Abstract
Purpose
To screen novel mutations in LHCGR responsible for empty follicle syndrome and explore the pathological mechanism of mutations.
Methods
Four affected individuals diagnosed with infertility-associated anovulation or oligo-ovulation from three independent families were recruited. Sanger sequencing was used to identify the LHCGR mutations in affected individuals. Western blot was performed to evaluate the effects of mutations on LHCGR protein levels. Immunofluorescence was done to explore the effects of mutations on LHCGR subcellular localization. The ATP levels were measured to infer the functional effects of the mutations on LHCGR.
Results
In the present study, three novel biallelic mutations in LHCGR were identified in four affected individuals from three independent families with empty follicle syndrome or oligo-ovulation. All biallelic mutations were inherited from the proband of their parents. The western blot showed that the identified mutations decreased LHCGR protein level and altered the glycosylation pattern. The immunofluorescence showed an ectopic subcellular localization of LHCGR in cultured HeLa cells. Besides, the mutations in LHCGR also reduced the cellular ATP consumption.
Conclusion
These findings confirm previous studies and expand the mutational spectrum of LHCGR, which will provide genetic diagnostic marker for patients with empty follicle syndrome.
Electronic supplementary material
The online version of this article (10.1007/s10815-020-01931-2) contains supplementary material, which is available to authorized users.
Keywords: LHCGR, Mutations, Empty follicle syndrome, Reproduction
Introduction
The luteinizing hormone/choriogonadotropin receptor (LHCGR, MIM:152790) is a transmembrane receptor mainly expressed in the ovary and testis and is necessary for normal hormonal responses during human reproduction [1, 2]. LHCGR consists of a signal peptide domain, an extracellular hormone-binding domain, a seven-helix transmembrane domain, and an intracellular C-terminal domain [3]. It is activated by luteinizing hormone (LH) and human chorionic gonadotropin (hCG) [4]. In females, LHCGR is expressed in granulosa cells, theca cells, and luteal cells and is necessary for estradiol production, ovulation, and luteal formation [1]. In males, LHCGR has been identified in the Leydig cells. It binds with high affinity to hCG, which is critical for the stimulation of testosterone production and secretion and thus supports spermatogenesis [5, 6]. Therefore, normal LHCGR functioning is critical for reproduction in both females and males.
In males, gain-of-function mutations in LHCGR are associated with familial male precocious puberty [7] (MIM:176410), while biallelic inactivation mutations in LHCGR cause Leydig cell hypoplasia (LCH, MIM:238320), which leads to male disorders of sexual differentiation [8]. In females, it has been reported that homozygous or compound heterozygous mutations in LHCGR cause LH resistance (MIM:238320) leading to female infertility characterized by primary amenorrhea, oligomenorrhea, and anovulation but without any effect on the sex characteristics [9–14]. Mutations in LHCGR lead to partial or complete loss of response to LH thus causing LH resistance [15–17]. Although several mutations in LHCGR have been identified, novel mutations and their corresponding mechanisms are worthy of being investigated.
In this study, we aimed to screen novel mutations in LHCGR responsible for empty follicle syndrome and explore the pathological mechanism of mutations. We recruited four affected individuals diagnosed with infertility-associated anovulation or oligo-ovulation from three independent families. The two affected individuals from families 1 and 3 had no oocytes retrieved during in vitro fertilization (IVF) attempts, while the patient in family 2 had a few oocytes retrieved. Sanger sequencing was used to identify the LHCGR mutations in affected individuals. Western blot and immunofluorescence were performed to evaluate the effects of mutations on LHCGR protein levels and subcellular localization. The ATP levels were measured to infer the functional effects of the mutations on LHCGR. This study provides a comprehensive understanding of mutations in LHCGR that are responsible for empty follicle syndrome and abnormal ovulation and will help in selecting the proper treatment for these patients.
Materials and methods
Clinical samples and genetic studies
Patients diagnosed with infertility-associated anovulation or oligo-ovulation were recruited from the Shanghai Ji Ai Genetics and IVF Institute, the Ninth Hospital Affiliated with Shanghai Jiao Tong University, and the First Affiliated Hospital with Nanjing Medical University. Genomic DNA samples were extracted from the patients’ peripheral blood using the QIAamp DNA Blood Mini Kit (Qiagen, Germany). Sanger target sequencing was then performed to identify new mutations in LHCGR. All studies on human subjects were approved by the ethics committee of the Medical College of Fudan University, and written informed consent was obtained from the affected individuals.
