Abstract
IRE1α endonuclease is a key regulator of endoplasmic reticulum (ER) stress that controls cell survival/apoptosis in cancers. Inhibition of IRE1α endonuclease leads to decreased splice XBP1 which decreases cell proliferation and increases cell death in cancer cells. Therefore, this study investigated the effects and mechanism of STF-083010 (an IRE1α inhibitor) on the cell growth/apoptosis of ovarian malignant cells via the XBP1-CHOP-Bim pathway following the induction of ER stress (ERS). ERS in OVCAR3 and SKOV3 cells was measured using Thioflavin T staining. The expression of ER stress response genes was evaluated by QRT-PCR. The levels of XBP1(s), PERK, phospho-PERK, p-PP2A, ATF4, BIP/GRP78, CHOP, and Bim proteins were evaluated using western blotting. Cell viability and apoptosis in STF-083010 and Tunicamycin (Tm) co-treated cells were assessed using BrdU, MTT, Annexin V-FITC/PI staining, and caspases-12 and -3 activity assays. The results showed increased XBP1, CHOP, and ATF-4 mRNA expression levels as well as high protein aggregation in STF-083010 and Tm co-treated cells. The IRE1α inhibitor down-regulated sXBP1 and BIP proteins, while XBP-1, p-PERK, ATF-4, CHOP, and Bim proteins were up-regulated. STF-083010 reduced cell proliferation and induced apoptosis through the activation of caspases-12 and -3 and Bax/Bcl-2 protein expression. In summary, the present data revealed the effects of STF-083010 in ER stress and apoptosis as well as signaling via XBP1/CHOP/Bim mediators. Thus, STF-083010 is proposed as a new target for the control of ERS in ovarian cancer cells.
Keywords: IRE1α, ER stress, Apoptosis, Ovarian cancer
Introduction
Ovarian cancer encompasses 2.5% of all malignancies in women and accounts for 5% of female cancer mortality (Torre et al. 2018). Ovarian cancer is a recurrent tumor and relatively resistant to current chemotherapy drugs (Pokhriyal et al. 2019); thus, novel therapeutic agents are needed. In tumor cells, the balance between synthesis and protein secretion sometimes fails or proteins cannot be properly degraded. The accumulation of non-folded or mis-folded proteins within the ER cause the disruption of ER homeostasis, referred to as ER stress (ERS). The unfolded protein response (UPR) is activated to return normal ER hemostasis and allows the cell to survive (Wang et al. 2010). This suggests that manipulation of UPR pathways can be considered as a potential therapeutic strategy. Because of the high production of protein, ovarian tumor cells display chronic ERS (G. Wang et al. 2010; M. Wang and Kaufman 2014).
The UPR has three signal transducers: PERK, IRE1α, and ATF6α. In the physiological state, the ER chaperone immunoglobulin heavy chain-binding protein (BIP/GRP78) binds with transducers and is maintained in an inactive monomeric form. In response to ERS, BIP is released from transducers and results in their dimerization and auto-phosphorylation and the activation of UPR signaling. The UPR pathway is an integrated pro-adaptive and pro-apoptotic signal. The primary purpose of the UPR is to restore ER homeostasis and adaptation. However, if the adaptive programs fail and ERS remains unresolved, the UPR activates apoptosis programs (G. Wang et al. 2010). IRE1α is one of the most important switching components between cell survival and death signaling, and it regulates the dynamic signaling of the UPR (Hetz 2012; Wu et al. 2016). Previous studies have demonstrated that manipulation of IRE1α can be a good candidate for cancer therapy (Karagöz et al. 2017; Suh et al. 2012). IRE1α consists of two domains with kinase and endoribonuclease (RNase) activity. IRE1α-RNase sites slice the X-Box Binding Protein 1 (XBP1) mRNA and produce the transcription factor sXBP1 (spliced XBP1) that encodes a wide variety of UPR target genes (Mimura et al. 2012).
The sXBP1 mainly encodes genes which promote ER protein folding capacity and expansion of the ER membrane under ERS (Hetz 2012). Therefore, IRE1α causes adaptation through sXBP1 (Márquez et al. 2017). The sXBP1 induces the expression of ER chaperone genes such as GRP78/BIP (T.-H. Chen et al. 2017; Lee et al. 2003). The GRP78/BIP chaperone protects the cells against ER stress-induced apoptosis by reducing the activity of caspase-7 and -12, inhibiting pro-apoptotic BIK and Bax proteins, and blocking the release of cytochrome c (Racek et al. 2008). Moreover, overexpression of GRP78 is correlated with high proliferation and poor prognosis in many cancers (Li et al. 2011) and is known as a central pro-survival component of UPR (Luo et al. 2006). The sXBP1 can also reduce expression of the CHOP protein by activating ERK1/2 pathways. CHOP is the main protein required for ERS-mediated apoptosis, but the precise mechanism of sXBP1 is not yet fully understood. Recent therapeutic cancer research has focused on IRE1–XBP1 signaling inhibitors (Guo et al. 2012; Mimura et al. 2012; Namba et al. 2015).
STF-083010 is a novel inhibitor of IRE1α that directly binds to the RNase site. The reduction of sXBP1 by STF-083010 has been shown to have apoptotic effects in breast and pancreatic cancers as well as multiple myeloma cells (L. Chen et al. 2016; Chien et al. 2014; Ming et al. 2015a; Papandreou et al. 2011a). There is a limited number of studies on the modulation of sXBP1, and the therapeutic efficiency of STF-08310 in human ovarian cancer cells remains unknown. The current study aimed to investigate the effects of STF-08310 and reduced sXBP1 on the apoptosis rates in SKOV3 and OVCAR3 cancer cells throughout the sXBP1-CHOP-Bim pathway.
Methods and materials
Chemical
STF-083010 as an IRE1α inhibitor was obtained from Tocris Bioscience (UK). Tunicamycin (Tm) and Thioflavin T were purchased from Sigma-Aldrich (St. Louis, MO). The cell culture supplements were bought from Gibco. Antibodies against XBP1 (sc-8015), ATF4/CREB-2 (sc-390,063), p-PERK (Thr 981) (sc-32,577), p-PP2A (sc-271,903), Bcl-2 (sc-492), Bax (sc-7480), Bim (sc-374,358), and B-Actin (sc-47,778) were obtained from Santa Cruz (CA). The sXBP1 (12782S) antibody and ER Stress Antibody Sampler Kit (9956 T) were purchased from Cell Signaling.
Cell culture
All assessments were performed on OVCAR3 and SKOV3 (NCBI codes: C430, C209) human ovarian cancer cell lines. Cells were cultured in RPMI-1640 media with 10% fetal bovine serum (FBS), 100 U/mL penicillin G, and 100 U/mL streptomycin.
MTT assay
MTT assay was used to assess cell viability. In brief, 10× 103 cells/well were cultured in 96-well plates. After 24 h, cells were exposed to Tm (3 μg/ml) with or without various dosages (0.1, 1, 10, 50 and 100 μM) of STF-083010 for 18 h. After treatment, cells were incubated with MTT dye (5 mg/ml) for 4 h. Then, DMSO (200 μl/well) was added to dissolve formazan crystals. Optical density was determined to be 570 nm using a Synergy H1 plate reader.
Bromodeoxy Uridine cell proliferation measurement
Cell proliferation was carried out with a BrdU kit following the manufacturer’s protocol. During cell proliferation, BrdU, a pyrimidine analog, was incorporated into replicating DNA rather than thymidine. To detect BrdU, a BrdU mAb that is recognized by HRP-linked antibodies reacts effectively reacts with it. The cells were transferred into 96-well plates (5 × 103 cells per well). When the confluence reached about 80%, the cells were treated by Tm (3 μg/ml) with or without different concentrations (0.1, 1, 10, 50, and 100 μM) of STF-083010 for 18 h. Then, BrdU labeling solution was added (10 μL/well). After 4 h, the solution was aspirated, and the cells were incubated with secondary anti-BrdU antibodies that conjugated with peroxidase. After washing, the color reaction was formed by adding substrate solution for 3–5 min. Finally, the reaction was stopped by adding sulfuric acid (1 M), and the optical density was determined to be 370 nm.
