Abstract
Dopamine loss in Parkinson’s disease (PD) is associated with abnormal oscillatory activity in the cortico-basal ganglia network. However, the oscillatory pattern of striatal neurons in PD remains poorly defined. Here, we analyzed the local field potentials in one untreated and five MPTP-treated non-human primates (NHP) with chronic, advanced parkinsonism. Oscillatory activities in the alpha (8–13 Hz) and low-beta (13–20 Hz) frequency bands were found in the striatum similarly to the motor cortex and globus pallidus of the NHP model of PD. Both alpha and low-beta frequency band oscillations of the striatum were highly coherent with the cortical and pallidal oscillations, confirming the presence of abnormal 8–20 Hz oscillatory activity in the cortico-basal ganglia network in parkinsonian NHPs. The reversal of parkinsonism induced by acute levodopa administration was associated with reduced 8–20 Hz oscillations in the striatum. These findings indicate that pathological oscillations at alpha and low-beta bands are also present in the striatum concordant with basal ganglia network changes in the primate model of PD.
Keywords: local field potentials, striatal projection neurons, Parkinson’s disease, dopamine, nonhuman primates
Introduction
Abnormal oscillatory activities in the basal ganglia network are correlated with motor impairment in Parkinson’s disease (PD) (Brown, 2003; Singh et al., 2013; Singh, 2018). The degeneration of nigral dopaminergic neurons that project to the striatum is the pathological hallmark of PD (Damier et al., 1999), but the striatal participation in oscillatory activities of the network has remained unclear. Our previous studies have shown aberrant striatal neuronal spiking activity in parkinsonian non-human primates (NHPs) and patients with PD (Liang et al., 2008; Singh et al., 2015; Singh et al., 2016; Beck et al., 2019). Indeed, chronic dopamine loss leads to major changes in the firing of striatal projection neurons (SPNs), i.e. hyperactivity up to more than 10-fold increase in mean firing rate compared to the normal state, and unstable neuronal responses to dopaminergic stimulation, i.e. firing rate changes in response to L-Dopa that cannot be sustained during the peak-dose effect of the drug (Singh et al., 2015; Singh et al., 2018; Beck et al., 2019). These significant changes in the SPN spiking are likely to be accompanied by altered local inputs and oscillatory activity congruent with the network. In extrastriatal basal ganglia structures, increased oscillations in the 8–30 Hz frequency band are consistently present in animal models and patients with PD (Devergnas et al., 2014; de Hemptinne et al., 2015; Singh et al., 2020). Furthermore, improvement of parkinsonian motor disability by high frequency deep brain stimulation or L-Dopa is accompanied by suppression of the synchronized 8–35 Hz oscillations in the basal ganglia (Kuhn et al., 2006). These data have led to establish the increased oscillatory activity in the cortico-basal ganglia network as a key feature of PD pathophysiology (Stein and Bar-Gad, 2013).
In contrast to the more typical 13–30 Hz (beta-band) basal ganglia oscillation found in human studies, synchronized oscillations in the 8–13 Hz frequency band are detected in parkinsonian NHPs (Stein and Bar-Gad, 2013; Devergnas et al., 2014), and the improvement of parkinsonism in NHP models is also correlated with a decrease in these oscillations (Brown, 2007). These studies suggest that 8–13 Hz rhythms may be a subclass of, or similar to, the beta-band observed in patients with PD; however, the current understanding of this oscillatory activity is limited. The function of these lower frequency band (8–13 Hz) oscillations in the mechanisms of parkinsonian motor deficits remains unknown. Recent data have suggested that certain parameters of the beta-band oscillations, such as duration, are associated specifically with PD since oscillations in this frequency can also be found in normal animals (Deffains et al., 2018). Additionally, the source(s) that generates the basal ganglia network oscillations has been elusive throughout diverse animal and human studies. Clearly, the oscillatory pattern of the striatum, which is the basal ganglia recipient structure, is essential to understand the mechanisms underlying the pathological oscillations in the whole cortico-basal ganglia network developed following dopamine loss in PD.
Here, we examined the striatum and other basal ganglia nuclei for oscillatory activities in normal and parkinsonian NHPs. In addition, we analyzed the striatal oscillations before and after acute L-Dopa administration in correlation with improvement of motor deficits. This study shows that abnormal oscillations in the alpha (8–13 Hz) and low-beta (13–20 Hz) bands are present in the striatum of parkinsonian primates, and that these striatal oscillations are modulated by dopamine replacement.
