Abstract
Primary care provider (PCP) perceptions of colorectal cancer (CRC) screening test effectiveness and their recommendations for testing intervals influence patient screening uptake. Few large studies have examined providers’ perceptions and recommendations, including their alignment with evidence suggesting comparable test effectiveness and guideline recommendations for screening frequency. Providers (n=1281) within 4 healthcare systems completed a survey in 2017–2018 regarding their perceptions of test effectiveness and recommended intervals for colonoscopy and fecal immunochemical testing (FIT) for patients ages 40–49, 50–74, and ≥75 years. For patients 50–74 (screening eligible), 82.9% of providers rated colonoscopy as very effective versus 59.6% for FIT, and 26.3% rated colonoscopy as more effective than FIT. Also, for this age group, 77.9% recommended colonoscopy every 10 years and 92.4% recommended FIT annually. For patients ages 40–49 and ≥75, over one-third of providers believed the tests were somewhat or very effective, although >80% did not routinely recommend screening by either test for these age groups. Provider screening test interval recommendations generally aligned with CRC guidelines; however, 25% of providers believed colonoscopy was more effective than FIT for mortality reduction, which differs from some modeling studies that suggest comparable effectiveness. The latter finding may have implications for health systems where FIT is the dominant screening strategy. Only one-third of providers reported believing these screening tests were effective in younger and older patients (i.e., <50 and ≥75 years). Evidence addressing these beliefs may be relevant if cancer screening recommendations are modified to include older and/or younger patients.
INTRODUCTION
Colorectal cancer (CRC) is the second leading cause of cancer death in the United States (US) [1]. Screening can prevent CRC and reduce deaths through removal of precancerous adenomatous polyps and early detection and treatment of cancer [2, 3]. Colonoscopy and fecal immunochemical testing (FIT) are the most commonly used CRC screening tests worldwide [4, 5], and guidelines recommend screening colonoscopy every 10 years or FIT annually in average-risk patients 50–75 years of age [6]. Also, modeling studies suggest that colonoscopy and FIT have comparable effectiveness for reducing CRC-associated mortality assuming complete adherence [7].
The US National CRC Roundtable set a goal to have ≥80% of the screening-eligible US population up to date with CRC screening by 2018 [4]. However, there is wide variability in screening participation within the US. Approximately 83% of screening-eligible individuals were up to date with CRC screening in a large integrated healthcare setting that combined organized FIT outreach with on-request colonoscopy screening [8]. A study involving 4 healthcare systems reported 77.5% of eligible patients were up to date with CRC screening [9], yet even this relatively high proportion is lower than the 84.6% reported for cervical cancer screening participation across 5 healthcare systems [10]. Also, nationwide, only 63% of screening-eligible individuals overall and fewer than 50% of some racial/ethnic groups were estimated to be up to date with CRC screening [11].
Patient awareness and provider recommendations for screening may influence CRC screening participation. In a recent systematic review, screening participation was noted to be dependent on patient awareness of CRC screening, and a key factor mediating awareness was primary care providers recommending screening to their patients [12]. The importance of providers recommending screening has also been reported for breast and cervical cancer screening [13], and consistent with these reports, patients with greater numbers of annual primary care visits had higher CRC screening participation [14]. However, relatively few data exist regarding provider beliefs about colonoscopy and FIT effectiveness for screening, whether beliefs are associated with recommendations for screening test intervals, and if beliefs and recommendations differ across patient age groups (e.g., those 45–49 years for whom the American Cancer Society now recommends screening, or patients ≥75 years) [15].
To address these knowledge gaps, we investigated across 4 diverse healthcare systems, provider beliefs about colonoscopy and FIT effectiveness, provider recommendations for screening test intervals, if these beliefs and recommendations differed across patient age groups, and provider perceptions of barriers to CRC screening.
METHODS
Study Setting
The study was funded through the National Cancer Institute-funded Population-based Research Optimizing Screening through Personalized Regimens (PROSPR I) consortium (U54CA163262, U54CA163261, U54CA163308) and Population-based Research to Optimize the Screening Process II (PROSPR II) consortium (UM1 CA222035, UM1 CA221940), which conduct multisite, transdisciplinary research to evaluate and improve cancer screening processes.
The study was conducted within 4 PROSPR I sites: Kaiser Permanente Northern California (KPNC), Kaiser Permanente Southern California (KPSC), Kaiser Permanente Washington (KPWA) (formerly Group Health), and Parkland Health & Hospital System/University of Texas Southwestern (Parkland-UTSW). As previously described [16], these sites, located in the Southern and Western regions of the US, feature diverse healthcare settings and patient populations. KPNC and KPSC comprise 2 large integrated health care systems delivering care to over 8 million members in Northern and Southern California. KPWA is a mixed‐model, nonprofit health plan serving approximately 710,000 Washington State residents. Parkland-UTSW is a safety‐net provider for underinsured and uninsured Dallas County Texas residents, delivering ambulatory care to approximately 167,000 adults annually. KPNC and KPSC have implemented organized CRC screening programs that include a combination of annual mailing of FIT kits or on-request (opportunistic) colonoscopy screening for screening-eligible members 50–74 years of age who are not up to date with screening by another method [8, 16]. At KPWA, eligible patients are reminded about CRC screening needs in an annual birthday letter and during clinic visits. At Parkland-UTSW, colonoscopy and FIT are available opportunistically to all primary care patients ≥50 years during in-person clinic visits with the screening modality offered based on provider and patient preferences [16]. Descriptive information on patients 40–89 years of age at each site (i.e., age, sex, race/ethnicity, and health insurance) was obtained for patients at the time the survey was administered (2017) (Supplemental Table 1). The study was conducted in accordance with the ethical guidelines of the U.S. Common Rule and was performed after approval of the institutional review boards (IRBs) of each health care system. The IRBs determined that it was not necessary to obtain written informed consent from the subjects.
