Abstract
It has been demonstrated that two Golgi stacking proteins, GRASP55 and GRASP65, self-interact to form trans-oligomers that tether adjacent Golgi membranes into stacks and ribbons in mammalian cells. This ensures proper functioning of the Golgi apparatus in protein trafficking and processing. More recently, GRASP proteins have drawn extensive attention from researchers due to their diverse and essential roles in and out of the Golgi in different organisms. In this review, we summarize their established roles in Golgi structure formation and function under physiological conditions. We then highlight the emerging and divergent roles for individual GRASP proteins, focusing on GRASP65 in cell migration and apoptosis and GRASP55 in unconventional protein secretion and autophagy under stress or pathological conditions.
Keywords: apoptosis, autophagy, cell migration, Golgi stacking, membrane trafficking, unconventional protein secretion
Overview of Golgi structure and function
The Golgi apparatus (or “the Golgi” for simplicity), initially observed in 1898 by Camillo Golgi, functions as the center of the conventional exocytic pathway. To fulfill its essential roles, the Golgi has developed a unique stacked and ribbon-like structure in animal cells [1]. Its basic unit is a polarized stack of closely opposed and flattened cisternae (see Glossary) with fenestrated rims. Each stack is usually defined with three separate modules: the cis-Golgi network (CGN), which is close to the nucleus and receives almost the entire output of the endoplasmic reticulum (ER); the stacked cis-, medial-, and trans-Golgi cisternae that contain enzymes and process cargo proteins and lipids, including glycosylation, sulfation, phosphorylation and proteolysis; and the trans-Golgi network (TGN) that is often facing the plasma membrane and associated with sorting cargos for their final destinations inside or outside of the cell [2].
Although stacking is the most characteristic feature of the mammalian Golgi, different organisms show variations in the morphological organization of the Golgi. For instance, in the budding yeast Saccharomyces cerevisiae, the Golgi membranes do not normally form stacks [3]. For some unicellular eukaryotes, including parasites and algae, each cell contains a single Golgi stack. In the vast majority of organisms, including most fungi, plants and invertebrates, multiple Golgi stacks are scattered independently throughout the cytoplasm. Whereas in interphase mammalian cells, often about 100 Golgi stacks are laterally linked by tubular structures to form a compact Golgi ribbon located in the perinuclear region [4]. Therefore, stack and ribbon structures are evolutionarily developed features of the Golgi.
Despite its structured organization, the Golgi is a highly dynamic organelle, which undergoes morphological changes under physiological conditions such as the cell cycle progression [5] (Box 1), or under certain stress and pathological conditions such as inflammation, neurodegeneration and cancer [6]. The unique morphology and highly dynamic nature of the Golgi apparatus have prompted numerous studies aimed at understanding the molecular mechanisms that regulate Golgi structure and function. Early morphological and biochemical research revealed proteinaceous connections that bridge the adjacent cisternae [7, 8]. In 1994, Graham Warren and colleagues isolated a detergent and salt-resistant protein complex and introduced the concept of “Golgi matrix” [9]. The first identified component of the Golgi matrix was GM130, an extended rod-like protein on the cis-Golgi [10]. Numerous Golgi matrix proteins have since been discovered, including Golgi reassembly stacking proteins (GRASPs) and golgins, which are vital for Golgi structural organization and protein trafficking [11–14]. While golgins tether vesicles to Golgi cisternal membranes [15–18], GRASPs hold Golgi cisternae into stacks [19–21]; they work together to maintain Golgi structure and function [22]. So far, two Golgi stacking proteins, GRASP65 (the Golgi ReAssembly Stacking Protein of 65 kDa, also called GORASP1) and GRASP55 (GORASP2), have been identified and named for their specific roles in Golgi stack formation [20, 23]. In addition to Golgi structure formation, these GRASP proteins also play essential roles outside of the Golgi, such as in unconventional protein secretion and autophagy. In this review, we attempt to summarize the emerging roles of GRASP55 and GRASP65 in the Golgi and beyond.
Box 1. Overview of Golgi structure and dynamics during the mammalian cell cycle.
The mammalian Golgi is a highly dynamic membrane structure, with a unique and highly regulated disassembly and reassembly process during the cell cycle. The whole process can be divided into three distinct stages to disassemble and reassemble the Golgi cisternae, stacks, and ribbon. It requires the cooperation of many factors, including Golgi matrix proteins, membrane tethers, vesicle budding and fusion machineries, and the cytoskeleton. Notably, reversible post-translational modifications, such as acetylation, phosphorylation and ubiquitination, play essential roles in regulating this sophisticated process [2, 5, 58].
During interphase, the Golgi cisternae are held together by GRASP65 and GRASP55 trans-oligomers, multiple Golgi stacks are often laterally linked by tubular structures to form a Golgi ribbon. Assembly of Golgi ribbon in the perinuclear region requires an intact microtubule network; and the microtubule minus end-directed motor, the dynein/dynactin complex, is required to bring Golgi membranes into the cell center. COPI transport vesicles bud from the Golgi membranes, and then are targeted to the acceptor membranes by the giantin-p115-GM130 tethering complex, which facilitates vesicle fusion catalyzed by SNARE proteins.
Early in mitosis, phosphorylation of GM130 by Cdk1 prevents its binding to p115, which blocks vesicle tethering and subsequent fusion. GRASP65 and GRASP55 are phosphorylated by Cdk1, Plk1 and MEK1/ERK2, respectively, which disrupts GRASP oligomerization and results in ribbon unlinking and cisternal unstacking. Unstacking increases the membrane surface for COPI vesicle budding, while disruption of the tethering complex attenuates membrane fusion, both lead to extensive disassembly of the Golgi into vesicles and tubular structures. Microtubules are also reorganized into a mitotic spindle. Most of the derived vesicles disassociate from the spindle microtubules, while some Golgi remnants remain associated with the spindle.
In late mitosis, the Golgi reforms by the fusion processes mediated by two AAA ATPases, N-ethylmaleimide-sensitive factor (NSF) and valosin-containing protein (p97), with their adaptor proteins. NSF and its cofactors, α/γ-SNAPs, are required for membrane fusion by mediating SNARE complex dissociation and thus recycling of SNARE proteins for the next round of membrane fusion. Monoubiquitination of the Golgi t-SNARE protein Syntaxin 5 by HACE1 in mitosis, as well as its deubiquitination by VCIP135 in late mitosis, is required for p97/p47-mediated membrane fusion [92]. Dephosphorylation of GM130 by PP2A resumes its interaction with p115 and helps restore membrane fusion and generate new cisternae. Reformed cisternae are stacked once GRASP65 is dephosphorylated by PP2A, while the phosphatase for GRASP55 remains unknown. Subsequently, microtubules generated from both the Golgi stacks and the centrosome direct Golgi stacks moving toward each other and to the cell center for the formation of a new Golgi ribbon.
GRASP55 and GRASP65 play complementary roles in Golgi stack and ribbon formation
GRASPs are evolutionally conserved; their orthologs and homologs have been identified in different species, including yeast, parasites, and flies, but not in plants [24]. The human GRASP55 gene (on chromosome 2) is believed to be duplicated from a region within chromosome 3 where GRASP65 is localized [25]. In mammalian cells, GRASP65 is present in cis-Golgi, while GRASP55 is more concentrated in the medial- and trans-cisternae [20]. Although most of these GRASPs are found localized to the Golgi, a subset is also detected on some other membranes. For instance, the single Drosophila GRASP ortholog dGRASP is also observed on the ER exit sites (ERES) and on the ER itself [26]. Grh1, the single GRASP ortholog of the budding yeast S. cerevisiae, is also found at the translational ER sites (tER) where it binds to COPII vesicles [27].
GRASP55 and GRASP65 tether Golgi membranes together by forming trans-oligomers
GRASP55 and GRASP65 are peripheral membrane proteins and anchored to the Golgi via a myristic acid attached to the N-terminal glycine residue (Figure 1) [28]. Both GRASPs contain an N-terminal highly conserved GRASP domain which is comprised of two bona fide PDZ domains in tandem. These PDZ domains allow GRASPs to form homodimers and then trans-oligomers that hold adjacent cisternae together into stacks [21, 29, 30]. In addition to N-terminal myristoylation, the GRASP domain of GRASP65 and GRASP55 proteins interact with their Golgi receptors, GM130 [19] and Golgin-45 [31], respectively. This dual anchoring of GRASPs onto the Golgi membranes restricts the orientation of the protein to form trans-oligomers rather than pairing over cis [28, 32]. Both GRASPs contain a C-terminal serine/proline-rich (SPR) domain that is quite divergent, but is enriched of serine, threonine and proline residues. Phosphorylation of the SPR domain regulates GRASP oligomerization and Golgi stacking [1, 30]. The crystal structure of the GRASP domain is globular, with a 6.5-nm diameter [33, 34], which fits well the 11-nm inter-cisternal gap in the stack. The crystal structure of full length GRASP55 or GRASP65 is still not available, and thus how phosphorylation at the SPR-domain affects oligomerization of the GRASP-domain remains unknown.