Material and Methods
Animals and MPTP Treatment
Six adult macaques (four female rhesus monkeys, Macaca Mulatta: Gl, Va, Na, Sa; and two male cynomolgus monkeys, Macaca Fascicularis: Br and Ch; all 5–8 kg) were used in this study. All experimental protocols were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (1996). We have used two species of macaques because they share behavioral similarities particularly regarding motor features of parkinsonism. Five animals received weekly intravenous injections of MPTP (0.2–0.6 mg/kg, see details in previous reports (Singh et al., 2015; Singh et al., 2016; Beck et al., 2019) until motor disability stabilized to a non-recoverable parkinsonism of moderate-severe degree, as measured by the standardized “Motor disability scale for MPTP-treated primates” (Potts et al., 2014; Singh et al., 2015). All five animals had sustained a chronic parkinsonism at the time of recordings 6 months or longer after last MPTP injection. One animal (Sa) was not treated with MPTP and used as a normal control. Three parkinsonian NHPs (Gl, Va, Na) were previously treated with daily L-Dopa regimen (Sinemet®, 100–200 mg/day) to improve their health, but these animals did not develop L-Dopa-induced dyskinesias. The L-Dopa treatment was withdrawn 24 hours prior to the recording time. The other two parkinsonian NHPs (Br and Ch) had never been exposed to daily L-Dopa administration before the recordings. All the demographics and clinical characteristics are shown in Table 1. L-Dopa doses used for acute subcutaneous injections during the recordings (L-Dopa methyl ester plus benserazide) in animals Br and Ch were selected based on our previous recordings of parkinsonian primates (Singh et al., 2015; Singh et al., 2018; Beck et al., 2019) where we used the dose sufficient to induce a measurable motor response compatible with the restraint of the animal in the primate chair (see doses in next section). MDS in the “off” state (basal parkinsonian motor disability) and the “on” state (reversal of parkinsonism by dopaminergic drug treatment) induced by L-Dopa administration during the recordings are shown in Table 1. The level of parkinsonism developed in all 5 animals was similar, as shown by motor disability scores (MDS) in “OFF” with minimal variation (MDS between 19 and 22, scale range 0–39).
Table 1.
Subject demographics and characteristics of the primate MPTP model of PD.
| Subject | Species (Macaca) | Sex | Age (years) | Weight (kg) | MPTP TCD (mg) | MDS OFF | MDS ON | Recording regions | Recording states |
|---|---|---|---|---|---|---|---|---|---|
| Sa | Mulatta | F | 6 | 5.5 | - | - | - | Putamen | Normal |
| Gl | Mulatta | F | 8 | 5.1 | 13.7 | 21 | - | Putamen, GPi | PD, OFF |
| Va | Mulatta | F | 7 | 7.6 | 10.8 | 20 | - | Motor Cortex, Putamen, GPi | PD, OFF |
| Na | Mulatta | F | 12 | 8.1 | 20.1 | 19 | - | Motor Cortex | PD, OFF |
| Br | Fascicularis | M | 6 | 5.8 | 7.6 | 22 | 9.5* | Putamen | PD, Off/ON |
| Ch | Fascicularis | M | 7 | 6 | 3 | 20 | 6.5* | Putamen | PD, Off/ON |
: L-Dopa treatment was not administered daily but only acutely for recording sessions. The L-Dopa methyl ester/benserazide doses given s.c during the recordings were 95/24 mg for Br and 90/22.5 mg for Ch. TCD = total cumulative dose of MPTP. MDS = motor disability score in the “OFF” or “ON” state (scale range 0–39). F = female; M = male; GPi = globus pallidus internus.
Surgical Procedures and LFP Recordings
Stainless steel recording chambers (19 mm diameter) were stereotaxically implanted in the coronal plane with a 15–20° angle in all NHPs to allow a trajectory of recording electrodes from the motor cortex to the striatum (putamen) and the internal pallidum (GPi). A head post was also implanted in surgery for head restraint in the primate chair during the recordings. Implant surgeries were conducted under general anesthesia in all animals. Following a period of recovery from surgery, the recordings began under head restraint in the primate chair. Recordings were performed lowering the recording electrode inside the recording chamber and to the brain using an electronic microdrive (NAN Instruments LTD, Israel). Data were collected using Blackrock Microsystems (sampling rate 40 kHz; spike data: high-pass filter at 750 Hz; LFP data: low-pass filter at 250 Hz). The electrophysiological mapping of the motor cortex, striatum (putamen), and GPi was performed by extracellular recordings of spiking activity using tungsten microelectrodes (FHC, Bowdoinham, ME; impedance = 0.1–0.3 MΩ at 1 kHz) (Liang et al., 2008). We determined the target locations on the basis of their signature neuronal firing pattern (see Fig. S1). We did not apply imaging techniques to determine the target location, as our mapping techniques have successfully identified target regions in basal ganglia in our previous studies (Singh et al., 2015; Singh et al., 2018; Beck et al., 2019). The histological verification of our target regions at the end of the studies were provided in our previous reports (Singh et al., 2018; Beck et al., 2019). Striatal LFP signals were collected in the post-commisural, dorsolateral areas of the putamen after electrophysiologic mapping of the basal ganglia. As unipolar LFP reference, we used the recording chamber implanted on the skull or the connection to the amplified electrical ground. To determine striatal LFP changes following dopamine depletion, LFPs were recorded in the striatum (putamen) in three NHPs. Putamenal LFP were recorded in two parkinsonian NHPs in the ‘off’ state (Gl, n=9; Va, n=7), and compared to the putamenal LFPs in one normal NHP (Sa, n=54 recordings). LFPs were then recorded in parkinsonian NHPs in the ‘off’ state in motor cortex and GPi to determine the correlated oscillatory activities across animals in all three structures (in motor cortex: Na, n=12 and Va, n=8 recordings; in GPi: Gl, n=9 and Va, n=11 recordings). Due to low variability in these LFPs across animals, we analyzed the presence of coherent oscillatory activity within the cortico-basal ganglia network in one parkinsonian NHP with simultaneous recordings of LFP signals in (i) motor cortex and striatum (Va, n=13 recordings), and (ii) striatum and GPi (Va, n=11 recordings). To determine the relation between the observed oscillatory activity and the dopaminergic state, striatal LFPs were recorded before and after acute L-Dopa administration (“off” and “on” states, respectively) in two additional parkinsonian NHPs (Br, n=17; Ch, n=5 recordings). Doses of L-Dopa methyl ester/benserazide given s.c. for these experiments were 95/24 mg in Br and 90/22.5 mg in Ch. Typically, the “on” state was evidenced by behavioral changes approximately 20 minutes after the acute systemic (s.c.) injection of L-Dopa. LFP signals (sampling frequency: 2000 Hz, Blackrock Microsystems; sampling frequency: 20 kHz, Multichannel Acquisition Processor, Plexon Inc.) were amplified and low pass filtered with 750 Hz. LFPs were recorded continuously for at least 3 min in the awake condition (open eyes). Animals were monitored during the recordings to ensure that transition to the behavioral state after L-Dopa injection (“on” state) correlated with our LFP data collection. A potential limitation to the current dataset includes the lack of vehicle control injections. However, any changes in LFP induced by agitation of the animal due to the injection itself was not affecting our results because data were collected 20 min after the injection at the time of the “on” state induced by the subcutaneous L-Dopa dose. Any agitation of the animal caused by the injection disappeared after a few minutes, and the animal returned to its quiet “off” state before turning “on” 20 min later. Collected LFPs were stored for offline analysis.
