Abstract
Erlotinib is an oral tyrosine kinase inhibitor of epidermal growth factor receptor (EGFR) pathway. Although our previous study has proved the efficacy of Erlotinib in head and neck squamous cell carcinoma (HNSCC), it has also demonstrated poor clinical response rates and disappointing results in clinical trials for HNSCC to date. In this study, we discovered elevated cell proliferation and invasion ability in erlotinib-resistant HNSCC cells. The contributions of miRNAs within extracellular vesicles (EVs) during the formation of chemoresistance were investigated in this study. Among up-regulated miRNAs in EVs derived from resistant cells, miR-7704, miR-21-5p and miR-3960 showed the most pro-tumorigenic alterations after transfection. Conversely, let-7i-5p, miR-619-5p and miR-30e-3p demonstrated tumor suppressive effects. By performing qRT-PCR and Western blot analysis, we found Vimentin played a pivotal role in modulating erlotinib resistance. Additionally, immune system was highlighted in the GO and KEGG analyses. Transfection of miR-7704, miR-21-5p significantly elevated CTLA-4 and LAG3 mRNA levels. Meanwhile, miR-3960 increased the relative mRNA expression of TIM3 in HNSCC cells. Transfection of let-7i-5p, miR-619-5p and miR-30e-3p decreased these checkpoint factors. To conclude, the present study described the roles of EVs-transmitted miRNAs on erlotinib resistance. Targeting the disregulated immune system could be the effective method to overcome erlotinib-resistance in HNSCC cells.
Electronic supplementary material
The online version of this article (10.1007/s12079-020-00546-7) contains supplementary material, which is available to authorized users.
Keywords: HNSCC, Erlotinib, miRNAs, Extracellular vesicles (EVs), EMT
Introduction
Head and neck cancer is the 6th most common cancer worldwide (Zibelman and Mehra 2016). Squamous cell carcinomas are by far the most common subtype, accounting for >90% of head and neck tumors (Zibelman and Mehra 2016). Despite initial chemoradiation combined surgery and postoperative adjuvant radiotherapy, the overall survival of patients with HNSCC has improved only marginally over the past 3 decades (Pilborough et al. 2019; Suh et al. 2014). The increasing understanding of the molecular biology of HNSCC has led to an interest in the development of targeted therapies (Leemans et al. 2011).
Overexpression of epidermal growth factor receptor (EGFR) and its ligands have been observed in nearly 80–90% of cases of head and neck squamous cell carcinoma (HNSCC) and correlates with poor prognosis and resistance to radiation (Bauman et al. 2017; Cassell and Grandis 2010). Erlotinib is an oral tyrosine kinase inhibitor (TKI) of EGFR, approved in the United States as treatment for advanced pancreatic and non–small-cell lung cancer (Yao et al. 2016). Preclinical models suggest a significant radiotherapy-sensitizing effect and a potential synergism with cisplatin (Martins et al. 2013). In patients with recurrent/metastatic HNSCC, erlotinib showed a 70% disease control rate when combined with cisplatin in patients with recurrent/metastatic disease. In a phase 1/2, single-arm trial, patients with advanced HNSCC treated with cisplatin-based chemoradiotherapy plus erlotinib experienced a 74% overall complete response rate (CRR) and 3-year overall survival (OS) of 72% (William et al. 2018). However, erlotinib has also demonstrated poor clinical response rates (5–10%) and thus disappointing results in clinical trials for HNSCC to date (Cohen et al. 2009). Although EGFR was the first validated molecular target in HNSCC, absolute improvement in a reliable clinical endpoint is limited to 10%–20% of patients, suggesting intrinsic resistance to EGFR inhibition, despite overexpression of the purported target in the majority of patients (Gross et al. 2014). Several studies have proposed the activation of compensatory signaling pathways for the acquisition of erlotinib resistance, but the picture remains unclear (Cufi et al. 2013; Zheng et al. 2018a, b). On the other hand, few prognostic factors have been validated as predictive substance of erlotinib response. Thus, it is urgent to elucidate the underlying mechanisms of erlotinib resistance and discover reliable markers that can predict erlotinib response in HNSCC patients.
It is reported that primary tumours release extracellular vesicles (EVs), including exosomes, promotes tumor proliferation, metastasis and drug resistance by transferring their genetic information, such as messenger RNAs (mRNAs) and short non-coding microRNAs (miRNAs), to surrounding cells or distant organs (Keklikoglou et al. 2019; Montermini et al. 2015; Takahashi et al. 2017). However, whether EVs derived from resistant cancer cells can confer drug resistance to sensitive cells needs to be elucidated. Moreover, components embedded in circulating EVs may serve as easily accessible factors for the evaluation of drug response in patients.
MiRNAs are part of a large family of non-coding RNAs that regulate many important cellular functions (Liu et al. 2014; Mahlab-Aviv et al. 2018). MiRNAs modulate gene expression by decreasing target mRNA stability or repressing translational efficiency. Some of the miRNAs are partly packaged into extracellular vesicles or included by an RNA-induced simple complex (RISC) with the Agonature2 protein to protect against the elimination of RNase (Mahlab-Aviv et al. 2018; Van Roosbroeck and Calin 2017). Emerging evidence supports the notion that circulating miRNAs modulate numerous hallmarks of cancer, including proliferation, apoptosis and metastasis in many cancers (Boelens et al. 2014).
Recent studies have indicated that microenvironment modified by EVs from erlotinib-resistant cells mediated the aggressive character of cancer cells (Zhang et al. 2018). However, the differential miRNA expression profiles of erlotinib-resistant HNSCC cell lines have rarely been observed. In this study, we discovered an oncogenic phenotype in HNSCC cells administrated with erlotinib. We demonstrated that EVs genetic material is taken up by infiltrating erlotinib-resistant cells present in the tumor microenvironment, which indicates that the tumor microenvironment promotes tumor progression and chemo resistance through the help of cancer EVs.
In this study, a total of 114 EVs-transported miRNAs were identified to be dysregulated in resistant cells compared with the respective parental cells (including 44 down-regulated miRNAs and 70 up-regulated miRNAs). Among up-regulated miRNAs, miR-7704, miR-21-5p and miR-3960 showed the most pro-tumorigenic alterations after transfection. Conversely, let-7i-5p, miR-619-5p and miR-30e-3p demonstrated tumor suppressive effects after transfection. By performing qRT-PCR, we found Vimentin gene expression levels were significantly augmented in erlotinib-resistant cells. Moreover, immune system was highlighted in the GO and KEGG analyses. Transfection of miR-7704, miR-21-5p significantly elevated CTLA-4 and LAG3 mRNA levels. Meanwhile, miR-3960 increased the relative mRNA expression of TIM3 in HN6 cells. Transfection of let-7i-5p, miR-619-5p and miR-30e-3p decreased these checkpoint factors.
In summary, the present study determined the roles of EVs-transmitted miRNAs on erlotinib resistance. Further experiments needed to be conducted to verify the mechanism based on immune system to overcome erlotinib-resistance in HNSCC cells.
Materials and methods
Cell culture and inhibitor
WSU-HN6 (hereafter simplified to HN6) was obtained from Dr. Silvio Gutkind of NIH (Bethesda, MD). CAL27 was purchased from American Type Culture Collection (ATCC). Two cell lines have been authenticated at July, 2017 by short tandem repeat testing done in the Fragment Analysis Facility, Tian Lin Biology and Technology Company (Shanghai, China). Cells were recovered and maintained in Dulbecco’s Modified Eagle Medium containing 10% FBS. Erlotinib was purchased from Selleck (Selleck Chem, Houston, USA) and was dissolved in Dimethyl Sulphoxide (DMSO), which was also used for treatment controls.
To establish cells resistant to erlotinib, we treated parental HN6 cells (HN6-ErS cells) with escalating concentrations of the drug, starting from 10 nmol/L, until they reacquired the ability to grow in presence of erlotinib at the same rate or faster rate of parental cells (in absence of the drug); then the inhibitor concentration was doubled until the cells accomplished resistance to 10 μmol/L erlotinib. Following a similar protocol, we treated parental CAL27 cells (CAL27-ErS cells) with escalating concentrations of erlotinib, starting from 10 nmol/L, until establishing resistance to 5 μmol/L.