Figure 1. GRASP depletion accelerates protein trafficking but impairs protein glycosylation and sorting in mammalian cells.
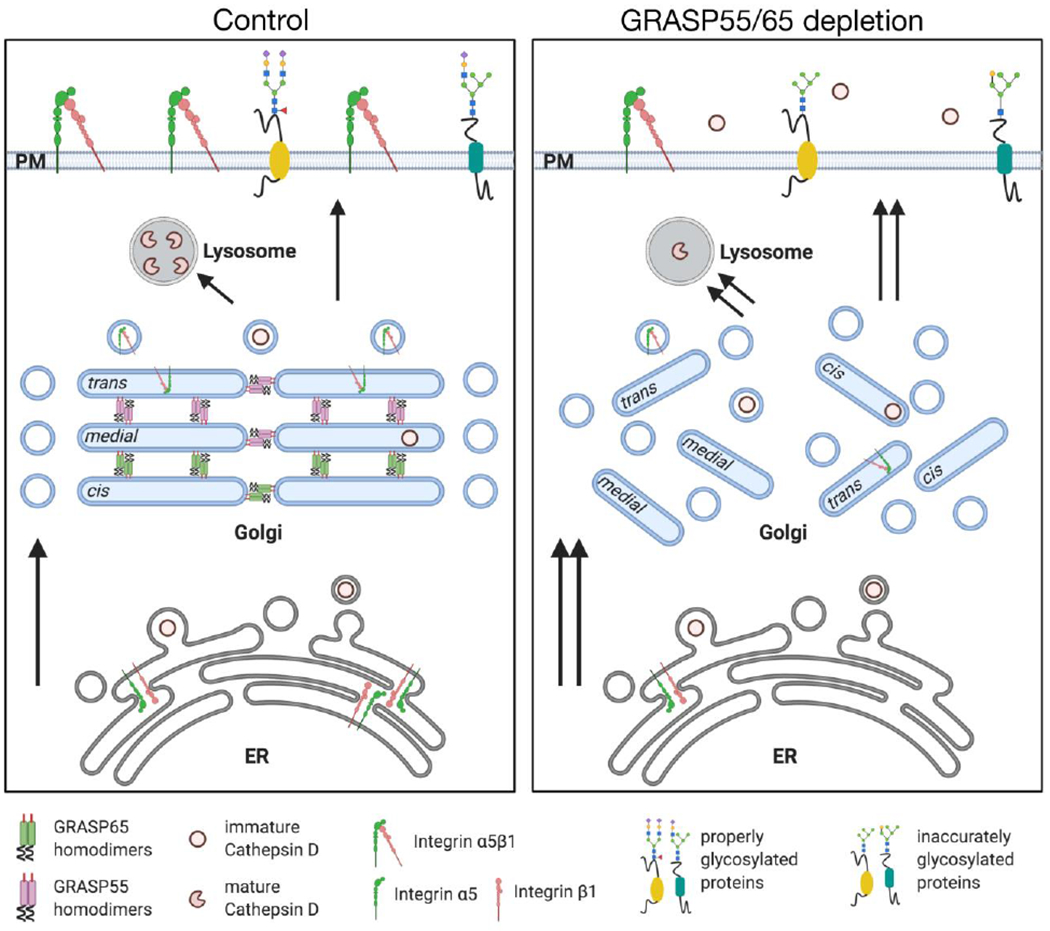
In mammalian cells, GRASP65 localizes to cis-Golgi, while GRASP55 is more concentrated in the medial- and trans-cisternae. GRASPs from adjacent cisternae form homodimers and then trans-oligomers that function as a “glue” to hold Golgi membranes into stacks and ribbon (left control panel). The schematic structure of GRASPs homodimers in this and other figures are as follows: the red bar indicates the myristic acid attached to the N-terminal glycine residue for Golgi membrane association; the N-terminal conserved GRASP domain (each contains two PDZ domains) is represented with rectangular shape, with GRASP65 in green and GRASP55 in pink; the curved line indicates the disordered C-terminal SPR domain. In control cells with both GRASPs (left), cargo molecules, such as Cathepsin D and integrin α5β1, exit the ER and travel through the Golgi stack step-by-step to be sequentially processed by different Golgi enzymes. At the TGN, they are sorted and delivered to their final destinations, including lysosomes or plasma membrane. While in GRASP55/65 depleted cells (right), disruption of the Golgi stack and ribbon increases membrane areas accessible for vesicles budding and fusion, and therefore accelerated cargo transport through the Golgi membranes. Significantly, Golgi destruction impairs accurate protein glycosylation and leads to mis-sorting of the Cathepsin D precursor into the extracellular space. Notably, the synthesis of integrin α5β1 has also been reduced with unknown reasons. The arrows and its numbers indicate the direction and speed of trafficking. The plasma membrane has been enlarged in this diagram for better recognition of the glycosylated transmembrane proteins. Abbreviations: ER, Endoplasmic reticulum; PM, plasma membrane.
Depletion of GRASP55 and GRASP65 proteins disrupts the Golgi structure in mammalian cells
The role of GRASPs in Golgi stack formation have been explored using a number of experimental approaches. The first evidence for GRASP65’s function as a stacking factor came from an in vitro cell-free Golgi reassembly assay in which inhibition of GRASP65, using recombinant proteins or antibodies, blocked the formation of Golgi stacks but not the generation of single cisternae [19]. Subsequently, inhibition of GRASP55/65 functions in cells by microinjection of antibodies, or by knockdown (KD, by RNAi) or knockout (KO, by CRISPR/Cas9) of the proteins, impairs Golgi stack formation [19–21, 35–37]. Depletion of a single GRASP protein reduces the number of cisternae per Golgi stack, whereas simultaneous removal of both GRASP55 and GRASP65 leads to the disassembly of the entire stack [37, 38]. These results demonstrate that GRASP55 and GRASP65 play complementary roles in Golgi stack formation.
In addition to stacking, GRASP55 and GRASP65 have also been shown to link Golgi stacks into a ribbon in mammalian cells. Acute GRASP65 inactivation causes Golgi ribbon unlinking specifically at the cis side, while GRASP55 inactivation affected the Golgi ribbon more at the trans side, which is consistent with their localization at specific cisternae [39]. This supports the non-redundant functions of GRASP65 and GRASP55 in Golgi ribbon linking.
GRASP55 and GRASP65 single knockout mice have been reported. However, only limited defects in Golgi structure and function were detected [40, 41]. One potential concern regarding the GRASP65 knockout mouse strain is that some mRNA encoding exon 1-3 is still present, which encodes a 115 aa N-terminal fragment of GRASP65 [40]. If translated, which is not confirmed, this fragment would be sufficient for Golgi stacking [36]. A similar concern exists in the GRASP55 knockout mouse. Alternatively, the lack of a more prominent effect of single GRASP knockout on the Golgi may be due to the compensation by the other GRASP protein. Most recently, a GRASP55 and GRASP65 double knockout mice was reported [42]. While conventional knockout of both GRASPs is embryonically lethal, the authors used tamoxifen-induced Cre recombinase to knockout GRASP55 in the GRASP65 knockout mouse described above. Analysis of small intestine cells in this GRASP55 and GRASP65 double knockout mouse revealed defects in Golgi ribbon unlinking. Golgi stacks remained intact but contained only 3 cisternae in each stack [42]. While related information in wild type mice was not shown in the report, this number is apparently lower than that of 5-8 cisternae/stack reported in normal animal cells and tissues [43–45]. Similar to GRASP65, a truncated GRASP55 protein harboring PDZ1, half of PDZ2 and a very short C-terminus may still be translated in this mouse [42], which may explain the observation that the cisternal core is still stacked. Therefore, a proper GRASP55 and GRASP65 double knockout mouse with careful investigation of the phenotype in different tissues is still needed to fully understand their roles in Golgi structure and function.
GRASP-interacting proteins may help GRASPs in Golgi structure formation
Despite the rigorous experimental validations, there is still debate as to whether GRASPs act as stacking factors. First, budding yeast Saccharomyces cerevisiae has a single GRASP homologue Grh1 but its Golgi does not normally form stacks. Second, knockdown of both dGRASP and GM130 in Drosophila S2 cells resulted in more dramatic Golgi disassembly than the depletion of dGRASP alone [26]. More recently, the Rothman lab showed that efficient stacking occurs in the absence of GRASP65/55 when either GM130 or Golgin-45 was overexpressed, and thus hypothesized that multiple proteins, including GRASP55/65, Golgin-45, GM130, and perhaps others, may work together and contribute to the adhesive force in Golgi stack formation [46]. This hypothesis indicates a higher complexity in Golgi stacking, and helps explain how Golgi stacking occurs in plants in which no GRASP proteins have been found.
