Abstract
Background:
Manual qualitative and quantitative measures of terminal duct lobular unit (TDLU) involution were previously reported to be inversely associated with breast cancer risk. We developed and applied a deep-learning method to yield quantitative measures of TDLU involution in normal breast tissue. We assessed the associations of these automated measures with breast cancer risk factors and risk.
Methods:
We obtained eight quantitative measures from whole slide images (WSIs) from a benign breast disease (BBD) nested case-control study within the Nurses’ Health Studies (287 breast cancer cases and 1083 controls). Qualitative assessments of TDLU involution were available for 177 cases and 857 controls. The associations between risk factors and quantitative measures among controls were assessed using analysis of covariance adjusting for age. The relationship between each measure and risk was evaluated using unconditional logistic regression, adjusting for the matching factors, BBD subtypes, parity, and menopausal status. Qualitative measures and breast cancer risk were evaluated accounting for matching factors and BBD subtypes.
Results:
Menopausal status and parity were significantly associated with all eight measures; select TDLU measures were associated with BBD histological subtype, BMI, and birth index (p<0.05). No measure was correlated with body size at ages 5-10 years, age at menarche, age at first birth, or breastfeeding history (p>0.05). Neither quantitative nor qualitative measures were associated with breast cancer risk.
Conclusions:
Among Nurses’ Health Studies women diagnosed with BBD, TDLU involution is not a biomarker of subsequent breast cancer.
Impact:
TDLU involution may not impact breast cancer risk as previously thought.
Keywords: breast cancer risk factors, breast lobule involution, breast lobule atrophy, deep learning, computational pathology
Introduction
Terminal duct lobular units (TDLUs) are the functional milk-producing structures of the breast that consist of an extra lobular terminal duct and a lobule composed of clusters of acini. TDLUs are the origin of most breast cancer precursors and cancers (1–3). Puberty, pregnancy, lactation, and menopausal transition mark important times of breast tissue alterations. TDLUs are traditionally assessed qualitatively and classified into four lobule types: type 1 (least developed; <12 acini), type 2 (intermediate; ~50 acini), type 3 (fully developed; >80 acini), and type 4 (occurs during pregnancy and lactation) (4). TDLU involution is a natural phenomenon that occurs with aging as lobules of types 2 and 3 regress to type 1. In quantitative terms, TDLU involution is reflected by decreases in the number and size of TDLUs, as well as the number of acini in the breast (3).
Using qualitative assessment of TDLU involution, we and others showed that among women with benign breast disease (BBD), those with less TDLU involution had a higher risk of developing breast cancer compared to those with increased involution (5,6). The manual assessment of TDLU involution is subjective and laborious, and is a major bottleneck to studying TDLU involution in large epidemiological studies. Research groups subsequently developed more quantitative and reliable measures (7–9).
In 2009, McKian et al. measured the number of acini per lobule and lobular area in women diagnosed with BBD (85 patients who developed breast cancer and 142 age-matched controls). The number of acini per lobule and lobular area were inversely associated with breast cancer risk, after adjusting for Gail model score, parity, histology, and family history (8). In 2014, Figueroa et al. developed three standardized measures of TDLU involution—number of TDLUs per tissue area, median TDLU span, and the median number of acini per TDLU (9). Their subsequent nested case-control study in 99 cases and 145 age-matched controls demonstrated that women in the highest quartile of TDLU counts and TDLU span had higher breast cancer risk compared to women in the lowest quartile, accounting for family history of breast cancer, menopausal hormone use, and BBD severity (10). These semi-quantitative measures were also associated with higher breast density in pre-menopausal Caucasian women (11) and post-menopausal Chinese women (12), and aggressive breast cancer subtypes in Chinese (13) and Polish women (14). Although these quantitative measures of TDLU involution developed by Figueroa et al. were an improvement over qualitative categories, they were considered semi-quantitative as they still relied on pathologists to conduct histological assessment of the breast tissues and acquire measurements.
In 2013, Rosebrock et al. pioneered a computational method to quantify the number of acini in a TDLU using classical medical imaging techniques (15). Their method was limited to images that only contain one TDLU each, and not whole slide images (WSIs) with multiple TDLUs. In 2019, our group developed a fully-automated deep learning computational pathology method to segment TDLUs, detect acini, and quantify TDLUs and acini on WSIs (7,16). In this manuscript, we applied our automated method to the BBD nested case-control study within the Nurses’ Health Study (NHS) and NHSII to obtain quantitative TDLU involution measures for 287 cases and 1083 controls. We then assessed the associations of these quantitative measures with established breast cancer risk factors and subsequent breast cancer risk. This study is one of the first to apply an artificial intelligence WSI analysis method to a large breast cancer epidemiological study. The number of participants in this study is larger than similar BBD nested case-control studies (8,10).
Materials and Methods
Study population
The NHS was established in 1976 with 121,700 US female registered nurses aged 30-55 years. NHSII was established in 1989 with 116,429 nurses aged 25-42 years. NHS and NHSII participants completed baseline questionnaires that provided a medical history as well as extensive information about demographic, lifestyle, reproductive, and dietary risk factors for breast cancer (17). Participants provide updated information biennially via follow-up questionnaires, and also report new diagnoses of BBD or breast cancer. Participants who reported a diagnosis of BBD were contacted for consent to obtain pathology records and tissue specimens pertaining to the BBD lesion from the diagnosing hospital. Participants who reported breast cancer were confirmed via medical record review, verbally by the participant, or via the cancer registry.
A nested case-control study of women with biopsy-confirmed BBD was created within the NHS and NHSII (5,18–25). Cases were women who reported a diagnosis of invasive breast cancer after the cohort baseline (through 1998 for NHS, through 1999 for NHSII) and had previously reported a BBD diagnosis (either prior to study entry or after study baseline). Cases were excluded if the time between BBD and breast cancer diagnoses was less than six months or if there was evidence of carcinoma (invasive or in situ) during centralized histopathological review of the BBD lesion. Tumor estrogen receptor (ER) status was retrieved from pathology reports. Controls were women diagnosed with BBD who did not develop breast cancer. Cases and controls were matched 1:4 on year of BBD diagnosis, age at breast cancer diagnosis (index date for controls), and years between BBD and breast cancer diagnosis (or index date). The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health, and those of participating registries as required.
Whole slide image acquisition
Hematoxylin and eosin (H&E) breast tissue slides were retrieved for biopsy-confirmed BBD patients who gave permission to review their biopsy records (18,20,21). H&E slides were available for 488 cases and 2124 controls (i.e., full nested case-control study group) for centralized pathology review (5,18,19). Within this group, a total of 3836 slides were digitized into WSIs at 20× (n=234) or 40× (n=3602) magnification using the Panoramic SCAN 150 (3DHISTECH Ltd, Budapest, Hungary). For women with good quality slides, up to six slides from different tissue blocks were digitized. H&E slides that could not be digitized were due to poor quality, slides too thick to fit into scanner, and plastic mounting covers. Attempts to create new H&E slides were not always possible due to missing (or returned to hospital) blocks, old-style blocks not created using tissue cassettes, or poor-quality blocks.
Quantifying TDLUs and acini
We previously published our deep learning computational pathology method that detects and quantifies normal acini, segments and quantifies normal TDLUs, and segments adipose tissue (Figures 1A and 1B) (7,16). Briefly, each task was developed using a separate U-Net convolutional neural network architecture, and the networks were integrated into a single automated method. A total of 92 WSIs were annotated for normal acini, TDLU, and adipose tissue to train the networks. The training images were annotated in reference to the pathological assessment criteria as described by Figueroa and colleagues (7,9,10,16)—TDLUs with proliferative or metaplastic changes were not annotated but remained as background; acini with elongated shapes, epithelial proliferation, apocrine metaplasia, or without lumina were also not annotated. We validated and reported that the three standardized quantitative measures (established by Figueroa et al. (9)) when derived using our automated method were highly correlated with manually acquired data in an independent set of 40 WSIs (7).
