Abstract
Obstructive sleep apnea (OSA) is a chronic prevalent condition characterized by intermittent hypoxia (IH) and sleep fragmentation (SF). Evidence suggests that OSA can alter the gut microbiome (GM) diversity and composition that may then promote the occurrence of some of the OSA-associated morbidities. However, it is unclear whether perturbations in the GM caused by IH can elicit sleep disturbances that underlie the increased sleep propensity that occurs in IH-exposed mice. To evaluate this issue, we exposed C57Bl/6J mice to IH or room air (RA) for 6 weeks, and fecal matter was collected and frozen. C57Bl/6J naïve mice were then randomly assigned to a fecal microbiota transfer (FMT) protocol for 3 weeks with either IH or RA fecal slur, and their GM was then analyzed using 16s rRNA sequencing. In addition, FMT recipients underwent sleep recordings using piezoelectric approaches for 3 consecutive days. As anticipated, FMT-IH and FMT-RA mice showed different taxonomic profiles that corresponded to previous effects of IH on GM. Furthermore, FMT-IH mice exhibited increased sleep duration and the frequency of longer sleep bouts during the dark cycle, suggesting increased sleepiness (p<0.0001 vs. FMT-RA mice). Thus, alterations of GM diversity induced by IH exposures can elicit sleep disturbances in the absence of concurrent IH, suggesting that sleep disturbances can be mediated, at least in part, by IH-induced alterations in GM.
Keywords: sleep apnea, intermittent hypoxia, fecal matter transfer, gut microbiome, sleep
Introduction
Obstructive sleep apnea (OSA) is a chronic condition affecting a billion people around the world (Benjafield et al., 2019), characterized by episodes of repetitive airway collapse during sleep that in turn induce intermittent hypoxia (IH), and hypercapnia, as well as disrupted sleep due to recurrent arousals resulting in sleep fragmentation (SF) (Almendros et al., 2020; Badran et al., 2015). As a result, OSA patients frequently manifest excessive daytime sleepiness (He and Kapur, 2017; Zinchuk et al., 2017), and may develop metabolic (Gileles-Hillel et al., 2016), cognitive (Gagnon et al., 2014) and cardiovascular morbidities (Manrique-Acevedo et al., 2020). Evidence from animal models shows that IH exposures can impair sleep architecture via shortening total wakefulness and increasing sleepiness in a sex-dependent manner (Sanfilippo-Cohn et al., 2006). These changes in sleep patterns can be persistent, even two weeks after cessation of IH exposures (Veasey et al., 2013).
Over 100 trillion microorganisms are harbored as an integrated and highly regulated ecosystem in the human intestine (Guinane and Cotter, 2013). This finely tuned and responsive network plays an essential role in regulating host immunity (Rooks and Garrett, 2016), metabolism (Velagapudi et al., 2010) and circadian rhythms (Parkar et al., 2019) . Changes in the gut microbial community ecosystems that result in altered proportion and diversity of commensal bacteria, also known as dysbiosis, can be induced by a variety of environmental factors (Phillips, 2009), medications (Walsh et al., 2018) and lifestyle including variations in nutritional intake (Yang et al., 2020). Dysbiosis has been linked to chronic diseases, such as inflammatory bowel disease (Ni et al., 2017), metabolic syndrome (Boulangé et al., 2016), cancer (Sheflin et al., 2014), depression and cognitive dysfunction (Paiva et al., 2020; Pascale et al., 2020), and cardiovascular disease (Lau et al., 2017). Evidence points to a bidirectional association between sleep disturbances and dysbiosis through disruption of the brain-gut-microbiota axis (BGMA) (Smith et al., 2019). For instance, short-term partial sleep deprivation can alter gut microbiome in humans (Benedict et al., 2016), while high sleep quality is associated with higher proportions of Phyla associated with enhanced performance on cognitive tasks (Anderson et al., 2017). Furthermore, SF mimicking OSA has been shown to alter the gut microbiome (GM), increase gut permeability and ultimately induce insulin resistance (Poroyko et al., 2016). However, the mechanisms underlying the interrelationships between the GM and sleep are still poorly explored.
The cumulative evidence to date shows that OSA in humans and exposing animals to chronic IH recapitulating the oxygenation patterns of patients with OSA can reproducibly alter the GM diversity and abundance. Indeed, fecal matter collected from OSA patients revealed gut microbial dysbiosis varying with the severity of OSA, and the type of enterotypes that were affected seemed to further suggest that some of the OSA-induced changes in GM may underlie some of the sleep and metabolic perturbations in such patients (C.-Y. Ko et al., 2019; C. Y. Ko et al., 2019). In animals, exposures to IH resulted in profound alterations in gut microbiota (Lucking et al., 2018; Moreno-Indias et al., 2016; TIAN et al., 2018; Tripathi et al., 2019, 2018) that was persistent even after returning to normoxic conditions for six weeks (Moreno-Indias et al., 2016). Evidently, dysbiosis triggered by IH may mediate cardiometabolic disease in OSA patients (N. Farré et al., 2018; Mashaqi and Gozal, 2019). However, there are no studies exploring whether IH-mediated GM alterations can affect sleep characteristics per se in the absence of concurrent IH. Therefore, we hypothesized that transplantation of fecal microbiota from mice exposed to IH to naïve mice may alter their gut microbiota and disrupt their sleep patterns.
Methods
All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Missouri. Male C57BL/6J mice (8 weeks old) were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). All animals were allowed to recover within the animal care facility for 7 days upon arrival. Animals were housed in a controlled environment with 12 h light–dark cycles (07.00 h–19.00 h) at constant temperature (24 ± 0.2°C) with ad libitum access to food (normal chow) and water. At the end of the experimental period, mice were euthanized using carbon dioxide (1–2 min) followed by cervical dislocation.
Intermittent hypoxia
The IH exposure protocol used has been described in detail previously (Gozal et al., 2017). Briefly, room air (RA) mice were housed in standard housing conditions, and intermittent hypoxia (IH) mice were exposed to IH for 6 weeks. IH consisted of exposures alternating 21% FIO2 and 6.1% FIO2, 20 cycles h−1 for 12 h day−1 during daylight (07:00 h – 19:00 h) using a commercially available system (80 × 50 × 50 cm; Oxycycler A44XO, BioSpherix, Redfield, NY, USA). These exposures recapitulate nadir oxyhemoglobin saturations in the range of 68–75%, which are the dominant correlate of moderate to severe OSA in humans (R. Farré et al., 2018). The rest of the day (19.00 h–07.00 h), the mice were in normoxic conditions (21% FIO2).
Fecal Microbiota Transplantation (FMT) in Naïve Mice
Fecal samples from mice exposed to RA or IH were collected, and FMT was performed by oral gavage of a fecal slurry into naïve mice (C57BL/6, Jackson Lab) as described (Stebegg et al., 2019). Recipient lean mice were fasted for 2 hours prior to FMT, and the fecal slurry were obtained from fecal pellets of 5 donor mice suspended by vortexing in 1 mL PBS per 100 mg of feces. Fecal mixtures were centrifugated at 500g for 5 min and the supernatants were collected for FMT. Each recipient mouse received 100 μl of fecal slurry by oral gavage twice/week for 3 weeks in both groups: i) FMT-RA and ii) FMT-IH. At the end of this period, fecal samples were collected from recipient mice were collected for further analyses.
