Abstract
During the last stages of wound healing, myofibroblasts differentiate mainly from fibroblasts. Myofibroblasts from normal skin wounds (Wmyo) can communicate with its surrounding using secreted factors. They also have the capacity to produce microvesicles (MVs), a type of extracellular vesicles, as mediators of intercellular communication. MVs cargo are potentially capable of regulating the behavior of targeted cells and tissues. The aim of this study is to evaluate the effect of Wmyo-derived MVs on dermal fibroblasts and to determine the responsible signaling molecule. Microvesicles were obtained from culture media of myofibroblasts and characterized using protein quantification, dynamic light scattering and transmission electron microscopy. Uptake of fluorescent MVs in fibroblasts was assessed by flow cytometry. Cytokines concentrations were quantified in MV samples by a multiplex ELISA. Different concentration of MVs or a selected cytokine were used as treatments over fibroblasts culture for 5 days. Following the treatments, parameters linked to the extracellular matrix were studied. Lastly, the selected cytokine was neutralized within MVs before evaluating collagen production. We showed that Wmyo derived-MVs were internalized by dermal fibroblasts. Cytokine array analysis revealed that a large amount of placental growth factor 1 (PLGF-1) (0.88 ± 0.63 pg/μg proteins in MVs) could be detected in MVs samples. Cutaneous fibroblasts treated with MVs or PLGF-1 showed significantly stimulated procollagen I level production (Fold change of 1.80 ± 0.18 and 2.07 ± 0.18, respectively). Finally, the neutralization of PLGF-1 in MVs significantly inhibited the production of procollagen I by fibroblasts. Our study shows that Wmyo derived-MVs are involved in intercellular communication by stimulating collagen production by fibroblasts during wound healing. This effect is possibly attained through PLGF-1 signalling. These findings represent a promising opportunity to gain insight into how MVs and Wmyo may mediate the healing of a skin wound.
Keywords: Collagen, Fibroblasts, Microvesicles, Myofibroblasts, PLGF-1, Wound healing
Introduction
Cells are able to communicate by exchanging information that can influence cell characteristics. This communication may occur by either direct membrane contact or through soluble secreted factors such as cytokines and growth factors (Schonherr and Hausser 2000). Cells may also produce extracellular vesicles and release them into the extracellular environment to act as carriers (Laberge et al. 2018). These vesicles can be released by virtually all cell types (Yanez-Mo et al. 2015).
Microvesicles (MVs) are a type of extracellular vesicle, along with exosomes and apoptotic bodies (Yanez-Mo et al. 2015). These vesicles are characterized by a phospholipid bilayer structure and are produced following a cell stimulus. MVs are heterogeneous spherical vesicles and are produced by budding of the cell membrane (Yanez-Mo et al. 2015). They can transfer their contents, composed of molecules such as proteins, cytokines, lipids and RNAs, to other cells. The composition of the MV membrane varies according to the producing cell (Choi et al. 2015; Colombo et al. 2014; Laberge et al. 2018; Raposo and Stoorvogel 2013; Yanez-Mo et al. 2015). MVs can contribute to pathological or physiological phenomena such as angiogenesis (Merjaneh et al. 2017) during normal wound healing. However, the exact composition and cell targets of MVs as well as their mechanisms of action remain little known. Despite this, MVs are now considered as vectors for the intercellular transfer of bioactive molecules during wound healing (Laberge et al. 2018).
Wound healing is a complex process involving many biological mechanisms that occurs in four partially overlapping interdependent stages (hemostasis, inflammation, granulation tissue formation, and remodeling) and involves cells, extracellular matrix (ECM) components and other factors (Shaw and Martin 2009; Takeo et al. 2015). Depending on the tissue repair environment, different cell types contribute to wound healing by producing MVs: MVs released by platelets have shown a therapeutic effect on wound healing by promoting angiogenesis (Sun et al. 2017); MVs derived from human non-diabetic adipose tissue-derived mesenchymal stem cells alter pathological cell function by improving the migration, survival, inflammation and angiogenesis (Trinh et al. 2016). In addition, MVs derived from keratinocytes regulate the gene expression of dermal fibroblasts (Huang et al. 2015).
The granulation tissue formation phase is mainly characterized by robust angiogenesis, ECM production, and the contraction of the wound edges. Wound myofibroblasts (Wmyos) are cells that appear during this phase and differentiate mainly from fibroblasts (Desmouliere et al. 1993; Hinz 2016) triggered by environmental factors such as inflammatory cell-secreted transforming growth factor-ß (TGFß1) (Postlethwaite et al. 2004). Wmyos have morphological and biochemical characteristics of both fibroblasts and smooth muscle cells (Véronique Moulin et al. 2012). They are characterized by a heightened expression of alpha-Smooth Muscle Actin (α-SMA), which is a recognized marker of these cells (Hinz et al. 2007). This differentiation of fibroblasts into Wmyos allows the fibroblasts to acquire the new properties necessary for ECM regulation following their migration and proliferation in the damaged zone (Moulin et al. 2000). Wmyos synthesize a large amount of type I collagen and also produce a large number of cytokines and growth factors (Grotendorst et al. 2004; Moulin et al. 1998). They are also characterized by their ability to contract the edges of the wound (Hinz et al. 2007). Our team demonstrated that serum, and more precisely, alpha-2-macroglobulin (A2M), does dependently stimulate MV production by human Wmyos (Moulin et al. 2010; Laberge et al. 2019). We have also demonstrated that Wmyo-derived MVs can stimulate angiogenesis (Merjaneh et al. 2017), highlighting the importance of MVs during wound healing.
The fibroblasts are the main producers of ECM structural proteins (such as collagen I) and adhesion proteins (such as fibronectin), as well as other substances (such as glycoproteins) (Xue and Jackson 2015). It is recognized that fibroblasts participate in angiogenesis, granulation tissue formation and reepithelialisation (Bainbridge 2013; Darby et al. 2014; Stunova and Vistejnova 2018). The fibroblasts are also able to respond to many autocrine and paracrine signals such as cytokines and growth factors (Mehrnaz Gharaee-Kermani 2004). During the granulation tissue and remodeling phases, fibroblasts synthesize the majority of the new ECM. They are also the main source of the matrix metalloproteinases (MMPs) that degrade the ECM (Xue and Jackson 2015). The activation and differentiation of fibroblasts into myofibroblasts play a vital role during wound healing (Kendall and Feghali-Bostwick 2014).
The objective of this study was to evaluate whether Wmyo-derived MVs interact with dermal fibroblasts, and whether their contents affect one or more ECM-related parameters. We also further characterized MVs using a cytokine multiplex assay. We thereby found that MVs were rich in placental growth factor (PLGF-1), a cytokine of the VEGF family. We also demonstrated that MVs as well as the soluble form of PLGF-1 can stimulate collagen production. Neutralizing PLGF-1 in MVs inhibited collagen production by fibroblasts. Taken together, our data provide evidence that MVs can regulate ECM production during wound healing via PLGF-1.
