Abstract
Antiviral proteins (AVPs) from plants possess multiple activities, such as N-glycosidase, RNase, DNase enzymatic activity, and induce pathogenesis-related proteins, salicylic acid, superoxide dismutase, peroxidase, and catalase. The N-glycosidase activity releases the adenine residues from sarcin/ricin (S/R) loop of large subunit of ribosomes and interfere the host protein synthesis process and this activity has been attributed for antiviral activity in plant. It has been shown that AVP binds directly to viral genome-linked protein of plant viruses and interfere with protein synthesis of virus. AVPs also possess the RNase and DNase like activity and may be targeting nucleic acid of viruses directly. Recently, the antifungal, antibacterial, and antiinsect properties of AVPs have also been demonstrated. Gene encoding for AVPs has been used for the development of transgenic resistant crops to a broad range of plant pathogens and insect pests. However, the cytotoxicity has been observed in transgenic crops using AVP gene in some cases which can be a limiting factor for its application in agriculture. In this review, we have reviewed various aspects of AVPs particularly their characteristics, possible mode of action and application.
Keywords: Antiviral protein, N-glycosidase activity, Virus infection, Host defense, Agriculture, Transgenic crop
Introduction
The agricultural crop production is always under pressure due to environmental factors including biotic and abiotic stresses. Among biotic stresses, fungi, bacteria, and insect pests are managed to a great extent by employing the integrated disease management strategy, but very little or no effective control method is currently available for plant viruses. This is mainly because viral pathogens depend on host cellular resources, particularly on ribosomes for protein synthesis for their replication and multiplication. Approximately 250 agriculturally important viruses are characterized, which are known to cause significant yield losses world wide (Briddon and Markham 2000). The various viral disease management strategies including host resistance developed through rigorous breeding programme often fail because viruses have ability for quick changes in their genome, develop complex virus–host–vector relationship, and has diversity of infection mechanism (Verma and Varsha 1995). Nevertheless, some of the management practices for plant viruses like control of insect vector population, virus-free propagating material, cultural practices and use of resistant cultivars are adopted as integrated approach, but could not achieve desired success. The gene silencing mechanism of host defense has also been exploited with viral coat protein gene, replicase protein, movement protein and helper component protein to develop virus resistant crops (Lomonossoff 2015). These approaches may also have limitations like potential risk of genetic recombination between a transgene and an infecting virus, emergence of new pathogenic viruses and change in the host preference (Greene and Allison 1994).
Antiviral proteins (AVPs) have been reported as an endogenous, nonstress induced proteins from large number of plants, which are capable of virus inhibition. The AVPs are synthesized as pro-proteins consisting of signal peptide and C-terminal extension to follow secretory pathway that allows plant cells to segregate the cytotoxic proteins into vacuoles or possibly in another extracytoplasmic compartment (VanDamme et al. 2001). The immunolocalization study shows that AVPs are majorly located in cell wall matrix and a small amount is found in vacuoles in leaves of Phytolacca americana (Monzingo et al. 1993; Ready et al. 1986).
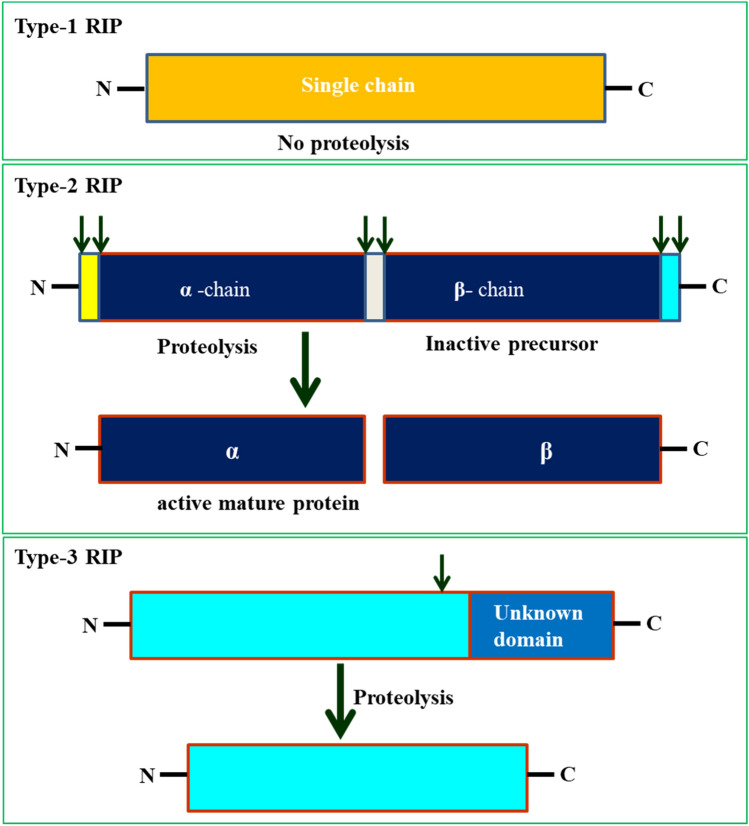
The AVPs are classified into three types based on their structure and composition viz. type 1, type 2, and type 3 (Fig. 1). Type 1 are holo-protein that consists only a single chain protein of approx. 30 kDa, whereas type-2 and type-3 are chimero protein consists 2 chain. The type 2 chimero protein of approx. 60 kDa has one α-chain polypeptide with RIP activity and another one β-chain with galactose binding lectin domain, both are linked through a disulfide bond. The type 3 chimero protein also contain one α-chain polypeptide with RIP activity and another domain with unknown function, however β-chain polypeptide must be removed to become active RIP (Nielsen and Boston 2001). These AVPs have rRNA N-glycosidase activity, which depurinate the large subunit of ribosome of host cells upon virus infection and therefore AVPs are also known as ribosome-inactivating proteins (RIPs) (Irvin 1975). Type-1 protein possess multiple enzymes activities like N-glycosidase, RNase, DNase, and antioxidant activities. The rRNA N-glycosidase activity is the main antiviral function of AVPs, which inactivates the host ribosomes and block their participation in protein synthesis (Meng et al. 2014; Zhu et al. 2013). In addition to enzymatic activity, AVPs have nonenzymatic antiviral activity like direct interaction to bind virus particles, interfere with viral infection process and also alter the host cell metabolism (Tumer 2015). Some AVPs are also characterized for their antibacterial, antifungal and insecticidal property (Rumiyati et al. 2014; Hamshou et al. 2016, 2017; Ajji et al. 2016; Zhu et al. 2013, 2018; Lodha et al. 2011; Tumer 2015; Kim et al. 2003). To show the antiviral activity, aqueous crude extract of leaves containing AVP was rubbed on to the leaf of nonhost healthy plants like Nicotiana glutinosa and Cyamopsis tetragonoloba, which were preinoculated with tobacco mosaic virus (TMV) and sunnhemp rosette virus (SRV). These plants showed ~ 95% lesser local lesions as compared to control plants that proved the antiviral property of AVP (Verma and Baranwal 1983; Roy et al. 2006; Stripe et al. 1981; Straub et al. 1986; Dutt et al. 2003). Some AVPs also induce antioxidant molecules that provide systemic resistance in plant upon virus infection (Bhatia and Lodha 2005; Srivastava et al. 2015; Straub et al. 1986). Like native AVPs, the recombinant AVP expressed and purified from E. coli also showed antiviral activity against viruses (Begam et al. 2006; Roy et al. 2006; Choudhary et al. 2008a; Sipahioglu et al. 2017; Shang et al. 2016; Zhu et al. 2013). An AVP can provide resistance to multiple viruses and other plant pathogens at low concentration that promises that AVPs can be a potential candidate in management of plant pathogens (Chen et al. 2002). However, the mechanism of AVPs against plant pathogens is not known completely. The researchers need to conduct depth study to understand the cell entry mechanism, specificity, stability, dosages of AVP for its wide application in agriculture (Puri et al. 2012).
Fig. 1.
Structure and processing of type- 1, 2 and 3 AVPs/RIPs. Type 1 RIP is a single chain holo-RIP and not required enzymatic processing to become active. Type 2 RIP is a chimero-RIPs and consists two parts, one chains has the enzymic properties and other part usually has lectin domain. Type 3 RIP is also chimero-RIPs and contain two parts, one chain has enzymatic properties, but it become active only if the other unknown domain removed
Source of AVPs
AVPs are found in leaves, seeds, flowers, fruits, roots, rhizomes or bark of plants belonging to plant taxonomic families like Amaranthaceae, Chenopodiaceae, Compositae, Cucurbitaceae, Euphorbiaceae, Graminae, Nyctaginaceae, Pinaceae, Rosaceae, Solanaceae, Verbenaceae (Verma and Varsha 1995). Most of the plants express only one type of AVP except for Iridaceae, Euphorbiaceae and Cucurbitaceae family, which can express more than one type of AVP. The source of different AVPs/RIPs with their activities is presented in Table 1.
Table 1.