Survey Design
Between 2017 and 2018, 6807 providers within the 4 study sites were invited to participate in a one-time survey (Supplemental Figure 1). The survey instrument was adapted from a prior smaller survey at 3 PROSPR I breast cancer research centers [17, 18]. Survey questions varied slightly across sites. Providers from KPNC, KPSC, and KPWA were sent either an advanced email announcing the survey or an email with a link to the survey, and up to 4, 2 and 3 email reminders, respectively. KPWA providers also received an advance letter with $5 pre-incentive and a paper copy of the study information sheet. UTSW providers were sent an email with a link to the survey followed by up to 4 email reminders, which included an incentive lottery to win 1 of several $50 gift cards. KPNC providers who completed the survey were eligible for a random drawing to win 1 of several $25 gift cards.
The survey addressed screening practices, beliefs, and barriers for colorectal, breast, cervical, and lung cancers. For CRC, the survey included questions on provider beliefs about colonoscopy and FIT effectiveness for screening and their recommended screening intervals by patient age category (40–49, 50–74 and ≥75 years), and provider characteristics such as age, sex, medical specialty, medical degree/certification, and average number of office visits per week (Supplemental Table 2). The age range of 50–74 was selected as the screening-eligible age range (rather than 50–75) because KPNC and KPSC discontinue CRC screening the year a patient turns 75 years of age.
Statistical Analyses
Pearson chi-square tests were used to compare survey response proportions and responses across sites. Descriptive statistics were calculated to summarize provider characteristics (age, sex, specialty, average number of weekly office visits, and healthcare system site), provider beliefs about the effectiveness of colonoscopy and FIT, and provider recommendations for screening intervals. Multivariable logistic regression was used to assess provider characteristics associated with providers’ believing screening tests (colonoscopy and FIT) are effective in reducing mortality and providers’ recommending colonoscopy and FIT at guideline-recommended intervals. Covariates in the model included provider age, sex, specialty, average number of weekly office visits, and healthcare system site. Provider belief was included as an independent variable in the model for guideline-consistent screening interval recommendations.
RESULTS
Among 6807 providers contacted (Supplemental Figure 1), 1887 completed the survey (27.6%). The response proportions differed across sites: 29.1% (983 / 3384) at KPNC, 21.6% (662 / 2886) at KPSC, 53.9% (171 / 317) at KPWA, and 47.7% (105 / 220) at Parkland-UTSW (p<0.001). We subsequently excluded 356 respondents who self-reported not having ordered CRC screening tests in the prior 12 months and 250 respondents who were not primary care providers, which resulted in an analytic dataset of 1281 providers, including 603 (47.1%) at KPNC, 430 (33.6%) at KPSC, 165 (12.9%) at KPWA, and 83 (6.5%) at Parkland-UTSW (Supplemental Table 1). Among these 1281 providers, 34.0% were 40–49 years of age, 56.5% were female, 52.9% were family practice physicians, 42.9% were general internal medicine physicians, 4.2% were another type of primary care provider, and 31.6% averaged 76–100 patient visits per week. Parkland-UTSW providers were more frequently younger; 60.2% were <40 years of age vs. 18.4%−34.5% for the Kaiser Permanente sites. In addition, they averaged fewer office visits per week; 66.3% had ≤50 office visits per week vs. 12.1%−40.0% for Kaiser Permanente sites.
As shown in Supplemental Table 1, among patients 40–89 years of age across sites in 2017, the majority were female (52.3%−63.2%) and 50–74 years of age (60.2%−66.1%). The percent non-Hispanic White ranged from 13.6% for Parkland-UTSW to 71.9% for KPWA, non-Hispanic Blacks ranged from 4.6% for KPWA to 30.8% for Parkland-UTSW, Hispanics ranged from 4.5% at KPWA to 49.8% at Parkland-UTSW, and Asian/Pacific Islanders ranged from 4.9% at Parkland-UTSW to 18.7% for KPNC.
Provider Beliefs about CRC Screening Test Effectiveness
Patients 50–74 years of age
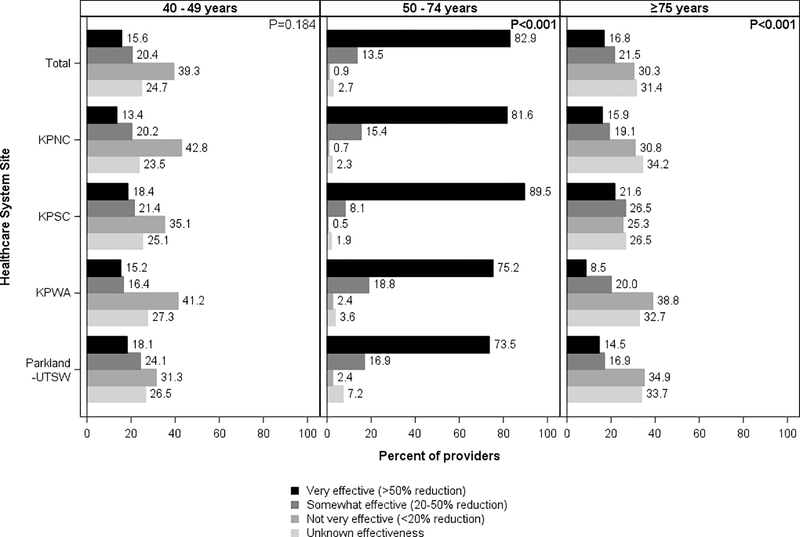
For patients of conventional screening age, 82.9% of providers believed colonoscopy screening was very effective and 13.5% somewhat effective (Figure 1). Across all sites, very effective was the predominant belief (range across sites: 73.5%−89.5%), though response distributions varied across sites (p<0.001).
