Abstract
Purpose
Tubulin beta eight class VIII (TUBB8) is essential for oogenesis, fertilization, and pre-implantation embryo development in human. Although TUBB8 mutations were recently discovered in meiosis-arrested oocytes of infertile females, there is no effective therapy for this gene mutation caused infertility. Our study aims to further reveal the infertility-causing gene mutations in the patient’s family and to explore whether the infertility could be rescued by optimizing the conditions of embryo culture and finally achieve the purpose of making the patient pregnant.
Methods
Whole-exome sequence analysis and Sanger sequencing were performed on patients’ family members to screen and identify candidate mutant genes. Construction of plasmids, in vitro transcription, microinjection of disease-causing gene cRNA, and immunofluorescence staining were used to recapitulate the infertility phenotype observed in patients and to understand the pathogenic principles. Simultaneously, overexpression of mutant and wild-type cRNA of the candidate gene in mouse oocytes at either germinal vesicle (GV) or metaphase II (MII) stage was performed in the rescue experiment.
Results
We first identified a novel heritable TUBB8 mutation (c.1041C>A: p.N347K) in the coding region which specifically affects the first mitosis and causes the developmental arrest of early embryos in a three-generation family. We further demonstrated that TUBB8 mutation could lead to abnormal spindle assemble. And moreover, additional expression of wild-type TUBB8 cRNA in the mouse oocytes in which the mutant TUBB8 were expressed can successfully rescue the developmental defects of resulting embryo and produce full-term offspring.
Conclusions
Our study not only defines a novel mutation of TUBB8 causing the early cleavage arrest of embryos, but also provides an important basis for treating such female infertility in the future.
Electronic supplementary material
The online version of this article (10.1007/s10815-020-01945-w) contains supplementary material, which is available to authorized users.
Keywords: TUBB8, Mutation, Embryo development arrest, Infertility, Rescue
Introduction
Successful reproduction requires proper oocyte maturation for subsequent fertilization and early embryo development [1, 2]. Oocyte maturation requires two meiotic divisions. During meiosis, bipolar meiotic spindles form and homologous chromosomes align at the metaphase I plate via microtubule-organizing centers (MTOCs) [3–5]. Following spindle assembly and during the extrusion of the first polar body, oocytes extrude half of their genetic material and then proceed directly to metaphase II (MII); oocytes then arrest at MII until fertilization [6, 7]. In humans, spindle assembly and chromosome separation are especially error-prone and lead to numerous aneuploid events and even oocyte maturation arrest [8, 9]. In mice, some studies have identified genes that cause the meiosis spindle dysfunction and oocyte maturation arrest phenotype; however, the genetic etiology of human oocyte aneuploid is still largely unknown.
Tubulin plays an important role in spindle assembly and chromosome separation. The tubulin gene has many mutations in humans and mammals that affect tubule dynamics and spindle function. TUBB8, which is a special β-tubulin isotype, plays an important role in human oocytes [10]. At present, TUBB8 has only been found in primates [10, 11]. Many studies have reported that different mutations and structural deletions in TUBB8 are associated with broad-spectrum failures in oocyte maturation, fertilization, or early embryonic development, most of which appear to be barriers to oocyte maturation [10–17]. According to previous reports, mutations in TUBB8 account for approximately 30% of the individuals with MI oocyte arrest [11, 15, 18, 19]. These observations suggest that different mutations might lead to different structural abnormalities and might alter TUBB8 interactions with kinesin or TUBB8 binding to other microtubule-associated proteins [14, 20]. Although multiple TUBB8 mutations were found in infertile patients, efficient treatment has not been reported.
In our study, we identified a novel TUBB8 mutation (c.1041C>A: p.N347K) in three generations of a family that specifically affects mitosis and causes arrest during early embryonic development. Animal experiments show that mutant TUBB8 mainly affects the mitotic ability of embryos after fertilization. We also carried out a rescue experiment by injecting wild-type TUBB8 cRNA into mouse oocytes in which the mutant TUBB8 was expressed, and we acquired the mouse offspring. These results not only define a novel mutation of TUBB8 that causes arrest at the early cleavage stage after fertilization but also provide a basis for the treatment of patients with infertility caused by a TUBB8 mutation.
Methods
Human subject
All oocytes were collected from a patient who was referred from the reproductive medicine center of Shanghai First Maternity and Infant Hospital affiliated with Tongji University. Thirty-nine oocytes were obtained from the patient throughout five IVF/ICSI cycles. All human subject procedures were approved by the Shanghai First Maternity and Infant Hospital Institutional Medical Review Board. The study was approved by the Reproductive Study Ethics Committee of Shanghai First Maternity and Infant Hospital affiliated with Tongji University.
Mice
All specific pathogen-free (SPF) mice were housed in the animal facility of Tongji University. The animal study procedures were consistent with the Laboratory Animal Care Guidelines. B6D2F1 (C57BL/6 × DBA2) female mice that were 7–8 weeks of age were used as oocyte donors. Nine- to 15-week-old ICR female mice were used as recipients for embryo transplantation.