Source of AVPs and their biochemical properties
| Source of AVPs | AVP/RIP | M.W. (kDa) | Biochemical property | Activity against plant pathogens, insect pest and others | References |
|---|---|---|---|---|---|
| Type 1 AVP | |||||
| Amaranthus tricolor (lal chaulai) | AAP | 27 | N-glycosidase and RNase | Virus [sunnhemp rosette virus (SRV)] | Roy et al. (2006) |
| Amaranthus viridis | Amaranthin | 30 | N-glycosidase activity | Viruses (tobacco mosaic virus) | Kwon et al. (1997, 2000) |
| Beta vulgaris L. (Sugar beet) | Beetins BE27 | 27, 29 | RNA depurination, and SAR | Viruses (TMV, artichoke mottled crinkle virus), Fungi (Penicillium digitatum) | Iglesias et al. (2005), Citores et al. (2016) |
| Boerhaavia diffusa L. | BDP-30 | 30 | N-glycosidase activity | Viruses (TMV) | Srivastava et al. (2015) |
| Bougainvillea × buttiana |
BAP-28 & BAP-24 BBAP1(recombinant) |
28 & 24 35.49 |
N-glycosidase, RNase, DNase | Viruses (TMV and SRV) |
Choudhary et al. (2008b) |
| Bougainvillea spectabilis (Roots, Leaves) | BAP 1, Bouganin |
28 26.2 |
N-glycosidase | Bacteria (Staphycococcus aureus, Bacillus subtilis) | Umamaheswari and Nuni (2008), Hartog et al. (2002) |
| Celosia cristata | CCP-25 & CCP-27 | 25 & 27 | N-glycosidase, RNase, DNase, and antioxidant activity | Viruses (TMV and SRV) | Begam et al. (2006), Gholizadeh et al. (2004) |
| Chenopodium album (bathua) | CAP-I and CAP-II (Isoform) | 24 | N-glycosidase | Viruses (TMV and SRV) | Dutt et al. (2003) |
| Chenopodium album | CAP-30 | 30 | N-glycosidase | Viruses (TMV) and anti-microbial activity | Park et al. (2004b) |
| Clerodendrum aculeatum | CA-SRI | 34 | Induce systemic resistance | Viruses | Kumar et al. (1997) |
| Clerodendrum inerme | CIP | 29 | N-glycosidase, induce SAR | Viruses (SRV, TMV, and Papaya ringspot virus) | Prasad et al. (2014) |
| Dianthus caryophyllus | Dianthin | 30 & 32 | N-glycosidase | Viruses (TMV) | Stripe et al. (1981) |
| Elaeis guineensis (Oil palm) | EgRIP-1a and EgRIP-1b | 28–30 | N-glycosidase | Fungus (Ganoderma boninense) | Sargolzaei et al. (2016) |
| Mirabilis expansa | ME1 and ME2 | 27.0 & 27.5 | N-glycosidase | Bacteria (Pseudomonas syringae, Agrobacterium tumefaciens), Fungus (Pythium irregulare, Fusarium oxysporum solani) | Vivanco et al. (1999) |
| Mirabilis jalapa | MAP and MAP-4 | 27.78 & 29.34 | Adenine polynucleotide glycosylase activity | Viruses (TMV), Bacteria (Propionibacterium acnes and Staphylococcus epidermidis) | Bolognesi et al. (2002), Rumiyati et al. (2014) |
| Momordica charantia | MAP, α-MC | 30 | N-glycosidase | Viruses (Zucchini Yellow Mosaic virus), Bacteria Anti-cancer activity | Puri et al. (2009), Meng et al. (2014), Wang et al. (2016) |
| Phytolacca americana | PAP | 29 | N-glycosidase | Viruses (Potato virus X, Zucchini yellow mosaic virus), Fungus (Rhizoctonia solani) | Tumer et al. (1997), Zoubenko et al. (1997), Sipahioglu et al. (2017) |
| Phytolacca dioica | PDL | 29.40 | Polynucleotide: adenosine glycosidases | Viruses | Di Maro et al. (1999) |
| Salsola longifolia | SLP-32 | 32 | DNase, N-glycosidase | Viruses (Tobacco necrosis virus, Bean yellow mosaic virus) | Torky (2012) |
| Spinacia oleracea | SoRIP1 and SoRIP2 | 31 & 29 | N-glycosidase activity, systemic resistance | Viruses (TMV) | Straub et al. (1986), Kawade et al. (2008) |
| Trichosanthes kirilowii | Trichosanthin | 30 | N-glycosidase activity | Viruses (TMV and CMV) | GuoYong et al. (1999) |
| Zea mays L. | maize RIP | 30 | N-glycosidase | Insect pest (cigarette beetle, Lasioderma serricorne (F.), and the tobacco hornworm, Manduca sexta (L.), Helicoverpa zea) and fungus sheath blight (Rhizoctonia solani) | Dowd et al. (2003, 2006, 2012), Kim et al. (2003) |
| Type-2 AVP | |||||
| Abrus precatorius | Abrin | 63 (30 + 33) | N-glycosidase | Viruses | Singh (2018) |
| Malus domestica Borkh) Apple | Type II | 30 | N-glycosidase |
Insect pest Pea aphids (Acyrthosiphon pisum) and green peach aphids (Myzus persicae) |
Hamshou et al. (2016) |
| Ricinus communis | Ricin A & B | 32 & 34 | N-glycosidase | Biomedical application | Polito et al. (2019) |
| Sambucus ebulus | Ebuin I | 56 (26 + 30) | N-glycosidase | Viruses | Citores et al. (1997) |
| Sambucus nigra | SNA-I | 24 + 23.5 | N-glycosidase | Insect pests (Acyrthosiphon pisum, Myzus nicotianae) beet armyworm (Spodoptera exigua) | Chen et al. (2002), Shahidi-Noghabi et al. (2009) |
|
Viscum album Himalayan |
Hm RIP | 65 | N-glycosidase | Anti-cancer, antigenicity and pharmacological properties | Mishra et al. (2004) |
Type 1 AVP/RIP
The type 1 AVP of 28 kDa and 24 kDa was found in leaves of B. xbuttiana (Narwal et al. 2001a; Bhatia and Lodha 2005) and CAP-I and CAP-II of 24 kDa in leaves of Chenopodium album cv Pusa Bathua 1 (Dutt et al. 2003). The SoRIP1 isolated from the roots of Spinacia oleracea L. cv. Jiromaru, accumulates primarily in pre-embryos stage and peripheral meristem of somatic embryos during early development. Its expression was very low in the callus, but high in the epidermis of somatic embryos suggesting that the expression of spinach RIP genes is differentially regulated in a development‐dependent fashion during somatic embryogenesis in spinach (Kawade et al. 2008). Some AVPs are present in isoforms and may be localized in different plant parts viz. AVP of 26.2 kDa in leaves and 28 kDa in roots of B. spectabilis (Rajesh et al. 2005; Umamaheswari and Nuni 2008). Seven isoforms of saporin was found in seeds, leaves and roots of soapwort (Saponaria officinalis L.) (Ferreras et al. 1993) while two isoforms of PAP have been reported in leaves and roots of Phytolacca americana (Duggar and Armstrong 1925; Irvin 1975, 1983; Barbieri et al. 1982). AVPs may also express differently in different season like PAP-II of 30 kDa was expressed in summer, while 29 kDa was observed in spring (Irvin et al. 1980). Similarly, CCP-25 expresses at pre-flowering stage, whereas CCP-27 expressed at postflowering stage in Celosia cristata (Balasubrahmanyam et al. 2000). Some well characterized type 1 AVPs are presented in Table 1.
Type 2-AVP/RIP
Ricin is the first AVP isolated from seeds of Ricinus communis L. which was characterized as a type 2 AVP. Type 2 protein contain the characteristic N-glycosidase activity and an additional an additional sugar-binding moiety, which helps this protein to cross the cell membrane (Polito et al. 2019; VanDamme et al. 2001; Bertholdo-Vargas et al. 2009). In a study, it was found that one ricin molecule can interfere the function of approximately 1500 ribosome molecules per minute in host plant. Therefore, type 2 AVP characterized as more toxic protein than type 1. Ricin is considered a threat for bioterrorism because no antidote is available to neutralize ricin (May et al. 2013; Tumer 2015). Abrin, a type II RIP isolated from seeds of Abrus precatorius, rosary pea induces the apoptosis process in cells triggering intrinsic mitochondrial pathway. Abrin toxin might inhibit the large subunit of ribosome, which block the protein synthesis and chain elongation in eukaryotes (Singh 2018). Ricin and abrin are a toxic RIP. A nontoxic type 2 RIP, ebulin was isolated from rhizomes of dwarf elder (Sambucus ebulus L.), which only depurinates sensitive ribosomes and interferes protein synthesis in mammal, but not in plant system (Citores et al. 1997). Himalayan mistletoe ribosome-inactivating protein II (HmRIP-II), a disulfide linked toxin and lectin subunits was purified from Viscum album. HmRIP-II occurs in four isoforms, HmRIP 1 and 2 of MW 28 and 34 kDa and HmRIP 3 and 4 of MW 28 and 32 kDa having isoelectric points of 6.6, 6.1, 5.2, and 4.7 (Mishra et al. 2004). Some well characterized type 2 AVPs are presented in Table 1.
Type 3 AVP/RIP
The jasmonate-induced protein (JIP60) is the well-known type 3 AVP found intracellularly in vacuoles, peroxisomes, and in the nucleoplasm of barley crop (Hause et al. 1994). The N-terminal and C terminal of JIP60 protein contain the homologous catalytic domain of ribosome-inactivating protein and eukaryotic translation initiation factor 4E (eIF4E) domain, respectively (Chaudhry et al. 1994). JIP60 synthesized as a precursor, which undergoes proteolytically processing and releases their C-terminal domain. The intact N-terminal domain of JIP function as classical N-glycosidase activity to inhibit the cellular translation. However, RIP function of JIP for plant immune response is dependent on host jasmonate hormones level (Przydacz et al. 2020; Reinbothe et al. 1994; VanDamme et al. 2001).
Enzymatic activities
AVPs possess the multiple enzymatic activities, however, the domain for enzymatic activities and recognition site of AVPs can be different for antiviral activity. Some of the enzymatic activities of AVPs are characterized as: (1) N-glycosidase activity; (2) specific depurination of capped mRNA; (3) depurination of supercoiled double-stranded DNA, and (4) RNase activity of AVPs.
N-glycosidase activity
A specific adenine conserved at nucleotide position 4324 (A4324) in α-S/R loop of large ribosomal subunit, is important for stability and structure of ribosome in plant species. The S/R loop is a critical element and it belongs to GTPase associated center for unidirectional route for translational apparatus by translational GTPases (trGTPase) activity upon GTP hydrolysis. GTP hydrolysis does not get activated by trGTPase enzyme in absence of intact S/R loop and hinders translation process (Grela et al. 2019). Upon virus infection, the basic amino acid present on surface structure of AVP interacts with the C-terminal of S/R loop to activate the N-glycosidase activity (Shi et al. 2016). The N-glycosidase activity releases the adenine (A4324) from S/R loop to damage the ribosome, which do not support the binding of initiation (eIF) and elongation factors (eEF), and thus protein synthesis stops (Wang and Tumer 1999). The PAP function is known to inhibit eEF1 dependent binding of amino acyl-tRNA to acceptor site (A site) and EF-1-mediated GTP hydrolysis. PAP also inhibits formation of EF2-GDP-ribosome complex and stimulates the ribosome-dependent hydrolysis of GTP (Irvin 1995). This is called ‘local suicide model’, which is widely accepted antiviral mechanism of AVP (Gessner and Irvin 1980). In noninfected cells, the ribosomes are protected from endogenous AVP because of compartment and predominant localization in extracellular matrix of mesophyll cells of leaf (Ready et al. 1986). Similar N-glycosidase activity also reported with AVPs like ME, Saporin, MAP, CIP-29, BAP 1 (Balasaraswathi et al. 2001; Barbieri et al. 1996, 1997; Bolognesi et al. 2002; Narwal et al. 2001b; Kwon et al. 1997, 2000; Olivieri et al. 1996; Park et al. 2004a; Roy et al. 2006; Kataoka et al. 1991). The catalytic activities of AVPs are also found at A4324 in 28S rRNA of rat liver, A2660 of 23S rRNA in E. coli, A3017 of 25S rRNA in plants, A4321 from large ribosome of yeast and A4323 from tobacco ribosome (Endo et al. 1987; Endo and Tsurugi 1987; Prestle et al. 1992; Hudak et al. 2000).
