Abstract
Objectives:
Ambulatory blood pressure monitoring measures 24-hour blood pressure, night-time blood pressure, and impaired dipping of nocturnal blood pressure, parameters that better predict cardiovascular risk than standard office blood pressure measurements. Systemic lupus erythematosus is characterized by immune system hyperactivity, elevated cardiovascular risk and high prevalence of hypertension; however, little is known about ambulatory blood pressure in lupus patients and its relationship to immune activation.
Methods:
We studied 26 patients with lupus and 26 control subjects. We obtained ambulatory 24-hour blood pressure measurements and report plasma concentrations of 77 markers of immune activation using a multiplex immunoassay and assessed their association with blood pressure measurements.
Results:
Despite similar office blood pressure measurements in patients with lupus and controls, lupus patients had higher 24-hour systolic [median (interquartile range) 129 (113 – 140) vs. 116 (111 – 121) mmHg, p=0.03] and diastolic blood pressure [80 (69 – 86) vs. 72 (64 – 75) mmHg, p=0.006] as well as less nocturnal dipping [7.8% (5.1 – 14.2%) vs. 12.0% (8.1-20.0%)] p=0.03], compared to controls. In patients with lupus, markers of the innate (monocyte chemotactic protein-3) and adaptive immune systems [CUB domain-containing protein-1 and Interleukin-15 receptor subunit-α,] were associated with nocturnal blood pressure measurements and attenuated nocturnal dipping. In conclusion, 24-hour systolic and diastolic blood pressure was higher and nocturnal blood pressure dipping was attenuated in patients with lupus compared to control subjects.
Conclusion:
In patients with SLE, nocturnal blood pressure and attenuated nocturnal blood pressure dipping were significantly associated with several innate and adaptive immune system biomarkers.
Keywords: systemic lupus erythematosus, 24-hour blood pressure, nocturnal blood pressure dipping, immune system
INTRODUCTION
Elevated blood pressure (BP) is the single largest risk factor for all-cause and cardiovascular mortality in the general population.(1, 2) When clinicians seek to define and treat hypertension, they rely almost exclusively on blood pressure measurements obtained in the clinic. However, increasing evidence indicates that ambulatory blood pressure monitoring (ABPM), which provides BP readings over the course of 24 hours, is a better predictor of long-term outcomes than office blood pressure.(3) Indeed, ABPM offers several advantages compared to traditional office BP measures.
ABPM provides measures of nocturnal blood pressure. Blood pressure follows a circadian rhythm; usually systolic blood pressure (SBP) drops during the night. Absence or attenuation of this phenomenon—“non-dipping”—is a cardiovascular risk factor independent of hypertension.(4) Moreover, non-dipping is a better predictor of cardiovascular events than blood pressure measured in the clinic and has been associated with albuminuria and decreased renal function.(5, 6) Also, ABPM can detect masked hypertension—normal BP in the clinic but elevated 24-hour BP.(7) The risk of cardiovascular events(8) and target organ damage in individuals with masked hypertension ranges from 61% to 81% higher than in normotensive subjects.(9) Finally, the availability of ABPM information counteracts the potential overtreatment of patients with isolated clinic hypertension but normal 24-hour BP.2
Hypertension is associated with both the innate and adaptive immune systems through macrophages, T-cells, and multiple cytokines which contribute to the pathogenesis of hypertension.(10) Furthermore, the activation of immune cells causes vasoconstriction and remodeling, leading to increased vascular resistance and blood pressure.(11)
Patients with SLE are at higher risk for developing hypertension and resistant hypertension.(12, 13) In addition, multiple dysregulations in the immune system—at the level of T-cells, macrophages, immunoglobulin production, and complement activation—play important roles in SLE pathogenesis and clinical severity.(14),(15) Despite these dysregulations related to SLE, we know little about the relationship between blood pressure, particularly 24-hour blood pressure measurements, and markers of immune activation in patients with SLE. Thus, we first tested the hypothesis that ABPM measurements are higher in patients with SLE than in control subjects. Second, we performed exploratory analysis to test the hypothesis that higher ABPM measurements and impaired nocturnal BP dipping were associated with markers of immune activation in SLE.