Exome sequencing and variant screening
WES sequencing and analysis protocol refers to the previous study [21]. All genomic DNA was extracted from peripheral blood from the patient and the patient’s family members using a TIANamp Genomic DNA Kit (Tiangen, Beijing, China). The whole exomes of the patient (Fig. 1c III-17), the patient’s parents (Fig. 1c II-3, II-4), and one unaffected aunt (Fig. 1c, II-9) were sequenced using Agilent SureSelect Whole Exome sequencing and Illumina sequencing technology. The variants were considered based on the following principles: the gene is expressed in oocytes and the variant frequency is less than 0.001 (http://gnomad.broadinstitute.org/); in the meanwhile, the mutation of the gene was not detected in the patient’s mother and fertile aunt (Supplementary Table 2).
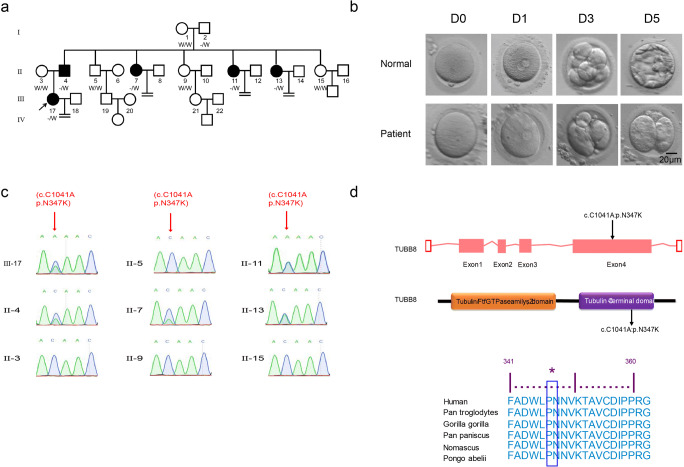
Fig. 1.
Identification of TUBB8 mutation in the family of the infertile patient. a Pedigree of the family. Squares indicate male family members, circles indicate female family members, solid symbols indicate affected members, open symbols indicate unaffected family members, and equal signs denote no offspring. b Clinical characteristics of oocytes retrieved from the infertile patient (III-17). The oocytes retrieved from the patient at 24 h, and most of the embryos were arrested following IVF after 72 h. c Sanger sequence analysis in the coding region of TUBB8 in family members is implicated with a heterozygous missense mutation (c.1041 C>A: p.N347K) (II-4, II-7, II-11, II-13, III-17). d The missense mutation in TUBB8 causes an amino acid substitution of the encoded protein, and that amino acid is conserved across multiple species
Sanger sequencing
Exon 4 of the TUBB8 gene contained a candidate mutation that was examined in patients and immediate family members by Sanger sequencing. The primer sequences used for screening are shown in Supplementary Table 1. PCR amplification was performed using Q5 High-Fidelity Polymerase (5×, NEB, MA, USA). The PCR products were sequenced for the initial screening by GENEWIZ Biological Technology Limited Corporation. More than five individual clones containing the suspected mutated exons in a pLB vector were sequenced to confirm the mutation.
Expression constructs of mutant and wild-type TUBB8 with fluorescent protein
To investigate whether the identified TUBB8 mutation (c.1041C>A: p.N347K) affected spindle formation, the human wild-type and mutant TUBB8 cDNA were cloned into the eukaryotic expression vector pcDNA3.1, which contains yellow fluorescent protein (YFP). The fluorescent tag was fused to the C-terminus of the wild-type or mutant TUBB8; the amplification and point-mutation primer sequences are shown in Supplementary Table 1.
Oocyte culture and microinjection
GV oocytes were isolated from female mice 48 h after PMSG injection, and then, the oocytes were incubated in MEM (Millipore, Billerica, MA) supplemented with IBMX (3-isobutyl-1-methylxanthine) at 37 °C before microinjection. Wild-type and mutant TUBB8 cRNAs were synthesized with an mMESSAGE T7 Ultra Kit (Ambion, Thermo Fisher, USA) according to the manufacturer’s instructions. The storage concentration of each cRNA was 1000 ng/μL. Oocytes or zygotes were microinjected with approximately 10 pL of cRNA using a Piezo-driven micromanipulator (Eppendorf). Surviving oocytes or zygotes were cultured (37 °C/5% CO2). The zygotes were cultured in CZB containing 0.05 μg/mL demecolcine (DC) 28–32 h post-hCG injection. Zygotes arrested in mitosis were collected and washed three times with drops of CZB and then transferred into CZB containing 2.5 μM MG-132 (Sigma) for 25 min. Manipulations were performed in a chamber containing oil-covered HCZB supplemented with 5 μg/mL cytochalasin B (CB) (Sigma) and 2 μM MG-132. Spindles were arrested at metaphase.
In vitro fertilization of wild-type and mutant mouse oocytes
Female BDF1 mice were super-ovulated by injecting 5 IU of pregnant mare serum gonadotropin (PMSG), which was followed by injection of 5 IU of human chorionic gonadotropin (hCG) (San-Sheng, China) after 48 h. The cumulus oocyte complexes were released from the oviducts at 14 h. Sperm were obtained from the caudal epididymis of 10-week-old BDF1 male mice. Briefly, cauda was cut several times with clippers and transferred into HTF medium in 5% CO2. Sperm were passively released into the culture medium through slits in the base of the cauda. Sperm were capacitated for 0.5 h before IVF. The oocytes treated with TUBB8 cRNA or untreated were placed in the sperm suspension for 4–6 h and then cultured in CZB medium.