EVs isolation and characterization
EVs were isolated from parental and erlotinib-resistant cell culture supernatants. In brief, culture supernatants were collected and differentially centrifuged at 300 g for 10 min, 1000×g for 20 min and 10,000×g for 30 min. Next, the supernatants were filtered using 0.22 μm filter units (Millex-GP; EMD Millipore, Darmstadt, Germany) and ultracentrifuged at 100,000×g for 3 h at 4 °C. After removing the supernatant, the pellets were resuspended in ice-cold PBS. Then the suspension was centrifuged at 100,000×g for another 3 h at 4 °C. EVs pellets were resuspended in PBS and stored at −80 °C. EVs were visualized by transmission electron microscopy and confirmed by the expression of TSG101, CD9 and CD63, which are specific proteins of EVs. CANX was viewed as negative control.
miRNA transfection
HN6 and CAL27 cells were seeded at 4 × 104 cells/mL in 6-well plates and transfected at 50–60% confluence with 20 miRNA mimics (50 nM, GemmaPharma) or negative control (NC) using HiperFect transfection reagent (Qiagen). After transfection, cells were incubated in complete medium for 72 h and processed for each experiment.
Western blot analysis
Total protein was lysed using lysis buffer (Beyotime, China) containing phosphatase inhibitor and protease inhibitor cocktails. Coomassie Brilliant Blue was utilized to quantify the protein lysates, and bovine serum albumin (BSA) was used as the standard. All proteins (10 μg) were separated using SDS-polyacrylamide gels and transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore), which were then blocked with 5% BSA at room temperature for 2 h and hybridized with primary antibodies (diluted 1:1000) specific for CD9, CD63, EGFR, p-EGFR, AKT, p-AKT, PI3K, mTOR, p-mTOR, CDK2, CDK4, CDK6, cyclin D1, β-catenin, E-cadherin, Vimentin, N-cadherin, snail, slug (purchased from CST); TSG101 and HES-1 (purchased from Abcam); Calnexin (CANX, purchased from Santa Cruz Biotechnology); Erk1/2, p-Erk1/2 and β-actin (purchased from Bioworld, China) overnight at 4 °C followed by incubation with anti-goat IgG HRP-conjugated secondary antibodies (Zhongshan Goldenbridge Bio, China) for 1 h at room temperature. Immunoreactive bands were detected using an Immobilon Western Chemiluminescent HRP Substrate (Millipore) and visualized using the ImageQuantLAS4000 mini imaging system (General Electric). Protein expression levels were counted as gray values relative to Beta-actin according to the analyses of the bands using ImageJ software. Three independent experiments were analyzed for quantification.
RNA extraction and quantitative reverse transcription PCR (RT-qPCR)
According to the manufacturer’s protocol, total RNA was extracted from cells using TRIzol reagent (Invitrogen) and was converted to cDNA using 5 × PrimeScript RT Master Mix (TaKaRa) at 37 °C for 15 min and 85 °C for 5 s. Primer sequences were obtained from the Primer Bank and were used in quantitative real-time PCR (RT-qPCR) with a 7900HT Real-Time PCR System (Applied Biosystems). The delta delta Ct method for quantitation of relative gene expression was used to determine the mean expression of each target gene normalized to the geometric mean of GAPDH. All primers were designed and synthesized to target the specific sequences of the genes as follows:
β-catenin: 5′- AAAGCGGCTGTTAGTCACTGG-3′
R: 5′- CGAGTCATTGCATACTGTCCAT −3’
Vimentin: F: 5′- GACGCCATCAACACCGAGTT-3′
R: 5′- CTTTGTCGTTGGTTAGCTGGT-3′
E-cadherin: F: 5′- CGAGAGCTACACGTTCACGG -3′
R: 5′- GGGTGTCGAGGGAAAAATAGG −3’
N-cadherin: F: 5′- TCAGGCGTCTGTAGAGGCTT-3′
R: 5′- ATGCACATCCTTCGATAAGACTG-3′
snail: F: 5′- TCGGAAGCCTAACTACAGCGA-3′
R: 5′- AGATGAGCATTGGCAGCGAG-3′
slug: F: 5′- CGAACTGGACACACATACAGT-3′
R: 5′- CTGAGGATCTCTGGTTGTGGT-3’
CTLA-4: F: 5′- GCCCTGCACTCTCCTGTTTTT-3′
R: 5′- GGTTGCCGCACAGACTTCA-3′
LAG3: F: 5′-GCGGGGACTTCTCGCTATG-3′
R: 5’-GGCTCTGAGAGATCCTGGGG-3′
TIM3: F: 5′-CTGCTGCTACTACTTACAAGGTC-3′
R: 5′-GCAGGGCAGATAGGCATTCT-3′
PD-1: F: 5′-CCAGGATGGTTCTTAGACTCCC-3′
R: 5′-TTTAGCACGAAGCTCTCCGAT-3′
PD-L1: F: 5′-TGGCATTTGCTGAACGCATTT-3′
R: 5′-TGCAGCCAGGTCTAATTGTTTT-3′
Cell viability and colony formation assay
Cells were seeded in 96-well microplates at a density of 2 × 103 cells per well. Cells were incubated in new medium containing 10% CCK-8 reaction solution (DOJINDO, Japan). After incubation for 2 h, the absorbance was measured on a spectrophotometer microplate reader (Multiskan MK3, Thermo) at a wavelength of 450 nm according to the manufacturer’s instructions. Five independent experiments were performed.
For the colony formation assay, cells were seeded in 6-well plates and cultured for approximately 2 weeks until colony formation was observed. Cell colonies were stained with 0.005% crystal violet and analyzed by microscopy.
Immunofluorescence staining
Briefly, parental and erlotinib-resistant cells were seeded onto sterile glass-coverslips in 24-well plates at approximately 20% confluence. After cultured for 24 h, cells were fixed in 4% paraformaldehyde and permeabilized in 1% Triton. After incubation overnight with β-catenin, E-cadherin, Vimentin or N-cadherin antibodies (diluted 1:100, CST), cells were stained for 1 h with goat anti-rabbit IgG antibody Cy3 (Proteintech, China) and counterstained with DAPI (Sigma, St Louis, MO). Cell morphology was determined by phalloidin staining (diluted 1:1000, ApexBio). Cells were subsequently viewed by fluorescence microscopy (ZEISS, Germany).
Wound healing assays
HN6 and CAL27 cells were respectively seeded on six-well plates at densities of 1 × 105 cells per well. Cells were grown for 24 h and thereafter the wounds were created with a wound maker tool. Cells were cultured in serum-free DMEM-F12 medium with mitomycin C for 12 h. Cells were then washed twice with DMEM-F12 medium. Imaging was conducted with an optical microscope (Leica, Germany). The wound area analysis was conducted with ImageJ software.
Transwell invasion assays
According to the manufacturer’s instructions, Transwell chambers (8 μm pore size; Millipore) were used to detect cell invasive ability. A total of 5 × 104 cells were seeded into the upper chamber of each insert and incubated at 37 °C for 24 h. Similar inserts coated with Matrigel (BD Biosciences) were used to determine invasive potential in cell invasion assays. Chambers were fixed in 4% paraformaldehyde for 30 min and then dyed with crystal violet staining. The non-invaded cells on the upper chamber surface were removed, and the invaded cells on the surface were subsequently viewed and counted by microscopy (ZEISS, Germany).
Flow cytometry
For cell-cycle analysis, stable cells were harvested and washed in phosphate-buffered saline (PBS) and fixed in 75% ice-cold ethanol for 30 min at 4 °C. Cells were then washed twice in PBS, stained with propidium iodide (50 μg/ml) in the presence of 50 μg/ml RNase A (Sigma-Aldrich) and incubated for 1 h at room temperature. The cell-cycle analysis was performed on a FACSCalibur flow cytometer (BD Biosciences) and CellQuest Pro software (BD Biosciences). Flow cytometric analysis of apoptotic cells was performed by staining the cells using the Annexin V Apoptosis Detection Kit (BD Pharmingen) according to the manufacturer’s protocol. The percentages of cells in specific cell-cycle stages in erlotinib-resistant cells were compared with those in parental cells.