METHODS
Subjects
Patients with SLE were eligible for the study if they had a diagnosis which fulfilled the American College of Rheumatology revised criteria for the classification of SLE(16) and were 18 years of age or older. Control subjects could not have a diagnosis of SLE or other autoimmune disease and were frequency matched by age, race, and sex. Exclusion criteria included the following: inability to provide informed consent, inability to operate the ABPM device, history of atrial fibrillation, use of anticoagulants, or the presence of a condition that could be exacerbated by blood pressure cuff inflation (e.g. lymphedema). Eligible consecutive patients with SLE from the Vanderbilt University Medical Center Rheumatology clinic were invited to participate and those who consented were enrolled. Control subjects were recruited from patients' referrals as well as a database of volunteers maintained by the General Clinical Research Center at Vanderbilt University School of Medicine and surrounding areas. The Vanderbilt University Medical Center Institutional Review Board approved this study and all participants provided written informed consent.
Study procedures
Demographic and clinical information, including details about medications and comorbidities, were obtained directly from subjects and their medical records. Blood pressure was measured by either a nurse or medical staff while patients were seated comfortably, as part of a routine clinic visit for SLE patients and by the study staff during the first study visit for controls. As previously done,(17, 18) we defined hypertension as a blood pressure measure >140/90 mmHg.(19) Treated hypertension was defined as the use of antihypertensive medications (e.g., diuretics, ACE inhibitors, beta blockers, and calcium channel blockers) prescribed for the treatment of hypertension.
Ambulatory blood pressure was monitored for 24 hours using a Card(x)plore blood pressure monitor (Meditech, Budapest, Hungary) at 15-minute intervals during the day and 30-minute intervals between 10 pm and 6 am. We included subjects with at least 50% of the readings available for both day (i.e., ≥32 readings) and night (i.e., ≥8 readings). We defined diurnal and nocturnal blood pressure measures according to each patient’s reported sleep period. The nocturnal SBP fall (%), termed “percent nocturnal dip” was calculated with the following formula 100x[1-(average nocturnal/average diurnal blood pressure)] and subjects who dipped less than 10% were classified as “non-dippers.”(20, 21) Dipping ratio is defined as the ratio of mean nocturnal SBP to mean diurnal SBP.
Masked hypertension was defined as a normal office blood pressure (<140/90 mmHg) but a high ambulatory blood pressure (i.e., at least one of the following three criteria: mean 24-hour blood pressure ≥130/80 mmHg, mean diurnal blood pressure ≥135/85 mmHg, or nocturnal hypertension). Nocturnal hypertension was defined as mean nocturnal blood pressure measures ≥120/70 mmHg.(22)
Immune activation was assessed using a multiplex immunoassay consisting of 92 inflammation-related markers (Inflammation panel; Olink Bioscience, Uppsala, Sweden) from plasma samples of patients with SLE and control subjects. (Supplemental table 2) We excluded markers of immune activation from the analysis if ≥25% of subjects had concentrations below the limit of detection.
Statistical Analyses
Sample size estimation was based on the outcome of nocturnal systolic blood pressure, using the PS-Power program for an unpaired t-test with a 2-sided test at a significance level of 5%.(23) Based on previous findings, the mean +/− standard deviation (SD) of nighttime systolic blood pressure was 120.2 +/−15.8 mmHg;(24) as such, a sample size of 26 subjects in each group would provide 80% power to detect a difference of 12.5 mm Hg between patients with SLE and control subjects. Because blood pressure measurements are not independent, no adjustment was made for multiple comparisons; the associations with markers of immune activation were regarded as exploratory.
Continuous and categorical variables are presented as median and interquartile range (IQR) or count and percentages, respectively. Between-group comparisons were assessed using either Mann-Whitney U tests, or Chi-square tests for continuous or categorical variables, as appropriate. Tests of hypotheses concerning within-group comparisons were performed using the Wilcoxon signed-rank test for continuous covariates, or Fisher’s exact Chi-square test for categorical covariates. We used Spearman correlations to assess the relation between blood pressure and markers of immune activation. Heat maps with hierarchical clustering were constructed with selected ABPM measures and markers of immune activation using Spearman correlation coefficients. Statistical significance was set to p-values < 0.05, and 95% confidence intervals for all pre-specified comparisons among study groups. All analyses were performed using STATA Statistical Software: Release 14 (College Station, TX: StataCorp LP), R version 3.6.1, or PS Power and Sample Size Calculation Software version 3.1.2.