Materials and methods
Cell culture
Populations of primary cells were isolated from normal skin and wounds of volunteer donors aged 20–40 years. All subjects gave written informed consent. Wmyos (Germain et al. 1994; Moulin et al. 1999) and skin fibroblasts (Moulin et al. 1996) were isolated following a previously described method. The numbers mentioned after each cell population or after MVs derived from a cell population refer to the individuals from whom the cells were isolated. Cells were grown in Dulbecco’s Modified Eagle’s medium (DME) supplemented with 20% fetal bovine serum (FBS) for Wmyo and 10% FBS for fibroblasts, 100 U/mL penicillin G and 25 mg/mL gentamicin (Schering Inc., Pointe Claire, Canada). Both Wmyos and fibroblast cells were grown at 37 °C in a humidified incubator with 8% CO2. Wmyos were used at passages between 3 and 8, and fibroblasts between passages 3 and 6. All procedures involving patients were reviewed and approved by the Research Ethical Committee of the Centre Hospitalier Universitaire (CHU) de Québec-Université Laval and followed the Declaration of Helsinki protocols.
Cell transduction
To generate Red Fluorescent Protein (RFP-670)-expressing Wmyos, cells were transduced for 6 h with a third generation self-inactivating RFP-670-encoding lentivector at a MOI of 30, being the same method used for Green Fluorescent Protein gene transduction in previous studies (Alexandra Laberge et al. 2019; Merjaneh et al. 2017).
Wmyo-derived MV isolation
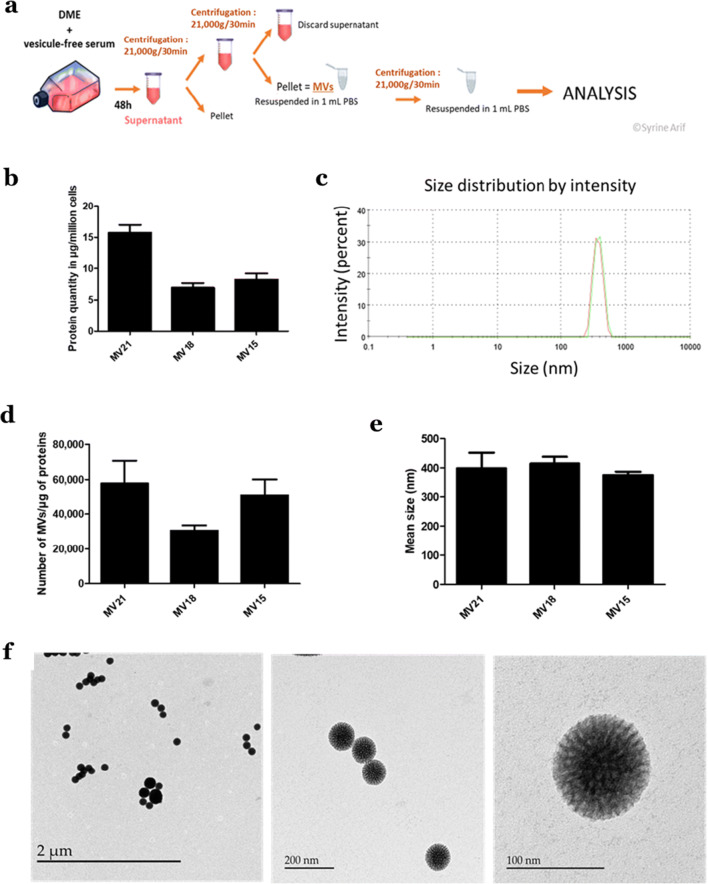
MVs were isolated by differential centrifugation as previously described (Merjaneh et al. 2017). FBS was depleted from extracellular vesicles using ultracentrifugation at 100,000 x g for 18 h at 4 °C. Confluent Wmyos were cultured during 48 h with DME + 20% vesicle-free FBS. Conditioned medium was collected and centrifuged at 300 x g for 10 min at 4 °C to remove cells and large debris. The supernatant was then centrifuged at 21,000 x g for 30 min at 4 °C. The pellet, containing MVs, was then washed three times with phosphate-buffered saline (PBS) and further centrifuged at 21,000 x g for 20 min (Fig. 1a).
Fig. 1.
MV isolation and characterization. a Wmyos were cultured with 20% vesicle-free serum during 48 h before MV isolation from conditioned medium by differential centrifugation. a Protein quantity of MVs (in μg) produced by one million Wmyos during 48 h measured using NanoDrop 1000 spectrophotometer at 280 nm. N = 3 populations of Wmyos, n = 6 samples of MVs from each population. c, d, e Dynamic light scattering (DLS) analysis of MV samples, their hydrodynamic size distribution and their number per μg of protein. N = 3 populations of Wmyos, n = 3 samples of MVs per population. f Transmission electron microscopy (TEM) analysis of MVs. The difference in MV size observed between DLS analysis and TEM may be related to the shrinkage of MVs during the fixation of samples for TEM
Protein assay
The concentration of proteins contained in MV samples was evaluated using a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Mississauga, ON, Canada) at 280 nm.
Transmission electron microscopy
MVs were fixed with 2.5% glutaraldehyde (Canemco, Lakefield, QC, Canada) overnight at 4 °C and then kept in a 0.1 M cacodylate buffer (Mecalab, Montreal, QC, Canada). Samples were stained with 3% uranyl acetate (Sigma, Oakville, QC, Canada) and processed for observation by transmission electron microscopy (80 kV, JEOL® electron microscope 1230, Akishima, Tokyo; Institut de biologie intégrative et des systèmes, Microscopy Platform at Université Laval, Quebec City, QC). Multiple pictures were taken at multiple spots, and those deemed to be representative of all the events observed within the samples were selected.
Nanosizer technology
The size and count distribution of MVs were measured using Nanosizer technology with the Zetasizer Nano-ZS dynamic light scattering (DLS) measurement system (Malvern, Montréal, QC, CA) according to the manufacturer’s instructions.
Cytokine measurement
Cytokines were analyzed with a multiplex ELISA method using the ProcartaPlex™ Human Cytokine/Chemokine/Growth Factor Convenience 45-Plex Panel 1 (Invitrogen EPXR450–12171-901, eBioscience, Carlsbad, CA) following the manufacturer’s instructions. Detection of the relative levels of 45 different cytokines, chemokines and growth factors were analyzed (BDNF; Eotaxin/CCL11; EGF; FGF-2; GM-CSF; GRO alpha/CXCL1; HGF; NGF beta; LIF; IFN alpha; IFN gamma; IL-1 beta; IL-1 alpha; IL-1RA; IL-2; IL-4; IL-5; IL-6; IL-7; IL-8/CXCL8; IL-9; IL-10; IL-12 p70; IL-13; IL-15; IL-17A; IL-18; IL-21; IL-22; IL-23; IL-27; IL-31; IP-10/CXCL10; MCP-1/CCL2; MIP-1 alpha/CCL3; MIP-1 beta/CCL4; RANTES/CCL5; SDF-1 alpha/CXCL12; TNF alpha; TNF beta/LTA; PDGF-BB; PLGF; SCF; VEGF-A; VEGF-D). Analyses were done on MVs, Wmyos and their 48 h-conditioned medium (MV-free). The TGF-β1 level was assayed using ELISA (Human TGF-beta 1 DuoSet ELISA, R&D systems, Minneapolis, MN) according to the manufacturer’s instructions.