Data Analysis
Continuous LFP data were analyzed using Matlab software (MathWorks, Natick, MA), with custom codes on the basis of standardized signal analysis functions (signal analysis toolbox). Power spectral analysis of LFP signals was computed applying the “pwelch” method. All signals were resampled to 1024 Hz and band passed 1–90 Hz (Butterworth zero phase filter). A 60 Hz notch filter was also used to eliminate line-noise. Signals were segmented into 5 sec epochs, which were transformed into the power spectrum domain (pwelch method: 1024 samples window size overlapped by 50%). A frequency range of 1–90 Hz was selected to compute relative power spectrum to reduce the inter-recording variation. Subsequently, all spectra were averaged. Visual examination was initially performed to remove time periods with obvious artifacts. We did not notice any movement artifacts in the LFPs during the “off” state. However, small movement artifacts were noticed in the LFPs during the “on” state. We eliminated epochs from the analysis whose amplitude was above 2 standard deviation from the mean of all epochs. Previous studies of the basal ganglia oscillations in MPTP-treated NHPs detected increases in the 8–20 Hz frequency range (Stein and Bar-Gad, 2013; Devergnas et al., 2014). As we observed two distinct spectral peaks in the 8–13 Hz and 13–20 Hz frequency ranges, we computed the mean relative LFP-power at these two frequency bands. For graphic visualization of the relative power spectrum, we used 4–50 Hz range. Coherence analysis between cortico-striatal and striato-pallidal LFPs was computed using the “csd” matlab function with a window size of 1024, which provided a frequency resolution of ~1 Hz with 50% overlap. Differences between relative power of striatal LFPs (8–13 Hz and 13–20 Hz) recorded in normal and parkinsonian NHPs were analyzed with unpaired t-test. Differences between “off” and “on” states for 8–13 Hz and 13–20 Hz frequency bands were analyzed with paired t-tests.
The relative power of 8–13 Hz and 13–20 Hz frequency band oscillations were computed to differentiate normal from parkinsonian NHPs, and “off” from “on” states using logistic regression (LR). Since the LFP analysis revealed peaks in 8–13 Hz and 13–20 Hz frequency bands, we selected those band power values as features for further analysis. We examined the accuracy of the relation (predictor or classifier) using a two-class problem in the support vector machine (SVM) method on the average of 10-fold cross-validation tests on 8–13 Hz and 13–20 Hz frequency bands values. Data were divided into ten groups and then trained with nine groups and tested with one group. This was repeated ten times while each group was used as a test group. Subsequently, single performance estimation was calculated. We used ‘svmtrain’ and ‘svmclassify’ matlab functions for classification, and ‘classperf’ matlab function was used to estimate the performance or accuracy of the classifier. Accuracy of the classifier was displayed using a Receiver Operating Characteristic (ROC) curve, a graphical plot of the sensitivity vs. (1-specificity).
Results
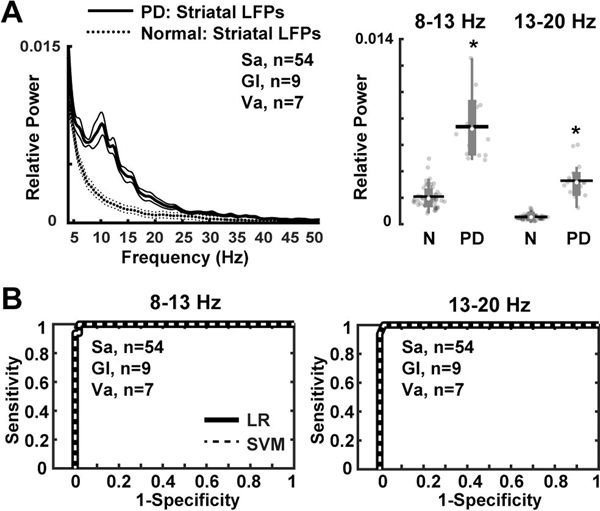
Parkinsonian NHPs exhibited aberrant striatal oscillations with relative power at 8–13 Hz and 13–20 Hz frequency bands as compared to the normal condition (P = 0.001; Fig. 1A). In the normal NHP, there is no predominant oscillatory activity in any frequency range in the striatum. The choice of boundaries for frequency bands was based on pervious data from PD models and patients, where 8–13 Hz and 13–20 Hz are the frequency bands of the oscillatory activity usually found (Stein and Bar-Gad, 2013; Devergnas et al., 2014). However, comparison of the power spectra of these rhythms indicates predominance of low frequencies (8–13 Hz), which seems more characteristic of the primate (Devergnas et al., 2014). Further analysis of the relation between the relative power of 8–13 Hz and 13–20 Hz frequency bands to normal and parkinsonian states in NHPs revealed a strong correlation with notable sensitivity (for both bands 8–13 Hz and 13–20 Hz: 99% LR and SVM methods), and accuracy (8–13 Hz: 97%; 13–20 Hz: 98%) for these parameters to classify MPTP-treated NHPs versus control (Fig. 1B). These results were highly constant across algorithms (LR and SVM) and cross-validation procedure.