Statistical analyses
Statistical analyses were performed using SPSS 25.0 (SPSS Inc., Chicago, IL). All experiments were repeated at least three times. Each data point represents the mean ± SD of data from 3 independent trials. The Student’s t test was used to evaluate the statistical significance of the results. All P values represented two-sided tests of statistical significance. (*P < 0.05, **P < 0.01, ***P < 0.001).
Results
Erlotinib inhibited canonical EGFR signaling and increased proliferation in resistant cells
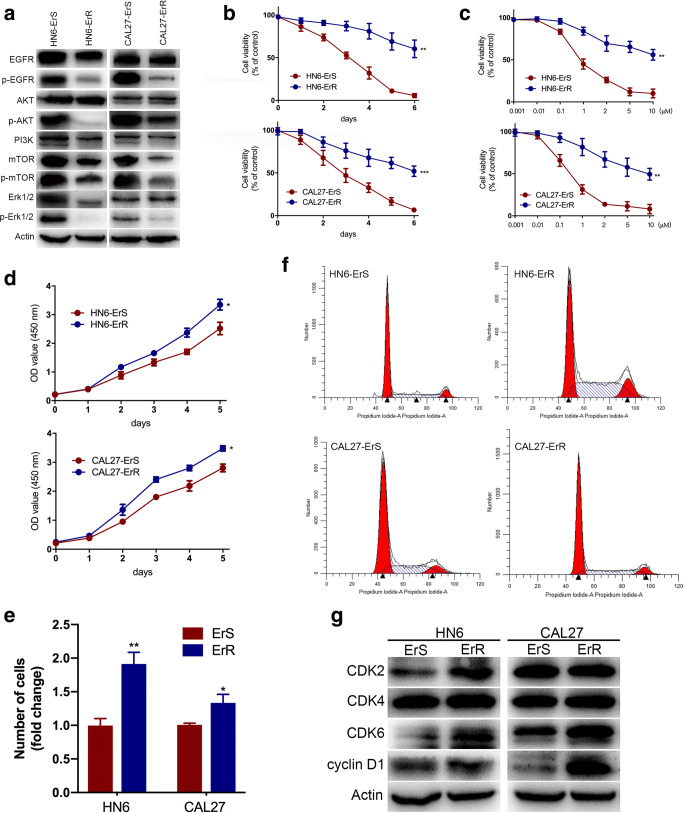
To investigate the underlying regulatory mechanism of erlotinib resistance, two erlotinib-resistant sublines derived from HN6 and CAL27 parental cell lines were established (HN6-ErR and CAL27-ErR, respectively), as described before. Cells without erlotinib treatment were demonstrated as controls (HN6-ErS and CAL27-ErS, respectively). The signaling inhibitory efficiency of erlotinib was detected by assessing the expression of EGFR pathway. Unlike the results in glioblastoma patients where lack of response to TKI occurs in the presence of unphosphorylated EGFR and active ERK and AKT pathways (Hegi et al. 2011), phosphorylation of EGFR, AKT, mTOR and Erk1/2 was markedly decreased, indicating the effective inhibition of erlotinib (Fig. 1a).
Fig. 1.
Erlotinib inhibited canonical EGFR signaling and increased proliferation in resistant cells. a Total and phosphorylation of EGFR, AKT, mTOR and Erk1/2 were analyzed by Western blot. Phosphorylation of EGFR, AKT, mTOR and Erk1/2 was markedly decreased in both HN6 and CAL27 resistant cells. b Cell viability was tested in sensitive and resistant HN6 and CAL27 cells. c The half-maximal inhibitory concentration value of erlotinib was detected for sensitive and resistant cells by cell viability assay. d CCK-8 assays showed that erlotinib-resistant cells exhibited higher tumorigenicity than the parental cells. e colony-formation assays displayed enhanced proliferative abilities in HN6-ErR and CAL27-ErR cells after two-week incubation. f Cell-cycle analysis was performed by flow cytometry in sensitive and insensitive cells. ErR cells presented a significantly lower percentage in the G1 phase and higher percent of cells in the S and G2 phase than ErS cells. *P < 0.05, **P < 0.01, ***P < 0.001
HN6-ErR and CAL27-ErR cells exhibited elevated cell viability, in contrast to the parental cells, when incubated with culture medium containing 5 to 10 μM erlotinib for 48 h (Fig. 1b). The concentration-effect curve indicated that the half-maximal inhibitory concentration (IC50) of erlotinib for HN6-ErR and CAL27-ErR cells was much higher than their parental cells (Fig. 1c).
In order to explore the biological property of resistant cells, we performed CCK-8 assays and colony formation experiments. CCK-8 assays showed that erlotinib-resistant cells exhibited higher tumorigenicity than the parental cells (Fig. 1d). In addition, colony-formation assays displayed enhanced proliferative abilities in HN6-ErR and CAL27-ErR cells after two-week incubation (Fig. 1e).
Moreover, we performed cell-cycle analysis by flow cytometry. The results demonstrated that HN6-ErR and CAL27-ErR cells presented a significantly lower percentage in the G1 phase and higher percent of cells in the S and G2 phase than control cells (Fig. 1f). The D-type cyclins (including cyclin D1) are reported to be induced as cells enter the G1 phase of the cell cycle and can activate cyclin-dependent kinases (CDKs), CDK2, CDK4 and CDK6, which can drive the cell into S phase (Casimiro et al. 2014; Zwijsen et al. 1997). In this study, changes in the expression of cell-cycle-specific proteins were selectively analyzed by Western blot. Consistently, HN6-ErR and CAL27-ErR cells showed the up-regulation of CDKs (2, 6) and cyclins (D1) (Fig. 1g).
Erlotinib enhanced invasion and induced epithelial-to-mesenchymal transition (EMT)
It has been increasingly recognized that cancer drug resistance is frequently accompanied with EMT in diverse cancers (Du and Shim 2016; Shibue and Weinberg 2017). To determine whether erlotinib resistance altered invasion of HNSCC cells, we utilized wound healing and Transwell invasion assays. Erlotinib-resistant cells exhibited drastically increased migrative and invasive ability compared to the parental cells (Fig. 2a-b).
Fig. 2.
