines for Biomedical Research Involving Human Subjects (CIOMS).
Measurement of plasma VDBP, total 25(OH)D, and albumin
Blood samples were shipped by overnight courier in chilled containers. On receipt, bloods were centrifuged, aliquoted, and stored in continuously-monitored liquid nitrogen freezers at -130°C or below. More than 95% of the blood samples arrived in our laboratory within 24 hours of phlebotomy.
Plasma VDBP was measured at Heartland Assays in 2013 by a monoclonal antibody-based, enzyme-linked immunosorbent assay (ELISA) (R&D Systems). Plasma total 25(OH)D was measured in the laboratory of Dr. Bruce Hollis (The Medical University of South Carolina, Charleston, SC) and Heartland Assays (Ames, IA) by radioimmunoassay (32). Plasma albumin was measured by a colorimetric assay (Roche Diagnostics) in the laboratory of Dr. Nader Rifai (Children’s Hospital, Boston, MA). Although all samples were assayed at the same laboratory, cases identified from different questionnaires were assayed in different batches, which are detailed in Supplementary Table S1. The mean intra-assay coefficients of variation for VDBP, total 25(OH)D, and albumin were ≤14.8%, ≤11.8%, and ≤4.0%, respectively.
Calculation of plasma bioavailable and free 25(OH)D
Bioavailable and free 25(OH)D were calculated by the following equations:
where KaAlbumin is the affinity of albumin for 25(OH)D (6×105), KaVDBP is the affinity of VDBP for 25(OH)D (7×108) (11), and all concentrations are in mol/L (18).
Using the TaqMan OpenArray SNP Genotyping Platform (Applied Biosystems), we successfully genotyped 2 common single-nucleotide polymorphisms (SNPs) in VDBP, rs4588 and rs7041, for 548 patients of our study population. The 2 SNPs give rise to 3 predominant haplotypes: GC1F, GC1S, and GC2. Regarding whether the affinity of VDBP for 25(OH)D is affected by these haplotypes, 1 study found the affinity of GC1F to be 4 times higher than that of GC2 and double that of GC1S (33), while 3 studies demonstrated no difference in the affinity (34–36). Therefore, we calculated bioavailable and free 25(OH)D using a constant affinity of VDBP for 25(OH)D, but our conclusions were essentially unchanged by using the genotype-specific affinities.
Mortality outcome
Patients were observed until date of death or last follow-up (June 2014 for NHS; January 2014 for HPFS), whichever came first. Ascertainment of deaths included reporting by family or postal authorities, and interrogation of names of persistent nonrespondents in the National Death Index, which has been shown to capture approximately 98% of deaths (37). The primary outcome was overall mortality, and the secondary outcome was CRC-specific mortality. Because deaths from CRC mostly occur within the first 5 years after diagnosis, we evaluated 5-year overall mortality as an additional outcome by censoring patients who were alive at the end of the first 5 years.
Covariates
Cancer stage, grade of tumor differentiation, location of primary tumor, and year of diagnosis (as a surrogate for treatment) were extracted from medical records. Body mass index (BMI) and physical activity were obtained from the questionnaire returned before blood collection.
Statistical analyses
Plasma vitamin D-related markers were categorized into quartiles by batch (Supplementary Table S1) and analyzed (21). Follow-up time was calculated from CRC diagnosis to death or censoring. Cox proportional hazards regression was used to calculate hazard ratios (HRs) and 95% CIs for 3 outcomes: overall mortality, CRC-specific mortality, and 5-year overall mortality. We tested for a linear trend across quartiles using an ordinal variable. A priori, we included other prognostic markers and potential confounders in multivariable models, including age at diagnosis, sex, BMI, physical activity, cancer stage, grade of tumor differentiation, location of primary tumor, and year of diagnosis. We additionally adjusted for season of blood collection when the exposure was total, bioavailable, or free 25(OH)D. Interaction between VDBP and the potential effect modifier was assessed by entering in the model the cross product of the quartile of the biomarker and the stratification variable, evaluated by the likelihood ratio test. The Cox models were tested for and met the proportional hazards assumption. All analyses were performed with SAS software, version 9.4 (SAS Institute, Cary, NC). All P values are 2 sided.
Results
Baseline characteristics
Plasma samples were collected at a median of 9.3 years (interquartile range [IQR]: 6.1–13.3 years) before CRC diagnosis. The median VDBP level was 250 μg/mL (IQR: 175–311 μg/mL) and the median total 25(OH)D level was 27.3 ng/mL (IQR: 20.4–33.0 ng/mL), and both were modestly correlated (r = 0.12; P < 0.01; Supplementary Table S2). The median bioavailable 25(OH)D level was 3.36 ng/mL (IQR: 2.26–4.66 ng/mL). As expected, bioavailable 25(OH)D levels were positively correlated with total 25(OH)D (r = 0.59; P < 0.0001) and albumin levels (r = 0.23; P < 0.0001) and negatively correlated with VDBP levels (r = −0.67; P < 0.0001). The median free 25(OH)D level was 8.25 pg/mL (IQR, 5.66–11.19 pg/mL), and was nearly perfectly correlated with bioavailable 25(OH)D levels (r = 0.99; P < 0.0001); thus, we focused our analyses on bioavailable 25(OH)D that was much more abundant in the circulation.
Patient characteristics were well balanced by quartile of VDBP, except that patients with higher VDBP levels had a lower BMI (Table 1). Patients with higher total 25(OH)D levels had a lower BMI and higher physical activity, compared to those with lower levels (Supplementary Table S3). In addition, patients with higher total or bioavailable 25(OH)D levels were more likely to have their blood collected in the summer or fall.
Table 1.
