Abstract
Embryo implantation is accompanied by a potent inflammatory response, and a gradient of cytokines and chemokines produced by endometrial cells supports the embryo-endometrial interaction. C-reactive protein (CRP) serves as an early marker of inflammation and recent studies have illustrated that controlled ovarian hyperstimulation (COH) could increase its levels. Interestingly, a high chance of pregnancy has been reported in women who had an elevated CRP level on the day of embryo transfer. It seems an elevated systemic inflammation in the in vitro fertilization (IVF) cycle can increase the implantation and pregnancy rates. However, the results regarding the association of CRP with ART outcomes are controversial. Therefore, in this review, we aimed to describe how CRP levels change during a cycle of IVF treatment and which factors can potentially affect this pattern of change. Furthermore, the association of CRP with ART outcomes has been discussed.
Keywords: ART, CRP, Reproductive, Biomarker, Embryo implantation
Introduction
Assisted reproductive technologies (ART) include all the techniques that are applied to treat infertile couples [1]. However, embryo implantation failure is one of the most important obstacles in ART. The statistics have shown that approximately 85% of the embryos could not be implanted after transfer. Embryo implantation is possible in a receptive uterus that occurs on days 19 to 23 of the menstrual cycle in humans, called window of implantation (WOI) [2]. Implantation associates with elevated levels of endometrial cytokines, prostaglandins, and leukocytes [3]. A gradient of cytokines and chemokines produced by endometrial cells can guide the embryo for implantation and support the embryo-endometrial interaction [4]. Based on the expression pattern of cytokines produced by CD4+ T helper (Th) cells, these cells are classified into two subsets, Th1 and Th2. The Th1 can induce cell-mediated immunity, while Th2 is involved in humoral immune responses and antibody production [5]. Studies have indicated that initiation of both implantation and pregnancy is accompanied by a potent inflammatory response of T helper (Th1) and production of inflammatory cytokines such as interleukin (IL) 6, IL8, leukemia inhibitory factor (LIF), and tumor necrosis factor (TNF) [6]. Regarding the difference in the function and pattern of cytokine secretion between the Th1 and Th2, it has been hypothesized that a balance is required between these cells to coordinate the immune system [5]. Further studies revealed that a successful pregnancy also requires a balance between Th1 and Th2 activities. Any abnormal increase in the Th1/Th2 ratio can result in elevated Th1-produced cytokines that consequently cause implantation disorders such as repeated implantation failure (RIF) and repeated pregnancy loss (RPL) [7].
C-reactive protein (CRP) is synthesized by the liver and serves as an early marker of inflammation or infection. This acute-phase protein is normally found at a concentration of less than 10 mg/L in the blood [8]. However, during infectious or inflammatory diseases, CRP levels rapidly rise within the first 6 to 8 h and peak up to 350–400 mg/L after 48 h [9]. CRP can activate the classical helper cascade of the immune system and enhance the activity of phagocytic cells [10].
Recent studies have illustrated that controlled ovarian hyperstimulation (COH (can positively affect CRP concentration [11]. On the other hand, no significant difference has been observed between serum and follicular levels of CRP [12, 13], indicating a direct correlation between the systemic and reproductive system inflammation. Interestingly, higher pregnancy rates have been reported in women with elevated CRP levels on the transfer day compared with women who had decreased CRP levels [14]. Furthermore, it has been seen that pregnant women following frozen embryo replacement (FER) had higher CRP levels compared with women who did not become pregnant. Therefore, the higher systemic inflammation in the in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) treatment can increase the chance of implantation and pregnancy. However, the results regarding the association of CRP with ART outcomes are controversial [15]. Therefore, the purpose of this review paper was to describe the changes in CRP levels during IVF treatments and the factors that can influence it. Moreover, the association between CRP with ART outcomes has been discussed.