The type 2-AVP ricin releases guanine (G4323) from S/R loop of large subunit of ribosomes of rat liver (Endo and Tsurugi 1987). The AVP-ME1 has been reported to release guanine from wobble base pair from hairpin stems (Park et al. 2004a). In some cases, PAP also releases guanine from ribosomes of rabbit reticulocytes, tobacco, yeast and E. coli (Rajamohan et al. 1999b; Hudak et al. 2000). However, the mechanism of release of guanine from ribosome is unknown and it is different than the N-glycosidase activity (VanDamme et al. 2001).
Depurination of capped mRNA
Viral genome-linked protein (VPg), an analogue of m7G cap structure is covalently linked at 5′ end via a tyrosine residue in many plant virus mRNAs and it is required for infectivity, replication, cell-to-cell movement, and other function of virus. PAP complex specifically binds to translational enhancer (TE) structure of VPg, which interacts with host eIF4E isoform (eIFiso4E) and eIF4F (eIFiso4F) factors at position between 511 and 624 for protein synthesis (Wang and Hudak 2006). A mutant AVP was created by deleting the 25 amino acids from C-terminal end (Tumer et al. 1997; Zoubenko et al. 1997). The depurination of capped viral mRNA was studied with mutant AVP, which do not have N-glycosidase activity. The mutant AVP was found to have four times more binding affinity to viral VPg than rRNA in an in vitro study (Hudak et al. 2002). However, PAPc mutants did not differentiate between viral and own capped RNA (Parikh et al. 2002). ME1 and saporin have different affinities with diverse types of capped viral RNAs, which suggest that specific depurination activity is limited to selective cap of RNA (Vivanco and Tumer 2003). This study shows a powerful antiviral strategy of intact PAP, which may interact with viral VPg to prevent replication of viral mRNA along with ribosome depurination (Domashevskiy et al. 2012; Domashevskiy and Goss 2015).
Depurination of DNA
The active site of AVP has apurinic/apyrimidinic (AP) region for the depurination activity, which removes the adenine nucleotide from dsDNA however, the molecular mechanism is still unknown. It was demonstrated that an active site mutant of PAP, which failed to perform the depurination activity in an in vitro study with PAPx (Wang and Tumer 1999; He and Liu 2004). AVPs-PD-S2 and PD-L4 isolated from Phytolacca dioica shows DNA polynucleotide: adenosine glycosylase activities on supercoiled plasmid DNA. Similarly, AVPs like BBAP1, CCP-27, PAP, tricosanthin AVP, momorcharin, ricin, luffin, cinnamomin, and camphorin having depurination activity, were found to alter the conformation of ds supercoiled plasmid DNA into nicked, circular and linear form at lower and higher concentration of AVPs, respectively. The typical conformation of DNA is essential for depurination activity because no further changes were observed in DNA with extended incubation time (Li et al. 1991; Thomas et al. 1992; Day et al. 1998; Ling et al. 1994; Choudhary et al. 2008b; Gholizadeh et al. 2005a; Bhatia and Lodha 2005; Begam et al. 2006; Torky 2012).
Ribonucleases (RNase) activity
N-terminal amino acids of AVP-figaren were sequenced and analyzed, which revealed that few conserved amino acids have similarity with RNase-like enzymes and were suspected to act on viral RNA genome to release a fragment (Masayuki et al. 2001).
RNase activity of AVPs were demonstrated in a gel assays containing RNA, which ruled out any external contamination of nucleases (Vivanco and Tumer 2003; Hudak et al. 2000). RNase activity of momorcharin-AVP releases the small RNA fragment from naked rRNA but not from polyU nucleotide sequence to supports ribonucleolytic cleavage (Mock et al. 1996). The CCP-25 releases a 360-ntd fragment from ribosomal RNA and inhibits the protein translation from RNA of Brome mosaic virus (BMV) (Baranwal et al. 2002). The recombinant PAP and nontoxic PAP mutant also depurinate BMV-RNA. In an in vivo experiment, decreased level of BMV-RNAs was found in barley protoplast pretreated with BMV-RNA3, which indicated that replicase enzyme stop synthesis at missing base of depurinated template. This depurination activity affects directly the replication of viral RNA and also the formation of subgenomic RNA (Picard et al. 2005). Similarly, BAP-24, BBAP1, CCP-27, PAP isoforms PAP-I, PAP-II, and PAP-III also showed RNase activity against the RNA of Torula yeast, CMV, TMV, and SRV (Masayuki et al. 2001; Choudhary et al. 2008b; Begam et al. 2006; Roy et al. 2006; Rajamohan et al. 1999a).
Mechanism of action of AVP
The antiviral mechanism of AVP has not yet been decoded completely at biochemical and molecular level, which is essential for its application in crop science to enhance quality and production (Bolognesi et al. 2016). Some in vitro and in vivo studies helped to understand the mechanism of antiviral activity of AVP. It believed that AVP expresses as soon as virus infects the plant host and remains active fighting at various stages of virus pathogenesis process to prevent them to establish inside host. AVP might interfered the virus infection process by blocking the entry of virus to host cell. In an experiment, it was demonstrated that the inhibitory response may persist up to 6 days (Verma and Baranwal 1983; Baranwal and Verma 1992). The AVPs-PAP I and PAP II facilitate to adsorb viruses and aggregate to form loose complex, which helped inhibit the pathogenicity of viruses. However, such experiment cannot justify completely the extent of inhibition of virus infection process (Grasso and Shepherd 1978; Kumon et al. 1990). The site-directed mutagenesis experiment shows that 16 amino acids of N-terminal of AVP are required for its toxicity and ribosome depurination. The ribosome depurination activity gradually decreases in case of sequential deletion of amino acids from C-terminal AVP. In another experiment, introducing stop codon at Glu-244 position or mutation at Tyr-123 of active site domain of PAP, interfered the ribosome depurination, but cytotoxicity and systemic acquired resistance (SAR) induction were stopped (Tumer et al 1997; Zoubenko et al. 2000). This study suggests that active site and C-terminal of AVP are essential for cytotoxicity and ribosome depurination activity, and antiviral activity of AVP might be different from ribosome-inactivating property (Hudak et al. 2004). AVPs might induces the apoptosis process for local cell death by probable action of cellular stress integration. AVP may also mitigate the virus induced hypersensitive response of cell death to control virus growth and maintain normal growth of plant (Gholizadeh et al. 2005b; Narayanan et al. 2005).
In another study, it was reported that AVP–BAP modifies the antioxidant level in TMV infected leaves. The level of superoxide dismutase (SOD) and peroxidase (POD) was also found increased, whereas catalase (CAT) level deceased as compared to noninfected leaves. It was believed that AVP may scavenge the reactive oxygen species (ROS) as well as alter host cell metabolism to maintain its antioxidant status to fight against viral pathogens (Bhatia et al. 2004; Zhu et al. 2016; Yang et al. 2016). Bioinformatics study analysis revealed that approx. 15 amino acid of AVP has similarity with Fe-SOD, which is known for strong antimicrobial activity against viruses, fungi and bacteria (Sharma et al. 2004). The systemic acquired resistance (SAR) has also been reported as an antiviral activity by AVP against plant viruses. In an experiment, it was shown that AVP-beetins application interfere the infection of artichoke mottled crinkle virus (ACMV) at local as well as distal leaves by inducing expression of salicylic acid (SA), a plant defense mediator molecule. It is also believed that AVP improves the damaged photosystem upon virus infection and enhances the glutathione disulfide ratio (Iglesias et al. 2005; Verma and Awasthi 1980).
Some AVPs showed antifungal activity by damaging the ribosome, inhibiting the protein synthesis and growth of fungus (Citores et al. 2016; Park et al. 2004a, 2009; Sargolzaei et al. 2016; Vivanco et al. 1999; Kim et al. 2003). The AVPs like BE27, BAP1, have shown antibacterial activity too by unknown mechanism in in vitro study against bacteria like Staphycococcus aureus, Bacillus subtilis, Streptococcus faecalis, Micrococcus luteus, Escherichia coli, Pseudomonas aeruginosa, Salmonella typhii, Klebsiella pneumoniae, Proteus vulgaris, Serratia marcescens, Shigella flexneri and Vibrio cholera (Umamaheswari and Nuni 2008), MAP-4 against Propionibacterium acnes and Staphylococcus epidermidis (Rumiyati et al. 2014). It is suspected that different functions of AVP might play role to minimize the local lesion formation on leaves after infection with pathogenic viruses, such as SRV, TMV, and potato virus X (Noronha et al. 1980).
AVP/RIP possess the insecticidal property and function against crop insect pest in concentration-dependent manner. The different concentrations of SNA-I, a type II RIP from Sambucus nigra, decreased the fecundity and survival of pea aphid Acyrthosiphon pisum (Shahidi-Noghabi et al. 2008, 2010). The transgenic tobacco expressing SNA-I demonstrated to show similar observation against green peach aphids Myzus nicotianae (Shahidi-Noghabi et al. 2009) and similar observation found with type I and type II RIPs of apple (Malus domestica Borkh) against wild type Anticarsia gemmatalis Hübner and Spodoptera frugiperda (Bertholdo-Vargas et al. 2009; Hamshou et al. 2016). In addition, the overexpression of a maize RIP in tobacco plants enhanced resistance against corn earworms, Helicoverpa zea (Dowd et al. 2003, 2012). How different AVPs/RIPs function as anti-insect pest is not yet known. However, some studies suggest that apoptosis process might be induced by RIP in the insect pest midgut through the activation of caspase 3 (Narayanan et al. 2005; Sikriwal and Batra 2010; Das et al. 2012; Shahidi-Noghabi et al. 2010).