Localisation of PLGF-1 in MV by flow cytometry
To evaluate any PLGF-1 association with MVs, immunolabelling of MV was performed with PLGF-1 antibody (Human PLGF Antibody, MAB264, R&D systems) or with an isotype control IgG1 (Negative control mouse IgG1, X0931, Dako, Denmark) followed by phycoerythrin-conjugated anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA). Using flow cytometry, the immunolabelled MV relative size (FSC) was compared with 500 nm diameter beads (Fluorescent microsphere, 0.50 μm (660/690), Bangs Laboratories, Inc., IN, USA).
Uptake of fluorescent MVs by fibroblasts
Confluent fibroblasts were treated with fluorescent and non-fluorescent MVs at a concentration of 150 μg of MV proteins/mL for 6 h. After treatments, cells were washed, dissociated with trypsin and resuspended in PBS + 2% vesicle-free FBS + 0.5 μM EDTA. The fluorescence of MVs and fibroblasts was evaluated using flow cytometry (Becton Dickinson, Mississauga, ON, Canada).
Cell migration
A scratch was created on confluent fibroblasts using a 1 mL sterile pipette tip. Culture medium was immediately removed and replaced with DME containing MVs (10 μg/mL), PLGF-1 (10 ng/mL) (Recombinant Human PLGF-1, PeproTech, Rocky Hill, NJ), TGF-β1 (10 ng/mL) (Recombinant Human TGF-β1, PeproTech), or DME. Phase contrast images (magnification of 40) were recorded on a digital camera at 0 (T0) and 24 h (T24) post-scratch. Cell migration was measured using ImageJ™ software (https://imagej.nih.gov/ij/) and expressed as the difference of the wound surface at T0 and T24. All scratch assays were performed in triplicate.
Fibroblast treatment
Fibroblasts were plated at 5 × 103 fibroblasts/well in a six-well plate with their usual culture medium. After 24 h, treatments with MVs (1/5/10 μg protein of MVs/mL), PLGF-1 (10 ng/mL) or TGFß1 (10 ng/ml) as control were performed for 5 days with medium changes every other day. All treatments contained DME + ascorbic acid (Sigma-Aldrich) at 50 μg/mL and 1% BSA (Bovine Serum Albumin, Sigma-Aldrich). After treatments, the conditioned medium was centrifuged at 21,000 x g for 20 min at 4 °C and the supernatant aliquoted for further analysis. Cells were dissociated with trypsin, counted using a Coulter ® counter (Beckman-Coulter) and fixed with ice-cold 100% methanol.
α-SMA expression analysis
The presence of α-SMA in methanol-fixed fibroblasts was evaluated by flow cytometry: cells were incubated for 45 min with an α-SMA antibody (monoclonal mouse anti-human α-SMA, clone 1A4, Sigma-Aldrich) followed by phycoerythrin-conjugated anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) as previously described (Moulin et al. 2010).
MMP activity measurement
Total MMP activity was determined in the cell culture supernatant using the SensoLyteTM 520 Generic MMP assay kit (Anaspec, Fremont, CA, USA). The kit can simultaneously detect the activity of MMP-1, −2, −7, −8, −9, −12, −13, and − 14. The results reflect the overall activity of MMPs present in the culture medium.
Procollagen I assay
Collagen type I secretion was indirectly evaluated in conditioned medium using a Procollagen type I ELISA assay (Human procollagen I a-1/COLIA1, R&D systems), following the manufacturer’s instructions.
Cytokine neutralization
MVs (10 μg/mL) were pretreated with PLGF-1 neutralizing antibodies (PLGF-1-NAb) (Human PLGF Antibody, R&D systems) at 20 ng/mL for 30 min before being added to the fibroblasts. IgG1 (Negative control mouse IgG1, X0931, Dako, Denmark) was used as an isotypic control.
Statistical analysis
Statistical analysis was performed with GraphPad 6 Prism Software (GraphPad Software Inc., La Jolla, CA, USA). The ANOVA (nonparametric) test was used for multiple comparisons. Data were compared using Dunnett’s test for nonparametric values. P < 0.05 was considered significant. All data are presented as the mean of triplicates (error bars: ± standard deviation). All figures are representative results of independent experiments performed with fibroblasts and MVs produced by different Wmyo (noted N in Figure legends).
Results
Wmyo-derived vesicles display the main characteristics of MVs
MVs were isolated from the supernatant of different cultures of Wmyo populations using differential centrifugation (Fig. 1a). The concentration of proteins in MVs samples showed that 106 Wmyos produces10.32 ± 3.87 μg of proteins of MVs (N = 3) (Fig. 1b).
MV size was determined using dynamic light scattering (DLS) analysis via NanoSizer®. MV samples presented a unique peak corresponding to a diameter of 396.56 ± 51.16 nm (N = 3) (Fig. 1c,e). This size range is usually characteristic of large extracellular vesicles obtained from the 20,000 x g fraction (after differential centrifugation) that corresponds to MVs (Hromada et al. 2017; Szatanek et al. 2015). We have previously demonstrated that Wmyos did not produce detectable amounts of the small vesicles isolated using 100,000 x g ultracentrifugation and referred to as exosomes (Merjaneh et al. 2017).
The number of MVs evaluated using the NanoSizer® was 46,255 ± 11,526 MVs/μg of MV proteins (N = 3) (Fig. 1d). The number of cells that produced MVs in each sample was counted (not shown here), allowing us to calculate a mean of 0.85 ± 0.26 MVs/cell. These results are consistent with our previous study using a flow cytometry approach (Alexandra Laberge et al. 2019).
MV samples were analyzed using TEM, which revealed large, rounded vesicles with a distinct dense appearance (Fig. 1f). In TEM, MVs seem to have a smaller diameter in comparison with the NanoSizer diameter evaluation because of the alteration due to sample preparation (Hromada et al. 2017).
Fibroblasts can uptake Wmyo-derived MVs
MVs produced by Wmyos interact with a variety of cells including endothelial cells to contribute to angiogenesis (Laberge et al. 2018; Merjaneh et al. 2017). Little is known about the internalization or effect of Wmyo-derived MVs in dermal fibroblasts during normal wound healing.