Expression constructs, western blot, and immunofluorescence
Full-length coding sequence of human LHCGR (NM_000233) was amplified and cloned into the GV141 vector with a flag tag. Mutations in LHCGR (c.T32C (p.Leu11Pro), c.C1936T (p.Arg646Cys), c.661dupG (p.Ala221Glyfs*63), and c.32_58dupTGAAGCTGCTGCTGCTGCTGCAGCCGC (p.Leu11_Pro19dup)) were introduced by using the site-directed KOD-Plus-Mutagenesis Kit (Toyobo Life Science) according to the manufacturer’s instructions. The HeLa cell line was obtained from the Cell Bank of Shanghai Institute for Biological Sciences, the Chinese Academy of Sciences (Shanghai, China). Cells were cultured in high-glucose Dulbecco’s minimum essential medium (Gibco) supplemented with 10% FBS and 1% penicillin/streptomycin at 37 °C in a humidified 5% CO2 incubator. For western blotting, cells were harvested at 36 h after quantification with the bicinchoninic acid assay (Shanghai Biocolor Biosciences & Technology Co.), and cell extracts were denatured in SDS loading buffer. The samples were then separated by SDS-PAGE and transferred to nitrocellulose membranes (Pall Corporation) and probed with mouse anti-FLAG antibodies (1:1000 dilution; Cell Signaling Technology) or rabbit anti-α-tubulin antibodies (1:1000 dilution; Cell Signaling Technology). The secondary antibodies were goat anti-rabbit immunoglobulin G (IgG) (1:5000 dilution; Abmart) or goat anti-mouse IgG (1:5000 dilution; Abmart) conjugated to horseradish peroxidase. For immunofluorescence, cells were fixed and stained as described previously [18]. Briefly, cells were fixed with 4% paraformaldehyde for 30 min and stained with an Alexa Fluor 594-labeled monoclonal anti-FLAG antibody for 1 h at room temperature. After washing with PBS, DAPI was added for 10 min to label the DNA.
ATP measurements
Wild-type (WT) and mutated LHCGR constructs were transfected into HeLa cells. At 36 h, cells were lysed in 400 μL of lysis buffer for 10 min and centrifuged at 4000×g at room temperature for 30 s in cell lysis buffer. The relative ATP content was determined in a mixture of 50 μL of supernatant and 50 μL of luciferine-luciferase using an ATP Bioluminescence Assay Kit HS II (Roche Applied Science) according to the manufacturer’s instructions.
Minigene assay
The c.384-2A>T splicing variant was located at the donor splice site of intron 4. Due to the large size of intron 4, we failed to clone the full sequence of exon 4, exon 5, and exon 6 into the minigene vector. We amplified the exon 4 and exon 5–exon 6 including 200–300 bp intron sequences before or after it, then two products were integrated into a modified pcDNA3 plasmid. The WT and c.384-2A>T plasmids were transfected into HeLa cells. At 24 h, total RNA was extracted using a RNeasy Mini Kit (Qiagen), and cDNA was obtained with the PrimeScript RT reagent kit (Takara, Osaka, Japan). The alternative splicing isoforms were detected by Sanger sequencing and agarose gel electrophoresis.
Statistical analysis
All the data were analyzed and graphed by GraphPad software. Brown-Forsythe test was performed to compare the homogeneity of variances. If the data had homogeneous variances, one-way ANOVA test followed by Tukey test was used for multiple comparisons of means. If the data did not have similar variances, the nonparameter Kruskal-Wallis test was performed. The P < 0.05 was considered significant.
Results
Clinical characterization
All four affected individuals had been diagnosed with primary infertility for several years characterized by anovulation or oligo-ovulation. The family pedigrees are shown in Fig. 1, and the clinical characteristics of the patients’ retrieved oocytes are summarized in Table 1. The patient in family 1 was 41 years old and had been diagnosed with primary infertility for 13 years and had undergone four failed IVF attempts, and no oocytes had been retrieved (Fig. 1; Table 1). Her sister was also diagnosed with primary infertility, but her clinical information was not available. The phenotype of the patient in family 2 was less severe. She was 33 years old, and of her four IVF/intracytoplasmic sperm injection (ICSI) cycles, a total of 14 oocytes were retrieved. Seven of them were successfully fertilized, but only one normally cleaved embryo was obtained and she failed to establish pregnancy after implantation (Fig. 1; Table 1). The patient from family 3 was 27 years old, and in her two IVF/ICSI attempts, no oocytes were retrieved (Fig. 1; Table 1).
