Abstract
Purpose
The objective of our meta-analysis was to estimate the effect of VTS on obstetric outcomes of ART singletons.
Methods
PubMed, Embase, MEDLINE, and ClinicalTrials.gov were searched up to January 2019 to find studies reporting the obstetric outcomes of ART singletons with VTS. Dichotomous data were expressed as odds ratios (OR) with 95% confidence intervals (CI). Continuous data were expressed as weighted mean difference (WMD) with 95% CI.
Results
A total of 17 observational studies encompassing more than 60,000 ART singletons were included in this meta-analysis. The impact of VTS on singletons was highly dependent on the definition of VTS, precisely, the vanishing timing and intrauterine growth stage. When VTS happened at or before 14 weeks, regardless of intrauterine growth stage, there were no differences in terms of gestational age (GA) [WMD = − 0.08, 95% CI = − 0.27, 0.10], preterm birth (< 37 weeks) (PTB) [OR = 1.23, 95% CI = 0.89, 1.70], and low birth weight (< 2.5 kg) (LBW) [OR = 1.56, 95% CI = 1.00, 2.43] in original singletons versus singleton with VTS. On the contrary, VTS occurred after 14 weeks was associated with significantly shorter GW and lower BW, as well as higher risks of PTB and LBW. When the sac reduced in VTS was an empty gestational sac, there would be no differences in GW, PTB, and LBW between singletons versus singletons with VTS, whereas the loss of a fetus with cardiac-activity was associated with adverse obstetric outcomes.
Conclusions
This meta-analysis suggests whether or not VTS is harmful to obstetric outcomes is highly dependent on the vanishing timing and intrauterine growth stage.
Keywords: Vanishing twin syndrome, VTS, Assisted reproductive technique, Obstetric outcome
Introduction
Over the past three decades, assisted reproductive technologies (ART) have resulted in many thousands of successful pregnancies each year. With the advanced achievements in ART treatments, the obstetric outcomes of children born after ART have been gradually improved [32]. However, there is still a great gap in terms of perinatal and obstetric outcomes between pregnancies assisted by ART and the naturally conceived [40]. The increased risks of adverse outcomes following ART pregnancies cannot be explained entirely by the high incidence of multiple births, for even singletons after ART are associated with higher risks of adverse outcomes when compared with singletons conceived naturally [24, 39].
On the management of ART pregnancies, several studies have suggested that the conception rate of multiple gestations was greater than their birth rate [22, 28]. This phenomenon may be explained by the loss of embryos or gestational sacs during the gestation period. In recent years, the clinical application of ultrasound enables visualization and confirmation of these losses, name them as the vanishing twin syndrome (VTS). A study published in 2002 has shown that the incidence of VTS increases with initial number of gestational sacs. It is estimated that VTS occurs in 36% of pregnancies with two sacs, 53% of three sacs, and 65% of four or more sacs. With regard to IVF/ICSI pregnancies, 2 studies published in 2005–2006 estimated that VTS occur in 12–30% of twin pregnancies in weeks 7–8, reducing them to singleton pregnancies [10, 28].
It is clinically important to understand the effects of VTS on ART singleton pregnancies. However, studies focusing on this topic reported rather conflicting results [1, 33, 43]. Some studies reported that there was no difference between VTS singletons and original singleton in terms of preterm birth and low birthweight, while other studies reported that singletons with VTS were associated with higher risks of adverse obstetric outcomes than original singletons [7, 24, 33, 43]. The main reason for these conflicting results may be that the definition of VTS varies among different authors. Gestational sacs can “vanish” at several time points, from empty gestational sac to gestational sac with evidence of cardiac activity [7, 43] or from the first trimester to all three trimesters [28].
The objectives of our meta-analysis were to estimate the effects of VTS on obstetric outcomes of ART singletons and to evaluate whether vanishing timing and intrauterine growth stage aspects in the VTS definition contribute to this effect. The results of this study should allow clinicians to adequately inform patients diagnosed with VTS of the potential adverse obstetric outcomes.
Methods
Search strategy
This study was performed according to the checklist provided in the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) statement for writing systematic reviews and meta-analyses [25]. The following electronic databases were searched up to January 2019: PubMed, Embase, MEDLINE, and ClinicalTrials.gov. The search strategy used the following relevant medical subject heading (MeSH) terms, keywords, and word variants: singleton pregnancies, spontaneous reduction, spontaneous fetal reduction, vanishing twins, vanishing twin syndrome, and VTS. We also examined the reference lists of all known primary studies and review articles to obtain additional references.
Study selection
Studies that compared the obstetric outcomes between ART singletons with VTS and original ART singletons were considered eligible. In the case of double publication, studies that reported the largest sample size or the most obstetric outcomes were included. Only studies published on peer-reviewed journals in English language were included. Exclusion criteria were as follows: (1) case report, case series, or reviews; (2) only abstract available; (3) studies that included women who underwent selective fetal reduction; (4) studies that did not report our interested obstetric outcomes; and (5) studies that did not restrict the live birth number of pregnancies with VTS to singleton. Studies were selected independently by two reviewers and checked by a third.
Data extraction
The following data were extracted from each included study: author’s names, location, time period, sample size, VTS definitions and descriptions, and information about the participants. The following outcomes were included: gestational age at delivery (GA), mean birth weight (BW), preterm birth (< 37 weeks) (PTB), very preterm birth (< 32 weeks) (VPTB), low birth weight (< 2500 g) (LBW), very low birth weight (< 1500 g) (VLBW), small for gestational age (SGA, as defined by the authors of the included studies), antepartum hemorrhage (APH) (combination of placenta previa, placental abruption, and other bleeding), gestational hypertensive disorders (GHD) (including pregnancy-induced hypertension, pre-eclampsia, and eclampsia), mortality, and congenital anomalies. Data were extracted independently by two reviewers and checked by a third. Any disagreements were resolved by discussion between the reviewers.
Quality assessment
Two authors independently assessed the risk of the included studies. The quality of each included study was assessed using the Newcastle–Ottawa Scale (NOS) for the observational studies, which is recommended by the Cochrane Collaboration. NOS is a critical appraisal tool consisting of 9 questions to appraise an observational study systematically in three broad domains: selection of the study group, comparability between groups, and outcomes of study group (cohort study)/ascertainment of exposed or not exposed cohorts (case-control study). A score is then allocated out of 9. A study earning zero to three stars was considered to be poor quality, four to six stars were considered to be fair quality, and seven or more stars were considered to be of high quality.
Overall quality of the body of evidence
We also assessed the quality of the evidence of the included studies using the GRADE approach, which in turn is based on the number of studies, design of studies, consistency of associations between studies, study limitations, directness, precision, publication bias, effect size, and relative and absolute effect [15]. We can categorize the quality as high, moderate, low, or very low, accordingly to predefined criteria. The quality levels are high level, we are very confident that the true effect lies close to the observed in this review (very confident); moderate level, moderately confidence; low, limited confidence; and very low, very little confidence. It must be emphasized GRADE stars of an observational study started at low level (level 2) but might be upgraded (or downgraded) [15].