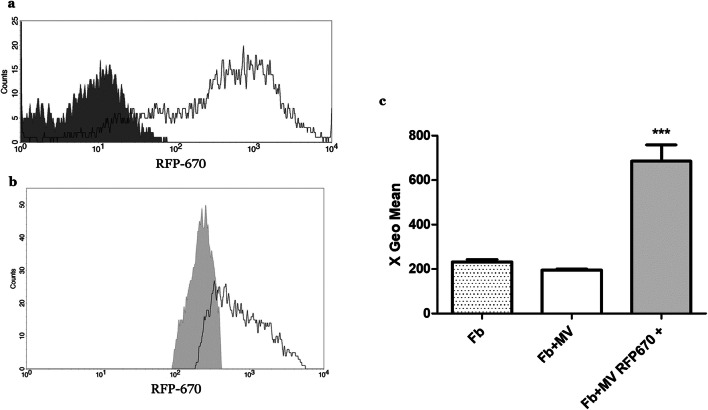
To model cell-to-cell communication between Wmyos and fibroblasts, we studied fibroblast uptake of fluorescent Wmyo-derived MVs. When stimulated with serum, RFP-670-transduced Wmyos produced fluorescent MVs (Fig. 2a). The fluorescent MVs were then added to fibroblast culture medium for 6 h and quantification of the fibroblast fluorescence was performed using flow cytometry. A large proportion of fibroblasts demonstrated a significant increase in their level of fluorescence (Fig. 2b-c).
Fig. 2.
Wmyo-derived MV uptake by fibroblasts. Fluorescent MVs were added to the culture medium of fibroblasts (Fb) in order to determine the capacity of Fb to uptake MVs. a Fluorescence evaluation of MVs produced by Wmyos (dark grey peak) or by RFP-670-transduced Wmyos (white peak). b Fluorescence evaluation of Fb stimulated by MVs produced by Wmyos (light grey peak) or RFP-670-transduced Wmyos (white peak). c Geometric mean of the fluorescence intensity of Fb in the absence of MVs (Fb), in the presence of non-fluorescent MVs (Fb + MV) or in the presence of fluorescent MVs (Fb + MV RFP-670+). N = 2 populations of Fb stimulated with 2 different Wmyo-derived MVs. One-way ANOVA (nonparametric), Dunnett’s test (control: Fb culture with non-fluorescent MVs (Fb + MV)), ***P < 0.0002
PLGF-1 cytokine was present in large amounts in Wmyo-derived MVs
During the process of wound healing, Wmyos secrete different elements such as cytokines in order to communicate with cells and induce signalling (Baum and Duffy 2011; Hinz et al. 2007; Werner and Grose 2003). Wmyo-derived MVs might thus contain cytokines that generate a more direct/focused and specific action in order to accelerate wound healing.
Cytokine array analysis showed that Wmyo-derived MVs contained several cytokines. PLGF-1 was the cytokine that was present in the greatest quantity (0.88 ± 0.63 pg/μg proteins in MVs, N = 6 MVs produced by 6 different Wmyo populations) (Fig. 3a). LTA, VEGFα and IL-23 were also detectable in large amounts in MVs (Fig. 3a-b). Besides the 45 cytokines of the array, we also used an ELISA kit to assay TGF-β1, a well-known cytokine that is secreted by Wmyos. TGF-β1 was not detectable in MV samples (Fig. 3b).
Fig. 3.
Cytokine distribution in MVs, conditioned media and mother cells. a Cytokines in MV samples derived from Wmyos. Forty-five cytokines were quantified. Cytokines are expressed as pg of cytokines per μg of protein in MVs ± SEM. Results are reported as protein levels in MVs that have been previously analyzed with Nanodrop. N = 6 samples of MVs derived from 6 different Wmyo populations. b PLGF-1, LTA, VEGF-A and IL-23 distribution in MVs, their parent cells and their conditioned media depleted of MVs (CM depleted of MVs), N = 6 samples of MVs derived from 6 different Wmyo populations. TGF-β1 was quantified by a separate ELISA and was not detected (ND) in either Wmyos or MV samples (N = 3 samples of MVs derived from 3 different Wmyo populations). c PLGF-1 association with MVs was evaluated by comparing, using flow cytometry assay, the size of PLGF1-associated MVs with 500 nm diameter beads. 500 nm beads dot plot analysis region were gated and were merged with MVs in the presence of PLGF-1 antibody or with an isotype control (N = 2 samples of MVs derived from 2 different Wmyo populations, n = 4). *P < 0.05, ***P < 0.0001, One-way ANOVA (nonparametric), Dunnett’s test with MVs as control
We also compared the quantity of cytokines in MVs, in their parent Wmyos cells and in the medium of Wmyo culture depleted of MVs (Fig. 3b). Results were calculated for 1 cm2 of culture surface to allow comparison between the three evaluations. Interestingly, LTA was only present in MVs while VEGF-A, IL-23 and TGF-β1 were detected mostly in conditioned media (Fig. 3b). To further evaluate PLGF-1 association with MVs, we compared, using flow cytometry assay, the size of MV detaining PLGF-1 with those of 500 nm diameter beads (Fig.3c). MVs size matches the range of 500 nm beads supporting the fact that PLGF-1 was bound to MVs.
MVs and PLGF-1 stimulated fibroblast growth and migration
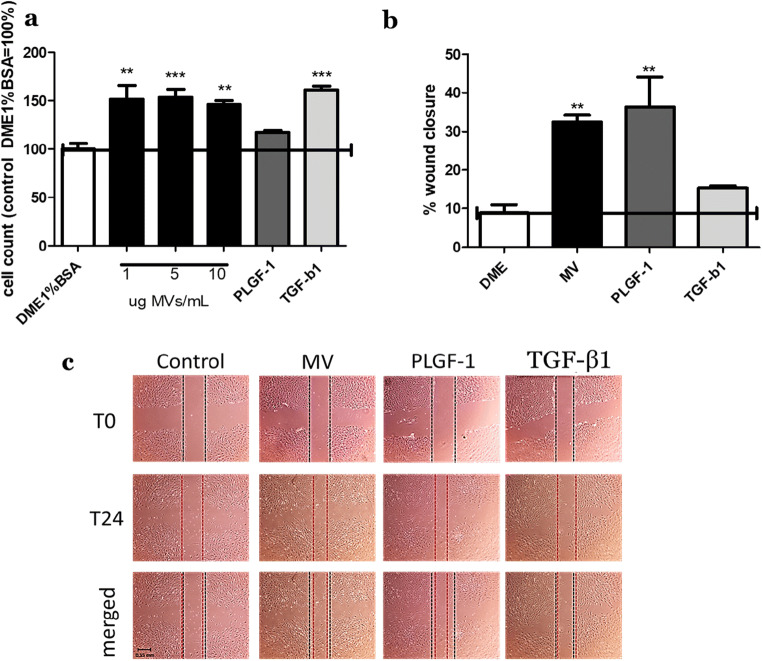
To study the impact of Wmyo-derived MVs on fibroblasts, we first evaluated growth rates following fibroblast stimulation with MVs. Fibroblasts were incubated with MVs at different concentrations (1, 5 and 10 μg of MV proteins), PLGF-1 (10 ng/mL) or TGFß1 (10 ng/mL) for 5 days. The growth rate of fibroblasts treated with MVs (10 μg /mL) and TGFß1 increased significantly with a fold change of respectively 1.46 ± 0.05 and 1.62 ± 0.05 versus control (DME + 1% BSA), while the soluble cytokine PLGF-1 did not stimulate cell growth (Fig. 4a).