Fig. 1.
Pedigrees of the three families carrying mutations in LHCGR. All four affected individuals were diagnosed with infertility-associated empty follicle syndrome or oligo-ovulation from three independent families. All of them carried homozygous or compound heterozygous mutations in LHCGR with a recessive inheritance pattern. The affected individuals from families 1 and 3 were diagnosed with empty follicle syndrome. The phenotype in family 2 was oligo-ovulation. Sanger sequencing confirmation is shown beside the pedigrees. The equal sign indicates infertility, and black circles represent the affected individuals
Table 1.
Clinical characteristics of the affected individuals and their retrieved oocytes
| Families | Age (years) | Duration of infertility (years) | IVF/ICSI cycles | Total No. of oocytes retrieved | Fertilized oocytes | Normal cleavage embryos | Outcomes |
|---|---|---|---|---|---|---|---|
| 1 (II-1) | 41 | 13 | 4 | 0 | 0 | 0 | 0 |
| 2 (II-1) | 33 | 8 | 4 | 14 | 7 | 1 | Failed pregnancy |
| 3 (II-1) | 27 | 3 | 2 | 0 | 0 | 0 | 0 |
Identification of mutations in LHCGR
Because LHCGR is a known causative gene for empty follicle syndrome [19], Sanger sequencing of the gene was performed directly. As indicated in Fig. 1 and Table 2, we identified homozygous or compound heterozygous mutations in LHCGR from the four affected individuals. The two sisters in family 1 had a homozygous c.T32C (p.Leu11Pro) missense mutation in LHCGR. The patient in family 2 had compound heterozygous mutations c.C1936T (p.Arg646Cys) and c.661dupG (p.Ala221Glyfs*63). In family 3, the patient had compound heterozygous mutations, including the splicing mutation c.384-2A>T and a 27-bp duplication c.32_58dupTGAAGCTGCTGCTGCTG CTGCAGCCGC (p.Leu11_Pro19dup). LHCGR is a transmembrane receptor, and it consists of a signal peptide domain, a Leu-rich domain, and a seven-helix transmembrane domain (Fig. 2). The mutations c.T32C (p.Leu11Pro) and c.32_58dupTGAAGCTGCTGCTGCTGCTGCAGCCGC (p.Leu11_Pro 19dup) are located in exon 1, which is close to the signal peptide domain, and it was inferred that both mutations might affect LHCGR localization in the membrane (Fig. 2). The c.661dupG (p.Ala221Glyfs*63) mutation is located in exon 8 within the Leu-rich domain, while the splicing area of c.384-2A>T comes just after exon 5. The missense mutation c.C1936T (p.Arg646Cys) is located in exon 11, the last exon of LHCGR. The missense mutation c.T32C (p.Leu11Pro) occurs at a conserved residue among different species, while the missense mutation p.Arg646Cys does not occur at a conserved residue (Fig. 2). Detailed information on the locations of the mutations in LHCGR, the minor allele frequency, and the predicted effect is provided in Table 2.
Table 2.
Mutations in LHCGR in the three families
| Families | Genomic position (chr2) | cDNA change | Protein change | Mutation type | SIFT a | PPH2a | ExAC Eb | gnomADc |
|---|---|---|---|---|---|---|---|---|
| 1 | 48982779 | c.T32C | p.Leu11Pro | Missense | D | B | 0 | 7.53 × 10−6 |
| 2 | 48915000 | c.C1936T | p.Arg646Cys | Missense | T | D | 0 | 4.6 × 10−5 |
| 48936105 | c.661dupG | p.Ala221Glyfs*63 | Frameshift insertion | NA | NA | NA | NA | |
| 3 | 48950837 | c.384-2A>T | – | Splicing | NA | NA | NA | NA |
| 48982753_48982779dup | c.32_58dupTGAAGCTGCTGCTGCTGCTGCAGCCGC | p.Leu11_Pro19dup | In-frame insertion | NA | NA | 0 | 1.56 × 10−5 |
B, benign; T, tolerated; D, damaging; NA, not available
aMutation assessment by SIFT and polyPhen-2 (PPH2)
bFrequency of the corresponding mutations in the East Asian population of the ExAC Browser
cFrequency of the corresponding mutations in gnomAD
Fig. 2.