Similarly, a number of new GRASP65-interacting proteins have recently been identified to contribute to Golgi ribbon linking. DjA1 interacts with GRASP65 and enhances Golgi structure formation through promoting GRASP65 trans-oligomerization [47]. Mena is recruited to the Golgi membranes by GRASP65 to facilitate actin polymerization and GRASP65 oligomerization, and thus it functions with actin as bridging proteins of GRASP65 for Golgi ribbon linking [48]. Taken together, GRASP55, GRASP65 and their interacting proteins play central roles in Golgi stack and Golgi ribbon formation in mammalian cells.
GRASP55 and GRASP65 regulate Golgi membrane dynamics during the cell cycle progression
In mitosis, GRASP65 and GRASP55 are the major targets of mitotic kinases for Golgi disassembly (Figure 2). GRASP65 phosphorylation by Cdk1 and Plk1 and GRASP55 phosphorylation by MEK1/ERK2 on multiple sites in their SPR domain lead to the disassembly of GRASP oligomers and subsequent Golgi ribbon unlinking and cisternal unstacking [21, 29]. GRASP65 phosphorylation is also required for cell entry into mitosis and G2/M transition [49–52]. Unstacking dissociates the Golgi cisternae into single layer membranes and provides more membrane surfaces for vesicle budding [53, 54], while phosphorylation of GM130 and other membrane tethers inhibits membrane tethering and reduces vesicle fusion [55, 56]. These together lead to Golgi disassembly in mitosis. At the end of mitosis, GRASP65 dephosphorylation by the protein phosphatase PP2A allows the reformation of GRASP trans-oligomers and restacking of the newly formed cisternae (Box 1) [57]. Consistently, expression of non-phosphorylatable mutants of GRASP55 and GRASP65 in mammalian cells increases the number of cisternae per stack in interphase and inhibits Golgi disassembly in mitosis [36, 38].
Figure 2. GRASP65 plays central roles in Golgi stack formation, mitosis and apoptosis.
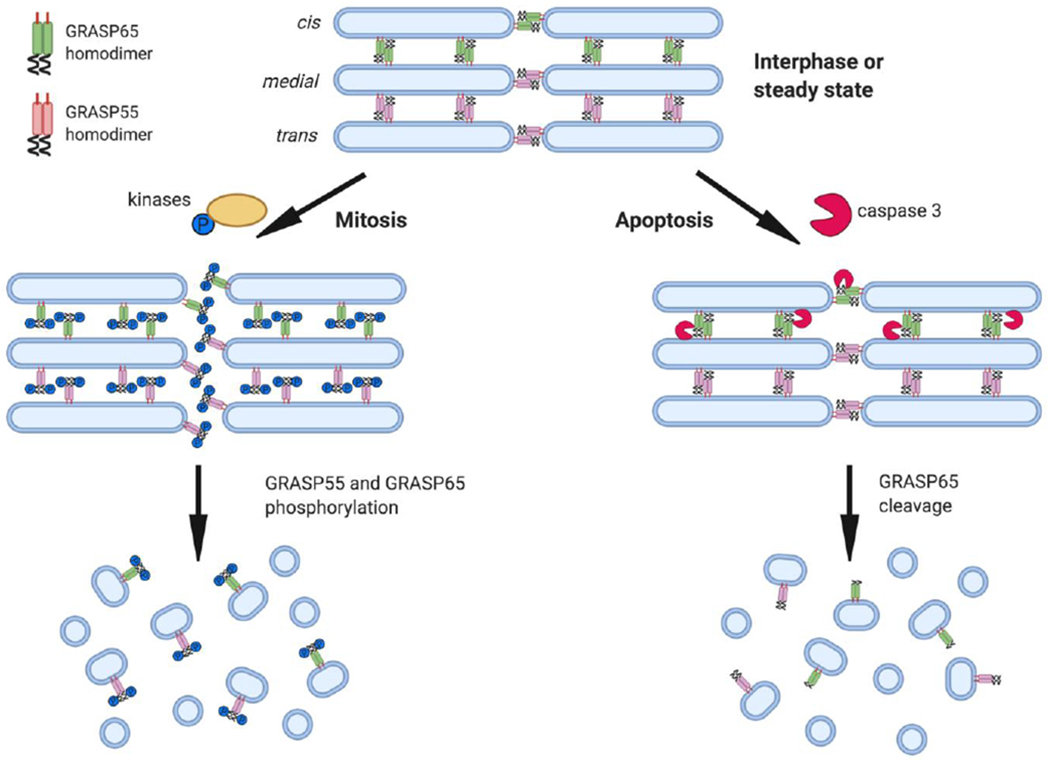
In interphase mammalian cells, GRASP65 and GRASP55 primarily act as membrane tethers for Golgi stack and ribbon formation. During mitosis, both GRASP65 and GRASP55 are phosphorylated at the C-terminal SPR domain by mitotic kinases, which impairs trans-oligomerization of the GRASP domain and facilitates mitotic Golgi disassembly. In apoptosis, activated caspase-3 cleaves the C-terminal SPR domain of GRASP65 but not GRASP55, leading to apoptotic Golgi fragmentation.
In addition to phosphorylation, GRASP55 acetylation also functions in Golgi structure regulation in the cell cycle. GRASP55 is highly acetylated on K50 in mitosis, which inhibits GRASP55 oligomerization and facilitates mitotic Golgi disassembly. Deacetylation of the same site by Sirtuin 2 (SIRT2) is required for GRASP55 self-interaction and proper Golgi reassembly at the mitotic exit [58].
Taken together, self-interaction and post-translational modifications (e.g. phosphorylation and acetylation) are key properties of GRASP55 and GRASP65 that control their roles in Golgi structure formation in interphase and Golgi disassembly in mitosis.
GRASP55 and GRASP65 modulate Golgi function
It has been a long mystery how the unique Golgi architecture is essential for its primary function in protein trafficking and processing. The identification of the Golgi stacking proteins GRASP55 and GRASP65 allows for the manipulation of the Golgi structure and thereby examination of the biological significance of Golgi stack and ribbon formation in mammalian cells (Figure 1).
GRASP55 and GRASP65 regulate protein trafficking through the Golgi
Destruction of the Golgi structure by GRASP depletion accelerates protein trafficking [37, 53, 54]. Hypothetically, the close spatial arrangement of cisternae in stacks minimizes the distance that molecules must travel. Thus, it was long thought that Golgi stacking should enhance protein trafficking. However, depletion of both GRASPs yielded the opposite results for cargo molecules such as VSV-G (vesicular stomatitis virus glycoprotein protein) and integrin [54]. A plausible explanation for these results is that stacking reduces the accessibility of coat proteins to the rims of the stacked Golgi membranes, which decreases the rate of vesicle budding and transport. In support of this hypothesis, unstacking increased the rate of COPI vesicle formation from Golgi membranes [53]. The effect of Golgi dysfunction on protein trafficking has also been observed under pathological conditions. In Alzheimer’s disease, GRASP65 phosphorylation by activated Cdk5 results in Golgi fragmentation, which subsequently accelerates APP (amyloid precursor protein) trafficking and increases Aβ (amyloid-beta peptide) production. Significantly, expression of the non-phosphorylatable GRASP-domain of GRASP65 rescues the Golgi structure and reduces Aβ production [59].
In addition to the regulation of Golgi stack formation and vesicle budding, which indirectly affect protein trafficking, GRASPs may also control trafficking of some cargo molecules via direct interaction. Proteins, such as TGFα, CD83, CD8α, and Frizzled4, contain a C-terminal valine residue that interacts with the PDZ domains of the GRASP proteins. Here, GRASPs function as cargo receptors or chaperones, and are required for efficient trafficking of these transmembrane proteins to the cell surface [2]. More recently, it has been shown that some lipid droplet (LD)-associated proteins, such as ATGL (adipose triglyceride lipase) and MGL (monoglyceride lipase), reach LDs via a Golgi- and GRASP55-dependent route, in which a direct interaction between GRASP55 and ATGL is required, although these proteins do not contain a C-terminal valine [60]. In addition to cargo proteins in the secretory pathway, GRASP55 can also directly bind Golgi enzymes, such as GCS (glucosylceramide synthase) and LCS (lactosylceramide synthase), and promote their anterograde transport and proper enzyme compartmentalization in the Golgi [61]. Taken together, GRASP proteins may regulate protein trafficking either through direct interaction with specific molecules, or via modulating the Golgi stack structure and vesicle budding.
