Essential elements like nitrogen and sulfur greatly affect rumen fermentation and metabolism in ruminants. However, little knowledge is available on the effects of sulfur on the rumen microbiota and plasma metabolome. The results of the present trial demonstrated that supplementing the steer ration with sodium sulfate markedly improved rumen fermentation, fiber digestibility, and metabolism of amino acids, purine derivatives, and vitamins through effects on the ruminal microbiome. The facts obtained from the present trial clarified the possible mechanisms of the positive effects of sulfur on rumen fermentation and nutrient utilization.
KEYWORDS: steers, cattle, metabolomics, microbiome, nutrient digestibility, rumen, sulfur
ABSTRACT
Six steers were used to study the effects of dietary supplementation with sodium sulfate (Na2SO4) on rumen fermentation, nutrient digestion, rumen microbiota, and plasma metabolites. The animals were fed a basal ration with Na2SO4 added at 0 g/day (sulfur [S] content of 0.115% dry matter [DM]), 20 g/day (S at 0.185% DM), or 40 g/day (S at 0.255% DM) in a replicate 3-by-3 Latin square design. The results indicated that supplementing with Na2SO4 increased the ruminal concentration of total volatile fatty acids, the molar proportions of acetate and butyrate, the ruminal concentrations of microbial protein, SO42−-S, and S2−-S, and the digestibility of fiber, while it decreased the molar proportion of propionate and the ruminal concentration of ammonia nitrogen. Supplementing with Na2SO4 increased the diversity and the richness of rumen microbiota and the relative abundances of the phylum Firmicutes and genera Ruminococcus 2, Rikenellaceae RC9 gut group, and Desulfovibrio, whereas it decreased the relative abundances of the phylum Bacteroidetes and genera Prevotella 1, Prevotellaceae UCG-001, and Treponema 2. Supplementing with Na2SO4 also increased the plasma concentrations of amino acids (l-arginine, l-methionine, l-cysteine, and l-lysine), purine derivatives (xanthine and hypoxanthine), vitamins (thiamine and biotin), and lipids (acetylcarnitine and l-carnitine). It was concluded that supplementing the steer ration with Na2SO4 was beneficial for improving the rumen fermentation, fiber digestibility, and nutrient metabolism through modulating the rumen microbial community.
IMPORTANCE Essential elements like nitrogen and sulfur greatly affect rumen fermentation and metabolism in ruminants. However, little knowledge is available on the effects of sulfur on the rumen microbiota and plasma metabolome. The results of the present trial demonstrated that supplementing the steer ration with sodium sulfate markedly improved rumen fermentation, fiber digestibility, and metabolism of amino acids, purine derivatives, and vitamins through effects on the ruminal microbiome. The facts obtained from the present trial clarified the possible mechanisms of the positive effects of sulfur on rumen fermentation and nutrient utilization.
INTRODUCTION
Sulfur (S) is the crucial part of some B vitamins and amino acid (AA) metabolism and has been recognized as an essential element for animals (1). S is closely related to nitrogen (N) metabolism in the rumen (2), and the suitable N/S ratio of the ration for beef cattle was suggested to be between 12:1 and 15:1 (3). A low dietary S concentration can lead to an imbalanced N/S ratio and results in an inability to synthesize adequate quantities of S-containing AA like cysteine (Cys) and methionine (Met) (4) and rumen microbial crude protein (MCP) (5), depresses the ruminal concentration of total volatile fatty acids (VFA) (6) and fiber digestion (4, 7), and negatively affects rumen functions and animal performance (8).
Corn silage and corn grains are typical feed ingredients of the ration for beef cattle and usually account for the larger part of steer rations in China (9). Because of the low S content and the imbalanced N/S ratio, corn-based rations for cattle are not able to meet the S requirement of 0.15% dry matter (DM) recommended by National Academies of Sciences, Engineering, and Medicine (1).
Ruminal bacteria use sulfide (S2−) derived from ruminal sulfate (SO42−)-reducing bacteria (SRB) for synthesizing S-containing compounds (10). McSweeney et al. (7) and McSweeney and Denman (11) reported the stimulating effects of dietary supplementation with S on anerobic fungi and Fibrobacter succinogenes in steers fed low-S diets. Anderson et al. (12) demonstrated the interaction between virally encoded auxiliary metabolic genes and ruminal S metabolism and found that the ruminal viruses could be involved in SO42− reduction by affecting Cys desulfurase, which may serve as a fitness factor during times of S limitation. However, little is known about the effects of S on the ruminal microbiome and nutrient utilization. It is hypothesized that supplementing the steer ration with S and balancing the dietary N/S ratio would improve the rumen fermentation and nutrient digestibility in steers through modulating the ruminal microbial community.
The objectives of the trial were to study the effects of supplementing the steer ration with sodium sulfate (Na2SO4) on rumen fermentation, nutrient digestion, plasma biochemical indices, and the ruminal microbial community and, further, to clarify the mechanisms of the effects through the changes in the rumen microbiota and the plasma metabolites.
RESULTS
Rumen fermentation.
The results presented in Table S3 in the supplemental material indicated that dietary supplementation with Na2SO4 did not affect the ruminal pH (P > 0.05), increased the ruminal concentration of total VFA (P = 0.005), the molar proportions of acetate (P = 0.013) and butyrate (P = 0.022), and the molar ratio of acetate to propionate (P = 0.005), and decreased the molar ratio of propionate (P = 0.019) in a linear manner. Dietary supplementation with Na2SO4 decreased the ruminal concentration of ammonia N (NH3-N) (P = 0.019), while it increased that of MCP (P = 0.017) in an opposite manner. Dietary supplementation with Na2SO4 tended to decrease the ruminal concentration of lactate linearly (P = 0.087). Dietary supplementation with Na2SO4 increased the ruminal concentrations of SO42−-S (P = 0.006) and S2−-S (P = 0.003), while it decreased (P < 0.001) the SO42−-S/S2−-S ratio in a linear manner.
Nutrient digestibility.
The results presented in Table S4 indicated that dietary supplementation with Na2SO4 tended to increase the apparent digestibility of DM (P = 0.054) and increased the apparent digestibility of neutral detergent fiber (NDF) (P = 0.023), acid detergent fiber (ADF) (P = 0.013), or S (P < 0.001) in a linear manner, whereas it did not affect the digestibility of organic matter (OM) or crude protein (CP) (P > 0.05).
Rumen bacterial communities. (i) Rumen bacterial diversity.
The amplicon sequencing of the rumen fluid samples generated a total of 928,868 high-quality sequences with an average of 51,607 ± 1,483 (mean ± standard error of the mean [SEM]) sequences per sample, and the average sequencing read length was 418 bp. High-quality reads were clustered into 2,462 microbial operational taxonomic units (OTUs) at 3% divergence, among which 2,005 OTUs were found in all groups and accounted for 81.4% of the total OTUs, indicating the presence of an extensive common microbiome (Fig. S1A). The numbers of exclusive OTUs from the three treatments, CON (0 g/day Na2SO4), S1 (20 g/day Na2SO4), and S2 (40 g/day Na2SO4), were 42, 49, and 58, respectively. The rarefaction curves are shown in Fig. S1B. All curves reached a plateau, indicating that the sequencing depth was sufficient and adding more OTUs would not affect the slope of the curve.
The alpha diversity indices of the ruminal bacterial community are in Table 1. Dietary supplementation with Na2SO4 increased the species richness indices of the Chao1 (P = 0.005), abundance-based coverage estimator (ACE) (P = 0.012), and Shannon (P = 0.003) and decreased the Simpson index (P = 0.018) linearly. The Good’s coverage of all groups was above 98.5%, indicating that the sequencing amount of each sample well reflected the diversity of the bacterial community types and structures.
TABLE 1.
Effects of dietary supplementation with Na2SO4 on the diversity of the rumen bacterial community in steers
| Alpha diversity index | Mean value fora
: |
SEM |
P value |
||||
|---|---|---|---|---|---|---|---|
| CON | S1 | S2 | Treatment | Linearb | Quadraticb | ||
| Richness | |||||||
| ACE | 1,171 b | 1,793 ab | 1,848 a | 27.583 | 0.017 | 0.012 | 0.735 |
| Chao1 | 1,720 b | 1,802 ab | 1,880 a | 29.211 | 0.010 | 0.005 | 0.968 |
| Diversity | |||||||
| Shannon | 5.64 c | 5.80 b | 5.95 a | 0.040 | 0.009 | 0.003 | 0.864 |
| Simpson | 0.012 a | 0.010 ab | 0.009 b | 0.0005 | 0.021 | 0.018 | 0.681 |
| Good’s coverage (%) | 98.58 | 98.54 | 98.51 | 0.019 | 0.147 | 0.192 | 0.922 |
Within a row, mean values without a common letter differ significantly (n = 6). CON, 0 g/day Na2SO4; S1, 20 g/day Na2SO4; S2, 40 g/day Na2SO4.
Orthogonal polynomial contrasts.