The location and conservation analysis of altered residues in LHCGR. The distribution of mutations in LHCGR exons and in the protein structure of LHCGR. The mutations are shown as blue circles. The conservation of the mutated residues is marked in yellow
Effects of the mutations on LHCGR expression, glycosylation, subcellular localization, and ATP consumption in cultured cells
To detect the effect of the c.384-2A>T variant on LHCGR splicing, we performed the minigene assay (Fig. S1a). Agarose gel electrophoresis showed a lower-size band and a similar-size band for the c.384-2A>T compared with wild type, indicating the abnormal alternative splicing isoforms with this mutation (Fig. S1b). To make clear the exact splicing isoforms, all the bands of the WT and c.384-2A>T were extracted and cloned into a PGMT vector. The Sanger sequence analysis showed that c.384-2A>T mutation led to three different alternative splicing isoforms. The first isoform caused a 3-bp deletion in Exon 5 and generated a c.384_386delGAG (p.Leu128Phe, Ser129del) abnormal transcript. The second isoform led to a 17-bp deletion in exon 5 and generated a c.384_400delGAGCATCTGTAACACAG (p.Ser129Hisfs*14) product. While the third isoform at least jumped the exon 5 and exon 6 (Fig. S1c).
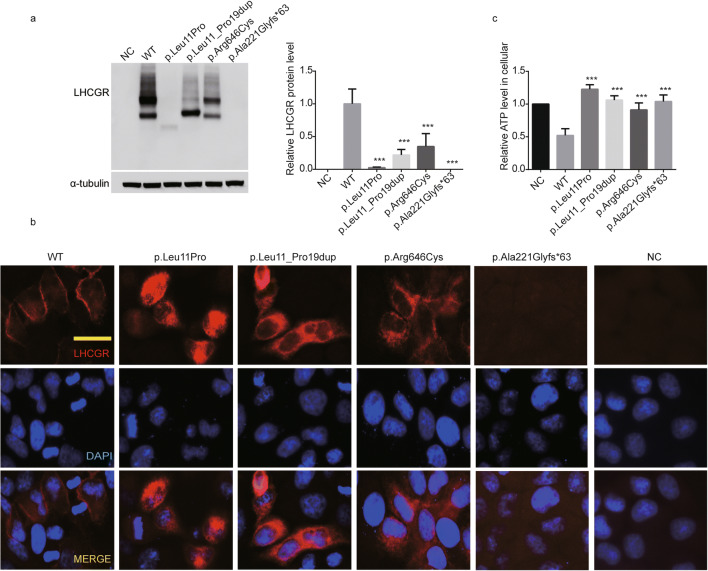
LHCGR is a highly N-linked glycosylated protein [20]. To explore the functional effects of the mutations in LHCGR, we transfected WT or mutant LHCGR constructs into HeLa cells for western blot analysis. As shown in Fig. 3a, WT LHCGR had two bands, while the mutations c.T32C (p.Leu11Pro) and c.32_58dupTGAAGCTGCTGC TGCTGCT GCAGCCGC (p.Leu11_Pro19dup) had one main band. This may indicate abnormal glycosylation of LHCGR (Fig. 3a). In addition, all the mutations resulted in different decreases in protein levels. The c.661dupG (p.Ala221Glyfs*63) mutation resulted in no detectable LHCGR protein. For the c.T32C (p.Leu11Pro) mutation, LHCGR protein level decreased by 90%, while mutation c.32_58dupTGAAGCTGCTGCTGCTGCTGCAGCC GC (p.Leu11_Pro19dup) and c.C1936T (p.Arg646Cys) resulted in 80 and 70% of LHCGR down-regulation, respectively (Fig. 3a).
Fig. 3.