Baseline characteristics among colorectal cancer patients by quartile of plasma vitamin D binding protein
|
Characteristic |
Vitamin D binding protein | |||
|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |
| No. of patients | 149 | 151 | 152 | 151 |
| Age at blood collection, mean (SD), years | 61.2 (7.8) | 62.3 (8.1) | 60.9 (8.3) | 60.9 (8.5) |
| Age at diagnosis, mean (SD), years | 71.3 (7.6) | 71.9 (8.5) | 71.0 (8.4) | 70.9 (8.8) |
| Time from blood collection to diagnosis, mean (SD), years | 10.1 (4.9) | 9.7 (4.6) | 10.1 (4.7) | 9.9 (5.1) |
| Sex, No. (%) | ||||
| Female | 89 (59.7) | 89 (58.9) | 90 (59.2) | 89 (58.9) |
| Male | 60 (40.3) | 62 (41.1) | 62 (40.8) | 62 (41.1) |
| Body mass index, mean (SD), kg/m2 | 26.9 (5.1) | 25.8 (3.7) | 25.6 (4.3) | 25.2 (3.6) |
| Physical activity, mean (SD), MET-h/wk | 22.1 (21.0) | 26.4 (36.8) | 24.5 (33.2) | 25.7 (29.8) |
| Cancer stage, No. (%) | ||||
| I | 39 (26.2) | 40 (26.5) | 39 (25.7) | 41 (27.2) |
| II | 39 (26.2) | 37 (24.5) | 34 (22.4) | 38 (25.2) |
| III | 33 (22.1) | 30 (19.9) | 30 (19.7) | 31 (20.5) |
| IV | 16 (10.7) | 21 (13.9) | 30 (19.7) | 21 (13.9) |
| Unknown | 22 (14.8) | 23 (15.2) | 19 (12.5) | 20 (13.2) |
| Grade of tumor differentiation, No. (%) | ||||
| Well differentiated | 16 (10.7) | 14 (9.3) | 17 (11.2) | 17 (11.3) |
| Moderately differentiated | 86 (57.7) | 90 (59.6) | 89 (58.6) | 84 (55.6) |
| Poorly differentiated | 24 (16.1) | 19 (12.6) | 18 (11.8) | 25 (16.6) |
| Unknown | 23 (15.4) | 28 (18.5) | 28 (18.4) | 25 (16.6) |
| Location of primary tumor, No. (%) | ||||
| Proximal colon | 66 (44.3) | 57 (37.7) | 75 (49.3) | 72 (47.7) |
| Distal colon | 42 (28.2) | 43 (28.5) | 36 (23.7) | 42 (27.8) |
| Rectum | 32 (21.5) | 38 (25.2) | 33 (21.7) | 27 (17.9) |
| Unknown | 9 (6.0) | 13 (8.6) | 8 (5.3) | 10 (6.6) |
| Year of diagnosis, No. (%) | ||||
| 1991–2000 | 68 (45.6) | 78 (51.7) | 66 (43.4) | 67 (44.4) |
| 2001–2011 | 81 (54.4) | 73 (48.3) | 86 (56.6) | 84 (55.6) |
| Season of blood collection, No. (%) | ||||
| Summer (June, July, August) | 45 (30.2) | 46 (30.5) | 48 (31.6) | 53 (35.1) |
| Fall (September, October, November) | 41 (27.5) | 47 (31.1) | 43 (28.3) | 41 (27.2) |
| Winter (December, January, February) | 33 (22.1) | 26 (17.2) | 28 (18.4) | 26 (17.2) |
| Spring (March, April, May) | 30 (20.1) | 32 (21.2) | 33 (21.7) | 31 (20.5) |
Abbreviations: MET, metabolic equivalent; SD, standard deviation.
Causes of death
The median time of follow-up among patients who were alive at the end of follow-up was 12.4 years (IQR: 8.0–15.3 years). During the follow-up, we documented 328 deaths, 187 (57.0%) of which were due to CRC. Non-CRC causes of death included other malignancies (n = 26), cardiovascular disease (n = 33), neurological disorders (n = 21), cerebrovascular disease (n = 13), respiratory disease (n = 12), and other or unknown reasons (n = 36). A summary of causes of death by follow-up period after diagnosis is presented in Supplementary Table S4. Of the 187 deaths due to CRC, 163 (87.2%) occurred within the first 5 years after diagnosis.
Association between prediagnostic VDBP levels and patient survival
Higher VDBP levels were significantly associated with improved overall (Ptrend = 0.001) and CRC-specific survival (Ptrend = 0.02) (Table 2). Compared to patients in the lowest quartile, those in the highest quartile of VDBP had a multivariable HR of 0.58 (95% CI, 0.41–0.80) for overall mortality and 0.58 (95% CI, 0.37–0.91) for CRC-specific mortality. The HRs were not materially changed after further adjustment for total 25(OH)D levels. In addition, higher VDBP levels were associated with improved 5-year overall survival (Ptrend = 0.001), with a multivariable HR of 0.50 (95% CI, 0.32–0.76) comparing extreme quartiles.
Table 2.
Hazard ratios for overall mortality, colorectal cancer-specific mortality, and 5-year overall mortality among colorectal cancer patients by quartile of plasma vitamin D binding protein
| Hazard ratio (95% confidence interval) | Ptrend | ||||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||
| Mean (SD), μg/mL | 125.2 (41.2) | 213.5 (34.8) | 274.6 (46.6) | 383.5 (79.9) | |
| No. of patients | 149 | 151 | 152 | 151 | |
| Overall mortality | |||||
| No. of events | 81 | 84 | 76 | 70 | |
| Base modela | Referent | 0.93 (0.68–1.26) | 0.89 (0.65–1.21) | 0.76 (0.55–1.04) | 0.08 |
| Multivariable modelb | Referent | 0.77 (0.57–1.06) | 0.69 (0.50–0.96) | 0.58 (0.41–0.80) | 0.001 |
| Model further adjusted for total 25(OH)Dc | Referent | 0.78 (0.57–1.06) | 0.70 (0.50–0.96) | 0.57 (0.41–0.79) | 0.001 |
| Colorectal cancer-specific mortality | |||||
| No. of events | 45 | 48 | 50 | 39 | |
| Base modela | Referent | 1.02 (0.68–1.53) | 1.08 (0.72–1.62) | 0.80 (0.52–1.23) | 0.39 |
| Multivariable modelb | Referent | 0.76 (0.50–1.15) | 0.73 (0.48–1.11) | 0.58 (0.37–0.91) | 0.02 |
| Model further adjusted for total 25(OH)Dc | Referent | 0.75 (0.49–1.15) | 0.73 (0.48–1.12) | 0.56 (0.36–0.88) | 0.02 |
| 5-year overall mortality | |||||
| No. of events | 53 | 54 | 51 | 39 | |
| Base modela | Referent | 0.96 (0.66–1.40) | 0.95 (0.65–1.40) | 0.68 (0.45–1.02) | 0.08 |
| Multivariable modelb | Referent | 0.74 (0.50–1.10) | 0.68 (0.46–1.01) | 0.50 (0.32–0.76) | 0.001 |
| Model further adjusted for total 25(OH)Dc | Referent | 0.74 (0.50–1.09) | 0.69 (0.46–1.02) | 0.49 (0.32–0.75) | 0.001 |
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; SD, standard deviation.