16S rRNA Sequencing of gut microbiota
Feces from FMT-RA and FMT-IH mice were processed using PowerFecal kits (Qiagen, Germany) according to the manufacturer’s instructions (Poroyko et al., 2016). Briefly, bacterial 16S rRNA amplicons were constructed via amplification of the V4 region of the 16S rRNA gene with universal primers (U515F/806R) previously developed against the V4 region, flanked by Illumina standard adapter sequences (Caporaso et al., 2011). The final amplicon pool was evaluated using the Advanced Analytical Fragment Analyzer automated electrophoresis system, quantified using quant-iT HS dsDNA reagent kits (Invitrogen, Carlsbad, CA, USA), and diluted according to Illumina’s standard protocol for sequencing on the MiSeq instrument (Ilumina, San Diego, CA, USA). Paired DNA sequences were merged using FLASH software (Magoč and Salzberg, 2011). Cutadapt (https://github.com/marcelm/cutadapt) was used to remove the primers at both ends of the contig and cull contigs that did not contain both primers. To assess the structure and integration of the microbial community, taxonomy was assigned to selected operating taxonomic units (OTUs) using BLAST against the SILVA database v128 of 16S rRNA sequences and taxonomy. OTUs where then used to calculate dissimilarity matrix for Principal Coordinate analysis (PCoA).
Sleep scoring
Sleep/wake activity was acquired and recorded using a piezoelectric monitoring system as described previously (Donohue et al., 2008). The system records pressure changes using a piezoelectric film, the duration and intensity of which are automatically scored by computer algorithms and classified as wake or sleep states. The system has been previously validated and exhibits 90% accuracy when compared to EEG/EMG-based sleep acquisition and scoring approaches (Mang et al., 2014). Data collected were binned over 5 mins using a rolling average of percentage sleep, and by individual sleep bout length from which mean bout lengths were calculated. Sleep bouts were defined by any contiguous sleep pattern that remained uninterrupted by arousal periods of more than 30 s. In addition, bout length counts were only initiated when a 30-second interval contained greater than 50% sleep and were terminated when a 30-second interval contained less than 50% sleep (Paulose et al., 2019).
Statistical analysis
For gut microbiome analysis, two-tailed P values were calculated for all pairwise multiple comparison procedures using the Student-Newman-Keuls test among groups. Statistical analyses were conducted using SPSS 21.0 software for Windows (IBM, Armonk, NY). Multivariate statistical analyses such as PLS-DA, ANOVA, box plots, and volcano plots were performed with the MetaboAnalyst 3.0 program after data pre-treatments, i.e., normalization to the sum, log transformation and Pareto scaling (http://www.metaboanalyst.ca/). Changes in metabolite abundances were considered statistically significant when their p values were <0.05. Statistical significance of the obtained PLS-DA model was evaluated with a permutation test (permutation number: 1,000). The PLS-DA model was considered statistically significant if the permutation test p value <0.05. Data was expressed as mean ± SD.
For sleep scoring, statistical analysis was performed using Prism 8 (GraphPad, San Diego, Ca, USA). Two-way ANOVA with repeated measures and Sidak post-hoc test, and unpaired t-test were used as appropriate. The data were expressed as mean ± SEM. A p value < 0.05 was considered statistically significant.
Results
Changes in gut microbiome composition in naïve mice subjected to FMT from mice exposed to RA and IH
The PCoA plot reveals marked differences in fecal bacterial composition in FMT-RA and FMT-IH groups (Figure 1A). As in other mammalian hosts, Bacteroidetes and Firmicutes were the predominant phyla with phylum Bacteroidetes dominated by class Bacteroida, order Bacteroidales and Phylum Firmicutes being dominated by class Clostridia, order Clostridiales (Table 1). The relative abundance of bacterial taxa in FMT-IH group shows higher abundance of Lachnospiraceae from the phylum Firmicutes, Prevotella and Muribacululaceae from the phylum Bacteroidetes, and lower abundance of Alistipes from the phylum Bacteroidetes when compared to FMT-RA (Figure 1B). Twenty-nine taxa detected had significantly greater relative abundance in both groups (Figure 1C, 1D). The OTUs achieving significance are listed in Table 1. Thus. FMT procedures using different fecal GM as obtained from IH-exposed and RA-exposed mice generated alteration in the GM of naïve mice to recapitulate the previously documented differences in GM induced by IH.
Figure 1:
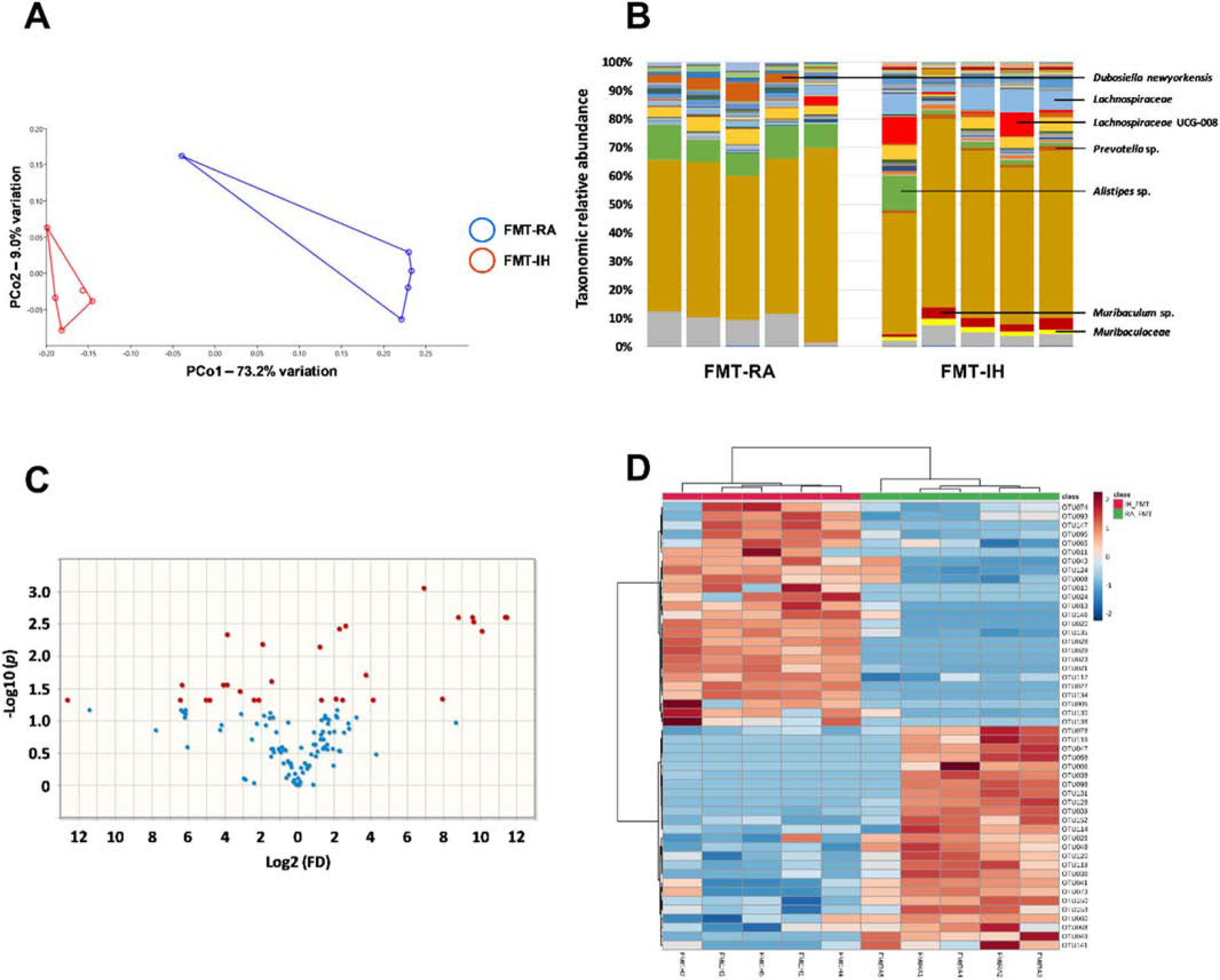
Alterations in fecal bacterial composition between FMT-IH and FMT-IA. A) principal coordinate analysis plot ordinated using Jaccard similarities. B) Stacked bar chart showing the mean relative abundance of bacterial taxa, C) Volcano plot showing fold difference (FD, x-axis) and p-value (Y-axis) associated with taxa detected at significantly greater relative abundance in FMT-IH (left) and FMT-RA (right), D) Hierarchical cluster analysis of the 50 consistently detected OTUs in the gut microbiota. FMT: fecal microbiota transplantation, IH: intermittent hypoxia, RA: room air, OUT: operational taxonomic unit
Table 1:
OTUs achieving statistical significance in mice receiving FMT from mice exposed to IH and RA
| Phylum | Class | Order | Family | Genus | Taxon | OTUs | t.stat | p.value | FDR |