Statistical analysis
We conducted all data analyses through STATA software 12 (Stata Corp, College Station, TX, USA). For dichotomous data, including PTB, VPTB, LBW, VLBW, SGA, APH, GHD, and congenital anomalies, we calculated the odds ratio (OR) with 95% confidence intervals (CI). For continuous data, including GA and BW, weighted mean difference (WMD) with 95% CI were calculated. Heterogeneity was assessed using I2 statistics. If heterogeneity was moderate (I2 > 30%) or substantial (I2 > 50%), we further conducted subgroup analyses and sensitivity analyses [17]. Meta-analysis was conducted using a random effects model. Risk of bias across studies was assessed using visual inspection of funnel plots and the Egger’s test [11]. We have limited our assessment of publication bias to two important obstetric outcomes, namely, GA and BW, as they were widely reported in the literature, allowing comparisons between different studies.
Other analyses
We performed sensitivity analyses to evaluate whether the conclusions would have differed if we restricted eligibility to (1) studies that deemed to be of high quality; (2) studies that only included women who underwent autologous embryo(s) transfer; (3) studies that excluded all monochorionic twin pregnancies; and (4) studies where VTS and singleton groups had no significant differences in maternal ages.
Since no limitation on the initial gestational sac number of singleton pregnancies with VTS was established, we performed the first subgroup analysis based on initial number of gestational sac. Compared with gestational age, ultrasonic visualization is a more reliable way to confirm the intrauterine growth of the fetus. Therefore, studies that identify the intrauterine growth stage by ultrasound when VTS occurred were not furtherly subdivided according to the vanishing timing. If a study did not give a clear explanation on the intrauterine growth stage, those studies were subdivided according to the vanishing timing. In line with this strategy, four groups were established: (1) intrauterine growth stage, gestational sac with cardiac activity; vanishing timing, not limited; (2) intrauterine growth stage, empty gestational sacs; vanishing timing, not limited; (3) intrauterine growth stage, not limited; vanishing timing, ≤ 14 weeks; (4) intrauterine growth stage, not limited; vanishing timing, not limited. Therefore, the second subgroup analysis was performed, dividing studies into these four groups. To eliminate the potential influence of initial number of gestational sac, we performed the third subgroup analysis based on the same four groups but excluded studies that did not restrict the initial number of gestational sac to two. The forth subgroup analysis was performed based on mean women’s age of VTS group. If the mean maternal age of VTS group was less than 35 years, this study would be regarded as “mean maternal age < 35 years”: otherwise, the study would be regarded as “mean maternal age ≥ 35 years.” In order to exactly explore the impact of maternal age, only studies where VTS and singleton groups had no significant differences in maternal ages were included.
One additional subgroup analysis was performed stratifying studies that defined SGA as birth weight below the 10th percentile according to gestational age at delivery and those defined SGA as birth weight less than 2 standard deviations
Results
Results of the searches
The search strategy yielded a total of 2374 articles. After removing the duplicates, 1413 articles were screened by title and abstract. Of these, 1388 articles were excluded because they were undoubtedly not relevant to the present meta-analysis (Fig. 1). The full text of the remaining 25 articles was assessed for eligibility, of which 8 were excluded: four for including women who underwent artificial fetal reduction [4, 5, 31, 38], two for estimating the impact of VTS by comparing outcomes between singletons with VTS and original twins [14, 16], one for not restricting the live birth number of pregnancies with VTS to singletons [2], and one for overlapping a population reported by other included studies [29]. Finally, 17 studies were included for meta-analyses [1, 7–13, 19, 21–24, 27, 28, 33–35, 42, 43]. The main characteristics and assessment for risk of bias of included studies are presented in Table 1.
Fig. 1.
Flow diagram of study selection for the meta-analysis
Table 1.
Characteristics and Newcastle–Ottawa scale score of studies assessing ART singleton with VTS versus original singletons
| Study | Setting | Enrollment | Groups | Maternal age | N a | VTS definition | Chorionicity assessment | Transfer policy | NOS |
|---|---|---|---|---|---|---|---|---|---|
| [21] | (Single center) China | Jan 2009 to Jan 2016 | VTS group | 31.2 ± 3.8 | 759 | One gestational sac without identification of intrauterine growth stage was lost during the whole pregnancy period | Dichorionic twins | Not mentioned | 8 |
| Original singletons | 31.5 ± 4.9 | 14,551 | |||||||
| [33] | (Single center) USA | 2007–2015 | VTS group | 37.2 ± 3.7 | 100 | One gestational sac without identification of intrauterine growth stage was lost at or before 14 weeks of gestation | Dichorionic twin | Autologous embryos | 7 |
| Original singletons | 35.2 ± 3.8* | 798 | |||||||