Figure 1. Striatal LFPs in parkinsonian non-human primates (NHPs).
(A) Relative power in the 8–13 Hz and 13–20 Hz frequency bands showing significant increases in the dopamine depleted striatum of NHPs (Gl, n=9; Va, n=7 recordings) as compared with the intact striatum in the normal NHP (Sa, n=54 recordings). (B) Classification of control (Sa, n=54 recordings) versus parkinsonian NHPs (9 Gl and 7 Va, total n=16 recordings) based on power values of 8–13 Hz and 13–20 Hz frequency bands. Receiver operating characteristic plots show the true (sensitivity) vs. false positive rates (1-specificity) of MPTP-treated vs. normal NHPs discrimination for each frequency band separately using logistic regression (LR) and support vector machines (SVM) methods. Thick and thin lines represent mean and standard error of mean, respectively. Significant difference is denoted by *P<0.01. N = Normal NHP; PD = MPTP-treated NHP. The black horizontal lines and white circles in the box plots represent the mean and median values, respectively.
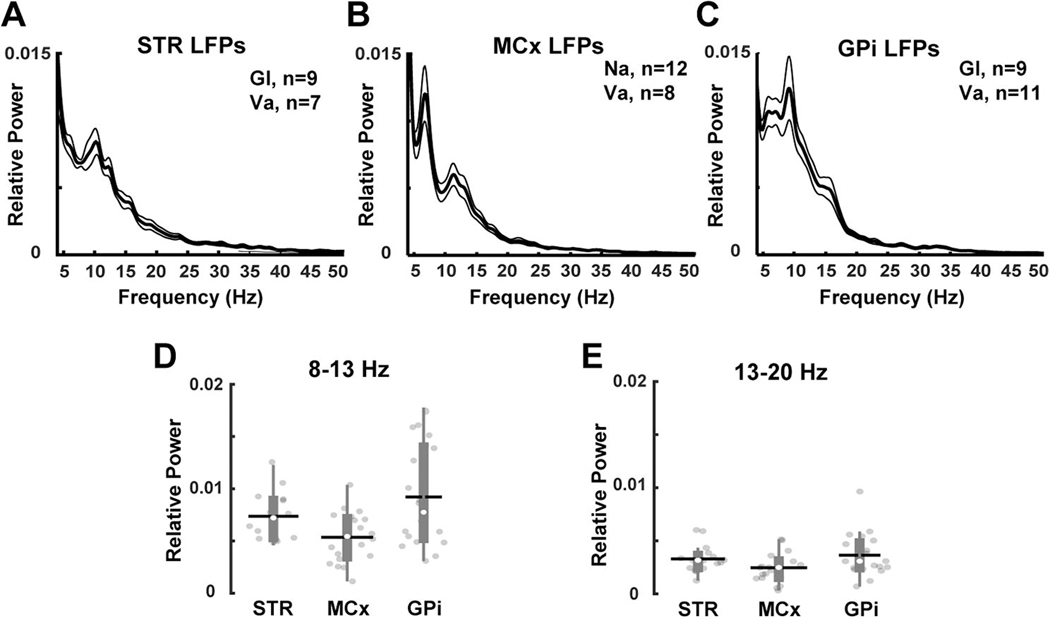
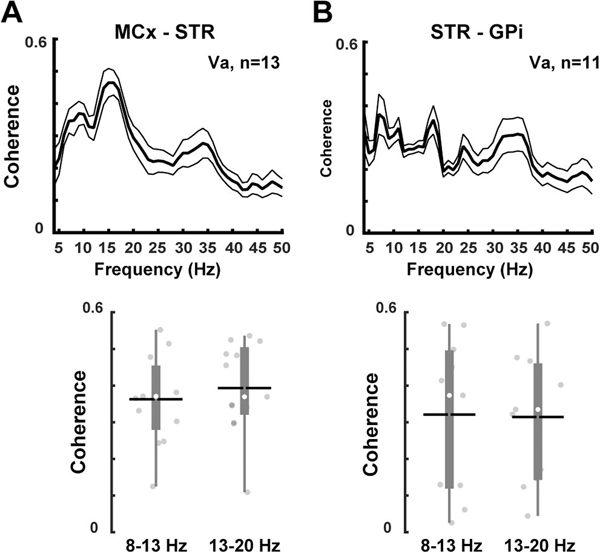
In parkinsonian NHPs, the relative powers of 8–13 Hz and 13–20 Hz frequency bands in the striatum were similar to those recorded in the motor cortex and GPi (Fig. 2). In the normal NHP, fixed electrodes in the striatum prevented recordings of LFPs in the cortex and GPi. LFPs recorded simultaneously in the motor cortex and the striatum or the striatum and GPi show increased coherence in the 8–13 Hz and 13–20 Hz frequency bands in parkinsonian NHPs (Fig. 3). Similarly, previous human and rodent studies have demonstrated an increase in coherence at the same frequency bands between cortical and basal ganglia regions in the parkinsonian state (Sharott et al., 2005; Litvak et al., 2011). Overall, these results confirm that synchronized striatal oscillations in the 8–20 Hz frequency band may reflect a network pattern that occurs after dopamine loss in the primate.