CRP levels during IVF/ICSI cycle
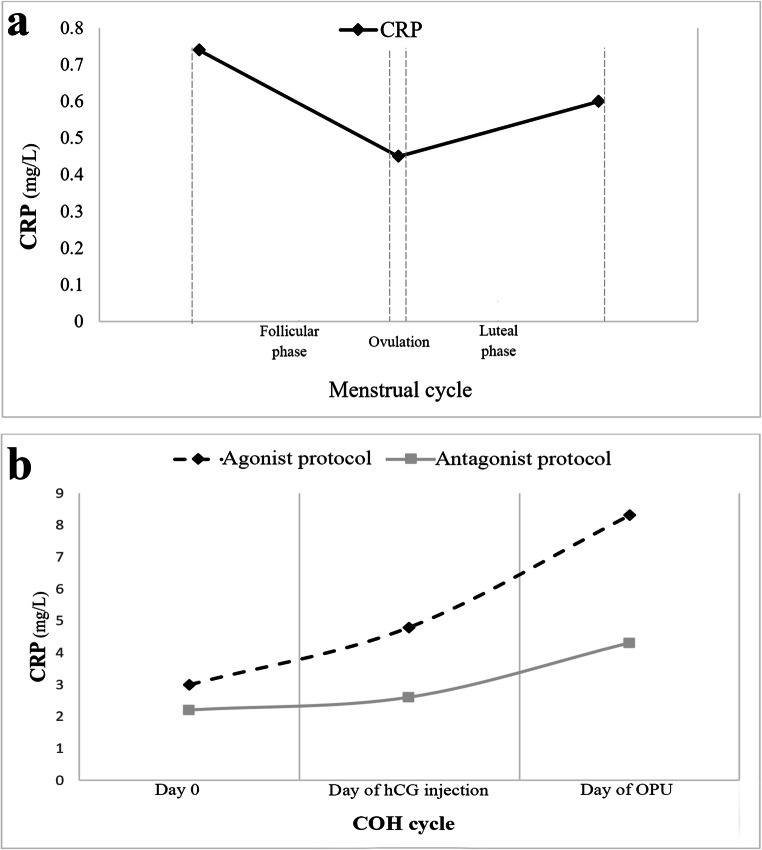
The results of menstrual cycle studies showed that the serum levels of CRP significantly vary across the menstrual cycle. It has been reported that the CRP decreases during the follicular phase and reaches to the lowest levels on the expected day of ovulation (reported median: 0.45 mg/L). Then, CRP increases in the luteal phase and reaches its highest levels during menses (reported median: 0.74 mg/L) (Fig. 1a). It has been observed that the variation of CRP is 50% higher during menses in comparison with the rest of the cycle (0.82 vs. 1.23 (mg/L) [16]. Previous studies have also indicated that the level of serum CRP was highly variable in the COH cycle as the serum CRP level of 2.6 mg/L on the stimulation day reached 3.7 mg/L on the human chorionic gonadotropin (hCG) injection day and had a peak (6.3 mg/L) on the oocyte pick-up (OPU) day. These results indicate an increasing trend for serum CRP levels during the COH [11]. Numerous studies have compared serum CRP levels between the natural and COH cycles and indicated higher levels of CRP throughout the COH cycles than the natural cycles. Such higher levels of CRP during the COH cycle could be due to hyperstimulation that subsequently causes an increase in endogenous estradiol levels, as well as, hCG injection, and OPU-induced inflammation [15].
Fig. 1.
C-reactive protein (CRP) levels during a) a natural menstrual cycle and b) controlled ovarian hyperstimulation (COH) cycle using the agonist or antagonist protocols. Figures 1a and b have been designed based on the data reported by Gaskins et al. [16] and Orvieto et al. [11], respectively. hCG, human chorionic gonadotropin; OPU, oocyte pick-up
Different treatment protocols can be applied in ART cycles using GnRH agonists and antagonists. The GnRH agonist is prescribed in both short- and long-term protocols. In the long-term protocol, the prescription of GnRH agonist begins at the previous luteal phase (day 21), whereas the treatment will be delayed or discontinued earlier at the short-term agonist. At the antagonist protocols, the GnRH antagonist is usually prescribed on days 4–7 of the stimulation [17]. Previous studies have illustrated that the type of treatment protocol can affect CRP levels in COH (Fig. 1b). For instance, Orvieto et al. [11] compared serum levels of CRP between the agonist and antagonist protocols in IVF cycles. They reported that in both treatment protocols, serum levels of CRP were significantly higher in OPU day than hCG injection day and day 0. They also found that the CRP levels were higher on hCG injection day compared with the day 0; however, the difference was statistically significant only when the agonist protocol was used. Moreover, serum levels of CRP on OPU day were significantly higher in the patients who received agonist protocol than those treated with antagonist protocol [11]. Liu et al. [18] also showed that the serum CRP levels gradually increased on the second day of the cycle, the day of hCG injection, and the day of embryo transfer (ET) in the short-term GnRH agonist protocol. It has been shown that serum levels of CRP increased on day 7 after ET compared with the day of stimulation in both pregnant and non-pregnant women who received long-term GnRH agonist treatment protocol [19]. Almagor et al. [20] conducted a study between July 2001 and October 2002 and evaluated serum levels of CRP in women who underwent the IVF cycle. They demonstrated that CRP levels were significantly increased at the day 5 to 7 after ET compared with the first day of the treatment, while its levels decreased on the day 12 of post-transfer compared with day 5 to 7 of post-transfer [20].