GRASP55 and GRASP65 depletion impairs accurate glycosylation
In mammalian cells, Golgi destruction by GRASP depletion decreases the overall glycan abundance and glycan complexity, altering the glycoprotein composition at the plasma membrane [54]. In the budding yeast, glycosylation in the Golgi mainly involves the addition of mannoses [62]. In multicellular organisms, glycosylation of membrane and secretory proteins is more complex and critical. Accurate glycosylation is essential for multiple cellular activities [63]. This explains why stacking is more important in high multicellular organisms. The Golgi harbors various glycosyltransferases, glycosidases and nucleotide sugar transporters in different subcompartments, but unlike ER that contains a high concentration of folding chaperones that retain improperly modified cargos [64], the Golgi lacks a rigorous system to control the fidelity of its biosynthetic processes. An ordered structure and a controlled cargo flow through the Golgi are likely required to carry out precise, sequential modifications as cargo proteins pass between cisternae [1, 2, 30].
Based on this idea, the cause of the glycosylation defects in GRASP-depleted cells can be explained in several ways. First, Golgi cisternal unstacking accelerates vesicle budding upon GRASPs depletion, which reduces the time that cargo molecules are modified by glycosylation enzymes. Alternatively, Golgi cisternal unstacking and partial vesiculation may cause disorganization of glycosylation enzymes in the Golgi membranes, and therefore disrupts the sequential modifications. In addition, it was proposed that each GRASP plays a cisterna-specific role in linking adjacent stacks to ensure Golgi compartmentalization, enzymes localization, and proper glycosylation [39]. These results indicate that GRASPs play essential roles in linking the ordered Golgi structure with its proper functions.
GRASP55 and GRASP65 depletion results in protein missorting at the TGN
Golgi destruction also causes missorting of Cathepsin D precursor to the extracellular space, suggesting that Golgi stacking may ensure that sorting occurs only when cargo molecules reach the TGN. The exact mechanism of how GRASP depletion causes protein missorting is still unknown, but the defect could involve the cargo molecule Cathepsin D itself or its sorting machinery (e.g. M6P receptors) [65–67]. Since GRASP depletion impairs accurate glycosylation, it may affect Cathepsin D glycosylation and subsequent tagging of mannose-6 phosphate (M6P) on the associated glycan chains. Alternatively, GRASP depletion may affect the localization and trafficking of CI-MPR, the specific M6P receptor which Cathepsin D utilizes for endosome targeting. These possibilities require further investigations.
GRASP55 and GRASP65 depletion affects other cellular activities
GRASP depletion also reduces cell adhesion and cell migration through the reduction of α5β1 integrins, a major and well-characterized cell adhesion complex, via decreasing α5β1 integrin protein synthesis than accelerating its degradation [68]. How exactly GRASP depletion reduces α5β1 integrins remains unknown. Interestingly, GRASP depletion significantly increases total protein synthesis and accelerates cell proliferation [68], consistent with a previous report that inhibition of mitotic Golgi fragmentation by the expression of non-regulatable GRASP65 mutants delays mitotic entry and inhibits cell proliferation [36]. It is possible that the increase of protein trafficking caused by GRASP depletion upregulates overall protein synthesis and accelerates cell proliferation.
In summary, GRASP55 and GRASP65 function together to organize the Golgi architecture and thereby ensure a proper flux of protein trafficking as well as accurate glycosylation and processing.
GRASP65 plays more specific roles in cell migration and apoptosis
Emerging evidences have revealed additional functions that are more specific to GRASP65, such as Golgi reorientation during cell migration and its breakdown in apoptosis.
GRASP65 is more essential for cell attachment and migration than GRASP55
Comparative studies indicate that depletion of GRASP65 results in more dramatic defects on cell attachment and migration than GRASP55 [68]. During directed cell migration of some cell types, temporal Golgi disassembly is required for the orientation of the Golgi toward the leading edge. This rearrangement is needed for the polarized transport of vesicles that provide new membranes to the leading edge. In this process, ERK-mediated GRASP65 phosphorylation at S277 leads to the disassembly of GRASP65 oligomers and causes at least partial Golgi cisternal unstacking [69]. Expression of a GRASP65 phosphorylation-deficient mutant prevents Golgi orientation to the leading edge, indicating that Golgi remodeling through GRASP65 phosphorylation is critical for the establishment of cell polarity in migrating cells [69].
GRASP65-specific roles in apoptosis
GRASP65 also appears to be a key target of caspases on the Golgi for its breakdown during apoptosis. GRASP65 cleavage in the C-terminus by caspase-3 is required for Golgi fragmentation in early apoptosis (Figure 2). The caspase-3 cleavage sites in GRASP65 are not conserved in GRASP55, and GRASP55 is not cleaved in apoptosis [70, 71]. Proteolytic cleavage of GRASP65 and other Golgi structural proteins by caspases provides the main driving force for apoptotic Golgi fragmentation. Accordingly, expression of a caspase-resistant GRASP65 mutant inhibits Golgi fragmentation and protects cells from Fas-mediated apoptosis [71], while overexpression of GRASP65 attenuates oxidized low-density lipoprotein-induced endothelial cell apoptosis [72]. Conversely, knockdown of GRASP65 increases Dihydromyricetin-induced ovarian cancer cell apoptosis [73].
In summary, GRASP65 but not GRASP55 plays essential roles in Golgi rearrangement during cell migration and apoptosis.
GRASP55 plays diverse roles in Golgi stress response, unconventional protein secretion and autophagy
GRASP55 also has some unique functions such as in unconventional protein secretion (UPS) and autophagy, which occur more often under stress conditions.
GRASP55 appears to be more important in Golgi stress response
In a recent study to characterize Golgi stress response, cells were treated acutely with ER stress inducers, thapsigargin (TG), tunicamycin (Tm), or dithiothreitol (DTT). Interestingly, only the TG treatment resulted in Golgi fragmentation, with fewer cisternae per stack and shorter cisternae as seen in GRASP-depleted cells [37, 38], but this occurs independently of ER stress [74]. Further experiments demonstrated that TG induces Golgi fragmentation through elevating intracellular Ca2+ and protein kinase Cα (PKCα) activity, which phosphorylates GRASP55 (but not GRASP65) that likely disrupts GRASP55 oligomerization and Golgi integrity. Significantly, activation of PKCα by the inflammatory agent histamine modulates Golgi structure in a similar fashion [74]. Hence, increased cytosolic Ca2+ may modulate Golgi structure and function through GRASP55 phosphorylation by PKCα.
GRASP55 is required for unconventional protein secretion
Recently, an increasing number of soluble signal peptide-free (or leaderless) cargo proteins and transmembrane proteins have been found to be secreted out of the cell or to reach the plasma membrane in an unconventional manner that bypass the Golgi apparatus (Box 2). Unexpectedly, the secretion of quite a few of these cargos is dependent on GRASPs, especially mammalian GRASP55, although it is unknown why such Golgi-independent protein secretion requires the Golgi structural protein GRASP55 [75–77].
Box 2. Overview of the unconventional protein secretion pathway.
The definition of unconventional protein secretion (UPS) is relative to conventional protein secretion or classical protein secretion (CPS), which has been a consensus for more than 50 years. In eukaryotes, CPS is the trafficking route that secretory proteins reach their destinations via the ER-Golgi-targeted organelle axis. These proteins contain a signal peptide and/or a transmembrane domain that direct their translocation into the ER lumen or on the ER membrane, from which they exit through the coat protein complex II (COPII)-coated vesicles to reach the Golgi apparatus for complex processing. In contrast, the newly identified UPS utilizes a pathway bypassing the Golgi apparatus and is found in all organisms examined, including yeast, fungi, plants, flies and mammals [97, 103].
According to the classification by Rabouille and colleagues, UPS is divided into four types with distinct mechanisms, including Type I, II and III UPS of cytoplasmic leaderless proteins (without a signal peptide), and Type IV UPS of Golgi-bypassing transmembrane proteins [97]. Type I, the leaderless proteins are secreted directly out of the cell through plasma membrane pores. Fibroblast growth factor 2 (FGF2) and HIV transactivator of transcription (TAT) are constitutively secreted through self-made lipidic pores, while cytokine IL-1β is released upon inflammation probably through Gasdermin-N-terminus formed pores at macrophage plasma membrane, followed by cell death. Type II, the ABC transporter-mediated secretion is not well studied and needs further investigation. Type III, the leaderless proteins are first translocated across the membrane of the sequestering ‘secretory’ organelles, such as autophagosomes and endosomes, which are then fused with the plasma membrane for protein release. The best-studied cargo is the cytokine IL-1β but the trigger is starvation, not inflammation as in Type I. IL-1β translocates across the ERGIC membrane into the lumen, and is delivered into secretory vesicles that finally fuse with the plasma membrane, thus ensuring its release as a soluble protein [104]. Type IV UPS, under certain stresses such as ER or mechanical stress, transmembrane proteins, such as ΔF508-CFTR and H723R-pendrin, enter the ER but bypass the Golgi on their way to the cell surface via GRASPs and Hsp70, respectively. Notably, out of the four types mechanisms proposed, GRASP proteins, especially GRASP55 in mammalian cells, participate in both Type III and Type IV pathways that are elicited by cellular stresses. The details have been described in the main text.