Transgenic crop development with AVP gene
The promising antimicrobial and anti-insecticidal property of AVPs with no risk of genetic recombination due to their plant origin, they are better candidate for management of viruses and other pests of agriculture crops (Dasgupta et al. 2003; Akkouh et al. 2015; Chen et al. 2002; Park et al. 2004b; Hassan and Ge 2018). Some of the isolated AVP genes presented in Table 2 have been used or could be used for the development of transgenic crops.
Table 2.
List of isolated genes encoding for AVPs/RIPs
| Source | Name of AVPs | MW (kDa) | No. of a.a | References |
|---|---|---|---|---|
| Adenia volkensii (Roots) | Volkensin-type-2 RIP | 29 | 523 | Chambery et al. (2004) |
| Amaranthus viridis | Amaranthin | 30 | 270 | Kwon et al. (2000) |
| Amaranthus tricolor | AAP | 27 | 297 | Roy et al. (2006) |
| Bougainvillea spectabilis (Leaves) | Bougainin | 26.2 | 250 | Hartog et al. (2002) |
| Bougainvillea spectabilis (Roots) | AP 1 | 28 | 315 | Balasaraswathi et al. (1998) |
| Bougainvillea × buttiana | BBAP | 28 | 319 | Choudhary et al. (2008a, b) |
| Clerodendrum aculeatum | CA-SRI | 32 | 302 | Kumar et al. (1997) |
| Celosia cristata | CCP-27 | 27 | 283 | Begam et al. (2006), Gholizadeh et al. (2005a) |
| Dianthus caryophyllus | Dianthin 30 | 29.5 | 293 | Legname et al. (1993) |
| Dianthus sinensis | Dianthus AVP | 23 | 294 | Cho et al. (2000) |
| Himalayan misteletoe | Hm RIP- a type-2 RIP | 65 | 552 | Mishra et al. (2004) |
| Mirabilis jalapa | MAP | 24.2 | 250 | Kataoka et al. (1991) |
| Mirabilis expansa | ME1 | 29.5 | 317 | Vepachedu et al. (2003) |
| Phytolacca americana | PAP | 29 | 291 | Poyet and Hoeveler (1997) |
|
PAP-S PAPII |
30 | 293 | ||
| Sechium elude | Sechiumin | 27 | 285 | Wu et al. (1998) |
| Trichosanthes kirilowii | TCS | 30 | 293 | GuoYong et al. (1999) |
NU nucleic acid, Pr protein
The transgenic tobacco crops developed with full-length cDNA of PAP shows resistance against mechanical or aphid-transmitted viruses from their early stage of pathogenesis and antifungal property against fungus Rhizoctonia solani (Lodge et al. 1993; Poyet and Hoeveler 1997; Wang et al. 1998). The constitutive expression of pathogenesis related protein (PR-1) was also reported but the expression of SA was unchanged in transgenic tobacco. The double mutant PAP at position L20R and Y49H was found less toxic than the full-length cDNA of PAP. The transformation efficiency of mutant PAP was also found enhanced by 7–18% with mutant PAP (Di and Tumer 2015). The transgenic creeping bent grass developed with mutant PAPII gene do not show antifungal property against Clerotinia homoeocarpa, a causal agent of dollar spot disease because of unstable and very less protein expression (Dai et al. 2003). The active form of Maize RIP when co-expressed with basic chitinase gene in transgenic rice helped to increase resistance against the fungus sheath blight (R. solani) (Kim et al. 2003). The transgenic crops developed with mutant PAP (amino acid substitution G75D) did not depurinate ribosome and was nontoxic but still provided antiviral resistance. It was suspected that mutant AVP induces the defense proteins like PR proteins, wound-inducible protein kinase and protease inhibitor II for antiviral resistance (Zoubenko et al. 2000).
In another experiment, transgenic tomato transformed with cDNA of trichosanthin (TCS) shows resistance to TMV and cucumber mosaic virus (CMV) (GuoYong et al. 1999). The C. tetragonoloba plants transformed with CIP-29 gene induces the systemic resistance and shows resistance to SRV, TMV, and papaya ringspot virus (Prasad et al. 2014). The tobacco plants transformed with cDNA of type-2 AVP gene, SNA-1, SNA-V, and SNLRP show reduced local lesion upon TMV infection (Venbussche et al. 2004; Chen et al. 2002).
Transgenic grapevine plant expressing chitinase AVP showed resistance to fungus Uncinula necator and Plamopara viticola under field conditions (Bornhoff et al. 2005); transgenic black gram expressing chitinase AVP showed enhanced resistance upto 47% to fungus Corynespora cassiicola (Chopra and Saini 2014); tobacco crop expressing barley AVP provided resistance to soil-borne fungus R. solani (Logemann et al. 1992); transgenic rice expressing α-MC provide resistance to rice blast disease (Wang et al. 2016; Qian et al. 2014); and transgenic potato expressing PAP provided resistant to Botrytis cinerea and R. solani (Gonzales-Salazar et al. 2017). The cDNA encoding PD-S2 was transferred to tobacco crops displays the antipathogenic properties against viruses, fungi and insects (Iglesias et al. 2016).
Conclusion and future prospects
Higher plants contain the antiviral proteins (AVPs) in an inactive state in localized compartment, which become active to protect host cells upon plant virus infection. AVPs are known for their multiple enzymatic activities like N-glycosidase, RNase, DNase, and also induce the PR proteins, SA, JA, SOD, and CAT in host cells. They play an antiviral role and provide protection from viruses at local and distal sites with unexplained mechanism. The N-glycosidase activity is the principal function of AVPs and is widely accepted for the antiviral activity. The N-glycosidase activity depurinates the host ribosome of infected cells and interferes with the host protein synthesis as well as of virus genome. As a result, survival of viruses inside the host cells get disturbed as they are an obligate parasite and dependent on host cells resources for their replication and protein synthesis for multiplication. The transgenics crops generated with single AVP gene have shown expected antiviral activity against several viruses and also displays antifungal, antibacterial and antiinsecticidal activity. However, cytotoxicity is the major concern, and due to this reason AVP genes have not been in use for agriculture crops against plant viruses. AVP isolated from bougainvillea plant was found less toxic among all characterized type 1 AVPs, therefore, it can be a candidate for agricultural uses (Barbieri et al. 1993; Hartog et al. 2002; Tumer 2015). Bougainvillea AVP, a lysine-rich protein of pI ~ 10.0–10.5 having characteristic enzymatic activity and also induces systemic resistance against plant viruses (Baranwal and Verma 1992; Narwal et al. 2001a; Bolognesi et al. 1997; Balasaraswathi et al. 1998; Narwal et al. 2001a; Bhatia and Lodha 2005; Straub et al. 1986). The mechanism of AVP for its antiviral, antibacterial, antifungal, and anti-insecticidal activity is still not fully understood and it requires the integrated approach of molecular biology, biochemistry, genomics and structural biology for better understanding. The detailed study of AVP at structure and function level will reveal the degree of binding, intracellular trafficking, ribosome interactions, and toxin activity. In addition, the complete understanding of AVP should help translate it into spray bioformulation for easy application on agricultural and horticultural crops against plant pathogens to enhance the quality production.
Compliance with ethical standards
Conflict of interest
The author(s) declare that they have no competing interests.