Figure 1.
Provider beliefs about the effectiveness of colonoscopy in reducing colorectal cancer moratality, by patient age and healthcare system site
KPNC, Kaiser Permanente Northern California; KPNC, Kaiser Permanente Southern California; KPWA, Kaiser Permanente Washington; Parkland Health and Hospital System/University of Texas, Southwestern
Chi-square p-values at the top right corner of each panel column compare provider responses across sites (α= 0.05).
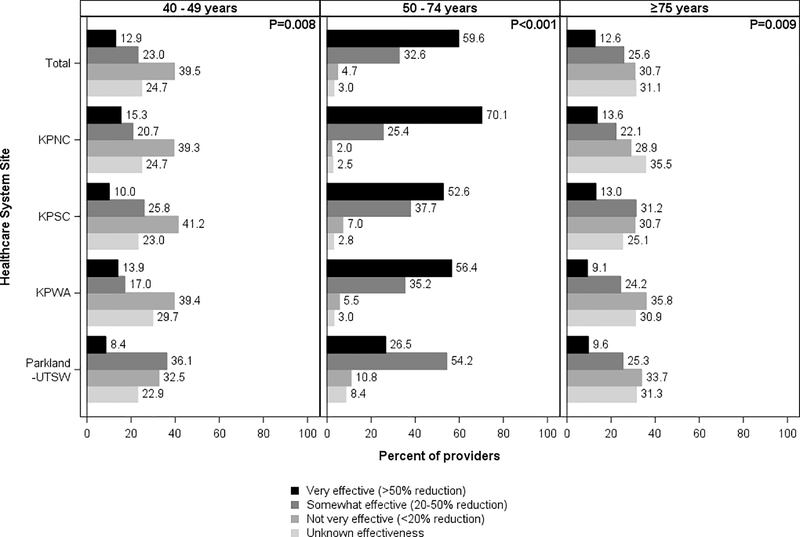
For FIT, 59.6% of providers believed it was very effective, while 32.6% believed it was somewhat effective (Figure 2). Across 3 sites (KPNC, KPSC, and KPWA), very effective was the predominant belief (range across 3 sites: 52.6%−70.1%), though response distributions differed (p<0.001). At Parkland-UTSW, 54.2% believed that FIT was somewhat effective.
Figure 2.
Provider beliefs about the effectiveness of fecal immunochemical testing in reducing colorectal cancer mortality, by patient age and healthcare system site
KPNC, Kaiser Permanente Northern California; KPNC, Kaiser Permanente Southern California; KPWA, Kaiser Permanente Washington; Parkland Health and Hospital System/University of Texas, Southwestern
Chi-square p-values at the top right corner of each panel column compare provider responses across sites (α= 0.05).
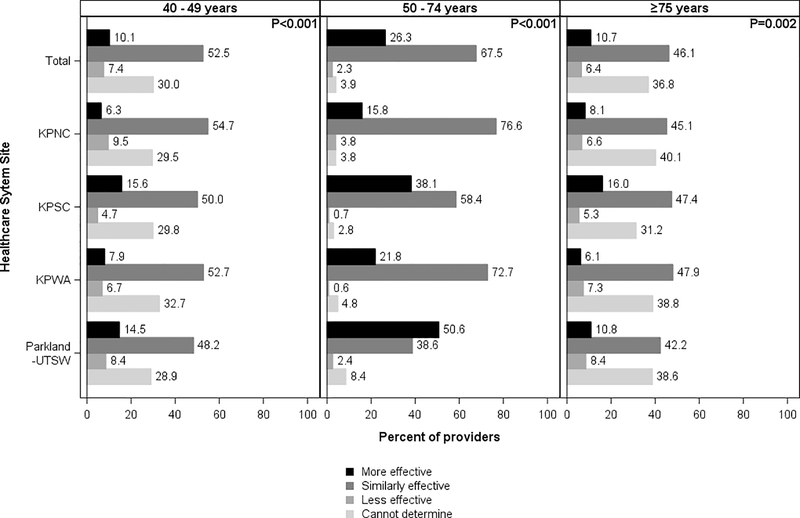
Overall, 67.5% of providers (range across sites: 38.6%−76.6%) reported believing colonoscopy and FIT had the same level of effectiveness, while 26.3% believed colonoscopy was more effective than FIT (range across sites: 15.8%−50.6%) (Figure 3). Distributions of responses for both tests varied significantly across sites (p<0.001)
Figure 3.
Distribution of providers who believe colonoscopy is more effective, similarly effective, or less effective than fecal immunochemical testing in reducing colorectal cancer mortality, by patient age and healthcare system site
KPNC, Kaiser Permanente Northern California; KPNC, Kaiser Permanente Southern California; KPWA, Kaiser Permanente Washington; Parkland Health and Hospital System/University of Texas, Southwestern
Chi-square p-values at the top right corner of each panel column compare provider responses across sites (α= 0.05).
Patients 40–49 years of age
For younger patients, not very effective was the predominant overall provider belief about colonoscopy (39.3%, range across sites: 31.3%−42.8%) (Figure 1) and FIT (39.5%, range across sites: 32.5%−41.2%) (Figure 2). However, 36.0% of providers believed colonoscopy was either somewhat effective or very effective (range across sites: 31.6–42.2; p=0.184), and 35.9% believed the same about FIT (range across sites: 30.9%−44.5%; p=0.008). Differences in responses across sites were statistically significant for FIT (p=0.008) but not colonoscopy (p=0.184).