Figure 2. LFPs recorded from motor cortex and basal ganglia nuclei.
(A) Striatal (Gl, n=9; Va, n=7 recordings), (B) motor cortical (Na, n=12; Va, n=8 recordings), and (C) internal pallidal (Gl, n=9; Va, n=8 recordings) oscillations recorded in parkinsonian NHPs. (D-E) Comparison of the relative power of 8–13 Hz and 13–20 Hz frequency bands in each of the studied regions. The relative powers of LFPs at 8–13 Hz and 13–20 Hz were similar across the motor cortex, the striatum and the GPi. Thick and thin lines represent mean and standard error of mean, respectively. STR= striatum; MCx= motor cortex; GPi= globus pallidus internus. The black horizontal lines and white circles in the box plots represent the mean and median values, respectively.
Figure 3. Coherence of oscillatory activity in the cortico-striato-pallidal network in parkinsonian non-human primates.
(NHPs). (A) Increased coherence of LFPs (upper graph) between the motor cortex and the striatum (Va, n=13 recordings) similarly shown at both 8–13 Hz and 13–20 Hz frequency bands (bottom graph). (B) Increased coherence of LFPs also between the striatum and the GPi (Va, n=11 recordings) at both frequency bands. These results demonstrate the presence of augmented oscillatory activity at 8–20 Hz in the basal ganglia circuits after dopamine loss. Thick and thin lines represent mean and standard error of mean, respectively. MCx=motor cortex; STR=striatum; GPi=globus pallidus internus. The black horizontal lines and white circles in the box plots represent the mean and median values, respectively.
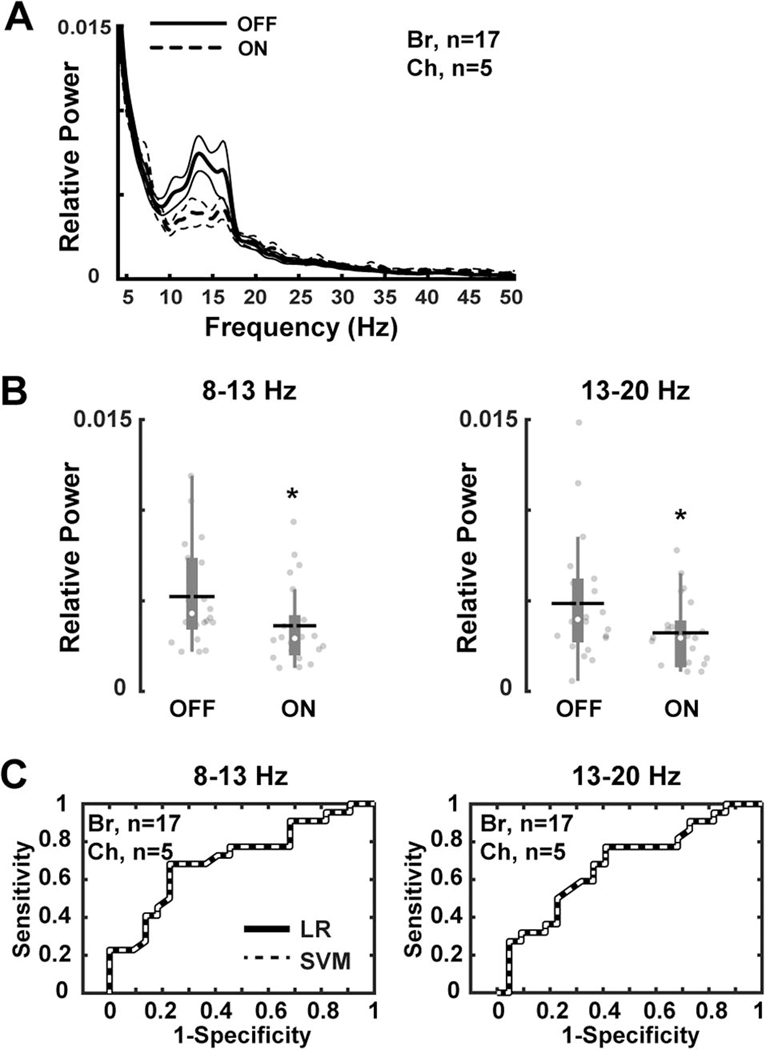
Power spectral analysis of the striatal LFPs recorded in NHPs after acute L-Dopa injection inducing a motor response (“on” state) revealed markedly reduced relative power in the 8–13 Hz (P = 0.001) and 13–20 Hz (P = 0.01) frequency bands (Fig. 4A and B). Reductions of both 8–13 Hz and 13–20 Hz frequency band oscillations following dopamine replacement were highly sensitive (8–13 Hz: 71% and 13–20 Hz: 68% LR and SVM methods; Fig. 4C) and accurate (8–13 Hz: 70%; 13–20 Hz: 66%) to distinguish the “off” and “on” states in parkinsonian NHPs. These results are in line with previous human and animal studies that have shown reduced alpha and beta-band power in extrastriatal regions after L-Dopa administration (Kuhn et al., 2006; Devergnas et al., 2014).