Fig. 4.
Effect of MVs and cytokines on cell growth and migration. a Fibroblasts were grown for 5 days with different treatments (DME1%BSA, different concentrations of MVs (1, 5 or 10 μg/mL), PLGF-1 (10 ng/mL) and TGF-β1(10 ng/mL) during the entire 5 days with media replacement. Treatments with MVs at 10 μg/mL significantly stimulated cell growth (fold change of 1.46 ± 0.05 versus control). b, c Fibroblasts were plated to 100% confluence then scraped with a 1 mL pipette tip. Cells were washed with PBS then treated with different conditions (DME, MVs at 10 μg/mL, PLGF-1 at 10 ng/mL and TGF-β1 at 10 ng/mL). Pictures were taken right after the scratch (T0) and 24 h after treatment (T24). MVs and PLGF-1 significantly enhanced migration as compared with the control (DME). The migration rate is represented as percentage of wound closure. Results are representative of N = 3 samples of MVs derived from 3 different Wmyo populations, and N = 3 different populations of fibroblasts in triplicate. Black lines represent the scratch delimitation for time 0 h of the scratch, red represents the scratch delimitation for time 24 h after the scratch and the last row represents the merge of both lines of the scratch after 24 h of treatment. *P < 0.05, **P < 0.001, ***P < 0.0002 One-way ANOVA (nonparametric), Dunnett’s test with DME1%BSA or DME as control
Migration capacity was determined using a scratch test assay (Liang et al. 2007). The migration rate of fibroblasts after MV or PLGF-1 treatments was found to be significantly increased compared to the control (DME + 1% BSA) while TGFß1 did not stimulate cell migration (Fig. 4b-c). The percentage of wound closure was 32.48% ± 2.51 in the presence of MVs, 36.40% ± 10.95 with PLGF-1, 15.35% ± 0.80 with TGF-β1 and 8.96% ± 2.95 for the control. These represent a fold change versus control of 3.67 ± 0.28 following MV treatment and 4.11 ± 1.24 after PLGF-1 treatment.
Wmyo-derived MVs did not affect fibroblast differentiation
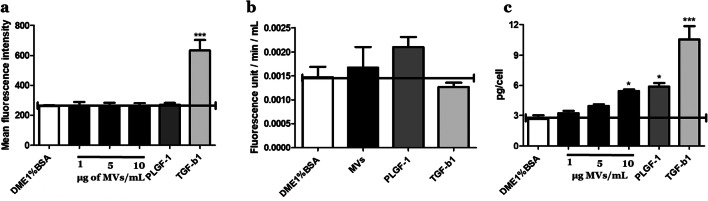
Fibroblasts can differentiate into Wmyos following stimulation by secreted molecules such as TGF-β1 (Stunova and Vistejnova 2018). Fibroblast α-SMA expression following MV or cytokine treatment was analyzed using flow cytometry. The treatment of cells with MVs or PLGF-1 for 5 days did not modify the phenotype of fibroblasts (Fig. 5a) while TGF-β1 treatment significantly increased the expression of this marker.
Fig. 5.
MV effect on ECM parameters. a Alpha-SMA study. Comparison of the geometric mean of fluorescence of the cells treated with DME1%BSA, different concentrations of MVs (1, 5 or 10 μg/mL), PLGF-1 (10 ng/mL) and TGF-β1 (10 ng/mL). b MMP activity level of fibroblast-conditioned medium following stimulation with DME1%BSA, 5 μg of MVs/mL, PLGF-1 (10 ng/mL) or TGF-β1 (10 ng/mL). Results were reported as fluorescence unit/min/mL. (c) Procollagen I evaluation in fibroblast-conditioned medium following stimulation with DME1%BSA, different concentrations of MVs (1, 5 or 10 μg/mL), PLGF-1 (10 ng/mL) or TGF-β1 (10 ng/mL). Results were expressed as pg of procollagen I per cell. All data are expressed as mean ± SD, averaged from three separate experiments. Results are representative of N = 3 different populations of fibroblasts treated with 3 different samples of MVs derived from 3 different Wmyo populations. *P < 0.05, ***P < 0.0001, One-way ANOVA (nonparametric), Dunnett’s test with DME1%BSA as control
MVs stimulated ECM production rather than its degradation
Throughout normal wound healing, the ECM undergoes renewal that occurs by a concomitant degradation (especially by MMPs) and production (mostly collagen I). Dermal fibroblasts can participate in both processes by secreting either MMPs or collagen (Xue and Jackson 2015).
The secretion of MMPs was evaluated using an enzymatic assay, which indicates the global matrix degradation capacity of the cells. Treatment with either MVs or PLGF-1 did not significantly modify MMP activity (Fig. 5b).
The level of collagen I secretion can be determined by the detection of its pro-peptides in culture supernatants (Cutroneo 2003). Treatment of fibroblasts with MVs significantly stimulated the level of procollagen I production with a fold change of 1.80 ± 0.18 when 10 μg of MVs were added to the culture medium (Fig. 5c) while PLGF-1 (10 ng/mL) and TGFß1 increased collagen secretion with a fold change of 2.07 ± 0.18 and 3.11 ± 0.95, respectively.
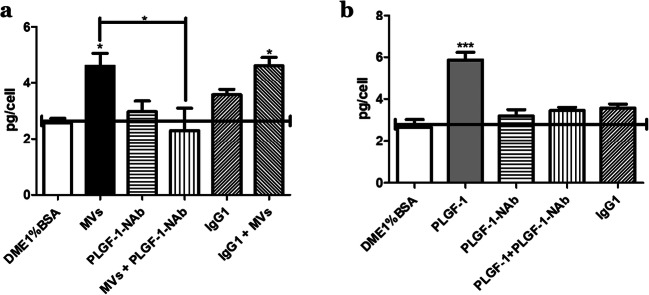
Neutralization of PLGF-1 inhibited collagen production induced by MVs
When PLGF-1-Neutralizing antibodies (PLGF-1-Nab) were added along with soluble PLGF-1, the action of PLGF-1 on procollagen secretion was neutralized (Fig. 6b). Pre-incubation of MVs with PLGF-1-NAb before adding them to fibroblasts also neutralized the positive effect of MVs on procollagen secretion (Fig. 6a) while the use of a corresponding isotypic control of PLGF-1 NAb, an IgG1 antibody type, had no effect.
Fig. 6.