Additionally, 52.5% of providers believed colonoscopy and FIT had the same level of effectiveness among younger individuals (range across sites: 48.2%−54.7%), with the response distributions differing somewhat across sites (p<0.001) (Figure 3).
Patients ≥75 years of age
For older patients, a similar percentage of providers reported unknown effectiveness (31.4%) and not very effective (30.3%) as the predominant beliefs for colonoscopy (Figure 1). The findings were similar for FIT, with 31.1% reporting unknown effectiveness and 30.7% reporting not very effective as the predominant beliefs (Figure 2).
In addition, 46.1% of providers (range across sites: 42.2%−47.9%) believed colonoscopy and FIT had the same level of effectiveness among older patients (Figure 3), with the response distributions differing somewhat across sites (p=0.002).
Provider Recommendations for Screening Intervals
Patients 50–74 years of age
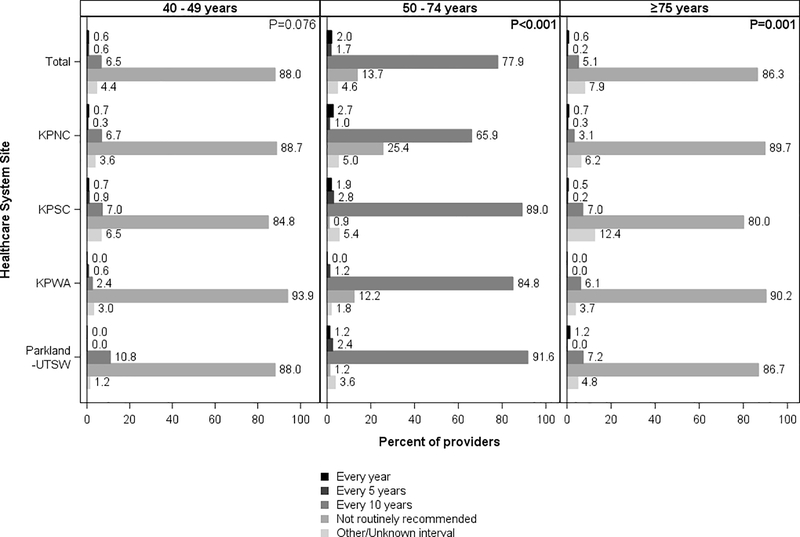
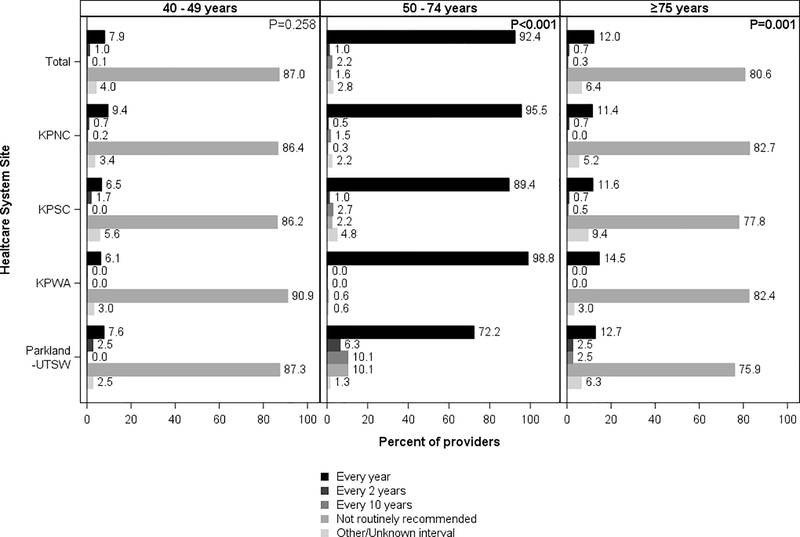
For patients of conventional screening age, 77.9% (range across sites: 65.9%−91.6) of providers reported recommending screening colonoscopy every 10 years, with differences across sites (p<0.001) (Figure 4), and 92.4% recommended FIT annually (range across sites: 72.2%−98.8%; p<0.001 for differences in the distribution across sites) (Figure 5).
Figure 4.
Provider-recommended screening intervals for colonoscopy, by patient age and healthcare system site
KPNC, Kaiser Permanente Northern California; KPNC, Kaiser Permanente Southern California; KPWA, Kaiser Permanente Washington; Parkland Health and Hospital System/University of Texas, Southwestern
Chi-square p-values at the top right corner of each panel column compare provider responses across sites (α= 0.05).
Figure 5.
Provider-recommended screening intervals for fecal immunochemical testing, by patient age and healthcare system site
KPNC, Kaiser Permanente Northern California; KPNC, Kaiser Permanente Southern California; KPWA, Kaiser Permanente Washington; Parkland Health and Hospital System/University of Texas, Southwestern
Chi-square p-values at the top right corner of each panel column compare provider responses across sites (α= 0.05).
Of the providers who did not routinely recommend colonoscopy, 98.9% reported recommending FIT annually (Supplemental Figure 2). And of the providers who did not routinely recommend FIT, 88.9% reported recommending colonoscopy every 10 years (Supplemental Figure 3).
Patients 40–49 years of age
For younger patients, 88.0% of providers (range across sites: 84.8%−93.9%) reported not routinely recommending colonoscopy screening Figure 4) and 87.0% of providers reported not routinely recommending FIT (range across sites: 86.2%−90.9%) (Figure 5). Responses were similar across sites (p=0.076 for colonoscopy and p=0.258 for FIT)
Among providers who did not routinely recommend colonoscopy to this age group, 94.5% reported not recommending FIT either (Supplemental Figure 2). Among providers who reported not routinely recommending FIT to this age group, 95.9% reported not recommending colonoscopy either (Supplemental Figure 3).