Adjusted for age at diagnosis (continuous).
Addtionally adjusted for sex, body mass index (continuous), physical activity (continuous), cancer stage (I to IV or unknown), grade of tumor differentiation (well differentiated, moderately differentiated, poorly differentiated, unknown), location of primary tumor (proximal colon, distal colon, rectum, unknown), and year of diagnosis (continuous).
Additionally adjusted for total 25-hydroxyvitamin D levels (quartiles).
To further address concerns about the possible influence of occult cancer on VDBP levels, we performed sensitivity analyses by excluding patients who developed CRC within 3, 4, and 5 years after blood collection, respectively. Although statistical power was diminished, the association between VDBP levels and patient survival remained largely unchanged (Table 3).
Table 3.
Multivariable hazard ratios for overall mortality, colorectal cancer-specific mortality, and 5-year overall mortality among colorectal cancer patients by quartile of plasma vitamin D binding protein and time interval from blood collection to diagnosis
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | Ptrend | |||||
|---|---|---|---|---|---|---|---|---|---|
| No. of patients/events | HR (95% CI)a | No. of patients/events | HR (95% CI)a | No. of patients/events | HR (95% CI)a | No. of patients/events | HR (95% CI)a | ||
| Overall mortality | |||||||||
| ≥2 years | 149/81 | Referent | 151/84 | 0.77 (0.57–1.06) | 152/76 | 0.69 (0.50–0.96) | 151/70 | 0.58 (0.41–0.80) | 0.001 |
| ≥3 years | 138/72 | Referent | 141/80 | 0.83 (0.60–1.15) | 141/71 | 0.72 (0.51–1.01) | 140/61 | 0.61 (0.43–0.87) | 0.004 |
| ≥4 years | 130/66 | Referent | 131/74 | 0.84 (0.59–1.19) | 134/65 | 0.72 (0.51–1.03) | 131/58 | 0.63 (0.44–0.91) | 0.01 |
| ≥5 years | 124/60 | Referent | 125/71 | 0.87 (0.61–1.24) | 125/59 | 0.77 (0.53–1.11) | 125/56 | 0.68 (0.47–1.00) | 0.04 |
| Colorectal cancer-specific mortality | |||||||||
| ≥2 years | 149/45 | Referent | 151/48 | 0.76 (0.50–1.15) | 152/50 | 0.73 (0.48–1.11) | 151/39 | 0.58 (0.37–0.91) | 0.02 |
| ≥3 years | 138/40 | Referent | 141/45 | 0.76 (0.49–1.19) | 141/46 | 0.73 (0.46–1.13) | 140/33 | 0.60 (0.37–0.96) | 0.04 |
| ≥4 years | 130/35 | Referent | 131/42 | 0.77 (0.48–1.24) | 134/41 | 0.74 (0.46–1.19) | 131/30 | 0.58 (0.35–0.97) | 0.05 |
| ≥5 years | 124/35 | Referent | 125/38 | 0.70 (0.43–1.14) | 125/37 | 0.76 (0.47–1.23) | 125/29 | 0.58 (0.35–0.98) | 0.07 |
| 5-year overall mortality | |||||||||
| ≥2 years | 149/53 | Referent | 151/54 | 0.74 (0.50–1.10) | 152/51 | 0.68 (0.46–1.01) | 151/39 | 0.50 (0.32–0.76) | 0.001 |
| ≥3 years | 138/46 | Referent | 141/51 | 0.79 (0.52–1.20) | 141/47 | 0.71 (0.47–1.08) | 140/35 | 0.54 (0.34–0.85) | 0.007 |
| ≥4 years | 130/42 | Referent | 131/48 | 0.78 (0.50–1.20) | 134/43 | 0.71 (0.46–1.10) | 131/32 | 0.50 (0.31–0.80) | 0.005 |
| ≥5 years | 124/42 | Referent | 125/45 | 0.72 (0.46–1.13) | 125/39 | 0.71 (0.46–1.12) | 125/30 | 0.47 (0.29–0.77) | 0.004 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
Adjusted for age at diagnosis (continuous), sex, body mass index (continuous), physical activity (continuous), cancer stage (I to IV or unknown), grade of tumor differentiation (well differentiated, moderately differentiated, poorly differentiated, unknown), location of primary tumor (proximal colon, distal colon, rectum, unknown), and year of diagnosis (continuous).
We next evaluated the associations between 2 VDBP polymorphisms and patient survival. Neither rs7041 nor rs4588 was significantly associated with overall or CRC-specific survival (P ≥ 0.08; Supplementary Table S5). In models additionally adjusted for these polymorphisms, the significant association between VDBP levels and patient survival remained unchanged (Ptrend = 0.001 and 0.008 for overall and CRC-specific mortality, respectively).
The association of VDBP levels with overall and CRC-specific survival was examined across strata of potential effect modifiers, including age at diagnosis, time from blood collection to diagnosis, sex, BMI, physical activity, cancer stage, grade of tumor differentiation, location of primary tumor, year of diagnosis, and total 25(OH)D levels, and remained largely unchanged in most subgroups (Pinteraction ≥ 0.18; Fig. 1).
Figure 1.