Unfortunately, there is no information about the CRP changes in the ovarian follicles during the IVF/ICSI cycle; however, due to the direct correlation reported between serum and follicular fluid levels of CRP, it can be postulated that the levels of CRP in follicles change similar to its serum levels. In supporting this hypothesis, Orvieto and colleagues [11] found that the follicular fluid levels of CRP did not differ from its serum amounts on the Day-OPU. Furthermore, Herzberger et al. [13] and Wunder et al. [21] found a positive correlation between serum and follicular levels of CRP during assisted reproductive cycles.
Effect of reproductive hormones on CRP
Studies have revealed that both endogenous and exogenous hormones of the reproductive system can affect the CRP levels. Endogenous progesterone can increase serum levels of CRP during a normal menstrual cycle. It has been reported that a 10-fold increase in progesterone levels was associated with a 19.4% increase in the serum levels of CRP at the luteal phase [16]. It has also been seen that CRP concentration is inversely correlated with estradiol during the menstrual cycle while it is positively correlated with luteal progesterone levels. These findings support the hypothesis that endogenous estradiol can act as an anti-inflammatory agent [16]. In this regard, the negative effect of estradiol on inflammatory cell migration in a variety of nonproductive and nonimmune tissues has been reported [22]. Estradiol also decreases TNF-α and many inflammatory cytokines [23], while progesterone increases neutrophil chemotaxis and production of inflammatory mediators by monocytes [24].
Interestingly, exogenous estradiol or estradiol-inducing hormones demonstrated a stimulatory effect on CRP production. A study on IVF patients demonstrated that CRP levels had a 30% increase on OPU day compared with hCG injection day with decreased E2 levels [12]. Studies on the effect of COH treatment (exogenous hormones) on CRP have revealed that CRP levels did not increase in the first day of stimulation and the day of hCG injection; however, its serum levels were doubled between hCG injection and OPU days [21]. Furthermore, it has been shown that there was a positive correlation between basal levels of estradiol and CRP in patients who underwent ART [13]. It seems the effect of exogenous estradiol on the CRP levels depends on the method of hormone replacement therapy (HRT). In this regard, it has been indicated that the oral estradiol could be rapidly and completely absorbed through the gastrointestinal tract and reach the liver at high concentrations through portal blood. This so-called first-pass effect is responsible for enhancing the hepatic synthesis of several proteins, including CRP [25]. A study by Raoul Orvieto et al. [26] compared the effects of hCG and gonadotropin-releasing hormone agonist on serum levels of CRP during a COH cycle and showed that the amount of CRP change on Day-hCG compared with Day-OPU was higher when hCG was administrated (96%) than GnRH administration (23%). So it can be concluded that COH using hCG could stimulate systemic inflammation more than GnRH agonist administration. However, the reason for this finding is not clear [26]. In another study, the stimulatory effect of hCG on CRP levels has also been documented [27].
Ovarian hyperstimulation syndrome (OHSS), one of the complications mainly in IVF, is divided into early and late types by time of incidence. Early OHSS occurs within 1 week after ovum pick-up, which is associated with the prescription of exogenous hCG, while late OHSS is induced by endogenous hCG secretion in pregnancy and occurs 10 days after OPU [28]. Comparison of CRP levels in early and late OHSS showed that its serum levels were significantly higher in patients with early OHSS than late OHSS or healthy women [29]. Furthermore, 12 mg/L CRP levels were introduced as a cut-off for early OHSS with 69% sensitivity and 71% specificity [29].
Studies have demonstrated that total testosterone was negatively correlated with CRP, whereas free testosterone had a direct relationship with CRP [30]. On the other hand, Martin K.C. et al. [31] reported that treatment with exogenous androgens, with or without elevated estradiol levels, did not affect serum levels of inflammatory markers in men. Furthermore, no correlation has been found between CRP and androgen levels during COH in PCOS women who underwent IVF cycle [32].