| [22] | (National registry) Norway | Jan 1984 to Dec 2013 | VTS group | Age > 35 (44%) | 583 | One or more gestational sac without identification of intrauterine growth stage was lost during whole pregnancy period | Not mentioned | Not mentioned | 6 |
| Original singletons | Age > 35 (37%)* | 16,038 | |||||||
| [9] | (Single center) Australia | Jan 1986 to Dec 2002 | VTS group | Unknown | 253 | One gestational sac without identification of intrauterine growth stage was lost at or before 14 weeks of gestation | Not mentioned | Not mentioned | 6 |
| Original singletons | Unknown | 3838 | |||||||
| [24] | (Single center) Hungary | Jan 1094 to Nov 2014 | VTS group | 34.8 ± 3.71 | 78 | One gestational sac without identification of intrauterine growth stage was lost at or before 14 weeks of gestation | Dichorionic twins | Not mentioned | 9 |
| Original singletons | 35.4 ± 3.74 | 234 | |||||||
| [27] | (Single center) USA | Jan 2004 and Jun 2013 | VTS group | 36.1 ± 4.08 | 853 | One or more gestational sacs without identification of intrauterine growth stage lost before 7 weeks of gestation | Dichorionic twins | Autologous embryos | 8 |
| Original singletons | 35.8 ± 4.51 | 3196 | |||||||
| [43] | (Single center) China | Jan 2004 to Apr 2014 | VTS | 32.04 ± 3.62 | 138 | One gestational sac/fetal with cardiac activity lost before 12 weeks of gestation | Dichorionic twins | Not mentioned | 8 |
| Original singletons | 31.57 ± 3.90 | 1936 | |||||||
| [42] | (Single center) China | Jan 1998 to Dec 2010 | VTS group | Unknown | 243 | One gestational sac without identification of intrauterine growth stage was lost during whole pregnancy period | Not mentioned | Not mentioned | 6 |
| Original singletons | Unknown | 2553 | |||||||
| [34] | (National registry) Sweden | 2002–2006 | VTS group | 35.2 ± 4.2 | 175 | One or more gestational sacs without identification of intrauterine growth stage were lost during whole pregnancy period | Not mentioned | Autologous embryos | 8 |
| Original singletons | 35.5 ± 3.9 | 2715 | |||||||
| [1] | (Single center) Israel | 1999–2007 | VTS group | 32.8 ± 5.1 | 57 | One gestational sac/fetal with cardiac activity lost before 12 weeks of gestation | Dichorionic twins | Not mentioned | 8 |
| Original singletons | 32.4 ± 5.3 | 171 | |||||||
| [23] | (Single center) Egypt | 2003–2005 | VTS group | 30.7 ± 4.5 | 206 | One empty gestational sacs or a fetus with no heartbeat was lost before 12 weeks of gestation | Dichorionic twins | Not mentioned | 7 |
| Original singletons | 31.2 ± 5.1 | 1764 | |||||||
| [13] | (National registry) Denmark | Apr 2004 to Jan 2006 | VTS group | 33.6 ± 4.3 | 47 | One gestational sac without identification of intrauterine growth stage was lost before 8 weeks of gestation | Not mentioned | Not mentioned | 8 |
| Original singletons | 32.8 ± 4.0 | 897 | |||||||
| [35] | (Single center) Austria | Jan 1999 to Dec 2005 | VTS group | 33.9 ± 4.3 | 46 | One gestational sac without identification of intrauterine growth stage was lost during whole pregnancy period | Not mentioned | Not mentioned | 6 |
| Original singletons | 33.9 ± 4.1 | 92 | |||||||
| [19] | (national registry) Italy | Jan 1992 to Jun 2004 | VTS group | Unknown | 84 | One gestational sac without identification of intrauterine growth stage was lost at or before 14 weeks | Dichorionic twins | Donor unknown | 6 |
| Original singletons | Unknown | 602 | |||||||
| [7] | (National registry) USA | 2003–2005 | VTS group | 37.6 ± 5.3 | 55 | One empty gestational sacs or a fetus with no heartbeat was lost at or before 14 weeks | Dichorionic twins | Not mentioned | 8 |
| Original singletons | 38.4 ± 4.6 | 168 | |||||||
| [28] | (Single center) Denmark | Jan 1995 to Dec 2001 | VTS group | 33.3 ± 3.9 | 642 |
Early vanishing One empty gestational sac or a fetus without fetal heart beat was lost before 8 weeks of gestation All vanish One gestational sac/fetus without identification of intrauterine growth stage was lost during whole pregnancy period |
Not mentioned | Not mentioned | 8 |
| Original singletons | 33.3 ± 3.7 | 5237 | |||||||
| [10] | (Single center) USA | Jul 1976 to Aug 2000 | VTS group | Age matched | 140 | One embryo without identification of intrauterine growth stage was lost during whole pregnancy period | Dichorionic twins | Autologous embryos | 7 |
| Original singletons | 4683 |
VTS, vanishing twin syndrome; NOS, Newcastle–Ottawa Scale; UK, the United Kingdom; USA, the United States of America
*The maternal age is significant different between two groups
aThe number of participants
Characteristics of included studies
Three studies provided information on the demographic characteristics of neither singleton pregnancies with VTS nor original singletons [9, 19, 42]. Four studies only included women who underwent autologous embryo(s) transfers [10, 27, 33, 34]. Ten studies excluded monochorionic twin pregnancies [1, 7, 10, 19, 21, 23, 24, 27, 33, 43]. In included twelve studies, there is no significant difference in maternal age between VTS and original singletons groups [1, 7, 10, 13, 21, 23, 24, 27, 28, 34, 35, 43].