The first indication that GRASP proteins participate in unconventional secretion of cytosolic proteins came from a study on AcbA secretion in Dictyostelium discoideum [78]. Due to the lack of an ER signal peptide, AcbA is secreted through an unconventional pathway. This process requires GrpA, the ortholog of the mammalian GRASPs in D. discoideum [78]. Similarly, in yeast Scicchciromyces cerevisiae and Pichia pastoris, the AcbA ortholog Acb1 is released from the cytosol to extracellular space upon starvation, which also depends on the GRASP homologue Grh1 [79, 80]. Subsequently, GRASP homologues have been indicated in the secretion of a variety cytosolic molecules in different species and cell types, such as Udp2 in Drosophila fat cells [81], IL-1β, IL-18, TGFB1 and HMGB1 in mammalian macrophages (Figure 3A) [82, 83], and IDE (insulin-degrading enzyme) secretion in Alzheimer disease neurons [84]. In addition to GRASP55, autophagy is also required for the secretion of some of these proteins [85–87]. Besides proteins, polysaccharide [88] and RNAs [89] can also be secreted in Cryptococcus neoformans in a GRASP-dependent manner. Collectively, despite the identification of multiple substrates in stress-induced, autophagy- and GRASP55-dependent unconventional secretion, no direct interactions between GRASP55 and the cargo molecules (except TGFB1) have been reported (Table 1) [87]. How GRASP55 senses cellular stresses, recruits cytosolic proteins, and sends them to the extracellular space remain as major unanswered questions in the field.
Figure 3. GRASP55 functions in unconventional protein secretion and autophagy.
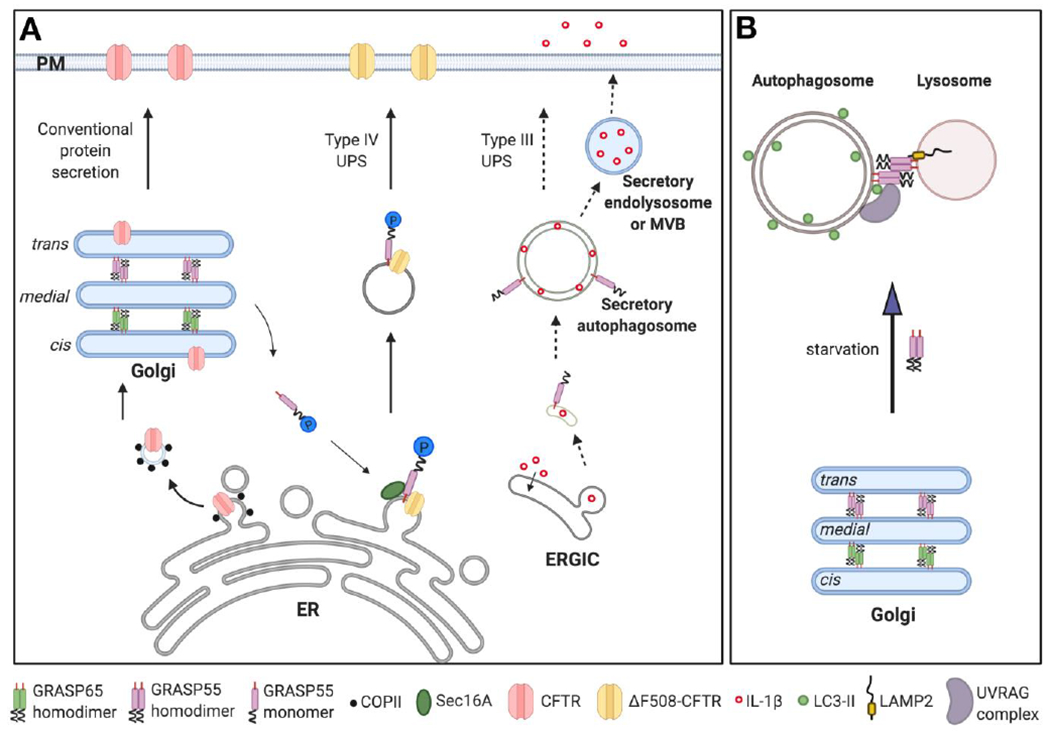
(A) Schematic model of GRASP55 function in unconventional protein secretion (UPS). Most transmembrane proteins, such as CFTR, are conventionally transported from the ER to the Golgi, and then to the plasma membrane (left, Conventional protein secretion pathway). Transmembrane ΔF508-CFTR can be delivered to the plasma membrane via a GRASP55-dependent and Golgi-independent pathway (middle, classified as Type IV UPS). ΔF508-CFTR UPS is induced by ER stress or inhibition of conventional protein secretion, which causes phosphorylation and monomerization of GRASP55. GRASP55 monomer then binds to ΔF508-CFTR and Sec16A at the ER exit sites and facilitates UPS. GRASP55 is also involved in unconventional secretion of some leaderless cytosolic proteins (right, classified as Type III UPS). IL-1β is one of the well-characterized cargo under starvation conditions. Once its mature form is produced, IL-1β is translocated into the ERGIC lumen, and then delivered by unconfirmed membrane carriers, possibly secretory autophagosomes, endolysosome or multi-vesicular bodies (MVB), to the plasma membrane for secretion. Here the exact role of GRASP55 is still unclear. (B) Schematic model of GRASP55 function in Golgi stacking and autophagosome-lysosome tethering. In growth conditions, GRASP55 localizes in medial-trans Golgi to glue adjacent cisternae together into stacks. Upon glucose- or amino acid-starvation, GRASP55 is translocated to the autophagosome and lysosome interface, where it binds LC3-II on autophagosomes and LAMP2 on lysosomes, and thereby facilitates the fusion of these two membrane organelles. Additionally, it promotes the targeting and assembly of the PtdIns3K UVRAG complex on autophagosomes to facilitate autophagosome maturation. For better visibility not all membranes are drawn on the same scale. Abbreviations: ER, Endoplasmic reticulum; ERGIC, the ER-Golgi intermediate compartment; MVB, multi-vesicular bodies; PM, plasma membrane; UPS, unconventional protein secretion.
Table 1.
List of cargo molecules in GRASPs-dependent unconventional protein secretion.
| Name | Species | GRASP binding | Cargo type | Reference |
|---|---|---|---|---|
| AcbA | Dictyostelium | Not reported | cytosolic | [78] |
| Acb1 | Yeast | Not reported | cytosolic | [79, 80] |
| SOD1 | Yeast | Not reported | cytosolic | [105] |
| Udp2 | Drosophila | Not reported | cytosolic | [81] |
| IL-1β | Human | Not reported | cytosolic | [82, 106] |
| IL-18 | Human | Not reported | cytosolic | [82, 83] |
| HMGB1 | Human | Not reported | cytosolic | [83] |
| IDE | Human | Not reported | cytosolic | [84] |
| TGFB1 | Human | Yes | cytosolic | [87] |
| αPS1 integrin | Drosophila | Not reported | transmembrane | [90] |
| αPS2 integrin | Drosophila | Not reported | transmembrane | [90, 107] |
| CLRN1N48K | Zebrafish | Not reported | transmembrane | [96] |
| ΔF508-CFTR | Human | Yes | transmembrane | [91] |
| Mpl | Human | Not reported | transmembrane | [94] |
| TACE | Human | Not reported | transmembrane | [95] |
Abbreviations: AcbA, acyl coenzyme A (CoA) binding protein; SOD1, superoxide dismutase 1; IL-1β, Interleukin-1β; IL-18, Interleukin-18; HMGB1, high mobility group box 1; IDE, insulin-degrading enzyme; TGFB1, transforming growth factor beta 1; CLRN1, Clarin-1; CFTR, cystic fibrosis transmembrane conductance regulator; Mpl, myeloproliferative leukemia virus oncogene; TACE, tumor necrosis factor-α converting enzyme.
Some transmembrane proteins are also trafficked from the ER to the plasma membrane through a GRASP55-dependent but Golgi-independent unconventional protein secretion pathway. The first example came from the study of Drosophila follicle cells at stage 10B, in which the epithelium stretches and the open zone of contact (ZOC) forms. The mechanical stress increases the dGRASP protein locally translation at the open ZOC, where dGRASP acts as a tethering factor for the fusion between ER-derived αPS1 integrin carriers and the plasma membrane [90]. Later, CFTR (cystic fibrosis transmembrane conductance regulator) was identified as the first mammalian transmembrane cargo in GRASP55-dependent unconventional secretion. Phe508 deletion (ΔF508), the most prevalent disease-causing mutant of CFTR, fails to fold properly and is retained in the ER. However, ΔF508-CFTR cell surface expression could be rescued by GRASP55 dependent unconventional secretion [91]. In response to ER stress or ER-to-Golgi blockade, GRASP55 is phosphorylated on serine 441, monomerized, and redistributed from the Golgi to the ER, where it interacts with ΔF508-CFTR via a PDZ1-based interaction [92]. In this process, GRASP55 also associates with Sec16A, which may help deliver ΔF508-CFTR to ER-derived vesicles that depart for the plasma membrane (Figure 3A) [93]. Importantly, components for autophagosome formation are also involved in this process [91]. In addition to CFTR, Mpl (myeloproliferative leukemia virus oncogene) [94], TACE (tumor necrosis factor-α converting enzyme) [95] and CLRN1N48K [96] also traffic through the unconventional, GRASP55-dependent pathway (Table 1).