Figure 4. Striatal LFPs before and after acute L-Dopa administration in parkinsonian nonhuman primates (NHPs).
(A and B) Relative power of the striatal oscillations (Br, n=17; Ch, n=5 recordings) at 8–13 Hz and 13–20 Hz frequency range showing significant decreases after L-Dopa injection. (C) Classification of “off” versus “on” states in parkinsonian NHPs based on power values of 8–13 Hz and 13–20 Hz frequency band oscillations (Br, n=17; Ch, n=5 recordings). Receiver operating characteristic plots show the true (sensitivity) vs. false positive rates (1-specificity) of “off” vs. “on” state discrimination for each frequency band separately using logistic regression (LR) and support vector machines (SVM) methods. Thick and thin lines represent mean and standard error of mean, respectively. Significant difference is denoted by *P<0.01. The black horizontal lines and white circles in the box plots represent the mean and median values, respectively.
Discussion
The present results showed augmented 8–13 Hz and 13–20 Hz oscillations in the striatum of MPTP-treated NHPs with chronic, advanced parkinsonism. It has been previously reported that the acute reduction of dopamine in the striatum of rodents amplifies striatal beta oscillations (Costa et al., 2006). However, the lack of significant striatal oscillatory activity in the normal primate indicates a pronounced change in the primate MPTP model. These data are consistent with findings in extrastriatal regions of basal ganglia showing the relationship between oscillatory activity in the 8–13 Hz and 13–20 Hz frequency bands and dopamine depletion in NHP (Stein and Bar-Gad, 2013; Devergnas et al., 2014). In rodents, low-beta band oscillations (14–20 Hz) has also been associated with dopamine depletion in a recent experiment (West et al., 2018), as opposed to earlier data showing abnormally amplified high-beta band oscillation (15–30 Hz) in the cortico-subthalamic nucleus (STN) network (Mallet et al., 2008). Interestingly, West et al. study presents a comparative analysis of these frequency bands showing changes in the network oscillations following “chronic” dopamine depletion. Plastic changes may occur due to progressive dopamine depletion where the oscillatory activity in the network synchronizes at a lower-beta band frequency. In patients with PD, basal ganglia oscillations were also reported in the 13–20 Hz frequency range with LFP recordings (Brown, 2003; Singh et al., 2013; Singh, 2018), and 10–25 Hz frequency range with single-unit recordings (Levy et al., 2002). Altogether, data indicate that oscillatory dynamics in the cortico-basal ganglia circuitry extend mostly from 8 to 20 Hz frequencies in the parkinsonian state.
Different neuronal populations in the striatum may participate in the changes of field potentials observed after dopamine loss. In dopamine-depleted rodents, SPNs in the indirect pathway exhibit increased spiking activity and abnormal phase-locked spiking to ongoing cortical oscillations in the 15–30 Hz frequency band (Sharott et al., 2017). Although these data indicate spectral differences of oscillatory activities between rodent and primate models, there is consistency with respect of unit activity changes. In Sharott et al. study, the synchronization of indirect striatal neurons to the 15–30 Hz oscillation revealed a cell-type selective entrainment of striatal spiking to parkinsonian beta oscillations. Nevertheless, the role of cell subtypes in basal ganglia regions in the generation, propagation, and interaction of oscillatory dynamics throughout the network is still unclear. The present study reports the network coherence of striatal oscillation in the primate model of advanced, chronic parkinsonism. The spiking synchronization to oscillatory activity in the striatum of this primate model remains to be investigated.
A recent primate study by Deffains et al. (Deffains et al., 2016) reported that STN, not striatal, activity in the 8–15 Hz correlates with basal ganglia downstream activity in parkinsonian NHPs. In their study, unit recordings showed no oscillatory activity of SPNs but did show 10 Hz oscillatory activity of striatal tonically active neurons (TANs). The study also showed 8–15 Hz oscillations in striatal LFP recordings, which were exaggerated in parkinsonian monkeys compared to normal monkeys. This striatal oscillation only entrained the spiking activity of TANs, but not SPNs. In both single- and multi-unit spiking activity only TANs were phase locked to the striatal 8–15 Hz LFP oscillations. Consistently, we found similar striatal oscillatory activity in the present LFP recordings in monkeys with advanced and chronically developed parkinsonism. However, we did not determine what type of striatal neuron (SPNs or TANs) synchronizes with the global synaptic input, which is represented by the LFP oscillations. While it is possible that in our model entraining of striatal neurons by global synaptic inputs develops differently, at present we lack the data to make assumptions on the impact of the striatal LFP oscillation in the downstream activity, which is driven by the SPNs.
McCarthy et al. (McCarthy et al., 2011) have combined computational and experimental methods to analyze the origin of striatal oscillations in the rodent model. Their results showed the participation of striatal cholinergic tone through interaction of muscarinic mediated voltage-gated K+ channels (M-currents) with GABAa currents from interconnected SPNs. Other studies have also shown the relation of the striatal cholinergic mechanism to the generation of beta oscillations and parkinsonian motor deficits (Kondabolu et al., 2016). Regarding SPN interconnections, GABAa synapses between SPNs are weakened in PD models (Taverna et al., 2008), and thus, the pattern of beta oscillations is likely regulated by modulators of the SPN excitability. Of note, striatal oscillations in the analyzed models were independent of GABAergic interneurons. Taken together, the available data support that the striatal network can augment or generate beta oscillations upon increased excitability of SPNs in the parkinsonian state.