Figure 1.
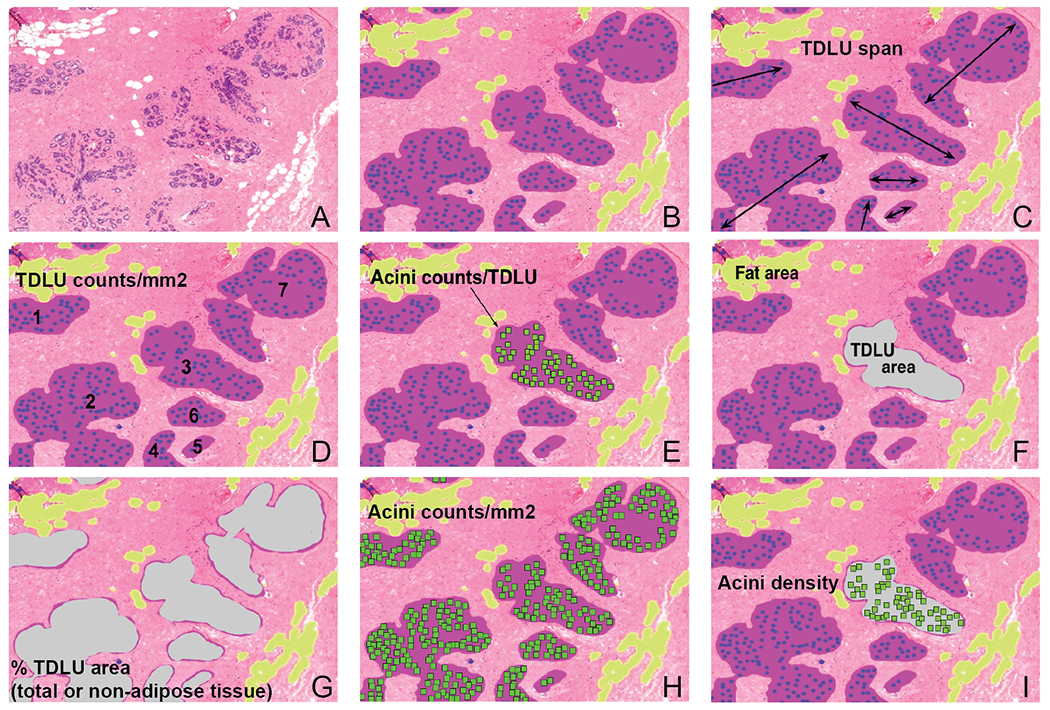
A panel of a region of a whole slide image describing our method and how the eight quantitative terminal duct lobular unit (TDLU) measures are calculated. (A) A region of a whole slide image. (B) Our computational pathology method segments TDLUs (purple areas), detects acini (blue dots), and segments adipose tissue (yellow areas). Quantitative TDLU involution measures investigated in this study consisted of the three standardized measures (median TDLU span (C), TDLU counts per non-adipose tissue area (D), and median acini counts per TDLU (E)), and five novel measures (median TDLU area (F), total TDLU area as a percentage of tissue area and non-adipose tissue area (G), total number of acini (detected in TDLUs) per non-adipose tissue area (H), and median acini density (I)).
We applied our method to the WSIs in this study. For each WSI, our method computed 1) total, adipose, and non-adipose tissue areas (mm2); 2) TDLU counts; 3) TDLU area (mm2); 4) TDLU span (μm); and 5) number of acini per TDLU. Of 3836 WSIs, 129 WSIs from women who did not satisfy study inclusion criteria were excluded; 12 WSIs could not be assessed by the automated method because of blurriness or artefacts; and 205 WSIs with fewer than six TDLUs were removed because previous work reported that at least six TDLUs should be evaluated to obtain reliable TDLU involution measures (8,10,14). Therefore, TDLU involution measures were obtained from 3490 WSIs representing 287 cases and 1083 controls (total n=1370). Among these participants with quantitative data, cases were diagnosed with breast cancer a median of 7.75 years after BBD diagnoses (interquartile range 4.42 to 11.92 years). Each participant contributed between one and five WSIs (median WSIs n=3).
Multiple WSIs for each participant were combined to obtain eight TDLU involution measures—three standardized measures established by Figueroa et al. (median TDLU span, TDLU counts per non-adipose tissue area, and median acini counts per TDLU; Figures 1C–1E) (9) and five novel measures (median TDLU area, TDLU area as a percentage of total tissue area (% TDLU area (total)), TDLU area as a percentage of non-adipose tissue area (% TDLU area (non-adipose)), acini counts per non-adipose tissue area, and median acini density; Figures 1F–1I; Supplementary Table S1). Acini density was calculated by dividing the number of acini within a TDLU by its TDLU area. Since the amount of adipose tissue is inversely correlated with TDLU counts (9,10), TDLU and acini counts were adjusted by dividing by non-adipose tissue area. Acini counts per non-adipose tissue area only included acini detected in TDLUs.
In Figueroa et al., while the median TDLU spans and median acini counts were restricted to WSIs with at least six TDLUS, all WSIs were included when measuring TDLU counts (9,10). We found that the relationships between breast cancer risk factors or breast cancer risk and TDLU counts/mm2 were highly similar regardless of whether all WSIs were included or WSIs with less than six TDLUs were excluded. For consistency, we computed all the TDLU measures in this study by excluding WSIs with less than six TDLUs.
Qualitative assessment of TDLU involution by pathologists
In prior BBD analyses within the NHS and NHSII, breast lobules were manually classified into type 1 (<12 acini), type 2 (~50 acini), and type 3 (~80 acini) (4,5). The presence of any type 1 or any type 3 lobules in normal TDLUs as well as the predominant lobule type for each participant were noted. Participants were grouped into three qualitative categories: no type 1 lobules (i.e., minimal involution), mixed lobule types (i.e., partial involution), and predominant type 1 and no type 3 lobules (i.e., complete involution) (5). Among the participants with automated quantitative data, 177 cases and 857 controls (total n=1034) had accompanying qualitative TDLU involution measurements.
Breast cancer risk factors
The histological type of the BBD lesion (non-proliferative, proliferative without atypia, and atypical hyperplasia) was determined by central pathology review. Participant body mass index (BMI), age at menarche, parity, age at first birth, breastfeeding history, and menopausal status were ascertained by the closest questionnaire prior to BBD biopsy. Body sizes at ages 5 and 10 were reported by cohort participants using a nine-level pictogram (Level 1 as leanest) (23), and the mean of the two reports was used to reflect childhood body size. Birth index, a metric reflecting the timing and spacing of births, was calculated as previously described (26). A higher birth index indicates a higher number of births occurring at earlier ages.
Statistical Analysis
Correlations between quantitative TDLU involution measures and between involution measures and age at BBD biopsy were evaluated among controls using Spearman’s rho. The relationships between qualitative TDLU categories and age at BBD biopsy or quantitative involution measures were evaluated among controls using the one-sided Jonckheere-Terpstra test to determine an increasing or decreasing trend (PMCMR R package version 4.3 (27)). The associations between breast cancer risk factors and quantitative involution measures (natural log-transformed) among controls were assessed using analysis of covariance (ANCOVA) adjusting for age at BBD biopsy (emmeans R package version 1.4.4 (28)).