While it is generally believed that GRASP65 also plays a role in unconventional secretion, at least in mammalian cells, but is less important than GRASP55 that has been more extensively studied. For instance, overexpression of GRASP65 alone increased surface expression of ΔF508-CFTR in HEK293 cells [91]. Consistently, unconventional secretion is heavily induced by cellular stresses such as ER stress [97]; whereas pharmacological induction of ER stress upregulates the expression of GRASP55, but not GRASP65 [98]. This provides a mechanism that places GRASP55 in a more important role in stress-induced autophagy and unconventional protein secretion. It should be noted that there are a number of cargos that do not depend on GRASP55 for their UPS pathway, such as transmembrane protein H723R-pendrin which is Hsp70-dependent, and some cytosolic cargos have not been tested for their GRASP55 dependency yet [75, 97].
GRASP55 plays essential roles in autophagy
To search for energy sensors in the Golgi, we recently surveyed a number of Golgi proteins for O-GlcNAcylation, the single sugar glycosylation that reflects the glucose level. Our results revealed that GRASP55, but not any other Golgi matrix proteins examined, including GRASP65, is O-GlcNAcylated by the O-GlcNAc transferase (OGT) under growth condition. Upon glucose starvation, GRASP55 is de-O-GlcNAcylated and forms puncta outside of the Golgi area. Further investigations demonstrated that de-O-GlcNAcylated GRASP55 is targeted to the autophagosome-lysosome interface, where it binds LC3-II on autophagosomes and LAMP2 on lysosomes, and functions as a membrane tether to facilitate autophagosome-lysosome fusion [99, 100], similar to its role in Golgi stacking (Figure 3B).
The role of GRASP55 in autophagosome maturation is not limited to glucose starvation, but also applies to amino acid starvation. Upon amino acid starvation, GRASP55 not only physically interacts with LC3-II and LAMP2, but also interacts with BECN1 to facilitate the assembly and membrane association of the PtdIns3K (phosphatidylinositol 3-kinase) UVRAG complex, which is known to facilitate autophagosome-lysosome fusion (Figure 3B) [101]. These findings are important as they provide not only the mechanism of how GRASP55 senses cellular stresses such as energy and nutrient deprivation, but also how GRASP55 reacts to help cells cope with the related stresses by increasing the autophagic flux.
Taken together, GRASP55 may serve as both a stress and nutrient sensor and an effector to balance Golgi-mediated intracellular trafficking and autophagy (Figure 3) [100, 102]. Future studies will investigate how GRASP55 responds to different cellular stressors as well as how it controls autophagic flux to facilitate unconventional protein secretion.
Concluding Remarks
Extensive studies have provided mounting evidence that GRASP55 and GRASP65 display essential roles inside and outside of the Golgi. While some of the functions are evolutionally conserved, others are less apparent. Most lower organisms contain a single GRASP ortholog whose function in unconventional protein secretion appears to be conserved, while its role in Golgi stack formation remains unproved. In mammalian cells, duplication of the gene provides both GRASP55 and GRASP65 proteins to maintain unconventional secretion and satisfy the needs for better control of Golgi structure and function. In parallel, the Golgi has evolved to form a more specialized polarized stack and laterally linked ribbon structure for its critical and complex roles in protein trafficking, processing and sorting, especially glycosylation. Here, GRASP65 and GRASP55 have gained their cisterna specific roles as membrane tethers for Golgi stack formation and ribbon linking to ensure Golgi sub-compartmentalization, enzymes localization, and proper glycosylation. However, the question remains as to the adhesive machinery for stack formation in lower eukaryotic organisms, and in plants where GRASP homologues are still missing (see Outstanding Questions).
Outstanding Questions.
What is the phenotype of GRASP55 and GRASP65 double knockout mice in Golgi structure and function?
What proteins other than GRASPs provide the adhesive force in Golgi stack formation in lower organisms as well as in plants where a GRASP orthologue is still missing?
What do the crystal structures of full length GRASP55 or GRASP65 look like? How do post-translational modifications of the SPR domain affect oligomerization of the GRASP domain?
How is GRASP55 targeted to different membrane structures? How is it affected by certain stresses?
Why is GRASP55 required for unconventional secretion of some specific proteins but not others?
What determines the cargo specificity in GRASP55-dependent unconventional protein secretion?
In mammalian cells, it seems that the two GRASPs have evolved to gain different functions. GRASP65 largely maintains its housekeeping roles in modulating the Golgi structure and function as well as other cellular activities related to the Golgi structure, such as cell cycle progression, apoptosis and cell migration. In comparison, although GRASP55 plays essential roles in holding medial- and trans-Golgi cisternae into stacks, it also plays important roles in unconventional protein secretion and autophagy in response to stresses. Consistently, GRASP55 is more frequently redistributed to other membrane organelles, such as the ER and autophagosomes. How GRASP55 is targeted to different membranes under different conditions remains as an outstanding question in the field. Considering the significance of GRASP55-dependent unconventional protein secretion under pathological conditions, identifying GRASP55-specific binding partners under specific stress conditions in the future may shed light on the entire signaling event in pathology (see Outstanding Questions).
Highlights.
GRASP55 and GRASP65 play complementary roles in maintaining the Golgi structure by forming trans-oligomers in mammalian cells.
Depletion of GRASP55 and GRASP65 in cells impairs Golgi functions in protein trafficking, sorting and glycosylation, and reduces cell adhesion and cell migration.
GRASP-interacting proteins may help GRASPs in Golgi structure formation and function.
GRASP65 is the target of signaling events leading to Golgi reorientation during cell migration and breakdown during apoptosis.
GRASP55 participates in unconventional protein secretion of both cytosolic and transmembrane proteins in response to certain stresses.
GRASP55 facilitates autophagosome maturation under starvation conditions.
Acknowledgements
We thank Wang Lab members, particularly Erpan Ahat and Jie Li, for their insightful discussions. This work was supported by the National Institutes of Health (Grants GM112786 and GM130331), M-Cubed, and the Fastforward Protein Folding Disease Initiative of the University of Michigan to YW. The figures are created with BioRender.com.