Effects of the mutations on LHCGR expression, glycosylation, subcellular localization, and ATP level in cultured cells. a The western blot analysis of WT and mutated LHCGR proteins in HeLa cells. α-Tubulin was used as the loading control. b The subcellular localization of WT and mutated LHCGR protein in HeLa cells. LHCGR is shown in red, and the DNA is shown in blue. Scale bar, 20 μm. c The cellular ATP levels of HeLa cells in the negative control (NC) and after transfection with WT and mutant LHCGR constructs. Data are shown as the means ± SEM, n = 3 biological replicates, one-way ANOVA, followed by Tukey test for more than two groups, ***P ≤ 0.001
Because LHCGR is a transmembrane receptor [3], we next determined whether the mutations in LHCGR affect its subcellular localization by transfecting WT or mutant constructs into HeLa cell lines and performing immunofluorescence experiments. WT LHCGR was mainly located in the cell membrane, while the c.T32C (p.Leu11Pro), c.32_58dupTGAAGCTGCTGCTGCTGCTGCAGCCGC (p.Leu11_Pro19dup), and c.C1936T (p.Arg646Cys) mutants had different degrees of ectopic localization in the cytoplasm (Fig. 3b). The effect of c.C1936T (p.Arg646Cys) was less severe than the others, while the frameshift mutation c.661dupG (p.Ala221Glyfs*63) had no LHCGR signal. These results were consistent with the western blot results (Fig. 3a and b).
LHCGR activates adenylyl cyclase via G proteins and causes ATP consumption and increased cAMP levels [16, 21, 22]. To determine the effects of the mutations on ATP consumption, we measured the ATP level in cultured HeLa cells transfected with WT and mutant LHCGR constructs. Compared with the negative control, the WT LHCGR caused an obvious ATP decrease, while the mutations in LHCGR had reduced ATP consumption, suggesting the signal transduction may be influenced by mutations in LHCGR (Fig. 3c).
Discussion
In the present study, we identified one homozygous and two compound heterozygous mutations in LHCGR that might be responsible for empty follicle syndrome or oligo-ovulation in three independent families. All of the mutations were inherited from the parents in a recessive pattern. The mutations in LHCGR caused abnormal LHCGR glycosylation, decreased protein level, ectopic subcellular localization, and impaired ATP consumption, which indicate the signal transduction may be affected.
The affected individuals from families 1 and 3 had typical empty follicle syndrome, and no oocytes were retrieved in their IVF cycles. The patient in family 2 had a few oocytes retrieved and some of these could be fertilized, but only one normal cleavage embryo was obtained and no pregnancy was established. This suggests that the phenotype of the patient from family 2 was less severe compared with the other patients, and this might be associated with the severity of the effects of the mutations. The biallelic mutations in LHCGR from families 1 and 3 caused severe impairment of LHCGR function in both alleles. In the patient from family 2, the allele c.661dupG (p.Ala221Glyfs*63) caused severe loss of function of LHCGR, while the missense mutation c.C1936T (p.Arg646Cys) had only a slight effect on the subcellular localization and the LHCGR glycosylation pattern. The ATP assay also showed that the c.C1936T (p.Arg646Cys) mutation had a less-severe effect on ATP consumption. This might explain why the patient in family 2 had a mild phenotype and had some oocytes retrieved during her IVF attempts.
In recent years, several inactivating mutations in LHCGR have been reported to cause female infertility [5, 10–12, 23–29], and attempts have been made to try to find treatments for women carrying mutations in LHCGR [24, , 29]. Recently, Lu et al. reported a successful treatment for patients with mutations in LHCGR. By using combined transvaginal ultrasound and adjusted human menopausal gonadotropin stimulation, they retrieved oocytes and obtained high-quality embryos from three women with mutations in LHCGR. Two of them successfully established pregnancies and had live births [30]. Lu et al. reported three homozygous mutations c.1753_1756delATCT (p.Ile585Leufs*16), c.846_847insT (p.Arg283*), and c.1129A>G (p.Asn377Asp) in LHCGR. All three mutations caused abnormal LHCGR glycosylation, decreased protein level, ectopic subcellular localization, and impaired cAMP levels which is similar with c.T32C (p.Leu11Pro), c.32_58dupTGAAGCTGCTGCTGCTGC TGCAGCCGC (p.Leu11_Pro19dup), and c.661dupG (p.Ala221Glyfs*63) mutations in this study [30]. Their phenotypes were also similar; no oocytes were obtained before using combined transvaginal ultrasound and adjusted human menopausal gonadotropin stimulation. The c.C1936T (p.Arg646Cys) mutation from family 2 in this study had a less-severe effect on LHCGR glycosylation and ATP consumption. So, the phenotype of patient from family 2 had a mild phenotype and had some oocytes retrieved during her IVF attempts in our study. It is likely that the severity of the functional impairment caused by mutations will affect the outcome of the treatment. Patients carrying less-severe mutations may have a better outcome of treatment. Their research sheds light on the treatment of patients carrying mutations in LHCGR, and thus screening for novel mutations in LHCGR can help the clinician to make the right choice in terms of treatment strategy.