|---|---|---|---|---|---|---|---|---|---|
| Bacteroidetes | Bacteroidia | Bacteroidales | Muribaculaceae | Ambiguous_taxa | Ambiguous_taxa | OTU020 | 13.785 | 7.30E-06 | 0.000898 |
| Bacteroidetes | Bacteroidia | Bacteroidales | Prevotellaceae | Prevotella 9 | uncultured bacterium | OTU023 | 18.198 | 5.36E-05 | 0.002534 |
| Bacteroidetes | Bacteroidia | Bacteroidales | Muribaculaceae | Muribaculum | uncultured bacterium | OTU021 | 10.9 | 7.14E-05 | 0.002534 |
| Bacteroidetes | Bacteroidia | Bacteroidales | Rikenellaceae | Rikenella | uncultured bacterium | OTU027 | 15.666 | 9.70E-05 | 0.002534 |
| Bacteroidetes | Bacteroidia | Bacteroidales | Rikenellaceae | Rikenellaceae RC9 gut group | Ambiguous_taxa | OTU028 | 15.428 | 0.000103 | 0.002534 |
| Patescibacteria | Saccharimonadia | Saccharimonadales | Saccharimonadaceae | Candidatus Saccharimonas | Ambiguous_taxa | OTU134 | 12.759 | 0.000146 | 0.002983 |
| Actinobacteria | Coriobacteriia | Coriobacteriales | Eggerthellaceae | uncultured | Ambiguous_taxa | OTU013 | 6.8291 | 0.000196 | 0.003449 |
| Patescibacteria | Saccharimonadia | Saccharimonadales | Saccharimonadaceae | Candidatus Saccharimonas | uncultured bacterium | OTU135 | 8.0021 | 0.000249 | 0.003832 |
| Bacteroidetes | Bacteroidia | Bacteroidales | Tannerellaceae | Parabacteroides | uncultured bacterium | OTU029 | 11.715 | 0.000304 | 0.004149 |
| Firmicutes | Clostridia | Clostridiales | Clostridiales vadinBB60 group | Ambiguous_taxa | Ambiguous_taxa | OTU048 | −6.6173 | 0.000381 | 0.004683 |
| Proteobacteria | Gammaproteobacteria | Betaproteobacteriales | Burkholderiaceae | Parasutterella | uncultured bacterium | OTU150 | −5.6107 | 0.000594 | 0.006637 |
| Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | Ruminococcaceae NK4A214 group | uncultured bacterium | OTU112 | 6.475 | 0.00071 | 0.007281 |
| Proteobacteria | Deltaproteobacteria | Desulfovibrionales | Desulfovibrionaceae | Desulfovibrio | uncultured bacterium | OTU146 | 4.5261 | 0.002093 | 0.019803 |
| Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | Ruminococcaceae UCG-014 | Ambiguous_taxa | OTU120 | −4.2698 | 0.002827 | 0.024833 |
| Firmicutes | Bacilli | Bacillales | Staphylococcaceae | Jeotgalicoccus | Ambiguous_taxa | OTU036 | −4.8013 | 0.003423 | 0.028068 |
| Firmicutes | Clostridia | Clostridiales | Clostridiales vadinBB60 group | uncultured bacterium | uncultured bacterium | OTU049 | −5.9213 | 0.003813 | 0.028222 |
| Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | Ruminococcaceae UCG-013 | uncultured bacterium | OTU119 | −5.1049 | 0.003901 | 0.028222 |
| Firmicutes | Bacilli | Lactobacillales | Lactobacillaceae | Lactobacillus | Lactobacillus gasseri | OTU041 | −4.6872 | 0.005179 | 0.035387 |
| Proteobacteria | Deltaproteobacteria | Desulfovibrionales | Desulfovibrionaceae | uncultured | uncultured Desulfovibrionales bacterium | OTU147 | 5.0068 | 0.007454 | 0.046383 |
| Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | Lachnoclostridium | Ambiguous_taxa | OTU065 | 3.547 | 0.007542 | 0.046383 |
| Firmicutes | Erysipelotrichia | Erysipelotrichales | Erysipelotrichaceae | Dubosiella | Dubosiella newyorkensis | OTU128 | −4.8467 | 0.00834 | 0.048129 |
| Tenericutes | Mollicutes | Anaeroplasmatales | Anaeroplasmataceae | Anaeroplasma | Ambiguous_taxa | OTU152 | −3.9595 | 0.00917 | 0.048129 |
| Actinobacteria | Coriobacteriia | Coriobacteriales | Eggerthellaceae | Enterorhabdus | uncultured bacterium | OTU008 | 3.6846 | 0.009439 | 0.048129 |
| Firmicutes | Bacilli | Bacillales | Staphylococcaceae | Staphylococcus | bacterium R78 | OTU039 | −4.4948 | 0.010041 | 0.048129 |
| Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | Lachnospiraceae UCG-004 | uncultured bacterium | OTU073 | −3.9841 | 0.010346 | 0.048129 |
| Tenericutes | Mollicutes | Mollicutes RF39 | Ambiguous_taxa | Ambiguous_taxa | Ambiguous_taxa | OTU153 | −3.4495 | 0.010391 | 0.048129 |
| Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | Marvinbryantia | uncultured bacterium | OTU078 | −3.8306 | 0.011001 | 0.048129 |
| Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | [Eubacterium] coprostanoligenes group | uncultured bacterium | OTU124 | 3.9647 | 0.011287 | 0.048129 |
| Firmicutes | Erysipelotrichia | Erysipelotrichales | Erysipelotrichaceae | Faecalibaculum | uncultured bacterium | OTU130 | 3.4462 | 0.011348 | 0.048129 |
FDR: false discovery rate, IH: intermittent hypoxia, OUT: operational taxonomic unit, RA: room air
Naïve mice receiving FMT from mice exposed to IH sleep more during the dark-phase
We assessed sleep-wake profiles in FMT-RA and FMT-IH groups for 3 days starting at 12pm. As shown in representative recordings (Figure 2A), the sleep-wake profiles were markedly different between the two groups, especially during the dark-phase. Wake time quantification showed that the percentage of wake time in the dark-phase decreased in the FMT-IH group (45.2 ± 1.6%) when compared to FMT-RA (58.3 ± 1.6%, p < 0.0001, Figure 2B), while the percentage of wake time in the light-phase increased, but was not significantly different in FMT-IH (32.9 ± 1.9%) when compared to FMT-RA (28.5 ± 1.2%, p > 0.05). In light of the modest increase in wake duration during the light-phase and the considerable decrease in wake duration during the dark-phase, the total wake time percentage over the 24-hour cycle showed significant decreases in the FMT-IH group (61.1 ± 1.1%) when compared to FMT-RA group (57.2 ± 1.1, p < 0.05).