Neutralization of PLGF-1 and the aftermath on collagen production. Procollagen I production in fibroblast-conditioned medium following stimulation. a Cells were treated with MVs, PLGF-1-neutralizing antibodies (NAb) (PLGF-1-NAb), MVs that were preincubated with PLGF-1-neutralizing antibodies (MVs + PLGF-1-NAb), an isotypic control antibody (igG1), or MVs preincubated with an isotypic antibody (IgG1 + MVs). b Fibroblasts were treated with the soluble form of PLGF-1, PLGF-1-Nab, the soluble form of PLGF-1 treated with its neutralizing antibody, or an isotypic control. Results are expressed as pg of procollagen I per cell ±SD. Results are representative of N = 2 different populations of fibroblasts treated with 3 different Wmyo-derived MV samples. *P < 0.05, ***P < 0.0002 One-way ANOVA (nonparametric), Dunnett’s test with DME1%BSA as control. Newman-Keuls multiple comparison test was also used after one-way ANOVA (nonparametric) for (A) to compare MVs with the other columns
Discussion
Wmyos appear in wounded tissues during the late stages of healing. In skin, they differentiate mainly from fibroblasts following stimulation by environmental factors such as TGF-β1. Wmyos are characterized by their expression of α-SMA, which is a recognized marker of these cells (Hinz et al. 2007). They play a central role during wound healing by contributing to ECM formation and wound contraction (Hinz 2016; Moulin et al. 2012, 1998, 1999). Their actions in the different wound healing mechanisms are well studied but the means by which they can communicate with other cells remain poorly known.
Our laboratory has previously demonstrated that these differentiated cells can produce MVs following stimulation with serum proteins or A2M (Alexandra Laberge et al. 2019; Merjaneh et al. 2017; Moulin et al. 2010). These MVs have been shown to alter some characteristics of target cells, such as endothelial cells, during wound healing (Merjaneh et al. 2017). The proteomic analysis has also unveiled elements that could be present in MVs and that are linked to the ECM, which suggests that MVs could intervene in the regulation of the ECM by communicating with cells such as fibroblasts (Merjaneh et al. 2017). We therefore undertook a first comprehensive study of cytokine association with MVs in an attempt to determine which cytokines are detected in MVs, and if fibroblast action on ECM homeostasis can be modulated by MVs. We thus analyzed the action of MVs on fibroblasts that affect five parameters linked to ECM homeostasis: cell growth, migration and differentiation as well as MMP and collagen production.
We first completed the characterization of the MVs by evaluating their size and morphology. All of our data were consistent with those reported in the literature: MVs are homogeneous and well-defined vesicles with a 400 nm diameter (Burger et al. 2011; Laberge et al. 2018). We then evaluated cytokine patterns in MVs. We have demonstrated that several cytokines can be detected in MV samples, but the most abundant cytokine was PLGF-1, a member of the VEGF family. PLGF-1 was first identified in the placenta but is also known to be present in other organs (Roy et al. 2006). This cytokine is known to be pro-angiogenic and can positively regulate wound healing (Roy et al. 2006), (Carmeliet et al. 2001; Freitas-Andrade et al. 2012). In the skin, PLGF-1 is expressed by keratinocytes and endothelial cells and is involved in inflammation (Hattori et al. 2002) and angiogenesis (Adini et al. 2002; Nagy et al. 2003). It has also been shown that PLGF-1 can directly stimulate the migration of fibroblasts (Barrientos et al. 2008).
To further ensure that PLGF-1 was not an impurity in the sample, it is to mention that differential centrifugation rate (21,000 x g followed by three PBS washes) used to isolate MVs could not precipitate soluble cytokines in the pellet. The diameter of PLGF-1-associated particles was around 500 nm, further supporting the fact that PLGF-1 was bound to MVs (Fig. 3c). In parallel, MVs were treated with proteinase K and PLGF1 presence on MVs was then evaluated using flow cytometry. PK treatment resulted in the decrease of surface-bound cytokines on MVs (data not shown). This suggests that PLGF-1 is, at least partially, membrane-bound to MVs as also previously concluded by Fitzgerald et al. (2018) for other cytokines.
By demonstrating the uptake of Wmyo derived MVs by fibroblasts, we simulated an indirect interaction/communication between Wmyo and dermal fibroblasts. Thus, additional studies will be required to determine the intracellular mechanisms induced by these extracellular vesicles, whether it be endocytosis (Costa Verdera et al. 2017), membrane fusion (Parolini et al. 2009) or by binding interaction (via integrins for example (Chen et al. 2018)) depending on the target cell.
In this study, we noticed that MVs significantly stimulated cell migration as well as collagen I secretion, but not cell differentiation or MMP secretion. We also evaluated whether the effect of the MVs could be mimicked by soluble PLGF-1. Then, we determined that the MVs positive effect on collagen secretion could be inhibited by PLGF-1 neutralizing antibodies, further demonstrating the role of this cytokine in MVs and in ECM homeostasis. To our knowledge, this is the first demonstration of the action of this cytokine on ECM production. This result can explain the increase in granulation tissue formation in the presence of PLGF-1 (Barrientos et al. 2008) in parallel with the increase in fibroblast migration shown by these authors and by our results.
Other cytokines have been detected in MVs, such as LTA, VEGF and IL-23. These cytokines could also have an impact on wound healing mechanisms. VEGF and PLGF-1 are both cytokines involved in the stimulation of angiogenesis (Ferrara et al. 2003; Roy et al. 2006; Nagy et al. 2003), which could explain the pro-angiogenic role of MVs, previously demonstrated by our laboratory (Merjaneh et al. 2017). LTA and IL-23 are mostly pro-inflammatory cytokines (Mehrnaz Gharaee-Kermani 2004; Parham 2005) that could be involved at the end of the inflammation phase, when Wmyos appear.
The evaluation of cytokines in MVs versus conditioned media or their reference cells shows that the distribution of cytokines can be highly variable. While LTA was exclusively present in MVs, others were similarly present in MVs and conditioned media, such as PLGF-1, or mainly in conditioned medium, such as IL-23 or VEGF. These results are in accordance with the results obtained by Fitzgerald et al. demonstrating that cytokine distribution in MVs versus conditioned medium is variable according to the cytokines and strongly associated with the parent cell type (Fitzgerald et al. 2018).
It is becoming increasingly apparent that extracellular vesicles are an important topic for the understanding of intercellular communication, whether it be pathological or physiological phenomena. The discovery of the mechanisms of action of MVs on the synthesis and remodeling of ECM would be a great advance in the field of tissue regeneration. Our work is the first study that has demonstrated the role of Wmyo-derived MVs, and more precisely, of PLGF-1 associated with MVs, in collagen production during wound healing. Although studies on PLGF-1 mainly focus on angiogenesis, it seems that we are the first to report its action on the production of collagen I. MV production by Wmyos could thus be an important means of modulating granulation tissue formation, from angiogenesis to the formation of new ECM. Wmyos have thus a central role during this healing phase.
Acknowledgements
The authors thank Caroline Gilbert from the CHU of the Université Laval for the use of the Nanosizer® apparatus, Richard Janvier from IBIS of the Université Laval for the use of their TEM microscope and, Annie Karakeussian-Rimbaud from the CHUM for the handling of the multiplex ELISA device.