Erlotinib enhanced migration and invasion in HNSCC cells. a Wound healing experiments were performed to determine the alteration of cell migration in HN6 resistant cells. b Cell invasion ability was detected by Matrigel Transwell assay for erlotinib-resistant and -sensitive cells (×20 magnification). c Microscopic-observation and phalloidin staining showed that the erlotinib-resistant cells acquired a spindle-cell morphology compared to parental cells
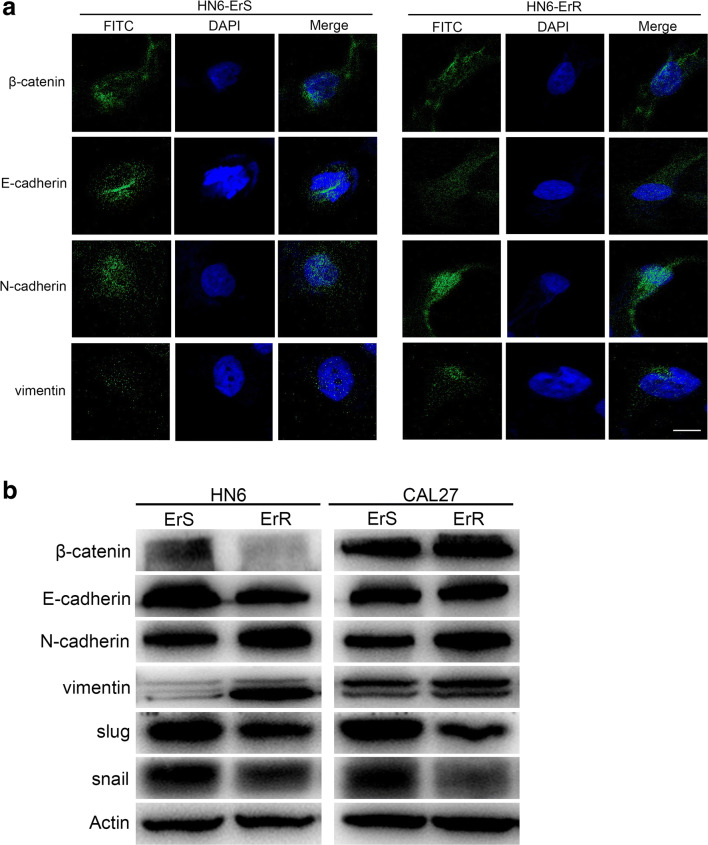
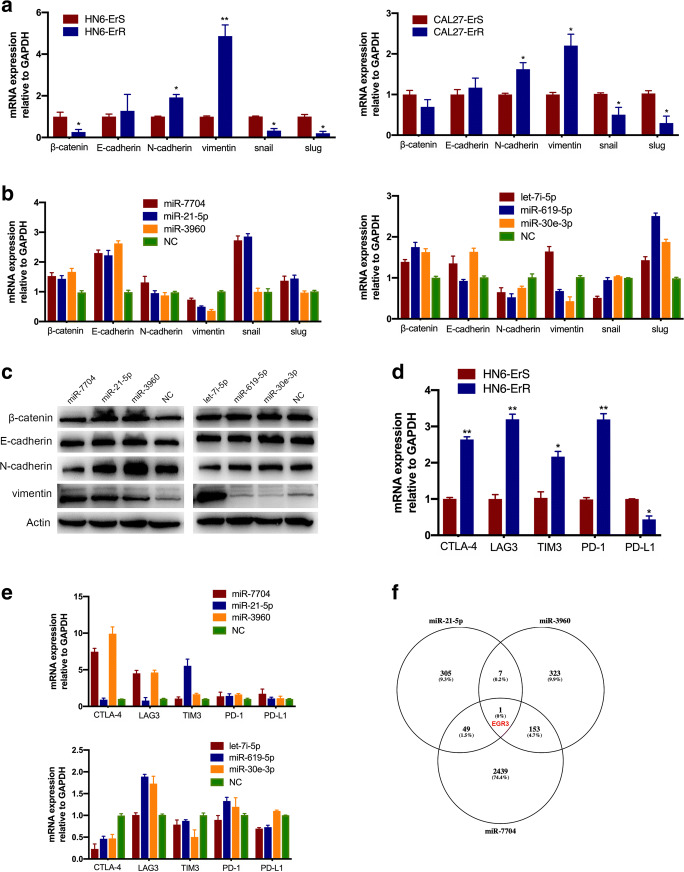
During drug screening process, we noticed that erlotinib-incubated cells exhibited specific morphological alterations consistent with EMT. Upon activation of EMT, carcinoma cells lose many of their epithelial characteristics, including the presence of epithelial cell junctions and apical–basal polarity, and instead acquire mesenchymal attributes, such as an elongated, fibroblast-like morphology as well as an increased capacity for migration and invasion (Goossens et al. 2017). These hybrid phenotypes are facilitated in collective cell migration, are associated with cell migration, the acquisition of stem-like properties, chemoresistance and aggressiveness. Phalloidin staining showed that the erlotinib-resistant cells acquired a spindle-cell morphology, with increased intercellular separation signifying the loss of intercellular adhesion, and increased formation of pseudopodia (Fig. 2c). Immunofluorescence staining showed that the epithelial markers β-catenin and E-cadherin were downregulated in HN6-ErR cells, accompanied by an increase in the expression of N-cadherin and Vimentin (Fig. 3a).
Fig. 3.
Epithelial-to-mesenchymal transition (EMT) was observed in resistant cells. a Immunofluorescence staining showed that mesenchymal markers Vimentin and N-cadherin were increased in HN6-ErR cells, with the downregulation of epithelial markers β-catenin and E-cadherin. b Western blot analysis was performed to assess the expression of EMT-specific markers at the protein level
Additionally, we performed Western blot analysis to assess the expression of EMT-specific markers at the protein level. As expected, erlotinib markedly increased the expression of the mesenchymal marker Vimentin and N-cadherin and slightly reduced the expression of the epithelial marker E-cadherin and β-catenin compared to the control cells (Fig. 3b). The Snail family transcription factors are representative EMT regulators, and they have been previously implicated as inducers of EMT in many types of malignancies (Wang et al. 2013). To further elucidate the potential molecular mechanisms, we analyzed the protein levels of the classic EMT-inducers, snail and slug. As shown in Fig. 3b, inconsistent with the high Vimentin protein levels detected in ErR cells, we discovered a decrease in both snail and slug protein expression in resistant cells.
Characterization of EVs-transported miRNAs secreted from erlotinib-resistant and parental cells
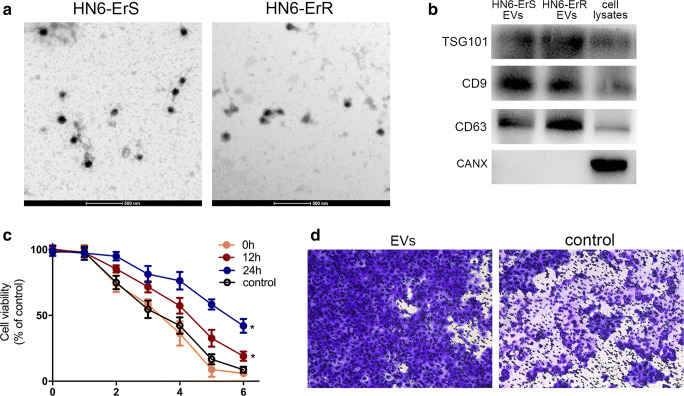
EVs may be actively secreted from a variety of cell types, including cancer cells (Bach et al. 2017; Keklikoglou et al. 2019). To determine whether EVs may be secreted from head and neck cancer cells, and whether the secreted EVs are able to regulate erlotinib resistance, HN6-ErR and the parental cells were incubated in EVs-free medium containing EVs-free FBS. As presented in Fig. 4a, transmission electron microscopy revealed similar morphological characteristics between the HN6-ErS and HN6-ErR EVs, with a homogeneous structure. In addition, their size was within the characteristic diameter range of 40–120 nm, with a median diameter of 100 nm. The presence of EVs protein markers, including TSG101, CD9 and CD63, were further confirmed by western blot analysis. The results demonstrated specific bands in isolated EVs fraction, and not in the whole cell lysate (Fig. 4b).
Fig. 4.
Characterization of EVs secreted from erlotinib-resistant and parental cells. a Transmission electron microscopy revealed similar morphological characteristics between the HN6-ErS (left panel) and HN6-ErR (right panel) EVs, with a homogeneous structure. b EV protein markers, including TSG101, CD9 and CD63, were confirmed by western blot analysis in HN6-ErS EVs, HN6-ErR EVs and HN6-ErR cell lysates. CANX protein level was viewed as negative control. c Cell viability was assessed in HN6 parental cells with incubation of EVs derived from HN6 erlotinib-resistant cells for 0 h, 12 h or 24 h. Group control was HN6 parental cells with incubation of EVs derived from their own cell supernatant. d Transwell assay showed the increased invasive ability in cells cultured with EVs derived from resistant cells. Control represented EVs derived from parental cells
To determine the function of EVs in erlotinib resistance, we collected EVs secreted from erlotinib-resistant cells and incubated with parental cells. We noticed that parental cells appeared more resistance to erlotinib after 24-h incubation with EVs (Fig. 4c). Moreover, cells became more aggressive compared with control (Fig. 4d), suggesting the active transmission of EVs to erlotinib-sensitive cells.