Although all included studies reported the obstetric outcomes of singleton pregnancies diagnosed with VTS, the VTS definition varied in the aspect of intrauterine growth stage. VTS in two studies was defined as cases where two gestational sacs with cardiac activity were detected between 6 and 7 weeks and only one with cardiac activity was detected thereafter [1, 43]. VTS in three studies was defined as the spontaneous reduction of an empty gestational sac or fetus with no cardiac activity [7, 23, 28]. The VTS definition also varied in aspect of vanishing timing. Among remaining studies that did not elaborate the intrauterine growth stage, eight excluded cases in which VTS happened after 14 weeks [7, 10, 13, 19, 23, 24, 28, 33]. Moreover, the definition of VTS also varied in terms of initial number of gestational sac. Three studies included both triple-to-singleton VTS and twin-to-singleton VTS [10, 27, 42]; two studies did not clarify the initial number of gestational sac in VTS group [22, 34]. The remaining studies only included VTS with initial number of two.
SGA was defined as birth weight less than 2 standard deviations of the mean for that gestation [34] or less than the 10th percentile [1, 7, 10, 22, 24, 35] according to gestational age in a reference population.
Synthesis of results
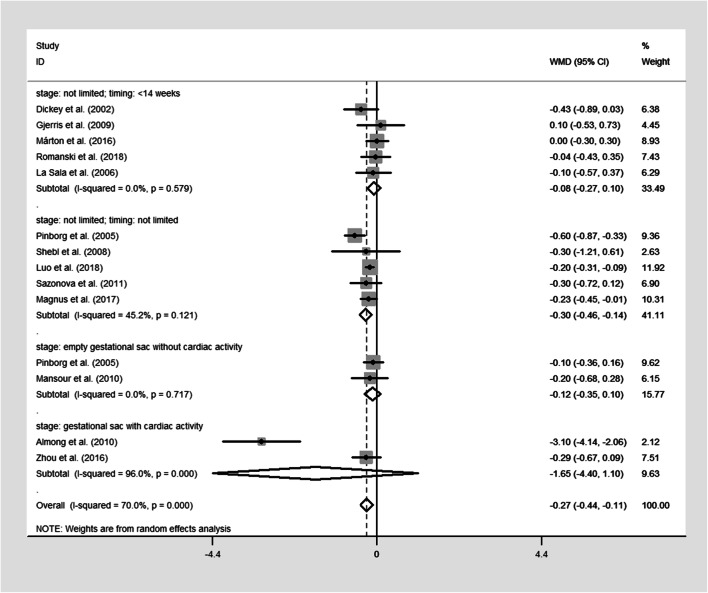
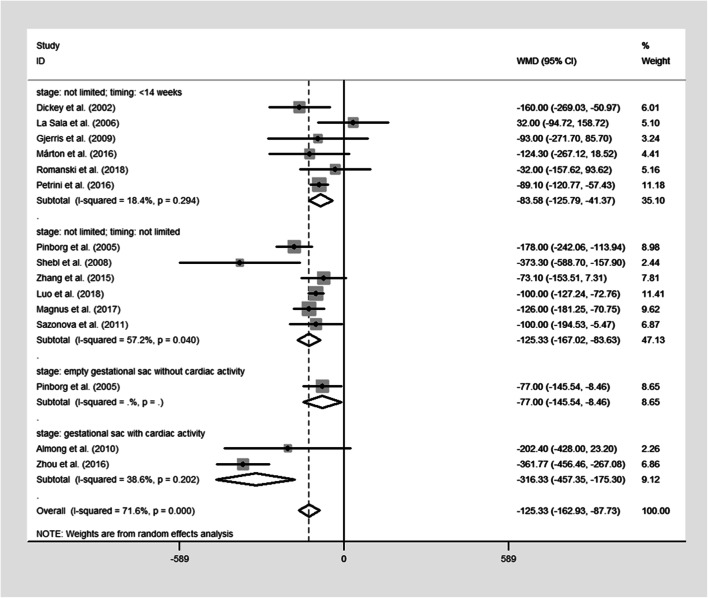
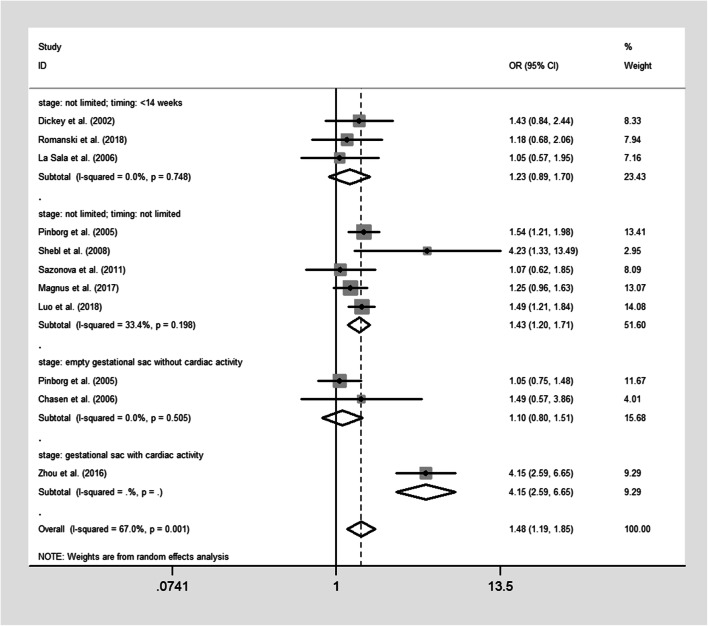
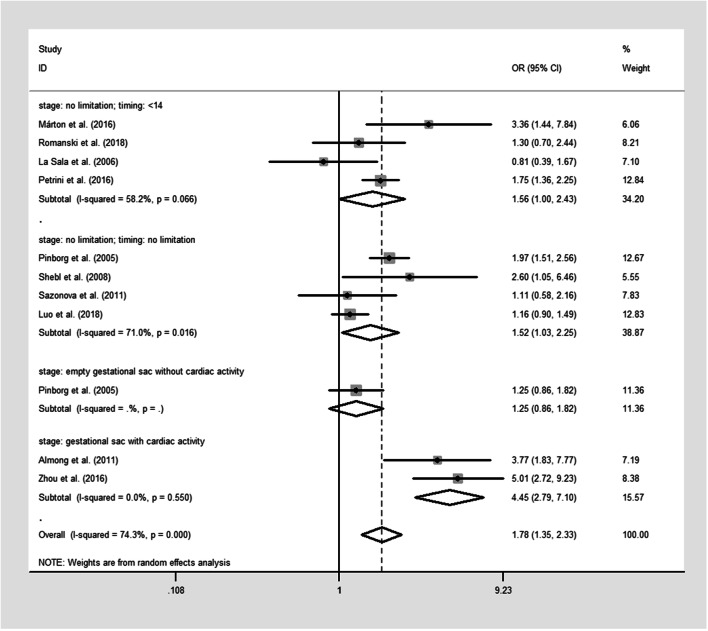
The pooled results were reported in Table 2 and Figs. 2, 3, 4, and 5. Singleton pregnancies with VTS are associated with significantly shorter GA [WMD, − 0.27; 95% CI, − 0.44, − 0.11 I2 = 72%; Fig. 2] and lower BW [WMD, − 125.33; 95% CI, − 162.93, − 87.73; I2 = 73%; Fig. 3] than original singleton pregnancies. Moreover, the risks of PTB [OR, 1.48; 95% CI, 1.19, 1.85; I2 = 69.5%; Fig. 4], VPTB [OR, 2.04; 95% CI, 1.43, 2.51; I2 = 15%], LBW [OR, 1.78; 95% CI, 1.35, 2.33; I2 = 76%; Fig. 5], VLBW [OR, 2.09; 95% CI, 1.39, 3.14; I2 = 32%], SGA [OR, 1.81; 95% CI, 1.14, 2.87; I2 = 77%], and mortality [OR, 3.07; 95% CI, 1.63, 5.80; I2 = 0%] were higher in the singletons with VTS than original singletons. No significant difference was observed between two groups for APH [OR, 1.53; 95% CI, 0.83, 2.88; I2 = 0%], GHD [OR, 1.20; 95% CI, 0.67, 2.18; I2 = 35%], and congenital anomalies [OR, 1.23; 95% CI, 0.88, 1.73; I2 = 37.9%]
Table 2.
Pooled results and judgments of the QoE for obstetrical outcomes following singletons with VTS versus original singletons
| All studies | Sensitivity analysisa | |||||||||
| N |
Patients, n VTS vs. controlb |
WMD (95% CI)c | I2 | N |
Patients, n VTS vs. control |
WMD (95% CI) | I2 | Interpretation | QoEd | |
| GA | 13 | 3780 vs. 49,763 | − 0.27 [− 0.44, − 0.11] | 72 | 10 | 3067 vs. 32,986 | − 0.34 [− 0.58, − 0.10] | 78 | Reductions are worse | Very lowf |
| BW | 14 | 4719 vs. 54,819 | − 125.33 [− 162.93, − 87.73] | 73 | 10 | 3714 vs. 34,418 | − 141.07 [− 189.04, − 93.11] | 75 | Reductions are worse | Very lowf |
|
Events/patients, n VTS vs. control |
OR (95% CI) e |
Events/patients, n VTS vs. control |
OR (95% CI) | |||||||
| PTB | 10 | 363/2722 vs. 4177/46810 | 1.48 [1.19, 1.85] | 70 | 7 | 276/2009 vs. 2633/30078 | 1.74 [1.28, 2.36] | 73 | Reductions are worse | Very lowf |
| VPTB | 7 | 54/1993 vs. 382/29892 | 2.04 [1.43, 2.51] | 15 | 6 | 52/1909 vs. 367/29290 | 2.13 [1.49, 3.05] | 16 | Reductions are worse | Low |
| LBW | 10 | 335/2932 vs. 2135/29532 | 1.78 [1.35, 2.33] | 76 | 8 | 314/2802 vs. 2046/28838 | 1.96 [1.42, 2.72] | 78 | Reductions are worse | Very lowf |
| VLBW | 9 | 67/2835 vs. 261/28734 | 2.09 [1.39, 3.14] | 32 | 7 | 54/2705 vs. 231/28040 | 2.18 [1.42, 3.34] | 30 | Reductions are worse | Low |
| SGA | 8 | 159/1393 vs. 1875/26742 | 1.81 [1.14, 2.87] | 77 | 6 | 74/679 vs. 420/9189 | 1.90 [0.96, 3.74] | 79 | Reductions are worse | Very lowf |
| Congenital anomalies | 4 | 100/1110 vs. 827/11245 | 1.23 [0.88, 1.73] | 35 | 3 | 67/858 vs. 521/7407 | 1.01 [0.77, 1.32] | 0 | No difference | Very lowg |
| Mortality | 3 | 13/955 vs. 44/9888 | 3.07 [1.63, 5.80] | 0 | 3 | 13/955 vs. 44/9888 | 3.07 [1.63, 5.80] | 0 | Reductions are worse | Lowg h |
| GHD | 4 | 25/433 vs. 215/4130 | 1.20 [0.67, 2.18] | 35 | 4 | 25/433 vs. 215/4130 | 1.20 [0.67, 2.18] | 35 | No difference | Very lowg |
| APH | 3 | 14/367 vs. 88/3835 | 1.54 [0.83, 2.88] | 0 | 3 | 14/367 vs. 88/3835 | 1.54 [0.83, 2.88] | 0 | No difference | Very lowg |
N, no. of studies; OR, odds ratio; WMD, weight mean difference; CI, confidence interval; GA, gestational age at delivery; BW, birth weight; PTB, preterm birth (< 37 weeks); VPTB, very preterm birth (< 32 weeks); LBW, low birth weight (< 2.5 kg); VLBW, very low birth weight (< 1.5 kg); SGA, small for gestational age, APH, antepartum hemorrhage; GHD, gestational hypertensive diseases
aSensitivity analysis restricted eligibility to studies deemed to be of high quality
bThe number of participants, events, and proportions considered all women included in these studies
cA WMD < 0 suggest an significantly lower of this outcome in singleton with VTS compared with original singleton
dAll outcomes were downgraded two levels because evidence come from observational studies
eA OR > 1.0 suggest an increased risk of this outcome in singleton with VTS compared with original singleton
fDowngraded one level because of inconsistency
gDowngraded one level because of imprecision
hUpgraded one level because of large effects
Fig. 2.