Abbreviations
- Wmyo
Myofibroblasts from normal skin wounds
- MVs
microvesicles
- PLGF-1
Placental growth factor 1
- ECM
Extracellular matrix
- TGFß1
Transforming growth factor-ß
- α-SMA
Alpha-Smooth Muscle Actin
- A2M
Alpha-2-macroglobulin
- MMPs
Matrix metalloproteinases
- DME
Dulbecco’s Modified Eagle’s medium
- FBS
Fetal bovine serum
- RFP-670
Red Fluorescent Protein
- PBS
Phosphate-buffered saline
- DLS
Dynamic light scattering
- PLGF-1-NAb
PLGF-1 neutralizing antibodies
- LTA
Lymphotoxin alpha/ tumor necrosis beta
Authors contributions
S.A. performed the experiments and wrote the manuscript, S.L conceived the transduction of Wmyo with a fluorescent protein, V.J.M conducted the project and wrote the manuscript. All authors reviewed the manuscript.
Funding information
This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) (RGPIN2014–04404); les Fonds de recherche du Québec-Santé (FRQS) (Research Centre funding grant); the Quebec Cell, Tissue and Gene Therapy Network–ThéCell (a thematic network supported by FRQS).
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code Availability
Not applicable.
Compliance with ethical standards
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethics approval
All procedures involving patients were reviewed and approved by the Research Ethical Committee of the Centre Hospitalier Universitaire (CHU) de Québec-Université Laval, 2014–04404.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adini A, Kornaga T, Firoozbakht F, Benjamin LE. Placental growth factor is a survival factor for tumor endothelial cells and macrophages. Cancer Res. 2002;62(10):2749–2752. [PubMed] [Google Scholar]
- Bainbridge, P. (2013). Wound healing and the role of fibroblasts. J Wound Care, 22(8), 407-408, 410-412. 10.12968/jowc.2013.22.8.407 [DOI] [PubMed]
- Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16(5):585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- Baum J, Duffy HS. Fibroblasts and Myofibroblasts: what are we talking about? J Cardiovasc Pharmacol. 2011;57(4):376–379. doi: 10.1097/FJC.0b013e3182116e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger D, Montezano AC, Nishigaki N, He Y, Carter A, Touyz RM. Endothelial microparticle formation by angiotensin II is mediated via Ang II receptor type I/NADPH oxidase/ rho kinase pathways targeted to lipid rafts. Arterioscler Thromb Vasc Biol. 2011;31(8):1898–1907. doi: 10.1161/atvbaha.110.222703. [DOI] [PubMed] [Google Scholar]
- Carmeliet, P., Moons, L., Luttun, A., Vincenti, V., Compernolle, V., De Mol, M., ... Persico, M. G. (2001). Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med, 7(5), 575–583. doi:10.1038/87904 [DOI] [PubMed]
- Chen L, Chen R, Kemper S, Brigstock DR. Pathways of production and delivery of hepatocyte exosomes. J Cell Commun Signal. 2018;12(1):343–357. doi: 10.1007/s12079-017-0421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DS, Kim DK, Kim YK, Gho YS. Proteomics of extracellular vesicles: Exosomes and ectosomes. Mass Spectrom Rev. 2015;34(4):474–490. doi: 10.1002/mas.21420. [DOI] [PubMed] [Google Scholar]
- Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- Costa Verdera H, Gitz-Francois JJ, Schiffelers RM, Vader P. Cellular uptake of extracellular vesicles is mediated by clathrin-independent endocytosis and macropinocytosis. J Control Release. 2017;266:100–108. doi: 10.1016/j.jconrel.2017.09.019. [DOI] [PubMed] [Google Scholar]
- Cutroneo KR. How is type I procollagen synthesis regulated at the gene level during tissue fibrosis. J Cell Biochem. 2003;90(1):1–5. doi: 10.1002/jcb.10599. [DOI] [PubMed] [Google Scholar]
- Darby IA, Laverdet B, Bonte F, Desmouliere A. Fibroblasts and myofibroblasts in wound healing. Clin Cosmet Investig Dermatol. 2014;7:301–311. doi: 10.2147/CCID.S50046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122(1):103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Fitzgerald W, Freeman ML, Lederman MM, Vasilieva E, Romero R, Margolis L. A system of cytokines encapsulated in ExtraCellular vesicles. Sci Rep. 2018;8(1):8973. doi: 10.1038/s41598-018-27190-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas-Andrade M, Carmeliet P, Charlebois C, Stanimirovic DB, Moreno MJ. PlGF knockout delays brain vessel growth and maturation upon systemic hypoxic challenge. J Cereb Blood Flow Metab. 2012;32(4):663–675. doi: 10.1038/jcbfm.2011.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain L, Jean A, Auger FA, Garrel DR. Human wound healing fibroblasts have greater contractile properties than dermal fibroblasts. J Surg Res. 1994;57(2):268–273. doi: 10.1006/jsre.1994.1143. [DOI] [PubMed] [Google Scholar]
- Grotendorst GR, Rahmanie H, Duncan MR. Combinatorial signaling pathways determine fibroblast proliferation and myofibroblast differentiation. FASEB J. 2004;18(3):469–479. doi: 10.1096/fj.03-0699com. [DOI] [PubMed] [Google Scholar]
- Hattori K, Heissig B, Wu Y, Dias S, Tejada R, Ferris B, Hicklin DJ, Zhu Z, Bohlen P, Witte L, Hendrikx J, Hackett NR, Crystal RG, Moore MAS, Werb Z, Lyden D, Rafii S. Placental growth factor reconstitutes hematopoiesis by recruiting VEGFR1(+) stem cells from bone-marrow microenvironment. Nat Med. 2002;8(8):841–849. doi: 10.1038/nm740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B. Myofibroblasts. Exp Eye Res. 2016;142:56–70. doi: 10.1016/j.exer.2015.07.009. [DOI] [PubMed] [Google Scholar]
- Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The Myofibroblast: one function, multiple origins. Am J Pathol. 2007;170(6):1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hromada C, Mühleder S, Grillari J, Redl H, Holnthoner W (2017) Endothelial extracellular vesicles—promises and challenges. Front Physiol 8. 10.3389/fphys.2017.00275 [DOI] [PMC free article] [PubMed]
- Huang P, Bi J, Owen GR, Chen W, Rokka A, Koivisto L, Heino J, Häkkinen L, Larjava H. Keratinocyte microvesicles regulate the expression of multiple genes in dermal fibroblasts. J Invest Dermatol. 2015;135(12):3051–3059. doi: 10.1038/jid.2015.320. [DOI] [PubMed] [Google Scholar]