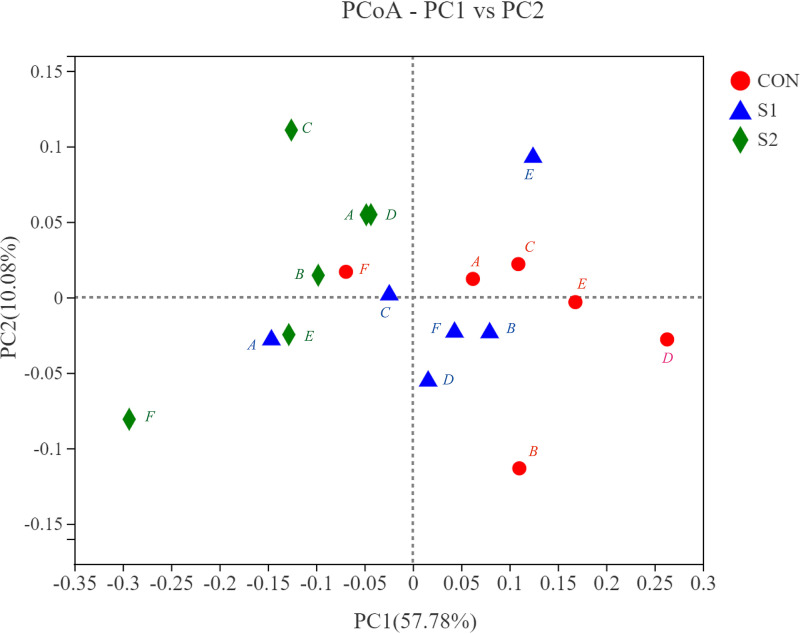
Principal-coordinate analysis (PCoA) based on the weighed UniFrac method was used to characterize the beta diversity of the rumen bacterial community (Fig. 1). The distances between different points indicated the degrees of similarity and variation of the samples, and the values for principal coordinate 1 (PC1) (57.78%) and PC2 (10.08%) represented the percentages of variation between samples. The results of the analysis of similarity (ANOSIM) showed that there was a significant difference between groups CON and S2 (P = 0.012).
FIG 1.
Principal-coordinate analysis (PCoA) based on weighted UniFrac distances of rumen bacterial communities in steers fed Na2SO4 at 0 g/day (CON), 20 g/day (S1), and 40 g/day (S2). Analysis of similarity (ANOSIM) results were as follows: CON versus S1, P = 0.072; CON versus S2, P = 0.012; and S1 versus S2, P = 0.140. A, B, C, D, E, and F are steers’ identifiers (n = 6).
(ii) Divergence of rumen bacterial communities.
At the phylum level, a total of 24 bacterial phyla were identified in the rumen fluid samples. Among these phyla, the average relative abundances of Bacteroidetes and Firmicutes were 55.6% ± 2.7% (mean ± SEM) and 35.5% ± 2.9%, respectively, higher than the abundances of other phyla (Fig. S2A). The results in Table 2 showed that dietary supplementation with Na2SO4 decreased the relative abundance of Bacteroidetes (q = 0.024) and increased that of Firmicutes (q = 0.031) in a linear manner and affected the relative abundance of Proteobacteria in a quadratic manner (q = 0.028).
TABLE 2.
Main microbiota that changed significantly among different treatmentsa
| Taxon | Mean abundance (%) in steers that receivedb
: |
SEM |
q valuec
|
||||
|---|---|---|---|---|---|---|---|
| CON | S1 | S2 | Treatment | Lineard | Quadraticd | ||
| Phyla | |||||||
| Bacteroidetes | 60.55 a | 57.80 a | 48.38 b | 2.741 | 0.028 | 0.024 | 0.304 |
| Firmicutes | 29.93 b | 33.88 ab | 42.67 a | 2.917 | 0.039 | 0.031 | 0.606 |
| Proteobacteria | 1.56 a | 0.83 b | 1.35 ab | 0.187 | 0.047 | 0.704 | 0.028 |
| Actinobacteria | 0.59 b | 1.06 ab | 1.12 a | 0.172 | 0.035 | 0.030 | 0.647 |
| Families | |||||||
| Prevotellaceae | 40.67 a | 33.60 ab | 23.48 b | 3.850 | 0.031 | 0.026 | 0.721 |
| Ruminococcaceae | 12.68 b | 14.73 ab | 19.76 a | 2.140 | 0.044 | 0.037 | 0.465 |
| Lachnospiraceae | 6.71 | 7.65 | 9.82 | 0.423 | 0.068 | 0.045 | 0.694 |
| Family XIII | 0.89 b | 1.03 b | 1.47 a | 0.134 | 0.025 | 0.018 | 0.552 |
| Succinivibrionaceae | 0.92 a | 0.32 b | 0.50 b | 0.111 | 0.022 | 0.044 | 0.154 |
| Genera | |||||||
| Prevotella 1 | 33.01 a | 26.14 ab | 18.87 b | 2.226 | 0.011 | 0.008 | 0.947 |
| Rikenellaceae RC9 gut group | 6.62 | 8.53 | 9.53 | 0.728 | 0.058 | 0.034 | 0.779 |
| Ruminococcus 2 | 1.84 b | 2.71 ab | 4.34 a | 0.413 | 0.039 | 0.028 | 0.659 |
| Prevotellaceae UCG-001 | 3.18 a | 2.51 ab | 1.77 b | 0.326 | 0.037 | 0.026 | 0.845 |
| Ruminococcaceae UCG-010 | 0.98 b | 1.17 ab | 1.44 a | 0.090 | 0.040 | 0.034 | 0.739 |
| norank f_Bacteroidales UCG-001 | 1.51 | 0.96 | 0.85 | 0.169 | 0.064 | 0.047 | 0.347 |
| Treponema 2 | 1.37 a | 0.96 ab | 0.75 b | 0.118 | 0.031 | 0.024 | 0.654 |
| Butyrivibrio 2 | 0.43 b | 0.66 ab | 0.74 a | 0.113 | 0.027 | 0.021 | 0.635 |
| Succinivibrionaceae UCG-002 | 0.66 a | 0.21 b | 0.36 b | 0.115 | 0.034 | 0.030 | 0.582 |
| Ruminococcaceae UCG-002 | 0.20 b | 0.49 a | 0.44 a | 0.094 | 0.026 | 0.013 | 0.412 |
| Pseudobutyrivibrio | 0.24 b | 0.25 b | 0.45 a | 0.091 | 0.021 | 0.035 | 0.524 |
Main microbiota are sequences summarized at phylum, family, and genus levels at an average abundance of >0.1% in at least one of the dietary treatments.
Within a row, means without a common letter differ significantly (n = 6). CON, 0 g/day Na2SO4; S1, 20 g/day Na2SO4; S2, 40 g/day Na2SO4.
q value, false discovery rate; q < 0.05 is considered significant.
Orthogonal polynomial contrasts.
At the family level, a total of 153 families were identified in the rumen fluid samples, of which Prevotellaceae (32.6% ± 3.8%), Ruminococcaceae (15.7% ± 2.7%), Rikenellaceae (8.8% ± 0.9%), and Lachnospiraceae (7.5% ± 0.9%) were the dominant families (Fig. S2B). Supplementing with Na2SO4 linearly decreased the relative abundances of Prevotellaceae (q = 0.026) and Succinivibrionaceae (q = 0.044), while it increased the relative abundances of Ruminococcaceae (q = 0.037), Lachnospiraceae (q = 0.045), and Family XIII (q = 0.018).
At the genus level, a total of 313 bacterial genera were identified in the rumen fluid samples, and 256 genera were present in all samples, which was indicative of the core microbiome in the present trial (Fig. S2C); many of these were unclassified genera and nonranked genera. Among these, Prevotella 1 (26.0% ± 2.2%), Rikenellaceae RC9 gut group (8.2% ± 0.5%), norank f F082 (6.65% ± 0.7%), Ruminococcaceae NK4A214 group (4.5% ± 0.4%), unclassified o Clostridiales (3.7% ± 0.3%), Christensenellaceae R-7 group (3.0% ± 0.3%), and Ruminococcus 2 (3.0% ± 0.4%) were considered the high-abundance taxa. Genera that were at levels higher than 0.1% of the total sequences were selected for further analysis. The results in Table 2 showed that dietary supplementation with Na2SO4 significantly affected the main microbiota at the genus level by linearly decreasing the relative abundances of Prevotella 1 (q = 0.008), Prevotellaceae UCG-001 (q = 0.026), norank f Bacteroidales UCG-001 (q = 0.047), Treponema 2 (q = 0.024), and Succinivibrionaceae UCG-002 (q = 0.030) and increasing those of Rikenellaceae RC9 gut group (q = 0.034), Ruminococcus 2 (q = 0.028), Ruminococcaceae UCG-010 (q = 0.034), Butyrivibrio 2 (q = 0.021), Ruminococcaceae UCG-002 (q = 0.013), and Pseudobutyrivibrio (q = 0.035).
The relative abundances of the main SRB and lactate metabolism bacteria are shown in Fig. 2. Dietary supplementation with Na2SO4 linearly increased the relative abundances of the genera Desulfovibrio (q = 0.018) and Desulfobulbus (q = 0.025) and decreased that of Selenomonas 1 (q = 0.038), whereas it did not affect the relative abundances of the genera Desulfuromonas, Lactobacillus, and Streptococcus (q > 0.05).
FIG 2.
Effects of supplementing Na2SO4 at 0 g/day (CON), 20 g/day (S1), and 40 g/day (S2) on the relative abundances of the main sulfate-reducing bacteria (SRB) (A, Desulfovibrio; B, Desulfobulbus; C, Desulfuromonas) and lactate metabolism bacteria (D, Streptococcus; E, Lactobacillus; F, Selenomonas 1) at the genus level. Boxes represent the interquartile range (IQR) between the 1st and 3rd quartiles (25th and 75th percentiles, respectively), and the horizontal line inside each box defines the median value. Whiskers represent the lowest and highest values within 1.5 times the IQR from the 1st and 3rd quartiles, respectively. Dashed lines connect the mean values of treatments. Boxes with different letters above their whiskers are significantly different (q < 0.05, n = 6) among treatments. q values in brackets indicate the treatment (T) and linear (L) effects; no quadratic effects were observed.
(iii) Biomarker taxa within the microbial community.
Linear discriminant analysis (LDA) effect size (LEfSe) was used to combine rank sum tests and taxonomic information to identify the biomarker taxa (logarithmic LDA score > 3.0) with the greatest impact on the structure of the community. The LDA histogram (Fig. 3) showed that there were 26 biomarkers, including 12 in group CON, 2 in group S1, and 13 in group S2.
FIG 3.
Linear discriminant analysis (LDA) effect size (LEfSe) (P < 0.05 by Kruskal-Wallis test; P < 0.05 by pairwise Wilcoxon test; logarithmic LDA score of >3.0) of the ruminal microbiota of steers fed Na2SO4 at 0 g/day (CON), 20 g/day (S1), or 40 g/day (S2). Taxonomic rank labels are provided before bacterial names; p_, c_, o_, f_, and g_ indicate phylum, class, order, family, and genus, respectively. The greater the LDA score of the biomarker taxon mean value, the greater the influence of species abundance on the difference in the microbial community in the different treatments.
(iv) Correlations between bacterial genera and rumen fermentation parameters.
The results shown in Fig. 4 indicated that both the ruminal concentration of total VFA and the molar proportion of acetate were positively correlated with the relative abundances of Rikenellaceae RC9 gut group (r = 0.52, q = 0.024) and Ruminococcus 2 (r = 0.63, q = 0.041) and negatively correlated with that of Prevotellaceae UCG-001 (r = 0.67, q = 0.034). The results shown in Fig. 4 also indicated that the molar proportion of propionate was positively correlated with the relative abundances of Prevotella 1 (r = 0.74, q = 0.028), Prevotellaceae UCG-001 (r = 0.54, q = 0.044), and norank f Bacteroidales UCG-001 (r = 0.51, q = 0.043), while it was negatively correlated with the relative abundances of Ruminococcus 2 (r = −0.73, q = 0.036), Ruminococcaceae NK4A214 group (r = −0.52, q = 0.037), and Christensenellaceae R-7 group (r = −0.54, q = 0.040). The molar proportion of valerate was positively correlated with the relative abundances of Succiniclasticum (r = 0.53, q = 0.038) and “Candidatus Saccharimonas” (r = 0.57, q = 0.029). The ruminal concentration of NH3-N was positively correlated with the relative abundances of Prevotella 1 (r = 0.72, q = 0.041) and Prevotellaceae UCG-001 (r = 0.58, q = 0.042), whereas it was negatively correlated with that of Ruminococcus 2 (r = −0.64, q = 0.045). The ruminal concentration of S2− was positively correlated with the relative abundance of Desulfovibrio (r = 0.60, q = 0.020).
FIG 4.
Spearman’s correlation matrix between the major classified genera (each having a relative abundance of ≥0.1% in at least one of the dietary treatments) and the rumen fermentation parameters. Only those with significant and strong correlation coefficients (|r| > 0.5, q < 0.05) are visualized. The degree of each correlation is shown by both the size of the bubble and the intensity of the color as shown in the color key on the right. Acetate, propionate, valerate, and isovalerate are presented as molar proportions relative to that of TVFA (total volatile fatty acids).
Plasma metabolic profiling. (i) Plasma conventional indices.
The results presented in Table S5 indicated that dietary supplementation with Na2SO4 did not affect the plasma concentration of globulin, growth hormone (GH), uric acid, creatinine, or hippuric acid (P > 0.05), whereas it increased the plasma concentrations of total protein (P = 0.031), albumin (P = 0.028), and insulin-like growth factor-1 (IGF-1) (P = 0.007), decreased the plasma concentration of urea (P = 0.010) in a linear manner, and tended to increase the plasma concentration of total AA (P = 0.057).
(ii) Plasma metabolome.
(a) Principal-component analysis (PCA) and orthogonal partial least squares discriminant analysis (OPLS-DA). In total, liquid chromatography-mass spectrometry (LC-MS) detected 766 valid peaks that were unique and nonoverlapping in the plasma samples. After strict quality control and identification, 640 compounds were obtained from all samples, mainly comprising lipids, lipid-like molecules, organic acids, derivatives, and organic oxygen compounds. Multivariate analysis was performed to explore the differences among treatments in the plasma metabolic profile.
The PCA score plot revealed that the variations of the first and the second principal components were 33.8% and 11.8%, respectively (Fig. S4). There was no clear distinction in plasma metabolites between CON and S1, while the principal-component analysis (PCA) indicated clear separations between CON and S2. For further analysis, orthogonal partial least squares discriminant analysis (OPLS-DA) was performed to investigate the differences among the treatments. The results presented in Fig. S4 indicated that the plasma metabolites of the intergroup were clearly distinguished from each other. All samples in the score plots were within 95% of the Hotelling’s T2 ellipse. The results of the permutation test of the three groups were in a reliable range, with the R2 values of the three groups all being >0.90, indicating a satisfactory effectiveness of the model, which can be used to identify the difference between two groups.
(b) Plasma metabolites. Combined with statistical analysis and the variable importance in projection (VIP) values obtained from the OPLS-DA analysis, 13 metabolites were identified as being different between groups S1 and CON, 29 between S2 and CON, and 22 between S2 and S1 (q < 0.05, fold change [FC] > 1.5 or < 0.67, and VIP > 1). The differential plasma metabolites, mainly organic acids, derivatives, lipids, and lipid-like molecules, were classified according to the properties of the compounds (Tables S6 to S8).
Compared with CON, supplementing Na2SO4 at 20 g/day (S1) increased the plasma relative concentrations of l-arginine (Arg) (q = 0.041), Met (q = 0.037), Cys (q = 0.025), S-adenosyl-l-methionine (q = 0.011), acetylcarnitine (q = 0.022), biotin (q = 0.047), the lysophosphatidylethanolamine lysoPE(0:0/18:1(11Z)) (q = 0.026), and xanthine (q = 0.040), whereas it decreased the plasma relative concentrations of l-pipecolic acid (q = 0.022), succinic acid (q = 0.009), azelaic acid (q = 0.024), lysoPE(0:0/22:0) (q = 0.019), and the lysophosphatidylcholine lysoPC(20:1(11Z)) (q = 0.033).
Compared with CON, supplementing Na2SO4 at 40 g/day (S2) increased the plasma relative concentrations of Arg (q = 0.018), Met (q = 0.010), Cys (q = 0.035), lysine (Lys) (q = 0.044), l-phenylalanine (Phe) (q = 0.047), taurine (q = 0.031), sulfate (q = 0.002), l-carnitine (q = 0.015), stearic acid (q = 0.034), lysoPC(16:0) (q = 0.012), lysoPE(15:0/0:0) (q = 0.027), xanthine (q = 0.024), hypoxanthine (q = 0.009), guanine (q = 0.015), thiamine pyrophosphate (q = 0.036), and biotin (q = 0.022), but S2 had lower relative concentrations of glycine (Gly) (q = 0.026), l-serine (Ser) (q = 0.047), succinic acid (q = 0.041), inositol phosphate (q = 0.031), pyruvate (q = 0.016), d-lactate (q = 0.024), palmitic acid (q = 0.008), the monoradylglycerol (MG) (P-18:0e/0:0/0:0) (q = 0.015), lysoPE(0:0/22:0) (q = 0.007), lysoPC(14:0/0:0) (q = 0.035), procurcumadiol (q = 0.019), atrolactic acid (q = 0.042), and indoleacetic acid (q = 0.039).
Compared with S1, supplementing Na2SO4 at 40 g/day (S2) increased the plasma relative concentrations of Arg (q = 0.038), Met (q = 0.021), Cys (q = 0.047), l-isoleucine (Ile) (q = 0.039), betaine (q = 0.022), sulfate (q = 0.022), stearic acid (q = 0.034), lysoPC(16:0) (q = 0.042), lysoPC(18:2(9Z,12Z)) (q = 0.033), xanthine (q = 0.024), uracil (q = 0.009), and thiamine pyrophosphate (q = 0.036), but S2 had lower relative concentrations of Gly (q = 0.032), Ser (q = 0.047), d-lactate (q = 0.038), glucosamine 6-sulfate (q = 0.041), valeric acid (q = 0.012), MG(P-18:0e/0:0/0:0) (q = 0.015), lysoPE(0:0/22:0) (q = 0.007), lysoPC(16:1(9Z)/0:0) (q = 0.044), lysoPC(20:0/0:0) (q = 0.036), and curdione (q = 0.029).
(c) Pathway analysis. Tables S3 and S4 show the P values and the impact values of pathway analysis in which the metabolites that were significantly different between two treatments. The results in Fig. 5A show that three main metabolic pathways of S1 were enriched versus CON, including “cysteine and methionine metabolism,” “arginine and proline metabolism,” and “biotin metabolism.” The results in Fig. 5B show that seven main metabolic pathways of S2 were enriched versus CON, including “aminoacyl-tRNA biosynthesis,” “thiamine metabolism,” “glycine, serine, and threonine metabolism,” “pyruvate metabolism,” “biotin metabolism,” “taurine and hypotaurine metabolism,” and “cysteine and methionine metabolism.” The results in Fig. 5C show that four main metabolic pathways of S2 were enriched versus S1, including “cysteine and methionine metabolism,” “aminoacyl-tRNA biosynthesis,” “glycine, serine, and threonine metabolism,” and “thiamine metabolism.”
FIG 5.
Overview of pathway analysis of the differential metabolites from the three treatments. (A) Steers fed Na2SO4 at 20 g/day (S1) versus 0 g/day Na2SO4 (CON). (B) Steers fed Na2SO4 at 40 g/day (S2) versus 0 g/day Na2SO4 (CON). (C) Steers fed Na2SO4 at 40 g/day (S2) versus Na2SO4 at 20 g/day (S1). The x axis represents a pathway impact value in the topology analysis, and larger bubbles represent higher pathway impact values. The y axis represents the P value (−lnP) of the metabolic pathway in the enrichment analysis, and darker bubbles represent higher levels of pathway enrichment.
(d) Correlations between bacterial genera and plasma metabolites. The results in Fig. 6 indicate that the plasma concentration of Met was positively correlated (r = 0.75, q = 0.022) with the relative abundance of Ruminococcus 2 and negatively correlated (r = −0.53, q = 0.041) with that of Treponema 2. The plasma concentrations of Cys (r = 0.61, q = 0.039), sulfate (r = 0.78, q = 0.012), and thiamine pyrophosphate (r = 0.58, q = 0.046) were all positively correlated with the relative abundance of Desulfovibrio. The plasma concentration of Ser was positively correlated with the relative abundance of Prevotellaceae UCG-001 (r = 0.54, q = 0.031), whereas it was negatively correlated with that of Treponema 2 (r = −0.57, q = 0.047). The plasma concentration of xanthine was positively correlated with the relative abundance of Ruminococcus 2 (r = 0.55, q = 0.029) and negatively correlated with those of Prevotellaceae UCG-001 (r = −0.56, q = 0.032) and Prevotella 1 (r = 0.70, q = 0.031). The plasma concentration of d-lactate was positively correlated with the relative abundance of Succinivibrionaceae UCG-002 (r = 0.61, q = 0.039) and negatively correlated with that of Butyrivibrio 2 (r = −0.58, q = 0.036).
FIG 6.
Spearman’s correlation matrix between major classified genera (each having a relative abundance of ≥0.1% in at least one of the dietary treatments) and the plasma metabolites. Only those with significant and strong correlation coefficients (|r| > 0.5, q < 0.05) are visualized. The degree of each correlation is shown by both the size of the bubble and the intensity of the color as shown by the color key on the right.
DISCUSSION
Ruminal microbiome. (i) Microbiota richness and diversity.
Gut microorganisms differ in their functionality and their ability to use the substrate resources in the digestive tract (13). High microbiota richness and diversity are considered to be beneficial to improve the stability of the microbiota and allow it to use resources more efficiently (14). The alpha diversity analysis indicated that supplementation with Na2SO4 increased the diversity and the richness of the rumen microbiota, suggesting improvement of microbiota activity and function in rumen fermentation. The beta diversity analysis indicated that supplementation with Na2SO4 affected the rumen bacterial composition, while the LEfSe analysis indicated that supplementation with Na2SO4 resulted in a marked shift in the ruminal microbiota. Results of the present trial indicated that supplementation with Na2SO4 impacted the phyla Bacteroidetes, Firmicutes, Proteobacteria, and Actinobacteria and families Prevotellaceae, Ruminococcaceae, and Lachnospiraceae. In the present trial, Bacteroidetes and Firmicutes were the predominant ruminal bacteria. The results were in agreement with previous reports in beef cattle (15, 16). It was reported that the members of Bacteroidetes were the major rumen microorganisms in degrading nonstructural carbohydrates and nonfibrous polysaccharides, while Firmicutes were the major rumen microorganisms in degrading structural carbohydrates (14).
The recommended and the maximum tolerable S concentrations in steer rations are 0.15% and 0.40% DM, respectively (1), and the ideal dietary N/S ratio required for rumen microorganisms is between 12:1 and 15:1 in cattle (3). The dietary S concentration and the N/S ratio of CON in the present trial were 0.115% DM and 19.3:1, respectively, which were apparently not suitable for rumen microorganisms. Hence, supplementation with Na2SO4 increased the dietary concentration of S and balanced the dietary N/S ratio. The changes of the rumen bacterial communities should be attributed to dietary supplementation of Na2SO4.
(ii) S metabolism in rumen and SRB.
Bacterial SO42− reduction may be classified as either dissimilatory or assimilatory (17). In assimilatory reduction, bacteria reduce SO42− to H2S, which is then utilized to produce S-containing AA (Met and Cys) or vitamins (thiamine and biotin), while in dissimilatory reduction, the bacteria reduce SO42− and produce H2S as an end product of their metabolism (10). SRB, which primarily use the dissimilatory pathway (10), are thought to compose a very small portion (less than 1%) of the ruminal bacterial population (18). Desulfovibrio bacteria are considered to be the most prominent SRB, while Desulfobulbus and Desulfuromonas are both identified as SRB in the rumen (8). In the present trial, supplementation with Na2SO4 at 20 and 40 g/day increased the relative abundances of Desulfovibrio and Desulfobulbus. Therefore, the increased ruminal concentration of S2− could be primarily attributed to the increased abundance of Desulfovibrio.
The ruminal concentrations of S2− of different groups in the present trial ranged from 1.87 to 6.83 mg/liter, which was above the minimum level of 0.96 mg/liter required by rumen microorganisms (19). Results of the present trial indicated that dietary supplementation with Na2SO4 decreased the SO42−-S/S2−-S ratio in rumen fluid, indicating that Na2SO4 increased the reduction rate of SO42− (6, 59). The results also indicated that Na2SO4 improved the S digestibility. The results could be attributed to the increased abundance of Desulfovibrio. Results of the present trial also indicated that the steers supplemented with 40 g Na2SO4/day had higher S digestibility than those supplemented with 20 g Na2SO4/day. The results indicated that dietary S levels higher than required could be beneficial to steers in utilizing S.
(iii) Ruminal fermentation of carbohydrates and cellulolytic bacteria.
Ruminococcus spp. are typical hemicellulolytic, cellulolytic, and pectinolytic bacteria (21, 22), and the members of family Ruminococcaceae are recognized as acetate producers (20). The bacteria belonging to family Rikenellaceae are associated with either the primary or secondary degradation of structural carbohydrate (23). Results of the present trial indicated that dietary supplementation with Na2SO4 increased the relative abundances of ruminal Ruminococcus 2 and Ruminococcaceae UCG-010, belonging to the family Ruminococcaceae, and also increased the relative abundance of genus Rikenellaceae RC9 gut group. The results also indicated that Na2SO4 increased the ruminal concentration of total VFA, the molar proportion of acetate, and the digestibility of dietary NDF and ADF. The results could be attributed to the increased relative abundances of Ruminococcus 2 and Rikenellaceae RC9 gut group. The effects of supplementing Na2SO4 on increasing fiber digestibility were consistent with the increased ruminal concentration of total VFA.
Butyrivibrio fibrisolvens and Pseudobutyrivibrio spp. are important butyrate-producing bacteria in the rumen (22). Results of the present trial indicated that dietary supplementation with Na2SO4 increased the molar proportion of butyrate in rumen fluid, as well as the relative abundances of Butyrivibrio and Pseudobutyrivibrio. Therefore, the increased molar proportion of butyrate in rumen fluid could be attributed to the increased relative abundances of genera Butyrivibrio and Pseudobutyrivibrio.
Most of the propionate in the rumen is produced by the succinate-producing bacteria and the succinate-to-propionate reducing bacteria (24). The ruminal Fibrobacter produces succinate as one of its products, and Prevotella, as the major propionate producer, is able to convert succinate into propionate (25, 26). Results of the present trial indicated that supplementation with Na2SO4 decreased the relative abundance of Prevotella 1, whereas it did not influence the relative abundance of Fibrobacter. Since Prevotella 1 is the dominant genus in the rumen, the decrease in the molar proportion of propionate in rumen fluid could be attributed to the decreased relative abundance of Prevotella 1.
Streptococcus and Lactobacillus are regarded as the major lactate producers in the rumen (27). Desulfobulbus and Desulfuromonas are the SRB, which utilize lactate in the dissimilatory pathway of SO42− reduction (8), while Selenomonas is also a lactate utilizer (28) in the rumen. Results of the present trial indicated that supplementation with Na2SO4 increased the relative abundances of Desulfovibrio and Desulfobulbus and decreased that of Selenomonas 1, whereas it did not affect those of Streptococcus and Lactobacillus. Hence, the results of supplementation with Na2SO4 of tending to decrease the ruminal concentration of lactate could be attributed to the increased relative abundances of Desulfovibrio and Desulfobulbus.
(iv) Ruminal N metabolism and related bacteria.
The loss of NH3-N in the rumen is the main reason for low N utilization efficiency in ruminants (29). Results of the present trial indicated that dietary supplementation with Na2SO4 decreased the ruminal concentration of NH3-N, whereas it increased that of MCP. The results indicated that more NH3-N had been used for the ruminal MCP synthesis that resulted from a better-balanced dietary N/S ratio.
Prevotella spp. are the predominant bacteria in the rumen, and one of their most important roles is to degrade protein and peptides in an animal’s gut (30). Since dietary supplementation with Na2SO4 decreased the relative abundance of Prevotella 1 in the rumen, the lowered ruminal concentration of NH3-N could partly be the result of the decreased protein degradation in a rumen impacted by the relative abundance of Prevotella 1.
Plasma metabolomics. (i) Effects of S on plasma protein, hormones, and AA.
Results of the present trial indicated that dietary supplementation with Na2SO4 increased the plasma concentrations of total protein and albumin and tended to increase the plasma concentration of total AA. The results were in accordance with the increased ruminal concentration of MCP. The results indicated that dietary supplementation with Na2SO4 increased the ruminal MCP supply to the steers.
IGF-1 and GH play important roles in the regulation of protein metabolism in ruminants (31), and Met and Lys are the key limiting AA involved in the expression of IGF-1 in the liver of ruminants (32). Results of the present trial indicated that dietary supplementation with 40 g Na2SO4/day increased the plasma concentrations of IGF-1 and Met, Lys, Arg, Cys, and Phe. The results indicated that supplementing S effectively modulated the AA and protein metabolism in steers. The increased plasma concentration of IGF-1 was in accordance with the increased plasma concentrations of AA. The enriched pathways observed in the present trial, including “biosynthesis of aminoacyl-tRNA,” “cysteine and methionine metabolism,” and “arginine and proline metabolism,” also confirmed the effects of supplementing Na2SO4 on the AA and protein metabolism in steers. Results also indicated that the plasma concentrations of essential AA, e.g., Arg, Met, and Cys, of steers fed Na2SO4 at 40 g/day were higher than in steers fed 20 Na2SO4 at g/day. The results indicated that supplementation with Na2SO4 above the S requirement also increased the supply of the metabolizable Met and other essential AA.
Results of the present trial indicated that dietary supplementation with Na2SO4 decreased the plasma concentration of urea. The results could be attributed to the decreased ruminal concentration of NH3-N, because the plasma urea is positively correlated with the ruminal concentration of NH3-N in ruminants (33). Since a lower concentration of plasma urea is beneficial to decrease the urinary excretion of urea (34), dietary supplementation with Na2SO4 would be effective to improve the N retention rate in steers.
(ii) Effects of S on plasma pyruvate, lactate, thiamine, and biotin.
Propionate absorbed from the digestive tract can be transformed into glucose through gluconeogenesis, whereas glucose can be degraded into pyruvate in the liver (35). Results of the present trial indicated that dietary supplementation with Na2SO4 decreased the plasma concentration of pyruvate. Evidently the decreased plasma concentration of pyruvate resulted from the decreased molar proportion of propionate in the rumen of steers.
Pyruvate can be converted to lactate by lactate dehydrogenase (LDH) or to acetyl coenzyme A (CoA) and formate by pyruvate formate-lyase (PFL), which is composed of two cofactors, S-adenosyl-methionine and thiamine pyrophosphate (TPP) (36). Results of the present trial indicated that dietary supplementation with Na2SO4 increased the plasma concentrations of S-adenosyl-methionine and TPP. Evidently Na2SO4 improved the activity of PFL in degrading pyruvate to produce more acetyl-CoA, resulting in a decreased plasma concentration of lactate.
Thiamine and biotin are essential cofactors for various enzymes required for the metabolism of carbohydrate, fatty acids, AA, and nucleic acids (37, 38). Results of the present trial indicated that dietary supplementation with Na2SO4 increased the plasma concentrations of thiamine and biotin. Since cattle are unable to synthesize thiamine and biotin in the body and they must be obtained from microorganisms in the digestive tract (39), dietary supplementation with Na2SO4 should have improved the microbial synthesis of thiamine and biotin in the rumen, as well as the absorption of these vitamins from the digestive tract. The increased plasma concentrations of thiamine and biotin were in accordance with the pathway analysis, which indicated that dietary supplementation with Na2SO4 enriched the pathways “thiamine metabolism” and “biotin metabolism.” The results also suggested that supplying more S than required was beneficial to improve the microbial synthesis of thiamine and biotin.
(iii) Effects of S on plasma purine derivatives and lipids.
Results of the present trial indicated that dietary supplementation with Na2SO4 increased the plasma concentrations of xanthine, hypoxanthine, and guanine, which are the metabolites of nucleic acids from the ruminal microorganisms.
Purine derivatives are used as the biomarkers of the ruminal MCP synthesis (40). The plasma concentrations of purine derivatives are positively correlated with the amounts of rumen microbial nucleic acids that enter the small intestine (41). Results of the present trial indicated that dietary supplementation with Na2SO4 increased the absorption of rumen microbial nucleic acids in steers. The results were in accordance with the increased ruminal concentration of MCP. The results also showed that the steers supplemented with Na2SO4 at 40 g/day had higher plasma concentrations of xanthine and uracil than the steers supplemented with Na2SO4 at 20 g/day. The results indicated that supplying S to steers at levels higher than required could be beneficial for ruminal MCP synthesis.
Results of the present trial indicated that dietary supplementation with Na2SO4 altered the plasma concentrations of lipids and lipid-like molecules. The results could be attributed to the increased plasma concentrations of acetylcarnitine and l-carnitine in the liver of steers, which facilitate the oxidation of lipids (42). However, the exact mechanisms by which S affected the metabolism of lipids and fatty acids are unclear and need to be investigated in further studies.
Overall, dietary supplementation with Na2SO4 increased the ruminal concentrations of total VFA and MCP and the fiber digestibility, and it also positively affected the plasma concentrations of metabolites, including AA, vitamins, purine derivatives, and lipids. The results demonstrated that balancing the dietary ratio of N/S by adding Na2SO4 to the steer ration was beneficial to improve the nutrient digestibility and nutrient supply in steers. The results could be attributed to the effects of the S in Na2SO4 on enhancing the richness and the diversity of the rumen bacterial community. It should be noted that although the correlations between the abundances of some rumen microorganisms and rumen VFA, NH3-N, and plasma metabolites were significant, the actual mechanisms by which the rumen microorganisms impact the rumen fermentation are still unclear. The only way to identify causal links between increased S content and the trends that are identified is to design subsequent experiments testing these phenomena specifically.
MATERIALS AND METHODS
The animal experimental procedures and protocols were approved by the Animal Care and Use Committee of China Agricultural University (Beijing, China) and in accordance with the University’s guidelines for animal research. This experiment was conducted on a commercial beef farm during the months of September and November 2019.
Animals and experimental design.
Six castrated Simmental steers (22 months old) with an initial liveweight of 449 ± 19 kg were used as the experimental animals. The animals were fed a basal ration prepared as a total mixed ration (TMR) composed of 488 g corn silage and 512 g corn grain-based concentrate mixture per kg dry matter (DM) (Table S1 in the supplemental material). Three levels of anhydrous Na2SO4 (purity of 99.9%; Sinopharm Chemical Reagent, Shanghai, China), i.e., 0 g/day (CON), 20 g/day (S1), and 40 g/day (S2), were added to the basal ration as experimental treatments. The animals and the treatments were allocated to a replicate 3-by-3 Latin square design. Each experimental period lasted 20 days, including 15 days for adaption and 5 days for sampling. The dietary nitrogen (N)/sulfur (S) ratios were 19.34:1, 12.11:1, and 8.72:1 for treatments CON, S1, and S2, respectively (Table S2).
The steers were housed in individual pens (10 m2) bedded with rubber mattresses in a metabolism barn. An amount of 6.65 kg DM of TMR, which was about 90% of the ad libitum feed intake obtained from a preliminary test, was supplied to each animal daily. The amount of TMR was divided into two equal parts and fed to the steers half at 0700 h and half at 1700 h. No orts were left throughout the trial. The amounts of Na2SO4 designed as treatments were also divided into two equal parts and were mixed with the TMR before feeding. The steers had free access to drinking water. Liveweights of the steers were recorded before morning feeding at the beginning of each experimental period.
Feed and fecal matter sampling, measurements, and analysis.
The TMR was sampled daily during each sampling period and stored at −20°C prior to chemical analyses. The feces from each steer were collected in total daily during each sampling period. The daily output of feces from each steer was recorded and homogenized. Then, 3% of the feces was sampled and supplemented with 20 ml of sulfuric acid (concentration, 10% [vol/vol]) to preserve the N, to analyze DM, organic matter (OM), crude protein (CP), neutral detergent fiber (NDF), and acid detergent fiber (ADF). Another 3% of the feces was also sampled but without adding H2SO4, to analyze the fecal S. All the samples were frozen at −20°C until analysis.
The samples of TMR and feces were lyophilized in a freeze dryer (LGJ-12; Songyuan Huaxing Technology Development Co., Ltd., Beijing, China) for 72 h and then ground to pass through a 40-mesh sieve. The DM was determined by drying at 105°C for 4 h (Association of Official Analytical Chemists [AOAC] method 930.15 [43]). The N was determined by the Kjeldahl method, and CP content was calculated as N × 6.25 (AOAC method 981.10 [43]). The crude ash was measured by combustion in an electric muffle furnace at 550°C for 6 h (AOAC method 942.05 [43]), and the OM was calculated as DM minus crude ash. The NDF was determined on an Ankom200 fiber analyzer (Ankom Technology Corp., Macedon, NY, USA) using heat stable α-amylase and sodium sulfite according to the method of Van Soest et al. (44). The ADF was determined on an Ankom200 fiber analyzer (Ankom Technology Corp., Macedon, NY, USA) (AOAC method 973.18 [43]). The gross energy of feeds was measured by combustion in an adiabatic bomb calorimeter (Parr 6300 calorimeter; Parr Instrument Company, Moline, IL, USA). The S in TMR and feces was analyzed using the magnesium nitrate method of China National Standard GB/T 17776–2016 (45). The ruminal degradable protein (RDP) and ruminal undegradable protein (RUP) of feeds were determined and calculated using the methods of Licitra et al. (46) and the National Research Council (47). The amounts of Met and Lys of feeds were determined by using a Hitachi L-8900 amino acid analyzer (Hitachi Co., Tokyo, Japan).
Rumen fluid sampling, measurements, and analysis.
On the first day of each sampling period, rumen fluid was aspirated using an esophageal stomach tube 3 h after the morning feeding. The initial 200 ml of collected rumen fluid was discarded to avoid saliva contamination, and the subsequent 100 ml of rumen fluid was kept as the representative sample. The pH of the rumen fluid was measured immediately using a digital-type pH meter (PHS-3C; Shanghai Yueping Scientific Instrument Co., Ltd., Shanghai, China). The rumen fluid samples were strained through four layers of cheesecloth and divided into five portions. Four portion samples of 10 ml were frozen at −20°C for analyzing volatile fatty acids (VFA), ammonia N (NH3-N), microbial crude protein (MCP), and S components (sulfate [SO42−] and sulfide [S2−]), respectively. The 5th 10-ml portion was kept in a sterilized centrifuge tube and frozen at −80°C for DNA extraction.
The frozen rumen fluid samples were thawed and centrifuged at 20,000 × g for 15 min at 4°C. The ruminal concentration of VFA was determined using a gas chromatograph (GC-8600; Beifen Tianpu Instrument Technology Co., Ltd., Beijing, China). The ruminal concentration of NH3-N was determined using the phenol-hypochlorite method as described by Broderick and Kang (48). The ruminal concentration of MCP was determined according to the method of Makkar et al. (49). The ruminal concentrations of SO42− and S2− were determined according to the method described by Qi et al. (17). The lactate in rumen fluid was analyzed using a commercial kit (colorimetric method; Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Ruminal microbiota analysis. (i) Bacterial DNA extraction and sequencing.
The rumen bacterial DNA was extracted using the E.Z.N.A. soil DNA kit (Omega Bio-tek, Norcross, GA, USA). The purity and quality of DNA samples were examined by 1% agarose gel electrophoresis and a Nanodrop 2000 spectrophotometer (Thermo Scientific, Wilmington, NC, USA). The V3 and V4 hypervariable regions of the 16S rRNA genes of bacteria were amplified by PCR (95°C for 2 min, followed by 27 cycles at 98°C for 10 s, 62°C for 30 s, and 68°C for 30 s and a final extension at 68°C for 10 min) using barcoded primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′), where the barcode is an 8-base sequence unique to each sample. PCR of the samples was performed in triplicate in 20 μl of reaction mixture containing 4 μl of 5× FastPfu buffer, 2 μl of 2.5 mM deoxynucleoside triphosphates (dNTPs), 0.8 μl of each primer (5 μM), 0.4 μl of FastPfu polymerase, 10 ng of template DNA. PCR products were examined by electrophoresis with 2% agarose gel and then purified with the AxyPrep DNA gel extraction kit (Axygen Biosciences, Union City, CA, USA) according to the manufacturer’s instructions. Subsequently, purified PCR products were pooled to equimolar concentrations to generate the final DNA library, which was paired-end sequenced (2 × 300 bp) on an Illumina MiSeq platform (Illumina, San Diego, CA, USA).
(ii) Bioinformatics analysis.
Raw sequences were demultiplexed and quality filtered in Quantitative Insights into Microbial Ecology (QIIME, version 1.9.1), and bases with average quality scores of >20 were retained (50). Paired-end reads of the DNA fragments were merged using Fast Length Adjustment of Short reads (FLASH, version 1.2.11) with a minimum overlap of 10 bp and mismatch error rates of 2% (51). High-quality and clean sequences were then clustered into operational taxonomic units (OTUs) with a 3% dissimilarity cutoff using UPARSE (version 7.0.1090) (52). Taxonomic OTU assignments were performed using the Ribosome Database Project (RDP) Classifier (version 2.2) and then aligned against entries in the Silva 16S rRNA database (release 132; https://www.arb-silva.de/) (53). The OTU rarefaction curves were generated and plotted using QIIME. Alpha diversity indices of the bacterial communities, including Shannon, Simpson, Chao1, ACE, and Good’s coverage, were evaluated using the procedures of mothur (version 1.30.2) (54). Beta diversity was visualized using principal-coordinate analysis (PCoA) as calculated based on the weighted UniFrac matrix distances and plotted in R (version 3.2.4) (55). The significance of grouping in the PCoA plot was tested by ANOSIM in QIIME with 999 permutations using the vegan package in R (version 3.2.4) (54). The relative abundances of bacteria were expressed as percentages.
In addition, the linear discriminant analysis (LDA) effect size (LEfSe) was used to identify the most differentially abundant bacterial taxa among treatments. LEfSe uses a nonparametric factorial Kruskal-Wallis and Wilcoxon rank sum test followed by a linear discriminate analysis to estimate the effect size of each taxon. A significance level of P < 0.05 and effect size threshold of 3 were applied in the trial to identify the biomarker taxa.
Plasma sample collection and measurements.
Blood samples were taken from the jugular vein into 5-ml vacuum tubes containing K2EDTA for all steers 2 h after morning feeding on the last day of each sampling period and immediately centrifuged at 3,000 × g for 15 min at 4°C to obtain plasma samples, which were kept at −80°C prior to analysis.
The plasma albumin (bromocresol green method), total protein (Biuret method), urea (urease/Berthelot method), uric acid (uricase/peroxidase method), and creatinine (Jaffe’s method) were analyzed using an automatic biochemical analyzer (BS-420; Shenzhen Mindray Biomedical Electronic Co., Ltd., Shenzhen, China). The amount of plasma globulin was calculated as the difference between total protein and albumin.
Plasma allantoin (colorimetric method), hippuric acid (pyridine/benzene sulfonyl chloride method), total amino acids (colorimetric method), insulin-like growth factor-1 (enzyme linked immunosorbent assay [ELISA]), and growth hormone (ELISA) were analyzed on a semiautomatic biochemical analyzer (A6; Beijing Shining Sun Technology Co., Ltd., Beijing, China). The procedures for analyzing all parameters were according to the manufacturer’s instructions using commercially available kits (Beijing Sino-UK Institute of Biological Technology, Beijing, China).
Plasma metabolomics profiling. (i) LC-MS analysis.
The plasma sample preparation for LC-MS analysis was conducted according to the procedures of Wang et al. (56). Briefly, 100 μl plasma, 300 μl methanol, and 20 μl internal standard (dl-o-chlorophenylalanine) were mixed in an Eppendorf tube. The mixture was vortexed for 30 s and then centrifuged at 13,000 × g for 15 min at 4°C, and the supernatant was used for analysis.
The LC-MS analysis was performed using an ExionLC AD system (AB Sciex, Framingham, MA, USA) with a Waters Acquity BEH C18 column (1.7-μm particle size, 100 mm in length, 2.1-mm inner diameter; Waters Corporation, Milford, MA, USA) preheated to 40°C. The ultraperformance liquid chromatography (UPLC) system was coupled to a quadrupole-time-of-flight (TOF) mass spectrometer (TripleTOF 5600+; AB Sciex, Framingham, MA, USA) in positive and negative modes (Waters Corporation, Milford, MA, USA). The volume of plasma for injection was 20 μl. The mobile phase consisted of 0.1% (vol/vol) formic acid in water (solvent A), and 0.1% (vol/vol) formic acid in a mixture of acetonitrile and isopropanol (1:1, vol/vol) (solvent B) was used with an elution gradient as follows: 5% to 20% B for 0 to 3 min, 20% to 95% B for 3 to 9 min, 95% B for 9 to 13 min, 95% to 5% B for 13 to 13.1 min, and 5% B for 13.1 to 16 min at a flow rate of 0.4 ml/min. The parameters of the electron spray ionization (ESI) source were set according to the description of Liu et al. (57). To monitor the stability of the analysis, quality control (QC) samples were prepared by mixing all extracted plasma samples and injected at regular intervals (every 8 samples).
(ii) Metabolomics data processing.
LC-MS raw data were generated and processed through Waters MassLynx software (version 4.1; Waters Corporation, Milford, MA, USA) and Progenesis QI (version 2.3; Nonlinear Dynamics, Newcastle, UK) for peak detection and alignment. The human metabolome database (HMDB) (http://www.hmdb.ca/) and Metlin database (https://metlin.scripps.edu/) were used for metabolite identification. The data were subjected to multivariate analysis using Simca-P software package (version 14.1; MKS Data Analytics Solutions, Umeå, Sweden), including principal-component analysis (PCA) and orthogonal partial least squares discriminant analysis (OPLS-DA). Differential expression of metabolites between two treatments was identified based on the variable importance in projection (VIP) from OPLS-DA analysis and statistical analysis (VIP > 1 and q [false discovery rate {FDR}] < 0.05). The fold change (FC) value for each metabolite was calculated through comparing the mean values of the peak area of each metabolite for all samples within the same treatment, and the log2FC value was used to indicate the specific variable quantity in the comparison. The differential metabolites identified from the approach described above were used to perform the metabolic pathway analysis using MetaboAnalyst 4.0 (https://www.metaboanalyst.ca/) (58), and the pathways with greater impact values and lower P values were regarded as the key pathways.
Statistical analysis.
The data for rumen fermentation parameters, nutrient digestibility, alpha diversity indices, and conventional plasma metabolites were analyzed as a replicate Latin square design using the MIXED procedures of SAS (version 9.3; SAS Institute, Inc., Cary, NC, USA), with results presented as the least squares mean and standard error of the mean. The analytical model was as follows:
in which Yijkl is the dependent variable, μ is the overall mean, Ti is the fixed treatment effect, Pj is the random period effect, Sk is the random square effect, C(k)l is the random effect of the lth cattle within the kth square, T × Sik is the interaction between the ith treatment and the kth square, and eijkl is the error residual. Differences among treatment means were tested for significance using Tukey’s multiple range test. The effects of increasing Na2SO4 supplementation from 0 g/day to 20 and 40 g/day were examined through linear and quadratic orthogonal contrasts using the CONTRAST procedures of SAS. Differences among means were declared significant at an α level of ≤0.05, and trends were declared between P > 0.05 and P ≤ 0.10.
The software R (version 3.2.4) was used for the analysis of the rumen microbial taxa. The “lm” function of the Estimability package in R was used to evaluate the linear and quadratic effects of the dietary Na2SO4. All P values from the multiple comparison analyses of the rumen microbial community were adjusted by the FDR of the BonEV package in R. FDR-corrected P values below 0.05 (q < 0.05) were considered significantly different.
Spearman’s rank correlation was performed and plotted using the Hmisc and Corrplot packages in R (version 3.2.4) to examine the correlations between the rumen fermentation parameters, the plasma metabolites, and the relative abundances of the rumen microbial taxa at the genus level. P values were adjusted with FDR, and corrected P values below 0.05 (q < 0.05) were regarded as statistically significant.
Data availability.
All the raw sequences after assembling and filtering have been submitted to the NCBI Sequence Read Archive (SRA; https://www.ncbi.nlm.nih.gov/Traces/sra/) under accession number SRP254871.
Supplementary Material
ACKNOWLEDGMENTS
The research was financially supported by Ministry of Agriculture and Rural Affairs of China (grant number 16190051-03). The funders had no role in the experimental design, data collection and interpretation, or the decision to submit the work for publication.
We declare no conflict of interest.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.National Academies of Sciences, Engineering and Medicine. 2015. Nutrient requirements of beef cattle, 8th revised ed National Academies Press, Washington, DC. [Google Scholar]
- 2.Cherdthong A, Wanapat M, Wachirapakorn C. 2011. Influence of urea calcium mixture supplementation on ruminal fermentation characteristics of beef cattle fed on concentrates containing high levels of cassava chips and rice straw. Anim Feed Sci Technol 163:43–51. doi: 10.1016/j.anifeedsci.2010.10.003. [DOI] [Google Scholar]
- 3.Kandylis K. 1984. The role of sulphur in ruminant nutrition. Livest Prod Sci 11:611–624. doi: 10.1016/0301-6226(84)90075-7. [DOI] [Google Scholar]
- 4.Nezamidoust M, Alikhani M, Ghorbani GR, Edriss MA. 2014. Responses to betaine and inorganic sulphur of sheep in growth performance and fibre growth. J Anim Physiol Anim Nutr 98:1031–1038. doi: 10.1111/jpn.12166. [DOI] [PubMed] [Google Scholar]
- 5.Supapong C, Cherdthong A, Wanapat M, Chanjula P, Uriyapongson S. 2019. Effects of sulfur levels in fermented total mixed ration containing fresh cassava root on feed utilization, rumen characteristics, microbial protein synthesis, and blood metabolites in Thai native beef cattle. Animals 9:261. doi: 10.3390/ani9050261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amat S, McKinnon JJ, Penner GB, Hendrick S. 2014. Effects of dietary sulfur concentration and forage-to-concentrate ratio on ruminal fermentation, sulfur metabolism, and short-chain fatty acid absorption in beef heifers. J Anim Sci 92:712–723. doi: 10.2527/jas.2013-7254. [DOI] [PubMed] [Google Scholar]
- 7.McSweeney CS, Denman SE, Conlan LL, Prasad CS, Anandan S, Chandrasekharaiah M, Sampath KT. 2009. The stimulatory effect of the organic sulfur supplement, mercaptopropane sulfonic acid on cellulolytic rumen microorganisms and microbial protein synthesis in cattle fed low sulfur roughages. Animal 3:802–809. doi: 10.1017/S1751731109004108. [DOI] [PubMed] [Google Scholar]
- 8.Richter EL. 2011. The effect of dietary sulfur on performance, mineral status, rumen hydrogen sulfide, and rumen microbial populations in yearling beef steers. MSc dissertation. Iowa State University, Ames, IA.
- 9.Xue F, Zhou Z, Ren L, Meng Q. 2011. Influence of rumen-protected lysine supplementation on growth performance and plasma amino acid concentrations in growing cattle offered the maize stalk silage/maize grain-based diet. Anim Feed Sci Technol 169:61–67. doi: 10.1016/j.anifeedsci.2011.05.011. [DOI] [Google Scholar]
- 10.Bradley AS, Leavitt WD, Johnston DT. 2011. Revisiting the dissimilatory sulfate reduction pathway. Geobiology 9:446–457. doi: 10.1111/j.1472-4669.2011.00292.x. [DOI] [PubMed] [Google Scholar]
- 11.McSweeney CS, Denman SE. 2007. Effect of sulfur supplements on cellulolytic rumen micro-organisms and microbial protein synthesis in cattle fed a high fibre diet. J Appl Microbiol 103:1757–1765. doi: 10.1111/j.1365-2672.2007.03408.x. [DOI] [PubMed] [Google Scholar]
- 12.Anderson CL, Sullivan MB, Fernando SC. 2017. Dietary energy drives the dynamic response of bovine rumen viral communities. Microbiome 5:155. doi: 10.1186/s40168-017-0374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henderson G, Cox F, Ganesh S, Jonker A, Young W, Janssen PH, Global Rumen Census Collaborators. 2015. Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci Rep 5:14567. doi: 10.1038/srep14567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khafipour E, Li S, Tun HM, Derakhshani H, Moossavi S, Plaizier JC. 2016. Effects of grain feeding on microbiota in the digestive tract of cattle. Anim Front 6:13–19. doi: 10.2527/af.2016-0018. [DOI] [Google Scholar]
- 15.Kruger Ben Shabat S, Sasson G, Doron-Faigenboim A, Durman T, Yaacoby S, Berg Miller ME, White BA, Shterzer N, Mizrahi I. 2016. Specific microbiome-dependent mechanisms underlie the energy harvest efficiency of ruminants. ISME J 10:2958–2972. doi: 10.1038/ismej.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H, Li H, Wu F, Qiu X, Yu Z, Niu W, He Y, Su H, Cao B. 2019. Effects of dietary energy on growth performance, rumen fermentation and bacterial community, and meat quality of Holstein‐Friesians bulls slaughtered at different ages. Animals 9:1123. doi: 10.3390/ani9121123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qi K, Lu CD, Owens FN, Lupton CJ. 1992. Sulfate supplementation of Angora goats: metabolic and mohair responses. J Anim Sci 70:2828–2837. doi: 10.2527/1992.7092828x. [DOI] [PubMed] [Google Scholar]
- 18.Callaway TR, Dowd SE, Edrington TS, Anderson RC, Krueger N, Bauer N, Kononoff PJ, Nisbet DJ. 2010. Evaluation of bacterial diversity in the rumen and feces of cattle fed different levels of dried distillers grains plus solubles using bacterial tag-encoded FLX amplicon pyrosequencing. J Anim Sci 88:3977–3983. doi: 10.2527/jas.2010-2900. [DOI] [PubMed] [Google Scholar]
- 19.Suttle NF. 2010. Mineral nutrition of livestock, 4th ed CABI, New York, NY. [Google Scholar]
- 20.Varzaneh MB, Klevenhusen F, Zebeli Q, Petri R. 2018. Scrophularia striata extract supports rumen fermentation and improves microbial diversity in vitro compared to monensin. Front Microbiol 9:2164. doi: 10.3389/fmicb.2018.02164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kong Y, Teather R, Forster R. 2010. Composition, spatial distribution, and diversity of the bacterial communities in the rumen of cows fed different forages. FEMS Microbiol Ecol 74:612–622. doi: 10.1111/j.1574-6941.2010.00977.x. [DOI] [PubMed] [Google Scholar]
- 22.Biddle A, Stewart L, Blanchard J, Leschine S. 2013. Untangling the genetic basis of fibrolytic specialization by lachnospiraceae and ruminococcaceae in diverse gut communities. Diversity 5:627–640. doi: 10.3390/d5030627. [DOI] [Google Scholar]
- 23.Pitta DW, Pinchak WE, Dowd SE, Osterstock J, Gontcharova V, Youn E, Dorton K, Yoon I, Min BR, Fulford JD, Wickersham TA, Malinowski DP. 2010. Rumen bacterial diversity dynamics associated with changing from bermudagrass hay to grazed winter wheat diets. Microb Ecol 59:511–522. doi: 10.1007/s00248-009-9609-6. [DOI] [PubMed] [Google Scholar]