Glossary
- Autophagy
most often referred to as macroautophagy, is an evolutionarily conserved intracellular degradation process, which is crucial for clearance of protein aggregates and turnover of damaged organelles for cellular homeostasis; also important for survival of eukaryotic cells in response to stress conditions, such as nutrient and energy deprivation
- Cisterna (plural cisternae)
a flattened membrane disk of the endoplasmic reticulum (ER) and Golgi apparatus
- COPI vesicle
a type of coated vesicle that transports proteins from the Golgi apparatus to the ER, and between Golgi compartments
- COPII vesicles
a type of coated vesicles that transport proteins from the ER to the Golgi apparatus
- Golgi matrix
a collection of proteins involved in the structure and function of the Golgi apparatus; a mesh connecting Golgi cisternae and associated vesicles under electron microscopy (EM)
- Myristoylation
a lipidation modification where a myristoyl group, derived from myristic acid, is covalently attached to the alpha-amino group of an N-terminal glycine residue
- O-GlcNAc transferase (OGT)
an enzyme that catalyzes the addition of a single sugar N-acetylglucosamine (GlcNAc) through an O-glycosidic linkage to serine or threonine, or through a S-glycosidic linkage to cysteine residues of intracellular proteins
- PDZ domains
well-defined structural motifs of around 80-90 amino acids organized in six β-sheets and two α-helices allowing them for specific protein-protein interactions; PDZ is an initialism combining the first letters of the first three proteins discovered to share the domain - PSD95, Dlg1 and Zo-1
- Signal peptide
a short peptide (usually 16-30 amino acids long) present at the N-terminus of the majority of newly synthesized proteins that are targeted to the ER and eventually destined to be either secreted out of the cell, or transported to different membranous cellular compartments
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhang X and Wang Y (2016) Glycosylation Quality Control by the Golgi Structure. J Mol Biol 428 (16), 3183–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang S and Wang Y (2017) Golgi structure formation, function, and post-translational modifications in mammalian cells. F1000Res 6, 2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suda Y and Nakano A (2012) The yeast Golgi apparatus. Traffic 13 (4), 505–10. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y and Seemann J (2011) Golgi biogenesis. Cold Spring Harb Perspect Biol 3 (10), a005330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang D and Wang Y (2013) Cell cycle regulation of Golgi membrane dynamics. Trends Cell Biol 23 (6), 296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makhoul C et al. (2019) Golgi Dynamics: The Morphology of the Mammalian Golgi Apparatus in Health and Disease. Front Cell Dev Biol 7, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mollenhauer HH (1965) An Intercisternal Structure in the Golgi Apparatus. J Cell Biol 24 (3), 504–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franke WW et al. (1972) Inter- and intracisternal elements of the Golgi apparatus. A system of membrane-to-membrane cross-links. Z Zellforsch Mikrosk Anat 132 (3), 365–80. [DOI] [PubMed] [Google Scholar]
- 9.Slusarewicz P et al. (1994) Isolation of a matrix that binds medial Golgi enzymes. J Cell Biol 124 (4), 405–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakamura N et al. (1995) Characterization of a cis-Golgi matrix protein, GM130. J Cell Biol 131 (6 Pt 2), 1715–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lupashin V and Sztul E (2005) Golgi tethering factors. Biochim Biophys Acta 1744 (3), 325–39. [DOI] [PubMed] [Google Scholar]
- 12.Ramirez IB and Lowe M (2009) Golgins and GRASPs: holding the Golgi together. Semin Cell Dev Biol 20 (7), 770–9. [DOI] [PubMed] [Google Scholar]
- 13.Short B et al. (2005) Golgins and GTPases, giving identity and structure to the Golgi apparatus. Biochim Biophys Acta 1744 (3), 383–95. [DOI] [PubMed] [Google Scholar]
- 14.Xiang Y and Wang Y (2011) New components of the Golgi matrix. Cell Tissue Res 344 (3), 365–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura N et al. (1997) The vesicle docking protein p115 binds GM130, a cis-Golgi matrix protein, in a mitotically regulated manner. Cell 89 (3), 445–55. [DOI] [PubMed] [Google Scholar]
- 16.Malsam J et al. (2005) Golgin tethers define subpopulations of COPI vesicles. Science 307 (5712), 1095–8. [DOI] [PubMed] [Google Scholar]
- 17.Satoh A et al. (2003) Golgin-84 is a rabl binding partner involved in Golgi structure. Traffic 4 (3), 153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shorter J et al. (2002) Sequential tethering of Golgins and catalysis of SNAREpin assembly by the vesicle-tethering protein p115. J Cell Biol 157 (1), 45–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barr FA et al. (1997) GRASP65, a protein involved in the stacking of Golgi cisternae. Cell 91 (2), 253–62. [DOI] [PubMed] [Google Scholar]
- 20.Shorter J et al. (1999) GRASP55, a second mammalian GRASP protein involved in the stacking of Golgi cisternae in a cell-free system. Embo J 18 (18), 4949–4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y et al. (2003) A direct role for GRASP65 as a mitotically regulated Golgi stacking factor. Embo J 22 (13), 3279–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shorter J and Warren G (2002) Golgi architecture and inheritance. Annu Rev Cell Dev Biol 18, 379–420. [DOI] [PubMed] [Google Scholar]
- 23.Barr FA et al. (1998) Mapping the interaction between GRASP65 and GM130, components of a protein complex involved in the stacking of Golgi cisternae. Embo J 17 (12), 3258–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X and Wang Y (2015) GRASPs in Golgi Structure and Function. Front Cell Dev Biol 3, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Protopopov A et al. (2003) An integrated physical and gene map of the 3.5-Mb chromosome 3p21.3 (AP20) region implicated in major human epithelial malignancies. Cancer Res 63 (2), 404–12. [PubMed] [Google Scholar]
- 26.Kondylis V et al. (2005) dGRASP localization and function in the early exocytic pathway in Drosophila S2 cells. Mol Biol Cell 16 (9), 4061–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levi SK et al. (2010) The yeast GRASP Grh1 colocalizes with COPII and is dispensable for organizing the secretory pathway. Traffic 11 (9), 1168–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rabouille C and Linstedt AD (2016) GRASP: A Multitasking Tether. Front Cell Dev Biol 4, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y et al. (2005) Mapping the functional domains of the Golgi stacking factor GRASP65. J Biol Chem 280 (6), 4921–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang X and Wang Y (2015) GRASPs in Golgi Structure and Function. Frontiers in Cell and Developmental Biology 3, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Short B et al. (2001) A GRASP55-rab2 effector complex linking Golgi structure to membrane traffic. J Cell Biol 155 (6), 877–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bachert C and Linstedt AD (2010) Dual anchoring of the GRASP membrane tether promotes trans pairing. J Biol Chem 285 (21), 16294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Truschel ST et al. (2011) Structure of the membrane-tethering GRASP domain reveals a unique PDZ ligand interaction that mediates Golgi biogenesis. J Biol Chem 286 (23), 20125–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng Y et al. (2013) Structural insight into Golgi membrane stacking by GRASP65 and GRASP55 proteins. J Biol Chem 288 (39), 28418–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sutterlin C et al. (2005) The Golgi-associated protein GRASP65 regulates spindle dynamics and is essential for cell division. Mol Biol Cell 16 (7), 3211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang D et al. (2010) The Role of GRASP65 in Golgi Cisternal Stacking and Cell Cycle Progression. Traffic 11 (6), 827–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bekier ME 2nd et al. (2017) Knockout of the Golgi stacking proteins GRASP55 and GRASP65 impairs Golgi structure and function. Mol Biol Cell 28 (21), 2833–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiang Y and Wang Y (2010) GRASP55 and GRASP65 play complementary and essential roles in Golgi cisternal stacking. J Cell Biol 188 (2), 237–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jarvela T and Linstedt AD (2014) Isoform-specific tethering links the Golgi ribbon to maintain compartmentalization. Mol Biol Cell 25 (1), 133–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Veenendaal T et al. (2014) GRASP65 controls the cis Golgi integrity in vivo. Biol Open 3 (6), 431–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiritoiu M et al. (2019) GRASP55 and UPR Control Interleukin-1beta Aggregation and Secretion. Dev Cell 49 (1), 145–155 e4. [DOI] [PubMed] [Google Scholar]
- 42.Grond R et al. (2020) The function of GORASPs in Golgi apparatus organization in vivo. J Cell Biol 219 (9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rambourg A and Clermont Y (1990) Three-dimensional electron microscopy: structure of the Golgi apparatus. Eur J Cell Biol 51 (2), 189–200. [PubMed] [Google Scholar]
- 44.Klumperman J (2011) Architecture of the mammalian Golgi. Cold Spring Harb Perspect Biol 3 (7), 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ladinsky MS et al. (1999) Golgi structure in three dimensions: functional insights from the normal rat kidney cell. J Cell Biol 144 (6), 1135–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee I et al. (2014) Membrane adhesion dictates Golgi stacking and cisternal morphology. Proc Natl Acad Sci U S A 111 (5), 1849–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li J et al. (2019) DjA1 maintains Golgi integrity via interaction with GRASP65. Mol Biol Cell 30 (4), 478–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang D et al. (2016) Mena-GRASP65 interaction couples actin polymerization to Golgi ribbon linking. Mol Biol Cell 27 (1), 137–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duran JM et al. (2008) The Role of GRASP55 in Golgi Fragmentation and Entry of Cells into Mitosis. Mol Biol Cell 19 (6), 2579–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sutterlin C et al. (2002) Fragmentation and dispersal of the pericentriolar Golgi complex is required for entry into mitosis in mammalian cells. Cell 109 (3), 359–69. [DOI] [PubMed] [Google Scholar]