Patients ≥75 years of age
For older patients, 86.3% of providers reported not routinely recommending colonoscopy screening (range across sites: 80.0%−90.2%) (Figure 4) and 80.6% reported not routinely recommending FIT (range across sites: 75.9%−82.7%) (Figure 5). Differences in responses across sites were small but statistically significant (p<0.001).
Among providers who reported not routinely recommending colonoscopy screening for this age group, 90.3% reported not recommending FIT either (Supplemental Figure 2). And among providers who reported not routinely recommending FIT to this age group, 96.7% reported not recommending colonoscopy either (Supplemental Figure 3).
Provider Characteristics Associated with Believing Colorectal Cancer Screening Tests are Effective in Patients 50–74 Years
The provider characteristics associated with provider beliefs that colonoscopy and FIT are effective in reducing mortality are shown in Table 1. Male providers were less likely than female providers to believe colonoscopy is effective (odds ratio (OR): 0.34; 95% confidence interval (CI): 0.17–0.68). Also, compared to KPNC providers, KPWA providers were less likely (OR: 0.30; 95% CI: 0.11–0.83) to believe colonoscopy is effective. In contrast, compared to KPNC providers, providers from KPSC (OR: 0.43; 95% CI: 0.25–0.73), KPWA (OR: 0.29; 95% CI: 0.13–0.68), and Parkland-UTSW (OR: 0.17; 95% CI: 0.08–0.40) were less likely to believe FIT is effective.
Table 1.
Association of provider characteristics and beliefs that screening tests are effective at reducing colorectal cancer mortality and recommending screening at guideline-recommended intervals
| Belief in Test Effectiveness | Recommend Guideline-Recommended Screening Intervals | |||
|---|---|---|---|---|
| Colonoscopy OR [95% CI]† | FIT OR [95% CI]† | Colonoscopy every 10 years OR [95% CI]‡ | FIT annually OR [95% CI]‡ | |
| Provider beliefs about the effectiveness of the screening test in reducing colorectal cancer mortality | ||||
| Very Effective (>50% reduction) | - | - | 1.33 [0.87–2.03] | 1.65 [0.88–3.12] |
| Somewhat or Very Effective (20–50% reduction) | - | - | 1.00 [reference] | 1.00 [reference] |
| Not Very Effective (<20% reduction) | - | - | 2.21 [0.25–19.22] | 0.50 [0.19–1.31] |
| Effectiveness Not Known | - | - | 1.40 [0.47–4.15] | 1.76 [0.36–8.71] |
| Age, years | ||||
| <40 | 0.85 [0.37–1.97] | 0.97 [0.55–1.69] | 1.21 [0.80–1.83] | 0.94 [0.43–2.03] |
| 40–49 | 1.00 [reference] | 1.00 [reference] | 1.00 [reference] | 1.00 [reference] |
| 50–59 | 1.13 [0.46–2.81] | 1.26 [0.69–2.29] | 1.01 [0.68–1.52] | 1.10 [0.49–2.47] |
| ≥60 | 1.18 [0.41–3.36] | 1.63 [0.71–3.75] | 0.69 [0.41–1.16] | 0.70 [0.27–1.81] |
| Sex | ||||
| Male | 0.34 [0.17–0.68] | 0.72 [0.45–1.13] | 1.10 [0.79–1.54] | 1.02 [0.56–1.87] |
| Female | 1.00 [reference] | 1.00 [reference] | 1.00 [reference] | 1.00 [reference] |
| Medical specialty/provider type | ||||
| Family Medicine Physician | 1.00 [reference] | 1.00 [reference] | 1.00 [reference] | 1.00 [reference] |
| General Internal Medicine Physician | 0.67 [0.33–1.37] | 0.64 [0.39–1.03] | 1.53 [1.09–2.15] | 0.90 [0.49–1.64] |
| Other Primary Care Provider* | 2.79 [0.34–22.92] | 1.66 [0.45–6.10] | 0.60 [0.26–1.39] | 0.43 [0.09–1.93] |
| Average number of office visits/week | ||||
| ≤50 | 0.53 [0.20–1.36] | 1.27 [0.64–2.54] | 0.85 [0.54–1.36] | 0.46 [0.21–1.01] |
| 51–75 | 0.90 [0.34–2.38] | 1.25 [0.66–2.40] | 0.87 [0.58–1.30] | 1.15 [0.46–2.88] |
| 76–100 | 1.00 [reference] | 1.00 [reference] | 1.00 [reference] | 1.00 [reference] |
| >101 | 0.52 [0.18–1.47] | 0.63 [0.34–1.17] | 1.08 [0.67–1.74] | 0.95 [0.38–2.39] |
| Healthcare system site | ||||
| KPNC | 1.00 [reference] | 1.00 [reference] | 1.00 [reference] | 1.00 [reference] |
| KPSC | 1.60 [0.66–3.89] | 0.43 [0.25–0.73] | 8.20 [5.02–13.40] | 0.44 [0.21–0.92] |
| KPWA | 0.30 [0.11–0.83] | 0.29 [0.13–0.68] | 4.43 [2.43–8.06] | 3.36 [0.65–17.44] |
| Parkland-UTSW | 0.41 [0.13–1.26] | 0.17 [0.08–0.40] | 7.56 [2.85–20.03] | 0.13 [0.05–0.33] |
FIT, fecal immunochemical test; OR, odds ratio; CI, confidence interval; KPNC, Kaiser Permanente Northern California; KPSC, Kaiser Permanente Southern California; KPWA, Kaiser Permanente Washington; Parkland-UTSW, Parkland Health and Hospital System/University of Texas, Southwestern
Other primary care provider includes physician assistant (n=1), registered nurse (n=1), nurse practitioner (n=11) and other allied health professional (n=43)
Odds ratios adjusted for provider age, sex, medical specialty/provider type, average number of office visits per week, and healthcare system site.