Each quantitative measure was categorized into quartiles as defined by the distribution among the controls. The relationship between each quantitative measure (in quartiles) and breast cancer risk was evaluated using unconditional logistic regression models accounting for the matching factors to estimate odd ratios (ORs) and 95% confidence intervals (CI). Unconditional logistic regression models were used because there were incomplete matched case-control sets due to the inability to obtain pathology records and/or slides for all selected cases and controls. Model 1 adjusted for matching factors. Model 2 adjusted for matching factors and BBD histological subtypes. Model 3 adjusted for matching factors, BBD histological subtypes, parity, and menopausal status. Analyses were also conducted by stratifying the participants according to parity, menopausal status, or BBD histological subtype.
Qualitative TDLU involution measures and breast cancer risk were also evaluated using unconditional logistic regression models accounting for the matching factors (Model 1) and for matching factors and BBD histological subtypes (Model 2). The level of significance used for all statistical tests was p<0.05. We did not adjust for multiple comparisons. All statistical analyses were performed using R.
Results
Study population
The matching factors and BBD histopathological subtypes of the 287 breast cancer cases and 1083 controls with WSIs are shown in Table 1. The majority of the participants were diagnosed with proliferative breast disease without atypia. Cases were more likely to be diagnosed with atypical hyperplasia than controls (27.5% versus 14.3%). The mean (± standard deviation (SD)) age at breast cancer diagnosis among cases was 53.9±8.6. Among the 287 cases, 179 tumors were ER-positive, 51 were ER-negative, and 57 were unknown.
Table 1.
Participants’ characteristics in this study.
| Cases, n (%) | Controls, n (%) | ||
|---|---|---|---|
| n | 287 | 1083 | |
| Age at benign breast disease (BBD) biopsy | |||
| <40 years | 76 (26.5) | 244 (22.5) | |
| 40-49 years | 131 (45.6) | 431 (39.8) | |
| 50-59 years | 56 (19.5) | 272 (25.1) | |
| ≥60 years | 24 (8.4) | 136 (12.6) | |
| Year of BBD biopsy | |||
| Before 1970 | 30 (10.4) | 55 (5.1) | |
| 1970 to 1979 | 78 (27.2) | 224 (20.7) | |
| 1980 to 1989 | 128 (44.6) | 475 (43.9) | |
| After 1989 | 51 (17.8) | 329 (30.4) | |
| Age at breast cancer diagnosis/index date | |||
| <45 years | 41 (14.3) | 197 (18.2) | |
| 45-54 years | 111 (38.7) | 361 (33.3) | |
| ≥55 years | 135 (47.0) | 525 (48.5) | |
| Years between BBD biopsy and breast cancer diagnosis/index date | |||
| 0.5 to 4.9 years | 85 (29.6) | 501 (46.3) | |
| 5.0 to 9.9 years | 101 (35.2) | 274 (25.3) | |
| 10.0 to 14.9 years | 54 (18.8) | 169 (15.6) | |
| ≥15.0 years | 47 (16.4) | 139 (12.8) | |
| BBD histological subtype | |||
| Non-proliferative | 59 (20.6) | 303 (28.0) | |
| Proliferative without atypia | 149 (51.9) | 625 (57.7) | |
| Atypical hyperplasia | 79 (27.5) | 155 (14.3) | |
Confirming the inverse relationship between quantitative or qualitative involution measures and age
We observed an inverse relationship between age at BBD biopsy and TDLU involution among the 1083 controls (Supplementary Figure S1). All eight quantitative measures were inversely correlated with age with Spearman’s rho ranging from −0.42 for median TDLU area to −0.07 for median acini density. The quantitative measures were significantly positively correlated with each other apart from median acini density, which was inversely associated with median TDLU area (rho=−0.17) and median TDLU span (rho=−0.31).
Qualitative assessment of TDLU involution by central pathology review was available for 857 of 1083 controls. One hundred and fourteen participants were categorized as no type 1 lobules (i.e., minimal involution), 409 had mixed lobule types (i.e., partial involution), and 334 had predominant type 1 and no type 3 lobules (i.e., complete involution). Median age at BBD biopsy was higher among women with no type 3 lobules than among women with no type 1 lobules (p-trend <0.001; Figure 2A). Medians of all eight quantitative measures significantly decreased across qualitative categories of TDLU involution (all p-trend <0.001; Figures 2B–2I), indicating good concordance between the automated method with pathologists’ manual assessment.
Figure 2.
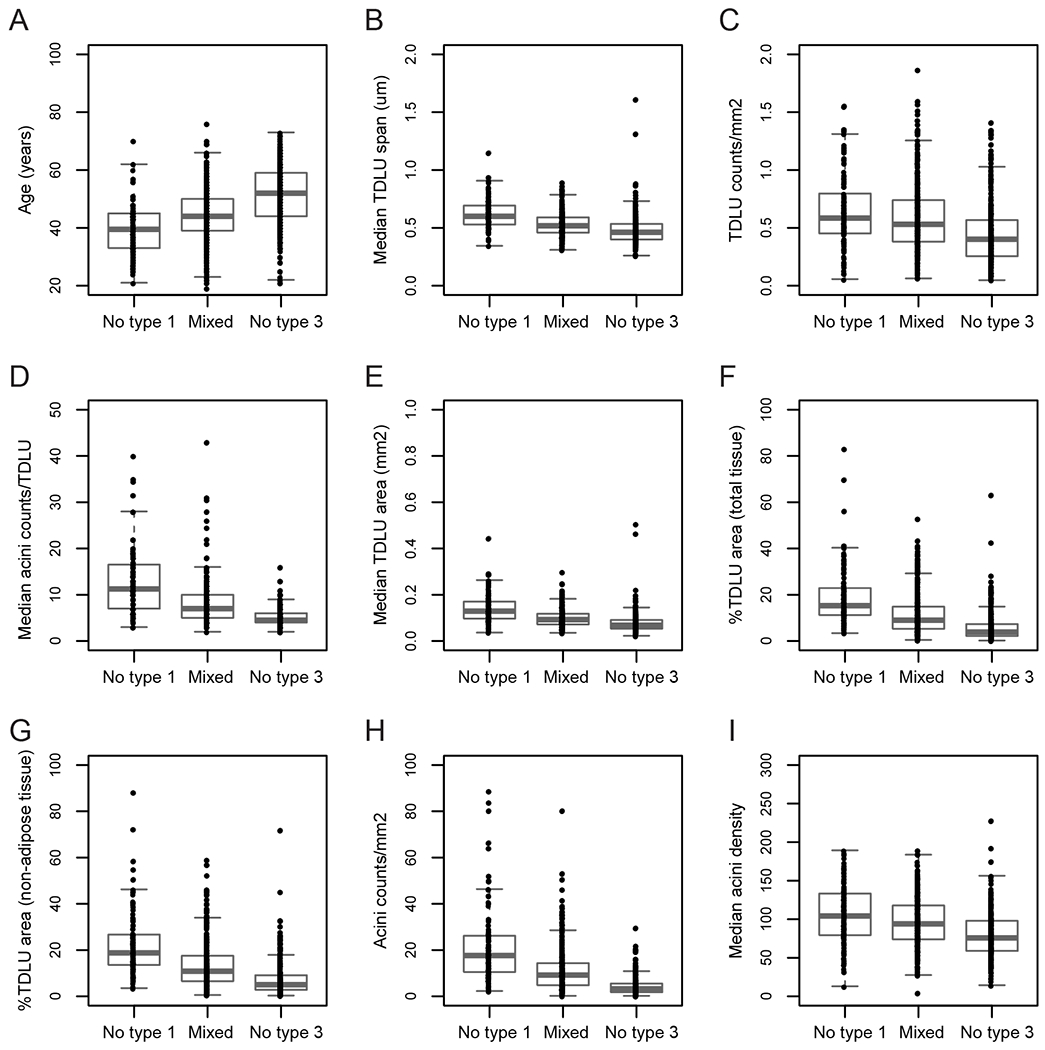
Terminal duct lobular unit (TDLU) involution was evaluated among 827 controls using qualitative categories—no type 1 lobules (n=114), mixed lobule types (n=409), and predominant type 1 and no type 3 lobules (n=334). TDLU involution was significantly correlated with age at BBD biopsy (p-trend <0.001; A) and significantly inversely correlated with the eight quantitative measures derived from our automated method (p-trend <0.001; B-I).