Immunofluorescent staining
About 30–50 GV oocytes or zygotes per group were collected, and about 10–20 embryos per group at the middle stage with spindle formation used for staining each time. Briefly, oocytes or zygotes were fixed with 4% paraformaldehyde and then permeabilized with 0.2% Triton X-100 for 15 min at room temperature. The primary antibody used for spindle identification was α-tubulin (1:500, Sigma), and the secondary antibody was AlexaFluor 594 (1:500, Sigma). The chromosomes were stained with DAPI (1:500, Merck). We set the line scanned between two poles of spindle as the diameter. For the measurement, we observed the shape of the spindle under laser confocal fluorescence microscopy, and we used the microinjection needle to adjust the position of oocytes or zygotes so that the two centroids of the spindle were on the same plate, and then, the diameter of the spindle could be considered to be at the longest cross section. All stained samples were imaged using an LSM880 Confocal Laser Scanning Microscope (Zeiss, Germany). Three-time repeated experiments were performed.
Image analysis
Measurement and analysis of spindle lengths were processed by ZEN imaging software (blue edition). The spindle length at the largest section was chosen and the maximum diameter was measured. The spindle length ratio of mutant group/wild-type group was used for analysis.
Statistical analysis
The data were shown as mean ± the standard error of the mean or n (%) unless otherwise stated. Single comparisons were performed with Student’s t test or one-way analysis of variance (ANOVA) for treatment effect. Differences were considered statistically significant at P < 0.05.
Results
Clinical description
The infertile patient (Fig. 1a III-17) received a diagnosis of primary infertility at 38 years of age after 10 years of cohabitation with her partner. Infertility-related examinations revealed abnormal development of embryos. The patient had five aunts; three of them were sterile (Fig. 1a II-7, II-11, II-13), and the other two were fertile (Fig. 1a II-9, II-15). The patient underwent two failed IVF attempts in another hospital. At our hospital, 39 oocyte cumulus complexes (COCs) were obtained from the patient from five IVF/ICSI cycles; 37 of the eggs matured on the first day, and 20 oocytes were fertilized normally. However, only 15 zygotes cleaved, and all of them arrested at an early embryonic stage (Fig. 1b).
Mutation of TUBB8
Whole-exome sequence analysis of the four family members (Fig. 1a II-3, II-4, II-9, III-17) implicated a heterozygous missense mutation (c.1041C>A: p.N347K) in the coding region of TUBB8 (Supplementary Table 2). Sanger sequencing further confirmed that the patient (Fig. 1c III-17) carried a heterozygous missense mutation (c.1041C>A: p.N347K) in the coding region of TUBB8 in exon 4. This mutation was transmitted from her father. The father (Fig. 1c II-4) and her aunts who were infertile (Fig. 1c II-7, II-11, II-13) showed a heterozygous mutation in TUBB8 that was in full accord with the patient. The patient’s mother (Fig. 1c II-3) and fertile aunts (Fig. 1c II-9, II-15) carried a normal TUBB8 gene. The analysis of the reading frame of the gene suggests that the missense mutation in TUBB8 causes an amino acid substitution of the encoded protein, in which the 347th aspartic acid was replaced by lysine, resulting in an abnormal tubulin structure (Fig. 1d). According to the analysis of the conserved domains of the NCBI database, the 347th aspartic acid of the TUBB8 protein is highly conserved in six primate species (Fig. 1d).
The mutation of TUBB8 affects mitosis during early embryonic development
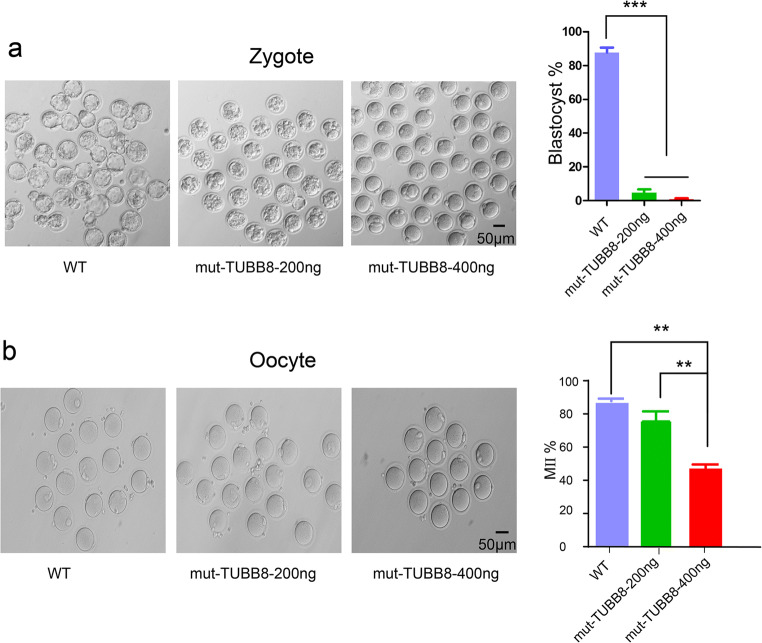