Enhanced beta frequency oscillations in PD have been attributed to the cortex-STN/globus pallidus externus (GPe) patterning in the network (Magill et al., 2001; Bevan et al., 2002). Noteworthy, this source of oscillations and other STN/GPe activity changes depend critically upon receiving increased striatal input via the indirect striatal output pathway (Terman et al., 2002; Chu et al., 2017). The striatal LFP analyzed here showed the network coherence of striatal oscillation in the primate model of advanced, chronic parkinsonism. It is important to recognize the limitations in these primate recordings avoiding the use of multiple electrodes lowered to deep brain structures due to safety reasons. The lack of multiple bipolar signals collected simultaneously limited the coherence analysis to unipolar signals, which can be affected by volume conduction. Yet, the present data are in keeping with the cumulative evidence supporting a key role of the striatum in the network changes associated with dopamine depletion in PD. However, the intrinsic striatal mechanisms and specific cell types involved in the generation of beta frequency oscillations remain unsettled. Previous studies consistently revealed SPN hyperactivity as one salient condition underlying striatal functional changes, which is congruent with the prediction of the computational model in McCarthy et al. (2011). The increase of SPN activity that underlies synchronized spiking in beta frequencies in the parkinsonian state also causes pathological (unstable) SPN responses to dopamine (Singh et al., 2015). Notably, this abnormal activity pattern can be reversed by reducing the excitatory glutamatergic signaling (Singh et al., 2018), indicating that basal SPN hyperexcitability is a major source of striatal network changes. Therefore, glutamatergic control of SPN spiking likely plays a key role in the generation of striatal beta band oscillations in PD.
The normal and pathological functions of beta rhythms are still unclear, but dopamine modulation of oscillatory activity concomitant with motor effects is significant. Consistent with previous results (Sharott et al., 2005; Mallet et al., 2008), the “on” state induced by levodopa administration was accompanied by reduced striatal 8–20 Hz oscillations in the present NHP experiments. Nevertheless, the present study does not address the specific relationship of striatal oscillations to parkinsonian motor impairment. These LFP recordings demonstrate that oscillatory dynamics develop in the striatum of primates with chronic, moderate parkinsonism. The study also reveals that the striatal oscillation between alpha (8–13 Hz) and low-beta (13–20 Hz) frequency bands is coherent with the rhythms found in the cortico-basal ganglia network in NHP models, thereby supporting a primary contribution of the striatum to the motor network changes in PD.
Supplementary Material
Highlights.
Alpha and low-beta oscillations were present in the striatum of MPTP-treated NHP.
These oscillations were highly coherent with the cortical and pallidal oscillations.
L-dopa reduced abnormal 8–20 Hz oscillations in the striatum of MPTP-treated NHP.
Striatal oscillations may play a key role in the motor network changes of PD.
Acknowledgements
This work was supported by NIH grants NS045962, NS073994, RR000165 and OD011132 (S.M.P). We thank the staff of the Veterinary Department at the YNPRC for their assistance in the care of primates with advanced PD.
Footnotes
Competing interests
The authors declare no competing interests.
Data Availability
The data that support the findings of this study are available on request from the corresponding author (A.S.).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beck G, Singh A, Zhang J, Potts LF, Woo JM, Park ES, et al. Role of striatal DeltaFosB in l-Dopa-induced dyskinesias of parkinsonian nonhuman primates. Proc Natl Acad Sci U S A 2019; 116: 18664–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan MD, Magill PJ, Terman D, Bolam JP, Wilson CJ. Move to the rhythm: oscillations in the subthalamic nucleus-external globus pallidus network. Trends Neurosci 2002; 25: 525–31. [DOI] [PubMed] [Google Scholar]
- Brown P. Oscillatory nature of human basal ganglia activity: relationship to the pathophysiology of Parkinson’s disease. Mov Disord 2003; 18: 357–63. [DOI] [PubMed] [Google Scholar]
- Brown P. Abnormal oscillatory synchronisation in the motor system leads to impaired movement. Curr Opin Neurobiol 2007; 17: 656–64. [DOI] [PubMed] [Google Scholar]
- Chu HY, McIver EL, Kovaleski RF, Atherton JF, Bevan MD. Loss of Hyperdirect Pathway Cortico-Subthalamic Inputs Following Degeneration of Midbrain Dopamine Neurons. Neuron 2017; 95: 1306–18 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa RM, Lin SC, Sotnikova TD, Cyr M, Gainetdinov RR, Caron MG, et al. Rapid alterations in corticostriatal ensemble coordination during acute dopamine-dependent motor dysfunction. Neuron 2006; 52: 359–69. [DOI] [PubMed] [Google Scholar]
- Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain 1999; 122 ( Pt 8): 1437–48. [DOI] [PubMed] [Google Scholar]