- 51.Truschel ST et al. (2012) Allosteric regulation of GRASP protein-dependent Golgi membrane tethering by mitotic phosphorylation. J Biol Chem 287 (24), 19870–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cervigni RI et al. (2015) JNK2 controls fragmentation of the Golgi complex and the G2/M transition through phosphorylation of GRASP65. J Cell Sci 128 (12), 2249–60. [DOI] [PubMed] [Google Scholar]
- 53.Wang Y et al. (2008) Golgi Cisternal Unstacking Stimulates COPI Vesicle Budding and Protein Transport. PLoS ONE 3 (2), e1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xiang Y et al. (2013) Regulation of protein glycosylation and sorting by the Golgi matrix proteins GRASP55/65. Nat Commun 4, 1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lowe M et al. (1998) Cdc2 kinase directly phosphorylates the cis-Golgi matrix protein GM130 and is required for Golgi fragmentation in mitosis. Cell 94 (6), 783–93. [DOI] [PubMed] [Google Scholar]
- 56.Witkos TM and Lowe M (2015) The Golgin Family of Coiled-Coil Tethering Proteins. Front Cell Dev Biol 3, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tang D et al. (2012) Sequential phosphorylation of GRASP65 during mitotic Golgi disassembly. Biol Open 1 (12), 1204–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang X et al. (2019) SIRT2 deacetylates GRASP55 to facilitate post-mitotic Golgi assembly. J Cell Sci 132 (21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Joshi G et al. (2014) Abeta-induced Golgi fragmentation in Alzheimer’s disease enhances Abeta production. Proc Natl Acad Sci U S A 111 (13), E1230–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim J et al. (2020) Grasp55(−/−) mice display impaired fat absorption and resistance to high-fat diet-induced obesity. Nat Commun 11 (1), 1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pothukuchi P et al. (2020) Regulated compartmentalization of enzymes in Golgi by GRASP55 controls cellular glycosphingolipid profile and function. bioRxiv doi: 10.1101/2020.05.03.074682. [DOI] [Google Scholar]
- 62.Wildt S and Gerngross TU (2005) The humanization of N-glycosylation pathways in yeast. Nat Rev Microbiol 3 (2), 119–28. [DOI] [PubMed] [Google Scholar]
- 63.Ohtsubo K and Marth JD (2006) Glycosylation in cellular mechanisms of health and disease. Cell 126 (5), 855–67. [DOI] [PubMed] [Google Scholar]
- 64.Hebert DN and Molinari M (2007) In and out of the ER: protein folding, quality control, degradation, and related human diseases. Physiol Rev 87 (4), 1377–408. [DOI] [PubMed] [Google Scholar]
- 65.Carvelli LF et al. (2010) Castration induces changes in the cation-dependent mannose-6-phosphate receptor in rat epididymis: possible implications in secretion of lysosomal enzymes. J Cell Biochem 110 (5), 1101–10. [DOI] [PubMed] [Google Scholar]
- 66.Chao HH et al. (1990) Mannose 6-phosphate receptor dependent secretion of lysosomal enzymes. EMBO J 9 (11), 3507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ghosh P et al. (2003) Mannose 6-phosphate receptors: new twists in the tale. Nat Rev Mol Cell Biol 4 (3), 202–12. [DOI] [PubMed] [Google Scholar]
- 68.Ahat E et al. (2019) GRASP depletion-mediated Golgi destruction decreases cell adhesion and migration via the reduction of alpha5beta1 integrin. Mol Biol Cell 30 (6), 766–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bisel B et al. (2008) ERK regulates Golgi and centrosome orientation towards the leading edge through GRASP65. J Cell Biol 182 (5), 837–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lane JD et al. (2002) Caspase-mediated cleavage of the stacking protein GRASP65 is required for Golgi fragmentation during apoptosis. J Cell Biol 156 (3), 495–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cheng JP et al. (2010) Caspase cleavage of the Golgi stacking factor GRASP65 is required for Fas/CD95-mediated apoptosis. Cell Death Dis 1, e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ji G et al. (2019) Golgi apparatus fragmentation participates in oxidized low-density lipoprotein-induced endothelial cell injury. J Cell Biochem 120 (11), 18862–18870. [DOI] [PubMed] [Google Scholar]
- 73.Wang F et al. (2019) Golgi reassembly and stacking protein 65 downregulation is required for the anti-cancer effect of dihydromyricetin on human ovarian cancer cells. PLoS One 14 (11), e0225450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ireland S et al. (2020) Cytosolic Ca(2+) Modulates Golgi Structure Through PKCalpha-Mediated GRASP55 Phosphorylation. iScience 23 (3), 100952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gee HY et al. (2018) Unconventional secretion of transmembrane proteins. Semin Cell Dev Biol 83, 59–66. [DOI] [PubMed] [Google Scholar]
- 76.Malhotra V (2013) Unconventional protein secretion: an evolving mechanism. EMBO J 32 (12), 1660–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rabouille C et al. (2012) Diversity in unconventional protein secretion. J Cell Sci 125 (Pt 22), 5251–5. [DOI] [PubMed] [Google Scholar]
- 78.Kinseth MA et al. (2007) The Golgi-associated protein GRASP is required for unconventional protein secretion during development. Cell 130 (3), 524–34. [DOI] [PubMed] [Google Scholar]
- 79.Manjithaya R et al. (2010) Unconventional secretion of Pichia pastoris Acb1 is dependent on GRASP protein, peroxisomal functions, and autophagosome formation. J Cell Biol 188 (4), 537–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bruns C et al. (2011) Biogenesis of a novel compartment for autophagosome-mediated unconventional protein secretion. J Cell Biol 195 (6), 979–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rajan A et al. (2017) A Mechanism Coupling Systemic Energy Sensing to Adipokine Secretion. Dev Cell 43 (1), 83–98 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dupont N et al. (2011) Autophagy-based unconventional secretory pathway for extracellular delivery of IL-1beta. EMBO J 30 (23), 4701–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang Z et al. (2020) Autophagy-based unconventional secretion of HMGB1 by keratinocytes plays a pivotal role in psoriatic skin in flammation. Autophagy, 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Son SM et al. (2016) Insulin-degrading enzyme secretion from astrocytes is mediated by an autophagy-based unconventional secretory pathway in Alzheimer disease. Autophagy 12 (5), 784–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ahat E et al. (2019) New Insights Into the Golgi Stacking Proteins. Front Cell Dev Biol 7, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li J et al. (2019) Golgi Structure and Function in Health, Stress, and Diseases. Results Probl Cell Differ 67, 441–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nuchel J et al. (2018) TGFB1 is secreted through an unconventional pathway dependent on the autophagic machinery and cytoskeletal regulators. Autophagy 14 (3), 465–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kmetzsch L et al. (2011) Role for Golgi reassembly and stacking protein (GRASP) in polysaccharide secretion and fungal virulence. Mol Microbiol 81 (1), 206–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Peres da Silva R et al. (2018) Golgi Reassembly and Stacking Protein (GRASP) Participates in Vesicle-Mediated RNA Export in Cryptococcus Neoformans. Genes (Basel) 9 (8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schotman H et al. (2008) dGRASP-mediated noncanonical integrin secretion is required for Drosophila epithelial remodeling. Dev Cell 14 (2), 171–82. [DOI] [PubMed] [Google Scholar]
- 91.Gee HY et al. (2011) Rescue of DeltaF508-CFTR Trafficking via a GRASP-Dependent Unconventional Secretion Pathway. Cell 146 (5), 746–60. [DOI] [PubMed] [Google Scholar]
- 92.Kim J et al. (2016) Monomerization and ER Relocalization of GRASP Is a Requisite for Unconventional Secretion of CFTR. Traffic 17 (7), 733–53. [DOI] [PubMed] [Google Scholar]
- 93.Piao H et al. (2017) Sec16A is critical for both conventional and unconventional secretion of CFTR. Sci Rep 7, 39887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cleyrat C et al. (2014) Mpl traffics to the cell surface through conventional and unconventional routes. Traffic 15 (9), 961–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhao Z et al. (2019) Tyrosine phosphorylation directs TACE into extracellular vesicles via unconventional secretion. Traffic 20 (3), 202–212. [DOI] [PubMed] [Google Scholar]
- 96.Gopal SR et al. (2019) Unconventional secretory pathway activation restores hair cell mechanotransduction in an USH3A model. Proc Natl Acad Sci U S A 116 (22), 11000–11009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rabouille C (2017) Pathways of Unconventional Protein Secretion. Trends Cell Biol 27 (3), 230–240. [DOI] [PubMed] [Google Scholar]
- 98.van Ziel AM et al. (2019) Unconventional secretion factor GRASP55 is increased by pharmacological unfolded protein response inducers in neurons. Sci Rep 9 (1), 1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang X et al. (2018) GRASP55 Senses Glucose Deprivation through O-GlcNAcylation to Promote Autophagosome-Lysosome Fusion. Dev Cell 45 (2), 245–261 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang X and Wang Y (2018) The Golgi stacking protein GORASP2/GRASP55 serves as an energy sensor to promote autophagosome maturation under glucose starvation. Autophagy 14 (9), 1649–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang X et al. (2019) GORASP2/GRASP55 collaborates with the PtdIns3K UVRAG complex to facilitate autophagosome-lysosome fusion. Autophagy 15 (10), 1787–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang X and Wang Y (2018) GRASP55 facilitates autophagosome maturation under glucose deprivation. Mol Cell Oncol 5 (4), e1494948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim J et al. (2018) Unconventional protein secretion - new insights into the pathogenesis and therapeutic targets of human diseases. J Cell Sci 131 (12). [DOI] [PubMed] [Google Scholar]
- 104.Zhang M et al. (2020) A Translocation Pathway for Vesicle-Mediated Unconventional Protein Secretion. Cell 181 (3), 637–652 e15. [DOI] [PubMed] [Google Scholar]
- 105.Cruz-Garcia D et al. (2017) A diacidic motif determines unconventional secretion of wild-type and ALS-linked mutant SOD1. J Cell Biol 216 (9), 2691–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang M et al. (2015) Translocation of interleukin-1beta into a vesicle intermediate in autophagy-mediated secretion. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang ZH et al. (2015) Loss of a Clueless-dGRASP complex results in ER stress and blocks Integrin exit from the perinuclear endoplasmic reticulum in Drosophila larval muscle. Biol Open 4 (5), 636–48. [DOI] [PMC free article] [PubMed] [Google Scholar]