Association of CRP with the oogenesis and embryogenesis in an ART cycle
It has been well documented that an appropriate inflammatory response is required for successful folliculogenesis, oocyte maturation, and ovulation. Therefore, several pro-inflammatory cytokines are produced throughout oogenesis as well as ovulation [33]. It has even been shown that inhibition of cyclooxygenase-2 (COX-2), an enzyme involved in prostaglandin-induced inflammation, could impair follicle rupture [34]. Studies have also demonstrated that non-steroidal anti-inflammatory drugs such as indomethacin could inhibit ovulation in 80% of the cases [34]. It should also be mentioned that excessive levels of inflammation can impair folliculogenesis and ovulation and consequently lead to infertility. An excessive inflammatory response has been observed in different types of female infertilities [33]. In this regard, it has been reported that serum and follicular levels of CRP were elevated in obese women who had impaired folliculogenesis [35]. One of the negative impacts of CRP on ovulation could be via increasing ROS levels. although ROS at physiological concentration is required for ovulation [36, 37]. On the other hand, Orvieto et al. [12] found no significant association between serum levels of CRP with the number of retrieved oocytes. Robinson et al. [38] also showed that there was no significant association between CRP with the number of collected and fertilized oocytes.
The oocyte quality is generally evaluated by different morphological factors including cytoplasm appearance, zona pellucida, and polar body [39]. It is well documented that the oocyte quality could predict the developmental potential and quality of embryos [39]. On the other hand, the embryo quality which is evaluated based on the different indicators such as pronuclear morphology; size, symmetry, and fragmentation of blastomeres; and cleavage and blastocyst formation rate can improve the IVF success [40]. Thus, previous studies evaluated the possible association between CRP levels with oocyte and embryo quality in an IVF cycle. In this regard, Selman et al. [41] reported that there were significant negative correlations between the CRP levels with the number of retrieved MII oocytes, fertilized oocytes, and grade I embryos. This study demonstrated that elevation in CRP levels could decrease the oocyte and embryo quality. Herzberger et al. [13] also found that patients with serum levels of CRP more than 0.5 mg dl−1 had low-quality embryos. They concluded that elevated serum levels of CRP on OPU day could negatively affect the embryo quality.
CRP is a predictive biomarker for ART success
Many studies have conducted to find a biomarker to predict ART outcomes. For this purpose, different factors in the blood, endometrial flushing, and follicular fluid have been evaluated, such as cytokines and growth factors [42]. CRP has also been considered as a predictor of ART success (summarized in Table 1). In this regard, it has been shown that pregnancy occurred in 68.4% of women who had an elevated CRP level on the day of ET compared with OPU day; therefore, serum levels of CRP on the day of ET has been suggested as a biomarker for success in IVF/ICSI patients [14]. Chun-Xia et al. [45] have also shown that serum levels of CRP on the day of ET were significantly higher in women who became pregnant compared with non-pregnant women. Furthermore, higher serum levels of CRP on the day of gonadotropin initiation, day of hCG administration, and day of OPU have been reported in non-pregnant women than those who became pregnant [45]. It has been suggested that an ET-day/hCG-day CRP ratio of less than 1.75 could be used as a predictive marker for ART outcome with sensitivity and specificity of 77.8% and 75%, respectively [18]. In a similar study, it has been reported that the CRP ratio of ET day/OPU day was 1.2 in women who became pregnant after IVF, while this ratio for non-pregnant women was 2.5. Using the CART (classification and regression trees) method, the ratio of less than 1.85 was considered as a predictive marker for IVF outcome with sensitivity and specificity of 86% and 44%, respectively [20].
Table 1.
CRP association with ART outcomes
| COH protocol | Day of CRP measurement | Sample | Mean levels of CRP (pregnant vs. non-pregnant; p value) | CRP as a predictive marker | Reference |
|---|---|---|---|---|---|
| Agonist of GnRH/long protocol | Day-S | Serum | 4.36 vs. 3.68 mg/L; NS | ET day/OPU day CRP ratio ≥ 1.23 results in pregnancy | [14] |
| hCG injection day | Serum | 5.10 vs. 4.82 mg/L; NS | |||
| OPU day | Serum | 5.90 vs. 5.27 mg/L; NS | |||
| ET day | Serum | 8.98 vs. 4.84 mg/L; p < 0.001 | |||
| Agonist of GnRH/long protocol | Day-S | Serum | 1.75 vs. 2.27 mg/L; p < 0.05 | NA | [43] |
| hCG injection day | Serum | 1.50 vs. 1.59 mg/L; p < 0.05 | |||
| OPU day | Serum | 1.82 vs. 2.75 mg/L; p < 0.05 | |||
| ET day | Serum | 4.96 vs. 4.74 mg/L; p < 0.05 | |||