In summary, we identified one homozygous and two compound heterozygous mutations in LHCGR. The mutations caused abnormal LHCGR glycosylation, decreased protein expression, ectopic subcellular localization of LHCGR, and reduced ATP consumption in HeLa cells. These findings confirm those of previous studies and expand the mutational spectrum of LHCGR and thus provide additional potential genetic diagnostic markers for empty follicle syndrome that might help the clinician develop a proper treatment strategy for these patients.
Electronic supplementary material
Effects of the c.384-2A>T splicing mutation on LHCGR alternative splicing. a The schematic diagram of minigene assay. b Agarose gel electrophoresis of WT and c.384-2A>T splicing mutation. c The Sanger sequence analysis of WT and c.384-2A>T splicing mutation. The c.384-2A>T mutation led to three different alternative splicing isoforms (PNG 461 kb)
Authors’ contributions
Lei Wang and Qing Sang conceived and designed the study. Zhihua Zhang, Ling Wu, and Lin Zhao performed the experiments. Biaobang Chen, Jian Mu, and Wenjing Wang performed the genetic study. Zhou Zhou, Jie Dong, and Yang Zeng organized the medical records. Feiyang Diao, Xiaoyan Mao, Zheng Yan, Bin Li, Jing Fu, Yanping Kuang, and Xiaoxi Sun collected the samples. Zhihua Zhang, Qing Sang, Lei Wang, Jing Du, and Lin He wrote the manuscript.
Funding
This work was supported by the Strategic Collaborative Research Program of the Ferring Institute of Reproductive Medicine, Ferring Pharmaceuticals, and Chinese Academy of Sciences (FIRMC200507).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All studies on human subjects were approved by the ethics committee of the Medical College of Fudan University.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhihua Zhang, Ling Wu, Feiyang Diao and Biaobang Chen contributed equally to this work.
Contributor Information
Qing Sang, Email: sangqing@fudan.edu.cn.
Lei Wang, Email: wangleiwanglei@fudan.edu.cn.
References
- 1.Ascoli M, Fanelli F, Segaloff DL. The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocr Rev. 2002;23(2):141–174. doi: 10.1210/edrv.23.2.0462. [DOI] [PubMed] [Google Scholar]
- 2.Jia XC, Oikawa M, Bo M, Tanaka T, Ny T, Boime I, et al. Expression of human luteinizing hormone (LH) receptor: interaction with LH and chorionic gonadotropin from human but not equine, rat, and ovine species. Molec Endocrinol (Baltimore, Md) 1991;5(6):759–768. doi: 10.1210/mend-5-6-759. [DOI] [PubMed] [Google Scholar]
- 3.Troppmann B, Kleinau G, Krause G, Gromoll J. Structural and functional plasticity of the luteinizing hormone/choriogonadotrophin receptor. Hum Reprod Update. 2013;19(5):583–602. doi: 10.1093/humupd/dmt023. [DOI] [PubMed] [Google Scholar]
- 4.Moyle WR, Campbell RK, Rao SN, Ayad NG, Bernard MP, Han Y, et al. Model of human chorionic gonadotropin and lutropin receptor interaction that explains signal transduction of the glycoprotein hormones. J Biol Chem. 1995;270(34):20020–20031. doi: 10.1074/jbc.270.34.20020. [DOI] [PubMed] [Google Scholar]
- 5.Chen C, Xu X, Kong L, Li P, Zhou F, Zhao S, et al. Novel homozygous nonsense mutations in LHCGR lead to empty follicle syndrome and 46, XY disorder of sex development. Human Reproduct (Oxford, England) 2018;33(7):1364–1369. doi: 10.1093/humrep/dey215. [DOI] [PubMed] [Google Scholar]
- 6.David R, Yoon DJ, Landin L, Lew L, Sklar C, Schinella R, et al. A syndrome of gonadotropin resistance possibly due to a luteinizing hormone receptor defect. J Clin Endocrinol Metab. 1984;59(1):156–160. doi: 10.1210/jcem-59-1-156. [DOI] [PubMed] [Google Scholar]