It is well established that miRNAs regulate EMT through the regulation of E-cadherin, Vimentin and other molecules, such as the transcription factor snail and ZEB, facilitating the process of EMT and reversing the efficacy of conventional cancer therapeutics. Having verified the release of EVs from erlotinib-resistant cells, the present study sought to define the specific EVs-transported miRNAs that may regulate erlotinib resistance. The erlotinib resistance associated EVs-transported miRNAs released in the conditioned media in HN6-ErR and HN6-ErS cells, were characterized by microarray analysis. A total of 114 EVs-transported miRNAs were identified to be dysregulated (P < 0.001) in resistant cells compared with the respective parental cells (including 44 down-regulated miRNAs and 70 up-regulated miRNAs). Among those, EVs-transported miR-7704, miR-21-5p, miR-3960, miR-22-3p, miR-151a-5p, miR-423-5p, miR-378a-3p, miR-1307-3p, miR-4488, miR-1268b and miR-378f were drastically augmented in erlotinib-resistant cells. On the contrary, the numbers of EVs-transported miR-1246, miR-3529-3p, let-7i-5p, miR-12,136, miR-182-5p, miR-619-5p, miR-30e-3p, miR-3184-3p and miR-7975 were significantly lower in resistant cells (Table 1). Additionally, IC50 values were analyzed in HN6 parental cells after transfection of up-regulating miRNA in Erlotinib-resistant cells or down-regulating miRNA in Erlotinib-resistant cells in the parental cells (Figure S1).
Table 1.
The top 20 upregulated and downregulated miRNAs in EVs collected from HN6-ErR and HN6-ErS cells screened by fold change and P value
| miRNA id | TPM(ErR) | TPM(ErS) | P value | Chrom | Regulation |
|---|---|---|---|---|---|
| hsa-miR-7704 | 156.05 | 624.94 | 0 | 2q31.1 | up |
| hsa-miR-21-5p | 603.27 | 1833.07 | 0 | 17q23.1 | up |
| hsa-miR-3960 | 14.27 | 39.87 | 4.73E-61 | 9q34.11 | up |
| hsa-miR-22-3p | 32.57 | 60.42 | 1.37E-41 | 17p13.3 | up |
| hsa-miR-151a-5p | 36.83 | 77.62 | 1.22E-71 | 8q24.3 | up |
| hsa-miR-423-5p | 0.28 | 15.75 | 7.98E-93 | 17q11.2 | up |
| hsa-miR-378a-3p | 22.2 | 196.39 | 0 | 5q32 | up |
| hsa-miR-1307-3p | 2.16 | 13.45 | 1.82E-44 | 10q24.33 | up |
| hsa-miR-4488 | 19.91 | 48.29 | 5.03E-59 | 11q12.2 | up |
| hsa-miR-1268b | 4.82 | 30.37 | 5.33E-99 | 17q25.3 | up |
| hsa-miR-378f | 4.5 | 28.16 | 1.44E-91 | 1p36.11 | up |
| hsa-miR-1246 | 289.48 | 104.52 | 0 | 2q31.1 | down |
| hsa-miR-3529-3p | 264.85 | 31.31 | 0 | 15q26.1 | down |
| hsa-let-7i-5p | 298.29 | 146.74 | 3.68E-248 | 12q14.1 | down |
| hsa-miR-12,136 | 42.84 | 1.03 | 3.28E-239 | 1p36.33 | down |
| hsa-miR-182-5p | 23.76 | 11.75 | 4.23E-21 | 7q32.2 | down |
| hsa-miR-619-5p | 7.25 | 0.14 | 1.65E-42 | 12q24.11 | down |
| hsa-miR-30e-3p | 22.52 | 8.09 | 6.50E-35 | 1p34.2 | down |
| hsa-miR-3184-3p | 5.27 | 0.94 | 3.99E-17 | 17q11.2 | down |
| hsa-miR-7975 | 5.05 | 1.6 | 2.56E-10 | 19q13.42 | down |
Identification of miRNAs as potential prognostic markers in erlotinib-resistant HNSCC
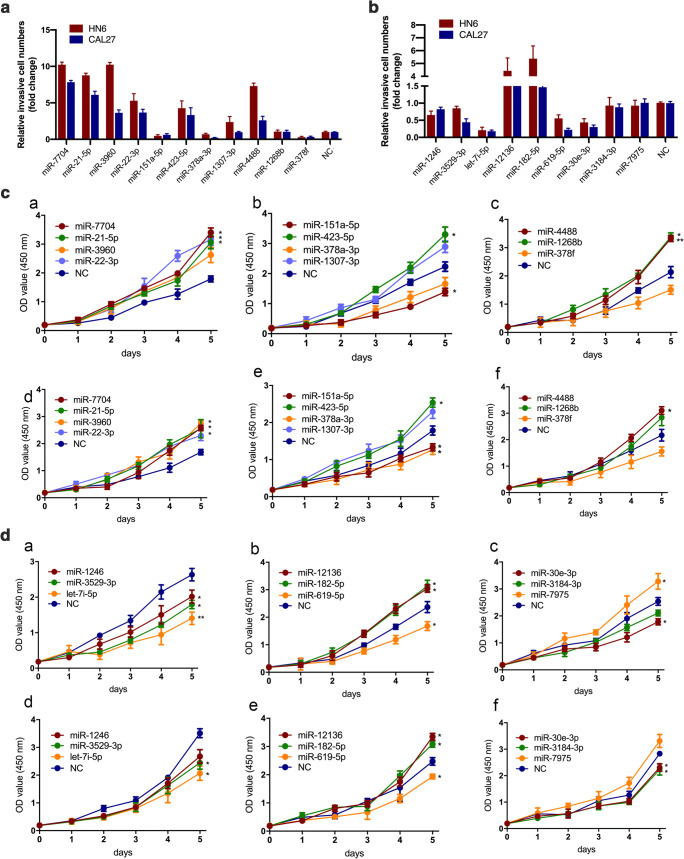
As EMT transition has been verified in resistant cells, we doubted whether the alteration of EVs-transported miRNAs secreted from resistant cells had an effect on sensitive cells which in turn acquired drug resistance and resulted in EMT. We transfected 20 miRNAs in resistant and parental cells respectively, in which 11 miRNAs were up-regulated and 9 miRNAs were down-regulated in erlotinib-resistant cells as described above. After 11 miRNAs were transfected in erlotinib-sensitive cells, 8 miRNAs (miR-7704, miR-21-5p, miR-3960, miR-22-3p, miR-423-5p, miR-1307-3p, miR-4488 and miR-1268b) exhibited elevated invasion compared with negative control (Fig. 5a, Figure S2). Cell proliferative potential was consistent with invasion abilities (Fig. 5b). MiR-7704, miR-21-5p and miR-3960 showed most prominent phenotypic alteration after gene transfection. In erlotinib-resistant cells, 6 out of 9 miRNAs transfected (miR-1246, miR-3529-3p, let-7i-5p, miR-619-5p, miR-30e-3p and miR-3184-3p) showed decreased invasive ability (Fig. 5c, Figure S3). Cell proliferation ability was in accord to the invasiveness (Fig. 5d). Let-7i-5p, miR-619-5p and miR-30e-3p showed most prominent phenotypic alteration after gene transfection.
Fig. 5.
Identification of miRNAs as prognostic markers in erlotinib-resistant HNSCC. a By performing transwell assays, 8 out of 11 up-regulated miRNAs were demonstrated elevated invasion compared with negative control in both HN6 and CAL27 erlotinib-sensitive cells. b Cell proliferation was determined in erlotinib-sensitive cells after transfection of 11 up-regulated miRNAs in HN6 (a-c) and CAL27 (d-f) erlotinib-sensitive cells. c 6 out of 9 down-regulated miRNAs were demonstrated reduced invasion compared with negative control in both HN6 and CAL27 erlotinib-resistant cells. d Cell proliferation was determined in erlotinib-resistant cells after transfection of 9 down-regulated miRNAs in HN6 (a-c) and CAL27 (d-f) erlotinib-resistant cells. *P < 0.05, **P < 0.01
As EMT-related protein levels showed the down-regulation of β-catenin, E-cadherin, snail and slug as well as the up-regulation of N-cadherin and Vimentin, we performed qRT-PCR by detecting EMT-related genes (Fig. 6a). The mRNA levels in β-catenin, snail and slug were lower in erlotinib-resistant cells compared to their parental cells. Vimentin gene expression levels were significantly augmented in erlotinib-resistant cells. Based on the results described above, we transfected 3 up-regulated and 3 down-regulated miRNAs in resistant cells to identify the potential mechanism on chemoresistance. Protein and gene expression levels showed that Vimentin could be a crucial modulating factor in erlotinib resistance (Fig. 6b-c).