Thioflavin T staining assay
Thioflavin T (ThT) is able to detect ERS 20 min after treatment with induction agents by binding to the aggregated proteins (Beriault and Werstuck 2013). For ThT assay, 5000 cells/well were grown in a 96-well plate and incubated for 24 h. Then, cells were treated with Tm (3 μg/ml) in the presence or absence of various dosages (0.1, 1, 10, 50 and 100 μM) of STF-083010 for 18 h. Next, the supernatant was removed and the cells were washed with PBS. For fixation, paraformaldehyde (4%) was added for 20 min; then, ThT (5 μM) was added to the wells (50 μl/well). Finally, cells were washed (3 times) and fluorescent images of live cells were acquired with Zeiss Axioplan 2 microscope. Fluorescence intensity was measured at 485 nm, excited with blue dye filter, and images were provided at 535 nm emission and semi-quantified by ImageJ.
ERS gene expression
The expression of ERS genes was assessed using the QRT-PCR method. In summary, 5 x 105 cells were grown in cell culture plates (6-well). Next, the cells were exposed to Tm (3 μg/ml) with or without STF-083010 (50 μM) for 18 h. After treatment, total RNA was extracted using the Cinnagen kit (PR891620). The cDNA was synthesized from total RNA using a first-strand cDNA synthesis kit (Takara Shuzo, Otsu, Japan). GAPDH was performed as an internal control. The primers are listed in Table 1. Amplification of cDNA started with 15 min at 95 °C, followed by 40 cycles of 15 s at 95 °C and 1 min at 55–60 °C using High ROX master mixed Green (AMPLIQON). A melting point curve was made at the termination of each reaction to confirm amplification specificity. The relative gene expression levels were assessed through the 2−ΔΔCt formula.
Table 1.
Primer sequences of ER stress gene that were used for RT-PCR
| Gene symbol | NCBI Accession number | Forward primer | Reverse Primer |
|---|---|---|---|
| GAPDH | NM_002046.7 | CTCAGACACCATGGGGAAGGTGA | ATGATCTTGAGGCTGTTGTCATA |
| XBP1 | NM_005080.3 | CTGGTTGCTGAAGAGGAG | ATGGGGAGATGTTCTGGAG |
| sXBP1 | NM_001079539.1 | CTGAGTCCGCAGCAGGTG | CCAGAATGCCCAACAGGA |
| CHOP | NM_004083.5 | AGGGCCAACAGAGGTCACAC | GGGCCATAGAACTCTGACTGGA |
| ATF4 | NM_182810.2 | GAAACCTCATGGGTTCTCCA | GCCAATTGGGTTCACTGTCT |
Western blots
Western blotting was performed to determine the protein levels of XBP1, sXBP1, BIP, Bim, CHOP, p-PERK, PERK, ATF4, p-PP2A, Bax, and Bcl-2 proteins. 5× 105 cells were grown in plates (six-well). The cells were treated with Tm (3 μg/ml) with or without STF-083010 (50 μM) for 18 h. To extract the total proteins, cells were lysed in a cocktail of RIPA (Sigma-Aldrich), whole protease inhibitors (Sigma-Aldrich), and phosphatase inhibitors (Sigma-Aldrich), and they were destroyed by sonication. The protein concentration was determined using Bradford Protein Assay. Equal amounts (20 to 30 μg of total protein per well) of total protein for each sample was electrophoresed using 12% SDS-PAGE. Then, the proteins were transferred onto a PVDF transfer membrane (Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom) using a Mini Trans-Blot® Cell Module. After blocking for 1 h, the transferred membrane was incubated overnight with mouse and rabbit monoclonal antibodies against (XBP1 1:1000, sXBP1 1:300, CHOP 1:300, Bim 1:200, BIP 1:300, ATF4 1:200, PERK 1:500, p-PERK 1:500, Bax 1:300, Bcl-2 1:200 and p-PP2A 1:200). Antibody binding was distinguished by conjugated secondary antibodies (1/5000 for 90 min) and captured on film via ECL Substrate (both from Amersham Corp., Arlington Heights, IL). Beta-actin was used as an internal control.
Detection of apoptosis using Annexin V/ PI staining
The detection of cell death was assessed through flow cytometry as previously described (Jafari et al. 2018). Briefly, 5× 105 cell/well were seeded in plates (6 well) and treated in a range of STF-083010 (0.1, 1, 10 and 50 μmol/L) with Tm (3 μg/ml) for 18 h. Next, cells were harvested and centrifuged. Then, the washed cells were re-suspended in the binding buffer (100 μL/well) and incubated for 15 min. Next, the cells were incubated with Annexin V-FITC and PI for 15 min and analyzed by FACS Calibur flow cytometer (BD Bioscience).
Caspase-12 and -3 fluorometric activity assay
To evaluate the caspase activity, SKOV3 and OVCAR3 cells (5 × 105105 cells in each well) were seeded into a six-well plate and treated with various ranges of STF-083010 (0.1, 1, 10, and 50 μmol/L) in the presence of Tm (3 μg/ml) for 18 h. Caspase-12 and -3 activity was determined based on the manufacturer’s instructions (Biovision Systems, USA). Briefly, the collected cells were lysed in lysis buffer (100 μl/well). Then, supernatants were incubated with caspase-12 fluorescence substrate (ATAD-AFC) or caspase-3 (DEVD-AFC) in new tubes. Samples were incubated for 2 h and absorbance was read at 400/505 nm ex/em using a Microplate Reader (Synergy H1 Hybrid MultiMode BioTek).
Statistical analysis
All data was processed and analyzed using Graph Pad Prism (version 5). The results are presented as the mean ± standard deviation (SD) of independent triplicate experiments (with 3 repeats). Statistical non-parametric one-way analysis of variance (ANOVA) test followed by the Tukey Post-hoc test were used to compare data, and a p value <0.05 was considered statistically significant.
Results
Inhibition of IRE1-XBP1 by STF-083010 increased the accumulation of aggregated proteins and intensified ERS
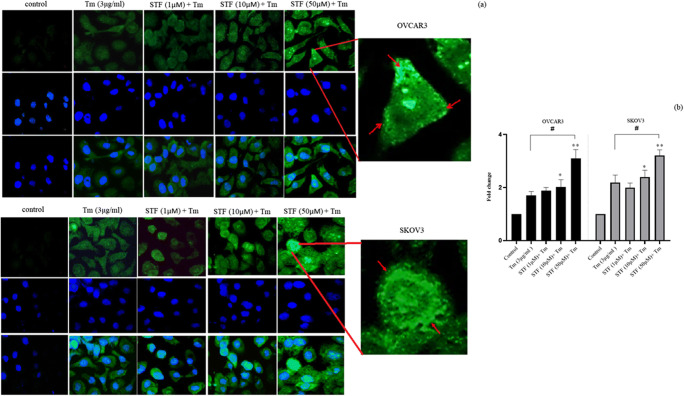
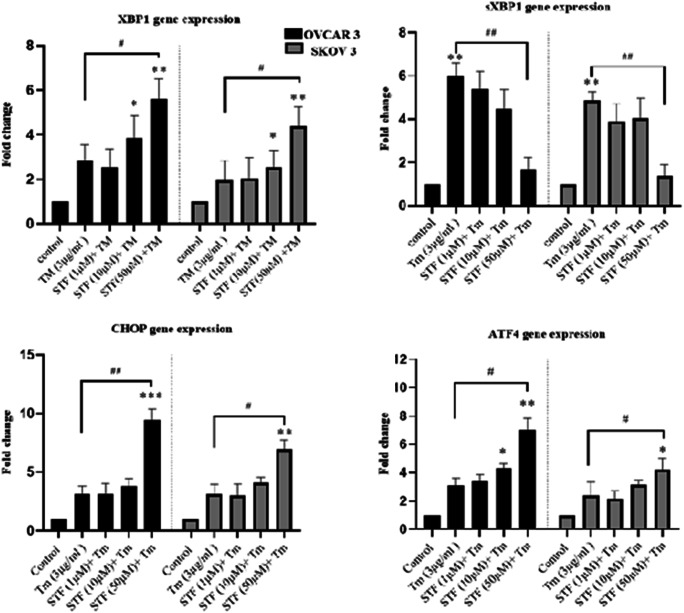
Previously, we demonstrated that ThT staining could show the aggregation of mis/unfolded proteins and ERS in cells (Shahrestanaki et al. 2019). To investigate the effects of IRE1 inhibitor (STF-083010) on ER stress, ovarian cancer cells were first co-treated with Tm and STF-083010 (1, 10, 50 μM) followed by ThT staining. The ThT fluorescence intensity was increased in a dose-dependent manner after 18 h incubation (Fig. 1). The cells co-treated with 50 μM of STF-083010 and Tm have remarkably higher ThT intensity than Tm-treated cells alone (1.71 ± 0.31 fold for OVCAR3; p˂0.05, and 1.65 ± 0.23 fold for SKOV3; p˂0.05) (Fig. 1a, b). Then, ERS gene response to co-treatment with STF-083010 and Tm were also evaluated. The results demonstrated significantly increased levels of ERS gene expression (at 50 μM) compared with Tm-treated cells (Fig. 2). The expression levels of XBP1, ATF4, and CHOP were increased 2.5 ± 0.75, 3 ± 0.73 and 4.1 ± 0.8 fold in OVCAR3 cells, as well, 2 ± 0.68, 1.98 ± 0.67 and 2.14 ± 0.48 fold in SKOV3 cells, compared with Tm-treated cells, respectively. Because sXBP-1 was generated by the RNase activity of IRE1α, sXBP-1 mRNA was significantly decreased (p˂0.01) in co-treated cells (0.33 ± 0.73 fold in OVCAR3 cells and 0.28 ± 0.53 fold in SKOV3 cells) compared with the Tm-treated cells. The results showed that the inhibition of IRE1-XBP1 (by STF-083010) increased ER stress.