Odds ratios adjusted for provider beliefs in effectiveness of test in reducing colorectal cancer mortality and provider age, sex, medical specialty/provider type, average number of office visits per week, and healthcare system site.
Provider Characteristics Associated with Recommending Screening Tests at Guideline-Recommended Screening Intervals in Patients 50–74 Years of Age
Provider characteristics associated with recommending colonoscopy every 10 years and FIT annually are shown in Table 1. Compared to family medicine physicians, general internal medicine physicians were more likely to recommend colonoscopy every 10 years (OR: 1.53; 95% CI: 1.09–2.15). Also, compared to KPNC providers, providers from KPSC (OR: 8.20; 95% CI: 5.02–13.40), KPWA (OR: 4.43; 95% CI: 2.43–8.06) and Parkland-UTSW (OR: 7.56; 95% CI: 2.85–20.03) were more likely to recommend colonoscopy every 10 years. Compared to KPNC providers, providers from KPSC (OR: 0.44; 95% CI: 0.21–0.92) and Parkland-UTSW (OR: 0.13; 95% CI: 0.05–0337) were less likely to recommend annual FIT screening. Provider beliefs about screening test effectiveness were not associated with the likelihood of recommending colonoscopy screening every 10 years or FIT annually (Table 1).
Provider Perceptions of Barriers to Screening
Compared to providers at Kaiser Permanente sites combined, Parkland-UTSW providers more frequently reported major barriers to screening, including patients being unable to afford screening (Parkland-UTSW: 39.8%; other sites combined: 17.4%; p<0.001), difficulty getting to the screening site (Parkland-UTSW: 50.6%; other sites combined: 22.4%; p<0.001), and not perceiving cancer as a threat (Parkland-UTSW: 31.3%; other sites combined: 22.0%; p=0.048); and the health system lacking resources to perform screening (Parkland-UTSW: 20.5%; other sites combined: 6.3%; p<0.001) (Supplemental Table 3).
DISCUSSION
A key finding from our study was that provider beliefs about test effectiveness and recommended screening intervals varied by healthcare system. For example, compared to KPNC providers, those from KPSC, KPWA, and Parkland-UTSW were less likely to believe FIT was very effective at reducing CRC mortality; providers at KPSC and Parkland-UTSW were also less likely to recommend FIT annually. These beliefs about test effectiveness and frequency mirrored differences in screening programs and screening delivery across sites. For example, while KPNC and KPSC both have well-established organized annual FIT-based screening programs, screening colonoscopies are more commonly performed at KPSC. The Parkland-UTSW providers may have had less confidence in the effectiveness of FIT because it was the only site that did not have an organized FIT-based screening program and it also had a larger number of trainees and academic physicians who practiced in a University health system that promoted a colonoscopy-based screening strategy. These findings suggest that healthcare system programs and policies may influence provider beliefs about screening test effectiveness.
Provider knowledge and/or perceptions of disparities in CRC by race/ethnicity and socioeconomic status may have also impacted their beliefs and recommendations. For example, there were substantial differences in the patient populations between the Kaiser Permanente sites as compared to Parkland-UTSW. The Kaiser Permanente sites, located in California and Washington, were limited to patients with medical insurance, they had a relatively stable membership base from year to year, and the majority of members (41.4%−74.2%) were non-Hispanic White and a much smaller minority (4.3%−9.0%) were non-Hispanic Black. In contrast, Parkland-UTSW is the sole safety-net provider for under-insured and uninsured Dallas County residents living at ≤200% of federal poverty level [16], and at the time of the survey in 2017, 13.6% were non-Hispanic White and 30.8% non-Hispanic Black. These differences between sites are potentially important because CRC incidence and mortality rates vary substantially by race and ethnicity, with Blacks having the highest rates, largely driven by differences in risk factor prevalence (e.g., smoking and obesity) and limited access to health care secondary to low socioeconomic status among Black individuals [1], Indeed, compared to providers at Kaiser Permanente sites, Parkland-UTSW providers more frequently reported perceiving factors such as patients being unable to afford screening, having difficulty getting to the screening site, and not perceiving cancer as a threat, as well as the health system lacking the resources to perform screening as major barriers to screening. Thus, disparities in provider perceptions of test effectiveness (versus efficacy) may stem from fundamental differences between sites in factors such as patient race/ethnicity, socioeconomic status, access to healthcare, and CRC incidence and mortality rates. For example, for providers of patients with limited access to healthcare, a one-and-done colonoscopy procedure that covers screening needs over multiple years and can remove precancerous polyps may be perceived as more effective than FIT which, after a positive test, requires an additional step (i.e., a follow-up colonoscopy) to complete the screening episode. Similarly, colonoscopy may be perceived by providers as more effective for patients that present more frequently with high-risk lesions.