Forest plot of the included studies reporting data on gestation age (GA)
Fig. 3.
Forest plot of the included studies reporting data on mean birthweight (BW)
Fig. 4.
Forest plot of the included studies reporting data on preterm birth (< 37 weeks) (PTB)
Fig. 5.
Forest plot of the included studies reporting data on low birth weight (< 2500 g) (LBW)
Risk of bias across studies
All included studies were of observational design. The risks of bias of included studies were reported in Table 1. The risk of significant bias across studies regarding the primary outcome was rejected by Egger’s test (P = 0.079 for GA and P = 0.381 for BW) and visual inspection of the funnel plots (Fig. 6).
Fig. 6.
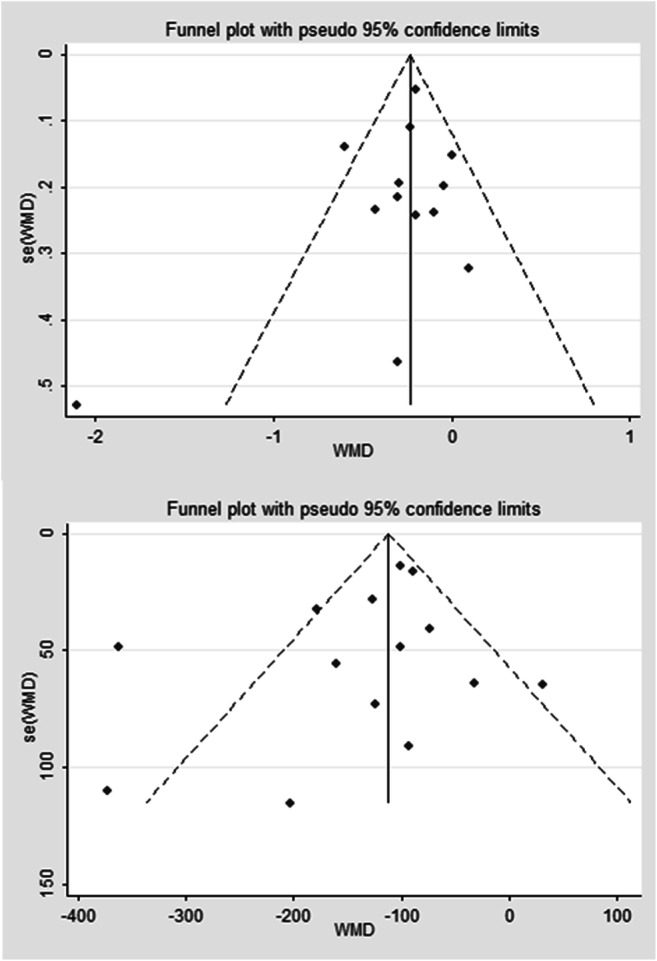
Funnel plot for publication bias for the studies included in this meta-analysis. a gestational age (GA); b mean birth weight (BW)
Additional analyses
The first sensitivity analysis restricting eligibility to studies deemed to be of high quality was reported in Table 2. The results from the first sensitivity analysis did not alter the direction or magnitude of the observed effects except for the observed effect of SGA.
The results from the second sensitivity analysis, which restricted to studies that only included women who underwent autologous embryo transfer, showed that there was no difference between singletons with VTS and original singletons for GA (three studies; 8611 participants; OR, − 0.23; 95% CI, − 0.48, 0.01; I2 = 0%) and PTB (three studies; 9220 participants; OR, 1.48; 95% CI, 0.58, 2.58; I2 = 67.5%). However, singletons with VTS were associated with significantly lower BW (four studies; 12,660 participants; OR, − 91.94; 95% CI, − 120.15, − 63.72; I2 = 0%) and higher risk of LBW (three studies; 8404 participants; OR, 1.60; 95% CI, 1.27, 2.01; I2 = 2.7%) than original singletons.
The results from the third sensitivity analysis, which restricted to studies that excluded all monochorionic twin pregnancies, showed that all outcomes did not alter the direction or magnitude of the observed effects: singletons with VTS were associated with significantly shorter GA (eight studies; 26,436 participants; WMD, − 0.41; 95% CI, − 0.71, − 0.11; I2 = 80.7%), lower BW (nine studies; 29,201 participants; WMD, − 140.86; 95% CI, − 202.28, − 79.43; I2 = 81.5%), and higher risks of PTB (seven studies; 26,340 participants; OR, 1.91; 95% CI, 1.26, 2.88; I2 = 75.1%) and LBW (seven studies; 25,449 participants; OR, 1.94; 95% CI, 1.27, 2.95; I2 = 81.7%) than original singletons.
The results from the fourth sensitivity analysis showed that singletons with VTS are associated with significantly shorter GA (ten studies; 35,296 participants; WMD, − 0.37; 95% CI, − 0.62, − 0.13; I2 = 78%), lower BW (ten studies; 37,372 participants; WMD, − 160.95; 95% CI, − 212.58, − 109.31; I2 = 77%), and higher risks of PTB (seven studies; 31,327 participants; OR, 1.78; 95% CI, 1.29, 2.44; I2 = 71%) and LBW (eight studies; 30,880 participants; OR, 2.11; 95% CI, 1.52, 2.94; I2 = 78%) than original singletons, which indicated that all outcomes did not alter the direction or magnitude of the observed effects.
The first subgroup based on initial number of gestational sac found that significantly shorter GA (WMD, − 0.25; 95% CI, − 0.44, − 0.06; I2 = 0%), lower BW (WMD, − 98.58; 95% CI, − 124.84, − 72.32; I2 = 0%), and significantly higher risk of LBW (OR, 1.56; 95% CI, 1.05, 2.29; I2 = 36%) were observed between VTS singletons with 3 or more initial gestational sacs versus original singleton. Moreover, restricting the initial number to two did not alter the direction or magnitude of the effects observed in the first subgroup.
The second subgroup analysis was able to explain the observed heterogeneity and/or identify a specific subgroup that alters the direction or magnitude of the observed effects. No significant difference in GA (Fig. 2), PTB (Fig. 4), and LBW (Fig. 5) between singletons with VTS and original singletons was revealed by the subgroup analysis on the studies that only defined VTS as the spontaneous reduction of empty gestational sac or fetus with no cardiac activity. Moreover, no difference in GA (Fig. 2), PTB (Fig. 4), and LBW (Fig. 5) was observed between singletons in which VTS occurred at or before 14 weeks without identification of intrauterine growth stage and original singletons.
The third subgroup analysis based on the vanishing timing and developmental stage, but excluded studies that did not restrict the initial number of gestational sac to two did not alter the direction or magnitude of the effects observed in the second subgroup.
The fourth subgroup analysis based on maternal age found that no difference in GA (WMD, − 0.30; 95% CI, − 0.72, 0.12) and PTB (OR, 1.16; 95% CI, 0.72, 1.86; I2 = 0%) was revealed by the subgroup analysis on studies in which mean women’s age is equal to or more than 35 years. However, significantly shorter GA (WMD, − 0.39; 95% CI, − 0.70, − 0.09; I2 = 82%), lower BW (WMD, − 196.66; 95% CI, − 285.26; − 108.05; I2 = 83%), and significantly higher risk of PTB (OR, 2.18; 95% CI, 1.39, 3.43; I2 = 83%) and LBW (OR, 2.51; 95% CI, 1.56; 4.07; I2 = 83%) were observed by the subgroup analysis on studies in which mean women’s age is less than 35 years.