References
- Ajji PK, Walder K, Puri M. Functional analysis of a type-I ribosome inactivating protein balsamin from Momordicabalsamina with anti-microbial and DNase activity. Plant Foods Hum Nutr. 2016;71:265–271. doi: 10.1007/s11130-016-0555-4. [DOI] [PubMed] [Google Scholar]
- Akkouh O, Ng TB, Cheung RC, Wong JH, Pan W, Ng CC, Sha O, Shaw PC, Chan WY. Biological activities of ribosome-inactivating proteins and their possible applications as antimicrobial, anticancer, and anti-pest agents and in neuroscience research. Appl Microbiol Biotechnol. 2015;99:9847–9863. doi: 10.1007/s00253-015-6941-2. [DOI] [PubMed] [Google Scholar]
- Balasaraswathi R, Sadasivam S, Chita HE, Raja JA. Inhibition of in vitro translation and cleavage of rRNA by Bougainvillea antiviral protein. Indian J Agric Biochem. 2001;14:67–68. [Google Scholar]
- Balasaraswathi R, Sadasivam S, Ward M, Walker JM. An antiviral protein from Bougainvilleaspectabilis roots; purification and characterization. Phytochemistry. 1998;47:1561–1565. doi: 10.1016/s0031-9422(97)00788-7. [DOI] [PubMed] [Google Scholar]
- Balasubrahmanyam A, Baranwal VK, Lodha ML, Varma A, Kapoor HC. Purification and properties of growth stage dependent antiviral proteins from the leaves of Celeosiacristata. Plant Sci. 2000;154:13–21. doi: 10.1016/s0168-9452(99)00192-2. [DOI] [PubMed] [Google Scholar]
- Baranwal VK, Verma HN. Localized resistance against virus infection by leaf extract of Celosiacristata. Plant Pathol. 1992;41:633–638. [Google Scholar]
- Baranwal VK, Tumer NE, Kapoor HC. Depurination of ribosomal RNA and inhibition of viral RNA translation by an antiviral protein of Celosiacristata. Indian J Exp Biol. 2002;40:1195–1197. [PubMed] [Google Scholar]
- Barbieri L, Aron GM, Irvin JD, Stirpe F. Purification and partial characterization of another form of the antiviral protein from the seeds of Phytolaccaamericana L. (pokeweed) Biochem J. 1982;203:55–59. doi: 10.1042/bj2030055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri L, Battelli MG, Stripe F. Ribosome-inactivating proteins from plants. Biochem Biophys Acta. 1993;1554:237–282. doi: 10.1016/0304-4157(93)90002-6. [DOI] [PubMed] [Google Scholar]
- Barbieri L, Valbonesi P, Bonora E, Gorini P, Bolognesi A, Stirpe F. Polynucleotide: adenosine glycosidase activity of ribosome-inactivating proteins: effect on DNA, RNA and poly (A) Nucl Acids Res. 1997;25:518–522. doi: 10.1093/nar/25.3.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri L, Valbonesi P, Gorini P, Pession A, Stripe F. Polynucleotide: adenosine glycosidase activity of saporin L1: effect on DNA RNA Poly(A) Biochem J. 1996;319:507–513. doi: 10.1042/bj3190507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begam M, Sharma SK, Roy S, Campanella JJ, Kapoor HC. Molecular cloning and functional identification of an antiviral/ribosome-inactivating protein from leaves of post-flowering stage of Celosiacristata. Phytochemistry. 2006;67:2441–2449. doi: 10.1016/j.phytochem.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Bertholdo-Vargas LR, Martins JN, Bordin D, Salvador M, Schafer AE, Barros NM, Barbieri L, Stirpe F, Carlini CR. Type 1 ribosome inactivating proteins entomotoxic oxidative and genotoxic action on Anticarsiagemmatalis (Hübner) and Spodopterafrugiperda (JE Smith) (Lepidoptera: Noctuidae) J Insect Physiol. 2009;55:51–58. doi: 10.1016/j.jinsphys.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Bhatia S, Lodha ML. RNase and DNase activities of antiviral proteins from leaves of Bougainvilleaxbuttiana. Ind J Biochem Biophys. 2005;42:152–155. [PubMed] [Google Scholar]
- Bhatia S, Kapoor HC, Lodha ML. Modification of antioxidant status of host cell in response to Bougainvilleaantiviral proteins. J Plant Biochem Biotechnol. 2004;18:113–119. [Google Scholar]
- Bolognesi A, Polito L, Lubelli C, Barbieri L, Parente A, Stirpe F. Ribosome-inactivating adenine polynucleotide glycosylase activities in Mirabilisjalapa L. tissues. J Biol Chem. 2002;277:13709–13716. doi: 10.1074/jbc.M111514200. [DOI] [PubMed] [Google Scholar]
- Bolognesi A, Bortolotti M, Maiello S, Battelli MG, Polito L. Ribosome-inactivating proteins from plants: a historical overview. Molecules. 2016;21:1627–1647. doi: 10.3390/molecules21121627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognesi A, Polioto L, Olivieri F, Valbonesi P, Barbieri L, Batteli MG, Carusi MV, Benvenuto E, Blanco FDV, Maro AD, Parente AL, Stirpe F. New ribosome-inactivating proteins with polynucleotide: adenosine glycosidase and antiviral activities from Basellarubra L. and Bougainvilleaspectabilis Willd. Planta. 1997;203:422–429. doi: 10.1007/s004250050209. [DOI] [PubMed] [Google Scholar]
- Bornhoff BA, Harst M, Zyprian E, Topfer R. Transgenic plants of Vitisvinifera cv Seyval blanc. Plant Cell Rep. 2005;24:433–438. doi: 10.1007/s00299-005-0959-3. [DOI] [PubMed] [Google Scholar]
- Briddon RW, Markham PJ. Cotton leaf curl virus disease. Virus Res. 2000;71:151–159. doi: 10.1016/s0168-1702(00)00195-7. [DOI] [PubMed] [Google Scholar]
- Chambery A, Di Maro A, Monti MM, Stirpe F, Parente A. Volkensin from Adeniavolkensii Harms (kilyambiti plant) a type 2 ribosome-inactivating protein. Eur J Biochem. 2004;271:108–117. doi: 10.1046/j.1432-1033.2003.03909.x. [DOI] [PubMed] [Google Scholar]
- Chaudhry B, Müller-Uri F, Cameron-Mills V, Gough S, Simpson D, Skriver K, Mundy J. The Barley 60 kDa jasmonate-induced protein (JIP60) is a novel ribosome-inactivating protein. Plant J. 1994;6:815–824. doi: 10.1046/j.1365-313x.1994.6060815.x. [DOI] [PubMed] [Google Scholar]
- Chen Y, Peumans WJ, Vamme Els JM. The Sambucus nigra type-2 ribosome-inactivating protein SNA-I’ exhibits in planta antiviral activity in transgenic tobacco. FEBS Lett. 2002;516:27–30. doi: 10.1016/s0014-5793(02)02455-9. [DOI] [PubMed] [Google Scholar]
- Chopra R, Saini R. Transformation of blackgram (Vignamungo (L.) Hepper) by barley chitinase and ribosome-inactivating protein genes towards improving resistance to Corynespora leaf spot fungal disease. Appl Biochem Biotechnol. 2014;174:2791–2800. doi: 10.1007/s12010-014-1226-2. [DOI] [PubMed] [Google Scholar]
- Choudhary N, Kapoor HC, Lodha ML. Cloning and expression of antiviral/ribosome-inactivating protein from Bougainvilleaxbuttiana. J Biosci. 2008;33:91–101. doi: 10.1007/s12038-008-0025-8. [DOI] [PubMed] [Google Scholar]
- Choudhary N, Yadav OP, Lodha ML. Ribonuclease, deoxyribonuclease and antiviral activity of Escherichiacoli-expressed Bougainvilleaxbuttiana antiviral protein 1. Biochem Mosc. 2008;73:273–277. doi: 10.1134/s000629790803005x. [DOI] [PubMed] [Google Scholar]
- Cho HJ, Lee SJ, Kim S, Kim BD. Isolation characterization of cDNAs encoding ribosome inactivating protein from Dianthussinensis L. Mol Cells. 2000;10(2):135–141. doi: 10.1007/s10059-000-0135-0. [DOI] [PubMed] [Google Scholar]
- Citores L, Iglesias R, Gay C, Ferreras JM. Antifungal activity of the ribosome-inactivating protein BE27 from sugar beet (Betavulgaris L.) against the green mould Penicilliumdigitatum. Mol Plant Pathol. 2016;17:261–271. doi: 10.1111/mpp.12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citores L, de Benito FM, Iglesias R, Ferreras JM, Argueso P, Jimenez P, Testera A, Camafeita E, Mendez E, Girbes T. Characterization of a new non-toxic two-chain ribosome-inactivating protein a structurally-related lectin from rhizomes of dwarf elder (Sambucusebulus L.) Cell Mol Biol. 1997;43:485–499. [PubMed] [Google Scholar]
- Dai WD, Bonos S, Guo Z, Meyer WA, Day PR, Belanger FC. Expression of pokeweed antiviral proteins in creeping bentgrass. Plant Cell Rep. 2003;21:497–502. doi: 10.1007/s00299-002-0534-0. [DOI] [PubMed] [Google Scholar]
- Das MK, Sharma RS, Mishra V. Induction of apoptosis by ribosome inactivating proteins Importance of N-glycosidase activity. Appl Biochem Biotechnol. 2012;166:1552–1561. doi: 10.1007/s12010-012-9550-x. [DOI] [PubMed] [Google Scholar]
- Dasgupta I, Malathi VG, Mukherjee SK. Genetic engineering for virus resistance. Curr Sci. 2003;84:341–354. [Google Scholar]
- Day PJ, Lord JM, Roberts LM. The deoxyribonuclease activity attributed to ribosome-inactivating proteins is due to contamination. Eur J Biochem. 1998;258:540–545. doi: 10.1046/j.1432-1327.1998.2580540.x. [DOI] [PubMed] [Google Scholar]
- Di Maro A, Valbonesi P, Bolognesi A, Stirpe F, DeLuca P, Siniscalco GG, Gaudio L, Delli BP, Ferranti P, Malorni A, Parente A. Isolation and characterization of four type-1 ribosome-inactivating proteins with polynucleotide:adenosine glycosidase activity from leaves of Phytolaccadioica L. Planta. 1999;208:125–131. doi: 10.1007/s004250050542. [DOI] [PubMed] [Google Scholar]
- Di R, Tumer NE. Pokeweed antiviral protein: its cytotoxicity mechanisms and applications in plant disease resistance. Toxins. 2015;7:755–772. doi: 10.3390/toxins7030755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domashevskiy AV, Goss DJ. Pokeweed antiviral protein, a ribosome inactivating protein: activity, inhibition and prospects. Toxins (Basel) 2015;7:274–298. doi: 10.3390/toxins7020274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domashevskiy AV, Miyoshi H, Goss DJ. Inhibition of pokeweed antiviral protein (PAP) by turnip mosaic virus genome-linked protein (VPg) J Biol Chem. 2012;287:29729–29738. doi: 10.1074/jbc.M112.367581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd PF, Holmes RA, Pinkerton TS, Johnson ET, Lagrimini LM, Boston RS. Relative activity of a tobacco hybrid expressing high levels of a tobacco anionic peroxidase and maize ribosome-inactivating protein against Helicoverpazea and Lasiodermaserricorne. J Agric Food Chem. 2006;54:2629–2634. doi: 10.1021/jf058180p. [DOI] [PubMed] [Google Scholar]