It is worth noting that the patient had a high proportion of PB1 oocytes and a high proportion of normal fertilization, which is different from previous reports of mutations in TUBB8. We first examined the effect of the mutant TUBB8 on fertilization and cleavage. The wild-type and mutant TUBB8 cRNA were transcribed in vitro and microinjected into the mouse MII oocytes. After in vitro fertilization, we observed that the MII oocytes injected with wild-type TUBB8 cRNA underwent normal fertilization and entered mitotic metaphase. However, the development of zygotes injected with the mutant TUBB8 was obviously decreased. We found that a low concentration (200 μg/mL) of mutant TUBB8 cRNA resulted in most embryos being blocked in the 4–8 cell stage. In addition, almost of oocytes injected with a high concentration (400 μg/mL) of mutant TUBB8 cRNA were arrested at the first mitotic metaphase (Fig. 2a). Next, we studied the effects of the mutant TUBB8 on oocyte meiosis. We microinjected wild-type and mutated TUBB8 cRNA into mouse GV oocytes. The oocytes microinjected with wild-type TUBB8 cRNA showed normal maturation approximately 16 h after in vitro maturation. The GV oocytes that were microinjected with a low concentration (200 μg/mL) of mutant TUBB8 cRNAs had little effect on the extrusion of the first polar body. Nevertheless, the development rate of oocytes microinjected with a high concentration (400 μg/mL) mutant TUBB8 cRNAs was relatively decreased (Fig. 2b). This suggests that compared with mitosis, the mutant TUBB8 cRNAs has little effect on oocyte maturation.
Fig. 2.
The mutant TUBB8 affects early embryonic development. a Few MII oocytes which injection with mutant TUBB8 cRNA could develop to zygotes. b The maturation rate of GV oocytes microinjected with wild-type or mutant TUBB8 cRNA. The data are represented as the mean ± SEM. *p < 0.05, **P < 0.01, ***p < 0.001
TUBB8 mutation leads to spindle malformation
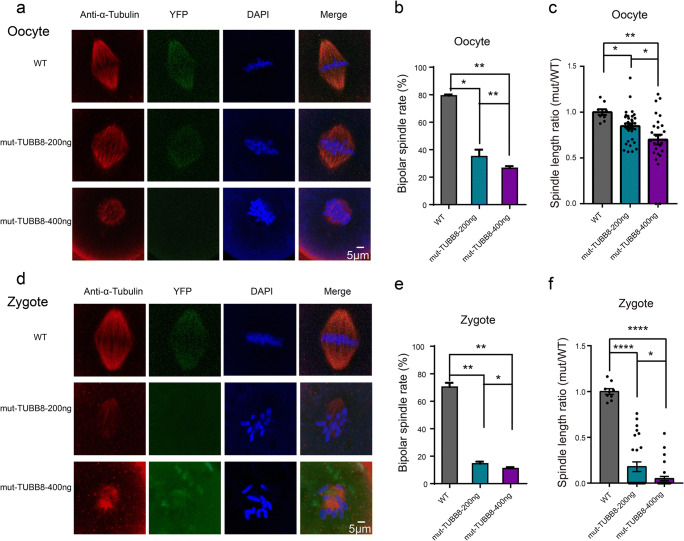
To establish the causal relationship between the mutation in TUBB8 and the disruption of meiosis and mitosis, we assessed the ability of the TUBB8 mutation to interfere with spindle microtubule dynamics. We set the spindle length ratio (abbreviated as S) which was the ratio of spindle length of oocyte/zygote injected with mutant TUBB8 cRNA to oocytes/zygotes injected with wild-type TUBB8 cRNA to assess the effects of mutant TUBB8 on spindle assembly. As observed, most of the mouse GV oocytes microinjected with wild-type TUBB8 cRNA developed to MII stage and had normal bipolar spindles (Fig. 3a). In contrast, 36% of the oocytes microinjected with a low concentration (200 μg/mL) of mutant TUBB8 cRNA could form bipolar spindles with lengths similar to normal spindle lengths (S = 0.8476 ± 0.03036, n = 33), whereas only 27% of the high concentration mutant treatment group could form spindles, and the spindles were shorter than normal spindles (S = 0.7006 ± 0.05276, n = 28) (Fig. 3a–c). Interestingly, zygotes expressing mutant TUBB8 resulted in severely impaired spindle assembly, regardless of whether the concentration was low (200 μg/mL) (S = 0.1793 ± 0.0521, n = 27) or high (400 μg/mL) (S = 0.04977 ± 0.02314, n = 32) TUBB8 cRNA injection, almost all the fertilized zygotes failed to form a normal bipolar spindle (Fig. 3d–f). This suggests that the mutant TUBB8 cRNA had more serious negative effects on the fertilized eggs, which could not form bipolar spindles, and the chromosomes were disorganized. Thus, these results suggest that the meiosis spindle assembly has stronger fault tolerance than mitosis. This model provides a unique perspective for studying the mechanism of spindle assembly and chromosome separation in meiosis and mitosis.
Fig. 3.