| Agonist of GnRH/short protocol | 2nd day of stimulation | Serum | 87.43 vs. 145.10 mg/mL; NA | ET-day/ hCG-day CRP ratio < 1.75 could predict IVF success (sensitivity 77.8% and specificity 75%) | [18] |
| hCG injection day | Serum | 144.83 vs. 157.39 mg/mL; NA | |||
| ET day | Serum | 243.74 vs. 280.81 mg/mL; NA | |||
| Agonist of GnRH/short protocol | 3rd day of stimulation | Serum | 2.20 vs. 6.20 mg/dL; p = 0.049 | NA | [44] |
| 8th day of stimulation | Serum | 1.30 vs. 4.60 mg/dL; NS | |||
| OPU day | Serum | 1.80 vs. 3.80 mg/dL; NS | |||
| 12 days after ET | Serum | 2.30 vs. 3.60 mg/dL; NS | |||
| NA | OPU day | Serum | 9.40 vs. 5.20 mg/L; NS | ET day/ OPU day CRP ratio < 1.85 could predict IVF success (sensitivity 86% and specificity 44%) | [20] |
| ET day | Serum | 6.90 vs. 6.90 mg/L; NS | |||
| 5–7 days after ET | Serum | 16.00 vs. 14.00 mg/L; NS | |||
| 12 days after ET | Serum | 11.50 vs. 9.90 mg/L; NS | |||
| Agonist of GnRH/long protocol | Day-S | Serum | 1.26 vs. 1.76 μg/mL; NS | NA | [21] |
| hCG injection day/hCG-1 | Serum | 1.25 vs. 1.40 μg/mL; NS | |||
| OPU day | Serum | 2.87 vs. 3.48 μg/mL; NS | |||
| OPU day | Follicular fluid | 1.48 vs. 1.32 μg/mL; NS |
NA, not available; NS, non-significant; Day-S, day of stimulation; OPU, oocyte pick-up; ET, embryo transfer
In contrary to the abovementioned studies, Seckin and colleagues [19] found no significant difference in serum levels of high-sensitivity C-reactive protein (hs-CRP) on the day of gonadotropin injection and 7 days after ET between pregnant and non-pregnant patients. In a study, it has also been indicated that CRP levels were not always different between pregnant and non-pregnant women during the ART cycle. Nevertheless, this study claimed that high CRP levels on day 3 of the cycle were associated with IVF failure [44]. Robinson et al. [38] also showed that serum hs-CRP concentration was not a good predictor for ART outcomes.
Although there is evidence showing that COH enhances systemic inflammation and ART success partially by increasing CRP on the day of ET [43], no definitive cut-off has been determined for serum and follicular levels of CRP to predict ART outcome, and so further studies with large sample size and uniform treatment protocol are required to derive a definite conclusion.
Conclusion
The inflammatory system can influence all functions of the female reproductive system from folliculogenesis to embryo implantation and pregnancy. It has been proved that the disruption of inflammatory response could inhibit ovulation and subsequently reduce the number of retrieved oocytes in ART cycles. It should be noted that low-grade inflammation is required for ART procedure and on the other hand, excessive inflammation can have detrimental effects on its outcomes. Previous studies suggested CRP as a potential biomarker for the prediction of ART outcomes. However, there are still conflicting data regarding the association between CRP and ART outcomes and its predictive value. Given the variation of CRP levels during menstruation and ART cycles, as well as the association between CRP and embryo implantation, it can be assumed that CRP is an important factor in ART success. However, there are several issues regarding the assessment of CRP levels during ART cycle and its interpretation for the treatment management. For example, evaluation of CRP levels in the female reproductive system is difficult and also its blood levels cannot accurately reflect its local levels. Furthermore, the blood levels of CRP can be easily affected by various factors, including endogenous and exogenous hormones, underlying inflammatory diseases, age, and body mass index (BMI). For example, it has been demonstrated that CRP levels were positively correlated with visceral adiposity and BMI [46]. This can be explained by the role of adipose tissue in the production of pro-inflammatory factors [47]. Moreover, studies confirmed that inflammatory factors such as CRP can be changed by age, as higher CRP levels were found in 60- to 70-year old people compared with younger individuals [48, 49]. Therefore, future studies are recommended to monitor CRP levels in uterine flushing and follicles at different maturation stages. Moreover, the interfering factors such as steroid hormones, age, BMI, and underlying inflammatory diseases should carefully be taken into account before the interpretation of CRP levels throughout ART treatment. It seems that CRP may serve as an indicator of the inflammatory and immune conditions of women during an IVF cycle.
Code availability
Not applicable
Authors’ contributions
Diba-Bagtash F. and Farshbaf-Khalili A. literature review and manuscript drafting; Ghasemzadeh A. and Lotz L. literature review and manuscript editing; Fattahi A, Shahnazi M, and Dittrich R study design and critical revisions.
Data availability
Not applicable
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Amir Fattahi, Email: amirfattahi@gmail.com.