Figure 2:
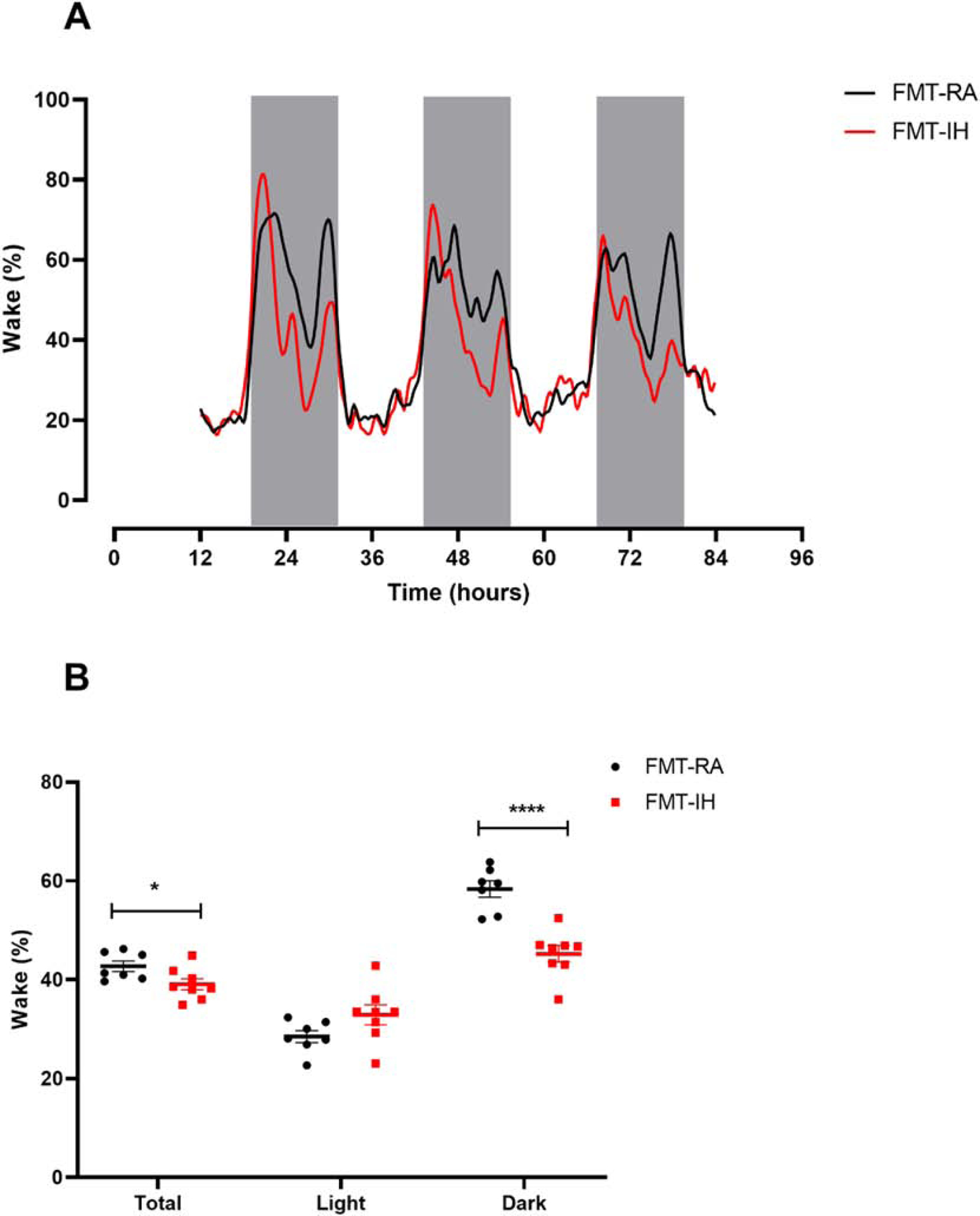
Changes in sleep patterns between FMT-IH and FMT-RA. A) Representative sleep-wake profiles of FMT-IH and FMT-IA groups over 3 days. B) Quantification of total, light-phase and dark-phase average wake percentages. Groups were compared using Student’s t test (n=7–8). *p < 0.05, ** p < 0.01, ***p < 0.001, ****p < 0.0001.
We also examined the mean sleep bout length, and significant increases in the dark phase in FMT-IH (672.1 ± 87.9 s) emerged when compared to FMT-RA (427.4 ± 58.3 s, p < 0.05, Figure 3A). However, the sleep bout length in the FMT-IH group during the light phase (1384.1 ± 247.1 s) did not differ from that of the FMT-RA group (909.3 ± 62.4 s, p > 0.05). The net result indicated higher but not significant total sleep bout length in the FMT-IH treated mice (951.9 ± 146.1 s) when compared to the FMT-RA group (618.4 ± 57.5 s, p > 0.05). Furthermore, the FMT-IH treated mice showed significantly lower numbers of short sleep bouts (i.e., 30 s duration; n=57.7 ± 5.3) and of 60 s sleep bouts (n=40.2 ± 4.7) when compared to FMT-RA mice (30s bouts – n=93.7 ± 16.6, p < 0.001; 60 s bouts – n=63.3 ± 9.9, p < 0.05, Figure 3D). The lower number of short bouts was primarily circumscribed to the dark phase in FMT-IH mice (Figure 3E, 3F).
Figure 3:
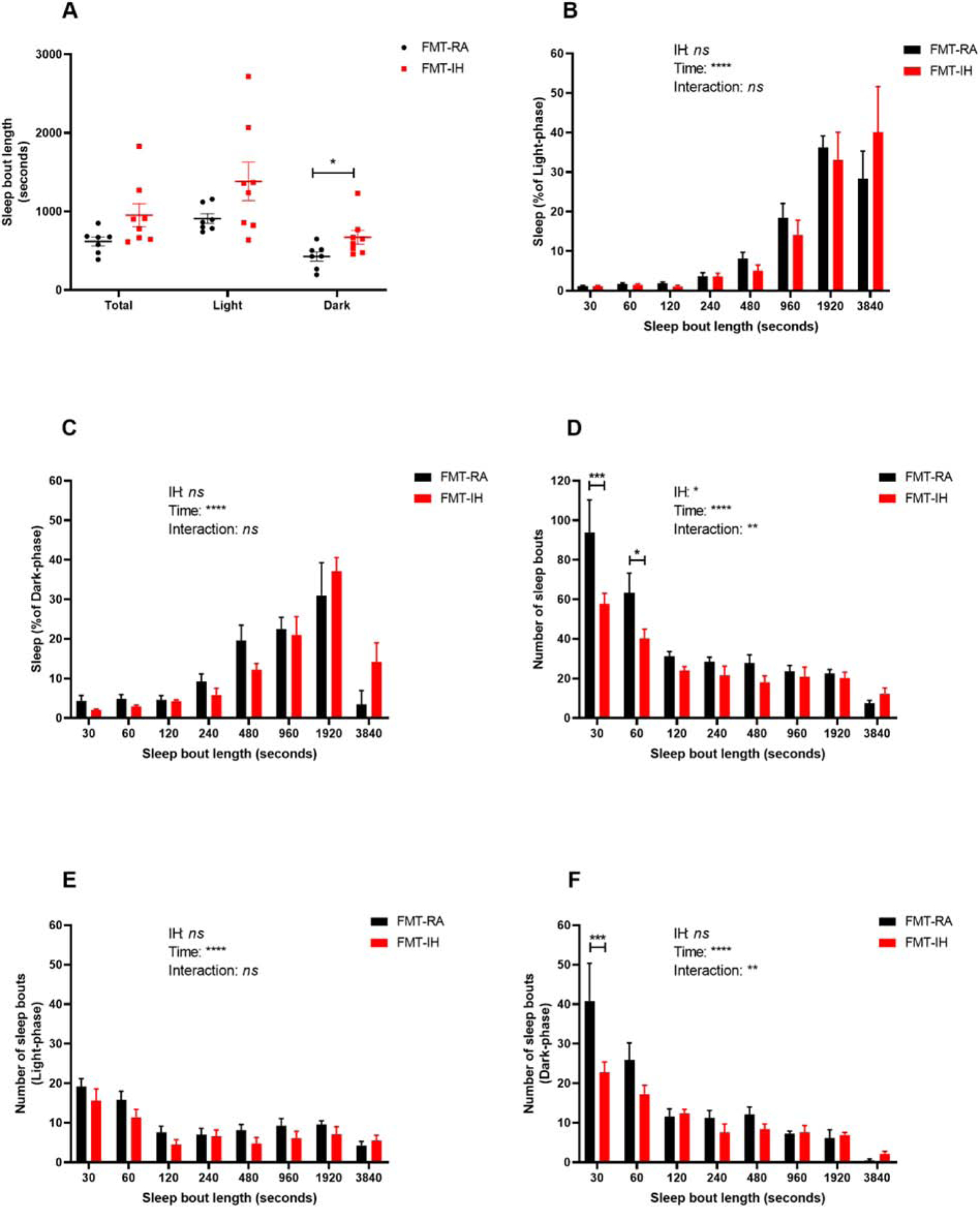
Changes is sleep bout lengths and numbers in FMT-IH and FMT-IA. A) Quantification of total, light-phase and dark-phase average sleep bouts. Groups were compared using Student’s t test (n=7–8). (B-C) sleep percent of average sleep bout lengths during the light-phase (B) and dark-phase (C). (D-F) number of sleep bouts lengths across in total (D), light-phase (E) and dark-phase (F). Groups were compared using Two-way ANOVA with repeated measurement with Sidak post hoc test (n=7–8). *p < 0.05, ** p < 0.01, ***p < 0.001, ****p < 0.0001.