Fig. 1.
IRE1α-XBP1 inhibition increased the accumulation of aggregated proteins. Ovarian cancer cells were co-treated with Tunicamycin (Tm) and STF-083010 (1, 10, 50 μM) for 18 h. After incubation time, the cells were stained with Thioflavin T (ThT) (a). The Thioflavin T results were quantified with Image J software (b). Results were manifested as mean ± SD and *p < 0.05, **p < 0.01 versus control. #p < 0.05, ##p < 0.01 versus Tm (3 μg/mL) considered as significant
Fig. 2.
1 IRE1α-XBP1 inhibition effected on ER stress genes. The cells were co-treated with Tm (3 μg/ml) and STF-083010 (1, 10, 50 μM) for 18 h and mRNA of CHOP, ATF4, XBP1, and XBP-1 s were assessed using QRT-PCR (c). Results were manifested as mean ± SD and *p < 0.05, **p < 0.01 versus control. #p < 0.05, ##p < 0.01 versus Tm (3 μg/mL) considered as significant
STF-080310 regulated UPR proteins pathway
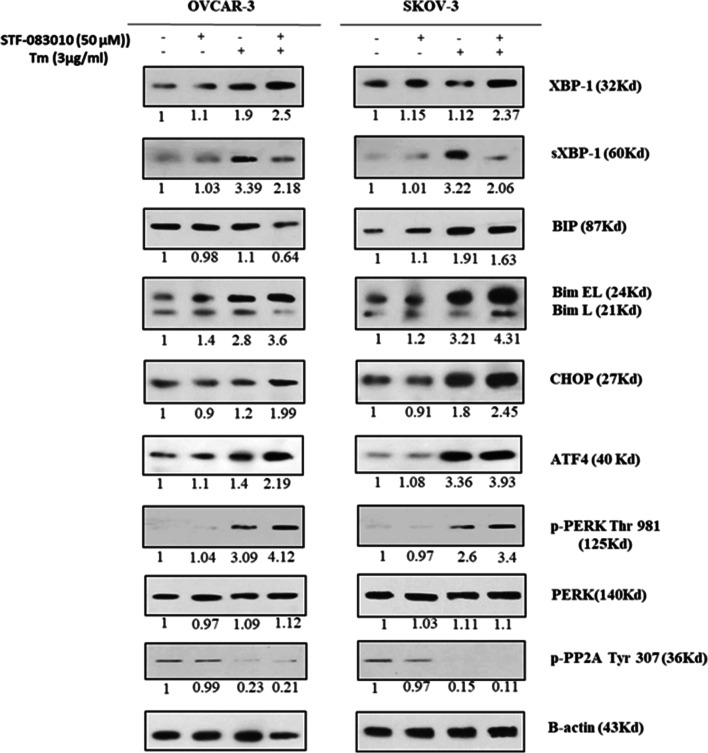
To discover the effects of IRE1α-XBP1 inhibition on expression levels of proteins related to UPR-mediated apoptosis, the levels of XBP1, sXBP1, BIP, ATF4, p-PERK, PERK, CHOP, Bim, and p-PP2A proteins in Tm and STF-083010 (50 μM) co-treated cells were evaluated (Fig. 3). These proteins are the most important components of UPR signaling in ER stress-mediated apoptosis. The results indicated that XBP1 protein was significantly increased in cells co-treated with STF-083010 and Tm compared with Tm-treated cells (1.37 ± 0.41 fold for OVCAR3 and 2.11 ± 0.24 fold for SKOV3), which reflected the ER stress state, whereas a reduction in sXBP1 protein levels was observed in co-treated cells (0.62 ± 0.13 fold for OVCAR3 and 0.58 ± 0.11 fold for SKOV3) compared with Tm-treated cells that demonstrated the inhibitory effects of STF-083010 on IRE1 RNase activity in both cells. BIP/Grp78 protein level was also decreased as the target of sXBP1 with anti-apoptotic effects (Fig. 3). Moreover, cells co-treated with STF-083010 and Tm exhibited an enhancement in CHOP, ATF4, p-PERK, PERK and Bim proteins. Because Bim is activated by two novel pathways involving phosphatase 2A and CHOP-/EBP-α proteins during overwhelming ER stress (Szegezdi et al. 2006), the phosphorylated form of PP2A (p-PP2A) in both cell lines was evaluated. When cells were treated with Tm, the p-PP2A protein level decreased compared with the control cells. The STF-083010 treatment, however, made no change in p-PP2A protein levels compared with Tm-treated cells. These results indicated that IRE1-XBP1 inhibition during ER stress increased the expression of pro-apoptotic proteins related to ER stress.
Fig. 3.
blockage of IRE1α with STF-083010 (50 μM) and its effect on ER stress-associated apoptotic factors (XBP1, sXBP1, BIP, Bim, CHOP, p-PERK, PERK, ATF4, p-PP2A) in ovarian malignancy cells (OVCAR3, SKOV3) using Western blot assay. Beta-actin was used as normalization
Anti-proliferative effects of STF-083010 underlying ER stress
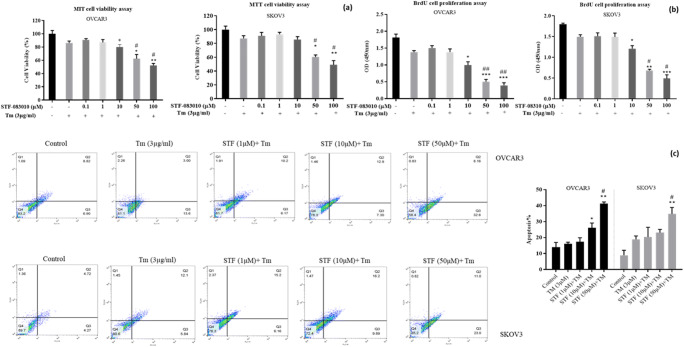
To determine whether the IRE1α inhibitor (STF-083010) induced apoptosis, SKOV3 and VOCAR3 cells were co-treated with various doses of STF-083010 (0.1, 1, 10, 50, and 100 μM) and Tm. Then, viability and proliferation were measured using the MTT and BrdU assays (Fig. 4a,b). As shown in Fig. 4a, cell viability was significantly decreased after co-treatment with STF-083010 compared with Tm in both OVCAR3 and SKOV3 cell lines. The BrdU results also showed that STF-083010 significantly decreased proliferation rates in both cell lines starting at 10 (p < 0.05) compared with Tm-treated cells (Fig. 4b). Overall, the results of the MTT and BrdU assays showed that STF-083010 developed a dramatic anti-proliferative effect at a concentration of 50 μM in SKOV3 and VOCAR3 cells. In the next step, the effects of STF-083010 on apoptosis were evaluated using flow cytometry. The results showed that STF-083010 increased annexin–FITC/PI positive cells at the concentration of 50 μM (p < 0.05) compared with Tm in both cell lines. Apoptosis ranged from 20.2 ± 2.5 to 40.23 ± 5.1% in OVCAR3 and from 21.36 ± 2.6 to 32.5 ± 2.2% in SKOV3 cells (Fig. 4c). The results indicated that STF-083010 had an apoptotic effect on ovarian malignancy cells under ER stress conditions.
Fig. 4.
STF-083010 enhances Tm-induced ER stress and displays cytotoxic impact in ovarian cancer cell lines (OVCAR3 and SKOV3). Viability and apoptosis were assessed in co-treated cells with Tm (3 μg/ml) and STF-083010 (0.1–100 μM) using MTT assay (a) and BrdU incorporation (b) and annexin–FITC/PI (c) analysis. Results were manifested as mean ± SD and *p < 0.05, **p < 0.01, ***p˂0,001 versus control. #p < 0.05, ##p < 0.01 versus Tm (3 μg/mL) considered as significant
STF-083010 induced apoptosis by activation of caspase-12 and -3 and by regulation of Bax/Bcl-2 ratio
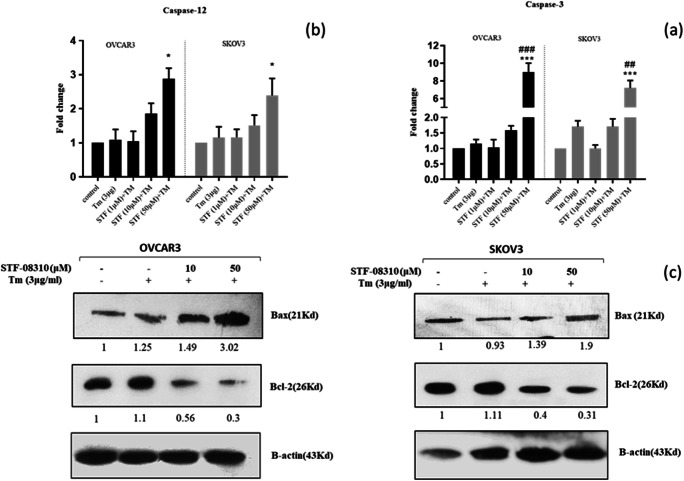
Previous studies have reported that caspase-12 is a pro-caspase placed in the ER membrane which plays a central role in ER stress-induced apoptosis (Tungkum et al. 2017). To clarify the molecular mechanism of the apoptotic effect of STF-083010, the activity of caspases-12 and -3 was evaluated. Then, the cells were co-treated with STF-080310 (at 1, 10, 50 μM) and Tm (Fig. 5a, b), and increased levels of caspases-3 (Fig. 5a, 8.2 ± 0.37 fold in OVCAR3; p˂0.001 and 6.3 ± 0.28 fold in SKOV3; p˂0.01) and − 12 (Fig. 5b, 2.73 ± 0.7 fold for OVCAR3; p˂0.05 and 2.3 ± 0.57 fold for SKOV3; p˂0.05) were observed compared with the Tm-treated cells.
Fig. 5.
STF-083010 enhances ER stress-mediated apoptosis in ovarian cancer cells (OVCAR3 and SKOV3). Caspase-3 and -12 activities measured by specific fluorometric assay kit (a, b) and measurement of Bax and Bcl-2 protein levels using western blotting (c). Results were manifested as mean ± SD and *p < 0.05, **p < 0.01, ***p˂0,001 versus control. #p < 0.05, ##p < 0.01, ###p˂0.001 versus Tm (3 μg/mL) considered as significant
To further confirm the STF-083010-mediated apoptosis in ovarian cancer cells, the levels of Bcl2 (anti-apoptotic) and Bax (pro-apoptotic) proteins in the Tm and STF-083010 (at 10 and 50 μM) co-treated cells were measured using western blotting. The data indicated that STF-083010-treatment increased Bax and decreased Bcl-2 protein expression in a dose-dependent manner (Fig. 4b). A significant increase in the Bax/Bcl-2 protein ratio was detected (p < 0.05) when cells were co-treated with STF-083010 and Tm in comparison to the Tm group, which revealed the apoptotic state of both ovarian cancer cell lines (Fig. 4b). These results indicated that inhibition of IRE1α-endonuclease through STF-083010 increased SKOV3 and OVCAR3 cell apoptosis during ER stress. STF-08310 enhanced apoptosis by activating caspases-12 and -3 and modulated the expression of Bcl-2 and Bax proteins.
Discussion
The present results showed that STF-083010, an inhibitor of IRE1α RNase-activity, had appropriate apoptotic effects on ovarian cancer cells. Tumor cells are dependent on optimal UPR signaling to survive in their environment (Jain 2017). Any interference in UPR signaling disrupts the transcriptional adaptive program. Recently, the IRE1α-XBP1 axis has been considered as a target for cancer therapy, especially in solid and secretory tumors such as those seen in ovarian cancer (M. Wang and Kaufman 2014). XBP1 is activated by the IRE1 RNase domain to generate an active/spliced form of XBP1 (sXBP1) with transcriptional activity. sXBP1 regulates the transcription of many genes, some of which restore the folding capacity of ER stress (Jin et al. 2016). The selective pharmacologic inhibitors of UPR transducers have prominent therapeutic potency in various cell types such as IRE1α inhibitors (Atkins et al. 2013). Several IRE1α RNase-specific inhibitors that are related to tumor development, progression, and chemotherapy responses have been examined in several cancer cells, including colorectal, pancreatic, breast cancer, and multiple myeloma cells, (X. Chen et al. 2014; Chien et al. 2014; Ming et al. 2015b; Sheng et al. 2015). However, IRE1α inhibitors may display a various mechanism within cancer cells. For example, STF-083010 is cytotoxic in a dose- and time-dependent manner in MM cells. Moreover, STF-083010 displays a preferentially cytotoxicity to CD138+ MM cells (Papandreou et al. 2011b). XBP1 has been addressed to be pivotal for MM pathogenesis (Carrasco et al. 2007). Resistance MCF-7 cells, STF-083010 can reverse tamoxifen sensitivity (Jie Ming et al., 2015). Also, IRE1α inhibitor-MKC8866 decreased production of protumorigenic factors in TNBC cells (Logue et al. 2018). In prostate cancer, MKC8866 reduces c-MYC levels and tumor regression (Sheng et al. 2019). Another IRE1α inhibitor-4 μ8 C enhances cell death through PERK dependent autophagy in U937 and BC3 cells (Storniolo et al. 2018).
In the current study, the anti-proliferative effect of IRE1α-XBP1 inhibition was determined in two ovarian cancer cell lines undergoing Tm-mediated ER stress that were co-treated by RNase inhibitor STF-083010. In addition, the molecular mechanism of STF-083010 on apoptosis was investigated. The results showed that the blockage of IRE1-RNase activity through STF-083010 has moderate toxicity on ovarian malignancy cells. An augmentation in ERS in STF-083010-treated cells was also found. The increase in both ERS marker genes and the rate of un/misfolded protein aggregation indicated the severity of ERS by inhibition of the IRE1-XBP1 axis. In this study, the most prominent changes in ERS apoptotic-associated proteins were the up-regulation of Bim and CHOP proteins and the elevation of caspase-3/12 activity in co-treated cells (Figs. 3 and 5a, b), the STF-enhanced expression of Bax and caspase-3 activity, and the decreased expression of Bcl-2 (Fig. 5c), BIP, and sXBP1 (Fig. 3) proteins in response to Tm treatment.
This study evaluated cell proliferation using the MTT and BrdU assays. The data indicated that these cells are sensitive at concentrations of STF-083010 in the range of 10 to 100 μM. STF-083010 moderately enhanced cytotoxicity of tunicamycin and significantly inhibited the growth of ovarian cancer cells. Additionally, a more sensitive examination was performed on annexin V/propidium iodide that showed good induction of apoptosis at an STF-083010 concentration of 50 μM. The annexin V/propidium iodide results revealed that STF-083010 induced both early and late apoptosis in ovarian cancer cells. Furthermore, both ovarian cancer cell lines were responsive to the apoptotic effect of STF-083010 in a dose-dependent manner. Consistent with this finding, the inhibition of IRE1-XBP1 by STF-083010 showed great toxicity in pancreatic cancer and multiple myeloma cells (Chien et al. 2014; Papandreou et al. 2011a). Similarly, the study of Logue et al. used EdU cell proliferation assay to show significantly inhibited proliferation of breast cancer cells (Logue et al. 2018).
In the further evaluation of the apoptotic effects of STF-083010, the levels of anti-apoptotic Bcl-2 and pro-apoptotic Bax in co-treated cells were examined. The results showed that Bcl-2 expression was decreased, while Bax expression and caspases-3 and -12 were increased in the co-treated cell lines. Primarily, it was believed that the activation of caspase-12 initiated ERS-induced apoptosis, but now this is a controversial issue. Murine procaspase-12 is well known as a caspase related to ER stress apoptosis located in the ER membrane (Bakhshi et al. 2008), which has high homology with caspases-4 and -5 in humans (Saleh et al. 2004). The human caspase-12 cDNA has 68% and 57% identity to mouse caspase-12 and human caspase-4, respectively. Human caspase-12 is known as a more dominant-negative regulator that inhibits the secretion of interleukin-1β (IL-1β) and IL-18. It is not clear, however, whether human caspase-12 can also mediate cell apoptosis specifically by endoplasmic reticulum (ER) stress (Saleh et al. 2004). Cellular studies suggest various mechanism of caspase-12 activation that may depend on the cell type. A study on the mechanism involved in the activation of caspases-12 in HEK 293 cells revealed that the kinase domain of human IRE1α forms a complex with human TRAF2 (tumor necrosis factor receptor-associated factor 2). In response to ER stress, IRE1α-TRAF2 complex interacts with transfected murine procaspase-12 and cleaves it, resulting in the activation of caspase-12. Murine caspase-12 can also be activated by the CHOP–ERO1α–IP3R–calcium–calpain pathway which is a new ER-specific apoptosis pathway mediated by caspase-12 in mice glial cells (Martinez et al. 2010; Tabas and Ron 2011). Another activation mechanism is cleavage pro-caspase-12 by caspase-7 which translocate from the cytosol to the ER to interact with caspase-12(Kalai et al. 2003; Tabas and Ron 2011). To date, the role of caspase-12 in human cells has not been clarified. There are few studies about the effects of the silencing of various branches of the UPR on caspase-12 activation and apoptosis. Moreover, some previous studies have shown that human caspase-12 can be induced in ER stress. For example, Mandic, A et al. reported that cisplatin induces ER stress and activates human caspase-12, resulting in ERS-induced apoptosis in a panel of nine melanoma cell lines (Mandic et al. 2003). It has also been shown previously that the apoptotic function of caspase-12 in HEK 293 and Sak2 cell lines are affected by ER stress inducers, including tapsigargin and brefeldin A (Bakhshi et al. 2008; Gan et al. 2017; Rao et al. 2004). Luthra et al. confirmed caspase-12 involvement in ER stress-mediated apoptosis in human retinal cells (Luthra et al. 2006). Chiu et al. reported that Tanshinone-IIA induced apoptosis in cancer cells and increased the protein expression levels of PERK, ATF6, caspase-12, IRE1α, elF2α, p-JNK, CHOP, and caspase-3 in BxPC-3-derived xenograft tumors in a dose-dependent manner (Chiu and Su 2017). Also, the activation of caspase-12 in apoptosis has been reported in colon cancer and CCRF-CEM-Jurkat cells (Tinhofer et al. 2002). Contrary to these results, more recent articles have mentioned that this caspase does not have a role in UPR-mediated apoptosis, and caspase-12 may be related to the mitochondrial apoptotic pathway (Ruiz-Vela et al. 2005; Saleh et al. 2006). For example, MEF deficiency in caspase-12 displays no sensitivity to ER stress inducers (Iurlaro and Munoz-Pinedo 2016). Shiraishi et al. have reported that Apaf-1 and the mitochondrial pathway of apoptosis play an important role in ER stress-induced apoptosis where Apaf-1 causes cleavage of caspase-3 during ER stress. Finally, caspase-3 cuts procaspase-12 leading to activation in MEF. Meanwhile, Shiraishi et al. have shown that neither overexpression nor knockdown of caspase-12 affect the cell death in MEF cell line during ER stress (2006). Recent studies have suggested that caspase-12 has no functional apoptosis role in the majority of human cells and may be an inflammatory caspase (Bian et al. 2008), while its relative, caspase-4, displays a functional role in the apoptosis related to ER stress in most human cells (Hitomi et al. 2004). The current data demonstrated that STF-083010 induced caspase-12 in both cell lines (Fig. 5a). Apoptotic performer caspase-3 activation was also assessed, and it was determined that its levels were increased in co-treated cell lines (Fig. 5b). Recently, the inhibition of IRE1 has been effective on the tumor secretome and production of pro-tumorigenic factors IL-6, IL-8, CXCL1, GM-CSF, and TGFβ2. Therefore, the increase in caspase-12 may be a response to this effect of IRE1 inhibition that was not evaluated in the current study.
A mechanistic study revealed that the pro-apoptotic protein Bim is the predominant protein up-regulated after treatment with STF-083010. Bim is a member of the Bcl-2 family of proteins that triggers apoptotic pathways in response to cytokine and particular stress conditions (Puthalakath et al. 2007). Consistent with the current results, Bim has also been up-regulated as a result of IRE1α-XBP1 inhibition in several cancer types. Bim has three isoforms: short (Bim-S), long (Bim-L), and extra-long (Bim-EL). Data in the current study displayed the up-regulation of both Bim-L and Bim-EL in two ovarian malignancy cell lines. When ER stress remains unresolved or is magnified, the apoptotic program in cells begins. Prolonged ER stress can induce Bim through two pathways, protein phosphatase 2A (PP2A) and CHOP (Puthalakath et al. 2007; Tay et al. 2012). The de-phosphorylation of Bim by PP2A is a novel activation pathway for Bim-EL that prevents its ubiquitination and degradation (Tay et al. 2012). The exact mechanism of PP2A activation through ER stress is unclear. Anette Hübner et al. demonstrated that Bim-EL consists of several phosphorylation sites, which are partially affected by JNK and ERK1/2 activity (Hübner et al. 2008). Phosphorylation through JNK enhances the pro-apoptotic function of Bim by increasing the Bim-Bax interaction, while ERK1/2-mediated phosphorylation reduces this interaction (Luciano et al. 2003; Tay et al. 2012). However, phosphorylation of catalytic subunits causes the inactivation of PP2A. Several studies have reported decreased p-PP2A (inactive form) in unresolved ER stress. Therefore, the p-PP2A (phosphorylated and inactivated) levels after inhibition of the IRE1α-XBP1 axis were investigated. The results showed a significant decrease in p-PP2A levels in Tm-treated cells, but the decrease was not significant after treatment with STF-083010 compared with the Tm-treated cells (Fig. 3).
CHOP is another key pro-apoptotic protein associated with ERS. It directly enhances the expression of Bim during prolonged ER stress (Marciniak et al. 2004) and inhibits the protein expression of Bcl-2 (Han et al. 2013; Jung et al. 2015). When the unfolded protein response exceeds a threshold, damaged cells become apoptotic through a mechanism that may involve the caspase-12-, PERK/ATF4-, and ATF6-mediated induction of the CHOP signaling pathway (Kim et al. 2006; Rasheva and Domingos 2009). In the present study, CHOP was up-regulated after the treatment of cells with STF-083010, suggesting CHOP was activated through ATF4, because PERK/ATF4s are the most powerful inducers of CHOP expression, and ATF4 directly targets CHOP promoters and causes translation induction. The protein expression of PERK/ATF4 was also investigated in the current study. The results demonstrated elevated ATF4 levels, while the expression of P-PERK and PERK proteins was not different after treatment with STF-083010. As well as ER stress, ATF4 can also be up-regulated independently through oxidative stress (Bagheri-Yarmand et al. 2019). Cellular studies have shown that CHOP interacts with ATF4 and directly up-regulates important genes involving protein synthesis leading to the depletion of ATP, induction of oxidative stress, and cell death (Han et al. 2013). The current data showed the ATF4 and CHOP were the main mediators of cell death after the inhibition of the IRE1α-XBP1 axis in OVCAR3 and SKOV3 cell lines. Similarly, multiple myeloma cells after exposure to MKC-3946, an IRE1-XBP1 inhibitor, enhanced apoptosis in response to ER stress by up-regulating both ATF4/PERK and CHOP (Sun et al. 2016). However, a previous study on a panel of 11 pancreatic cancer cell lines showed only the up-regulation of CHOP and decreased ATF4 mRNA expression after treatment with four IRE1α inhibitors (STF-083010, HNA, 3ETH, toyocamycin) (Chien et al. 2014). Chien et al. reported growth arrest at the G1 and G2/M phases by inhibition of IRE-RNase activity. Their results showed an increase in cleaved caspase 3 and PARP (Poly (ADP-ribose) polymerase), and prominent induction of BIM. (2014). Logue et al. demonstrated that constitutive IRE1 RNase activity has a more highlighted role in the maintenance of a pro-tumorigenic secretome than survival pathways directly. They also showed that the inhibition of IRE1-XBP1 is more effective in combination with chemotherapeutic agents and reduces only breast cancer cell proliferation. Their results showed there was no difference in either ATF6 processing or CHOP induction and PERK phosphorylation after treatment with MKC886 in triple negative breast cancer cells (Logue et al. 2018). This diversity may be due to differences in constitutive IRE1 RNase activity, or in molecular characteristics and complexities among tumor types and cell lines. Altogether, this suggests that the inhibition of IRE1 can display varies effect on UPR protein expression in different cancer types and other unreported mechanisms may also be involved in the apoptotic effects of STF-083010 in ovarian cancer cells such as autophagy and inflammatory responses. However, the current results showed that protein and mRNA levels of ATF4 and CHOP, as well as BIM protein, were mildly induced by STF-083010 in ovarian cancer cell lines, leading to apoptosis during ERS. Although there were no significant differences between the expressions of UPR-related proteins like BIM and CHOP (according to the amount of B-actin expression) in two cell lines, SKOV3 cells did show a greater survival rate compared with OVCAR3. Thus, SKOV3 has more resistance than OVCAR3 to IRE1 inhibition and ER stress. This difference in susceptibility may be due to the genotypic and phenotypic differences or the different STF-083010-metabolism in each cell line which it needs to more investigate. Generally, SKOV3 is a more invasive cell line compared with OVCAR3 that has shown more resistance to several cytotoxic drugs, such as cisplatin and flavonoids(Sak 2015). However, the pattern of changes was similar in both cell lines according to our results.
In this study, GRP78/BIP was investigated as the most important ER chaperone (A. S. Lee 2005). Indeed, sXBP1 is a transcription factor that promotes the expression of a variety of chaperones, such as GRP78 (A. H. Lee et al. 2003). The up-regulation of GRP78 is introduced as a worse prognostic marker in many cancer types such as ovarian cancer (Delie et al. 2012). Previously, it was reported that GRP78 acts as a central component in the survival, proliferation, and development of tumor cells, for which the reduction of GRP78 can be a pivotal therapy (Dong et al. 2008). However, the expression of GRP78 was mildly reduced after the exposure of ovarian cancer cells to STF-083010. This suggests that the inhibition of IRE1α-XBP1 enhances the accumulation of mis/unfolded proteins and may cause compensatory up-regulation of GRP78. Thus, we evaluated the intensity of accumulated mis/unfolded proteins by ThT assay (fluorescence dye-binding mis/unfolded proteins), and ER stress markers were measured using RT-PCR in STF-083010-treated cells in response to Tm treatment. The results showed increased aggregation of mis/unfolded proteins after IRE1-XBP1 inhibition which augmented ER stress.
Conclusion
In conclusion, the present study has shown that inhibition of IRE1-XBP1 (using STF-083010) can partially enhance the expression of pro-apoptotic ATF4, CHOP, and Bim proteins and modestly decrease BIP protein in response to tunicamycin in OVCAR3 and SKOV3 cell lines. STF-083010 may offer a therapeutic advantage for ovarian cancer by reducing the growth and viability of the cells. Further in vivo studies will be necessary to determine the effects of STF-083010 in the tumor microenvironment condition in ovarian cancer. However, we are a long way from designing a therapeutic approach which is specific for tumor cells by suitable pharmacokinetics. Therefore, future studies are needed to target the IRE1-XBP1 inhibitors.
Acknowledgments
Parts of this project was financially helped by Isfahan University of Medical Sciences (Grant Number: 396511).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Atkins C, Liu Q, Minthorn E, Zhang S-Y, Figueroa DJ, Moss K, et al. Characterization of a novel PERK kinase inhibitor with antitumor and antiangiogenic activity. Cancer Res. 2013;73(6):1993–2002. doi: 10.1158/0008-5472.CAN-12-3109. [DOI] [PubMed] [Google Scholar]
- Bagheri-Yarmand R, Sinha KM, Li L, Lu Y, Cote GJ, Sherman SI, Gagel RF. Combinations of tyrosine kinase inhibitor and ERAD inhibitor promote oxidative stress-induced apoptosis through ATF4 and KLF9 in medullary thyroid Cancer. Mol Cancer Res. 2019;17(3):751–760. doi: 10.1158/1541-7786.MCR-18-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhshi J, Weinstein L, Poksay KS, Nishinaga B, Bredesen DE, Rao RV. Coupling endoplasmic reticulum stress to the cell death program in mouse melanoma cells: effect of curcumin. Apoptosis. 2008;13(7):904–914. doi: 10.1007/s10495-008-0221-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beriault DR, Werstuck GH. Detection and quantification of endoplasmic reticulum stress in living cells using the fluorescent compound, Thioflavin T. Biochim Biophys Acta. 2013;1833(10):2293–2301. doi: 10.1016/j.bbamcr.2013.05.020. [DOI] [PubMed] [Google Scholar]
- Bian Z-M, Elner SG, Elner VM. Regulated expression of caspase-12 gene in human retinal pigment epithelial cells suggests its immunomodulating role. Invest Ophthalmol Vis Sci. 2008;49(12):5593–5601. doi: 10.1167/iovs.08-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco DR, Sukhdeo K, Protopopova M, Sinha R, Enos M, Carrasco DE, et al. The differentiation and stress response factor XBP-1 drives multiple myeloma pathogenesis. Cancer Cell. 2007;11(4):349–360. doi: 10.1016/j.ccr.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Li Q, She T, Li H, Yue Y, Gao S, et al. IRE1α-XBP1 signaling pathway, a potential therapeutic target in multiple myeloma. Leuk Res. 2016;49:7–12. doi: 10.1016/j.leukres.2016.07.006. [DOI] [PubMed] [Google Scholar]
- Chen TH, Chiang YH, Hou JN, Cheng CC, Sofiyatun E, Chiu CH, Chen W. (2017) Xbp1-mediated Bip/GRP78 upregulation copes with oxidative stress in mosquito cells during dengue 2 virus infection. BioMed research international, 2017 [DOI] [PMC free article] [PubMed]
- Chen X, Iliopoulos D, Zhang Q, Tang Q, Greenblatt MB, Hatziapostolou M, Lim E, Tam WL, Ni M, Chen Y, Mai J, Shen H, Hu DZ, Adoro S, Hu B, Song M, Tan C, Landis MD, Ferrari M, Shin SJ, Brown M, Chang JC, Liu XS, Glimcher LH. XBP1 promotes triple-negative breast cancer by controlling the HIF1alpha pathway. Nature. 2014;508(7494):103–107. doi: 10.1038/nature13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien W, Ding LW, Sun QY, Torres-Fernandez LA, Tan SZ, Xiao J, et al. Selective inhibition of unfolded protein response induces apoptosis in pancreatic cancer cells. Oncotarget. 2014;5(13):4881–4894. doi: 10.18632/oncotarget.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu TL, Su CC. Tanshinone IIA increases protein expression levels of PERK, ATF6, IRE1alpha, CHOP, caspase3 and caspase12 in pancreatic cancer BxPC3 cellderived xenograft tumors. Mol Med Rep. 2017;15(5):3259–3263. doi: 10.3892/mmr.2017.6359. [DOI] [PubMed] [Google Scholar]
- Delie F, Petignat P, Cohen M (2012) GRP78 protein expression in ovarian cancer patients and perspectives for a drug-targeting approach. Journal of oncology, 2012 [DOI] [PMC free article] [PubMed]
- Dong D, Ni M, Li J, Xiong S, Ye W, Virrey JJ, et al. Critical role of the stress chaperone GRP78/BiP in tumor proliferation, survival, and tumor angiogenesis in transgene-induced mammary tumor development. Cancer Res. 2008;68(2):498–505. doi: 10.1158/0008-5472.CAN-07-2950. [DOI] [PubMed] [Google Scholar]
- Gan PP, Zhou YY, Zhong MZ, Peng Y, Li L, Li JH. Endoplasmic reticulum stress promotes autophagy and apoptosis and reduces chemotherapy resistance in mutant p53 lung Cancer cells. Cell Physiol Biochem. 2017;44(1):133–151. doi: 10.1159/000484622. [DOI] [PubMed] [Google Scholar]
- Guo F-J, Liu Y, Zhou J, Luo S, Zhao W, Li X, Liu C. XBP1S protects cells from ER stress-induced apoptosis through Erk1/2 signaling pathway involving CHOP. Histochem Cell Biol. 2012;138(3):447–460. doi: 10.1007/s00418-012-0967-7. [DOI] [PubMed] [Google Scholar]
- Han J, Back SH, Hur J, Lin YH, Gildersleeve R, Shan J, Yuan CL, Krokowski D, Wang S, Hatzoglou M, Kilberg MS, Sartor MA, Kaufman RJ. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol. 2013;15(5):481–490. doi: 10.1038/ncb2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13(2):89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- Hitomi J, Katayama T, Eguchi Y, Kudo T, Taniguchi M, Koyama Y, Manabe T, Yamagishi S, Bando Y, Imaizumi K, Tsujimoto Y, Tohyama M. Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Abeta-induced cell death. J Cell Biol. 2004;165(3):347–356. doi: 10.1083/jcb.200310015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübner A, Barrett T, Flavell RA, Davis RJ. Multisite phosphorylation regulates Bim stability and apoptotic activity. Mol Cell. 2008;30(4):415–425. doi: 10.1016/j.molcel.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iurlaro R, Munoz-Pinedo C. Cell death induced by endoplasmic reticulum stress. FEBS J. 2016;283(14):2640–2652. doi: 10.1111/febs.13598. [DOI] [PubMed] [Google Scholar]
- Jafari SM, Joshaghani HR, Panjehpour M, Aghaei M. A2B adenosine receptor agonist induces cell cycle arrest and apoptosis in breast cancer stem cells via ERK1/2 phosphorylation. Cell Oncol. 2018;41(1):61–72. doi: 10.1007/s13402-017-0359-z. [DOI] [PubMed] [Google Scholar]
- Jain BP. An overview of unfolded protein response signaling and its role in cancer. Cancer Biother Radiopharm. 2017;32(8):275–281. doi: 10.1089/cbr.2017.2309. [DOI] [PubMed] [Google Scholar]
- Jin C, Jin Z, Chen NZ, Lu M, Liu CB, Hu WL, Zheng CG. Activation of IRE1alpha-XBP1 pathway induces cell proliferation and invasion in colorectal carcinoma. Biochem Biophys Res Commun. 2016;470(1):75–81. doi: 10.1016/j.bbrc.2015.12.119. [DOI] [PubMed] [Google Scholar]
- Jung KJ, Min KJ, Bae JH, Kwon TK. Carnosic acid sensitized TRAIL-mediated apoptosis through down-regulation of c-FLIP and Bcl-2 expression at the post translational levels and CHOP-dependent up-regulation of DR5, Bim, and PUMA expression in human carcinoma caki cells. Oncotarget. 2015;6(3):1556–1568. doi: 10.18632/oncotarget.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalai M, Lamkanfi M, Denecker G, Boogmans M, Lippens S, Meeus A, Declercq W, Vandenabeele P. Regulation of the expression and processing of caspase-12. J Cell Biol. 2003;162(3):457–467. doi: 10.1083/jcb.200303157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagöz GE, Acosta-Alvear D, Nguyen HT, Lee CP, Chu F, Walter P. An unfolded protein-induced conformational switch activates mammalian IRE1. Elife. 2017;6:e30700. doi: 10.7554/eLife.30700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim R, Emi M, Tanabe K, Murakami S. Role of the unfolded protein response in cell death. Apoptosis. 2006;11(1):5–13. doi: 10.1007/s10495-005-3088-0. [DOI] [PubMed] [Google Scholar]
- Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003;23(21):7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AS. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods. 2005;35(4):373–381. doi: 10.1016/j.ymeth.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Li X, Zhang K, Li Z. Unfolded protein response in cancer: the Physician's perspective. J Hematol Oncol. 2011;4(1):8. doi: 10.1186/1756-8722-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logue SE, McGrath EP, Cleary P, Greene S, Mnich K, Almanza A, et al. Inhibition of IRE1 RNase activity modulates the tumor cell secretome and enhances response to chemotherapy. Nat Commun. 2018;9(1):3267. doi: 10.1038/s41467-018-05763-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano F, Jacquel A, Colosetti P, Herrant M, Cagnol S, Pages G, Auberger P. Phosphorylation of Bim-EL by Erk1/2 on serine 69 promotes its degradation via the proteasome pathway and regulates its proapoptotic function. Oncogene. 2003;22(43):6785–6793. doi: 10.1038/sj.onc.1206792. [DOI] [PubMed] [Google Scholar]
- Luo S, Mao C, Lee B, Lee AS. GRP78/BiP is required for cell proliferation and protecting the inner cell mass from apoptosis during early mouse embryonic development. Mol Cell Biol. 2006;26(15):5688–5697. doi: 10.1128/MCB.00779-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthra S, Fardin B, Dong J, Hertzog D, Kamjoo S, Gebremariam S, Butani V, Narayanan R, Mungcal JK, Kuppermann BD, Kenney MC. Activation of Caspase-8 and Caspase-12 pathways by 7-Ketocholesterol in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2006;47(12):5569–5575. doi: 10.1167/iovs.06-0333. [DOI] [PubMed] [Google Scholar]
- Mandic A, Hansson J, Linder S, Shoshan MC. Cisplatin induces endoplasmic reticulum stress and nucleus-independent apoptotic signaling. J Biol Chem. 2003;278(11):9100–9106. doi: 10.1074/jbc.M210284200. [DOI] [PubMed] [Google Scholar]
- Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18(24):3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Márquez S, Fernández JJ, Terán-Cabanillas E, Herrero C, Alonso S, Azogil A, et al. Endoplasmic reticulum stress sensor IRE1α enhances IL-23 expression by human dendritic cells. Front Immunol. 2017;8:639. doi: 10.3389/fimmu.2017.00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez JA, Zhang Z, Svetlov SI, Hayes RL, Wang KK, Larner SF. Calpain and caspase processing of caspase-12 contribute to the ER stress-induced cell death pathway in differentiated PC12 cells. Apoptosis. 2010;15(12):1480–1493. doi: 10.1007/s10495-010-0526-4. [DOI] [PubMed] [Google Scholar]
- Mimura N, Fulciniti M, Gorgun G, Tai Y-T, Cirstea D, Santo L, et al. Blockade of XBP1 splicing by inhibition of IRE1α is a promising therapeutic option in multiple myeloma. Blood. 2012;119(24):5772–5781. doi: 10.1182/blood-2011-07-366633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming J, Ruan S, Wang M, Ye D, Fan N, Meng Q, et al. A novel chemical, STF-083010, reverses tamoxifen-related drug resistance in breast cancer by inhibiting IRE1/XBP1. Oncotarget. 2015;6(38):40692–40703. doi: 10.18632/oncotarget.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming J, Ruan S, Wang M, Ye D, Fan N, Meng Q, Tian B, Huang T. A novel chemical, STF-083010, reverses tamoxifen-related drug resistance in breast cancer by inhibiting IRE1/XBP1. Oncotarget. 2015;6(38):40692–40703. doi: 10.18632/oncotarget.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namba T, Chu K, Kodama R, Byun S, Yoon KW, Hiraki M, Mandinova A, Lee SW. Loss of p53 enhances the function of the endoplasmic reticulum through activation of the IRE1α/XBP1 pathway. Oncotarget. 2015;6(24):19990–20001. doi: 10.18632/oncotarget.4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papandreou I, Denko NC, Olson M, Van Melckebeke H, Lust S, Tam A, et al. Identification of an Ire1alpha endonuclease specific inhibitor with cytotoxic activity against human multiple myeloma. Blood. 2011;117(4):1311–1314. doi: 10.1182/blood-2010-08-303099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papandreou I, Denko NC, Olson M, Melckebeke V, Heleen L, Sofie T, Arvin, Niwa M. Identification of an Ire1alpha endonuclease specific inhibitor with cytotoxic activity against human multiple myeloma. Blood. 2011;117(4):1311–1314. doi: 10.1182/blood-2010-08-303099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokhriyal R, Hariprasad R, Kumar L, Hariprasad G (2019) Chemotherapy Resistance in Advanced Ovarian Cancer Patients. Biomarkers in cancer, 11, 1179299X19860815-11179299X19860815. doi: 10.1177/1179299X19860815 [DOI] [PMC free article] [PubMed]
- Puthalakath H, O'Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129(7):1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Racek T, Buhlmann S, Rüst F, Knoll S, Alla V, Pützer BM. Transcriptional repression of the prosurvival endoplasmic reticulum chaperone GRP78/BIP by E2F1. J Biol Chem. 2008;283(49):34305–34314. doi: 10.1074/jbc.M803925200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RV, Ellerby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ. 2004;11(4):372–380. doi: 10.1038/sj.cdd.4401378. [DOI] [PubMed] [Google Scholar]
- Rasheva VI, Domingos PM. Cellular responses to endoplasmic reticulum stress and apoptosis. Apoptosis. 2009;14(8):996–1007. doi: 10.1007/s10495-009-0341-y. [DOI] [PubMed] [Google Scholar]
- Ruiz-Vela A, Opferman JT, Cheng EH, Korsmeyer SJ. Proapoptotic BAX and BAK control multiple initiator caspases. EMBO Rep. 2005;6(4):379–385. doi: 10.1038/sj.embor.7400375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sak K. In vitro cytotoxic activity of flavonoids on human ovarian cancer cell lines. Cancer Sci Res. 2015;2(1):1–13. [Google Scholar]
- Saleh M, Mathison JC, Wolinski MK, Bensinger SJ, Fitzgerald P, Droin N, Ulevitch RJ, Green DR, Nicholson DW. Enhanced bacterial clearance and sepsis resistance in caspase-12-deficient mice. Nature. 2006;440(7087):1064–1068. doi: 10.1038/nature04656. [DOI] [PubMed] [Google Scholar]
- Saleh M, Vaillancourt JP, Graham RK, Huyck M, Srinivasula SM, Alnemri ES, Steinberg MH, Nolan V, Baldwin CT, Hotchkiss RS, Buchman TG, Zehnbauer BA, Hayden MR, Farrer LA, Roy S, Nicholson DW. Differential modulation of endotoxin responsiveness by human caspase-12 polymorphisms. Nature. 2004;429(6987):75–79. doi: 10.1038/nature02451. [DOI] [PubMed] [Google Scholar]
- Shahrestanaki MK, Arasi FP, Aghaei M. Adenosine protects pancreatic beta cells against apoptosis induced by endoplasmic reticulum stress. J Cell Biochem. 2019;120(5):7759–7770. doi: 10.1002/jcb.28050. [DOI] [PubMed] [Google Scholar]
- Sheng X, Arnoldussen YJ, Storm M, Tesikova M, Nenseth HZ, Zhao S, et al. Divergent androgen regulation of unfolded protein response pathways drives prostate cancer. EMBO Mol Med. 2015;7(6):788–801. doi: 10.15252/emmm.201404509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng X, Nenseth HZ, Qu S, Kuzu OF, Frahnow T, Simon L, et al. IRE1α-XBP1s pathway promotes prostate cancer by activating c-MYC signaling. Nat Commun. 2019;10(1):1–12. doi: 10.1038/s41467-018-08152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storniolo A, Alfano V, Carbotta S, Ferretti E, Renzo D, Livia IRE1α deficiency promotes tumor cell death and eIF2α degradation through PERK dipendent autophagy. Cell death discovery. 2018;4(1):1–12. doi: 10.1038/s41420-017-0002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh DH, Kim M-K, Kim HS, Chung HH, Song YS. Unfolded protein response to autophagy as a promising druggable target for anticancer therapy. Ann N Y Acad Sci. 2012;1271(1):20. doi: 10.1111/j.1749-6632.2012.06739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Lin DC, Guo X, Kharabi Masouleh B, Gery S, Cao Q, et al. Inhibition of IRE1alpha-driven pro-survival pathways is a promising therapeutic application in acute myeloid leukemia. Oncotarget. 2016;7(14):18736–18749. doi: 10.18632/oncotarget.7702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7(9):880–885. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13(3):184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay KH, Jin L, Tseng HY, Jiang CC, Ye Y, Thorne RF, Liu T, Guo ST, Verrills NM, Hersey P, Zhang XD. Suppression of PP2A is critical for protection of melanoma cells upon endoplasmic reticulum stress. Cell Death Dis. 2012;3:e337. doi: 10.1038/cddis.2012.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinhofer I, Anether G, Senfter M, Pfaller K, Bernhard D, Hara M, Greil R. Stressful death of T-ALL tumor cells after treatment with the anti-tumor agent Tetrocarcin-a. FASEB J. 2002;16(10):1295–1297. doi: 10.1096/fj.02-0020fje. [DOI] [PubMed] [Google Scholar]
- Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68(4):284–296. doi: 10.3322/caac.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tungkum W, Jumnongprakhon P, Tocharus C, Govitrapong P, Tocharus J. Melatonin suppresses methamphetamine-triggered endoplasmic reticulum stress in C6 cells glioma cell lines. J Toxicol Sci. 2017;42(1):63–71. doi: 10.2131/jts.42.63. [DOI] [PubMed] [Google Scholar]
- Wang G, Yang Z-Q, Zhang K. Endoplasmic reticulum stress response in cancer: molecular mechanism and therapeutic potential. Am J Transl Res. 2010;2(1):65–74. [PMC free article] [PubMed] [Google Scholar]
- Wang M, Kaufman RJ. The impact of the endoplasmic reticulum protein-folding environment on cancer development. Nat Rev Cancer. 2014;14(9):581–597. doi: 10.1038/nrc3800. [DOI] [PubMed] [Google Scholar]
- Wu J, He GT, Zhang WJ, Xu J, Huang QB. IRE1α signaling pathways involved in mammalian cell fate determination. Cell Physiol Biochem. 2016;38(3):847–858. doi: 10.1159/000443039. [DOI] [PubMed] [Google Scholar]