Mahnaz Shahnazi, Email: mshahnazi@tbzmed.ac.ir.
References
- 1.Sunderam S, Kissin DM, Zhang Y, Folger SG, Boulet SL, Warner L et al. Assisted reproductive technology surveillance-United States, 2016. Morbidity and mortality weekly report Surveillance summaries (Washington, DC: 2002). 2019;68:1–23. [DOI] [PMC free article] [PubMed]
- 2.Abrahams VM, Kim YM, Straszewski SL, Romero R, Mor G. Macrophages and apoptotic cell clearance during pregnancy. Am J Reprod Immunol. 2004;51:275–282. doi: 10.1111/j.1600-0897.2004.00156.x. [DOI] [PubMed] [Google Scholar]
- 3.Kelly RW, King AE, Critchley H. Cytokine control in human endometrium. Reproduction. 2001;121:3–19. doi: 10.1530/rep.0.1210003. [DOI] [PubMed] [Google Scholar]
- 4.Hajipour H, Nejabati HR, Latifi Z, Hamdi K, Bahrami-Asl Z, Fattahi A, et al. Lymphocytes immunotherapy for preserving pregnancy: mechanisms and challenges. Am J Reprod Immunol. 2018;80:e12853. doi: 10.1111/aji.12853. [DOI] [PubMed] [Google Scholar]
- 5.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 6.Dominguez F, Yanez-Mo M, Sanchez-Madrid F, Simon C. Embryonic implantation and leukocyte transendothelial migration: different processes with similar players? FASEB J. 2005;19:1056–1060. doi: 10.1096/fj.05-3781hyp. [DOI] [PubMed] [Google Scholar]
- 7.Miyazaki S, Tsuda H, Sakai M, Hori S, Sasaki Y, Futatani T, Miyawaki T, Saito S. Predominance of Th2-promoting dendritic cells in early human pregnancy decidua. J Leukoc Biol. 2003;74:514–522. doi: 10.1189/jlb.1102566. [DOI] [PubMed] [Google Scholar]
- 8.Kolb-Bachofen V. A review on the biological properties of C-reactive protein. Immunobiology. 1991;183:133–145. doi: 10.1016/S0171-2985(11)80193-2. [DOI] [PubMed] [Google Scholar]
- 9.Peisajovich A, Marnell L, Mold C, Du Clos TW. C-reactive protein at the interface between innate immunity and inflammation. Expert Rev Clin Immunol. 2008;4:379–390. doi: 10.1586/1744666X.4.3.379. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. C-reactive protein concentrations as a marker of inflammation or infection for interpreting biomarkers of micronutrient status. 2014.
- 11.Orvieto R, Volodarsky M, Hod E, Homburg R, Rabinson J, Zohav E, Anteby EY, Meltcer S. Controlled ovarian hyperstimulation using multi-dose gonadotropin-releasing hormone (GnRH) antagonist results in less systemic inflammation than the GnRH-agonist long protocol. Gynecol Endocrinol. 2007;23:494–496. doi: 10.1080/09513590701500994. [DOI] [PubMed] [Google Scholar]
- 12.Orvieto R, Chen R, Ashkenazi J, Ben-Harush A, Bar J, Fisch B. C-reactive protein levels in patients undergoing controlled ovarian hyperstimulation for IVF cycle. Hum Reprod. 2004;19:357–359. doi: 10.1093/humrep/deh089. [DOI] [PubMed] [Google Scholar]
- 13.Herzberger EH, Miller N, Ghetler Y, Yaniv RT, Keren KA, Shulman A, et al. High C-reactive protein levels in women undergoing IVF are associated with low quality embryos. Fertil Steril. 2016;106:e262. [Google Scholar]
- 14.Arefi S, Panahi PS, Saruiy LA, Zeraati H. C-reactive protein level and pregnancy rate in patients undergoing IVF/ICSI. Iranian J Reproduct Med. 2010;8:197–202. [Google Scholar]
- 15.El-Shawarby SA, Sacks GP, Seyani L, Lavery SA, Trew GH. Maternal C-reactive protein levels in patients undergoing frozen embryo replacement cycles: a prospective study. Fertil Steril. 2005;84:1053–1055. doi: 10.1016/j.fertnstert.2005.04.045. [DOI] [PubMed] [Google Scholar]
- 16.Gaskins AJ, Wilchesky M, Mumford SL, Whitcomb BW, Browne RW, Wactawski-Wende J, Perkins NJ, Schisterman EF. Endogenous reproductive hormones and C-reactive protein across the menstrual cycle: the BioCycle study. Am J Epidemiol. 2012;175:423–431. doi: 10.1093/aje/kwr343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huirne J, Homburg R, Lambalk C. Are GnRH antagonists comparable to agonists for use in IVF? Hum Reprod. 2007;22:2805–2813. doi: 10.1093/humrep/dem270. [DOI] [PubMed] [Google Scholar]