Association of breast cancer risk factors and quantitative measures among controls
Table 2 displays the age-adjusted means (95% CI) and the ANCOVA p-values of the associations between BBD histological subtypes, body size, and reproductive breast cancer risk factors and the quantitative measures of TDLU involution among the controls. Women with proliferative BBD subtypes (with or without atypia) appear to have less TDLU involution compared to controls with non-proliferative subtypes as their breast tissues consisted of a greater percentage of TDLUs (i.e., higher % TDLU area) and higher acini counts/mm2 (p<0.05); the remaining measures did not differ by BBD subtype.
Table 2.
Quantitative terminal duct lobular unit (TDLU) measures and breast cancer risk factors among 1083 controls. Data presented for age are means (95% confidence interval). Data for other variables are presented as age-adjusted means (95% confidence interval); age was adjusted as a continuous variable.
| n | Median TDLU span (μm) | TDLU counts/mm2 | Median acini counts/TDLU | Median TDLU area (mm2) | |
|---|---|---|---|---|---|
| Age at benign breast disease (BBD) biopsy | |||||
| <40 years | 244 | 0.56 (0.55,0.58) | 0.48 (0.44,0.52) | 7.56 (7.11,8.04) | 0.11 (0.10,0.11) |
| 40-49 years | 431 | 0.52 (0.51,0.53) | 0.49 (0.46,0.51) | 7.52 (7.18,7.87) | 0.09 (0.09,0.10) |
| 50-59 years | 272 | 0.47 (0.45,0.48) | 0.43 (0.40,0.46) | 5.32 (5.02,5.64) | 0.07 (0.07,0.07) |
| ≥60 years | 136 | 0.46 (0.44,0.47) | 0.38 (0.34,0.42) | 4.33 (3.99,4.71) | 0.07 (0.06,0.07) |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | |
| BBD histological subtype | |||||
| Non-proliferative | 303 | 0.49 (0.48,0.51) | 0.43 (0.40,0.46) | 6.40 (6.05,6.78) | 0.08 (0.08,0.09) |
| Proliferative without atypia | 625 | 0.51 (0.50,0.52) | 0.46 (0.44,0.48) | 6.36 (6.12,6.62) | 0.09 (0.08,0.09) |
| Atypical hyperplasia | 155 | 0.51 (0.50,0.53) | 0.48 (0.44,0.53) | 6.86 (6.33,7.43) | 0.09 (0.08,0.10) |
| p-value | 0.06 | 0.14 | 0.25 | 0.04 | |
| Body size at ages 5-10 years | |||||
| Level 1 | 308 | 0.51 (0.50,0.53) | 0.46 (0.43,0.49) | 6.58 (6.22,6.97) | 0.09 (0.08,0.09) |
| Level 1.5 to 2 | 276 | 0.51 (0.50,0.52) | 0.45 (0.42,0.48) | 6.66 (6.27,7.07) | 0.09 (0.08,0.09) |
| Level ≥2.5 | 353 | 0.50 (0.49,0.51) | 0.46 (0.44,0.49) | 6.12 (5.80,6.45) | 0.08 (0.08,0.09) |
| p-value | 0.44 | 0.84 | 0.07 | 0.10 | |
| Body mass index (kg/m2) | |||||
| <25 | 612 | 0.51 (0.50,0.52) | 0.45 (0.43,0.47) | 6.52 (6.26,6.78) | 0.09 (0.08,0.09) |
| 25 to <30 | 288 | 0.50 (0.49,0.52) | 0.45 (0.42,0.48) | 6.62 (6.24,7.01) | 0.08 (0.08,0.09) |
| ≥30 | 169 | 0.51 (0.49,0.52) | 0.48 (0.44,0.52) | 5.90 (5.47,6.36) | 0.08 (0.08,0.09) |
| p-value | 0.88 | 0.44 | 0.04 | 0.34 | |
| Age of menarche | |||||
| ≤12 years | 515 | 0.50 (0.49,0.51) | 0.46 (0.44,0.49) | 6.46 (6.19,6.75) | 0.08 (0.08,0.09) |
| 13 years | 317 | 0.51 (0.50,0.52) | 0.45 (0.42,0.48) | 6.51 (6.16,6.88) | 0.09 (0.08,0.09) |
| ≥14 years | 246 | 0.51 (0.50,0.53) | 0.45 (0.42,0.49) | 6.29 (5.91,6.70) | 0.09 (0.08,0.09) |
| p-value | 0.55 | 0.79 | 0.70 | 0.84 | |
| Parity | |||||
| Nulliparous | 101 | 0.48 (0.46,0.51) | 0.33 (0.30,0.38) | 5.20 (4.71,5.73) | 0.08 (0.07,0.08) |
| Parous | 978 | 0.51 (0.50,0.52) | 0.47 (0.45,0.49) | 6.58 (6.38,6.79) | 0.09 (0.08,0.09) |
| p-value | 0.03 | <0.001 | <0.001 | 0.03 | |
| Age at first birth among parous women | |||||
| <25 years | 535 | 0.50 (0.49,0.51) | 0.46 (0.44,0.49) | 6.55 (6.28,6.83) | 0.08 (0.08,0.09) |
| 25 to 29 years | 347 | 0.51 (0.50,0.52) | 0.49 (0.46,0.52) | 6.42 (6.10,6.76) | 0.09 (0.08,0.09) |
| ≥30 years | 99 | 0.52 (0.50,0.54) | 0.44 (0.39,0.50) | 6.77 (6.14,7.46) | 0.09 (0.08,0.10) |
| p-value | 0.22 | 0.28 | 0.63 | 0.20 | |
| Birth index among parous women | |||||
| ≤30 | 226 | 0.52 (0.50,0.53) | 0.47 (0.43,0.51) | 6.43 (5.99,6.91) | 0.09 (0.08,0.10) |
| 31 to 59 | 275 | 0.52 (0.50,0.53) | 0.48 (0.45,0.52) | 7.29 (6.86,7.74) | 0.09 (0.09,0.10) |
| ≥60 | 218 | 0.52 (0.50,0.53) | 0.51 (0.47,0.56) | 7.05 (6.56,7.58) | 0.09 (0.08,0.09) |
| p-value | 0.97 | 0.35 | 0.04 | 0.82 | |
| Breastfeeding among parous women | |||||
| Never | 390 | 0.50 (0.49,0.51) | 0.46 (0.43,0.49) | 6.39 (6.09,6.70) | 0.08 (0.08,0.09) |
| <6 months | 203 | 0.50 (0.49,0.52) | 0.48 (0.44,0.52) | 6.85 (6.41,7.32) | 0.09 (0.08,0.09) |
| ≥6 months | 292 | 0.51 (0.50,0.53) | 0.47 (0.44,0.51) | 6.50 (6.15,6.88) | 0.09 (0.08,0.09) |
| p-value | 0.43 | 0.70 | 0.25 | 0.65 | |
| Menopausal Status | |||||
| Pre | 667 | 0.52 (0.51,0.53) | 0.49 (0.46,0.52) | 7.35 (7.03,7.68) | 0.09 (0.09,0.09) |
| Post | 332 | 0.49 (0.47,0.50) | 0.39 (0.36,0.43) | 4.93 (4.59,5.29) | 0.08 (0.07,0.08) |
| p-value | <0.01 | <0.01 | <0.001 | <0.001 | |
| % TDLU area (total) | % TDLU area (non-adipose) | Acini counts/mm2 | Median acini density | ||
| Age at benign breast disease (BBD) biopsy | |||||
| <40 years | 11.15 (9.95,12.50) | 13.08 (11.69,14.64) | 9.27 (8.21,10.46) | 80.88 (76.82,85.16) | |