RESULTS
Baseline characteristics
We enrolled 26 SLE patients and 26 controls of similar age, race, sex, and BMI (Table 1). Among SLE patients, disease activity and organ damage were low, as assessed using a SLEDAI and SLICC scores, respectively [SLEDAI median and IQR: 4 (2 – 6); SLICC 1 (0 – 1)]. Renal function, measured by serum creatinine concentrations, and estimated glomerular filtration rate were within the normal range in patients with SLE, and their values were not statistically significantly different from those of the control group. The prevalence of treated hypertension was significantly higher among SLE patients (46.2%) compared to control subjects (7.7%).
Table 1:
Baseline Characteristics of SLE Patients and Controls
| CHARACTERISTICS | SLE (n=26) | CONTROLS (n=26) | p-value |
|---|---|---|---|
| Age, years | 35 (31 – 52) | 36 (27 – 54) | 0.93 |
| Sex, female | 22 (84.6) | 22 (84.6) | 1.0 |
| BMI, kg/m2 | 25.5 (21.3 – 33.8) | 24.3 (22.7 – 27.3) | 0.37 |
| White race | 16 (61.5) | 21 (80.8) | 0.27 |
| Hispanic ethnicity | 3 (11.5) | 2 (7.7) | 1.0 |
| Treated hypertension | 12 (46.2) | 2 (7.7) | 0.004 |
| Diabetes | 2 (7.7) | 0 (0.0) | 0.49 |
| Smoking (current) | 1 (4.2) | 2 (7.7) | 0.94 |
| Creatinine (mg/dL) | 0.8 (0.7 – 0.9) | 0.7 (0.7 – 0.8) | 0.42 |
| eGFR (mEq/L) | 94 (81 – 104) | 92 (86 – 99) | 0.91 |
Continuous and categorical data shown as medians (interquartile ranges) or number (percentages) respectively. Wilcoxon rank sum test and Fisher’s exact test used to compare study groups as appropriate.
BMI: body mass index; SLEDAI: systemic lupus erythematosus disease activity index; SLICC: Systemic Lupus International Collaborating Clinics damage index
Office and 24-hour Blood Pressure
Office systolic and diastolic blood pressures did not differ significantly among the SLE and control groups (Table 2). In contrast, the median 24-hour blood pressure as measured by ABPM was significantly higher in the SLE group compared to controls (SBP 129, IQR [113 – 140] vs 116, IQR [111 – 121] mmHg, respectively; p=0.03). This difference was present in both diurnal and nocturnal periods, but it was larger among nocturnal measures (SBP 117, IQR [105 – 138] vs 104, IQR [98 – 109] mmHg; p=0.004).
Table 2:
Blood Pressure Measurements in Patients with SLE and Control Subjects
| CHARACTERISTICS | SLE (n=26) | CONTROLS (n=26) | p-value |
|---|---|---|---|
| Office SBP mm Hg | 127 (114 – 149) | 121 (118 – 127) | 0.31 |
| Office DBP mm Hg | 84 (68 – 90) | 80 (72 – 85) | 0.60 |
| 24h SBP mm Hg | 129 (113 – 140) | 116 (111 – 121) | 0.03 |
| 24h DBP mm Hg | 80 (69 – 86) | 72 (64 – 75) | 0.006 |
| Day SBP mm Hg | 131 (115 – 140) | 119 (115 – 125) | 0.05 |
| Day DBP mm Hg | 83 (71 – 89) | 76 (69 – 79) | 0.02 |
| Night SBP mm Hg | 117 (105 – 138) | 104 (98 – 109) | 0.004 |
| Night DBP mm Hg | 69 (58 – 83) | 58 (52 – 66) | 0.002 |
| Percent nocturnal BP dip, * | 7.8 (5.1 – 14.2) | 12.0 (8.1 – 20.0) | 0.03 |
| Non-Dipper * (n, %) | 14 (60.9) | 9 (40.9) | 0.24 |
| Masked HTN* (n, %) | 5 (21.7) | 1 (4.6) | 0.19 |
| Night HTN* (n, %) | 13 (56.5) | 8 (36.4) | 0.24 |
SBP: systolic blood pressure, DBP: diastolic blood pressure, non–dipper defined as subjects with <10% nocturnal dip.