Discussion
This is the first study that evaluated sleep in naïve mice subjected to FMT from mice exposed to IH. Fecal microbiome analysis showed the anticipated taxonomic profile differences between FMT-IH and FMT-RA groups, indicating that the FMT procedures effectively altered the GM of the naïve recipients. Furthermore, we observed taxonomic similarities between FMT-IH and mice exposed to IH in previous studies (Moreno-Indias et al., 2015). Most importantly, the sleep recordings in unrestricted mice revealed that changes in the composition and diversity of the microbiome associated with IH-exposures elicited disturbances in sleep patterns in the FMT-IH mice, and manifested as increased sleep duration during the dark-phase, i.e., the phase during which mice generally are naturally more active and engage in feeding and play. Thus, current findings indicate that alterations of GM diversity caused by FMT from mice exposed to IH can elicit sleep disturbances even when in the absence of environmental IH.
FMT have been recently used as a therapeutic intervention to treat resistant Clostridium difficile in patients with or without inflammatory bowel disease (Gupta and Khanna, 2017). However, other studies showed that chronic conditions (such as hypertension) can be transferable through FMT from animals and humans to naïve animals (Adnan et al., 2017; Li et al., 2017). To test this hypothesis in OSA, Durgan et al. reported that rats intubated with inflatable endotracheal device (a model of OSA) and fed high fat diet (HFD) sustained significant increases in systolic blood pressure compared to rats fed HFD or OSA alone. After transferring fecal matter from OSA+HFD rats and HFD only rats to naïve rats, they reported that FMT from OSA+HFD induced significant elevations in systolic blood pressure compared to FMT from HFD rats alone. They also noticed changes in the Firmicutes/Bacteroidetes (F/B) ratio between the groups. There was a significant reduction in relative abundance of short chain fatty acid (SCFA)-producing bacteria, and increased relative abundance in lactate-producing bacteria among rats receiving FMT from OSA+HFD rats when compared to HFD alone. These findings suggest that OSA-induced hypertension can be transferred through FMT (Durgan et al., 2016).
Growing evidence shows that OSA can induce gut dysbiosis in both adults and children (Kheirandish-Gozal et al., 2014; C.-Y. Ko et al., 2019). Findings from animal models of OSA, such as IH and SF, revealed profound alterations in GM and suggested that such GM changes may be mechanistically associated with some of the major morbidities of OSA. Indeed, chronic SF for 4 weeks induced reversible GM changes that were characterized by the growth of Lachnospiraceae and Ruminococcaceae and a decrease of Lactobacillaceae families accompanied by visceral white adipose tissue inflammation and alterations in insulin sensitivity (Poroyko et al., 2016). In mice exposed to 6 weeks of IH, significant alterations in the GM diversity and composition emerged, with increases in obligate anaerobes, such as Prevotella, Desulfovibrio and Lachnospiraceae (Moreno-Indias et al., 2015). The main effector of such GM changes was attributed to overall reductions in oxygen content in the gut while the animals were exposed to IH. Our current results concur with such findings, as illustrated by the notable differences between FMT-IH and FMT-RA GM samples in the relative abundance of dominant microbiota families and genera, where obligate anaerobes such as Prevotella, Desulfovibrio and Lachnospiraceae increased in FMT-IH mice. Thus, FMT in naïve mice with fecal slurry from IH-exposed mice recapitulated the GM changes in the recipients similar to those documented in the IH-exposed donors. The concomitant presence of Prevotella and Desulfovibrio can operate synergistically, whereby Prevotella can degrade mucin glycoprotein present in the mucosal layer of the gut releasing sulphate, and Desulfovibrio removes the sulphate that inhibits mucin degradation (Arumugam et al., 2011; Wright et al., 2000). Bacterial degradation of mucin, which is protective to the host intestinal lumen, could initiate a variety of intestinal disorders, starting with increased intestinal permeability, but potentially also progressing to inflammatory bowel disease (IBD), as access of luminal antigens to the intestinal immune system is facilitated (Ganesh et al., 2013). On the other hand, the obligatory anaerobe Lachanospiraceae is an important source of the SCFA butyrate that can inhibit intestinal inflammation, maintain the intestinal barrier and modulate gut motility (Silva et al., 2018). The increase of Lachanospiraceae is likely a protective response to environmental stresses. The overall net effect of such changes in FMT-IH GM on specific intestinal function were not examined, but deserve future study since the overall metabolomic composition of the GM may facilitate transfer of sleep-promoting substances in the FMT-IH mice. Indeed, in experiments consisting of sleep disruption in mice, alterations in GM and gut metabolomic profiles were identified, with many of these substances potentially contributing to sleep regulation (Bowers et al., 2020).