The mutant TUBB8 leads to spindle malformation. a Representative images of spindles from GV oocytes treated in different concentration mutant TUBB8 cRNA. b Bipolar spindle rates of oocytes from WT, mut-TUBB8–200 ng group, and mut-TUBB8-400 ng group (WT = 79.2 ± 0.9, mut-TUBB8-200 ng = 35 ± 5, mut-TUBB8-400 ng = 26.5 ± 1.5, n = 3). c Spindle length ratio of each group, the value of WT was set at an arbitrary value = 1 (mut-TUBB8-200 ng, S = 0.8476 ± 0.03036, n = 33, mut-TUBB8-400 ng, S = 0.7006 ± 0.05276, n = 28, experiments repeated for 3 times). d Representative images of spindles from zygotes treated in different concentration mutant TUBB8 cRNA. e Bipolar spindle rates of oocytes from WT, mut-TUBB8-200 ng group, and mut-TUBB8-400 ng group (WT = 76.5 ± 1.2, mut-TUBB8-200 ng = 14.5 ± 1.5, mut-TUBB8-400 ng = 11 ± 1, n = 3). f Spindle length ratio of each group, the value of WT was set at an arbitrary value = 1 (WT, S = 1 ± 0.03162, n = 13, mut-TUBB8-200 ng, S = 0.1793 ± 0.0521, n = 27, mut-TUBB8-400 ng, S = 0.04977 ± 0.02314, n = 32, experiments repeated for 3 times). The data are represented as the mean ± SEM. **P < 0.01, ***p < 0.001, ****P < 0.0001. Scale bar = 5 μm
Rescue experiment in mouse oocytes
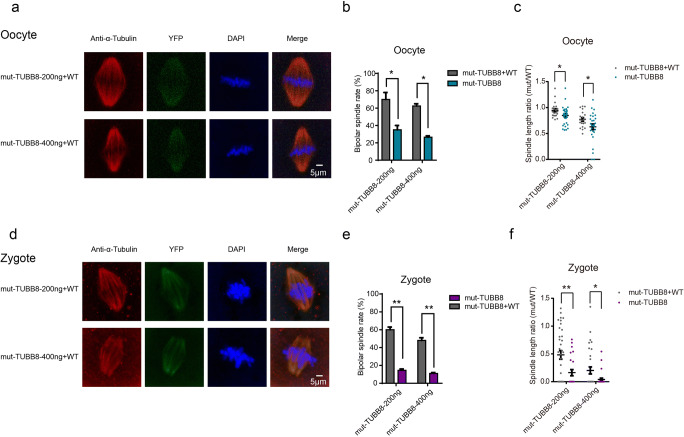
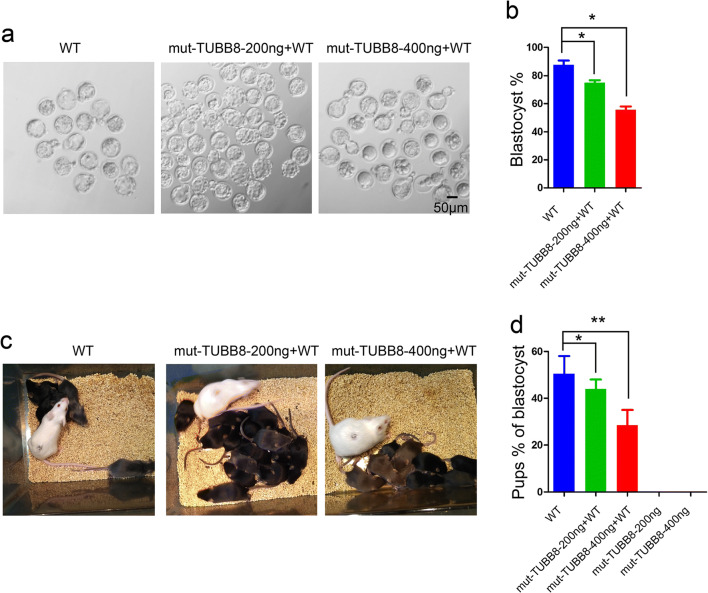
Recently, almost 30 various mutations of the TUBB8 gene were identified as responsible for arrest in oocyte maturation, fertilization, or early embryonic development [13]. Previous studies mainly focused on the missense types of mutations, but there is no effective potential treatment of the disease. To investigate whether the mutant oocytes can be successfully rescued by additional expression of wild-type TUBB8 cRNA, we co-injected mutant TUBB8 cRNA (200 or 400 μg/mL) with 400 μg/mL of wild-type TUBB8 cRNA into mouse MII oocytes followed by IVF. The similar expression levels of the mutant and wild-type TUBB8 cRNA are confirmed by fluorescence intensity (Supplementary Fig. 1). We first investigated the length ratio and formation rate of spindles of oocytes and zygotes which co-injected mutant and wild-type TUBB8 cRNA. We found that 70% of the oocytes co-injected with a low concentration (200 μg/mL) of the mutant and wild-type TUBB8 cRNA (400 μg/mL) could form bipolar spindles with lengths similar to normal spindle lengths (S = 0.9462 ± 0.03152, n = 21), and 62.5% of the high concentration mutant group (400 μg/mL) could form spindles with relatively increased length (S = 0.7712 ± 0.0333, n = 21) (Fig. 4a–c). To our surprise, 60% of the zygotes co-injected with a low concentration (200 μg/mL) mutant and 400 μg/mL wild-type TUBB8 cRNA could form bipolar spindles that were half of the length of normal spindles (S = 0.4822 ± 0.07717, n = 29). Spindle length in the high concentration (400 μg/mL) mutant and wild-type TUBB8 cRNA group approached 20% of normal spindles (S = 0.2019 ± 0.06314, n = 32) (Fig. 4d–f). This finding suggests that supplementation with exogenous wild-type TUBB8 could effectively improve spindle assembly abnormalities caused by mutant TUBB8. We further investigated whether additional expression of wild-type TUBB8 cRNA could improve embryo development in vitro and in vivo. The results showed that supplementing wild-type TUBB8 into the oocytes that were injected with the low or high concentration of mutant TUBB8 could significantly improve the blastocyst rate (Fig. 5a, b). More importantly, those embryos ultimately produced full-term offspring after transplantation into recipients (Fig. 5c, d). These results suggest that the dose-dependent effects of mutant TUBB8 that caused early embryo developmental arrest could be rescued by additional expression of wild-type TUBB8 cRNA.