| 40-49 years | 7.87 (7.22,8.57) | 9.64 (8.86,10.49) | 7.64 (6.97,8.37) | 90.92 (87.46,94.51) | |
| 50-59 years | 4.90 (4.39,5.45) | 6.19 (5.56,6.88) | 4.41 (3.93,4.95) | 83.00 (79.05,87.15) | |
| ≥60 years | 3.39 (2.91,3.95) | 4.45 (3.83,5.17) | 2.80 (2.38,3.29) | 73.01 (68.14,78.23) | |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | |
| BBD histological subtype | |||||
| Non-proliferative | 4.99 (4.52,5.52) | 6.26 (5.67,6.92) | 5.01 (4.49,5.58) | 87.47 (83.44,91.70) | |
| Proliferative without atypia | 7.59 (7.09,8.14) | 9.31 (8.69,9.97) | 6.52 (6.04,7.03) | 82.51 (79.86,85.25) | |
| Atypical hyperplasia | 7.94 (6.90,9.15) | 9.71 (8.45,11.17) | 7.10 (6.09,8.28) | 84.80 (79.34,90.64) | |
| p-value | <0.001 | <0.001 | <0.001 | 0.13 | |
| Body size at ages 5-10 years | |||||
| Level 1 | 7.45 (6.73,8.25) | 9.16 (8.29,10.13) | 6.66 (5.97,7.43) | 84.80 (80.90,88.88) | |
| Level 1.5 to 2 | 6.97 (6.26,7.75) | 8.49 (7.64,9.44) | 6.15 (5.48,6.91) | 83.78 (79.72,88.03) | |
| Level ≥2.5 | 6.41 (5.83,7.05) | 7.98 (7.27,8.76) | 5.82 (5.26,6.45) | 83.00 (79.44,86.72) | |
| p-value | 0.10 | 0.14 | 0.21 | 0.81 | |
| Body mass index (kg/m2) | |||||
| <25 | 6.84 (6.36,7.35) | 8.34 (7.77,8.96) | 6.10 (5.64,6.59) | 83.86 (81.12,86.69) | |
| 25 to <30 | 6.85 (6.17,7.61) | 8.46 (7.62,9.39) | 6.36 (5.68,7.13) | 87.02 (82.89,91.35) | |
| ≥30 | 6.49 (5.66,7.43) | 8.28 (7.24,9.48) | 5.73 (4.95,6.64) | 80.77 (75.84,86.02) | |
| p-value | 0.78 | 0.96 | 0.54 | 0.18 | |
| Age of menarche | |||||
| ≤12 years | 6.74 (6.24,7.29) | 8.33 (7.71,9.00) | 6.13 (5.64,6.67) | 85.23 (82.21,88.36) | |
| 13 years | 6.90 (6.25,7.63) | 8.53 (7.73,9.41) | 6.24 (5.61,6.95) | 84.30 (80.51,88.26) | |
| ≥14 years | 6.78 (6.06,7.59) | 8.31 (7.43,9.29) | 5.94 (5.26,6.71) | 81.53 (77.39,85.90) | |
| p-value | 0.94 | 0.92 | 0.83 | 0.39 | |
| Parity | |||||
| Nulliparous | 4.41 (3.70,5.26) | 5.44 (4.57,6.47) | 3.52 (2.92,4.25) | 73.92 (68.11,80.21) | |
| Parous | 7.10 (6.71,7.51) | 8.75 (8.28,9.25) | 6.48 (6.10,6.88) | 85.33 (83.14,87.58) | |
| p-value | <0.001 | <0.001 | <0.001 | <0.01 | |
| Age at first birth among parous women | |||||
| <25 years | 6.64 (6.15,7.16) | 8.21 (7.62,8.85) | 6.15 (5.67,6.66) | 86.92 (83.92,90.04) | |
| 25 to 29 years | 7.51 (6.83,8.24) | 9.31 (8.48,10.21) | 6.73 (6.09,7.45) | 84.03 (80.44,87.79) | |
| ≥30 years | 6.89 (5.78,8.22) | 8.36 (7.03,9.94) | 6.27 (5.19,7.56) | 81.34 (74.95,88.28) | |
| p-value | 0.13 | 0.11 | 0.38 | 0.24 | |
| Birth index among parous women | |||||
| ≤30 | 7.48 (6.60,8.48) | 9.20 (8.14,10.41) | 6.64 (5.79,7.61) | 80.05 (75.45,84.92) | |
| 31 to 59 | 7.77 (7.00,8.63) | 9.57 (8.63,10.61) | 7.24 (6.46,8.12) | 88.67 (84.38,93.18) | |
| ≥60 | 7.95 (7.01,9.02) | 9.74 (8.61,11.03) | 7.70 (6.71,8.84) | 90.27 (85.04,95.81) | |
| p-value | 0.82 | 0.83 | 0.37 | 0.01 | |
| Breastfeeding among parous women | |||||
| Never | 6.71 (6.14,7.33) | 8.33 (7.64,9.09) | 6.06 (5.51,6.66) | 84.89 (81.52,88.41) | |
| <6 months | 6.88 (6.09,7.78) | 8.48 (7.51,9.57) | 6.54 (5.74,7.46) | 89.52 (84.62,94.69) | |
| ≥6 months | 7.38 (6.67,8.18) | 9.11 (8.23,10.07) | 6.70 (6.01,7.48) | 84.58 (80.70,88.64) | |
| p-value | 0.37 | 0.41 | 0.35 | 0.25 | |
| Menopausal Status | |||||
| Pre | 7.76 (7.16,8.42) | 9.52 (8.78,10.31) | 7.49 (6.86,8.17) | 89.94 (86.64,93.35) | |
| Post | 5.40 (4.73,6.15) | 6.70 (5.89,7.63) | 4.13 (3.59,4.75) | 72.15 (67.93,76.63) | |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | |
Breast tissue of women who reported a larger childhood body size (Levels 1.5-2 and ≥2.5) had suggestively lower median acini counts/TDLU (p=0.07), smaller median TDLU area (p=0.10), and a lower percentage of TDLU area in total tissue (p=0.10) compared to women with body sizes of 1 or 1.5 to 2 at ages 5-10 years. Women with BMI ≥30 at the time of BBD biopsy had lower median acini counts per TDLU compared to women with lower BMI (p=0.04). BMI was not associated with the other seven measures (Table 2).
Parous women had less TDLU involution compared to nulliparous women. Parous women had higher TDLU counts/mm2, acini counts/TDLU, median TDLU area, % TDLU area in total and non-adipose tissue, acini counts/mm2, and median acini density (all p<0.05; Table 2). Results were similar when parous women were further subdivided into women who had one birth (primiparous) and women who had ≥2 births (multiparous). Both primiparous and multiparous women had less TDLU involution compared to nulliparous women, with multiparous women displaying the least amount of TDLU involution (Supplementary Table S2). Parous women were also subdivided into women whose last birth was <20 years or ≥20 years prior to BBD diagnosis. The observation of less TDLU involution in parous women was mostly driven by women who had their last birth <20 years prior to BBD diagnosis. The degree of TDLU involution in women who had their last birth ≥20 years prior to BBD diagnosis still remained higher than nulliparous women (Supplementary Table S2).