Emerging evidence proposes that the GM can influence health and sleep quality through the brain-gut-microbiome axis (BGMA) (Carabotti et al., 2015). BGMA signaling is bidirectional, whereby gut microbiome can impact health and behavior, while changes in the mental states can influence gut health. Perturbations of the BGMA can lead to gastrointestinal (Mayer et al., 2015), cognitive (Galland, 2014) and mental disorders (Valles-Colomer et al., 2019) through neural (Zhu et al., 2017), hormonal (Cani and Knauf, 2016), and immune responses (Belkaid and Hand, 2014) as well as changes in gut and blood brain barrier permeabilities (Braniste et al., 2014; Cani et al., 2009). In the context of IH, one study found that exposing mice to chronic IH (6 and 24 weeks) induced significant microglial changes in distinct dorsal region of the hippocampus, including increased density and morphological features of microglia priming. However, exposing mice to acute IH (1 day) increased pro-inflammatory interleukin (IL)-1β and chemokine ligand 5 (RANTES/CCL5) mRNA, while chronic IH plus lipopolysaccharide (LPS) intraperitoneal injections increased IL-6 and IL10 mRNA compared to LPS alone. These results support the theory that IH can induce neuroinflammation, and may contribute to IH-induced cognitive impairments (Sapin et al., 2015). Furthermore, Ganesh et al. showed that treating rats exposed to OSA with the probiotic Clostridium butyricum and the prebiotic Hylon VII increased SCFA-producing bacteria, reduced OSA-induced dysbiosis, attenuated epithelial goblet cell loss, mucus barrier thinning and overall diminished the degree of activation of brain microglia. These findings suggest that OSA-induced dysbiosis can cause neuroinflammation (Ganesh et al., 2018). However, sleep has been understudied in the context of the BGMA. Administration of broad-spectrum antibiotics depleted the GM, and reduced slow-wave sleep in rats (Brown et al., 1990). When given to healthy male students for three consecutive days, the antibiotic minocycline reduced slow-wave sleep, but did not affect sleep latency or alter wake after sleep onset (Nonaka et al., 1983). As mentioned, studies in OSA patients and in animals exposed to IH clearly demonstrate profound sleep disturbances and alterations to the gut microbiota (Gozal et al., 2001; C.-Y. Ko et al., 2019; Moreno-Indias et al., 2016; Polotsky et al., 2006; Veasey et al., 2004). Here, we show that transferring GM from mice exposed to IH will elicit sleep disturbances in the naïve recipient mice. Specifically, sleep duration increased in the dark-phase (likely corresponding to increased sleep propensity) as well as longer sleep bouts (likely corresponding to increased sleepiness) when compared to mice receiving fecal matter from mice exposed to room air. It is therefore plausible that gut dysbiosis can affect sleep structure and drive via the BGMA. However, more studies are needed to elucidate the mechanisms involved in the role of BGMA in sleep regulation. Furthermore, our findings support the conceptual framework that IH exposures can induce sleep disturbances, not only in the context of fluctuating oscillations in oxygen levels, but also through changes in GM diversity and composition. However, given the complexity of the GM in terms of composition and abundance, it is difficult to infer the role of specific bacterial groups in the regulation of sleep, let alone specific stages of sleep. Such studies will require more extensive and precise changes in GM. We should also point out that our study was limited to male mice, such that potential sex-related differences remain unexplored. Finally, the resolution of the sleep pattern alteration in FMT-IH after cessation of FMT was not explored, and potential interventions with pre- and pro-biotics were not implemented, all of which merit future study.
In summary, alterations in gut microbiome induced by IH simulating OSA can alter sleep patterns in naïve mice receiving FMT and are reminiscent of the excessive daytime sleepiness frequently occurring in OSA patients. Thus, IH-mediated GM alterations may play a role in the sleep dysregulation of OSA as demonstrated here in mice who were not exposed to IH. Thus, the present study provides initial compelling evidence for the GM as a major role player in sleep regulation, and may present an important therapeutic target for improving sleep and excessive daytime sleepiness in OSA patients.
Acknowledgments
This study was supported in part by NIH grants R01 HL130984, R01 HL140548, and R01 G061824 and by a Tier 2 Grant of the University of Missouri. The author appreciates the access to unrestricted use of the sleep recording system and software by Jazz Pharmaceuticals.
Abbreviations
- BGMA
brain-gut-microbiome axis
- FMT
fecal microbiota transplantation
- GM
gut microbiome
- IH
intermittent hypoxia
- RA
room air
- OSA
obstructive sleep apnea
- OTU
operational taxonomic unit
- SF
sleep fragmentation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interest
All authors declare that they have no competing interests.
References
- Adnan S, Nelson JW, Ajami NJ, Venna VR, Petrosino JF, Bryan RM, Durgan DJ, 2017. Alterations in the gut microbiota can elicit hypertension in rats. Physiol. Genomics 49, 96–104. 10.1152/physiolgenomics.00081.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almendros I, Martinez-Garcia MA, Farré R, Gozal D, 2020. Obesity, sleep apnea, and cancer. Int. J. Obes 10.1038/s41366-020-0549-z [DOI] [PubMed] [Google Scholar]
- Anderson JR, Carroll I, Azcarate-Peril MA, Rochette AD, Heinberg LJ, Peat C, Steffen K, Manderino LM, Mitchell J, Gunstad J, 2017. A preliminary examination of gut microbiota, sleep, and cognitive flexibility in healthy older adults. Sleep Med. 38, 104–107. 10.1016/j.sleep.2017.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, Bertalan M, Borruel N, Casellas F, Fernandez L, Gautier L, Hansen T, Hattori M, Hayashi T, Kleerebezem M, Kurokawa K, Leclerc M, Levenez F, Manichanh C, Nielsen HB, Nielsen T, Pons N, Poulain J, Qin J, Sicheritz-Ponten T, Tims S, Torrents D, Ugarte E, Zoetendal EG, Wang J, Guarner F, Pedersen O, De Vos WM, Brunak S, Doré J, Weissenbach J, Ehrlich SD, Bork P, 2011. Enterotypes of the human gut microbiome. Nature 473, 174–180. 10.1038/nature09944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badran M, Yassin BA, Fox N, Laher I, Ayas N, 2015. Epidemiology of Sleep Disturbances and Cardiovascular Consequences. Can. J. Cardiol 10.1016/j.cjca.2015.03.011 [DOI] [PubMed] [Google Scholar]
- Belkaid Y, Hand TW, 2014. Role of the microbiota in immunity and inflammation. Cell. 10.1016/j.cell.2014.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict C, Vogel H, Jonas W, Woting A, Blaut M, Schürmann A, Cedernaes J, 2016. Gut microbiota and glucometabolic alterations in response to recurrent partial sleep deprivation in normal-weight young individuals. Mol. Metab 5, 1175–1186. 10.1016/j.molmet.2016.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, Nunez CM, Patel SR, Penzel T, Pépin JLD, Peppard PE, Sinha S, Tufik S, Valentine K, Malhotra A, 2019. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir. Med 7, 687–698. 10.1016/S2213-2600(19)30198-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulangé CL, Neves AL, Chilloux J, Nicholson JK, Dumas ME, 2016. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 10.1186/s13073-016-0303-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers SJ, Vargas F, González A, He S, Jiang P, Dorrestein PC, Knight R, Wright KP, Lowry CA, Fleshner M, Vitaterna MH, Turek FW, 2020. Repeated sleep disruption in mice leads to persistent shifts in the fecal microbiome and metabolome. PLoS One 15 10.1371/journal.pone.0229001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, Korecka A, Bakocevic N, Guan NL, Kundu P, Gulyás B, Halldin C, Hultenby K, Nilsson H, Hebert H, Volpe BT, Diamond B, Pettersson S, 2014. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med 6 10.1126/scitranslmed.3009759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R, Price RJ, King MG, Husband AJ, 1990. Are antibiotic effects on sleep behavior in the rat due to modulation of gut bacteria? Physiol. Behav 48, 561–565. 10.1016/0031-9384(90)90300-S [DOI] [PubMed] [Google Scholar]