Fig. 4.
Overexpression of wild-type TUBB8 cRNA improves impaired spindle assembly. a–c Immunostaining, bipolar spindle formation rates, and spindle length in mouse oocytes at 14 h after germinal vesicle breakdown (GVBD). GV oocytes were injected with mutant or co-injected with mutant and wild-type TUBB8 cRNA. d–f Immunostaining, bipolar spindle formation rates, and spindle length of mouse zygotes at 14 h after fertilization; analysis followed injection with mutant or co-injection with mutant and wild-type TUBB8 cRNA. The data are represented as the mean ± SEM, *p < 0.05, **P < 0.01. Scale bar = 5 μm
Fig. 5.
Rescue experiments in mouse oocytes. a, b The development of MII oocytes supplemented with a low concentration (200 μg/mL) or a high concentration (400 μg/mL) of mutant TUBB8 and wild-type TUBB8 could develop into blastocysts in vitro. c, d Following injection of the wild-type cRNA, treated embryos were transplanted into recipients that ultimately delivered healthy pups. The data are represented as the mean ± SEM, *p < 0.05, **P < 0.01
Discussion
In this study, we revealed a novel heterozygous mutation (c.1041C>A: p.N347K) in TUBB8 that is responsible for mitosis division defects and causes early embryonic development arrest. This mutation specifically interferes with mitotic spindle assembly and chromosome arrangement, which does not block oocyte maturation and fertilization but does cause early embryonic development arrest. Finally, we found that the introduction of wild-type TUBB8 cRNA into mutant TUBB8-expressing oocytes can successfully rescue the developmental defects of the resulting embryo and produce full-term offspring.
Previous studies confirm that a high proportion of defects in oocyte maturation in infertile women are caused by mutants of TUBB8 [10]. The present study reported almost 30 different mutations in TUBB8, most of which were heterozygous mutations. Pathogenic missense TUBB8 mutations influence microtubule dynamics and impair oocyte meiotic spindle assembly via dominant-negative effects [19]. In our study, the p.N347K mutation in TUBB8 specifically interferes with mitosis and leads to early embryonic development arrest which is different from the phenotype previously reported. This indicates that the high genetic variability and phenotypic diversity of TUBB8. In the clinic, many patients exhibit atypical oocyte maturation, which is characterized by having only a few oocytes arrested at MI. The rest of the oocytes were morphologically recognizable as MII oocytes and could be fertilized and even cleaved, but these embryos later become developmentally arrested. This could be one of the underlying factors for the high proportion of TUBB8 mutations in infertile women. Given that fathers carrying the TUBB8 mutation were fertile, it is possible that the prevalence of fertile men with heterozygous TUBB8 mutations could be underestimated. Thus, TUBB8 mutation screening might not only be a genetic diagnostic marker for patients with oocyte maturation arrest but might also have clinical implications for patients with embryos repeatedly blocked in the early stage of cleavage [22].
Oocyte maturation and subsequent mitosis are critical periods for the normal development of an embryo. Aneuploidy resulting from abnormal spindle assembly is an important cause of early embryonic arrest. In our study, we identified a new mutation in TUBB8 that does not affect oocyte maturation and fertilization but does influence mitosis of the fertilized egg. Oocyte-injected mutant TUBB8 showed that mitosis was more susceptible to the influence of spindle assembly than meiosis. The latest research found that two bipolar spindles form in the zygote and then independently assemble the maternal and paternal genomes in mice and humans. This spindle assembly mechanism provides a potential rationale for zygote spindles being sensitive to microtubule gene mutation and reveals differences between the mechanisms of mitosis and meiosis during oocyte and zygotic transformation. This TUBB8 mutation may provide a unique perspective on the differences between meiotic and mitotic spindle assembly in the maternal-to-zygotic transition in mammals.