Fig. 6.
Immune checkpoints were related to erlotinib resistance. a RT-PCR was performed to detect EMT-related genes, including β-catenin, E-cadherin, N-cadherin, Vimentin, snail and slug. b Vimentin gene expression levels were tested in 3 up-regulated and 3 down-regulated miRNAs in resistant cells. c Protein levels of Vimentin were detected by western blot analysis. d Gene expression levels of CTLA-4, LAG3, TIM3, PD-1 and PD-L1 were determined in HN6 resistant and parental cells. e Immune checkpoints were detected in HN6 resistant cells after transfection of 3 up-regulated miRNAs. f Venn diagram displayed early growth response 3 (EGR3) might be the target of these 3 up-regulated miRNAs. *P < 0.05, **P < 0.01
Immune checkpoints were related to erlotinib resistance
Additionally, KEGG and GO analyses were assessed in Figure S4. Immune system was highlighted in the analyses. It has been proved that CTLA-4, PD-1 and other immunological factors are involved in EMT transition and drug resistance (Hahn et al. 2017; McNiel and Tsichlis 2017). CTLA-4 expression within tumors can be regulated by the oncogenic pathways on EMT and PD-L1 expression in tumor cells (Cufi et al. 2013). The tumor cells undergoing EMT also express immunological factors, which are one of the essential factors of immune evasion (Noman et al. 2017). We first detected immune checkpoint factors in resistant and parental cells. We noticed that CTLA-4, LAG3, TIM3 and PD-1 were highly expressed in resistant cells (Fig. 6d). We then examined the expression of immunological factors after transfection of miRNAs. Transfection of miR-7704 and miR-3960 significantly elevated CTLA-4 and LAG3 mRNA levels. Meanwhile, miR-21-5p increased the relative mRNA expression of TIM3 in HN6 cells (Fig. 6e). Transfection of let-7i-5p, miR-619-5p and miR-30e-3p decreased these checkpoint factors (Fig. 6e). To further explore the potential target genes of selected miRNAs, we used Targetscan to predict the direct binding sites of these miRNAs. Early growth response 3 (EGR3) was the only gene that had direct binding sites in all three up-regulated miRNAs (miR-7704, miR-21-5p and miR-3960) (Fig. 6f). It has been reported that EGR3 can act as a regulator of immune response(Li et al. 2012). We hypothesized the dysregulation of immune checkpoints was caused by the downregulation of EGR3. Further experiments needed to be conducted to verify the unique role of EGR3 in regulating immune system thus exploring the mechanism on overcoming erlotinib-resistance in HNSCC cells.
Discussion
There is considerable evidence linking EMT to cancer progression, metastasis, and EGFR inhibitor resistance in multiple tumor types (Sesumi et al. 2017). In our current study, we noticed that erlotinib-resistant cells exhibited significantly higher tumorigenicity than the parental cells. Compared to the parental cells, the obtained cells showed EMT-like features, with a spindle cell-like morphology, improved migration ability, and a decrease in E-cadherin, accompanied by increased N-cadherin and Vimentin expression. In this study, down-regulated snail and slug expression was detected in erlotinib-resistant cells although which exhibited an enhanced EMT phenotype. Interestingly, our previous study also discovered a paradox between snail family expression and EMT phenotype (Zheng et al. 2018a, b). We suspected that although snail family was involved in most EMT process, the alteration of invasion ability by the erlotinib resistance was not mainly attributed to the snail family. Moreover, the pivotal role for Vimentin in the erlotinib resistance need to be elucidated in the further study although the acquisition of a mesenchymal like phenotype and increased invasiveness in resistant cells is well substantiated.
Accumulating evidence supports a critical role for miRNAs in governing metastatic propensity (Chen et al. 2015; Van Roosbroeck and Calin 2017). MiRNAs target coding genes through complex regulatory networks mediating the multistep metastatic cascade. EVs are a novel class of extracellular vesicles that have gained enormous attention lately as facilitators of the progression of various tumors (Tauro et al. 2013). Erlotinib resistance in cancer cells can be reflected by extracellular vesicles (Al-Nedawi et al. 2008). To determine the function of EVs in erlotinib resistance, we collected EVs secreted from erlotinib-resistant cells and incubated with parental cells. We noticed that parental cells gained resistance to erlotinib and cells became more aggressive compared with control. This transmission of drug resistance suggested the function of EVs spreading from aggressive cells to non-aggressive cells. In cancer cells, some miRNAs are abundantly released in EVs, leading to its high levels in serum and low cellular levels (Takahashi et al. 2017). Selective release of such miRNAs in cancer EVs may underlie its diagnostic and predictive potential. In this article, we employed microarray analysis to perform EVs-transported miRNA profiling analyses from supernatant of resistant and their parental cells and discovered dysregulated EVs-transported miRNAs. Among up-regulated miRNAs, miR-7704, miR-21-5p and miR-3960 showed the most pro-tumorigenic alterations after transfection. Conversely, let-7i-5p, miR-619-5p and miR-30e-3p demonstrated tumor suppressive effects after transfection.
It was reported that miR-7704 and miR-21 were highly overexpressed in hepatocellular carcinoma (HCC) and cirrhosis and miR-7704 had an over 400-fold change in HCC compared to cirrhosis (Mahlab-Aviv et al. 2018). Additionally, miR-21 was considered to be an oncogenic miRNA that appears to control the EMT process and the cancer stem cell (CSC) phenotype by collaborating with the EMT transcription factor SNAIL (Cufi et al. 2013). MiR-3960 was over-expressed in triple negative breast cancers (TNBC) as well as bladder cancers and was related to cancer progression (Huang et al. 2019; Usuba et al. 2019). On the contrary, EVs-transported let-7i-5p and miR-30e-3p has been proved to be a cancer-suppressor gene in cervical cancer, hepatocellular carcinoma and oral cancers (Chhabra 2018; Falzone et al. 2019; Volinia et al. 2012). In non-small cell lung carcinoma (NSCLC), miR-619 selectively inhibited the ability of the long DNA polymerase eta (PolH) transcript and, subsequently, reversed cisplatin resistance (Zhang et al. 2019).
To explore the mechanism of drug resistance, we analyzed predicted target genes. Venn diagram displayed early growth response 3 (EGR3) might be the target of miR-7704, miR-21-5p and miR-3960. The EGR family comprises four members (EGR1, EGR2, EGR3 and EGR4), which share a high degree of homology at their DNA-binding zinc finger domains recognizing a GC-rich fragment in the promoter region of multiple target genes (Ehrengruber et al. 2000). EGR3 has previously been implicated as a regulator in neuro-development, autoimmunity, inflammation, angiogenesis, and cancer (Baron et al. 2015; Li et al. 2012; Morita et al. 2016). According to the GO and KEGG results, we detected immune checkpoints in obtained and miRNA-transfected cells. The mRNA levels of CTLA-4, LAG3 and TIM3 were dysregulated compared to control. As EGR3 was proved to act as a regulator of immune response, we hypothesized the dysregulation of immune checkpoints was caused by the downregulation of EGR3. Further experiments need to confirm the role of EGR3 in erlotinib resistance and the modulation of EMT.
In conclusion, the present study described the roles of EVs-transmitted miRNAs on erlotinib resistance. Targeting the disregulated immune system could be the effective method to overcome erlotinib-resistance in HNSCC cells.