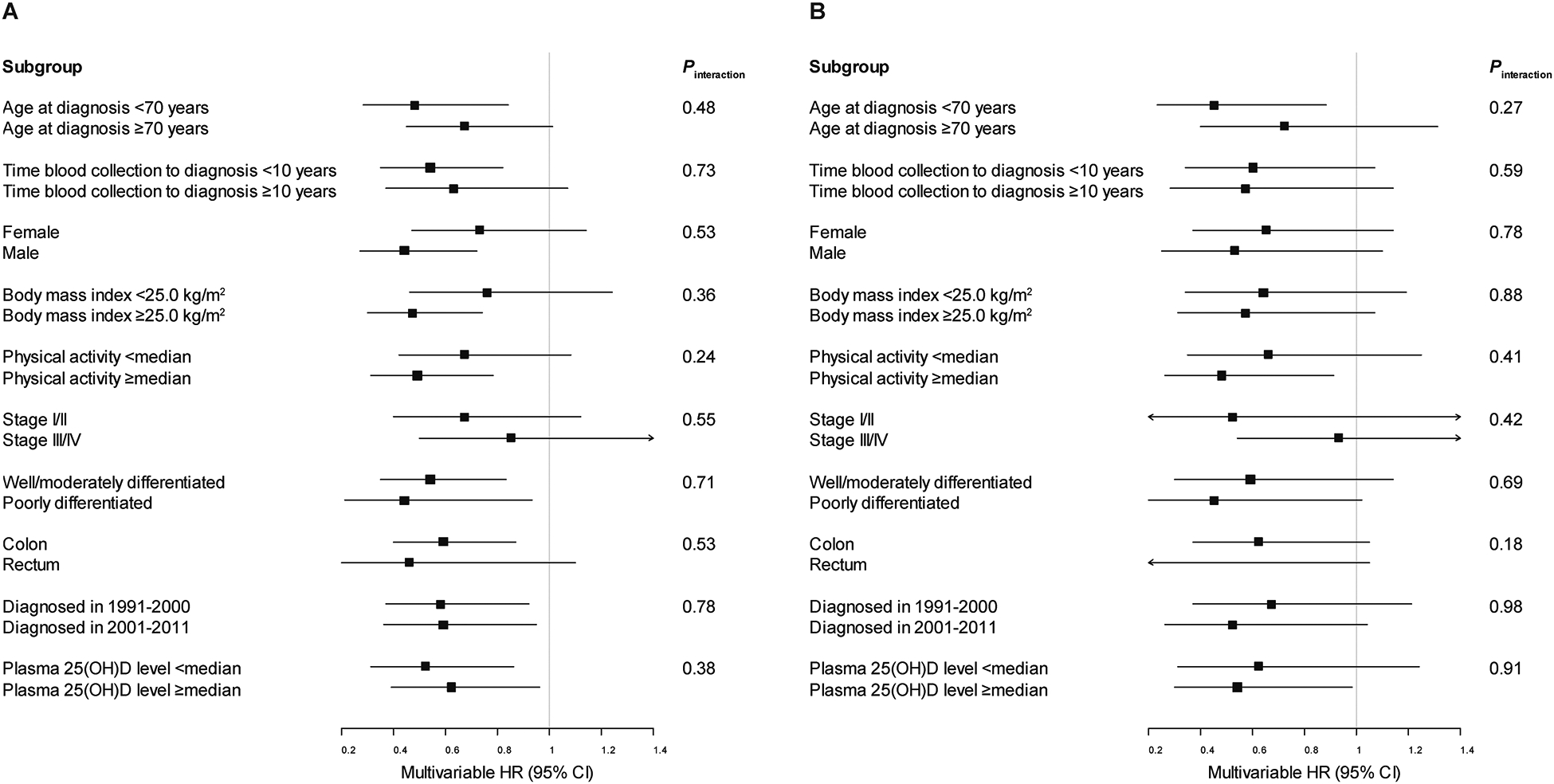
Multivariable hazard ratios (HRs) and 95% confidence intervals (CIs) for (A) overall and (B) colorectal cancer-specific mortality comparing the highest to the lowest quartile of plasma vitamin D binding protein among colorectal cancer patients, stratified by covariates. Adjusted for age at diagnosis (continuous), sex, body mass index (continuous), physical activity (continuous), cancer stage (I to IV or unknown), grade of tumor differentiation (well differentiated, moderately differentiated, poorly differentiated, unknown), location of primary tumor (proximal colon, distal colon, rectum, unknown), and year of diagnosis (continuous), excluding the stratification covariate. 25(OH)D, 25-hydroxyvitamin D.
Associations between prediagnostic levels of total, bioavailable, and free 25(OH)D and patient survival
Total 25(OH)D levels were not significantly associated with overall (Ptrend = 0.09) or CRC-specific survival (Ptrend = 0.08) (Table 4). However, higher total 25(OH)D levels were associated with improved 5-year overall survival (Ptrend = 0.01), with a multivariable HR of 0.48 (95% CI, 0.30–0.78) comparing the highest to the lowest quartile. The association remained significant after further adjustment for VDBP levels (Ptrend = 0.02).
Table 4.