- 18.Liu B, Zhang L, Guo R, Wang W, Duan X, Liu Y. The serum level of C-reactive protein in patients undergoing GnRH agonist protocols for in vitro fertilization cycle. Clin Exp Obstet Gynecol. 2014;41:190–194. [PubMed] [Google Scholar]
- 19.Seckin B, Ozaksit G, Batioglu S, Ozel M, Aydoğan M, Senturk B. The relationship between the change in serum high sensitivity C-reactive protein levels and IVF success. Gynecol Endocrinol. 2012;28:418–421. doi: 10.3109/09513590.2011.633653. [DOI] [PubMed] [Google Scholar]
- 20.Almagor M, Hazav A, Yaffe H. The levels of C-reactive protein in women treated by IVF. Hum Reprod. 2004;19:104–106. doi: 10.1093/humrep/deh036. [DOI] [PubMed] [Google Scholar]
- 21.Wunder D, Kretschmer R, Bersinger NA. Concentrations of leptin and C-reactive protein in serum and follicular fluid during assisted reproductive cycles. Hum Reprod. 2005;20:1266–1271. doi: 10.1093/humrep/deh767. [DOI] [PubMed] [Google Scholar]
- 22.Chakrabarti S, Lekontseva O, Davidge ST. Estrogen is a modulator of vascular inflammation. IUBMB Life. 2008;60:376–382. doi: 10.1002/iub.48. [DOI] [PubMed] [Google Scholar]
- 23.Srivastava S, Weitzmann MN, Cenci S, Ross FP, Adler S, Pacifici R. Estrogen decreases TNF gene expression by blocking JNK activity and the resulting production of c-Jun and JunD. J Clin Invest. 1999;104:503–513. doi: 10.1172/JCI7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Hum Reprod Update. 2005;11:411–423. doi: 10.1093/humupd/dmi008. [DOI] [PubMed] [Google Scholar]
- 25.Steingold K, Matt D, Deziegler D, Sealey J, Fratkin M, Reznikov S. Comparison of transdermal to oral estradiol administration on hormonal and hepatic parameters in women with premature ovarian failure. J Clin Endocrinol Metab. 1991;73:275–280. doi: 10.1210/jcem-73-2-275. [DOI] [PubMed] [Google Scholar]
- 26.Orvieto R, Zagatsky I, Yulzari-Roll V, La Marca A, Fisch B. Substituting human chorionic gonadotropin by gonadotropin-releasing hormone agonist to trigger final follicular maturation, during controlled ovarian hyperstimulation, results in less systemic inflammation. Gynecol Endocrinol. 2006;22:437–440. doi: 10.1080/09513590600881339. [DOI] [PubMed] [Google Scholar]
- 27.Orvieto R. Controlled ovarian hyperstimulation—an inflammatory state. J Soc Gynecol Investig. 2004;11:424–426. doi: 10.1016/j.jsgi.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Mathur RS, Akande AV, Keay SD, Hunt LP, Jenkins JM. Distinction between early and late ovarian hyperstimulation syndrome. Fertil Steril. 2000;73:901–907. doi: 10.1016/S0015-0282(00)00492-1. [DOI] [PubMed] [Google Scholar]
- 29.Korhonen KV, Savolainen-Peltonen HM, Mikkola TS, Tiitinen AE, Unkila-Kallio LS. C-reactive protein response is higher in early than in late ovarian hyperstimulation syndrome. Eur J Obstet Gynecol Reprod Biol. 2016;207:162–168. doi: 10.1016/j.ejogrb.2016.10.051. [DOI] [PubMed] [Google Scholar]
- 30.Sowers MR, Jannausch M, Randolph JF, Mcconnell D, Little R, Lasley B, et al. Androgens are associated with hemostatic and inflammatory factors among women at the mid-life. J Clin Endocrinol Metab. 2005;90:6064–6071. doi: 10.1210/jc.2005-0765. [DOI] [PubMed] [Google Scholar]
- 31.Ng MK, Liu PY, Williams AJ, Nakhla S, Ly LP, Handelsman DJ, et al. Prospective study of effect of androgens on serum inflammatory markers in men. Arterioscler Thromb Vasc Biol. 2002;22:1136–1141. doi: 10.1161/01.atv.0000022167.80130.a6. [DOI] [PubMed] [Google Scholar]