- Cani PD, Knauf C, 2016. How gut microbes talk to organs: The role of endocrine and nervous routes. Mol. Metab 5, 743–52. 10.1016/j.molmet.2016.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, Geurts L, Naslain D, Neyrinck A, Lambert DM, Muccioli GG, Delzenne NM, 2009. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 58, 1091–103. 10.1136/gut.2008.165886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, Fierer N, Knight R, 2011. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. U. S. A 108, 4516–4522. 10.1073/pnas.1000080107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carabotti M, Scirocco A, Maselli MA, Severi C, 2015. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol 28, 203–209. [PMC free article] [PubMed] [Google Scholar]
- Donohue KD, Medonza DC, Crane ER, O’Hara BF, 2008. Assessment of a non-invasive high-throughput classifier for behaviours associated with sleep and wake in mice. Biomed. Eng. Online 7 10.1186/1475-925X-7-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durgan DJ, Ganesh BP, Cope JL, Ajami NJ, Phillips SC, Petrosino JF, Hollister EB, Bryan RM, 2016. Role of the Gut Microbiome in Obstructive Sleep Apnea-Induced Hypertension. Hypertension 67, 469–474. 10.1161/HYPERTENSIONAHA.115.06672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farré N, Farré R, Gozal D, 2018. Sleep Apnea Morbidity: A Consequence of Microbial-Immune Cross-Talk? Chest 154, 754–759. 10.1016/j.chest.2018.03.001 [DOI] [PubMed] [Google Scholar]
- Farré R, Montserrat JM, Gozal D, Almendros I, Navajas D, 2018. Intermittent hypoxia severity in animal models of sleep apnea. Front. Physiol 9 10.3389/fphys.2018.01556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon K, Baril AA, Gagnon JF, Fortin M, Décary A, Lafond C, Desautels A, Montplaisir J, Gosselin N, 2014. Cognitive impairment in obstructive sleep apnea. Pathol. Biol 10.1016/j.patbio.2014.05.015 [DOI] [PubMed] [Google Scholar]
- Galland L, 2014. The gut microbiome and the brain. J. Med. Food 10.1089/jmf.2014.7000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesh BP, Klopfleisch R, Loh G, Blaut M, 2013. Commensal Akkermansia muciniphila Exacerbates Gut Inflammation in Salmonella Typhimurium-Infected Gnotobiotic Mice. PLoS One 8 10.1371/journal.pone.0074963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesh BP, Nelson JW, Eskew JR, Ganesan A, Ajami NJ, Petrosino JF, Bryan RM, Durgan DJ, 2018. Prebiotics, probiotics, and acetate supplementation prevent hypertension in a model of obstructive sleep apnea. Hypertension 72, 1141–1150. 10.1161/HYPERTENSIONAHA.118.11695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gileles-Hillel A, Kheirandish-Gozal L, Gozal D, 2016. Biological plausibility linking sleep apnoea and metabolic dysfunction. Nat. Rev. Endocrinol 10.1038/nrendo.2016.22 [DOI] [PubMed] [Google Scholar]
- Gozal D, Daniel JM, Dohanich GP, 2001. Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. J. Neurosci 21, 2442–2450. 10.1523/jneurosci.21-07-02442.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozal D, Gileles-Hillel A, Cortese R, Li Y, Almendros I, Qiao Z, Khalyfa AA, Andrade J, Khalyfa A, 2017. Visceral White Adipose Tissue after Chronic Intermittent and Sustained Hypoxia in Mice. Am. J. Respir. Cell Mol. Biol 56, 477–487. 10.1165/rcmb.2016-0243OC [DOI] [PubMed] [Google Scholar]
- Guinane CM, Cotter PD, 2013. Role of the gut microbiota in health and chronic gastrointestinal disease: Understanding a hidden metabolic organ. Therap. Adv. Gastroenterol 6, 295–308. 10.1177/1756283X13482996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Khanna S, 2017. Fecal microbiota transplantation. JAMA - J. Am. Med. Assoc 10.1001/jama.2017.6466 [DOI] [PubMed] [Google Scholar]
- He K, Kapur VK, 2017. Sleep-Disordered Breathing and Excessive Daytime Sleepiness. Sleep Med. Clin 10.1016/j.jsmc.2017.03.010 [DOI] [PubMed] [Google Scholar]
- Kheirandish-Gozal L, Peris E, Wang Y, Tamae Kakazu M, Khalyfa A, Carreras A, Gozal D, 2014. Lipopolysaccharide-binding protein plasma levels in children: effects of obstructive sleep apnea and obesity. J. Clin. Endocrinol. Metab 99, 656–63. 10.1210/jc.2013-3327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko C-Y, Liu Q-Q, Su H-Z, Zhang H-P, Fan J-M, Yang J-H, Hu A-K, Liu Y-Q, Chou D, Zeng Y-M, 2019. Gut microbiota in obstructive sleep apnea-hypopnea syndrome: disease-related dysbiosis and metabolic comorbidities. Clin. Sci. (Lond) 133, 905–917. 10.1042/CS20180891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko CY, Fan JM, Hu AK, Su HZ, Yang JH, Huang LM, Yan FR, Zhang HP, Zeng YM, 2019. Disruption of sleep architecture in Prevotella enterotype of patients with obstructive sleep apnea-hypopnea syndrome. Brain Behav. 9 10.1002/brb3.1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau K, Srivatsav V, Rizwan A, Nashed A, Liu R, Shen R, Akhtar M, 2017. Bridging the gap between gut microbial dysbiosis and cardiovascular diseases. Nutrients. 10.3390/nu9080859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zhao F, Wang Y, Chen J, Tao J, Tian G, Wu S, Liu W, Cui Q, Geng B, Zhang W, Weldon R, Auguste K, Yang L, Liu X, Chen L, Yang X, Zhu B, Cai J, 2017. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 5 10.1186/s40168-016-0222-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucking EF, O’Connor KM, Strain CR, Fouhy F, Bastiaanssen TFS, Burns DP, Golubeva AV, Stanton C, Clarke G, Cryan JF, O’Halloran KD, 2018. Chronic intermittent hypoxia disrupts cardiorespiratory homeostasis and gut microbiota composition in adult male guinea-pigs. EBioMedicine 38, 191–205. 10.1016/j.ebiom.2018.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magoč T, Salzberg SL, 2011. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27, 2957–2963. 10.1093/bioinformatics/btr507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mang GM, Nicod J, Emmenegger Y, Donohue KD, O’Hara BF, Franken P, 2014. Evaluation of a piezoelectric system as an alternative to electroencephalogram/ electromyogram recordings in mouse sleep studies. Sleep 37, 1383–92. 10.5665/sleep.3936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manrique-Acevedo C, Chinnakotla B, Padilla J, Martinez-Lemus LA, Gozal D, 2020. Obesity and cardiovascular disease in women. Int. J. Obes 10.1038/s41366-020-0548-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashaqi S, Gozal D, 2019. Obstructive sleep apnea and systemic hypertension: Gut dysbiosis as the mediator? J. Clin. Sleep Med 10.5664/jcsm.7990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer EA, Tillisch K, Gupta A, 2015. Gut/brain axis and the microbiota. J. Clin. Invest 10.1172/JCI76304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Indias I, Torres M, Montserrat JM, Sanchez-Alcoholado L, Cardona F, Tinahones FJ, Gozal D, Poroyko VA, Navajas D, Queipo-Ortuño MI, Farré R, 2015. Intermittent hypoxia alters gut microbiota diversity in a mouse model of sleep apnoea. Eur. Respir. J 45, 1055–65. 10.1183/09031936.00184314 [DOI] [PubMed] [Google Scholar]