(N SLE= 23, controls=22)
Continuous and categorical data are shown as median (interquartile range) and number (percentage), respectively.
The median percent nocturnal dip in the SLE group was 7.8% versus 12.0% in the control group (p=0.03). Although the prevalence of non-dippers was higher in the SLE group, this difference was not statistically significant: 60.9% vs 40.9%; p=0.24 (Table 2). The prevalence of masked hypertension was 21.7% in patients with SLE and 4.6% in controls (p=0.19) (Table 2). A sensitivity analysis excluded hypertensive patients from both study groups and the median nocturnal dip was 7.8 (5.6–12.9) % among patients with SLE and 12.0 (8.0–18.8) % among control subjects (p=0.09) (Supplemental table 1).
Markers of Immune Activation
Twenty-three of 77 markers of immune activation from the O-link array were associated with at least one ABPM measure in SLE patients (Table 3). Of these, six markers are linked to innate immune system activation (CSF-1, CCL-3, MCP3, CASP8, TRAIL, and SCF), eleven markers are related to the adaptive immune system (CUB, IL-15, TSLP, CCL23, IL-6, IL-18, IL-10RB, IL-18 R1, LIF, PDL-1, and SLAMF1), and three markers play a role in vascular function and/or remodeling (CX3CL1, OPG, and MMP10). Three other biomarkers correlated with elevated night systolic blood pressure, elevated night diastolic blood pressure, or percent nocturnal dip. Based on an exploratory hierarchical cluster analysis among patients with SLE, a group of adhesion molecules clustered positively with blood pressure measurements (Figure 1).
Table 3:
Correlation between Blood Pressure Measurements and Immune Mediators in SLE Patients (n=24)
| Biomarker | 24h SBP | 24h DBP | Day SBP | Day DBP | Night SBP | Night DBP | Dipping Ratio |
|---|---|---|---|---|---|---|---|
| Innate Immune System | |||||||
| CSF-1 | 0.12 | −0.02 | 0.1 | −0.04 | 0.3 | 0.3 | 0.6* |
| CCL3 | 0.3 | 0.2 | 0.3 | 0.2 | 0.4 | 0.4 | 0.4* |
| MCP3 | 0.1 | 0.1 | 0.05 | 0.07 | 0.3 | 0.4* | 0.5* |
| CASP8 | 0.1 | −0.03 | 0.01 | −0.1 | 0.3 | 0.3 | 0.5* |
| TRAIL | 0.4 | 0.3 | 0.3 | 0.3 | 0.4* | 0.5* | 0.3 |
| SCF | 0.3 | 0.4 | 0.2 | 0.3 | 0.4 | 0.4* | 0.2 |
| Adaptive Immune System | |||||||
| CDCP1 | 0.5* | 0.3 | 0.4 | 0.3 | 0.6* | 0.6* | 0.6* |
| IL-15 RA | 0.4 | 0.2 | 0.3 | 0.2 | 0.5* | 0.4* | 0.5* |
| TSLP | 0.3 | 0.4 | 0.2 | 0.3 | 0.4 | 0.5* | 0.3 |
| CCL23 | 0.1 | 0.04 | 0.1 | 0.0004 | 0.2 | 0.3 | 0.4* |
| IL-6 | 0.2 | 0.2 | 0.2 | 0.2 | 0.3 | 0.4 | 0.5* |
| IL-18 | −0.02 | −0.2 | −0.1 | −0.02 | 0.2 | 0.1 | 0.5* |
| IL-10 RB | 0.2 | −0.01 | 0.1 | −0.04 | 0.4 | 0.3 | 0.5* |
| IL 18 R1 | 0.3 | 0.1 | 0.2 | 0.02 | 0.4 | 0.3 | 0.5* |
| LIF | 0.3 | 0.5* | 0.4 | 0.5* | 0.3 | 0.6* | 0.2 |
| PDL1 | 0.03 | −0.1 | 0.01 | −0.1 | 0.2 | 0.2 | 0.4* |
| SLAMF1 | 0.3 | 0.1 | 0.2 | 0.1 | 0.3 | 0.3 | 0.5* |
| Response to Vascular Mechanical Stretch/Vascular Calcification or Tissue Remodeling | |||||||
| CX3CL1 | 0.4 | 0.3 | 0.3 | 0.3 | 0.5* | 0.5* | 0.6* |
| OPG | 0.4* | 0.3 | 0.4* | 0.3 | 0.5* | 0.3 | 0.3 |
| MMP10 | 0.3 | 0.3 | 0.2 | 0.3 | 0.5* | 0.5* | 0.5* |
| Other Mechanisms | |||||||
| NT3 | −0.4 | −0.5* | −0.4* | −0.5* | −0.3 | −0.4* | 0.02 |
| FGF23 | 0.1 | 0.2 | 0.1 | 0.2 | 0.2 | 0.5* | 0.2 |
| B-NGF | 0.1 | 0.1 | 0.1 | 0.1 | 0.3 | 0.4* | 0.4* |
The Table shows those markers of immune activation with at least one significant correlation with a blood pressure measurement.