Although multiple TUBB8 mutations were found in infertile women, there are currently no effective treatments available. Previous studies have reported that in recessive genetic diseases such as WEE2 mutations, injection of WEE2 cRNA into oocytes can result in normal blastocysts, but whether these embryos have the ability to develop into individuals is unknown [23]. We found that in vivo experiments where oocytes expressed mutant TUBB8, co-injection with wild-type TUBB8 cRNA could improve spindle assembly. More importantly, those embryos can support preimplantation development and produce live offspring. It is worth noting that although the regulatory mechanisms in oocytes and embryo division are similar, centrioles do not involve in mouse zygotes and mouse early embryo cleavage mode is different from that of human. Further studies in terms of safety and efficacy still need to be performed, but our present results suggest an efficient clinical method for these affected individuals with mutations in TUBB8.
Electronic supplementary material
(DOC 92 kb)
Funding
This project was supported by the National Natural Science Foundation of China (81630035, 31721003, 31601177, 31501197, 31501183, 81671521, and 31430056), the Ministry of Science and Technology of China (Grants 2016YFA0100400, 2015CB964800, 2018YFC1003102, 2014CB964601, 2016YFC1000600, and 2015CB964503), the Shanghai Natural Science Foundation (Grant 16ZR1427200), the Western Medicine Guidance Project of Shanghai Science and Technology Commission (1741196), the Youth Innovation Promotion Association of Chinese Academy of Sciences [2020104 to C.Z.], the Shanghai Shenkang Clinical Innovation Three-year Action Plan Project (16CR3069B).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Shanghai First Maternity and Infant Hospital of Tongji University.
Consent to participate
All subjects participating in this study signed their informed consents.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yanping Jia, Kunming Li, Caihong Zheng and Yuanyuan Tang contributed equally to this work.
Contributor Information
Wenqiang Liu, Email: liuwenqiang@51mch.com.
Xiaoming Teng, Email: tengxiaoming@51mch.com.
Shaorong Gao, Email: gaoshaorong@tongji.edu.cn.
References
- 1.Edwards RG, Bavister BD, Steptoe PC. Early stages of fertilization in vitro of human oocytes matured in vitro. Nature. 1969;221(5181):632–635. doi: 10.1038/221632a0. [DOI] [PubMed] [Google Scholar]
- 2.Li R, Albertini DF. The road to maturation: somatic cell interaction and self-organization of the mammalian oocyte. Nat Rev Mol Cell Biol. 2013;14(3):141–152. doi: 10.1038/nrm3531. [DOI] [PubMed] [Google Scholar]
- 3.Kimura N, Hoshino Y, Totsukawa K, Sato E. Cellular and molecular events during oocyte maturation in mammals: molecules of cumulus-oocyte complex matrix and signalling pathways regulating meiotic progression. Society of Reproduction and Fertility supplement. 2007;63:327–342. [PubMed] [Google Scholar]
- 4.Vogt E, Kirsch-Volders M, Parry J, Eichenlaub-Ritter U. Spindle formation, chromosome segregation and the spindle checkpoint in mammalian oocytes and susceptibility to meiotic error. Mutat Res. 2008;651(1–2):14–29. doi: 10.1016/j.mrgentox.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 5.Coticchio G, Dal Canto M, Mignini Renzini M, Guglielmo MC, Brambillasca F, Turchi D, Novara PV, Fadini R. Oocyte maturation: gamete-somatic cells interactions, meiotic resumption, cytoskeletal dynamics and cytoplasmic reorganization. Hum Reprod Update. 2015;21(4):427–454. doi: 10.1093/humupd/dmv011. [DOI] [PubMed] [Google Scholar]
- 6.Albertini DF. Regulation of meiotic maturation in the mammalian oocyte - interplay between exogenous cues and the microtubule cytoskeleton. Bioessays. 1992;14(2):97–103. doi: 10.1002/bies.950140205. [DOI] [PubMed] [Google Scholar]
- 7.Mehlmann LM. Stops and starts in mammalian oocytes: recent advances in understanding the regulation of meiotic arrest and oocyte maturation. Reproduction. 2005;130(6):791–799. doi: 10.1530/rep.1.00793. [DOI] [PubMed] [Google Scholar]
- 8.Pellestor F, Anahory T, Andreo B, Hedon B, Hamamah S. Maternal aging and aneuploidy: lessons from human oocytes. Fertil Steril. 2004;82:S281–S2S2. doi: 10.1016/j.fertnstert.2004.03.050. [DOI] [Google Scholar]