Women with a birth index ≤30 (i.e., fewer births at later ages) had lower median acini counts (p=0.04) and acini density (p=0.01) relative to women with higher birth indices; birth index was not associated with the other measures (Table 2). Menopausal status was associated with all eight measures after adjusting for age (p<0.01). As expected, post-menopausal women had fewer TDLUs, smaller TDLUs, and fewer acini in their breast tissues (i.e., more involution) compared to pre-menopausal women (Table 2). These eight measures were also selectively associated with age at menopause and/or elapsed time from menarche to menopause in post-menopausal women (Supplementary Table S3). No measures were significantly correlated with age of menarche, age at first birth, or breastfeeding.
TDLU involution measures and breast cancer risk
No quantitative TDLU involution metric was associated with subsequent breast cancer risk in crude BBD subtype-adjusted, or BBD subtype, parity, and menopausal status-adjusted models (all p-trend >0.05; Table 3 and Supplementary Table S4). Results remained null when stratified by parity (Supplementary Table S5), menopausal status (Supplementary Table S6), or BBD histological subtype (Supplementary Table S7). Polytomous logistic regression models assessed the association between the quantitative TDLU involution measures and risk of breast cancer defined by tumor ER expression, and demonstrated no heterogeneity (Supplementary Table S8).
Table 3.
The association between automated terminal duct lobular unit (TDLU) measures and breast cancer risk was evaluated using unconditional logistic regression models to estimate odd ratios (ORs) and 95% confidence intervals (CI).
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | p-trend | |
|---|---|---|---|---|---|
| Median TDLU span | |||||
| Cases/Controls, n | 65/271 | 72/270 | 73/271 | 77/271 | |
| Model 1 | Ref | 1.07 (0.73,1.57) | 0.98 (0.67,1.45) | 0.96 (0.65,1.43) | 0.75 |
| Model 2 | Ref | 0.94 (0.64,1.39) | 0.92 (0.62,1.37) | 0.89 (0.59,1.33) | 0.56 |
| TDLU counts/mm2 | |||||
| Cases/Controls, n | 67/271 | 73/270 | 71/271 | 76/271 | |
| Model 1 | Ref | 1.10 (0.75,1.60) | 1.06 (0.73,1.55) | 1.17 (0.80,1.71) | 0.45 |
| Model 2 | Ref | 1.04 (0.71,1.53) | 0.96 (0.65,1.41) | 1.15 (0.79,1.69) | 0.49 |
| Median acini counts/TDLU | |||||
| Cases/Controls, n | 26/121 | 79/348 | 89/311 | 93/303 | |
| Model 1 | Ref | 1.02 (0.63,1.71) | 1.21 (0.75,2.02) | 1.19 (0.73,1.99) | 0.40 |
| Model 2 | Ref | 0.94 (0.57,1.57) | 1.00 (0.61,1.69) | 1.05 (0.64,1.77) | 0.59 |
| Median TDLU area | |||||
| Cases/Controls, n | 58/271 | 78/270 | 66/271 | 85/271 | |
| Model 1 | Ref | 1.29 (0.88,1.91) | 0.99 (0.66,1.49) | 1.19 (0.79,1.78) | 0.72 |
| Model 2 | Ref | 1.15 (0.78,1.71) | 0.87 (0.57,1.31) | 1.10 (0.73,1.66) | 0.90 |
| % TDLU area (total) | |||||
| Cases/Controls, n | 58/271 | 82/270 | 63/271 | 84/271 | |
| Model 1 | Ref | 1.34 (0.92,1.98) | 0.99 (0.66,1.48) | 1.18 (0.79,1.78) | 0.82 |
| Model 2 | Ref | 1.15 (0.78,1.71) | 0.86 (0.57,1.30) | 1.04 (0.69,1.58) | 0.90 |
| % TDLU area (non-adipose) | |||||
| Cases/Controls, n | 58/271 | 87/270 | 57/271 | 85/271 | |
| Model 1 | Ref | 1.42 (0.97,2.08) | 0.89 (0.59,1.34) | 1.22 (0.82,1.82) | 0.81 |
| Model 2 | Ref | 1.23 (0.84,1.82) | 0.80 (0.52,1.21) | 1.10 (0.73,1.66) | 0.98 |
| Acini counts/mm2 | |||||
| Cases/Controls, n | 64/271 | 71/270 | 69/271 | 83/271 | |
| Model 1 | Ref | 1.09 (0.74,1.61) | 0.99 (0.67,1.47) | 1.10 (0.75,1.63) | 0.72 |
| Model 2 | Ref | 0.98 (0.67,1.45) | 0.86 (0.58,1.29) | 1.03 (0.69,1.53) | 0.83 |
| Median acini density | |||||
| Cases/Controls, n | 57/271 | 89/270 | 61/271 | 80/271 | |
| Model 1 | Ref | 1.59 (1.09,2.33) | 1.08 (0.72,1.62) | 1.33 (0.91,1.96) | 0.49 |
| Model 2 | Ref | 1.54 (1.05,2.27) | 1.05 (0.70,1.58) | 1.36 (0.92,2.01) | 0.41 |
Each quantitative TDLU measure was categorized into quartiles as defined by the distribution among the controls. Model 1 adjusted for matching factors. Model 2 adjusted for matching factors and BBD histological subtypes. The median value for each quartile was included as a continuous variable in the unconditional logistic regression for Model 1 and 2 to obtain the p-trend value (Wald test).
Qualitative categories of TDLU involution were also not associated with breast cancer risk among the subset of women with both quantitative and qualitative data (177 cases and 857 controls) or in the full BBD nested case-control study (288 cases and 1374 controls; Table 4). However, in the full BBD nested case-control study, women with predominant lobule type 1 no type 3 had lower breast cancer risk compared to combined women in the mixed type and no type 1 categories (crude OR=0.72, 95%CI 0.54-0.96). This association attenuated after adjusting for BBD histological subtypes (adjusted OR=0.80, 95%CI 0.59-1.07; Table 4).
Table 4.
The association between manual qualitative terminal duct lobular unit (TDLU) categories and breast cancer risk was evaluated using unconditional logistic regression models to estimate odd ratios (ORs) and 95% confidence intervals (CI). Model 1 adjusted for matching factors. Model 2 adjusted for matching factors and benign breast disease (BBD) histological subtypes.
| Cases/Controls n |
Model 1 | Model 2 | |
|---|---|---|---|
| Among women with both quantitative and qualitative data | |||
| No type 1 lobules | 23/114 | Ref | Ref |
| Mixed lobule types | 93/409 | 1.25 (0.76,2.14) | 1.15 (0.69,1.98) |
| Predominant type 1 and no type 3 lobules | 61/334 | 0.95 (0.54,1.70) | 0.95 (0.54,1.71) |
| No type 1 lobules or mixed lobule types | 116/523 | Ref | Ref |
| Predominant type 1 and no type 3 lobules | 61/334 | 0.79 (0.54,1.13) | 0.84 (0.58,1.22) |
| Women in full BBD nested case-control study with qualitative data | |||
| No type 1 lobules | 41/192 | Ref | Ref |
| Mixed lobule types | 151/640 | 1.17 (0.79,1.74) | 1.09 (0.74,1.65) |
| Predominant type 1 and no type 3 lobules | 96/542 | 0.82 (0.53,1.28) | 0.86 (0.56,1.35) |
| No type 1 lobules or mixed lobule types | 192/832 | Ref | Ref |
| Predominant type 1 and no type 3 lobules | 96/542 | 0.72 (0.54,0.96) | 0.80 (0.59,1.07) |
Discussion
In our nested case-control study within the NHS/NHSII, we applied our automated method to WSIs and captured eight quantitative measures of TDLU involution in normal tissue areas from BBD biopsies. We verified our data by confirming the inverse relationships between automated quantitative measures and age at BBD biopsy, as well as with qualitative categories of TDLU involution. We then evaluated the association of these quantitative TDLU involution measures with breast cancer risk factors and breast cancer risk. All eight quantitative measures were significantly higher (i.e., less involution) in parous women and pre-menopausal women; select measures were associated with BBD histopathological subtypes, BMI, and birth index. Neither quantitative nor qualitative measures of TDLU involution were associated with breast cancer risk in our study, suggesting that among NHS/NHSII women diagnosed with BBD, alterations in TDLU morphology is not a biomarker of subsequent breast cancer.