Electronic supplementary material
(DOCX 10101 kb)
Funding information
This research was supported by the National Natural Science Foundation of China (81402236), National Natural Science Foundation of China (81772887), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD, 2018–87), Jiangsu Provincial Medical Innovation Team (CXTDA2017036), Natural Science Foundation of Jiangsu Province of China (BK20171488) and Jiangsu Provincial Medical Youth Talent (QNRC2016854).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yang Zheng, An Song and Yan Zhou contributed equally to this work.
Contributor Information
Yunong Wu, Email: yunongwu@aliyun.com.
Xiaomeng Song, Email: xiaomengsong@njmu.edu.cn.
References
- Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, Rak J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10(5):619–624. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- Bach DH, Hong JY, Park HJ, Lee SK. The role of exosomes and miRNAs in drug-resistance of cancer cells. Int J Cancer. 2017;141(2):220–230. doi: 10.1002/ijc.30669. [DOI] [PubMed] [Google Scholar]
- Baron VT, Pio R, Jia Z, Mercola D. Early growth response 3 regulates genes of inflammation and directly activates IL6 and IL8 expression in prostate cancer. Br J Cancer. 2015;112(4):755–764. doi: 10.1038/bjc.2014.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman JE, Duvvuri U, Gooding WE, Rath TJ, Gross ND, Song J, Jimeno A, Yarbrough WG, Johnson FM, Wang L, Chiosea S, Sen M, Kass J, Johnson JT, Ferris RL, Kim S, Hirsch FR, Ellison K, Flaherty JT, Mills GB, Grandis JR. Randomized, placebo-controlled window trial of EGFR, Src, or combined blockade in head and neck cancer. JCI Insight. 2017;2(6):e90449. doi: 10.1172/jci.insight.90449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boelens MC, Wu TJ, Nabet BY, Xu B, Qiu Y, Yoon T, Azzam DJ, Twyman-Saint Victor C, Wiemann BZ, Ishwaran H, Ter Brugge PJ, Jonkers J, Slingerland J, Minn AJ. Exosome transfer from stromal to breast cancer cells regulates therapy resistance pathways. Cell. 2014;159(3):499–513. doi: 10.1016/j.cell.2014.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro MC, Velasco-Velazquez M, Aguirre-Alvarado C, Pestell RG. Overview of cyclins D1 function in cancer and the CDK inhibitor landscape: past and present. Expert Opin Investig Drugs. 2014;23(3):295–304. doi: 10.1517/13543784.2014.867017. [DOI] [PubMed] [Google Scholar]
- Cassell A, Grandis JR. Investigational EGFR-targeted therapy in head and neck squamous cell carcinoma. Expert Opin Investig Drugs. 2010;19(6):709–722. doi: 10.1517/13543781003769844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Gao DY, Huang L. In vivo delivery of miRNAs for cancer therapy: challenges and strategies. Adv Drug Deliv Rev. 2015;81:128–141. doi: 10.1016/j.addr.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhabra R. Let-7i-5p, miR-181a-2-3p and EGF/PI3K/SOX2 axis coordinate to maintain cancer stem cell population in cervical cancer. Sci Rep. 2018;8(1):7840. doi: 10.1038/s41598-018-26292-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen EE, Halpern AB, Kasza K, Kocherginsky M, Williams R, Vokes EE. Factors associated with clinical benefit from epidermal growth factor receptor inhibitors in recurrent and metastatic squamous cell carcinoma of the head and neck. Oral Oncol. 2009;45(10):e155–e160. doi: 10.1016/j.oraloncology.2009.05.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cufi S, Bonavia R, Vazquez-Martin A, Oliveras-Ferraros C, Corominas-Faja B, Cuyas E, Martin-Castillo B, Barrajon-Catalan E, Visa J, Segura-Carretero A, Joven J, Bosch-Barrera J, Micol V, Menendez JA. Silibinin suppresses EMT-driven erlotinib resistance by reversing the high miR-21/low miR-200c signature in vivo. Sci Rep. 2013;3:2459. doi: 10.1038/srep02459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du B, Shim JS (2016) Targeting epithelial-mesenchymal transition (EMT) to overcome drug resistance in cancer. Molecules 21(7). 10.3390/molecules21070965 [DOI] [PMC free article] [PubMed]
- Ehrengruber MU, Muhlebach SG, Sohrman S, Leutenegger CM, Lester HA, Davidson N. Modulation of early growth response (EGR) transcription factor-dependent gene expression by using recombinant adenovirus. Gene. 2000;258(1–2):63–69. doi: 10.1016/s0378-1119(00)00445-5. [DOI] [PubMed] [Google Scholar]
- Falzone L, Lupo G, La Rosa GRM, Crimi S, Anfuso CD, Salemi R, Rapisarda E, Libra M, Candido S (2019) Identification of novel MicroRNAs and their diagnostic and prognostic significance in oral cancer. Cancers (Basel) 11(5). 10.3390/cancers11050610 [DOI] [PMC free article] [PubMed]
- Goossens S, Vandamme N, Van Vlierberghe P, Berx G. EMT transcription factors in cancer development re-evaluated: beyond EMT and MET. Biochim Biophys Acta Rev Cancer. 2017;1868(2):584–591. doi: 10.1016/j.bbcan.2017.06.006. [DOI] [PubMed] [Google Scholar]
- Gross ND, Bauman JE, Gooding WE, Denq W, Thomas SM, Wang L, Chiosea S, Hood BL, Flint MS, Sun M, Conrads TP, Ferris RL, Johnson JT, Kim S, Argiris A, Wirth L, Nikiforova MN, Siegfried JM, Grandis JR. Erlotinib, erlotinib-sulindac versus placebo: a randomized, double-blind, placebo-controlled window trial in operable head and neck cancer. Clin Cancer Res. 2014;20(12):3289–3298. doi: 10.1158/1078-0432.CCR-13-3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn AW, Gill DM, Pal SK, Agarwal N. The future of immune checkpoint cancer therapy after PD-1 and CTLA-4. Immunotherapy. 2017;9(8):681–692. doi: 10.2217/imt-2017-0024. [DOI] [PubMed] [Google Scholar]
- Hegi ME, Diserens AC, Bady P, Kamoshima Y, Kouwenhoven MC, Delorenzi M, Lambiv WL, Hamou MF, Matter MS, Koch A, Heppner FL, Yonekawa Y, Merlo A, Frei K, Mariani L, Hofer S. Pathway analysis of glioblastoma tissue after preoperative treatment with the EGFR tyrosine kinase inhibitor gefitinib--a phase II trial. Mol Cancer Ther. 2011;10(6):1102–1112. doi: 10.1158/1535-7163.MCT-11-0048. [DOI] [PubMed] [Google Scholar]
- Huang D, Huang Y, Huang Z, Weng J, Zhang S, Gu W. Relation of AURKB over-expression to low survival rate in BCRA and reversine-modulated aurora B kinase in breast cancer cell lines. Cancer Cell Int. 2019;19:166. doi: 10.1186/s12935-019-0885-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keklikoglou I, Cianciaruso C, Guc E, Squadrito ML, Spring LM, Tazzyman S, Lambein L, Poissonnier A, Ferraro GB, Baer C, Cassara A, Guichard A, Iruela-Arispe ML, Lewis CE, Coussens LM, Bardia A, Jain RK, Pollard JW, De Palma M. Chemotherapy elicits pro-metastatic extracellular vesicles in breast cancer models. Nat Cell Biol. 2019;21(2):190–202. doi: 10.1038/s41556-018-0256-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11(1):9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- Li S, Miao T, Sebastian M, Bhullar P, Ghaffari E, Liu M, Symonds AL, Wang P. The transcription factors Egr2 and Egr3 are essential for the control of inflammation and antigen-induced proliferation of B and T cells. Immunity. 2012;37(4):685–696. doi: 10.1016/j.immuni.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Li J, Cairns MJ. Identifying miRNAs, targets and functions. Brief Bioinform. 2014;15(1):1–19. doi: 10.1093/bib/bbs075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahlab-Aviv S, Boulos A, Peretz AR, Eliyahu T, Carmel L, Sperling R, Linial M. Small RNA sequences derived from pre-microRNAs in the supraspliceosome. Nucleic Acids Res. 2018;46(20):11014–11029. doi: 10.1093/nar/gky791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins RG, Parvathaneni U, Bauman JE, Sharma AK, Raez LE, Papagikos MA, Yunus F, Kurland BF, Eaton KD, Liao JJ, Mendez E, Futran N, Wang DX, Chai X, Wallace SG, Austin M, Schmidt R, Hayes DN. Cisplatin and radiotherapy with or without erlotinib in locally advanced squamous cell carcinoma of the head and neck: a randomized phase II trial. J Clin Oncol. 2013;31(11):1415–1421. doi: 10.1200/JCO.2012.46.3299. [DOI] [PubMed] [Google Scholar]