- Dowd PF, Johnson ET, Price NP. Enhanced pest resistance of maize leaves expressing monocot crop plant-derived ribosome-inactivating protein agglutinin. J Agric Food Chem. 2012;60:10768–10775. doi: 10.1021/jf3041337. [DOI] [PubMed] [Google Scholar]
- Dowd PF, Zuo WN, Gillikin JW, Johnson ET, Boston RS. Enhanced resistance to Helicoverpazea in tobacco expressing an activated form of maize ribosome-inactivating protein. J Agric Food Chem. 2003;51:3568–3574. doi: 10.1021/jf0211433. [DOI] [PubMed] [Google Scholar]
- Duggar BM, Armstrong JK. The effect of treating the virus of tobacco mosaic with the juices of various plants. Ann Mo Botl Gard. 1925;12:359–366. [Google Scholar]
- Dutt S, Narwal S, Kapoor HC, Lodha ML. Isolation and characterization of two proteins isoforms with antiviral activity from Chenopodiumalbum L. leaves. J Plant Biochem Biotechnol. 2003;12:117–122. [Google Scholar]
- Endo Y, Tsurugi K. RNA N-glycosidase activity of ricin A-chain. Mechanism of action of the toxic lectin ricin on eukaryotic ribosomes. J Biol Chem. 1987;262:8128–8130. [PubMed] [Google Scholar]
- Endo Y, Mitsui K, Motizuki M, Tsurugi K. The mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes. The site and the characteristics of the modification in 28S ribosomal RNA caused by the toxins. J Biol Chem. 1987;262:5908–5912. [PubMed] [Google Scholar]
- Ferreras JM, Barbieri L, Girbes T, Battelli MG, Rojo MA, Arias FJ, Rocher MA, Soriano F, Mendez H, Stripe F. Distribution and properties of major ribosome-inactivating proteins (28S rRNA N glycosidases) of the plant Saponariaofficinalis L. (Caryophyllaceae) Biochem Biophys Acta. 1993;1216:31–42. doi: 10.1016/0167-4781(93)90034-b. [DOI] [PubMed] [Google Scholar]
- Gessner SL, Irvin JD. Inhibition of elongation factor 2-dependent translocation by the pokeweed antiviral protein and ricin. J Biol Chem. 1980;255:3251–3253. [PubMed] [Google Scholar]
- Gholizadeh A, Kumar M, Balasubrahmanyam A, Sharma S, Narwal S, Lodha ML, Kapoor HC. Antioxidant activity of antiviral proteins from Celosiacristata L. J Plant Biochem Biotech. 2004;13:13–18. [Google Scholar]
- Gholizadeh A, Kohnehrouz BB, Santha IM, Lodha ML, Kapoor HC. Cloning and expression of small cDNA fragment encoding strong antiviral peptide from Celosiacristata in E.coli. Biochemistry (Moscow) 2005;70:1005–1010. doi: 10.1007/s10541-005-0216-y. [DOI] [PubMed] [Google Scholar]
- Gholizadeh A, Santha IM, Kohnehrouz BB, Lodha ML, Kapoor HC. Cystatins may confer viral resistance in plants by inhibition of a virus-induced cell death phenomenon in which cysteine proteinases are active: cloning and molecular characterization of a cDNA encoding cysteine-proteinase inhibitor (celostatin) from Celosiacristata (crested cock’s comb) Biotechnol Appl Biochem. 2005;42:197–204. doi: 10.1042/BA20050029. [DOI] [PubMed] [Google Scholar]
- Gonzales-Salazar R, Cecere B, Ruocco M, Rao R, Corrado G. A comparison between constitutive and inducible transgenic expression of the PhRIP I gene for broad-spectrum resistance against phytopathogens in potato. Biotechnol Lett. 2017;39:10491058. doi: 10.1007/s10529-017-2335-0. [DOI] [PubMed] [Google Scholar]
- Grasso S, Shepherd RJ. Isolation and partial characterization of virus inhibitors from plant species taxonomically related to Phytolacca. Phytopathology. 1978;68:199–205. [Google Scholar]
- Greene AE, Allison RF. Recombination between viral RNA transgenic plant transcripts. Science. 1994;263:1423–1435. doi: 10.1126/science.8128222. [DOI] [PubMed] [Google Scholar]
- Grela P, Szajwaj M, Horbowicz-Drożdżal P, Tchórzewski M. How ricin damages the ribosome. Toxins (Basel) 2019;11:241. doi: 10.3390/toxins11050241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GuoYong J, Demin J, Man Li W, Guo B, Bin W, Jin DM, Weng ML, Guo BT, Wang B. Transformation and expression of trichosanthin gene in tomato. Acta Bot Sin. 1999;41:334–336. [Google Scholar]
- Hamshou M, Shang C, Zaeytijd JD, VanDamme EJM, Smagghe G. Expression of ribosome-inactivating proteins from apple in tobacco plants results in enhanced resistance to Spodopteraexigua. J Asia Pac Entomol. 2017;20:1–5. [Google Scholar]
- Hamshou M, Shang C, Smagghe G, VanDamme EJM. Ribosome-inactivating proteins from apple have strong aphicidal activity in artificial diet in planta. Crop Prot. 2016;87:19–24. [Google Scholar]
- Hartog MTD, Lubelli C, Boon L, Heerkins S, BuIjsee APO, Boer M, Stripe F. Cloning and expression of cDNA coding for bouganin: a type-I ribosome inactivating protein from Bougainvilleaspectabilis Willd. Eur J Biochem. 2002;269:1772–1779. doi: 10.1046/j.1432-1327.2002.02823.x. [DOI] [PubMed] [Google Scholar]
- Hassan Y, Ogg S, Ge H. Expression of novel fusion antiviral proteins ricin a chain-pokeweed antiviral protein (RTA-PAPs) in Escherichiacoli and their inhibition of protein synthesis and of hepatitis B virus in vitro. BMC Biotechnol. 2018;18:47. doi: 10.1186/s12896-018-0458-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hause B, Nieden UZ, Lehmann J, Wasternack C, Parthier B. Intracellular localization of jasmonate-induced proteins in barley leaves. Bot Acta. 1994;107(333):341. [Google Scholar]
- He WJ, Liu WY. Both N- and C-terminal regions are essential for cinnamomin A-chain to deadenylate ribosomal RNA and supercoiled double-strand DNA. Biochem J. 2004;377:17–23. doi: 10.1042/BJ20030703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudak KA, Bauman JD, Tumer NE. Pokeweed antiviral protein binds to the cap structure of eukaryotic mRNA and depurinates the mRNA downstream of the cap. RNA. 2002;8:1148–1159. doi: 10.1017/s1355838202026638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudak KA, Parikh BA, Di R, Baricevic M, Santana M, Seskar M, Tumer NE. Generation of pokeweed antiviral protein mutations in Saccharomycescerevisiae: evidence that ribosome depurination is not sufficient for cytotoxicity. Nucleic Acids Res. 2004;32:4244–4256. doi: 10.1093/nar/gkh757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudak KA, Wang P, Tumer NE. A novel mechanism for inhibition of translation by pokeweed antiviral protein: depurination of the capped RNA template. RNA. 2000;6:369–380. doi: 10.1017/s1355838200991337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias R, Citores L, Ragucci S, Russo R, Di Maro A, Ferreras JM. Biological and antipathogenic activities of ribosome-inactivating proteins from Phytolaccadioica L. Biochim Biophys Acta. 2016;1860:1256–1264. doi: 10.1016/j.bbagen.2016.03.011. [DOI] [PubMed] [Google Scholar]
- Iglesias R, Perez Y, Torre C, Ferreras JM, Antolin P, Jimenez P, Rojo MA, Mendez E, Girbes T. Molecular characterization and systemic induction of single-chain ribosome-inactivating proteins (RIPs) in sugar beet (Betavulgaris) leaves. J Exp Bot. 2005;56:1675–1684. doi: 10.1093/jxb/eri164. [DOI] [PubMed] [Google Scholar]
- Irvin JD. Pokeweed antiviral protein. Pharm Ther. 1983;21:371–387. doi: 10.1016/0163-7258(83)90061-x. [DOI] [PubMed] [Google Scholar]
- Irvin JD. Antiviral proteins from phytolacca. In: Chessin M, De Borde D, Zipf A, editors. Antiviral Proteins from higher plants. Boca Raton: CRC Press; 1995. pp. 65–69. [Google Scholar]
- Irvin JD. Purification and partial characterization of the antiviral protein from Phytolaccaamericana which inhibits eukaryotic protein synthesis. Arch Biochem Biophys. 1975;169:522–528. doi: 10.1016/0003-9861(75)90195-2. [DOI] [PubMed] [Google Scholar]
- Irvin JD, Kelly T, Robertus JD. Purification and properties of a second antiviral protein from Phytolaccaamericana which inactivates eukaryotic ribosomes. Arch Biochem Biophy. 1980;200:418–425. doi: 10.1016/0003-9861(80)90372-0. [DOI] [PubMed] [Google Scholar]
- Kawade K, Ishizaki T, Masud K. Differential expression of ribosome-inactivating protein genes during somatic embryogenesis in spinach (Spinaciaoleracea) Physiol Plant. 2008;134:270–281. doi: 10.1111/j.1399-3054.2008.01129.x. [DOI] [PubMed] [Google Scholar]
- Kataoka J, Habuka N, Furuno M, Miyano M, Takanami Y, Koiwai A. DNA sequence of Mirabilis antiviral protein (MAP) a ribosome-inactivating protein with an antiviral property from Mirabilisjalapa L. its expression in Escherichiacoli. J Biol Chem. 1991;266(13):8426–8430. [PubMed] [Google Scholar]
- Kim JK, Jang IC, Wu R, Zuo WN, Boston RS, Lee YH, Ahn IP, Nahm BH. Co-expression of a modified maize ribosome-inactivating protein a rice basic chitinase gene in transgenic rice plants confers enhanced resistance to sheath blight. Transgenic Res. 2003;12:475–484. doi: 10.1023/a:1024276127001. [DOI] [PubMed] [Google Scholar]
- Kumar D, Hridya N, Verma NT, Krishna KT. Cloning and characterization of a gene encoding an antiviral protein from Clerodendrumacculeatum L. Plant Mol Biol. 1997;33:745–751. doi: 10.1023/a:1005716103632. [DOI] [PubMed] [Google Scholar]
- Kumon K, Sasaki J, Sejima M, Takeuchi Y, Hayashi Y. Interactions between TMV, pokeweed antiviral protein, and tobacco cell wall. Phytopathology. 1990;80:636–641. [Google Scholar]
- Kwon YS, An CS, Liu JR, Peak K. A ribosome inactivating protein from Amaranthusviridis. Biosci Biotechnol Biochem. 1997;61:1613–1614. doi: 10.1271/bbb.61.1613. [DOI] [PubMed] [Google Scholar]