- 7.Shenker A, Laue L, Kosugi S, Merendino JJ, Jr, Minegishi T, Cutler GB., Jr A constitutively activating mutation of the luteinizing hormone receptor in familial male precocious puberty. Nature. 1993;365(6447):652–654. doi: 10.1038/365652a0. [DOI] [PubMed] [Google Scholar]
- 8.Kremer H, Kraaij R, Toledo SP, Post M, Fridman JB, Hayashida CY, et al. Male pseudohermaphroditism due to a homozygous missense mutation of the luteinizing hormone receptor gene. Nat Genet. 1995;9(2):160–164. doi: 10.1038/ng0295-160. [DOI] [PubMed] [Google Scholar]
- 9.Latronico AC, Anasti J, Arnhold IJ, Rapaport R, Mendonca BB, Bloise W, et al. Brief report: testicular and ovarian resistance to luteinizing hormone caused by inactivating mutations of the luteinizing hormone-receptor gene. N Engl J Med. 1996;334(8):507–512. doi: 10.1056/nejm199602223340805. [DOI] [PubMed] [Google Scholar]
- 10.Arnhold IJ, Lofrano-Porto A, Latronico AC. Inactivating mutations of luteinizing hormone beta-subunit or luteinizing hormone receptor cause oligo-amenorrhea and infertility in women. Horm Res. 2009;71(2):75–82. doi: 10.1159/000183895. [DOI] [PubMed] [Google Scholar]
- 11.Ben Hadj Hmida I, Mougou-Zerelli S, Hadded A, Dimassi S, Kammoun M, Bignon-Topalovic J, et al. Novel homozygous nonsense mutations in the luteinizing hormone receptor (LHCGR) gene associated with 46,XY primary amenorrhea. Fertil Steril. 2016;106(1):225–9.e11. doi: 10.1016/j.fertnstert.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Bentov Y, Kenigsberg S, Casper RF. A novel luteinizing hormone/chorionic gonadotropin receptor mutation associated with amenorrhea, low oocyte yield, and recurrent pregnancy loss. Fertil Steril. 2012;97(5):1165–1168. doi: 10.1016/j.fertnstert.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Toledo SP, Brunner HG, Kraaij R, Post M, Dahia PL, Hayashida CY, et al. An inactivating mutation of the luteinizing hormone receptor causes amenorrhea in a 46,XX female. J Clin Endocrinol Metab. 1996;81(11):3850–3854. doi: 10.1210/jcem.81.11.8923827. [DOI] [PubMed] [Google Scholar]
- 14.Toledo SP. Leydig cell hypoplasia leading to two different phenotypes: male pseudohermaphroditism and primary hypogonadism not associated with this. Clin Endocrinol. 1992;36(5):521–522. doi: 10.1111/j.1365-2265.1992.tb02256.x. [DOI] [PubMed] [Google Scholar]
- 15.Qiao J, Han B. Diseases caused by mutations in luteinizing hormone/chorionic gonadotropin receptor. Prog Mol Biol Transl Sci. 2019;161:69–89. doi: 10.1016/bs.pmbts.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Themmen AP, Martens JW, Brunner HG. Activating and inactivating mutations in LH receptors. Mol Cell Endocrinol. 1998;145(1-2):137–142. doi: 10.1016/s0303-7207(98)00180-4. [DOI] [PubMed] [Google Scholar]
- 17.Tsigos C, Latronico C, Chrousos GP. Luteinizing hormone resistance syndromes. Ann N Y Acad Sci. 1997;816:263–273. doi: 10.1111/j.1749-6632.1997.tb52150.x. [DOI] [PubMed] [Google Scholar]
- 18.Feng R, Sang Q, Kuang Y, Sun X, Yan Z, Zhang S, et al. Mutations in TUBB8 and human oocyte meiotic arrest. N Engl J Med. 2016;374(3):223–232. doi: 10.1056/NEJMoa1510791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yariz KO, Walsh T, Uzak A, Spiliopoulos M, Duman D, Onalan G, et al. Inherited mutation of the luteinizing hormone/choriogonadotropin receptor (LHCGR) in empty follicle syndrome. Fertil Steril. 2011;96(2):e125–30. 10.1016/j.fertnstert.2011.05.057. [DOI] [PMC free article] [PubMed]