The additional subgroup analysis separating studies according to SGA definition showed that no difference in SGA was observed between two groups when SGA was defined as birth weight less than 2 standard deviations of the mean for that gestation (two studies; 3788 participants; OR, 1.22; 95% CI, 0.70, 2.12; I2 = 8.7%). However, if SGA was defined as less than the 10th percentile according to gestational age in a reference population, singletons with VTS were associated with a higher risk of SGA than original singletons (six studies; 22,453 participants; OR, 2.11; 95% CI, 1.18, 3.77; I2 = 81.7%).
Discussion
Couples who underwent VTS often worry about the health of the survivors. Present study reveals that the prognosis of singletons with VTS is mainly determined by vanishing timing and intrauterine growth stage. Surviving singletons following VTS that happened before 14 weeks, regardless of growth stage, have similar obstetric outcomes, including GW, PTB, and LBW, than original singletons. On the contrary, VTS that happened after 14 weeks was associated with significantly lower shorter GA, lower BW, and higher risks of PTB and LBW than original singletons. When the sac reduced in VTS was an empty gestational sac, there would be no difference in GW, PTB, and LBW between singleton and singletons with VTS, whereas the loss of a fetus with cardiac activity is associated with significant adverse obstetric outcomes.
The underlying mechanisms of the fact that adverse outcomes were more prevalent following pregnancies with VTS should be discussed in two major aspects. The first and foremost aspect is the potential direct impacts including reabsorption of nonviable fetoplacental tissues [3]. Reabsorption of necrotic fetoplacental tissue not only results in increased release of proinflammatory cytokine and prostaglandin, initiating an inflammatory process, but also remodels the fetoplacental blood flow [9, 23]. Consequently, these changes temporarily decrease the nutrition supplement to the surviving fetus, resulting in relative placental insufficiency.
Taking the direct impact of VTS into consideration, several clinical or ultrasound variables seem to be important to the outcomes of pregnancies with VTS. The first is monochorionic twins, which was associated with a higher level of direct impact of VTS than dichorionic twins, for inter-twin vascular anastomoses are invariably present in all monochorionic placentas [6]. However, even when we restricted eligibility to studies that excluded all monochorionic twins, the singletons with VTS were still observed to be associated with higher risks of adverse outcomes than original singletons. The second variable is the vanishing timing [24]. Theoretically, the earlier VTS happens, the better the prognosis of survival co-twin gets. In line with this, we observed that no difference in GA, PTB, and LBW was observed between singletons in which VTS occurred at or before 14 weeks and original singletons. Recent studies have found that more than 90% VTS occurs within the first 12 weeks of pregnancy [21]; therefore, the prognosis of nearly all VTS cases may be similar to original singletons. The number of gestational sac vanished is the third variable influencing the impact of VTS. Although VTS can occur on any initial number of gestational sacs, the number of studies that included VTS singletons with 3 or more initial gestational sacs is limited. Given the unfavorable outcomes related to triplets and higher order pregnancies, twins are far more prevalent in pregnancies assisted by ART [8, 12]. Therefore, current clinical concern focuses more on the VTS in twin pregnancies. In the present analysis, only five included studies involved participants with 3 or more initial gestational sacs, and the number of these participants was very limited. Subgroup analysis based on initial numbers showed that for VTS singletons with an initial number of 3 or more and of 2, both situation were associated with high risk of adverse obstetric outcomes than original singletons. The fourth variable is the maternal age as advanced maternal age is well known to be associated with higher rates of adverse maternal and neonatal outcomes, including PTB and LBW [30]. The reasons we speculated why maternal age influence the effect of VTS are as follows. The first reason is the degradation of reproductive apparatus of women with advanced maternal age. It is well known that women with advanced maternal age are associated with a higher rate of adverse outcomes, including PTB, LBW, perinatal mortality, and stillbirth, which may be explained by the physio-pathological changes regarding the female reproductive apparatus that come with aging. In other words, temporary placental insufficiency caused by VTS may have less adverse effects on obstetric outcomes than physio-pathological changes of female reproductive apparatus caused by advanced maternal age. The temporary placental insufficiency may not have sufficient ability to significantly influence the outcomes of women with advanced maternal age. The second reason may explain this difference is that the VTS occurs earlier in women with advanced maternal age as physio-pathological changes of female reproductive apparatus of those women easily induce the VTS occurrence. The earlier the VTS occur, the smaller the tissues absorbed. The third reason is that the number of studies included in “mean maternal age ≥ 35 years” is limited. Therefore, further studies are needed to explore how maternal age influences the effect of VTS.
The second aspect that connects to the adverse obstetric outcomes following VTS is the occurrence of varying pathologies at different time points during the gestation [33]. Recent studies focusing on the mechanisms of VTS occurrence have indicated that the low quality of embryo transferred is attributed to losses of empty gestational sac [26, 41]. On the contrary, the demise of a gestational sac is more likely to be caused by factors [18, 20, 29, 36]. These mechanisms allow us to hypothesize that loss of gestational sac with cardiac activity is associated with longer period of insufficient nutrition supplement than the loss of empty gestational sac. Indeed, we first observed that the heterogeneity is dramatically reduced after excluding the studies that defined VTS as cases where two fetal pulses were detected between 6 and 7 weeks and only one fetal pulse was detected thereafter. Secondly, no differences in GA, PTB, and LBW were observed between original singletons and singletons survived after empty gestational sacs vanished.
Comparison with other meta-analysis or reviews
Only one meta-analysis evaluating the obstetric outcomes between singletons with VTS and original singletons was identified [37]. The Sun’s review concluded that singletons with VTS were associated with slightly higher risks of LBW and VPTB than original singletons. However, the Sun’s review did not include several of the observational studies included in our review [7, 13, 21, 22, 24, 28, 34], four of which [7, 13, 28, 34] were published before 2016, the end date of Sun’s search window. Additionally, Sun did not restrict eligibility to studies where only women who had autologous embryo(s) transfer were included or monochorionic twin pregnancies were excluded when drawing their conclusions. Finally, this review did not examine potential variables to understand their impacts on the effects of VTS, judging from the clinically and methodologically heterogeneous trials they reviewed. In contrast, the abundance of studies we obtained allowed us to examine the impact of variables.
Limitations
Our study does have certain limitations. The first limitation is that all included studies are observational studies. Due to the natural process of VTS, it would not be ethically justified to perform a randomized study to explore how VTS influences the obstetric outcomes. In other words, no randomized study can be made in the field of determining the effect of VTS. Due to the inherent methodological limitations of observational studies, some potential confounding factors are carefully scrutinized in order to avoid bias. Therefore, we performed several sensitivity analyses to eliminate the influence of confounding factors.
The second limitation is that the definition of VTS is different among included studies. Due to the uncertain nature of VTS itself, it is impossible to control the occurrence of VTS. Although no artificial design exists, nearly all studies on the topic of VTS artificially divided VTS cases according to the vanishing timing and developmental stage when VTS occurs. According to the definition of each study, we divided all studies into four groups, and the subgroup analysis based on these four groups not only explain the observed heterogeneity but also identify a specific subgroup that alters the direction or magnitude of the observed effects, which are helpful in clinical counseling.