Indicates p<0.05
Dipping ratio is defined as the ratio of mean nocturnal SBP to mean diurnal SBP.
Fig 1:
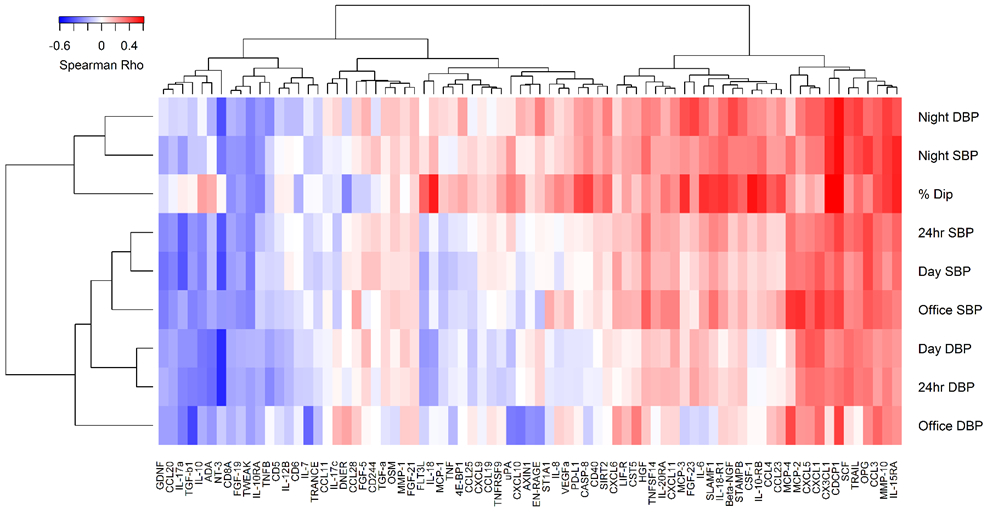
Association Between ABPM and Markers of Immune Activation in Patients with SLE
DISCUSSION
This study found that (1) despite having similar systolic and diastolic office blood pressure measures, all 24-hour blood pressure parameters were higher among patients with SLE than controls; and (2) in patients with SLE, nocturnal BP measures (including percent nocturnal dip) were associated with multiple markers of immune activation involved in both the innate and adaptive immune systems as well as vascular remodeling.
Our results indicate that patients with SLE have increased nocturnal blood pressure and less dipping than controls. This finding is important because two strategies target the management of nocturnal blood pressure in the general population and such interventions could have even a higher impact in patients with SLE. First, bedtime administration of antihypertensives reduced cardiovascular events by as much as 45%.(25) Second, angiotensin receptor blockers have demonstrated the capacity to revert non-dipping patterns.(20) Our findings are consistent with those of a previous study that reported similar office blood pressure, but higher prevalence of non-dipper status (61% vs 42%) and nocturnal hypertension (24% vs 6%) in patients with SLE compared to controls; however, that study did not examine the relationships between BP and inflammatory markers.(26) Together, these results underscores the notion that office measurements may not be adequate to monitor blood pressure in patients with SLE.
We found that several markers of immune activation among SLE patients were associated with at least one ABPM parameter. Although the function of immune mediators is highly interconnected and not mutually exclusive, we classified the biomarkers based on their role on: (1) the innate immune system; (2) the adaptive immune system; and (3) response to vascular stretch or vascular remodeling. Our results showed an association between the immune system and hypertension and while we cannot demonstrate directionality of the association, it is consistent with the cumulative knowledge supporting a bidirectional relationship between the immune system and hypertension. The innate immune system plays a role in the development of hypertension.(27) Our findings are consistent with this concept; they show a positive correlation between markers of macrophage, neutrophil, or mast cell activation (including CSF-1, CCL-3, MCP3, and SCF)(28–31) as well as molecules implicated in cell death and apoptosis (such as TRAIL and CASP8)(32, 33) with elevated nocturnal blood pressure and percent nocturnal dip in lupus.