- McNiel EA, Tsichlis PN (2017) Analyses of publicly available genomics resources define FGF-2-expressing bladder carcinomas as EMT-prone, proliferative tumors with low mutation rates and high expression of CTLA-4, PD-1 and PD-L1. Signal Transduct Target Ther 2. 10.1038/sigtrans.2016.45 [DOI] [PMC free article] [PubMed]
- Montermini L, Meehan B, Garnier D, Lee WJ, Lee TH, Guha A, Al-Nedawi K, Rak J. Inhibition of oncogenic epidermal growth factor receptor kinase triggers release of exosome-like extracellular vesicles and impacts their phosphoprotein and DNA content. J Biol Chem. 2015;290(40):24534–24546. doi: 10.1074/jbc.M115.679217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K, Okamura T, Inoue M, Komai T, Teruya S, Iwasaki Y, Sumitomo S, Shoda H, Yamamoto K, Fujio K. Egr2 and Egr3 in regulatory T cells cooperatively control systemic autoimmunity through Ltbp3-mediated TGF-beta3 production. Proc Natl Acad Sci U S A. 2016;113(50):E8131–E8140. doi: 10.1073/pnas.1611286114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noman MZ, Janji B, Abdou A, Hasmim M, Terry S, Tan TZ, Mami-Chouaib F, Thiery JP, Chouaib S. The immune checkpoint ligand PD-L1 is upregulated in EMT-activated human breast cancer cells by a mechanism involving ZEB-1 and miR-200. Oncoimmunology. 2017;6(1):e1263412. doi: 10.1080/2162402X.2016.1263412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilborough AE, Lambert DW, Khurram SA (2019) Extranodal extension in oral cancer: a role for the nodal microenvironment? Oral Pathol Med 48(10):863–870. 10.1111/jop.12870 [DOI] [PubMed]
- Sesumi Y, Suda K, Mizuuchi H, Kobayashi Y, Sato K, Chiba M, Shimoji M, Tomizawa K, Takemoto T, Mitsudomi T. Effect of dasatinib on EMT-mediated-mechanism of resistance against EGFR inhibitors in lung cancer cells. Lung Cancer. 2017;104:85–90. doi: 10.1016/j.lungcan.2016.12.012. [DOI] [PubMed] [Google Scholar]
- Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol. 2017;14(10):611–629. doi: 10.1038/nrclinonc.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh Y, Amelio I, Guerrero Urbano T, Tavassoli M. Clinical update on cancer: molecular oncology of head and neck cancer. Cell Death Dis. 2014;5:e1018. doi: 10.1038/cddis.2013.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi RU, Prieto-Vila M, Hironaka A, Ochiya T. The role of extracellular vesicle microRNAs in cancer biology. Clin Chem Lab Med. 2017;55(5):648–656. doi: 10.1515/cclm-2016-0708. [DOI] [PubMed] [Google Scholar]
- Tauro BJ, Mathias RA, Greening DW, Gopal SK, Ji H, Kapp EA, Coleman BM, Hill AF, Kusebauch U, Hallows JL, Shteynberg D, Moritz RL, Zhu HJ, Simpson RJ. Oncogenic H-ras reprograms Madin-Darby canine kidney (MDCK) cell-derived exosomal proteins following epithelial-mesenchymal transition. Mol Cell Proteomics. 2013;12(8):2148–2159. doi: 10.1074/mcp.M112.027086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usuba W, Urabe F, Yamamoto Y, Matsuzaki J, Sasaki H, Ichikawa M, Takizawa S, Aoki Y, Niida S, Kato K, Egawa S, Chikaraishi T, Fujimoto H, Ochiya T. Circulating miRNA panels for specific and early detection in bladder cancer. Cancer Sci. 2019;110(1):408–419. doi: 10.1111/cas.13856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Roosbroeck K, Calin GA. Cancer hallmarks and MicroRNAs: the therapeutic connection. Adv Cancer Res. 2017;135:119–149. doi: 10.1016/bs.acr.2017.06.002. [DOI] [PubMed] [Google Scholar]
- Volinia S, Galasso M, Sana ME, Wise TF, Palatini J, Huebner K, Croce CM. Breast cancer signatures for invasiveness and prognosis defined by deep sequencing of microRNA. Proc Natl Acad Sci U S A. 2012;109(8):3024–3029. doi: 10.1073/pnas.1200010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Shi J, Chai K, Ying X, Zhou BP. The role of snail in EMT and tumorigenesis. Curr Cancer Drug Targets. 2013;13(9):963–972. doi: 10.2174/15680096113136660102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- William WN, Jr, Tsao AS, Feng L, Ginsberg LE, Lee JJ, Kies MS, Glisson BS, Kim ES. Single arm, phase II study of Cisplatin, Docetaxel, and Erlotinib in patients with recurrent and/or metastatic head and neck squamous cell carcinomas. Oncologist. 2018;23(5):526–e549. doi: 10.1634/theoncologist.2017-0661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M, Woods C, Lavertu P, Fu P, Gibson M, Rezaee R, Zender C, Wasman J, Sharma N, Machtay M, Savvides P. Phase II study of erlotinib and docetaxel with concurrent intensity-modulated radiotherapy in locally advanced head and neck squamous cell carcinoma. Head Neck. 2016;38(Suppl 1):E1770–E1776. doi: 10.1002/hed.24313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Cai X, Yu J, Lu X, Qian Q, Qian W. Exosome-mediated transfer of lncRNA RP11838N2.4 promotes erlotinib resistance in non-small cell lung cancer. Int J Oncol. 2018;53(2):527–538. doi: 10.3892/ijo.2018.4412. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhang J, Sun W, Ren C, Kong X, Yan W, Chen X. A PolH transcript with a short 3'UTR enhances PolH expression and mediates Cisplatin resistance. Cancer Res. 2019;79(14):3714–3724. doi: 10.1158/0008-5472.CAN-18-3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Wang Z, Ding X, Dong Y, Zhang W, Zhang W, Zhong Y, Gu W, Wu Y, Song X. Combined Erlotinib and PF-03084014 treatment contributes to synthetic lethality in head and neck squamous cell carcinoma. Cell Prolif. 2018;51(3):e12424. doi: 10.1111/cpr.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Wang Z, Ding X, Zhang W, Li G, Liu L, Wu H, Gu W, Wu Y, Song X (2018b) A novel Notch1 missense mutation (C1133Y) in the Abruptex domain exhibits enhanced proliferation and invasion in oral squamous cell carcinoma. Cancer Cell Int 18(1) [DOI] [PMC free article] [PubMed]
- Zibelman M, Mehra R. Overview of current treatment options and investigational targeted therapies for locally advanced squamous cell carcinoma of the head and neck. Am J Clin Oncol. 2016;39(4):396–406. doi: 10.1097/COC.0000000000000283. [DOI] [PubMed] [Google Scholar]
- Zwijsen RM, Wientjens E, Klompmaker R, van der Sman J, Bernards R, Michalides RJ. CDK-independent activation of estrogen receptor by cyclin D1. Cell. 1997;88(3):405–415. doi: 10.1016/s0092-8674(00)81879-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 10101 kb)