- Kwon YS, Chung SA, Jang RL, Sang SK, Haeng SL, Jeong KK, Kyung HP. Molecular cloning of a cDNA encoding ribosome-inactivating protein from Amaranthusviridis and its expression in E.coli. Mol Cells. 2000;10:8–12. doi: 10.1007/s10059-000-0008-6. [DOI] [PubMed] [Google Scholar]
- Legname G, Gromo G, Lord JM, Monzini N, Modena D. Expression and activity of pre-dianthin 30 and dianthin 30. Biochem Biophys Res Commun. 1993;192(3):1230–1237. doi: 10.1006/bbrc.1993.1548. [DOI] [PubMed] [Google Scholar]
- Li MX, Yeung HW, Pan LP, Chan SI. Trichosanthin a potent HIV-1 inhibitor can cleave supercoiled DNA in vitro. Nucleic Acid Res. 1991;19:6309–6312. doi: 10.1093/nar/19.22.6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling J, Liu W, Wang TP. Cleavage of supercoiled double-stranded DNA by several ribosome-inactivating proteins in vitro. FEBS Lett. 1994;345:143–146. doi: 10.1016/0014-5793(94)00421-8. [DOI] [PubMed] [Google Scholar]
- Lodge K, Kaniewaski WK, Tumer NE. Broad-spectrum virus resistance in transgenic plants expression pokeweed antiviral protein. Proc Natl Acad Sci USA. 1993;90:7089–7093. doi: 10.1073/pnas.90.15.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodha ML, Choudhary N, Mahapatro GK, Singh B, Gupta GP. Purification and evaluation of antiviral proteins from Bougainvilleaxbuttiana against Helicoverpaarmigera. Indian J Agric Sci. 2011;81:74–78. [Google Scholar]
- Logemann J, Jach G, Tommerup H, Mundy J, Schell J. Expression of a barley ribosome-inactivating protein leads to increased fungal protection in transgenic tobacco plants. Nat Biotechnol. 1992;10:305–308. [Google Scholar]
- Lomonossoff GP. Pathogen-derived resistance to plant viruses. Annu Rev Phytopathol. 2015;33:323–343. doi: 10.1146/annurev.py.33.090195.001543. [DOI] [PubMed] [Google Scholar]
- Masayuki F, Takeshi K, Satoshi-TO TO. Purification and partial characterization of figaren and RNase-like novel anti-viral protein from Cucumisfigaren. J Gen Plant Pathol. 2001;67:152–158. [Google Scholar]
- May KL, Yan Q, Tumer NE. Targeting ricin to the ribosome. Toxicon. 2013;69:143–151. doi: 10.1016/j.toxicon.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y, Lin S, Liu SF, Fan X, Li GR, Meng YF. A novel method for simultaneous production of two ribosome-inactivating proteins a-MMC and MAP30 from Momordicacharantia L. PLoS One. 2014;9:e101998. doi: 10.1371/journal.pone.0101998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra V, Sharma RS, Yadav S, Babu CR, Singh TP. Purification and characterization of four isoforms of Himalayan mistletoe ribosome-inactivating protein from Viscumalbum having unique sugar affinity. Arch Biochem Biophy. 2004;423:288–301. doi: 10.1016/j.abb.2003.12.033. [DOI] [PubMed] [Google Scholar]
- Mock JW, Ng TB, Wong RN, Yao QZ, Yeung HW, Fong WP. Demonstration of ribonuclease activity in the plant ribosome-inactivating proteins alpha-and beta-Momorcharins. Life Sci. 1996;59:1853–1859. doi: 10.1016/s0024-3205(96)00532-2. [DOI] [PubMed] [Google Scholar]
- Monzingo AF, Collins EJ, Ernst SR, Irvin JD, Robertus JD. The 25 A structure of pokeweed antiviral protein. J Mol Biol. 1993;233:705–715. doi: 10.1006/jmbi.1993.1547. [DOI] [PubMed] [Google Scholar]
- Narayanan S, Surendranath K, Bora N, Surolia A, Kare AA. Ribosome inactivating proteins and apoptosis. FEBS Lett. 2005;579:1324–1331. doi: 10.1016/j.febslet.2005.01.038. [DOI] [PubMed] [Google Scholar]
- Narwal S, Balasubrahmanyam A, Lodha ML, Kapoor HC. Purification and properties of antiviral proteins from the leaves of Bougainvilleaxbuttiana. Indian J Biochem Biophys. 2001;38:342–347. [PubMed] [Google Scholar]
- Narwal S, Balasubrahmanyam A, Sadhna P, Kapoor HC, Lodha ML. A systemic resistance inducing antiviral protein with N-glycosidase activity from Bougainvilleaxbuttiana leaves. Indian J Exp Biol. 2001;39:600–603. [PubMed] [Google Scholar]
- Nielsen K, Boston RS. Ribosome-inactivating proteins: a plant perspective. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:785–816. doi: 10.1146/annurev.arplant.52.1.785. [DOI] [PubMed] [Google Scholar]
- Noronha B, Gil VL, Vincente M. Occurrence of plant virus inhibitors in species of Caryophyllales I. Alternaanthera ficoidea, Amaranthusdeflexus, Bougainvilleaspectabilis, Chenopodiumambrosoides and Mirabilisjalapa. Arg Inst Biol Sacpaulo. 1980;47:71–76. [Google Scholar]
- Olivieri F, Prasad V, Valbonesi P, Srivastava S, Chowdhury GP, Barbieri L, Bolognesi A, Stire F. A systemic antiviral resistance-inducing protein isolated from ClerodendruminermeGaertn is a polynucleotide: adenosine glycosidase (ribosome-inactivating proteins) FEBS Lett. 1996;396:132–134. doi: 10.1016/0014-5793(96)01089-7. [DOI] [PubMed] [Google Scholar]
- Parikh BA, Coetzer C, Tumer NE. Pokeweed antiviral protein regulates the stability of its own mRNA by a mechanism that requires depurination but can be separated from depurination of the α-Sarcin/Ricin loop of rRNA. J Biol Chem. 2002;277:41428–41437. doi: 10.1074/jbc.M205463200. [DOI] [PubMed] [Google Scholar]
- Park SW, Kim JY, Lee JK, Hwang I, Cheong H, Nah JW. Antifungal mechanism of a novel antifungal protein from pumpkin rinds against various fungal pathogens. J Agric Food Chem. 2009;57:9299–9304. doi: 10.1021/jf902005g. [DOI] [PubMed] [Google Scholar]
- Park SW, Vepachedu R, Owens RA, Vivanco JM. The N-glycosidase activity of the ribosome-inactivating protein ME1 target single-stranded region of nucleic acids independent of sequence or structural motifis. J Biol Chem. 2004;279:34165–34174. doi: 10.1074/jbc.M400105200. [DOI] [PubMed] [Google Scholar]
- Park JS, Hwang DJ, Lee SM, Kim YT, Choi SB, Cho KJ. Ribosome-inactivating activity and cDNA cloning of antiviral protein isoforms of Chenopodiumalbum. Mol Cells. 2004;17:73–80. [PubMed] [Google Scholar]
- Picard D, Kao CC, Hudak KA. Pokeweed antiviral protein inhibits brome mosaic virus replication in plant cells. J Biol Chem. 2005;280:20069–20075. doi: 10.1074/jbc.M413452200. [DOI] [PubMed] [Google Scholar]
- Polito L, Bortolotti M, Battelli MG, Calafato G, Bolognesi A. Ricin: an ancient story for a timeless plant toxin. Toxins (Basel) 2019;11:324–340. doi: 10.3390/toxins11060324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyet JL, Hoeveler A. Presence of an intron in a gene of PAP II the ribosome-inactivating protein from summer leaves of Phytolaccaamericana. Ann Bot. 1997;80:685–688. [Google Scholar]
- Prasad V, Mishra SK, Sivastava S, Srivastava A. A virus inhibitory protein isolated from Cyamopsistetragonoloba (L.) Taub upon induction of systemic antiviral resistance shares partial amino acid sequence homology with a lectin. Plant Cell Rep. 2014;33:1467–1478. doi: 10.1007/s00299-014-1630-7. [DOI] [PubMed] [Google Scholar]
- Prestle J, Schonfelder M, Adam G, Mundry KW. Type-I ribosome-inactivating proteins depurinate plant 25S rRNA without species specificity. Nucleic Acids Res. 1992;20:3179–3182. doi: 10.1093/nar/20.12.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przydacz M, Jones R, Pennington HG, Belmans G, Bruderer M, Greenhill R, Salter T, Wellham PAD, Cota E, Spanu PD. Mode of action of the catalytic site in the N-terminal ribosome-inactivating domain of JIP60. Plant Physiol. 2020;183:385–398. doi: 10.1104/pp.19.01029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri M, Kaur I, Kanwar RK, Gupta RC, Chauhan A, Kanwar JR. Ribosome inactivating proteins (RIPs) from Momordicacharantia for antiviral therapy. Curr Mol Med. 2009;9:1080–1094. doi: 10.2174/156652409789839071. [DOI] [PubMed] [Google Scholar]
- Puri M, Kaur I, Perugini MA, Gupta RC. Ribosome-inactivating proteins: current status and biomedical applications. Drug Discov Today. 2012;17:774–783. doi: 10.1016/j.drudis.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Qian Q, Huang L, Yi R, Wang SZ, Ding Y. Enhanced resistance to blast fungus in rice (Oryzasativa L.) by expressing the ribosome-inactivating protein alpha-momorcharin. Plant Sci. 2014;217–218:1–7. doi: 10.1016/j.plantsci.2013.11.012. [DOI] [PubMed] [Google Scholar]
- Rajamohan F, Kurinov IV, Venkatachalam JK, Ukun FN. Deguanylation of human immuno deficiency virus (HIV)-1 RNA by recombinant pokeweed antiviral protein. Biochem Biophys Res Commun. 1999;263:419–424. doi: 10.1006/bbrc.1999.1335. [DOI] [PubMed] [Google Scholar]
- Rajamohan F, Venkatachalam TK, Irvin JD, Uckun FN. Pokeweed antiviral protein isoforms PAPI PAPII and PAPIII depurinate RNA of human deficiency virus (HIV)-1. Biochem Biophys Res Commun. 1999;260:453–458. doi: 10.1006/bbrc.1999.0922. [DOI] [PubMed] [Google Scholar]
- Rajesh S, Balasaraswathi R, Doraisamy S, Sadasivam S. Synthesis and cloning of cDNA encoding an antiviral protein from the leaves of Bougainvilleaspectabilis Willd (Nyctaginaceae) World J Agri Sci. 2005;1:101–104. [Google Scholar]
- Ready MP, Brown DT, Robertus JD. Extracellular localization of pokeweed antiviral protein. Proc Natl Acad Sci USA. 1986;83:5053–5056. doi: 10.1073/pnas.83.14.5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinbothe S, Reinbothe C, Lehmann J, Becker W, Apel K, Partheir B. JIP60 a methyl jasmonates-induced ribosome inactivating protein involved in plant stress reactions. Proc Natl Acad Sci USA. 1994;91:7012–7016. doi: 10.1073/pnas.91.15.7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Sadhana P, Begum M, Kumar S, Lodha ML, Kapoor HC. Purification, characterization and cloning of antiviral/ribosome inactivating protein from Amaranthustricolor leaves. Phytochemistry. 2006;67:1865–1873. doi: 10.1016/j.phytochem.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Rumiyati NAW, Sismindari-Lukitaningsih E, Yuliati T. Potential of ribosome-inactivating proteins (RIPs) of Mirabilisjalapa L as an antiacne: effect on proliferation of cultured sebocyte cells its antibacterial activities against Propionibacteriumacnes and Staphylococcusepidermidis. Int J Pharm Chem. 2014;4:130–133. [Google Scholar]