- 9.Mullen TJ, Davis-Roca AC, Wignall SM. Spindle assembly and chromosome dynamics during oocyte meiosis. Curr Opin Cell Biol. 2019;60:53–59. doi: 10.1016/j.ceb.2019.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng R, Yan Z, Li B, Yu M, Sang Q, Tian G, Xu Y, Chen B, Qu R, Sun Z, Sun X, Jin L, He L, Kuang Y, Cowan NJ, Wang L. Mutations in TUBB8 cause a multiplicity of phenotypes in human oocytes and early embryos. J Med Genet. 2016;53(10):662–671. doi: 10.1136/jmedgenet-2016-103891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng RZ, Sang Q, Kuang YP, Sun XX, Yan Z, Zhang SZ, Shi J, Tian G, Luchniak A, Fukuda Y, Li B, Yu M, Chen J, Xu Y, Guo L, Qu R, Wang X, Sun Z, Liu M, Shi H, Wang H, Feng Y, Shao R, Chai R, Li Q, Xing Q, Zhang R, Nogales E, Jin L, He L, Gupta ML, Jr, Cowan NJ, Wang L. Mutations in TUBB8 and human oocyte meiotic arrest. New Engl J Med. 2016;374(3):223–232. doi: 10.1056/NEJMoa1510791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen B, Li B, Li D, Yan Z, Mao X, Xu Y, Mu J, Li Q, Jin L, He L, Kuang Y, Sang Q, Wang L. Novel mutations and structural deletions in TUBB8: expanding mutational and phenotypic spectrum of patients with arrest in oocyte maturation, fertilization or early embryonic development. Hum Reprod. 2017;32(2):457–464. doi: 10.1093/humrep/dew322. [DOI] [PubMed] [Google Scholar]
- 13.Chen B, Wang W, Peng X, Jiang H, Zhang S, Li D, Li B, Fu J, Kuang Y, Sun X, Wang X, Zhang Z, Wu L, Zhou Z, Lyu Q, Yan Z, Mao X, Xu Y, Mu J, Li Q, Jin L, He L, Sang Q, Wang L. The comprehensive mutational and phenotypic spectrum of TUBB8 in female infertility. Eur J Hum Genet. 2019;27(2):300–307. doi: 10.1038/s41431-018-0283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan P, Zheng LY, Liang H, Li Y, Zhao HJ, Li RQ, Lai L, Zhang Q, Wang W. A novel mutation in the TUBB8 gene is associated with complete cleavage failure in fertilized eggs. J Assist Reprod Gen. 2018;35(7):1349–1356. doi: 10.1007/s10815-018-1188-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang L, Tong X, Luo L, Zheng S, Jin R, Fu Y, Zhou G, Li D, Liu Y. Mutation analysis of the TUBB8 gene in nine infertile women with oocyte maturation arrest. Reprod BioMed Online. 2017;35(3):305–310. doi: 10.1016/j.rbmo.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 16.Xiang J, Wang W, Qian C, Xue J, Wang T, Li H, Li H. Human oocyte maturation arrest caused by a novel missense mutation in TUBB8. J Int Med Res. 2018;46(9):3759–3764. doi: 10.1177/0300060518778638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang AC, Zhang YS, Wang BS, Zhao XY, Wu FX, Zhai XH, Sun JX, Mei SY. Mutation analysis of the TUBB8 gene in primary infertile women with arrest in oocyte maturation. Gynecol Endocrinol. 2018;34(10):900–904. doi: 10.1080/09513590.2018.1464138. [DOI] [PubMed] [Google Scholar]
- 18.Chen BB, Zhang ZH, Sun XX, Kuang YP, Mao XY, Wang XQ, Yan Z, Li B, Xu Y, Yu M, Fu J, Mu J, Zhou Z, Li Q, Jin L, He L, Sang Q, Wang L. Biallelic mutations in PATL2 cause female infertility characterized by oocyte maturation arrest. Am J Hum Genet. 2017;101(4):609–615. doi: 10.1016/j.ajhg.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiang JJ, Wang W, Qian CF, Xue JY, Wang T, Li HB, Li H. Human oocyte maturation arrest caused by a novel missense mutation in TUBB8. J Int Med Res. 2018;46(9):3759–3764. doi: 10.1177/0300060518778638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang A-C, Zhang Y-S, Wang B-S, Zhao X-Y, Wu F-X, Zhai X-H, Sun JX, Mei SY. Mutation analysis of the TUBB8 gene in primary infertile women with arrest in oocyte maturation. Gynecol Endocrinol. 2018;34(10):900–904. doi: 10.1080/09513590.2018.1464138. [DOI] [PubMed] [Google Scholar]
- 21.Liu W, He X, Yang S, Zouari R, Wang J, Wu H, Kherraf ZE, Liu C, Coutton C, Zhao R, Tang D, Tang S, Lv M, Fang Y, Li W, Li H, Zhao J, Wang X, Zhao S, Zhang J, Arnoult C, Jin L, Zhang Z, Ray PF, Cao Y, Zhang F. Bi-allelic mutations in TTC21A induce asthenoteratospermia in humans and mice. Am J Hum Genet. 2019;104(4):738–748. doi: 10.1016/j.ajhg.2019.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen BB, Wang WJ, Peng XD, Jiang HF, Zhang SZ, Li D, Li B, Fu J, Kuang Y, Sun X, Wang X, Zhang Z, Wu L, Zhou Z, Lyu Q, Yan Z, Mao X, Xu Y, Mu J, Li Q, Jin L, He L, Sang Q, Wang L. The comprehensive mutational and phenotypic spectrum of TUBB8 in female infertility. Eur J Hum Genet. 2019;27(2):300–307. doi: 10.1038/s41431-018-0283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sang Q, Li B, Kuang Y, Wang X, Zhang Z, Chen B, Wu L, Lyu Q, Fu Y, Yan Z, Mao X, Xu Y, Mu J, Li Q, Jin L, He L, Wang L. Homozygous mutations in WEE2 cause fertilization failure and female infertility. Am J Hum Genet. 2018;102(4):649–657. doi: 10.1016/j.ajhg.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 92 kb)