- de Hemptinne C, Swann NC, Ostrem JL, Ryapolova-Webb ES, San Luciano M, Galifianakis NB, et al. Therapeutic deep brain stimulation reduces cortical phase-amplitude coupling in Parkinson’s disease. Nat Neurosci 2015; 18: 779–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deffains M, Iskhakova L, Katabi S, Haber SN, Israel Z, Bergman H. Subthalamic, not striatal, activity correlates with basal ganglia downstream activity in normal and parkinsonian monkeys. Elife 2016; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deffains M, Iskhakova L, Katabi S, Israel Z, Bergman H. Longer beta oscillatory episodes reliably identify pathological subthalamic activity in Parkinsonism. Mov Disord 2018; 33: 1609–18. [DOI] [PubMed] [Google Scholar]
- Devergnas A, Pittard D, Bliwise D, Wichmann T. Relationship between oscillatory activity in the cortico-basal ganglia network and parkinsonism in MPTP-treated monkeys. Neurobiol Dis 2014; 68: 156–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondabolu K, Roberts EA, Bucklin M, McCarthy MM, Kopell N, Han X. Striatal cholinergic interneurons generate beta and gamma oscillations in the corticostriatal circuit and produce motor deficits. Proc Natl Acad Sci U S A 2016; 113: E3159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn AA, Kupsch A, Schneider GH, Brown P. Reduction in subthalamic 8–35 Hz oscillatory activity correlates with clinical improvement in Parkinson’s disease. Eur J Neurosci 2006; 23: 1956–60. [DOI] [PubMed] [Google Scholar]
- Levy R, Ashby P, Hutchison WD, Lang AE, Lozano AM, Dostrovsky JO. Dependence of subthalamic nucleus oscillations on movement and dopamine in Parkinson’s disease. Brain 2002; 125: 1196–209. [DOI] [PubMed] [Google Scholar]
- Liang L, DeLong MR, Papa SM. Inversion of dopamine responses in striatal medium spiny neurons and involuntary movements. J Neurosci 2008; 28: 7537–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvak V, Jha A, Eusebio A, Oostenveld R, Foltynie T, Limousin P, et al. Resting oscillatory cortico-subthalamic connectivity in patients with Parkinson’s disease. Brain 2011; 134: 359–74. [DOI] [PubMed] [Google Scholar]
- Magill PJ, Bolam JP, Bevan MD. Dopamine regulates the impact of the cerebral cortex on the subthalamic nucleus-globus pallidus network. Neuroscience 2001; 106: 313–30. [DOI] [PubMed] [Google Scholar]
- Mallet N, Pogosyan A, Sharott A, Csicsvari J, Bolam JP, Brown P, et al. Disrupted dopamine transmission and the emergence of exaggerated beta oscillations in subthalamic nucleus and cerebral cortex. J Neurosci 2008; 28: 4795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Moore-Kochlacs C, Gu X, Boyden ES, Han X, Kopell N. Striatal origin of the pathologic beta oscillations in Parkinson’s disease. Proc Natl Acad Sci U S A 2011; 108: 11620–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts LF, Wu H, Singh A, Marcilla I, Luquin MR, Papa SM. Modeling Parkinson’s disease in monkeys for translational studies, a critical analysis. Exp Neurol 2014; 256: 133–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharott A, Magill PJ, Harnack D, Kupsch A, Meissner W, Brown P. Dopamine depletion increases the power and coherence of beta-oscillations in the cerebral cortex and subthalamic nucleus of the awake rat. Eur J Neurosci 2005; 21: 1413–22. [DOI] [PubMed] [Google Scholar]
- Sharott A, Vinciati F, Nakamura KC, Magill PJ. A Population of Indirect Pathway Striatal Projection Neurons Is Selectively Entrained to Parkinsonian Beta Oscillations. J Neurosci 2017; 37: 9977–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A. Oscillatory activity in the cortico-basal ganglia-thalamic neural circuits in Parkinson’s disease. Eur J Neurosci 2018; 48: 2869–78. [DOI] [PubMed] [Google Scholar]
- Singh A, Cole RC, Espinoza AI, Brown D, Cavanagh JF, Narayanan NS. Frontal theta and beta oscillations during lower-limb movement in Parkinson’s disease. Clin Neurophysiol 2020; 131: 694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Jenkins MA, Burke KJ Jr., Beck G, Jenkins A, Scimemi A, et al. Glutamatergic Tuning of Hyperactive Striatal Projection Neurons Controls the Motor Response to Dopamine Replacement in Parkinsonian Primates. Cell Rep 2018; 22: 941–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Liang L, Kaneoke Y, Cao X, Papa SM. Dopamine regulates distinctively the activity patterns of striatal output neurons in advanced parkinsonian primates. J Neurophysiol 2015; 113: 1533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Mewes K, Gross RE, DeLong MR, Obeso JA, Papa SM. Human striatal recordings reveal abnormal discharge of projection neurons in Parkinson’s disease. Proc Natl Acad Sci U S A 2016; 113: 9629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Plate A, Kammermeier S, Mehrkens JH, Ilmberger J, Bötzel K. Freezing of gait-related oscillatory activity in the human subthalamic nucleus. Basal Ganglia 2013; 3: 25–32. [Google Scholar]
- Stein E, Bar-Gad I. beta oscillations in the cortico-basal ganglia loop during parkinsonism. Exp Neurol 2013; 245: 52–9. [DOI] [PubMed] [Google Scholar]
- Taverna S, Ilijic E, Surmeier DJ. Recurrent collateral connections of striatal medium spiny neurons are disrupted in models of Parkinson’s disease. J Neurosci 2008; 28: 5504–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terman D, Rubin JE, Yew AC, Wilson CJ. Activity patterns in a model for the subthalamopallidal network of the basal ganglia. J Neurosci 2002; 22: 2963–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West TO, Berthouze L, Halliday DM, Litvak V, Sharott A, Magill PJ, et al. Propagation of Beta/Gamma Rhythms in the Cortico-Basal Ganglia Circuits of the Parkinsonian Rat. J Neurophysiol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.