- 32.Kjøtrød SB, Romundstad P, Von Düring V, Sunde A, Carlsen SM. C-reactive protein levels are unaffected by metformin during pretreatment and an IVF cycle in women with polycystic ovary syndrome. Fertil Steril. 2008;89:635–641. doi: 10.1016/j.fertnstert.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 33.Boots CE, Jungheim ES. Inflammation and human ovarian follicular dynamics. Semin Reprod Med. 2015;33:270–275. doi: 10.1055/s-0035-1554928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Espey LL. Current status of the hypothesis that mammalian ovulation is comparable to an inflammatory reaction. Biol Reprod. 1994;50:233–238. doi: 10.1095/biolreprod50.2.233. [DOI] [PubMed] [Google Scholar]
- 35.Zhou L, Li R, Wang R, Huang H-X, Zhong K. Local injury to the endometrium in controlled ovarian hyperstimulation cycles improves implantation rates. Fertil Steril. 2008;89:1166–1176. doi: 10.1016/j.fertnstert.2007.05.064. [DOI] [PubMed] [Google Scholar]
- 36.Wu LL-Y, Norman RJ, Robker RL. The impact of obesity on oocytes: evidence for lipotoxicity mechanisms. Reprod Fertil Dev. 2011;24:29–34. doi: 10.1071/RD11904. [DOI] [PubMed] [Google Scholar]
- 37.Shkolnik K, Tadmor A, Ben-Dor S, Nevo N, Galiani D, Dekel N. Reactive oxygen species are indispensable in ovulation. Proc Natl Acad Sci. 2011;108:1462–1467. doi: 10.1073/pnas.1017213108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robinson S, Pemberton P, Laing I, Nardo LG. Low grade inflammation, as evidenced by basal high sensitivity CRP, is not correlated to outcome measures in IVF. J Assist Reprod Genet. 2008;25:383–388. doi: 10.1007/s10815-008-9253-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coticchio G, Sereni E, Serrao L, Mazzone S, Iadarola I, Borini A. What criteria for the definition of oocyte quality? Ann N Y Acad Sci. 2004;1034:132–144. doi: 10.1196/annals.1335.016. [DOI] [PubMed] [Google Scholar]
- 40.Bączkowski T, Kurzawa R, Głąbowski W. Methods of embryo scoring in in vitro fertilization. Reprod Biol. 2004;4:5–22. [PubMed] [Google Scholar]
- 41.Selman MO, Jawad MA, Raghif ARA, Hussein MHO, Abbood MS, Al-Khafaji QM. A study of serum and follicular fluid C. reactive protein and antisperm antibody as predictive markers for human oocyte and embryo quality and ICSI outcome. Int J Adv Res. 2017;5:488–494. doi: 10.21474/ijar01/5794. [DOI] [Google Scholar]
- 42.Nejabati H, Mota A, Farzadi L, Ghojazadeh M, Fattahi A, Hamdi K, et al. Follicular fluid PlGF/sFlt-1 ratio and soluble receptor for advanced glycation end–products correlate with ovarian sensitivity index in women undergoing ART. J Endocrinol Investig. 2017;40:207–215. doi: 10.1007/s40618-016-0550-5. [DOI] [PubMed] [Google Scholar]
- 43.Wei C, Yu C, Li Y, Zhang Q, Mai M. AC-037 the levels of C-reactive protein in women treated by IVF–embryo transfer. Reprod BioMed Online. 2006;12:28. [Google Scholar]
- 44.Levin I, Gamzu R, Mashiach R, Lessing JB, Amit A, Almog B. Higher C-reactive protein levels during IVF stimulation are associated with ART failure. J Reprod Immunol. 2007;75:141–144. doi: 10.1016/j.jri.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 45.Wei C-X, Yu C-Y, Li Y. The levels of C-reactive protein in women treated by IVF-ET. Matern Child Health Care China. 2007;2007:49. [Google Scholar]
- 46.Park HS, Park JY, Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-α and IL-6. Diabetes Res Clin Pract. 2005;69:29–35. doi: 10.1016/j.diabres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 47.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 48.Wyczalkowska-Tomasik A, Czarkowska-Paczek B, Zielenkiewicz M, Paczek L. Inflammatory markers change with age, but do not fall beyond reported normal ranges. Arch Immunol Ther Exp. 2016;64:249–254. doi: 10.1007/s00005-015-0357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wener MH, Daum PR, Mcquillan GM. The influence of age, sex, and race on the upper reference limit of serum C-reactive protein concentration. J Rheumatol. 2000;27:2351. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable