Recent pieces of evidence suggest that Fe bound to the biofilm could assume at least two important functions, a local source of Fe for uptake and a support to extracellular metabolism, such as extracellular electron transfer. Our results show that B. subtilis can use biofilm-bound Fe for uptake only if it does not compromise Fe homeostasis of the biofilm, i.e., maintains a minimum Fe concentration in the biofilm for extracellular purposes. We propose a theoretical framework based on our results and recent literature to explain how B. subtilis manages biofilm-bound Fe and Fe uptake in response to environmental Fe availability. These results provide important insights into the management of biofilm-bound and environmental Fe by B. subtilis in response to Fe stress.
KEYWORDS: Bacillus subtilis, biofilm, homeostasis, iron, siderophores
ABSTRACT
Iron (Fe) is one of the most important micronutrients for most life forms on earth. While abundant in soil, Fe bioavailability in oxic soil is very low. Under environmental conditions, bacteria need to acquire sufficient Fe to sustain growth while limiting the energy cost of siderophore synthesis. Biofilm formation might mitigate this Fe stress, since it was shown to accumulate Fe in certain Gram-negative bacteria and that this Fe could be mobilized for uptake. However, it is still unclear if, and to what extent, the amount of Fe accumulated in the biofilm can sustain growth and if the mobilization of this local Fe pool is modulated by the availability of environmental Fe (i.e., Fe outside the biofilm matrix). Here, we use a nondomesticated strain of the ubiquitous biofilm-forming soil bacterium Bacillus subtilis and stable Fe isotopes to precisely evaluate the origin of Fe during growth in the presence of tannic acid and hydroxides, used as proxies for different environmental conditions. We report that this B. subtilis strain can accumulate a large quantity of Fe in the biofilm, largely exceeding Fe associated with cells. We also report that only a fraction of biofilm-bound Fe is available for uptake in the absence of other sources of Fe in the vicinity of the biofilm. We observed that the availability of environmental Fe modulates the usage of this pool of biofilm-bound Fe. Finally, our data suggest that consumption of biofilm-bound Fe relates to the efficacy of B. subtilis to transport Fe from the environment to the biofilm, possibly through siderophores.
IMPORTANCE Recent pieces of evidence suggest that Fe bound to the biofilm could assume at least two important functions, a local source of Fe for uptake and a support to extracellular metabolism, such as extracellular electron transfer. Our results show that B. subtilis can use biofilm-bound Fe for uptake only if it does not compromise Fe homeostasis of the biofilm, i.e., maintains a minimum Fe concentration in the biofilm for extracellular purposes. We propose a theoretical framework based on our results and recent literature to explain how B. subtilis manages biofilm-bound Fe and Fe uptake in response to environmental Fe availability. These results provide important insights into the management of biofilm-bound and environmental Fe by B. subtilis in response to Fe stress.
INTRODUCTION
Iron (Fe) is one of the most important elements for most microorganisms (1). Despite its high abundance in the earth crust and soil (2, 3), the low solubility of its common mineral forms (hydroxides and oxides) under oxic conditions, not greater than 10−18 M at pH 7 (4), results in very scarce bioavailability. To acquire Fe in bioavailable Fe-poor environments, microorganisms produce organic ligands with high affinity for Fe, called siderophores (5). Siderophores increase Fe availability by facilitating the dissolution of Fe oxides and competing with natural Fe complexes (i.e., organic matter) (6).
The presence of multicellular communities embedded in self-secreted matrices, or biofilms, is another important characteristic of most soil microorganisms (7). Biofilms provide many advantages for the microbial communities, such as protection against environmental stress (8). Importantly, biofilm formation and Fe acquisition are strongly intertwined. Fe availability (extracellular concentration and chemical form) has been shown to affect biofilm production and maturation in several bacteria, including the Gram-positive Bacillus subtilis (9, 10). Reciprocally, the formation of a biofilm could contribute to Fe acquisition. We recently showed that in Bacillus subtilis, both siderophore production and biofilm formation are required to support Fe acquisition from the medium and sustain Fe homeostasis during growth in static cultures (11). The extracellular biofilm matrix exhibits a wide range of properties (e.g., pH, redox potential, and composition) (8, 12, 13) that can influence metal speciation and siderophore efficiency and can facilitate metal uptake. In natural habitats, Fe sources in the vicinity of biofilm-forming bacteria are very diverse (e.g., minerals, organic matter, and xenosiderophores [14]), and Fe availability likely quickly evolves due to changes in water content, pH, and redox conditions in the bacterial microenvironment. Biofilm can sorb organic and inorganic compounds, including Fe (8, 12, 13). Biofilms of Pseudomonas aeruginosa were shown to accumulate important quantities of Fe that can be mobilized and taken up by the bacteria, possibly through siderophore complexation (12). The presence of Fe in the biofilm matrix could help bacteria cope with Fe stress by offering a local alternative to environmental Fe sources.
However, it is still unclear if, and to what extent, the amount of Fe accumulated in the biofilm can sustain growth and if the mobilization of this local Fe pool is modulated by the availability of environmental Fe (i.e., Fe outside the biofilm matrix). Here, we investigated how bacteria manage biofilm-bound Fe and environmental Fe sources to sustain Fe acquisition using the model bacterium B. subtilis strain NCIB3610. Our main objectives were (i) to quantify the accumulation of Fe by B. subtilis biofilms, (ii) to evaluate if the biofilm-bound Fe can be mobilized and to what extend it can support bacterial growth, and (iii) to test if the use of biofilm-bound Fe is modulated by the efficiency of cells to recruit Fe from environmental sources.
(This research was conducted by A. Rizzi in partial fulfillment of the requirements for a Ph.D. degree from the Université de Sherbrooke [Faculty of Sciences] [15].)
RESULTS
Quantitation of iron in Bacillus subtilis biofilm.
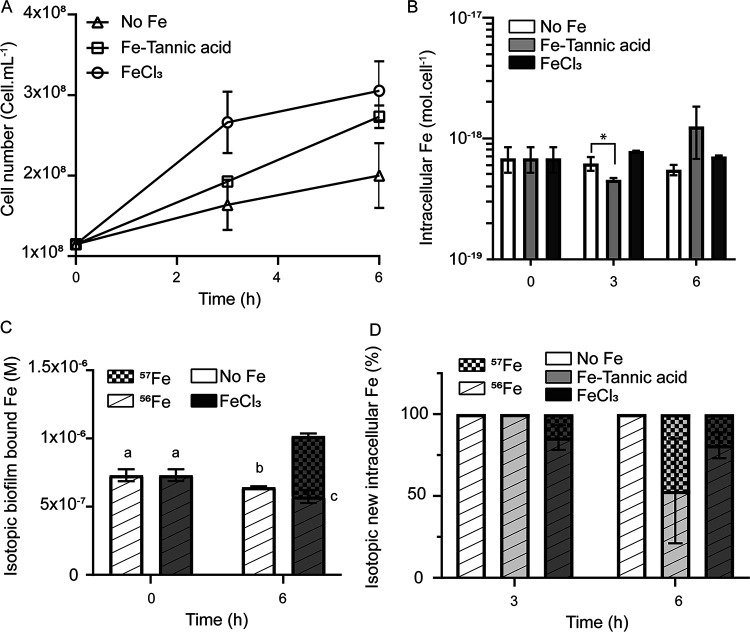
The capacity of microbial biofilms to accumulate various metals, including Fe, was reported in both laboratory settings and environmental samples for Gram-negative bacteria (12, 13, 16, 17). Thus, our first objective was to examine if biofilms formed by the Gram-positive bacteria Bacillus subtilis could also accumulate Fe. As shown in Fig. 1, under our experimental conditions, large quantities of Fe are stored in B. subtilis NCIB3610 biofilm collected after 22 h of growth (nearly 10−6 M) (Fig. 1A). To put this amount in perspective, after 22 h of growth the amount of Fe associated with the biofilm matrix was an order of magnitude higher than the amount of Fe associated with all B. subtilis cells (nearly 10−7 M) (Fig. 1A). Increasing the concentration of Fe in the medium (from 10−5 M to 10−4 M) and changing its chemical form (FeCl3 versus Fe-EDTA) did not significantly affect the amount of Fe bound to the biofilm after 28 h (Fig. 1B).
FIG 1.
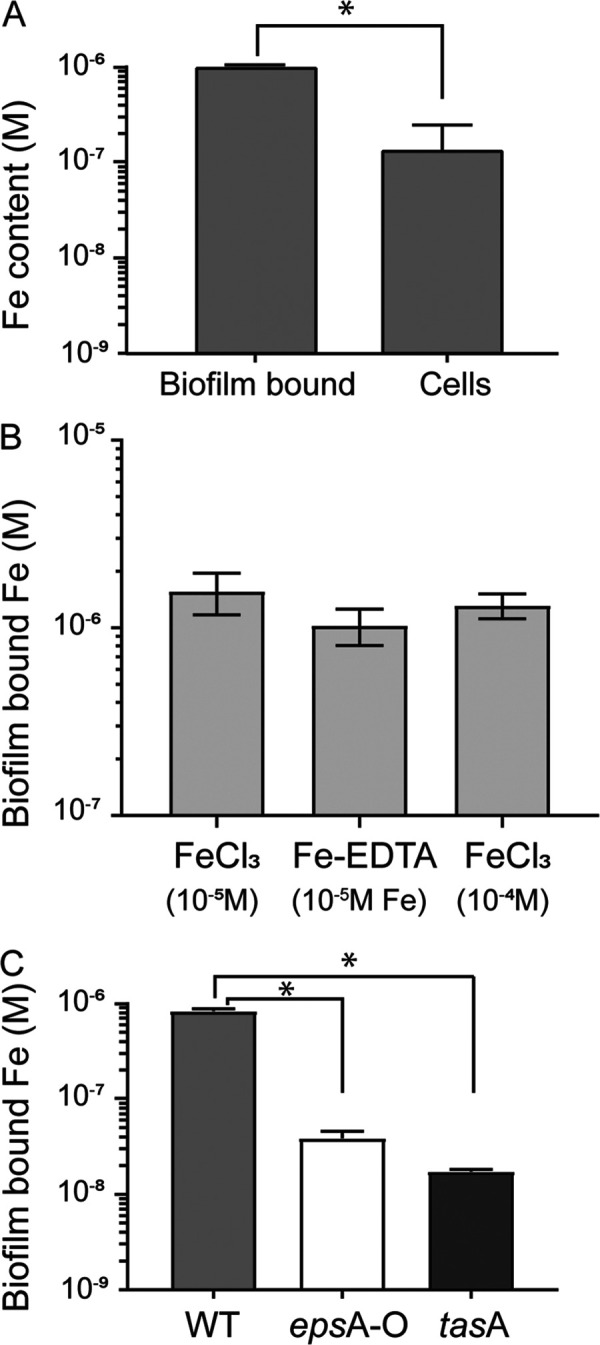
B. subtilis biofilm accumulates Fe. (A) Fe content (in molar) measured in B. subtilis cells and biofilm matrix after 22 h of growth at 30°C in MSgg supplemented with 10−4 M FeCl3 (an asterisk indicates significant difference by t test, P < 0.001). (B) Fe content (in molar) measured in B. subtilis cell biofilm matrix following 28 h of growth at 30°C in MSgg supplemented with Fe provided as FeCl3 (10−5 and 10−4 M) and Fe-EDTA (10−5 M FeCl3 + 10−4 M EDTA). Error bars are standard deviations. (C) Fe content of biofilms formed by the wild type (light gray bars), epsA-O (no exopolysaccharides, white bars), and tasA (no TasA fibers, dark gray bars) cells measured after 22 h of growth at 30°C in MSgg supplemented with 10−4 M FeCl3 (an asterisk indicates significant differences by ANOVA with post hoc Tukey’s test, P < 0.001).
To characterize which components of the biofilm matrix are involved in Fe sequestration, we examined the amount of Fe sequestered in B. subtilis deletion mutants epsA-O and tasA, producing only the protein (TasA) or the exopolysaccharide (EPS) component of the biofilm, respectively. As shown in Fig. 1C, under our experimental conditions both mutants accumulated significantly less Fe in their incomplete biofilms than the wild type (Fig. 1C) (data normalized to biofilm mass are presented in Fig. S2A in the supplemental material). Interestingly, the sum of Fe associated with the biofilms of mutants epsA-O and tasA (5.7 × 10−8 M) only accounted for 6.7% of the Fe associated with wild-type biofilms (8.4 × 10−7 M) (Fig. 1C). Coculture of epsA-O and tasA restored Fe storage in the biofilm at a level comparable to that of the wild type (Fig. S2B). These results suggest a synergic effect of biofilm components on Fe sequestration and that a complete and mature biofilm is required for optimal Fe binding.
Mobilization of biofilm-bound Fe by B. subtilis.
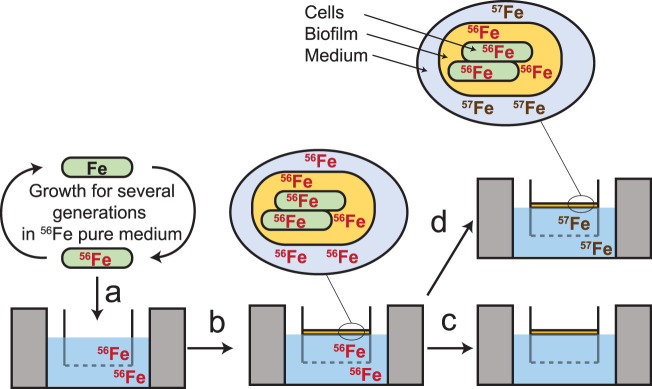
Since high levels of Fe are present in the biofilm, we evaluated if it could be mobilized by B. subtilis NCIB3610 to support its growth. To test this, we used Fe stable isotope labeling of B. subtilis cells and biofilms. With this technique, we were able to obtain cells and biofilms containing only 56Fe, which were then incubated in Fe-depleted medium or medium containing a different isotope, 57Fe. The contribution of Fe in cells, either from within (56Fe) or outside (57Fe) the biofilm, then was determined by analyzing the proportion of Fe isotopes in B. subtilis cells by inductively coupled plasma mass spectrometry (ICP-MS) (Fig. 2; also see Materials and Methods for details).
FIG 2.
Procedure for Fe isotopic labeling of B. subtilis cells and biofilms. Cells were precultured at 30°C in liquid MSgg medium containing pure 56Fe (10−4 M) for several generations to produce 56Fe-labeled cells (a), and then cells were inoculated in an MSgg medium containing pure 56Fe and grown for 22 h to produce the 56Fe-labeled robust biofilm (b). Following biofilm formation, the 56Fe-labeled cells and biofilm were transferred in a new MSgg medium containing no Fe (c) or pure 57Fe, provided as FeCl3 or Fe-tannic acid (d). Fe content (56Fe and 57Fe) in cells and cells plus biofilm was monitored for 6 h. Fe content in the biofilm matrix was calculated by subtracting cellular Fe from total biofilm Fe (cells plus matrix). Acquisition of new Fe was calculated by subtracting the amount of cell-bound Fe (number of cells × Fe cellular quotas) 6 h after transfer to the amount of cell-bound Fe at T0 (composed of 100% 56Fe).
As shown in Fig. 3A, when the biofilm was transferred to an Fe-depleted medium, B. subtilis was still able to grow to some extent (“no Fe” condition). However, this growth was not accompanied by a significant decrease in intracellular Fe levels (Fig. 3B, white bars), showing that cells were able to acquire Fe from the biofilm. Thus, biofilm-bound Fe sustained Fe acquisition and homeostasis, i.e., constant intracellular Fe concentration despite cellular division, and active growth in the absence of environmental Fe. However, the growth was slower than that of cells grown in the presence of environmental Fe (i.e., FeCl3 and Fe-tannic acid) (Fig. 3A). Importantly, after 6 h, the decrease in Fe content in the biofilm (8.6 × 10−8 M ± 3.6 × 10−8 M) (Fig. 3C, white bars) was consistent with the amount of Fe required to sustain growth (number of cells produced × cellular Fe quotas = 1.1 × 10−7 ± 0.1 × 10−7 M) (Fig. 3A and B). This result demonstrated that biofilm-bound Fe could be taken up by the bacteria, but in the absence of environmental Fe, only a fraction of total biofilm-bound Fe was mobilized for uptake, resulting in impeded growth.
FIG 3.
Influence of environmental Fe on B. subtilis growth and Fe acquisition strategy. Panels show cellular growth (A), total intracellular Fe concentration (B), isotopic composition of biofilm-bound Fe (C), and isotopic distribution of new cellular Fe (D) of B. subtilis cells 3 h and 6 h after transfer in a Fe-depleted MSgg medium (white bars in panels B, C, and D, and triangles in panel A), 10−4 M 57FeCl3 (dark gray bars in panels B, C, and D, and circles in panel A), or 10−4 M 57Fe-tannic acid (light gray bars in panels B and D, and squares in panel A).
Effect of environmental Fe on the acquisition of biofilm-bound Fe.
We next evaluated to what extent the use of biofilm-bound Fe could depend on the availability of environmental Fe. Biofilms grown in 56Fe-spiked medium were transferred in medium containing either freshly added 10−4 M 57FeCl3 (a proxy for hydroxides) or 10−4 M 57Fe-tannic acid (a proxy for Fe-natural organic matter complex). Under both conditions (FeCl3 and Fe-tannic acid), B. subtilis NCIB3610 achieved active growth (Fig. 3A) and Fe homeostasis (Fig. 3B). Cells grown in the presence of FeCl3 achieved a higher growth rate and reached stationary phase faster than cells grown in the presence of Fe-tannic acid (Fig. 3A). Interestingly, in the presence of environmental 57FeCl3, after 6 h of growth, 57Fe contributed less than a quarter (19.2% ± 7.9%) of the new Fe required for growth, while in the presence of 57Fe-tannic acid, it contributed close to half of the new Fe required for growth (46.8 ± 32.02) (Fig. 3D). While this difference between the 57FeCl3 and 57Fe-tannic acid treatments was not statistically significant within biological treatments, this trend was observed in all three biological replicates (Fig. S3). Considering that 6 h after transfer similar amount of cells (Fig. 3A) containing similar cellular Fe concentrations (Fig. 3B) were produced, this result implies that cells mobilized close to two times more 56Fe from the biofilm (2.9 × 10−7 ± 0.2 × 10−7 M) in the presence of 57FeCl3 than cells grown without environmental Fe or with 57Fe-tannic acid (1.1 × 10−7 ± 0.1 × 10−7 M and 1.9 × 10−7 ± 0.3 × 10−7 M for no Fe and 57Fe-tannic acid condition, respectively). Importantly, 57FeCl3 contributed significantly more to biofilm-bound Fe than to intracellular Fe (Fig. 3D). This experiment demonstrated that the environmental source of Fe had an important impact on the mobilization of biofilm-bound Fe by B. subtilis.
Efficiency of bacillibactin to compete with hydroxide and tannic acid for Fe.
As shown in Fig. 3B, Fe acquisition from the environment was delayed in the presence of 57Fe-tannic acid compared to 57FeCl3 (Fig. 3D). This delay could result from a lower availability of environmental Fe when present as Fe-tannic acid rather than FeCl3. Previously, we showed that siderophore production is absolutely required under our experimental conditions to support growth of B. subtilis NCIB3610 in standing liquid MSgg medium (5 mM potassium phosphate buffer, pH 7, 0.1 M morpholinepropanesulfonic acid, pH 7, 2 mM MgCl2, 0.05 mM MnCl2, 0.001 mM ZnCl2, 0.002 mM thiamine, 0.5%, vol/vol, glycerol, 0.5% [26.7 mM] glutamate, 0.7 mM CaCl2) (11). Consequently, we hypothesized that the earlier acquisition of environmental 57Fe under the FeCl3 condition reflected the higher efficiency of bacillibactin, the siderophore with the highest affinity for Fe produced by B. subtilis, to recruit Fe from Fe-hydroxides (coming from FeCl3) rather than from Fe-tannic acid. To test this hypothesis, we monitored the formation of Fe-bacillibactin complex in the presence of 10−5 M bacillibactin, the concentration produced by B. subtilis during biofilm formation (11), in MSgg medium containing 10−5 M Fe provided as FeCl3 or Fe-tannic acid. As presented in Fig. 4, after 4 h, 40% of Fe was complexed by bacillibactin in the medium containing FeCl3 compared to only 15% in the medium containing Fe-tannic acid. This observation confirmed the lesser accessibility of Fe-tannic acid than Fe-hydroxides to B. subtilis siderophores.
FIG 4.
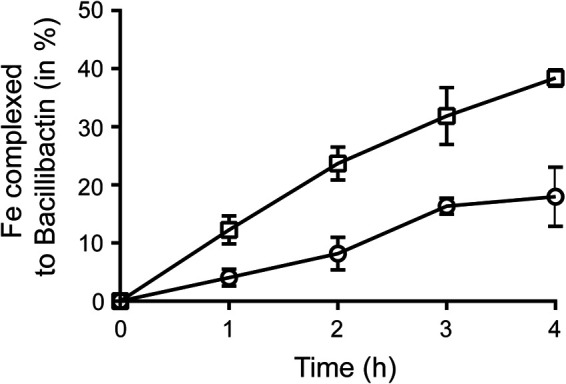
Complexation of Fe by the triscatechol siderophore bacillibactin in the presence of FeCl3 and Fe-tannic acid. Formation of Fe-bacillibactin complex, expressed as a percentage of Fe complexed to bacillibactin, in the presence of 10−5 M Fe-tannic acid (circles) and 10−5 M FeCl3 (squares). Complexation was assayed in Milli-Q water supplemented with 10−5 M bacillibactin.
DISCUSSION
Our results indicate that under static culture conditions, B. subtilis NCIB3610 biofilm can trap large quantities of Fe in its extracellular matrix. Our data also suggest that Fe complexation in the biofilm results from complex interactions between the various components of the matrix, reflecting the role of chemical interactions between exopolysaccharides and protein components in Fe binding. Since both exopolysaccharides and proteins are required for the complete maturation of the biofilm (18), the significantly higher sequestration of Fe by wild-type biofilms compared to those of mutants (Fig. 1C) also suggests that the three-dimensional structure of the biofilm significantly influences Fe sequestration. More research is required to decipher the role of biofilm chemical composition and physical structure on Fe sequestration.
Accumulation of Fe, and potentially other metals, in biofilm matrix might be widespread in environmental bacteria, as illustrated by similar Fe sequestration reported in biofilms of the Gram-negative P. aeruginosa (12). The presence of an Fe pool close to cells would constitute an efficient means of alleviating Fe stress in microorganisms exposed to fluctuations in the availability of elements in the natural environment.
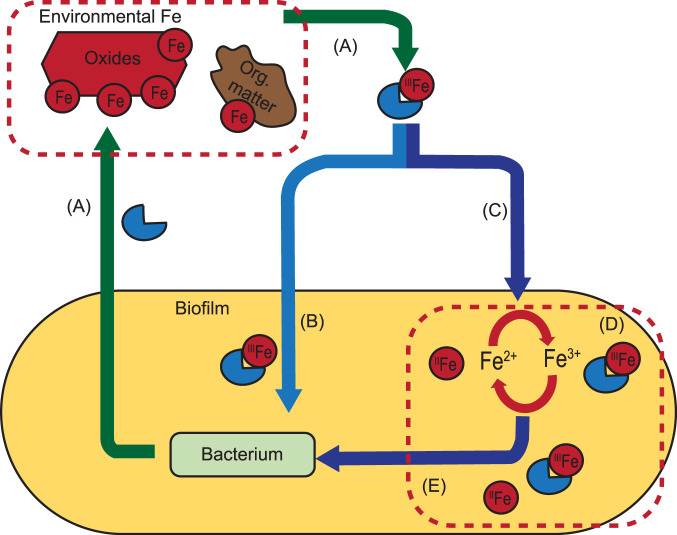
In the absence of environmental (i.e., from outside the biofilm matrix) Fe sources, only a fraction of biofilm-bound Fe is available for uptake, and this amount is insufficient to sustain normal growth. The similar concentrations of Fe bound to the biofilm of B. subtilis NCIB3610 cultures grown under contrasted Fe concentrations and chemical forms suggests a bacterial control over Fe accumulation in the biofilm. Recent studies reported that Fe in the biofilm can contribute to extracellular electron transport (EET) supporting bacterial metabolism (19–21). Thus, Fe bound to the biofilm seems to assume at least two important functions, a local source of Fe for uptake and an extracellular metabolic function, such as EET. Assuming that bacteria need to maintain a minimum pool of Fe in the biofilm for extracellular metabolic function (e.g., EET), we propose a theoretical framework to explain how B. subtilis manages environmental and biofilm-bound Fe for Fe uptake (Fig. 5). In the environment, three sources of Fe could support Fe uptake for growth: available Fe in the biofilm, not required for extracellular metabolism (Fig. 5E), direct Fe uptake from the environment, allowing the preservation of Fe stocks in the biofilm for extracellular needs (Fig. 5B), and uptake of Fe from a biofilm dynamically replenished by environmental Fe (Fig. 5C and E). Under our experimental conditions, mining biofilm-bound Fe for uptake quickly collides with the need to sustain the Fe requirement in the biofilm to support critical metabolic functions (e.g., EET and no Fe conditions). Once the small fraction of biofilm-bound Fe available for uptake is used, bacterial growth (Fe uptake) requires the contribution of external sources. In the presence of 57Fe-tannic acid, bacterial growth can be fully explained by the use of a small fraction of biofilm-bound Fe (similar to the amount used under the no Fe condition) followed by the direct uptake of Fe from the environment by cells (Fig. 5B and E). Indeed, the low accessibility of 57Fe-tannic acid could force cells to acquire Fe locally before accessing the external pool. Environmental 57FeCl3 is more easily accessible, providing an efficient replenishment of the biofilm with environmental 57Fe that matches or exceeds cellular Fe uptake, sustaining both intra- and extracellular Fe needs (Fig. 5C and E). This is illustrated by the higher acquisition of 56Fe from the biofilm (Fig. 3B) and the observed replenishment of the biofilm with environmental 57Fe under the FeCl3 condition (Fig. 3D). The mechanisms leading to the replenishment of Fe in the biofilm remain to be fully characterized.
FIG 5.
Schematic framework for biofilm-bound and environmental Fe management by B. subtilis. (A) Bacteria secrete siderophores in the environment to recruit Fe from natural sources (oxides and organic [Org.] matter). (B and C) Siderophore-Fe complexes can be directly taken up by cells (B) and/or contribute to refuel Fe in the biofilm (C). (D and E) The biofilm contains large amounts of Fe (an order of magnitude more than cells), a large fraction of which is used for extracellular metabolic functions (e.g., EET) (D), and the rest can be mobilized for uptake (E). The mechanisms underpinning the accumulation of Fe in the biofilm and the mobilization of Fe from the biofilm for uptake remain unclear.
Recently, we reported that siderophore production and biofilm formation are both essential for B. subtilis NCIB3610 growth and Fe homeostasis in static culture and that concentrations of bacillibactin in both the biofilm and the supernatant of B. subtilis standing cultures are similar (11). Thus, siderophores could contribute to refueling the biofilm by acting as a shuttle between environmental sources and the biofilm. The faster complexation of Fe from FeCl3 compared to that of Fe-tannic acid (Fig. 4) results in faster and higher availability of environmental Fe (Fe-siderophore complex) for uptake and subsequent biofilm refueling.
Nonetheless, our results, along with those of recent studies, suggest that Fe acquisition by biofilm-forming bacteria, such as B. subtilis, is complex and modulated by intra- and extracellular Fe requirements and environmental Fe availability (abundance and chemical form). More research on Fe requirements for extracellular metabolic functions and on the mechanisms underpinning Fe transport to the biofilm and to cells is warranted and would allow a more comprehensive understanding of Fe homeostasis in biofilm-forming bacteria. The high requirements of biofilm-bound Fe for normal growth observed in this study (Fig. 3) invite further research to decipher the real function and homeostasis of Fe in the biofilm.
MATERIALS AND METHODS
Strains, isotopic labeling of cells, and biofilm.
All experiments were conducted with Bacillus subtilis strain NCIB3610, except for those shown in Fig. 1, where strains SSB488 (3610 epsA-O::tet) (18) and CA017 (3610 tasA::kan) (22) were used. Biofilm medium used throughout this study was MSgg without Fe (5 mM potassium phosphate buffer, pH 7, 0.1 M morpholinepropanesulfonic acid, pH 7, 2 mM MgCl2, 0.05 mM MnCl2, 0.001 mM ZnCl2, 0.002 mM thiamine, 0.5%, vol/vol, glycerol, 0.5% [26.7 mM] glutamate, 0.7 mM CaCl2) (23). Milli-Q deionized water was used to prepare the medium. Prior to medium preparation, glassware was washed for 24 h with a 10% solution of HCl (trace metal reagent) and rinsed three times with Milli-Q water to prevent Fe contaminations of the MSgg. To prepare the inoculum for biofilm formation marked with intracellular 56Fe, B. subtilis cells were precultured from glycerol stocks for 5 h at 37°C with shaking at 150 rpm in MSgg medium containing 10−4 M pure 56FeCl (56FeCl3, 99% purity; Trace Sciences International Corp.). Cells were diluted 1:25, and this preculture was repeated 3 times to ensure maximum depletion of the other natural Fe isotopes. The culture was then diluted in 50 ml of MSgg medium without Fe added to generate an inoculum with an optical density at 595 nm of 1 ± 0.07. To prepare the 57Fe-labeled biofilm, wells (15.5 ml) from sterile 6-well plates were filled with a cell culture insert (Fisher Scientific) and 4.8 ml of MSgg medium with 10−4 M enriched 57FeCl3 (57FeCl3, 99% purity; Trace Sciences International Corp.) and inoculated with 150 μl of the 56Fe-marked B. subtilis inoculum suspension (time zero) (Fig. 2). Incubations were performed at 30°C until robust biofilm formation (∼22 h). As described previously (11), under these conditions growth is supported solely by the dilution of intracellular Fe stocks, in this case 56Fe (Fig. 2). The inserts containing 56Fe-labeled cells and 57Fe-labeled biofilm then were sterilely transferred into new wells containing MSgg with no Fe or 10−4 M pure 54Fe (54FeCl3, 95% purity; Trace Sciences International Corp.), provided as FeCl3 or Fe-tannic acid complex. When indicated, Fe was added to the medium from a solution of isotopic 54FeCl3 (37% HCl) or precomplexed with tannic acid (1 h). In the presence of tannic acid, 72.9% of Fe was complexed (calculated using the complexation constant reported by Sungur and Uzar [24]). The final Fe concentration is specified in the legend of each figure. Finally, intracellular Fe content, and its isotopic identity, was analyzed after 0 h, 3 h, and 6 h.
Cell isolation and Fe analysis.
The methodology and controls for cell and biofilm isolation and analysis are described in Rizzi et al. (11). For elemental analysis, cells were digested on an SCP Science Digiprep Jr with 1 ml of nitric acid (trace metal grade; Fisher Chemical) at 65°C for 45 min. After digestion, each tube was filled to 10 ml with Milli-Q water. Samples were analyzed for phosphorus and Fe content on an inductively coupled plasma mass spectrometer (ICP-MS; XSeries2; Thermo Scientific) as previously described (11). Cell numbers were determined based on cellular phosphorus content, which, under our experimental conditions, is linearly correlated with cell density in B. subtilis (11).
Fe complexation by bacillibactin.
Complexation of freshly precipitated Fe (10−5 M FeCl3) or precomplexed Fe-tannic acid (10−5 M) by bacillibactin (10−5 M) in water was monitored over time by UV-visible (UV-Vis) spectrometry (Genesys 10S; Thermo Scientific) at 330 nm. Bacillibactin was extracted and purified as described previously by Miethke et al. (25). This wavelength was selected to avoid spectral overlap between the spectrum of the Fe-bacillibactin complex and the spectra of apo-bacillibactin and Fe-tannic acid complex (see Fig. S1 in the supplemental material). Fe-tannic acid complexes were prepared by adding tannic acid and Fe (as FeCl3) in a 1/1 ratio (10−4 M) in Milli-Q water and allowing the complex to form for 2 h at room temperature on a rotating shaker. The concentration of Fe-bacillibactin complex formed over time was calculated using the extinction coefficient of Fe-bacillibactin at 330 nm (Ɛ = 12,800) (26).
Statistical analysis.
Statistical analyses were performed using GraphPad Prism, version 8. A t test was used to test the difference in Fe content between cells and the biofilm (P < 0.001) (Fig. 1A). The difference in Fe bound to the biofilm between the WT and the mutants (epsA-O and tasA) was tested by analysis of variance (ANOVA) (P < 0.001 by Tukey's post hoc test) (Fig. 1C). The difference in Fe contents (Fe bound and intracellular Fe) between treatments was tested using a two-way ANOVA (P < 0.001 by Benjamini-Kriger-Yekutieli test) (Fig. 3).
Supplementary Material
ACKNOWLEDGMENTS
We thank Olivier Savary for his critical help with the quantitation of isotopic iron.
This project was supported by the Fonds de Recherche du Québec–Nature et Technologies (FRQNT) through the Program de Recherche en Partenariat sur le Développement Durable du Secteur Minier (S.R. and J.-P.B.), the Canadian Research Chair in boreal biogeochemistry (J.-P.B.), and an NSERC discovery grant (P.B.B.).
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Frausto Da Silva JJR, Williams RJP. 1991. The biological chemistry of the elements: the inorganic chemistry of life. Oxford University Press, New York, NY. [Google Scholar]
- 2.Wedepohl KH. 1995. The composition of the continental crust. Geochim Cosmochim Acta 59:1217–1232. doi: 10.1016/0016-7037(95)00038-2. [DOI] [Google Scholar]
- 3.Kabata-Pendias A. 2000. Trace elements in soils and plants, 3rd ed CRC Press, Boca Raton, FL. [Google Scholar]
- 4.Andrews SC, Robinson AK, Rodríguez-Quiñones F. 2003. Bacterial iron homeostasis. FEMS Microbiol Rev 27:215–237. doi: 10.1016/S0168-6445(03)00055-X. [DOI] [PubMed] [Google Scholar]
- 5.Neilands J. 1995. Siderophores: structure and function of microbial iron transport compounds. J Biol Chem 270:26723–26726. doi: 10.1074/jbc.270.45.26723. [DOI] [PubMed] [Google Scholar]
- 6.Kraemer SM. 2004. Iron oxide dissolution and solubility in the presence of siderophores. Aquat Sci 66:3–18. doi: 10.1007/s00027-003-0690-5. [DOI] [Google Scholar]
- 7.Costerton JW, Cheng KJ, Geesey GG, Ladd TI, Nickel JC, Dasgupta M, Marrie TJ. 1987. Bacterial biofilms in nature and disease. Annu Rev Microbiol 41:435–464. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- 8.Flemming H-C, Wingender J, Szewzyk U, Steinberg P, Rice SA, Kjelleberg S. 2016. Biofilm: an emergent form of bacterial life matrix. Nat Rev Microbiol 14:563–575. doi: 10.1038/nrmicro.2016.94. [DOI] [PubMed] [Google Scholar]
- 9.Patriquin GM, Banin E, Gilmour C, Tuchman R, Greenberg EP, Poole K. 2008. Influence of quorum sensing and iron on twitching motility and biofilm formation in Pseudomonas aeruginosa. J Bacteriol 190:662–671. doi: 10.1128/JB.01473-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kolodkin-Gal I, Elsholz AKW, Muth C, Girguis PR, Kolter R, Losick R. 2013. Respiration control of multicellularity in Bacillus subtilis by a complex of the cytochrome chain with a membrane-embedded histidine kinase. Genes Dev 27:887–899. doi: 10.1101/gad.215244.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rizzi A, Roy S, Bellenger J, Beauregard PB. 2018. Iron homeostasis in Bacillus subtilis requires siderophore production and biofilm formation. Appl Environ Microbiol 85:1–10. doi: 10.1128/AEM.02439-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu S, Wei Q, Zhao T, Guo Y, Ma LZ. 2016. A survival strategy for Pseudomonas aeruginosa that uses exopolysaccharides to sequester and store iron to stimulate Psl-dependent biofilm formation. Appl Environ Microbiol 82:6403–6413. doi: 10.1128/AEM.01307-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papageorgiou SK, Kouvelos EP, Favvas EP, Sapalidis AA, Romanos GE, Katsaros FK. 2010. Metal-carboxylate interactions in metal-alginate complexes studied with FTIR spectroscopy. Carbohydr Res 345:469–473. doi: 10.1016/j.carres.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Miethke M, Kraushaar T, Marahiel MA. 2013. Uptake of xenosiderophores in Bacillus subtilis occurs with high affinity and enhances the folding stabilities of substrate binding proteins. FEBS Lett 587:206–213. doi: 10.1016/j.febslet.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 15.Rizzi A. 2020. Le biofilm de Bacillus Subtilis: un élément central dans la gestion de son homéostasie du fer. Ph.D. thesis Université de Sherbrooke, Sherbrooke, Quebec, Canada: https://savoirs.usherbrooke.ca/handle/11143/16425?show=full. [Google Scholar]
- 16.Zhiqiang H, Jing J, Héctor DA, Paul LH, Anthony GH, William CG, Michael LS, Gabriela H, Leonard WL. 2007. Spatial distributions of copper in microbial biofilms by scanning electrochemical microscopy. Environ Sci Technol 41:936–941. doi: 10.1021/es061293k. [DOI] [PubMed] [Google Scholar]
- 17.Hu Z, Hidalgo G, Houston PL, Hay AG, Shuler ML, Abruña HD, Ghiorse WC, Lion LW. 2005. Determination of spatial distributions of zinc and active biomass in microbial biofilms by two-photon laser scanning microscopy. Appl Environ Microbiol 71:4014–4021. doi: 10.1128/AEM.71.7.4014-4021.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Branda SS, Chu F, Kearns DB, Losick R, Kolter R. 2006. A major protein component of the Bacillus subtilis biofilm matrix. Mol Microbiol 59:1229–1238. doi: 10.1111/j.1365-2958.2005.05020.x. [DOI] [PubMed] [Google Scholar]
- 19.Li S, Li L, Qu Q, Kang Y, Zhu B, Yu D, Huang R. 2019. Biointerfaces extracellular electron transfer of Bacillus cereus biofilm and its effect on the corrosion behaviour of 316L stainless steel. Colloids Surf B Biointerfaces 173:139–147. doi: 10.1016/j.colsurfb.2018.09.059. [DOI] [PubMed] [Google Scholar]
- 20.Qin Y, He Y, She Q, Larese-Casanova P, Li P, Chai Y. 2019. Heterogeneity in respiratory electron transfer and adaptive iron utilization in a bacterial biofilm. Nat Commun 10:1–12. doi: 10.1038/s41467-019-11681-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keogh D, Lam LN, Doyle LE, Matysik A, Pavagadhi S, Umashankar S, Low PM, Dale JL, Song Y, Ng SP, Boothroyd CB, Dunny GM, Swarup S, Williams RBH, Marsili E, Kline KA. 2018. Extracellular electron transfer powers Enterococcus faecalis biofilm metabolism. mBio 9:1–17. doi: 10.1128/mBio.00626-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vlamakis H, Aguilar C, Losick R, Kolter R. 2008. Control of cell fate by the formation of an architecturally complex bacterial community. Genes Dev 22:945–953. doi: 10.1101/gad.1645008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Branda SS, Gonza E, Ben-Yehuda S, Losick R, Kolter R. 2001. Fruiting body formation by Bacillus subtilis. Proc Natl Acad Sci U S A 98:11621–11626. doi: 10.1073/pnas.191384198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sungur S, Uzar A. 2008. Investigation of the complexes tannic acid and myricetin with Fe(III). Spectrochim Acta Part A: Mol Biomol Spectrosc 69:225–229. doi: 10.1016/j.saa.2007.03.038. [DOI] [PubMed] [Google Scholar]
- 25.Miethke M, Klotz O, Linne U, May JJ, Beckering CL, Marahiel MA. 2006. Ferri-bacillibactin uptake and hydrolysis in Bacillus subtilis. Mol Microbiol 61:1413–1427. doi: 10.1111/j.1365-2958.2006.05321.x. [DOI] [PubMed] [Google Scholar]
- 26.Abergel RJ, Zawadzka AM, Hoette TM, Kenneth RN. 2009. Enzymatic hydrolysis of trilactone siderophores: where chiral recognition occurs in enterobactin and bacillibactin iron transport. J Am Chem Soc 131:12682–12692. doi: 10.1021/ja903051q. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.