Our novel data also reveals a positive correlation between increased concentrations of biomarkers of T-cell activation and ABPM measurements consistent with previous findings about the role of the adaptive immune system in the development of hypertension.(34) For example, CDCP1 is a cell-surface glycoprotein that regulates T-cell activation, and PD-1 is part of the co-stimulatory pathway in the initiation of cell-mediated immune response;(35) both were correlated with the percent nocturnal dip although their role in SLE is not fully defined. More directly related to SLE, IL-15RA binds to IL-15 and this linkage enhances the viability and proliferation of T cells(36) and its expression is enhanced in leukocytes from patients with this disease.(37) Finally, additional cytokines that affect the adaptive immune system, such as TSLP(38) and IL-6,(39) were all positively correlated with elevated nocturnal blood pressure and/or percent nocturnal dip.
Although these results show the relationship between the immune system and hypertension in patients with SLE, hypertension can also induce inflammation, potentially creating a cyclical pattern, and contributing to end-organ damage. For example, fractalkine (CX3CL-1) has been implicated in the pathogenesis of hypertension(40) and hypertension-related renal injury.(41) Our finding that this chemokine was associated with nocturnal systolic BP, diastolic BP, and percent nocturnal dip suggests that CX3CL-1 is not only a therapeutic target for multiple inflammatory conditions,(42) but also a potential therapeutic target for hypertension.
Finally, an exploratory clustering analysis suggests that several adhesion molecules are linked with both increased blood pressure measures and decreased nocturnal dipping. Further research will be needed to confirm these findings and to elucidate whether these adhesion molecules contribute to the pathogenesis of hypertension or are the result of the endothelial dysfunction secondary to hypertension in patients with SLE.
The present study has some limitations. The sample size offered limited power to detect statistically significant differences between study groups; as such, it is possible that other important immune pathways (e.g., IL-17 and certain chemokines) may be implicated although they were not statistically significant in this study. The cross-sectional nature of this study limits any conclusions related to the cause-effect relationship between blood pressure and markers of immune activation. Finally, the relatively low SLEDAI scores reflect well-controlled lupus disease activity among the patients in the SLE group and may limit the generalizability of our results.
In conclusion, office measurements were not different between groups, but ambulatory BP measures were higher in patients with SLE than controls. This difference was especially pronounced for nocturnal blood pressure. Several markers of immune activation were found to be significantly correlated with blood pressure measurements, including nocturnal blood pressure and dipping, strengthening our understanding of the relationship between inflammation and cardiovascular risk.
Supplementary Material
Acknowledgments
Sources of Funding
Supported by grants from the Lupus Research Alliance, NHLBI (HL140145), Rheumatology Research Foundation, and Veterans Health Administration CDA (IK2CX001269).
We acknowledge Dr. Laura Daniel and Ms. Alyson Dickson for editorial assistance with both the initial submission and the revised version.
Footnotes
Conflict(s) of Interest/Disclosure(s): none
References
- 1.Yang Q, Cogswell ME, Flanders WD, Hong Y, Zhang Z, Loustalot F, et al. Trends in Cardiovascular Health Metrics and Associations With All-Cause and CVD Mortality Among US Adults. JAMA. 2012;307(12):1273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piper MA, Evans CV, Burda BU, Margolis KL, O'Connor E, Whitlock EP. Diagnostic and Predictive Accuracy of Blood Pressure Screening Methods With Consideration of Rescreening Intervals: A Systematic Review for the U.S. Preventive Services Task ForceBlood Pressure Screening Methods and Consideration of Rescreening Intervals. Annals of Internal Medicine. 2015;162(3):192–204. [DOI] [PubMed] [Google Scholar]
- 3.Niiranen TJ, Maki J, Puukka P, Karanko H, Jula AM. Office, home, and ambulatory blood pressures as predictors of cardiovascular risk. Hypertension. 2014;64(2):281–6. [DOI] [PubMed] [Google Scholar]
- 4.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr., et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–52. [DOI] [PubMed] [Google Scholar]
- 5.Lurbe E, Redon J, Kesani A, Pascual JM, Tacons J, Alvarez V, et al. Increase in nocturnal blood pressure and progression to microalbuminuria in type 1 diabetes. N Engl J Med. 2002;347(11):797–805. [DOI] [PubMed] [Google Scholar]
- 6.Davidson MB, Hix JK, Vidt DG, Brotman DJ. Association of impaired diurnal blood pressure variation with a subsequent decline in glomerular filtration rate. Arch Intern Med. 2006;166(8):846–52. [DOI] [PubMed] [Google Scholar]
- 7.Pickering TG, Davidson K, Gerin W, Schwartz JE. Masked hypertension. Hypertension. 2002;40(6):795–6. [DOI] [PubMed] [Google Scholar]
- 8.Turner JR, Viera AJ, Shimbo D. Ambulatory blood pressure monitoring in clinical practice: a review. Am J Med. 2015;128(1):14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asayama K, Thijs L, Li Y, Gu YM, Hara A, Liu YP, et al. Setting thresholds to varying blood pressure monitoring intervals differentially affects risk estimates associated with white-coat and masked hypertension in the population. Hypertension. 2014;64(5):935–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caillon A, Paradis P, Schiffrin EL. Role of immune cells in hypertension. Br J Pharmacol. 2019;176(12):1818–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, et al. Inflammation, immunity, and hypertension. Hypertension. 2011;57(2):132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munguia-Realpozo P, Mendoza-Pinto C, Sierra Benito C, Escarcega RO, Garcia-Carrasco M, Mendez Martinez S, et al. Systemic lupus erythematosus and hypertension. Autoimmun Rev. 2019;18(10):102371. [DOI] [PubMed] [Google Scholar]
- 13.Gandelman JS, Khan OA, Shuey MM, Neal JE, McNeer E, Dickson A, et al. Increased Incidence of Resistant Hypertension in Patients With Systemic Lupus Erythematosus: A Retrospective Cohort Study. Arthritis Care Res (Hoboken). 2020;72(4):534–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhattacharya S, Aggarwal A. M2 macrophages and their role in rheumatic diseases. Rheumatol Int. 2019;39(5):769–80. [DOI] [PubMed] [Google Scholar]
- 15.Sharabi A, Tsokos GC. T cell metabolism: new insights in systemic lupus erythematosus pathogenesis and therapy. Nat Rev Rheumatol. 2020;16(2):100–12. [DOI] [PubMed] [Google Scholar]
- 16.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. [DOI] [PubMed] [Google Scholar]
- 17.Asanuma Y, Oeser A, Shintani AK, Turner E, Olsen N, Fazio S, et al. Premature coronary-artery atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349:2407–15. [DOI] [PubMed] [Google Scholar]
- 18.Chung C, Avalos I, Oeser A, Gebretsadik T, Shintani A, Raggi P, et al. High Frequency of the Metabolic Syndrome in Patients with Systemic Lupus Erythematosus: Association with Disease Characteristics and Cardiovascular Risk Factors. Ann Rheum Dis. 2005;66(2):208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension. 2020:HYPERTENSIONAHA12015026. [DOI] [PubMed] [Google Scholar]
- 20.Mahabala C, Kamath P, Bhaskaran U, Pai ND, Pai AU. Antihypertensive therapy: nocturnal dippers and nondippers. Do we treat them differently? Vasc Health Risk Manag. 2013;9:125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kario K, Pickering TG, Matsuo T, Hoshide S, Schwartz JE, Shimada K. Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension. 2001;38(4):852–7. [DOI] [PubMed] [Google Scholar]
- 22.Franklin SS, O'Brien E, Staessen JA. Masked hypertension: understanding its complexity. Eur Heart J. 2017;38(15):1112–8. [DOI] [PubMed] [Google Scholar]
- 23.Dupont WD, Plummer WD Jr. Power and sample size calculations. A review and computer program. Control Clin Trials. 1990;11(2):116–28. [DOI] [PubMed] [Google Scholar]
- 24.Banegas JR, Ruilope LM, de la Sierra A, Vinyoles E, Gorostidi M, de la Cruz JJ, et al. Relationship between Clinic and Ambulatory Blood-Pressure Measurements and Mortality. N Engl J Med. 2018;378(16):1509–20. [DOI] [PubMed] [Google Scholar]
- 25.Hermida RC, Crespo JJ, Dominguez-Sardina M, Otero A, Moya A, Rios MT, et al. Bedtime hypertension treatment improves cardiovascular risk reduction: the Hygia Chronotherapy Trial. Eur Heart J. 2019. [DOI] [PubMed] [Google Scholar]
- 26.Sabio JM, Martinez-Bordonado J, Sanchez-Berna I, Vargas-Hitos JA, Mediavilla JD, Navarrete-Navarrete N, et al. Nighttime Blood Pressure Patterns and Subclinical Atherosclerosis in Women with Systemic Lupus Erythematosus. J Rheumatol. 2015;42(12):2310–7. [DOI] [PubMed] [Google Scholar]
- 27.Harwani SC. Macrophages under pressure: the role of macrophage polarization in hypertension. Transl Res. 2018;191:45–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sweet MJ, Hume DA. CSF-1 as a regulator of macrophage activation and immune responses. Arch Immunol Ther Exp (Warsz). 2003;51(3):169–77. [PubMed] [Google Scholar]
- 29.Wolpe SD, Davatelis G, Sherry B, Beutler B, Hesse DG, Nguyen HT, et al. Macrophages secrete a novel heparin-binding protein with inflammatory and neutrophil chemokinetic properties. J Exp Med. 1988;167(2):570–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu LL, McVicar DW, Ben-Baruch A, Kuhns DB, Johnston J, Oppenheim JJ, et al. Monocyte chemotactic protein-3 (MCP3) interacts with multiple leukocyte receptors: binding and signaling of MCP3 through shared as well as unique receptors on monocytes and neutrophils. Eur J Immunol. 1995;25(9):2612–7. [DOI] [PubMed] [Google Scholar]
- 31.Huang B, Lei Z, Zhang GM, Li D, Song C, Li B, et al. SCF-mediated mast cell infiltration and activation exacerbate the inflammation and immunosuppression in tumor microenvironment. Blood. 2008;112(4):1269–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Falschlehner C, Schaefer U, Walczak H. Following TRAI’s path in the immune system. Immunology. 2009;127(2):145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tummers B, Green DR. Caspase-8: regulating life and death. Immunol Rev. 2017;277(1):76–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Norlander AE, Madhur MS, Harrison DG. The immunology of hypertension. J Exp Med. 2018;215(1):21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5(12):1365–9. [DOI] [PubMed] [Google Scholar]
- 36.Rowley J, Monie A, Hung CF, Wu TC. Expression of IL-15RA or an IL-15/IL-15RA fusion on CD8+ T cells modifies adoptively transferred T-cell function in cis. Eur J Immunol. 2009;39(2):491–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baranda L, de la Fuente H, Layseca-Espinosa E, Portales-Perez D, Nino-Moreno P, Valencia-Pacheco G, et al. IL-15 and IL-15R in leucocytes from patients with systemic lupus erythematosus. Rheumatology (Oxford). 2005;44(12):1507–13. [DOI] [PubMed] [Google Scholar]
- 38.Liu YJ, Soumelis V, Watanabe N, Ito T, Wang YH, Malefyt Rde W, et al. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol. 2007;25:193–219. [DOI] [PubMed] [Google Scholar]
- 39.Harrison DG. The immune system in hypertension. Trans Am Clin Climatol Assoc. 2014;125:130–38; discussion 8–40. [PMC free article] [PubMed] [Google Scholar]
- 40.Martynowicz H, Janus A, Nowacki D, Mazur G. The role of chemokines in hypertension. Adv Clin Exp Med. 2014;23(3):319–25. [DOI] [PubMed] [Google Scholar]
- 41.Ahadzadeh E, Rosendahl A, Czesla D, Steffens P, Prussner L, Meyer-Schwesinger C, et al. The chemokine receptor CX3CR1 reduces renal injury in mice with angiotensin II-induced hypertension. Am J Physiol Renal Physiol. 2018;315(6):F1526–F35. [DOI] [PubMed] [Google Scholar]
- 42.Jones BA, Beamer M, Ahmed S. Fractalkine/CX3CL1: a potential new target for inflammatory diseases. Mol Interv. 2010;10(5):263–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