- Sargolzaei M, Ho CL, Wong MY. Characterization of novel type I ribosome-inactivating proteins isolated from oil palm (Elaeisguineensis) inoculated with Ganodermaboninense the causal agent of basal stem rot. Physiol Mol Plant Pathol. 2016;94:53–61. [Google Scholar]
- Shang C, Rougé P, VanDamme EJM. Ribosome inactivating proteins from rosacea. Molecules. 2016;21:1105. doi: 10.3390/molecules21081105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidi-Noghabi S, VanDamme EJM, Mahdian K, Smagghe G. Entomotoxic action of Sambucusnigra agglutinin I in Acyrthosiphonpisum aphids, Spodopteraexigua caterpillars through caspase-3 like dependent apoptosis. Arch Insect Biochem Physiol. 2010;75:207–220. doi: 10.1002/arch.20387. [DOI] [PubMed] [Google Scholar]
- Shahidi-Noghabi S, VanDamme EJM, Smagghe G. Carbohydrate-binding activity of the type-2 ribosome-inactivating protein SNA-I from elderberry (Sambucusnigra) is a determining factor for its insecticidal activity. Phytochemistry. 2008;69:2972–2978. doi: 10.1016/j.phytochem.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Shahidi-Noghabi S, VanDamme EJM, Smagghe G. Expression of Sambucusnigra agglutinin (SNA-I') from elderberry bark in transgenic tobacco plants results in enhanced resistance to different insect species. Transgenic Res. 2009;18:249–259. doi: 10.1007/s11248-008-9215-2. [DOI] [PubMed] [Google Scholar]
- Sharma N, Park SW, Vepachedu R, Barbieri L, Ciani M, Stirpe F, Savary BJ, Vivanco M. Isolation and characterization of an RIP (ribosome-inactivating protein)-like protein from Tobacco with dual enzymatic activity. Plant Physiol. 2004;134:171–181. doi: 10.1104/pp.103.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi WW, Mak AN, Wong KB, Shaw PC. Structures and ribosomal interaction of ribosome-inactivating proteins. Molecules. 2016;21:1588. doi: 10.3390/molecules21111588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikriwal D, Batra JK. Ribosome inactivating proteins and apoptosis. In: Lord JM, Hartley MR, editors. Plant cell monographs: toxic plant proteins. Berlin: Springer; 2010. pp. 107–132. [Google Scholar]
- Singh S. A review-structure-function studies of abrin: a ribosome inactivation toxin. FASEB J. 2018;32:40. [Google Scholar]
- Sipahioglu HM, Kaya I, Usta M, Unal M, Ozcan D, Dilmen MO, Guller A, Pallas V. Pokeweed (Phytolaccaamericana L.) antiviral protein inhibits Zucchini yellow mosaic virus infection in a dose-dependent manner in squash plants. Turk J Agric For. 2017;41:256–262. [Google Scholar]
- Srivastava S, Verma HN, Srivastava A, Prasad V. BDP-30 a systemic resistance inducer from Boerhaaviadiffusa L. suppresses TMV infection and displays homology with ribosome-inactivating proteins. J Biosci. 2015;40:125–135. doi: 10.1007/s12038-014-9494-0. [DOI] [PubMed] [Google Scholar]
- Straub P, Adam G, Mundry KW. Isolation and characterization of a virus inhibitor from spinach (Spinaciaoleracea L.) J Phytopathol. 1986;115:357–367. [Google Scholar]
- Stripe F, Williams DG, Onyon LJ, Legg RF. Dianthins ribosome-damaging proteins with antiviral properties from Dianthuscaryophyllus L. (carnation) Biochem J. 1981;195:399–405. doi: 10.1042/bj1950399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas TMG, Yeung HW, Fong WP. Deoxyribonucleolytic activity of α and β-momorcharins. Life Sci. 1992;51:1347–1353. doi: 10.1016/0024-3205(92)90634-2. [DOI] [PubMed] [Google Scholar]
- Torky ZA. Isolation and characterization of antiviral protein from Salsolalongifolia leaves expressing polynucleotide adenosine glycoside activity. Online J Sci Technol. 2012;2:52–58. [Google Scholar]
- Tumer NE. Introduction to the toxins special issue on plant toxins. Toxins (Basel) 2015;7:4503–4506. doi: 10.3390/toxins7114503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumer NE, Hwang D, Bonness M. C-terminal deletion mutant of pokeweed antiviral protein inhibits viral infection but does not depurinate host ribosomes. Proc Natl Acad Sci USA. 1997;94:3866–3871. doi: 10.1073/pnas.94.8.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umamaheswari RS, Nuni A. In vitro antibacterial activity of Bougainvilleaspectabilis leaves extracts. Adv Biol Res. 2008;2:01–05. [Google Scholar]
- VanDamme EJM, Hao Q, Chen Y, Barre A, Venbussche F, Desmyter S, Rouge P, Peumans WJ. Ribosome-inactivating proteins: a family of plant proteins that do more than inactivate ribosomes. Crit Rev Plant Sci. 2001;20:395–465. [Google Scholar]
- Venbussche F, Desmyter S, Ciani M, Proost P, Peumans WJ, VanDamme EJM. Analysis of the in planta antiviral activity of elderberry ribosome-inactivating proteins. Eur J Biochem. 2004;271:1508–1515. doi: 10.1111/j.1432-1033.2004.04059.x. [DOI] [PubMed] [Google Scholar]
- Vepachedu R, Bais HP, Vivanco JM. Molecular characterization post-transcriptional regulation of ME1 a type-I ribosome-inactivating protein from Mirabilisexpansa. Planta. 2003;217(3):498–506. doi: 10.1007/s00425-003-1014-7. [DOI] [PubMed] [Google Scholar]
- Verma HN, Awasthi LP. Occurrence of highly antiviral agent in plants treated with Boerhaaviadiffusa inhibitor. Can J Bot. 1980;58:2141–2144. [Google Scholar]
- Verma HN, Baranwal VK. Antiviral activity and the physical properties of the leaf extract of Chenopodiumambrosoides. Proc Indian Acad Sci (Plant Sci) 1983;92:461–465. [Google Scholar]
- Verma HN, Varsha BVK. Agricultural role of endogenous antiviral substances of plant origin. In: Chessin M, DeBorde D, Zipf A, editors. Antiviral proteins in higher plants. Boca Raton: CRC Press; 1995. pp. 23–37. [Google Scholar]
- Vivanco JM, Tumer NE. Translation inhibition of capped and uncapped viral RNAs mediated by ribosome-inactivating proteins. Phytopathology. 2003;93:588–595. doi: 10.1094/PHYTO.2003.93.5.588. [DOI] [PubMed] [Google Scholar]
- Vivanco JM, Savary BJ, Flores HE. Characterization of two novel type I ribosome-inactivating proteins from the storage roots of the Andean crop Mirabilisexpansa. Plant Physiol. 1999;119:1447–1456. doi: 10.1104/pp.119.4.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Hudak KA. A novel interaction of pokeweed antiviral protein with translation initiation factors 4G and iso4G: a potential indirect mechanism to access viral RNAs. Nucleic Acids Res. 2006;34:1174–1181. doi: 10.1093/nar/gkj520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Tumer NE. Pokeweed antiviral protein cleaves double stranded supercoiled DNA using the same active site required to depurinate rRNA. Nucl Acids Res. 1999;27:1900–1905. doi: 10.1093/nar/27.8.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Zubenko O, Tumer NE. Reduced toxicity and broad-spectrum resistance to viral and fungal infection in transgenic plants expressing pokeweed antiviral protein II. Plant Mol Biol. 1998;38:957–964. doi: 10.1023/a:1006084925016. [DOI] [PubMed] [Google Scholar]
- Wang S, Zhang H, Zheng Y, Li Z, Xiang F, Ding Y, Xiang J. Environmental factors and phytohormones enhancing expression of a-momorcharin gene in Momordicacharantia. Biologia. 2016;71:155–160. [Google Scholar]
- Wu TH, Chow LP, Lin JY. Sechiumin a ribosome-inactivating protein from the edible gourd Sechiumedule Swartz–purification characterization molecular cloning and expression. Eur J Biochem. 1998;255:400–408. doi: 10.1046/j.1432-1327.1998.2550400.x. [DOI] [PubMed] [Google Scholar]
- Yang T, Meng Y, Chen LJ, Lin HH, Xi DH. The roles of alphamomorcharin and jasmonic acid in modulating the response of Momordicacharantia to Cucumbermosaic virus. Front Microbiol. 2016;7:1796. doi: 10.3389/fmicb.2016.01796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F, Zhou YK, Ji ZL, Chen XR. The plant ribosome-inactivating proteins play important roles in defense against pathogens and insect pest attacks. Front Plant Sci. 2018;9:146. doi: 10.3389/fpls.2018.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F, Yuan S, Zhang ZW, Qian K, Feng JG, Yang YZ. Pokeweed antiviral protein (PAP) increases plant systemic resistance to Tobacco mosaic virus infection in Nicotianabenthamiana. Eur J Plant Pathol. 2016;146:541–549. [Google Scholar]
- Zhu F, Zhang P, Meng YF, Xu F, Zhang DW, Cheng J, Lin HH, Xi DH. Alpha momorcharin a RIP produced by bitter melon enhances defense response in tobacco plants against diverse plant viruses and shows antifungal activity in vitro. Planta. 2013;237:77–88. doi: 10.1007/s00425-012-1746-3. [DOI] [PubMed] [Google Scholar]
- Zoubenko O, Hudak K, Timer NE. A non-toxic pokeweed antiviral protein mutant inhibits pathogen infection via a novel salicylic acid-independent pathway. Plant Mol Biol. 2000;44:219–229. doi: 10.1023/a:1006443626864. [DOI] [PubMed] [Google Scholar]
- Zoubenko O, Uckun F, Hur Y, Chet I, Tumer N. Plant resistance to fungal infection induced by nontoxic pokeweed antiviral protein mutants. Nat Biotechnol. 1997;15:992–996. doi: 10.1038/nbt1097-992. [DOI] [PubMed] [Google Scholar]