Another important finding was that providers reported differences in beliefs about the effectiveness of colonoscopy and FIT for CRC screening. While both tests were seen as either somewhat effective or very effective by >90% of providers, only about 60% reported believing FIT was very effective and more than a quarter reported believing colonoscopy was more effective than FIT, both of which contrast with findings from modeling studies commissioned by the USPSTF that suggest comparable mortality reductions, assuming high adherence [7]. In addition to the factors noted above, it is possible that differences in perceptions of test effectiveness reflect that colonoscopy involves a full structural examination of the colon and rectum during which precancerous polyps can be removed, whereas a positive FIT requires an additional step -- a follow-up colonoscopy -- to complete the screening episode. Also, approximately 40%−50% of FIT-positive tests are false-positives, where neither an adenoma nor CRC is detected at the follow-up colonoscopy [19], and this may have contributed to differences in perceptions of test effectiveness. Those possible explanations aside, we recently reported in a large, community-based integrated healthcare setting that supplementing opportunistic endoscopic screening with programmatic annual FIT outreach rapidly increased the percentage of patients up to date with screening from <40% to >80%, and was accompanied by substantial decreases in CRC incidence and mortality [8]. We also demonstrated that a program of mailed FIT and stepped levels of support can substantially improve CRC screening adherence, from 47.5% to 62.1% covered time over 5 years [20]. Also, in a randomized controlled trial at Parkland-UTSW, a program of mailed FIT doubled screening rates compared with usual visit-based care alone [21]. Thus, FIT as a screening modality can work in well-insured and uninsured populations, and in white and non-white populations. These findings underscore the potential for organized FIT-based outreach programs to rapidly increase screening uptake at the population level and improve CRC outcomes.
Prior studies have reported that physicians were more likely to prefer or recommend colonoscopy screening over fecal occult blood testing, potentially creating missed opportunities for some patients to get screened; however detailed evaluations of reasons for this preference (such as underlying beliefs) are few [22, 23]. Another study reported a low concordance between patient preferences for CRC screening tests and the actual test completed, suggesting that patient preference may not be fully incorporated into CRC screening discussions, which could contribute to reduced screening uptake [24]. A previous study also showed that primary care physicians with higher screening rates were more likely to offer screening test choices (i.e., colonoscopy and stool testing) and discuss the pros and cons of each with their patients [25]. A recent pilot study reported that a 2-hour interactive training seminar on offering a choice of CRC screening options increased the proportion of physicians with the intention to offer FIT and colonoscopy to their patients in equal proportions [26]. Conveying the potential effectiveness of both FIT and colonoscopy to primary care providers is a communication and health policy opportunity that may be especially important in healthcare settings that rely on a FIT-first CRC screening strategy. Efforts to increase provider awareness of the utility of both colonoscopy and FIT for screening – consistent with modeling studies of comparable effectiveness [7] – should be studied to see if they can increase provider willingness to recommend FIT where it is available as either an initial or alternative screening test. This may boost screening participation, including among the substantial portion of patients who decline or delay colonoscopy screening.
There was greater variability in provider beliefs about the effectiveness of colonoscopy screening and FIT for patients younger (40–49 years) and older (≥75 years) than for the guideline-recommended screening ages. Although not very effective and unknown effectiveness were the predominant beliefs about colonoscopy and FIT, respectively, an equal or even greater proportion of providers believed the tests were somewhat or very effective for these age groups. However, this did not translate to recommendations for screening in younger and older patients. Given new guidelines by some groups to start screening earlier [27], and the current USPSTF guidelines recommending shared decision-making in patients ages 76–85, further study of provider beliefs and practices in these age groups is warranted. Evidence regarding effectiveness in these age groups may be needed to convince practitioners to recommend screening, if guidelines change, given the study’s findings.
Our survey contrasts with an older (2006–2007) national survey of CRC screening practices among 1266 primary care physicians conducted by the National Cancer Institute which asked about practice patterns relative to age at screening initiation and testing intervals [15]. In that survey, initiation of colonoscopy screening at age 50 was recommended by 93.9% of providers. In contrast, 58.0% recommended initiating fecal occult blood testing at age 50, whereas 41.3% recommended starting at a younger age. Also, the screening interval for colonoscopy was 10 years for just 55.7% of respondents, whereas 44.3% recommended it more frequently. The screening interval for stool testing was 1 year for 88.5% of providers, while 11.3% recommended it less frequently. The differences in the findings appear to represent a shift toward consensus on 10 years as the recommended interval for a screening colonoscopy and 1 year as the interval for stool testing.
Study limitations include the low response proportions across sites and the inclusion of providers from only 4 healthcare systems, the majority being Kaiser Permanente healthcare system sites, which may limit the generalizability of the study findings. Individual patient-level data by provider were not available across sites; therefore, we were unable to validate provider beliefs and self-reported practices with their actual screening test ordering behaviors. Study strengths include the large number of providers surveyed; representation from 4 diverse, regional healthcare systems covering both well- and poorly-insured white and non-white populations; the ability to evaluate several potential predictors of beliefs and screening practices; and the evaluation of the two most commonly used CRC screening modalities in the US.
Current US Preventive Services Task Force (USPSTF) guidelines recommend screening in average-risk patients ages 50–75 years, including by screening colonoscopy every 10 years or high sensitivity fecal testing annually [6]. Consistent with these guidelines, providers in our study generally believed that both colonoscopy and FIT screening were effective for reducing mortality from CRC among screening-eligible patients. Also, provider recommendations for screening intervals for this age group aligned closely with guideline recommendations of every 10 years for screening colonoscopy and annually for FIT. However, in contrast to modeling studies and current guidelines, which suggest comparable test effectiveness for reducing CRC mortality with full test adherence, substantially fewer providers believed FIT was as effective as colonoscopy for reducing CRC mortality. Most providers believed screening was of unproven effectiveness for persons younger or older than conventional screening ages and few recommended it for these populations. If screening recommendations are extended to older and younger age groups, as recommended by some guidelines, targeting providers with relevant evidence-based screening test information should be evaluated as a strategy for increasing provider beliefs in test effectiveness and patient uptake of CRC screening.