The third limitation of the current review is that we were not able to estimate the longer-term survival and handicap rates among the surviving infants. Several studies reported that infant mortality and handicap rates are highly dependent on gestational age at delivery. Therefore, the influence of VTS over the mortality and handicap rates is likely to be reflected by the number of VPTB infants in each group.
The fourth limitation is the long time span of this meta-analysis. The publish year of included studies ranged from 2002 to 2019. In nearly two decades the ultrasonic diagnosis has been vastly improved and extensively utilized, allowing more accurate detection of early stage VTS before week 8. The existing studies focusing on the incidence of VTS are rendered obsolete. Meanwhile, increased clinical awareness to VTS and associated treatment strategy have been improving the prognosis. Therefore, further studies are required for providing more detailed information about frequency of VTS phenomenon and exploration into its treatment strategies, especially among IVF/ICSI pregnancies.
Conclusion
Current evidence shows that the obstetric outcomes of singletons with VTS are highly dependent on the definition of VTS, precisely, the vanishing timing and intrauterine growth stage. If VTS was defined as loss of an empty gestational sac or defined as loss of a gestational sac that occurs at or before 14 weeks without identification of intrauterine growth stage, no differences in the GA, PTB, and LBW would be observed between singletons with VTS versus original singletons. These results may help clinical counseling, for VTS at or before 14th week, or VTS with spontaneous reduction of an empty sac regardless of timing, no immediate measures would be required. On the contrary, significantly shorter GW and lower BW, as well as higher risks of PTB and LBW, were observed when VTS occurred after 14 weeks or a fetus with cardiac activity was reduced. In these scenarios, the patients should look for close obstetrical care.
Funding information
This research was financially supported by the National Natural Science Funding of China (Nos. 81673224; 81273018; 30700654), the Natural Science Funding of Shaanxi Province (Nos. 2019JM029; 2015JM8436), the Science Funding of Health Department, Shaanxi Province (2012D58), and the Fundamental Research Funds for the Central University (XJJ 2011024).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yi-xin Li and Tian-ze Sun contributed equally to this work.
References
- 1.Almog B, Levin I, Wagman I, Kapustiansky R, Lessing JB, Amit A, Azem F. Adverse obstetric outcome for the vanishing twin syndrome. Reprod BioMed Online. 2010;20:256–260. doi: 10.1016/j.rbmo.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 2.Barton SE, Missmer SA, Hornstein MD. Twin pregnancies with a ‘vanished’ embryo: a higher risk multiple gestation group? Hum Reprod. 2011;26:2750–2753. doi: 10.1093/humrep/der221. [DOI] [PubMed] [Google Scholar]
- 3.Bass HN, Oliver JB, Srinivasan M, Petrucha R, Ng W, Lee JES. Persistently elevated AFP and AChE in amniotic fluid from a normal fetus following demise of its twin. Prenat Diagn. 1986;6:33–35. doi: 10.1002/pd.1970060105. [DOI] [PubMed] [Google Scholar]
- 4.Bhandari S, Agrawal P, Ganguly I, Singh A, Gupta N. Perinatal outcome in assisted reproductive pregnancies: comparative analysis of reduced versus unreduced gestation. Int J Reprod Med. 2016;2016:7504609. doi: 10.1155/2016/7504609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brambati B, Tului L, Camurri L, Guercilena S. First-trimester fetal reduction to a singleton infant or twins: outcome in relation to the final number and karyotyping before reduction by transabdominal chorionic villus sampling. Am J Obstet Gynecol. 2004;191:2035–2040. doi: 10.1016/j.ajog.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Chang YL, Chao AS, Peng HH, Chang SD, Su SY, Chen KJ, Wang TH. Effects of inter-twin vascular anastomoses of monochorionic twins with selective intrauterine growth restriction on the contents of placental mitochondria DNA. BMC Pregnancy Childbirth. 2018;18:74. doi: 10.1186/s12884-018-1702-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chasen ST, Luo G, Perni SC, Kalish RB. Are in vitro fertilization pregnancies with early spontaneous reduction high risk? Am J Obstet Gynecol. 2006;195:814–817. doi: 10.1016/j.ajog.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 8.Connolly MP, Hoorens S, Chambers GM. ESHRE reproduction and society task force. The costs and consequences of assisted reproductive technology: an economic perspective. Hum Reprod Update. 2010;16(6):603–13. 10.1093/humupd/dmq013. [DOI] [PubMed]
- 9.Davies MJ, Rumbold AR, Whitrow MJ, Willson KJ, Scheil WK, Mol BW, Moore VM. Spontaneous loss of a co-twin and the risk of birth defects after assisted conception. J Dev Orig Health Dis. 2016;7:678–684. doi: 10.1017/S2040174416000301. [DOI] [PubMed] [Google Scholar]
- 10.Dickey RP, Taylor SN, Lu PY, Sartor BM, Storment JM, Rye PH, Pelletier WD, Zender JL, Matulich EM. Spontaneous reduction of multiple pregnancy: incidence and effect on outcome. Am J Obstet Gynecol. 2002;186:77–83. doi: 10.1067/mob.2002.118915. [DOI] [PubMed] [Google Scholar]
- 11.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed]
- 12.European IVF-Monitoring Consortium (EIM); European Society of Human Reproduction and Embryology (ESHRE), Kupka MS, et al. Assisted reproductive technology in Europe, 2011: results generated from European registers by ESHRE. Hum Reprod. 2016;31(2):233–48. 10.1093/humrep/dev319. [DOI] [PubMed]
- 13.Gjerris AC, Loft A, Pinborg A, Christiansen M, Tabor A. The effect of a ‘vanishing twin’ on biochemical and ultrasound first trimester screening markers for Down’s syndrome in pregnancies conceived by assisted reproductive technology. Hum Reprod. 2009;24:55–62. doi: 10.1093/humrep/den362. [DOI] [PubMed] [Google Scholar]
- 14.Gupta S, Fox NS, Feinberg J, Klauser CK, Rebarber A. Outcomes in twin pregnancies reduced to singleton pregnancies compared with ongoing twin pregnancies. Am J Obstet Gynecol. 2015;213:580.e1–580.e5. doi: 10.1016/j.ajog.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 15.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–94. 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed]
- 16.Haas J, Mohr Sasson A, Barzilay E, Mazaki Tovi S, Orvieto R, Weisz B, Lipitz S, Yinon Y. Perinatal outcome after fetal reduction from twin to singleton: to reduce or not to reduce? Fertil Steril. 2015;103:428–432. doi: 10.1016/j.fertnstert.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed]
- 18.La Sala GB, Nicoli A, Villani MT, Gallinelli A, Nucera G, Blickstein I. Spontaneous embryonic loss rates in twin and singleton pregnancies after transfer of top- versus intermediate-quality embryos. Fertil Steril. 2005;84:1602–1605. doi: 10.1016/j.fertnstert.2005.05.045. [DOI] [PubMed] [Google Scholar]