Hazard ratios for overall mortality, colorectal cancer-specific mortality, and 5-year overall mortality among colorectal cancer patients by quartile of plasma total 25-hydroxyvitamin D, bioavailable 25-hydroxyvitamin D, and free 25-hydroxyvitamin D
| Hazard ratio (95% confidence interval) | Ptrend | ||||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||
| Total 25-hydroxyvitamin D | |||||
| Mean (SD), ng/mL | 15.6 (4.3) | 23.7 (3.7) | 29.4 (3.8) | 40.5 (9.0) | |
| No. of patients | 155 | 158 | 158 | 156 | |
| Overall mortality | |||||
| No. of events | 74 | 88 | 89 | 77 | |
| Base modela | Referent | 1.19 (0.87–1.63) | 1.22 (0.89–1.67) | 0.93 (0.67–1.30) | 0.70 |
| Multivariable modelb | Referent | 1.18 (0.84–1.65) | 1.13 (0.80–1.59) | 0.72 (0.49–1.05) | 0.09 |
| Model further adjusted for VDBPc | Referent | 1.18 (0.84–1.65) | 1.14 (0.81–1.61) | 0.73 (0.50–1.07) | 0.11 |
| Colorectal cancer-specific mortality | |||||
| No. of events | 38 | 51 | 57 | 41 | |
| Base modela | Referent | 1.29 (0.85–1.98) | 1.47 (0.97–2.23) | 0.97 (0.61–1.53) | 0.97 |
| Multivariable modelb | Referent | 1.22 (0.77–1.93) | 1.45 (0.92–2.30) | 0.57 (0.34–0.97) | 0.08 |
| Model further adjusted for VDBPc | Referent | 1.23 (0.78–1.96) | 1.51 (0.95–2.40) | 0.60 (0.35–1.01) | 0.12 |
| 5-year overall mortality | |||||
| No. of events | 49 | 53 | 58 | 43 | |
| Base modela | Referent | 1.00 (0.68–1.48) | 1.12 (0.76–1.65) | 0.74 (0.48–1.12) | 0.25 |
| Multivariable modelb | Referent | 0.92 (0.60–1.40) | 1.05 (0.69–1.59) | 0.48 (0.30–0.78) | 0.01 |
| Model further adjusted for VDBPc | Referent | 0.92 (0.60–1.41) | 1.08 (0.71–1.65) | 0.50 (0.31–0.81) | 0.02 |
| Bioavailable 25-hydroxyvitamin Dd | |||||
| Mean (SD), ng/mL | 1.8 (0.7) | 2.9 (0.7) | 3.9 (0.8) | 6.5 (2.3) | |
| No. of patients | 146 | 154 | 151 | 152 | |
| Overall mortality | |||||
| No. of events | 65 | 85 | 84 | 77 | |
| Base modela | Referent | 1.22 (0.88–1.69) | 1.25 (0.90–1.74) | 1.16 (0.83–1.63) | 0.41 |
| Multivariable modelb | Referent | 1.11 (0.78–1.59) | 1.12 (0.78–1.61) | 1.19 (0.82–1.73) | 0.39 |
| Colorectal cancer-specific mortality | |||||
| No. of events | 38 | 50 | 47 | 47 | |
| Base modela | Referent | 1.21 (0.79–1.85) | 1.14 (0.74–1.76) | 1.17 (0.76–1.81) | 0.59 |
| Multivariable modelb | Referent | 1.07 (0.66–1.71) | 1.01 (0.61–1.65) | 1.26 (0.77–2.06) | 0.43 |
| 5-year overall mortality | |||||
| No. of events | 41 | 54 | 53 | 49 | |
| Base modela | Referent | 1.19 (0.79–1.79) | 1.16 (0.77–1.76) | 1.10 (0.72–1.69) | 0.72 |
| Multivariable modelb | Referent | 1.07 (0.68–1.69) | 1.03 (0.65–1.63) | 1.14 (0.71–1.82) | 0.66 |
| Free 25-hydroxyvitamin Dd | |||||
| Mean (SD), pg/mL | 4.5 (1.6) | 7.0 (1.6) | 9.4 (1.7) | 15.8 (5.5) | |
| No. of patients | 147 | 151 | 153 | 152 | |
| Overall mortality | |||||
| No. of events | 66 | 81 | 79 | 85 | |
| Base modela | Referent | 1.18 (0.85–1.63) | 1.14 (0.82–1.58) | 1.33 (0.96–1.85) | 0.12 |
| Multivariable modelb | Referent | 1.18 (0.82–1.69) | 1.11 (0.77–1.59) | 1.36 (0.94–1.95) | 0.15 |
| Colorectal cancer-specific mortality | |||||
| No. of events | 38 | 49 | 44 | 51 | |
| Base modela | Referent | 1.21 (0.79–1.86) | 1.04 (0.67–1.62) | 1.31 (0.85–2.01) | 0.35 |
| Multivariable modelb | Referent | 1.18 (0.73–1.90) | 1.05 (0.64–1.70) | 1.35 (0.83–2.18) | 0.32 |
| 5-year overall mortality | |||||
| No. of events | 42 | 50 | 48 | 57 | |
| Base modela | Referent | 1.09 (0.72–1.65) | 1.01 (0.67–1.54) | 1.28 (0.85–1.92) | 0.29 |
| Multivariable modelb | Referent | 1.03 (0.65–1.64) | 0.97 (0.61–1.53) | 1.29 (0.82–2.02) | 0.31 |
Abbreviations: SD, standard deviation; VDBP, vitamin D binding protein.
Adjusted for age at diagnosis (continuous) and season of blood collection (summer, fall, winter, spring).
Addtionally adjusted for sex, body mass index (continuous), physical activity (continuous), cancer stage (I to IV or unknown), grade of tumor differentiation (well differentiated, moderately differentiated, poorly differentiated, unknown), location of primary tumor (proximal colon, distal colon, rectum, unknown), and year of diagnosis (continuous).
Additionally adjusted for vitamin D binding protein levels (quartiles or missing).
Calculated by total 25-hydroxyvitamin D, vitamin D binding protein, and albumin levels and the constant affinity of vitamin D binding protein and albumin for 25-hydroxyvitamin D (6×105 and 7×108, respectively).
Bioavailable 25(OH)D levels were not associated with either overall (Ptrend = 0.39) or CRC-specific survival (Ptrend = 0.43), even in the analyses with 5-year overall survival as the outcome (Ptrend = 0.66; Table 4). Free 25(OH)D levels were also not associated with any of these outcomes (Ptrend = 0.15, 0.32, and 0.31, respectively; Table 4).
Discussion
We found that CRC patients with the highest prediagnostic plasma VDBP levels had a significant improvement in overall and CRC-specific survival, independent of total 25(OH)D levels. Bioavailable and free 25(OH)D levels were not associated with overall or CRC-specific mortality.
To date, only 1 study has examined the association between circulating VDBP levels and CRC survival. This analysis of 206 Chinese CRC patients measured VDBP at surgery and detected no association with overall survival (38). Additionally, a Canadian study found that the C allele at rs2282679 (a perfect proxy for rs4588) in VDBP was significantly associated with worse disease-free survival among 488 CRC patients (39). Our observation that higher VDBP levels were associated with increased survival is biologically plausible. As the major carrier protein of circulating 25(OH)D, VDBP may boost the anti-cancer effects of 25(OH)D by prolonging its half-life. In addition, VDBP has independent biological functions that may inhibit tumor growth. First, VDBP functions as an actin scavenger, binding to circulating actin released from tissue injury, and thereby preventing vascular occlusion and organ dysfunction (40). Second, VDBP plays a role in immune response through the inflammation-primed conversion to VDBP-macrophage activating factor, which has direct anti-angiogenic and anti-proliferative activities in addition to its ability to activate tumoricidal macrophages (41–44). Third, VDBP has an anti-inflammatory effect by directing neutrophils to sites of inflammation (neutrophil chemotaxis) (45, 46). In the current study, the association between VDBP levels and patient survival remained significant after controlling for total 25(OH)D levels. To minimize bias in the plasma VDBP levels by the presence of occult cancer, we excluded patients diagnosed within 2 years of blood collection in the main analyses, and continued to note an association even when extending this restriction to 5 years.