Supplementary Material
Acronyms
- CI
confidence interval
- CRC
colorectal cancer
- FIT
fecal immunochemical test
- IRBs
institutional review boards
- KPNC
Kaiser Permanente Northern California
- KPSC
Kaiser Permanente Southern California
- KPWA
Kaiser Permanente Washington
- OR
odds ratio
- PROSPR I
Population-based Research Optimizing Screening through Personalized Regimens I
- PROSPR II
Population-based Research to Optimize the Screening Process II
- US
United States
- USPSTF
US Preventive Services Task Force
- Parkland-UTSW
Parkland Health & Hospital System/University of Texas Southwestern
Footnotes
Conflict of Interest
The authors declare no potential conflicts of interest.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- 2.Brenner H, Chang-Claude J, Seiler CM, et al. Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med 2011;154:22–30. [DOI] [PubMed] [Google Scholar]
- 3.Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012;366:687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.About 80% by 2018: National Colorectal Cancer Roundtable. [Google Scholar]
- 5.Benson VS, Atkin WS, Green J, et al. Toward standardizing and reporting colorectal cancer screening indicators on an international level: The International Colorectal Cancer Screening Network. Int J Cancer 2012;130:2961–73. [DOI] [PubMed] [Google Scholar]
- 6.Force USPST, Bibbins-Domingo K, Grossman DC, et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2016;315:2564–75. [DOI] [PubMed] [Google Scholar]
- 7.Zauber AG, Lansdorp-Vogelaar I, Knudsen AB, et al. Evaluating test strategies for colorectal cancer screening: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med 2008;149:659–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levin TR, Corley DA, Jensen CD, et al. Effects of Organized Colorectal Cancer Screening on Cancer Incidence and Mortality in a Large Community-Based Population. Gastroenterology 2018;155:1383–1391 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singal AG, Corley DA, Kamineni A, et al. Patterns and predictors of repeat fecal immunochemical and occult blood test screening in four large health care systems in the United States. Am J Gastroenterol 2018;113:746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barlow WE, Beaber EF, Geller BM, et al. Evaluating screening participation, follow-up and outcomes for breast, cervical and colorectal cancer in the PROSPR consortium. J Natl Cancer Inst 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colorectal Cancer Facts & Figures 2017–2019. Atlanta: American Cancer Society, 2017. [Google Scholar]
- 12.Honein-AbouHaidar GN, Kastner M, Vuong V, et al. Systematic Review and Meta-study Synthesis of Qualitative Studies Evaluating Facilitators and Barriers to Participation in Colorectal Cancer Screening. Cancer Epidemiol Biomarkers Prev 2016;25:907–17. [DOI] [PubMed] [Google Scholar]
- 13.Breen N, Meissner HI. Toward a system of cancer screening in the United States: trends and opportunities. Annu Rev Public Health 2005;26:561–82. [DOI] [PubMed] [Google Scholar]
- 14.Halm EA, Beaber EF, McLerran D, et al. Association Between Primary Care Visits and Colorectal Cancer Screening Outcomes in the Era of Population Health Outreach. J Gen Intern Med 2016;31:1190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yabroff KR, Klabunde CN, Yuan G, et al. Are physicians’ recommendations for colorectal cancer screening guideline-consistent? J Gen Intern Med 2011;26:177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tiro JA, Kamineni A, Levin TR, et al. The colorectal cancer screening process in community settings: a conceptual model for the population-based research optimizing screening through personalized regimens consortium. Cancer Epidemiol Biomarkers Prev 2014;23:1147–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haas JS, Sprague BL, Klabunde CN, et al. Provider Attitudes and Screening Practices Following Changes in Breast and Cervical Cancer Screening Guidelines. J Gen Intern Med 2016;31:52–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haas JS, Barlow WE, Schapira MM, et al. Primary Care Providers’ Beliefs and Recommendations and Use of Screening Mammography by their Patients. J Gen Intern Med 2017;32:449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jensen CD, Corley DA, Quinn VP, et al. Fecal Immunochemical Test Program Performance Over 4 Rounds of Annual Screening: A Retrospective Cohort Study. Ann Intern Med 2016;164:456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green BB, Anderson ML, Cook AJ, et al. A centralized mailed program with stepped increases of support increases time in compliance with colorectal cancer screening guidelines over 5 years: A randomized trial. Cancer 2017;123:4472–4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singal AG, Gupta S, Tiro JA, et al. Outreach invitations for FIT and colonoscopy improve colorectal cancer screening rates: A randomized controlled trial in a safety-net health system. Cancer 2016;122:456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McQueen A, Bartholomew LK, Greisinger AJ, et al. Behind closed doors: physician-patient discussions about colorectal cancer screening. J Gen Intern Med 2009;24:1228–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zettler M, Mollon B, da Silva V, et al. Family physicians’ choices of and opinions on colorectal cancer screening modalities. Can Fam Physician 2010;56:e338–44. [PMC free article] [PubMed] [Google Scholar]
- 24.Hawley ST, McQueen A, Bartholomew LK, et al. Preferences for colorectal cancer screening tests and screening test use in a large multispecialty primary care practice. Cancer 2012;118:2726–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Farrell CM, Green BB, Reid RJ, et al. Physician-patient colorectal cancer screening discussions by physicians’ screening rates. J Am Board Fam Med 2012;25:771–81. [DOI] [PubMed] [Google Scholar]
- 26.Selby K, Cornuz J, Gachoud D, et al. Training primary care physicians to offer their patients faecal occult blood testing and colonoscopy for colorectal cancer screening on an equal basis: a pilot intervention with before-after and parallel group surveys. BMJ Open 2016;6:e011086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin 2018;68:250–281. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.