TDLUs are the sites of origin for breast cancer (1–3). TDLU involution was inversely associated with breast cancer risk in prior studies (6,8,10). This reduction of risk is related to decreased breast tissue cellularity that occurs with involution—decreased numbers and size of TDLUs that can be measured using TDLU counts/mm2, median TDLU span, median TDLU area, or TDLU area as a percentage of total or non-adipose tissue; and the number of acini in the breast that can be measured using median acini counts/TDLU, acini counts/mm2, or median acini density. We did not observe a significant association between TDLU involution and breast cancer risk in this current study. The method of involution measurement (automated versus manual) and the type of measurement (quantitative versus qualitative) may explain our discordant findings from prior studies (6,8,10). Although our automated method captured identical quantitative measures as reported by McKian et al. (8) and Figuoera et al. (10), our method analyzed entire tissue sections with a median of 76 TDLUs per WSI while the methods by McKian et al. (8) and Figuoera et al. (10) involved manually selecting a fixed-sized region on the tissue that contained up to 10 normal TDLUs for assessment. Data derived using our automated method were dependent on the ground truth images used to train our deep learning networks and thus the pathological assessment and annotation for our training dataset may differ from the assessment conducted by McKain et al. (8) and Figueroa et al. (10), even though when establishing our automated method, our training images were annotated in reference to the pathological assessment criteria as described by Figueroa et al. (7,9,10,16). Future collaborations are needed to further evaluate the TDLU involution measures captured using our automated method and breast cancer risk in normal, healthy women without BBD as well as in ethnically diverse epidemiological cohorts.
Two studies assessed qualitative measures of TDLU involution in relation to breast cancer risk, including a prior study in the NHS/NHSII (5,6). Milanese et al. assessed TDLU involution as none, partial, or complete involution in 8736 women and observed increased lobular involution to be associated with lower breast cancer risk (6). Although the authors observed that five to six lobules were adequate to assess the extent of involution and that one slide typically had at least 12 lobules, it is unclear how many slides per woman were assessed. Thus, the differences in the extent of assessment of involution between Milanese et al. and this current study may be contributing to the discordant finding between our current study and theirs. Our prior NHS/NHSII work assessed qualitative categories of TDLU involution for 200 cases and 915 controls, and found a suggestive inverse association with breast cancer risk in BBD subtype-adjusted models (predominant type 1 versus mixed or no type 1; adjusted OR=0.71, 95% CI 0.49-1.02) (5). Our current analysis for the full BBD nested case-control study included an additional 88 cases and 459 controls and found a comparable suggestive but non-significant inverse association with breast cancer (adjusted OR=0.80, 95% CI 0.59-1.07). Collectively, the findings from our current and prior studies using both quantitative and qualitative measures suggest that TDLU involution is, at best, weakly associated with breast cancer risk within the NHS/NHSII participants.
The association of TDLU involution with risk factors but not breast cancer risk warrants caution when interpreting data with regards to risk factors. In general, our data provided histopathological evidence to support epidemiological findings. Figueroa et al. reported higher TDLU counts (i.e., less involution) in women with lower BMI, parity, and younger age at first birth (10). We observed similar findings as Figueroa et al. albeit using different quantitative measurements. Most of the women in our control group consisted of younger women of <50 years old (62.3%) and pre-menopausal women (61.6%). Thus, our observation of higher acini counts (i.e., less TDLU involution) in women with lower BMI compared to women with higher BMI is in line with higher pre-menopausal breast cancer risk in women with BMI <25 compared to women with BMI ≥30 (29–31). Childbirth within the last 20 years had a pronounced effect on lobule morphology, as it was significantly inversely associated with TDLU involution for all eight quantitative measures. Birth index which summarizes age at first childbirth, number of childbirths, and the spacing between childbirths was also inversely associated with TDLU involution. Together, these results may partly explain why parous women who gave birth within 5 and 24 years prior have higher breast cancer risk compared to nulliparous women (32).
We observed less TDLU involution in normal breast tissues of women with proliferative lesions (with or without atypia) while Figueroa et al. did not (10). The study by Figueroa et al. may be underpowered to observe this phenomenon (without atypia n=90 and atypical hyperplasia n=19 in Figueroa et al. versus n=625 and n=155 in this study). However, our additional analyses suggested that lesser degrees of TDLU involution in women with proliferative lesions did not appear to influence their breast cancer risk. As such, we speculate that in women diagnosed with BBD, the molecular mechanisms associated with BBD or other underlying risk factors may have a greater influence on subsequent breast cancer risk than alterations in lobule morphology.
The strengths of our study include the application of an innovative automated method to assess TDLU involution in a large, well-established nested case–control study with detailed information on breast cancer risk factors (5,18,20–23). This study’s sample size was much larger than the two prior nested case-control studies from the Mayo BBD Cohort (8,10). Breast cancer cases were confirmed through review of medical records, and centralized pathology review of breast specimens was conducted to confirm and classify BBD. Our automated method eliminated the need for manual microscopic evaluation of the tissue, and captured TDLU measures for the entire tissue section instead of a fixed portion of the tissue. We corrected our quantitative measures for adipose tissue, as TDLU counts are inversely correlated with the amount of adipose tissue in the breast (9,10). The null association between quantitative TDLU involution measures and breast cancer risk correlated with traditional qualitative assessment.
Our study had some limitations. Our findings were limited to white women diagnosed with BBD. Women with ER-negative breast cancers have less TDLU involution compared with tumors that express hormone receptors (14). The null association between TDLU involution and breast cancer risk stratified by ER status in our study may be underpowered to observe that phenomenon. We were also underpowered to evaluate the association of TDLU involution and breast cancer subtypes (13,14), as well as mammographic density (11,12), as mammogram data were only available for 105 women (7.5%) in this study.
In conclusion, our study showed some association between breast cancer risk factors and quantitative TDLU involution measures. Automated and manual assessments of TDLU involution in normal tissue were not associated with breast cancer risk, suggesting that molecular mechanisms of BBD or risk factors may have more influence on subsequent breast cancer risk than TDLU morphology among women diagnosed with BBD. Future work can include evaluating automated TDLU involution measures and breast cancer risk in normal women without BBD or ethnically diverse epidemiological cohorts, and investigating the relationship between TDLU involution and mammographic density or breast cancer subtypes.
Supplementary Material
Acknowledgements
We thank the participants and staff of the Nurses’ Health Study and Nurses’ Health Study II for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
Funding: This work was supported by the National Institute of Health/National Cancer Institute R21 CA187642 (RMT), R01 CA175080 (RMT), UM1 CA186107 (AHE), and U01 176726 (AHE), Susan G. Komen for the Cure IIR13264020 (RMT), the Klarman Family Foundation (YJH), BIDMC High School Summer Research Program (EZL), and the Deep Learning for Medical Image Analysis research program by Netherlands Organization for Scientific Research P15-26 (SCW, MV, and JPWP) and Philips Research (SCW, MV, and JPWP).
Footnotes
Conflict of interest statement: The authors declare no conflicts of interest.