Multiple prospective cohort studies (21–24), as well as a phase II randomized clinical trial (26), have suggested a benefit of higher vitamin D levels on survival among CRC patients. In our previous analysis of 304 CRC patients from the same cohort studies, higher total 25(OH)D levels were associated with improved overall survival (21). In the current study with a larger sample size and extended follow-up, higher total 25(OH)D levels were associated with improved 5-year overall survival, but not with overall survival during the entire follow-up. One possible explanation is that vitamin D may not reduce excess mortality from causes other than CRC. In a recent meta-analysis of 52 randomized controlled trials, vitamin D supplementation was found to reduce the risk of cancer death by 16% without being associated with all-cause mortality (47). Another potential explanation is that some patients provided blood samples many years before their diagnosis. Whereas the prospective design of our study is advantageous in reducing bias that results from reverse causation, total 25(OH)D levels measured remotely from diagnosis may not accurately reflect the relevant vitamin D status that influences long-term CRC survival.
In this study, we found no association between bioavailable or free 25(OH)D levels and CRC survival even in analyses with 5-year overall survival as the outcome. As higher VDBP levels were associated with improved survival, and resulted in lower concentrations of bioavailable and free 25(OH)D, we would not expect that higher levels of bioavailable or free 25(OH)D would also be associated with improved survival. In a previous case-control study including participants from NHS, total 25(OH)D levels, but not bioavailable or free 25(OH)D levels, were inversely associated with CRC risk (15). Taken together, these data do not support the “free hormone hypothesis” within the context of circulating 25(OH)D and colorectal carcinogenesis, indicating that total 25(OH)D remains the best measure of clinically relevant vitamin D status.
Our study has several strengths, including the prospective design, long follow-up, high follow-up rate, and detailed data on potential confounders. The prospective design reduces reverse causation, as blood samples were collected years before inadequate nutrition and limited performance status that commonly develop at the time of CRC diagnosis. The comprehensive assessment of VDBP, total 25(OH)D, and bioavailable and free 25(OH)D, along with availability of albumin levels and VDBP genotype, allowed for a better understanding of the roles of vitamin D-related biomarkers in CRC survival.
Several limitations of our study deserve comment. We used a single measurement of VDBP and total 25(OH)D from plasma samples collected years before diagnosis, so we were unable to assess the influence of the dynamic changes of these markers. We did not directly measure bioavailable and free 25(OH)D; however, calculated and directly measured concentrations of these markers have been found to be well correlated (48). We used a monoclonal antibody-based ELISA to measure VDBP, which is inferior to a polyclonal assay and incapable of measuring VDBP levels in blacks (48, 49). To address this concern, we excluded non-white patients in the main analyses, and the results were similar after further adjustment for VDBP polymorphisms. Finally, information on treatment was not systematically collected in NHS and HPFS. However, treatment programs were unlikely to have varied by VDBP levels years before diagnosis.
In conclusion, higher prediagnostic plasma VDBP levels were associated with improved overall and CRC-specific survival among CRC patients. Bioavailable or free 25(OH)D levels were not associated with CRC survival. Additional efforts to understand the mechanisms through which the vitamin D pathway influences colorectal carcinogenesis and cancer progression are warranted.
Supplementary Material
Acknowledgments:
We would like to thank the participants and staff of the Nurses’ Health Study and the Health Professionals Follow-Up Study for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
Financial Support: The Nurses’ Health Study is supported by the National Institutes of Health (NIH) grants UM1 CA186107, P01 CA87969, and R01 CA49449. The Health Professionals Follow-Up Study is supported by the NIH grant U01 CA167552. This work was additionally supported by the Pussycat Foundation Helen Gurley Brown Presidential Initiative to C.Y. and K.N.; by the NIH grant R35 CA197735 to S.O.; by the NIH grants R01 CA137178 and K24 DK098311 and the Damon Runyon Cancer Research Foundation to A.T.C.; by the NIH grant P50 CA127003 to C.S.F.; by the NIH grants K07 CA148894 and R01 CA205406 and the Project P Fund to K.N.; and by the Entertainment Industry Foundation’s National Colorectal Cancer Research Alliance (NCCRA).
Footnotes
Potential Competing Interests:
B.M.W. declares research funding from Celgene and Eli Lilly and Company and consulting for BioLineRx, Celgene, G1 Therapeutics, and GRAIL. J.A.M. declares research funding from Boston Biomedical and consulting for Cota Healthcare, Ignyta, and Taiho Pharmaceutical. A.T.C. declares research funding from Bayer and consulting for Bayer and Pfizer. C.S.F. declares consulting for Agios, Bain Capital, Bayer, Celgene, Dicerna Pharmaceuticals, Eli Lilly and Company, Entrinsic Health Solutions, Five Prime Therapeutics, Genentech, Gilead Sciences, KEW, Merck & Co., Merrimack Pharmaceuticals, Pfizer, Sanofi, Taiho Pharmaceutical, and Unum Therapeutics. He also serves as a Director for CytomX Therapeutics and owns unexercised stock options for CytomX Therapeutics and Entrinsic Health Solutions. K.N. declares research funding from Evergrande Group, Genentech, Gilead Sciences, Pharmavite, Revolution Medicines, Tarrex Biopharma, and Trovagene; advisory board participation for Array Biopharma, Bayer, Eli Lilly and Company, Genentech, and Seattle Genetics; and consulting for Tarrex Biopharma. Other authors declare no conflicts of interest.
References
- 1.Meggouh F, Lointier P, Saez S. Sex steroid and 1,25-dihydroxyvitamin D3 receptors in human colorectal adenocarcinoma and normal mucosa. Cancer research. 1991;51:1227–33. [PubMed] [Google Scholar]
- 2.Vandewalle B, Adenis A, Hornez L, Revillion F, Lefebvre J. 1,25-dihydroxyvitamin D3 receptors in normal and malignant human colorectal tissues. Cancer letters. 1994;86:67–73. [DOI] [PubMed] [Google Scholar]
- 3.Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, et al. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. The Journal of clinical endocrinology and metabolism. 2001;86:888–94. [DOI] [PubMed] [Google Scholar]
- 4.Vandewalle B, Wattez N, Lefebvre J. Effects of vitamin D3 derivatives on growth, differentiation and apoptosis in tumoral colonic HT 29 cells: possible implication of intracellular calcium. Cancer letters. 1995;97:99–106. [DOI] [PubMed] [Google Scholar]
- 5.Diaz GD, Paraskeva C, Thomas MG, Binderup L, Hague A. Apoptosis is induced by the active metabolite of vitamin D3 and its analogue EB1089 in colorectal adenoma and carcinoma cells: possible implications for prevention and therapy. Cancer research. 2000;60:2304–12. [PubMed] [Google Scholar]
- 6.Scaglione-Sewell BA, Bissonnette M, Skarosi S, Abraham C, Brasitus TA. A vitamin D3 analog induces a G1-phase arrest in CaCo-2 cells by inhibiting cdk2 and cdk6: roles of cyclin E, p21Waf1, and p27Kip1. Endocrinology. 2000;141:3931–9. [DOI] [PubMed] [Google Scholar]
- 7.Iseki K, Tatsuta M, Uehara H, Iishi H, Yano H, Sakai N, et al. Inhibition of angiogenesis as a mechanism for inhibition by 1alpha-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 of colon carcinogenesis induced by azoxymethane in Wistar rats. International journal of cancer Journal international du cancer. 1999;81:730–3. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez-Garcia NI, Palmer HG, Garcia M, Gonzalez-Martin A, del Rio M, Barettino D, et al. 1alpha,25-Dihydroxyvitamin D3 regulates the expression of Id1 and Id2 genes and the angiogenic phenotype of human colon carcinoma cells. Oncogene. 2005;24:6533–44. [DOI] [PubMed] [Google Scholar]
- 9.Evans SR, Shchepotin EI, Young H, Rochon J, Uskokovic M, Shchepotin IB. 1,25-dihydroxyvitamin D3 synthetic analogs inhibit spontaneous metastases in a 1,2-dimethylhydrazine-induced colon carcinogenesis model. Int J Oncol. 2000;16:1249–54. [DOI] [PubMed] [Google Scholar]
- 10.Lamprecht SA, Lipkin M. Cellular mechanisms of calcium and vitamin D in the inhibition of colorectal carcinogenesis. Annals of the New York Academy of Sciences. 2001;952:73–87. [DOI] [PubMed] [Google Scholar]
- 11.Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, Haddad JG. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. The Journal of clinical endocrinology and metabolism. 1986;63:954–9. [DOI] [PubMed] [Google Scholar]
- 12.Bikle DD, Siiteri PK, Ryzen E, Haddad JG. Serum protein binding of 1,25-dihydroxyvitamin D: a reevaluation by direct measurement of free metabolite levels. J Clin Endocrinol Metab. 1985;61:969–75. [DOI] [PubMed] [Google Scholar]
- 13.Speeckaert M, Huang G, Delanghe JR, Taes YE. Biological and clinical aspects of the vitamin D binding protein (Gc-globulin) and its polymorphism. Clinica chimica acta; international journal of clinical chemistry. 2006;372:33–42. [DOI] [PubMed] [Google Scholar]
- 14.Tagliabue E, Raimondi S, Gandini S. Meta-analysis of vitamin D-binding protein and cancer risk. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2015;24:1758–65. [DOI] [PubMed] [Google Scholar]
- 15.Song M, Konijeti GG, Yuan C, Ananthakrishnan AN, Ogino S, Fuchs CS, et al. Plasma 25-Hydroxyvitamin D, Vitamin D Binding Protein, and Risk of Colorectal Cancer in the Nurses’ Health Study. Cancer prevention research. 2016;9:664–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weinstein SJ, Purdue MP, Smith-Warner SA, Mondul AM, Black A, Ahn J, et al. Serum 25-hydroxyvitamin D, vitamin D binding protein and risk of colorectal cancer in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. International journal of cancer. 2015;136:E654–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anic GM, Weinstein SJ, Mondul AM, Mannisto S, Albanes D. Serum vitamin D, vitamin D binding protein, and risk of colorectal cancer. PloS one. 2014;9:e102966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mendel CM. The free hormone hypothesis: a physiologically based mathematical model. Endocr Rev. 1989;10:232–74. [DOI] [PubMed] [Google Scholar]
- 19.Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, et al. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell. 1999;96:507–15. [DOI] [PubMed] [Google Scholar]
- 20.Ternes SB, Rowling MJ. Vitamin D transport proteins megalin and disabled-2 are expressed in prostate and colon epithelial cells and are induced and activated by all-trans-retinoic acid. Nutrition and cancer. 2013;65:900–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ng K, Meyerhardt JA, Wu K, Feskanich D, Hollis BW, Giovannucci EL, et al. Circulating 25-hydroxyvitamin d levels and survival in patients with colorectal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:2984–91. [DOI] [PubMed] [Google Scholar]
- 22.Ng K, Wolpin BM, Meyerhardt JA, Wu K, Chan AT, Hollis BW, et al. Prospective study of predictors of vitamin D status and survival in patients with colorectal cancer. British journal of cancer. 2009;101:916–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fedirko V, Riboli E, Tjonneland A, Ferrari P, Olsen A, Bueno-de-Mesquita HB, et al. Prediagnostic 25-hydroxyvitamin D, VDR and CASR polymorphisms, and survival in patients with colorectal cancer in western European ppulations. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2012;21:582–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mezawa H, Sugiura T, Watanabe M, Norizoe C, Takahashi D, Shimojima A, et al. Serum vitamin D levels and survival of patients with colorectal cancer: post-hoc analysis of a prospective cohort study. BMC cancer. 2010;10:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zgaga L, Theodoratou E, Farrington SM, Din FV, Ooi LY, Glodzik D, et al. Plasma vitamin D concentration influences survival outcome after a diagnosis of colorectal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32:2430–9. [DOI] [PubMed] [Google Scholar]