- Kendall RT, Feghali-Bostwick CA. Fibroblasts in fibrosis: novel roles and mediators. Front Pharmacol. 2014;5:123. doi: 10.3389/fphar.2014.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laberge A, Arif S, Moulin VJ. Microvesicles: intercellular messengers in cutaneous wound healing. J Cell Physiol. 2018;233(8):5550–5563. doi: 10.1002/jcp.26426. [DOI] [PubMed] [Google Scholar]
- Laberge A, Ayoub A, Arif S, Larochelle S, Garnier A, Moulin VJ. α-2-macroglobulin induces the shedding of microvesicles from cutaneous wound myofibroblasts. J Cell Physiol. 2019;234(7):11369–11379. doi: 10.1002/jcp.27794. [DOI] [PubMed] [Google Scholar]
- Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2(2):329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- Mehrnaz Gharaee-Kermani SHP. Role of fibroblasts and Myofibroblasts in idiopathic pulmonary fibrosis. In: Lynch JP, editor. Idiopathic pulmonary fibrosis. Boca Raton: CRC Press; 2004. pp. 507–561. [Google Scholar]
- Merjaneh M, Langlois A, Larochelle S, Cloutier CB, Ricard-Blum S, Moulin VJ. Pro-angiogenic capacities of microvesicles produced by skin wound myofibroblasts. Angiogenesis. 2017;20(3):385–398. doi: 10.1007/s10456-017-9554-9. [DOI] [PubMed] [Google Scholar]
- Moulin V, Castilloux G, Jean A, Garrel DR, Auger FA, Germain L. In vitro models to study wound healing fibroblasts. Burns. 1996;22(5):359–362. doi: 10.1016/0305-4179(95)00167-0. [DOI] [PubMed] [Google Scholar]
- Moulin V, Castilloux G, Auger FA, Garrel D, O'Connor-McCourt MD, Germain L. Modulated response to cytokines of human wound healing myofibroblasts compared to dermal fibroblasts. Exp Cell Res. 1998;238(1):283–293. doi: 10.1006/excr.1997.3827. [DOI] [PubMed] [Google Scholar]
- Moulin V, Garrel D, Auger FA, O'Connor-McCourt M, Castilloux G, Germain L. What's new in human wound-healing myofibroblasts? Curr Top Pathol. 1999;93:123–133. doi: 10.1007/978-3-642-58456-5_13. [DOI] [PubMed] [Google Scholar]
- Moulin V, Auger FA, Garrel D, Germain L. Role of wound healing myofibroblasts on re-epithelialization of human skin. Burns. 2000;26(1):3–12. doi: 10.1016/S0305-4179(99)00091-1. [DOI] [PubMed] [Google Scholar]
- Moulin VJ, Mayrand D, Messier H, Martinez MC, Lopez-Valle CA, Genest H. Shedding of microparticles by myofibroblasts as mediator of cellular cross-talk during normal wound healing. J Cell Physiol. 2010;225(3):734–740. doi: 10.1002/jcp.22268. [DOI] [PubMed] [Google Scholar]
- Moulin V, Bellemare J, Bergeron D, Genest H, Roy M, Lopez-Vallé C. Dupuytren’s disease and related Hyperproliferative disorders: principles, research, and clinical perspectives. Berlin: Springer Berlin Heidelberg; 2012. Myofibroblasts and interactions with other cells: contribution of the tissue engineering; pp. 69–75. [Google Scholar]
- Nagy JA, Dvorak AM, Dvorak HF. VEGF-A(164/165) and PlGF: roles in angiogenesis and arteriogenesis. Trends Cardiovasc Med. 2003;13(5):169–175. doi: 10.1016/S1050-1738(03)00056-2. [DOI] [PubMed] [Google Scholar]
- Parham, P. (2005). T-cell mediated immunity. In The immune system (2nd ed.) (pp. 172). New York: Garland science
- Parolini, I., Federici, C., Raggi, C., Lugini, L., Palleschi, S., De Milito, A., ... Fais, S. (2009). Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem, 284(49), 34211–34222. 10.1074/jbc.M109.041152 [DOI] [PMC free article] [PubMed]
- Postlethwaite AE, Shigemitsu H, Kanangat S. Cellular origins of fibroblasts: possible implications for organ fibrosis in systemic sclerosis. Curr Opin Rheumatol. 2004;16(6):733–738. doi: 10.1097/01.bor.0000139310.77347.9c. [DOI] [PubMed] [Google Scholar]
- Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy H, Bhardwaj S, Yla-Herttuala S. Biology of vascular endothelial growth factors. FEBS Lett. 2006;580(12):2879–2887. doi: 10.1016/j.febslet.2006.03.087. [DOI] [PubMed] [Google Scholar]
- Schonherr E, Hausser HJ. Extracellular matrix and cytokines: a functional unit. Dev Immunol. 2000;7(2–4):89–101. doi: 10.1155/2000/31748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw TJ, Martin P (2009) Wound repair at a glance. Journal of Cell Science 122(18):3209–3213. 10.1242/jcs.031187 [DOI] [PMC free article] [PubMed]
- Stunova A, Vistejnova L. Dermal fibroblasts-a heterogeneous population with regulatory function in wound healing. Cytokine Growth Factor Rev. 2018;39:137–150. doi: 10.1016/j.cytogfr.2018.01.003. [DOI] [PubMed] [Google Scholar]
- Sun C, Feng SB, Cao ZW, Bei JJ, Chen Q, Zhao WB, Xu XJ, Zhou Z, Yu ZP, Hu HY. Up-regulated expression of matrix Metalloproteinases in endothelial cells mediates platelet microvesicle-induced angiogenesis. Cell Physiol Biochem. 2017;41(6):2319–2332. doi: 10.1159/000475651. [DOI] [PubMed] [Google Scholar]
- Szatanek R, Baran J, Siedlar M, Baj-Krzyworzeka M. Isolation of extracellular vesicles: determining the correct approach (review) Int J Mol Med. 2015;36(1):11–17. doi: 10.3892/ijmm.2015.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeo M, Lee W, Ito M. Wound healing and skin regeneration. Cold Spring Harb Perspect Med. 2015;5(1):a023267. doi: 10.1101/cshperspect.a023267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh NT, Yamashita T, Tu TC, Kato T, Ohneda K, Sato F, Ohneda O. Microvesicles enhance the mobility of human diabetic adipose tissue-derived mesenchymal stem cells in vitro and improve wound healing in vivo. Biochem Biophys Res Commun. 2016;473(4):1111–1118. doi: 10.1016/j.bbrc.2016.04.025. [DOI] [PubMed] [Google Scholar]
- Werner S, Grose R (2003) Regulation of wound healing by growth factors and cytokines. Physiol Rev, 83(3), 835–870. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12843410, http://physrev.physiology.org/content/physrev/83/3/835.full.pdf [DOI] [PubMed]
- Xue, M., & Jackson, C. J. (2015). Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. In Adv Wound Care (New Rochelle) (Vol. 4, pp. 119-136) [DOI] [PMC free article] [PubMed]
- Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, ... De Wever O (2015) Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles, 4, 27066. doi:10.3402/jev.v4.27066 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Not applicable.