References
- 1.Russo J, Russo IH. Development of the human breast. Maturitas. 2004;49:2–15. [DOI] [PubMed] [Google Scholar]
- 2.Jensen HM. On the origin and progression of human breast cancer. Am J Obstet Gynecol. Elsevier; 1986;154:1280–4. [DOI] [PubMed] [Google Scholar]
- 3.Russo J, Hu YF, Yang X, Russo IH. Developmental, cellular, and molecular basis of human breast cancer. J Natl Cancer Inst Monogr. 2000;17–37. [DOI] [PubMed] [Google Scholar]
- 4.Russo J, Romero AL, Russo IH. Architectural pattern of the normal and cancerous breast under the influence of parity. Cancer Epidemiol Biomarkers Prev. 1994;3:219–24. [PubMed] [Google Scholar]
- 5.Baer HJ, Collins LC, Connolly JL, Colditz GA, Schnitt SJ, Tamimi RM. Lobule type and subsequent breast cancer risk: Results from the nurses’ health studies. Cancer. 2009;115:1404–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milanese TR, Hartmann LC, Sellers TA, Frost MH, Vierkant RA, Maloney SD, et al. Age-related lobular involution and risk of breast cancer. J Natl Cancer Inst. 2006;98:1600–7. [DOI] [PubMed] [Google Scholar]
- 7.Wetstein SC, Onken AM, Luffman C, Baker GM, Pyle ME, Kensler KH, et al. Deep learning assessment of breast terminal duct lobular unit involution : towards automated prediction of breast cancer risk. PLoS One. 2020;15:e0231653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKian KP, Reynolds CA, Visscher DW, Nassar A, Radisky DC, Vierkant RA, et al. Novel breast tissue feature strongly associated with risk of breast cancer. J Clin Oncol. 2009;27:5893–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Figueroa JD, Pfeiffer RM, Patel DA, Linville L, Brinton LA, Gierach GL, et al. Terminal duct lobular unit involution of the normal breast: Implications for breast cancer etiology. J Natl Cancer Inst. 2014;106:pii: dju286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Figueroa JD, Pfeiffer RM, Brinton LA, Palakal MM, Degnim AC, Radisky D, et al. Standardized measures of lobular involution and subsequent breast cancer risk among women with benign breast disease: a nested case–control study. Breast Cancer Res Treat. NIH Public Access; 2016;159:163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gierach GL, Patel DA, Pfeiffer RM, Figueroa JD, Linville L, Papathomas D, et al. Relationship of terminal duct lobular unit involution of the breast with area and volume mammographic densities. Cancer Prev Res. 2016;9:149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sung H, Guo C, Li E, Li J, Pfeiffer RM, Guida JL, et al. The relationship between terminal duct lobular unit features and mammographic density among Chinese breast cancer patients. Int J Cancer. 2019;145:70–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo C, Sung H, Zheng S, Guida J, Li E, Li J, et al. Age-related terminal duct lobular unit involution in benign tissues from Chinese breast cancer patients with luminal and triple-negative tumors. Breast Cancer Res. 2017;19:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang XR, Figueroa JD, Falk RT, Zhang H, Pfeiffer RM, Hewitt SM, et al. Analysis of terminal duct lobular unit involution in luminal A and basal breast cancers. Breast Cancer Res. 2012;14:R64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosebrock A, Caban JJ, Figueroa J, Gierach G, Linville L, Hewitt S, et al. Quantitative analysis of TDLUs using adaptive morphological shape techniques. Med Imaging 2013 Digit Pathol. 2013. page 86760N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wetstein SC, Onken AM, Baker GM, Pyle ME, Pluim JPW, Tamimi RM, et al. Detection of acini in histopathology slides: towards automated prediction of breast cancer risk. Proc SPIE 10956, Med Imaging 2019 Digit Pathol. 2019. page 109560Q. [Google Scholar]
- 17.Colditz GA, Hankinson SE. The Nurses’ Health Study: lifestyle and health among women. Nat Rev Cancer. 2005;5:388–96. [DOI] [PubMed] [Google Scholar]
- 18.Collins LC, Baer HJ, Tamimi RM, Connolly JL, Colditz GA, Schnitt SJ. The influence of family history on breast cancer risk in women with biopsy-confirmed benign breast disease: results from the Nurses’ Health Study. Cancer. 2006;107:1240–7. [DOI] [PubMed] [Google Scholar]
- 19.Collins LC, Baer HJ, Tamimi RM, Connolly JL, Colditz GA, Schnitt SJ. Magnitude and laterality of breast cancer risk according to histologic type of atypical hyperplasia: Results from the nurses’ health study. Cancer. 2007;109:180–7. [DOI] [PubMed] [Google Scholar]
- 20.Tamimi RM, Byrne C, Baer HJ, Rosner B, Schnitt SJ, Connolly JL, et al. Benign breast disease, recent alcohol consumption, and risk of breast cancer: a nested case-control study. Breast Cancer Res. 2005;7:R555–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collins LC, Aroner SA, Connolly JL, Colditz GA, Schnitt SJ, Tamimi RM. Breast cancer risk by extent and type of atypical hyperplasia: An update from the Nurses’ Health Studies. Cancer. 2016;122:515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aroner SA, Collins LC, Connolly JL, Colditz GA, Schnitt SJ, Rosner BA, et al. Radial scars and subsequent breast cancer risk: Results from the Nurses’ Health Studies. Breast Cancer Res Treat. 2013;139:277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oh H, Eliassen AH, Wang M, Smith-Warner SA, Beck AH, Schnitt SJ, et al. Expression of estrogen receptor, progesterone receptor, and Ki67 in normal breast tissue in relation to subsequent risk of breast cancer. npj Breast Cancer. 2016;2:16032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kensler KH, Beca F, Baker GM, Heng YJ, Beck AH, Schnitt SJ, et al. Androgen receptor expression in normal breast tissue and subsequent breast cancer risk. npj Breast Cancer. 2018;4:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beca F, Kensler K, Glass B, Schnitt SJ, Tamimi RM, Beck AH. EZH2 protein expression in normal breast epithelium and risk of breast cancer: Results from the Nurses’ Health Studies. Breast Cancer Res. 2017;19:R32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sisti JS, Collins LC, Beck AH, Tamimi RM, Rosner BA, Eliassen AH. Reproductive risk factors in relation to molecular subtypes of breast cancer: Results from the nurses’ health studies. Int J Cancer. 2016;138:2346–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pohlert T The Pairwise Multiple Comparison of Mean Ranks Package (PMCMR) [Internet]. R Packag. 2014. Available from: http://cran.r-project.org/package=PMCMR [Google Scholar]
- 28.Lenth R, Singmann H, Love J, Buerkner P, Herve M. Estimated Marginal Means, aka Least-Squares Means [Internet]. R Packag. 2020. Available from: https://cran.r-project.org/package=emmeans [Google Scholar]
- 29.Schoemaker MJ, Nichols HB, Wright LB, Brook MN, Jones ME, O’Brien KM, et al. Association of Body Mass Index and Age with Subsequent Breast Cancer Risk in Premenopausal Women. JAMA Oncol. 2018;4:e181771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang XR, Chang-Claude J, Goode EL, Couch FJ, Nevanlinna H, Milne RL, et al. Associations of breast cancer risk factors with tumor subtypes: A pooled analysis from the breast cancer association consortium studies. J Natl Cancer Inst. 2011;103:250–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78. [DOI] [PubMed] [Google Scholar]
- 32.Nichols HB, Schoemaker MJ, Cai J, Xu J, Wright LB, Brook MN, et al. Breast cancer risk after recent childbirth: A pooled analysis of 15 prospective studies. Ann Intern Med. 2019;170:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