- 26.Ng K, Nimeiri HS, McCleary NJ, Abrams TA, Yurgelun MB, Cleary JM, et al. Effect of High-Dose vs Standard-Dose Vitamin D3 Supplementation on Progression-Free Survival Among Patients With Advanced or Metastatic Colorectal Cancer: The SUNSHINE Randomized Clinical Trial. JAMA. 2019;321:1370–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan C, Sato K, Hollis BW, Zhang S, Niedzwiecki D, Ou FS, et al. Plasma 25-Hydroxyvitamin D Levels and Survival in Patients with Advanced or Metastatic Colorectal Cancer: Findings from CALGB/SWOG 80405 (Alliance). Clinical cancer research : an official journal of the American Association for Cancer Research. 2019;25:7497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. Journal of women’s health. 1997;6:49–62. [DOI] [PubMed] [Google Scholar]
- 29.Rimm EB, Giovannucci EL, Willett WC, Colditz GA, Ascherio A, Rosner B, et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991;338:464–8. [DOI] [PubMed] [Google Scholar]
- 30.Giovannucci E, Colditz GA, Stampfer MJ, Hunter D, Rosner BA, Willett WC, et al. A prospective study of cigarette smoking and risk of colorectal adenoma and colorectal cancer in U.S. women. Journal of the National Cancer Institute. 1994;86:192–9. [DOI] [PubMed] [Google Scholar]
- 31.Giovannucci E, Liu Y, Rimm EB, Hollis BW, Fuchs CS, Stampfer MJ, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. Journal of the National Cancer Institute. 2006;98:451–9. [DOI] [PubMed] [Google Scholar]
- 32.Hollis BW. Quantitation of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D by radioimmunoassay using radioiodinated tracers. Methods in enzymology. 1997;282:174–86. [DOI] [PubMed] [Google Scholar]
- 33.Arnaud J, Constans J. Affinity differences for vitamin D metabolites associated with the genetic isoforms of the human serum carrier protein (DBP). Human genetics. 1993;92:183–8. [DOI] [PubMed] [Google Scholar]
- 34.Boutin B, Galbraith RM, Arnaud P. Comparative affinity of the major genetic variants of human group-specific component (vitamin D-binding protein) for 25-(OH) vitamin D. Journal of steroid biochemistry. 1989;32:59–63. [DOI] [PubMed] [Google Scholar]
- 35.Kawakami M, Imawari M, Goodman DS. Quantitative studies of the interaction of cholecalciferol ((vitamin D3) and its metabolites with different genetic variants of the serum binding protein for these sterols. The Biochemical journal. 1979;179:413–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bouillon R, van Baelen H, de Moor P. Comparative study of the affinity of the serum vitamin D-binding protein. Journal of steroid biochemistry. 1980;13:1029–34. [DOI] [PubMed] [Google Scholar]
- 37.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. American journal of epidemiology. 1994;140:1016–9. [DOI] [PubMed] [Google Scholar]
- 38.Yang L, Chen H, Zhao M, Peng P. Prognostic value of circulating vitamin D binding protein, total, free and bioavailable 25-hydroxy vitamin D in patients with colorectal cancer. Oncotarget. 2017;8:40214–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu Y, Wang PP, Zhai G, Bapat B, Savas S, Woodrow JR, et al. Association of rs2282679 A>C polymorphism in vitamin D binding protein gene with colorectal cancer risk and survival: effect modification by dietary vitamin D intake. BMC cancer. 2018;18:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chishimba L, Thickett DR, Stockley RA, Wood AM. The vitamin D axis in the lung: a key role for vitamin D-binding protein. Thorax. 2010;65:456–62. [DOI] [PubMed] [Google Scholar]
- 41.Kanda S, Mochizuki Y, Miyata Y, Kanetake H, Yamamoto N. Effects of vitamin D(3)-binding protein-derived macrophage activating factor (GcMAF) on angiogenesis. Journal of the National Cancer Institute. 2002;94:1311–9. [DOI] [PubMed] [Google Scholar]
- 42.Kisker O, Onizuka S, Becker CM, Fannon M, Flynn E, D’Amato R, et al. Vitamin D binding protein-macrophage activating factor (DBP-maf) inhibits angiogenesis and tumor growth in mice. Neoplasia. 2003;5:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gregory KJ, Zhao B, Bielenberg DR, Dridi S, Wu J, Jiang W, et al. Vitamin D binding protein-macrophage activating factor directly inhibits proliferation, migration, and uPAR expression of prostate cancer cells. PloS one. 2010;5:e13428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pacini S, Punzi T, Morucci G, Gulisano M, Ruggiero M. Effects of vitamin D-binding protein-derived macrophage-activating factor on human breast cancer cells. Anticancer research. 2012;32:45–52. [PubMed] [Google Scholar]
- 45.Kew RR, Webster RO. Gc-globulin (vitamin D-binding protein) enhances the neutrophil chemotactic activity of C5a and C5a des Arg. The Journal of clinical investigation. 1988;82:364–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perez HD, Kelly E, Chenoweth D, Elfman F. Identification of the C5a des Arg cochemotaxin. Homology with vitamin D-binding protein (group-specific component globulin). The Journal of clinical investigation. 1988;82:360–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, Fang F, Tang J, Jia L, Feng Y, Xu P, et al. Association between vitamin D supplementation and mortality: systematic review and meta-analysis. BMJ. 2019;366:l4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nielson CM, Jones KS, Chun RF, Jacobs JM, Wang Y, Hewison M, et al. Free 25-Hydroxyvitamin D: Impact of Vitamin D Binding Protein Assays on Racial-Genotypic Associations. The Journal of clinical endocrinology and metabolism. 2016;101:2226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aloia J, Mikhail M, Dhaliwal R, Shieh A, Usera G, Stolberg A, et al. Free 25(OH)D and the Vitamin D Paradox in African Americans. The Journal of clinical endocrinology and metabolism. 2015;100:3356–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