- Moreno-Indias I, Torres M, Sanchez-Alcoholado L, Cardona F, Almendros I, Gozal D, Montserrat JM, Queipo-Ortuño MI, Farré R, 2016. Normoxic Recovery Mimicking Treatment of Sleep Apnea Does Not Reverse Intermittent Hypoxia-Induced Bacterial Dysbiosis and Low-Grade Endotoxemia in Mice. Sleep 39, 1891–1897. 10.5665/sleep.6176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni J, Shen TCD, Chen EZ, Bittinger K, Bailey A, Roggiani M, Sirota-Madi A, Friedman ES, Chau L, Lin A, Nissim I, Scott J, Lauder A, Hoffmann C, Rivas G, Albenberg L, Baldassano RN, Braun J, Xavier RJ, Clish CB, Yudkoff M, Li H, Goulian M, Bushman FD, Lewis JD, Wu GD, 2017. A role for bacterial urease in gut dysbiosis and Crohn’s disease. Sci. Transl. Med 9 10.1126/scitranslmed.aah6888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka K, Nakazawa Y, Kotorii T, 1983. Effects of antibiotics, minocycline and ampicillin, on human sleep. Brain Res. 288, 253–9. 10.1016/0006-8993(83)90101-4 [DOI] [PubMed] [Google Scholar]
- Paiva IHR, Duarte-Silva E, Peixoto CA, 2020. The role of prebiotics in cognition, anxiety, and depression. Eur. Neuropsychopharmacol 10.1016/j.euroneuro.2020.03.006 [DOI] [PubMed] [Google Scholar]
- Parkar SG, Kalsbeek A, Cheeseman JF, 2019. Potential role for the gut microbiota in modulating host circadian rhythms and metabolic health. Microorganisms. 10.3390/microorganisms7020041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascale A, Marchesi N, Govoni S, Barbieri A, 2020. Targeting the microbiota in pharmacology of psychiatric disorders. Pharmacol. Res 157, 104856 10.1016/j.phrs.2020.104856 [DOI] [PubMed] [Google Scholar]
- Paulose JK, Wang C, O’Hara BF, Cassone VM, 2019. The effects of aging on sleep parameters in a healthy, melatonin-competent mouse model. Nat. Sci. Sleep 11, 113–121. 10.2147/NSS.S214423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, 2009. Gut reaction: Environmental effects on the human microbiota. Environ. Health Perspect 117, A198 10.1289/ehp.117-a198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polotsky VY, Rubin AE, Balbir A, Dean T, Smith PL, Schwartz AR, O’Donnell CP, 2006. Intermittent hypoxia causes REM sleep deficits and decreases EEG delta power in NREM sleep in the C57BL/6J mouse. Sleep Med. 7, 7–16. 10.1016/j.sleep.2005.06.006 [DOI] [PubMed] [Google Scholar]
- Poroyko VA, Carreras A, Khalyfa A, Khalyfa AA, Leone V, Peris E, Almendros I, Gileles-Hillel A, Qiao Z, Hubert N, Farré R, Chang EB, Gozal D, 2016. Chronic Sleep Disruption Alters Gut Microbiota, Induces Systemic and Adipose Tissue Inflammation and Insulin Resistance in Mice. Sci. Rep 6, 1–11. 10.1038/srep35405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooks MG, Garrett WS, 2016. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol 10.1038/nri.2016.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfilippo-Cohn B, Lai S, Zhan G, Fenik P, Pratico D, Mazza E, Veasey SC, 2006. Sex differences in susceptibility to oxidative injury and sleepiness from intermittent hypoxia. Sleep. 10.1093/sleep/29.2.152 [DOI] [PubMed] [Google Scholar]
- Sapin E, Peyron C, Roche F, Gay N, Carcenac C, Savasta M, Levy P, Dematteis M, 2015. Chronic Intermittent Hypoxia Induces Chronic Low-Grade Neuroinflammation in the Dorsal Hippocampus of Mice. Sleep 38, 1537–1546. 10.5665/sleep.5042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheflin AM, Whitney AK, Weir TL, 2014. Cancer-Promoting Effects of Microbial Dysbiosis. Curr. Oncol. Rep 10.1007/s11912-014-0406-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva JPB, Navegantes-Lima KC, de Oliveira ALB, Rodrigues DVS, Gaspar SLF, Monteiro VVS, Moura DP, Monteiro MC, 2018. Protective Mechanisms of Butyrate on Inflammatory Bowel Disease. Curr. Pharm. Des 24, 4154–4166. 10.2174/1381612824666181001153605 [DOI] [PubMed] [Google Scholar]
- Smith RP, Easson C, Lyle SM, Kapoor R, Donnelly CP, Davidson EJ, Parikh E, Lopez JV, Tartar JL, 2019. Gut microbiome diversity is associated with sleep physiology in humans. PLoS One 14, e0222394 10.1371/journal.pone.0222394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebegg M, Silva-Cayetano A, Innocentin S, Jenkins TP, Cantacessi C, Gilbert C, Linterman MA, 2019. Heterochronic faecal transplantation boosts gut germinal centres in aged mice. Nat. Commun 10.1038/s41467-019-10430-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- TIAN YM, GUAN Y, TIAN SY, YUAN F, ZHANG L, ZHANG Y, 2018. Short-term Chronic Intermittent Hypobaric Hypoxia Alters Gut Microbiota Composition in Rats. Biomed. Environ. Sci 10.3967/bes2018.122 [DOI] [PubMed] [Google Scholar]
- Tripathi A, Melnik AV, Xue J, Poulsen O, Meehan MJ, Humphrey G, Jiang L, Ackermann G, McDonald D, Zhou D, Knight R, Dorrestein PC, Haddad GG, 2018. Intermittent Hypoxia and Hypercapnia, a Hallmark of Obstructive Sleep Apnea, Alters the Gut Microbiome and Metabolome. mSystems 3 10.1128/msystems.00020-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi A, Xu ZZ, Xue J, Poulsen O, Gonzalez A, Humphrey G, Meehan MJ, Melnik AV, Ackermann G, Zhou D, Malhotra A, Haddad GG, Dorrestein PC, Knight R, 2019. Intermittent Hypoxia and Hypercapnia Reproducibly Change the Gut Microbiome and Metabolome across Rodent Model Systems. mSystems 4 10.1128/msystems.00058-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valles-Colomer M, Falony G, Darzi Y, Tigchelaar EF, Wang J, Tito RY, Schiweck C, Kurilshikov A, Joossens M, Wijmenga C, Claes S, Van Oudenhove L, Zhernakova A, Vieira-Silva S, Raes J, 2019. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol 4, 623–632. 10.1038/s41564-018-0337-x [DOI] [PubMed] [Google Scholar]
- Veasey SC, Davis CW, Fenik P, Zhan G, Hsu Y-J, Pratico D, Gow A, 2004. Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep 27, 194–201. 10.1093/sleep/27.2.194 [DOI] [PubMed] [Google Scholar]
- Veasey SC, Lear J, Zhu Y, Grinspan JB, Hare DJ, Wang S, Bunch D, Doble PA, Robinson SR, 2013. Long-Term Intermittent Hypoxia Elevates Cobalt Levels in the Brain and Injures White Matter in Adult Mice. Sleep. 10.5665/sleep.3038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velagapudi VR, Hezaveh R, Reigstad CS, Gopalacharyulu P, Yetukuri L, Islam S, Felin J, Perkins R, Borén J, Orešič M, Bäckhed F, 2010. The gut microbiota modulates host energy and lipid metabolism in mice. J. Lipid Res 51, 1101–1112. 10.1194/jlr.M002774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh J, Griffin BT, Clarke G, Hyland NP, 2018. Drug–gut microbiota interactions: implications for neuropharmacology. Br. J. Pharmacol 10.1111/bph.14366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DP, Rosendale DI, Robertson AM, 2000. Prevotella enzymes involved in mucin oligosaccharide degradation and evidence for a small operon of genes expressed during growth on mucin. FEMS Microbiol. Lett 190, 73–9. 10.1111/j.1574-6968.2000.tb09265.x [DOI] [PubMed] [Google Scholar]
- Yang Q, Liang Q, Balakrishnan B, Belobrajdic DP, Feng QJ, Zhang W, 2020. Role of dietary nutrients in the modulation of gut microbiota: A narrative review. Nutrients. 10.3390/nu12020381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Han Y, Du J, Liu R, Jin K, Yi W, 2017. Microbiota-gut-brain axis and the central nervous system. Oncotarget 8, 53829–53838. 10.18632/oncotarget.17754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinchuk AV, Gentry MJ, Concato J, Yaggi HK, 2017. Phenotypes in obstructive sleep apnea: A definition, examples and evolution of approaches. Sleep Med. Rev 10.1016/j.smrv.2016.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]