- 20.Chambers AE, Stanley PF, Randeva H, Banerjee S. Microvesicle-mediated release of soluble LH/hCG receptor (LHCGR) from transfected cells and placenta explants. Reproduct Biol Endocrinol. 2011;9:64. doi: 10.1186/1477-7827-9-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ilgaz NS, Aydos OS, Karadag A, Taspinar M, Eryilmaz OG, Sunguroglu A. Impact of follicle-stimulating hormone receptor variants in female infertility. J Assist Reprod Genet. 2015;32(11):1659–1668. doi: 10.1007/s10815-015-0572-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newton CL, Anderson RC, Katz AA, Millar RP. Loss-of-function mutations in the human luteinizing hormone receptor predominantly cause intracellular retention. Endocrinology. 2016;157(11):4364–4377. doi: 10.1210/en.2016-1104. [DOI] [PubMed] [Google Scholar]
- 23.Xu Y, Chen Y, Li N, Hu X, Li G, Ding Y, et al. Novel compound heterozygous variants in the LHCGR gene identified in a subject with Leydig cell hypoplasia type 1. J Pediatr Endocrinol Metab. 2018;31(2):239–245. doi: 10.1515/jpem-2016-0445. [DOI] [PubMed] [Google Scholar]
- 24.Yuan P, He Z, Zheng L, Wang W, Li Y, Zhao H, et al. Genetic evidence of 'genuine' empty follicle syndrome: a novel effective mutation in the LHCGR gene and review of the literature. Human Reproduct (Oxford, England) 2017;32(4):944–953. doi: 10.1093/humrep/dex015. [DOI] [PubMed] [Google Scholar]
- 25.Qiao J, Han B, Liu BL, Chen X, Ru Y, Cheng KX, et al. A splice site mutation combined with a novel missense mutation of LHCGR cause male pseudohermaphroditism. Hum Mutat. 2009;30(9):E855–E865. doi: 10.1002/humu.21072. [DOI] [PubMed] [Google Scholar]
- 26.Bruysters M, Christin-Maitre S, Verhoef-Post M, Sultan C, Auger J, Faugeron I, et al. A new LH receptor splice mutation responsible for male hypogonadism with subnormal sperm production in the propositus, and infertility with regular cycles in an affected sister. Human Reproduct (Oxford, England) 2008;23(8):1917–1923. doi: 10.1093/humrep/den180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stavrou SS, Zhu YS, Cai LQ, Katz MD, Herrera C, Defillo-Ricart M, et al. A novel mutation of the human luteinizing hormone receptor in 46XY and 46XX sisters. J Clin Endocrinol Metab. 1998;83(6):2091–2098. doi: 10.1210/jcem.83.6.4855. [DOI] [PubMed] [Google Scholar]
- 28.Latronico AC, Chai Y, Arnhold IJ, Liu X, Mendonca BB, Segaloff DL. A homozygous microdeletion in helix 7 of the luteinizing hormone receptor associated with familial testicular and ovarian resistance is due to both decreased cell surface expression and impaired effector activation by the cell surface receptor. Molec Endocrinol (Baltimore, Md) 1998;12(3):442–450. doi: 10.1210/mend.12.3.0077. [DOI] [PubMed] [Google Scholar]
- 29.Mitri F, Bentov Y, Behan LA, Esfandiari N, Casper RF. A novel compound heterozygous mutation of the luteinizing hormone receptor-implications for fertility. J Assist Reprod Genet. 2014;31(7):787–794. doi: 10.1007/s10815-014-0249-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu X, Yan Z, Cai R, Khor S, Wu L, Sun L, et al. Pregnancy and Live Birth In Women With Pathogenic LHCGR Variants Using Their Own Oocytes. J Clin Endocrinol Metab. 2019;104(12):5877–5892. doi: 10.1210/jc.2019-01276. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effects of the c.384-2A>T splicing mutation on LHCGR alternative splicing. a The schematic diagram of minigene assay. b Agarose gel electrophoresis of WT and c.384-2A>T splicing mutation. c The Sanger sequence analysis of WT and c.384-2A>T splicing mutation. The c.384-2A>T mutation led to three different alternative splicing isoforms (PNG 461 kb)