- 19.La Sala GB, Villani MT, Nicoli A, Gallinelli A, Nucera G, Blickstein I. Effect of the mode of assisted reproductive technology conception on obstetric outcomes for survivors of the vanishing twin syndrome. Fertil Steril. 2006;86:247–249. doi: 10.1016/j.fertnstert.2005.11.073. [DOI] [PubMed] [Google Scholar]
- 20.Levy B, Sigurjonsson S, Pettersen B, Maisenbacher MK, Hall MP, Demko Z, Lathi RB, Tao R, Aggarwal V, Rabinowitz M. Genomic imbalance in products of conception: single-nucleotide polymorphism chromosomal microarray analysis. Obstet Gynecol. 2014;124:202–209. doi: 10.1097/AOG.0000000000000325. [DOI] [PubMed] [Google Scholar]
- 21.Luo L, Cai B, Jie HY, Gao Y, Chen M, Zhou CQ, et al. Influence of spontaneous fetal reduction on dichorionic diamniotic twin pregnancy outcomes after in vitro fertilization: a large-sample retrospective study. J Matern Fetal Neonatal Med. 2018:1–6. [DOI] [PubMed]
- 22.Magnus MC, Ghaderi S, Morken NH, Magnus P, Romundstad Bente L, Skjærven R, et al. Vanishing twin syndrome among ART singletons and pregnancy outcomes. Hum Reprod. 2017:1–7. [DOI] [PMC free article] [PubMed]
- 23.Mansour R, Serour G, Aboulghar M, Kamal O, Al-Inany H. The impact of vanishing fetuses on the outcome of ICSI pregnancies. Fertil Steril. 2010;94:2430–2432. doi: 10.1016/j.fertnstert.2010.02.058. [DOI] [PubMed] [Google Scholar]
- 24.Márton V, Zádori J, Kozinszky Z, Keresztúri A. Prevalences and pregnancy outcome of vanishing twin pregnancies achieved by in vitro fertilization versus natural conception. Fertil Steril. 2016;106:1399–1406. doi: 10.1016/j.fertnstert.2016.07.1098. [DOI] [PubMed] [Google Scholar]
- 25.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patrizio P, Bianchi V, Lalioti MD, Gerasimova T, Sakkas D. High rate of biological loss in assisted reproduction: it is in the seed, not in the soil. Reprod BioMed Online. 2007;14:92–95. doi: 10.1016/S1472-6483(10)60769-9. [DOI] [PubMed] [Google Scholar]
- 27.Petrini AC, Pereira N, Lekovich JP, Elias RT, Spandorfer SD. Early spontaneous multiple fetal pregnancy reduction is associated with adverse perinatal outcomes in in vitro fertilization cycles. Womens Health (Lond) 2016;12:420–426. doi: 10.1177/1745505716658898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinborg A, Lidegaard O, La Cour Freiesleben N, Andersen AN. Consequences of vanishing twins in IVF/ICSI pregnancies. Hum Reprod. 2005;20:2821–2829. doi: 10.1093/humrep/dei142. [DOI] [PubMed] [Google Scholar]
- 29.Pinborg A, Lidegaard O, Freiesleben N, Andersen AN. Vanishing twins: a predictor of small-for-gestational age in IVF singletons. Hum Reprod. 2007;22:2707–2714. doi: 10.1093/humrep/dem225. [DOI] [PubMed] [Google Scholar]
- 30.Pinheiro RL, Areia AL, Mota Pinto A, Donato H. Advanced maternal age: adverse outcomes of pregnancy, a meta-analysis. Acta Medica Port. 2019;32:219–226. doi: 10.20344/amp.11057. [DOI] [PubMed] [Google Scholar]
- 31.Razaz N, Avitan T, Ting J, Pressey T, Joseph KS. Perinatal outcomes in multifetal pregnancy following fetal reduction. Cmaj. 2017;189:E652–Ee58. doi: 10.1503/cmaj.160722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rienzi L, Gracia C, Maggiulli R, LaBarbera AR, Kaser DJ, Ubaldi FM, Vanderpoel S, Racowsky C. Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum Reprod Update. 2017;23:139–155. doi: 10.1093/humupd/dmw038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romanski PA, Carusi DA, Farland LV, Missmer SA, Kaser DJ, Walsh BW, Racowsky C, Brady PC. Perinatal and peripartum outcomes in vanishing twin pregnancies achieved by In vitro fertilization. Obstet Gynecol. 2018;131:1011–1020. doi: 10.1097/AOG.0000000000002595. [DOI] [PubMed] [Google Scholar]
- 34.Sazonova A, Källen K, Thurin-Kjellberg A, Wennerholm UB, Bergh C. Factors affecting obstetric outcome of singletons born after IVF. Hum Reprod. 2011;26:2878–2886. doi: 10.1093/humrep/der241. [DOI] [PubMed] [Google Scholar]
- 35.Shebl O, Ebner T, Sommergruber M, Sir A, Tews G. Birth weight is lower for survivors of the vanishing twin syndrome: a case-control study. Fertil Steril. 2008;90:310–314. doi: 10.1016/j.fertnstert.2007.06.048. [DOI] [PubMed] [Google Scholar]
- 36.Silver RM, Varner MW, Reddy U, Goldenberg R, Pinar H, Conway D, Bukowski R, Carpenter M, Hogue C, Willinger M, Dudley D, Saade G, Stoll B. Work-up of stillbirth: a review of the evidence. Am J Obstet Gynecol. 2007;196:433–444. doi: 10.1016/j.ajog.2006.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun L, Jiang LX, Chen HZ. Obstetric outcome of vanishing twins syndrome: a systematic review and meta-analysis. Arch Gynecol Obstet. 2017;295:559–567. doi: 10.1007/s00404-017-4289-9. [DOI] [PubMed] [Google Scholar]
- 38.Van de Mheen L, Everwijn SM, Knapen MF, Haak MC, Engels MA, Manten GT, Zondervan HA, Wirjosoekarto SA, van Vugt JM, Erwich JJ. Pregnancy outcome after fetal reduction in women with a dichorionic twin pregnancy. Hum Reprod. 2015;30:1807–1812. doi: 10.1093/humrep/dev132. [DOI] [PubMed] [Google Scholar]
- 39.Vermey BG, Buchanan A, Chambers GM, Kolibianakis EM, Bosdou J, Chapman MG, Venetis CA. Are singleton pregnancies after assisted reproduction technology (ART) associated with a higher risk of placental anomalies compared with non-ART singleton pregnancies? A systematic review and meta-analysis. Bjog. 2019;126:209–218. doi: 10.1111/1471-0528.15227. [DOI] [PubMed] [Google Scholar]
- 40.Woo I, Hindoyan R, Landay M, Ho J, Ingles SA, McGinnis LK, Paulson RJ, Chung K. Perinatal outcomes after natural conception versus in vitro fertilization (IVF) in gestational surrogates: a model to evaluate IVF treatment versus maternal effects. Fertil Steril. 2017;108:993–998. doi: 10.1016/j.fertnstert.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 41.Yang R, Yang S, Li R, Chen X, Wang H, Ma C, Liu P, Qiao J. Biochemical pregnancy and spontaneous abortion in first IVF cycles are negative predictors for subsequent cycles: an over 10,000 cases cohort study. Arch Gynecol Obstet. 2015;292:453–458. doi: 10.1007/s00404-015-3639-8. [DOI] [PubMed] [Google Scholar]
- 42.Zhang YL, Wang XY, Wang F, Su YC, Sun YP. Clinical analysis of spontaneous pregnancy reduction in the patients with multiple pregnancies undergoing in vitro fertilization/intracytoplasmic sperm injection-embryo transfer. Int J Clin Exp Med. 2015;8:4575. [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou L, Gao X, Wu Y, Zhang Z. Analysis of pregnancy outcomes for survivors of the vanishing twin syndrome after in vitro fertilization and embryo transfer. Eur J Obstet Gynecol Reprod Biol. 2016;203:35–39. doi: 10.1016/j.ejogrb.2016.04.014. [DOI] [PubMed] [Google Scholar]