- 24.Granja-Salcedo YT, Fernandes RMI, De Araujo RC, Kishi LT, Berchielli TT, De Resende FD, Berndt A, Siqueira GR. 2019. Long-term encapsulated nitrate supplementation modulates rumen microbial diversity and rumen fermentation to reduce methane emission in grazing steers. Front Microbiol 10:614. doi: 10.3389/fmicb.2019.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang J, Shi H, Wang Y, Li S, Cao Z, Ji S, He Y, Zhang H. 2017. Effect of dietary forage to concentrate ratios on dynamic profile changes and interactions of ruminal microbiota and metabolites in Holstein heifers. Front Microbiol 8:2206. doi: 10.3389/fmicb.2017.02206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.AlZahal O, Li F, Guan LL, Walker ND, McBride BW. 2017. Factors influencing ruminal bacterial community diversity and composition and microbial fibrolytic enzyme abundance in lactating dairy cows with a focus on the role of active dry yeast. J Dairy Sci 100:4377–4393. doi: 10.3168/jds.2016-11473. [DOI] [PubMed] [Google Scholar]
- 27.Owens FN, Secrist DS, Hill WJ, Gill DR. 1998. Acidosis in cattle: a review. J Anim Sci 76:275–286. doi: 10.2527/1998.761275x. [DOI] [PubMed] [Google Scholar]
- 28.Rumsey TS. 1978. Effects of dietary sulfur addition and synovex-S ear implants on feedlot steers fed an all-concentrate finishing diet. J Anim Sci 46:463–477. doi: 10.2527/jas1978.462463x. [DOI] [Google Scholar]
- 29.Tamminga S. 1992. Nutrition management of dairy cows as a contribution to pollution control. J Dairy Sci 75:345–357. doi: 10.3168/jds.S0022-0302(92)77770-4. [DOI] [Google Scholar]
- 30.Accetto T, Avguštin G. 2015. Polysaccharide utilization locus and CAZYme genome repertoires reveal diverse ecological adaptation of Prevotella species. Syst Appl Microbiol 38:453–461. doi: 10.1016/j.syapm.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Misciatteilli L, Kristensen VF, Vestergaard M, Welsbjerg MR, Sejrsen K, Hvelplund T. 2003. Milk production, nutrient utilization, and endocrine responses to increased postruminal lysine and methionine supply in dairy cows. J Dairy Sci 86:275–286. doi: 10.3168/jds.S0022-0302(03)73606-6. [DOI] [PubMed] [Google Scholar]
- 32.Stubbs AK, Wheelhouse NM, Lomax MA, Hazlerigg DG. 2002. Nutrient-hormone interaction in the ovine liver: methionine supply selectively modulates growth hormone-induced IGF-I gene expression. J Endocrinol 174:335–341. doi: 10.1677/joe.0.1740335. [DOI] [PubMed] [Google Scholar]
- 33.Rajabi M, Rouzbehan Y, Rezaei J. 2017. A strategy to improve nitrogen utilization, reduce environmental impact, and increase performance and antioxidant capacity of fattening lambs using pomegranate peel extract. J Anim Sci 95:499–510. doi: 10.2527/jas.2016.1069. [DOI] [PubMed] [Google Scholar]
- 34.Wang K, Nan X, Chu K, Tong J, Yang L, Zheng S, Zhao G, Jiang L, Xiong B. 2018. Shifts of hydrogen metabolism from methanogenesis to propionate production in response to replacement of forage fiber with non-forage fiber sources in diets in vitro. Front Microbiol 9:2764. doi: 10.3389/fmicb.2018.02764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ametaj BN, Zebeli Q, Saleem F, Psychogios N, Lewis MJ, Dunn SM, Xia J, Wishart DS. 2010. Metabolomics reveals unhealthy alterations in rumen metabolism with increased proportion of cereal grain in the diet of dairy cows. Metabolomics 6:583–594. doi: 10.1007/s11306-010-0227-6. [DOI] [Google Scholar]
- 36.Clemmons BA, Powers JB, Campagna SR, Seay TB, Embree MM, Myer PR. 2020. Rumen fluid metabolomics of beef steers differing in feed efficiency. Metabolomics 16:23. doi: 10.1007/s11306-020-1643-x. [DOI] [PubMed] [Google Scholar]
- 37.Seck M, Linton JAV, Allen MS, Castagnino DS, Chouinard PY, Girard CL. 2017. Apparent ruminal synthesis of B vitamins in lactating dairy cows fed diets with different forage-to-concentrate ratios. J Dairy Sci 100:1914–1922. doi: 10.3168/jds.2016-12111. [DOI] [PubMed] [Google Scholar]
- 38.Jenkins TP, Rathnayaka Y, Perera PK, Peachey LE, Nolan MJ, Krause L, Rajakaruna RS, Cantacessi C. 2017. Infections by human gastrointestinal helminths are associated with changes in faecal microbiota diversity and composition. PLoS One 12:e0184719. doi: 10.1371/journal.pone.0184719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayashi A, Mikami Y, Miyamoto K, Kamada N, Sato T, Mizuno S, Naganuma M, Teratani T, Aoki R, Fukuda S, Suda W, Hattori M, Amagai M, Ohyama M, Kanai T. 2017. Intestinal dysbiosis and biotin deprivation induce alopecia through overgrowth of Lactobacillus murinus in mice. Cell Rep 20:1513–1524. doi: 10.1016/j.celrep.2017.07.057. [DOI] [PubMed] [Google Scholar]
- 40.Singh M, Sharma K, Dutta N, Singh P, Verma AK, Mehra UR. 2007. Estimation of rumen microbial protein supply using urinary purine derivatives excretion in crossbred calves fed at different levels of feed intake. Asian Australas J Anim Sci 20:1567–1574. doi: 10.5713/ajas.2007.1567. [DOI] [Google Scholar]
- 41.Reynal SM, Broderick GA, Bearzi C. 2005. Comparison of four markers for quantifying microbial protein flow from the rumen of lactating dairy cows. J Dairy Sci 88:4065–4082. doi: 10.3168/jds.S0022-0302(05)73091-5. [DOI] [PubMed] [Google Scholar]
- 42.Zanton GI, Bowman GR, Vázquez-Añón M, Rode LM. 2014. Meta-analysis of lactation performance in dairy cows receiving supplemental dietary methionine sources or postruminal infusion of methionine. J Dairy Sci 97:7085–7101. doi: 10.3168/jds.2014-8220. [DOI] [PubMed] [Google Scholar]
- 43.AOAC. 2005. Official methods of analysis of AOAC International, 18th ed Association of Official Analytical Chemists, Gaithersburg, MD. [Google Scholar]
- 44.Van Soest PJ, Robertson JB, Lewis BA. 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci 74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- 45.General Administration of Quality Supervision, Inspection and Quarantine of the People 's Republic of China. 2016. National Standards of People’s Republic of China GB/T 17776–2016. Determination of sulfur in feedstuff—magnesium nitrate method. National Standard Press of China, Beijing, China.
- 46.Licitra G, Hernandez TM, Van Soest PJ. 1996. Standardization of procedures for nitrogen fractionation of ruminant feeds. Anim Feed Sci Technol 57:347–358. doi: 10.1016/0377-8401(95)00837-3. [DOI] [Google Scholar]
- 47.National Research Council. 2001. Nutrient requirements of dairy cattle, 7th revised ed National Academies Press, Washington, DC. [Google Scholar]
- 48.Broderick GA, Kang JH. 1980. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J Dairy Sci 63:64–75. doi: 10.3168/jds.S0022-0302(80)82888-8. [DOI] [PubMed] [Google Scholar]
- 49.Makkar HPS, Sharma OP, Dawra RK, Negi SS. 1982. Simple determination of microbial protein in rumen liquor. J Dairy Sci 65:2170–2173. doi: 10.3168/jds.S0022-0302(82)82477-6. [DOI] [PubMed] [Google Scholar]
- 50.Bokulich NA, Subramanian S, Faith JJ, Gevers D, Gordon JI, Knight R, Mills DA, Caporaso JG. 2013. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat Methods 10:57–59. doi: 10.1038/nmeth.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Magoč T, Salzberg SL. 2011. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Edgar RC. 2013. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- 53.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF. 2009. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen J, Bittinger K, Charlson ES, Hoffmann C, Lewis J, Wu GD, Collman RG, Bushman FD, Li H. 2012. Associating microbiome composition with environmental covariates using generalized UniFrac distances. Bioinformatics 28:2106–2113. doi: 10.1093/bioinformatics/bts342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang B, Ma MP, Diao QY, Tu Y. 2019. Saponin-induced shifts in the rumen microbiome and metabolome of young cattle. Front Microbiol 10:356. doi: 10.3389/fmicb.2019.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu C, Wu H, Liu S, Chai S, Meng Q, Zhou Z. 2019. Dynamic alterations in yak rumen bacteria community and metabolome characteristics in response to feed type. Front Microbiol 10:1116. doi: 10.3389/fmicb.2019.01116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chong J, Soufan O, Li C, Caraus I, Li S, Bourque G, Wishart DS, Xia J. 2018. MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res 46:W486–W494. doi: 10.1093/nar/gky310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Felix TL, Pyatt NA, Loerch SC. 2012. Effects of monensin supplementation on ruminal metabolism of feedlot cattle fed diets containing dried distillers grains. J Anim Sci 90:3905–3913. doi: 10.2527/jas.2011-5059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the raw sequences after assembling and filtering have been submitted to the NCBI Sequence Read Archive (SRA; https://www.ncbi.nlm.nih.gov/Traces/sra/) under accession number SRP254871.