Abstract
Purpose
As obesity becomes increasingly prevalent, its impact on fertility has been a subject of great debate. Nearly all prior research is retrospective and evaluates obesity utilizing body mass index (BMI), which may overestimate adiposity in individuals with a greater amount of lean muscle and underestimate adiposity in those with less muscle mass.
Methods
We prospectively evaluated 2013 couples undergoing infertility treatment with in vitro fertilization (IVF). Percent body fat (%BF) was measured by use of a bioelectric impedance analysis (BIA) scale at baseline. BMI was also determined. Ovarian reserve parameters, ovarian response to controlled ovarian hyperstimulation, and semen analyses were measured in correlation with their %BF and BMI.
Results
Females classified as obese based on %BF or BMI had lower serum FSH. However, when the analysis was limited to women without PCOS (n = 1706), obesity based on %BF or BMI was associated with lower serum AMH. Female obesity—regardless of a PCOS diagnosis—did not affect number of mature oocytes retrieved. Males who were in obese %BF category were found to have lower TMSC compared with normal weight counterparts (p < 0.05); however, the observed decrease was not significant enough to limit the success of assisted reproductive technologies.
Conclusions
These findings suggest that while obesity may affect ovarian reserve in women variably depending on presence of PCOS, it does not affect number of mature oocytes available after COH. Similarly, while a high %BF in males is associated with lower TMSC, the observed difference is unlikely to affect IVF outcomes.
Keywords: Obesity, Adiposity, Male infertility, Female infertility
Introduction
Obesity is a global epidemic that has worsened in severity over the past several decades [1]. This disease not only represents a major risk factor for cardiovascular disease, cancer, and diabetes mellitus, but the World Obesity Federation also declared obesity to be its own entity as a chronic progressive disease [2]. Besides increased morbidity and mortality, obesity has been found to lead to social stigma and also imposes increased economic burden [3, 4]. Nowhere is this more urgent or prevalent than in the USA where 42.8% of the adult population was obese (body mass index (BMI) ≥ 30 kg/m2) in 2018 [5].
With more than half of reproductive-aged women in the US overweight (BMI 25.0 to 29.9 kg/m2) or obese [6], the relationship between obesity and fertility must be considered. In women, there has been evidence that obesity increases risks of infertility and poor pregnancy outcomes, even outside of the polycystic ovarian syndrome (PCOS) patient population [6]. This highlights the intrinsic risk from excess weight, separate from the sequelae of irregular menses associated with increased insulin resistance. Obesity has also been noted to affect male reproduction through several mechanisms including hypogonadotropic-hyperestrogenic hypogonadism, increased inflammation from excess adipokines, elevated testicular temperature, sexual dysfunction, and epigenetic mechanisms [7, 8].
Within the context of reproduction, an essential question regarding obesity is whether it affects gamete reserve and production. Evidence from animal models have illuminated potential mechanisms of obesity’s effect on gametogenesis. Obese male mice have been noted to have altered Leydig cell function resulting in impaired spermatogenesis [9]. Also in mice, ovaries are found to be insulin sensitive under normal weight conditions but can progress to an insulin-resistant state secondary to obesity [10]. High-fat diets in mice have been identified as causing altered oocyte mitochondrial morphology and function [11]. This finding is significant because a number of mouse models with global or oocyte-targeted deletion of genes that are required for mitochondrial function result in infertility and a phenotype consistent with diminished ovarian reserve [12–14]. Moreover, the IGF-1/insulin pathway and mitochondrial mechanisms are implicated in cellular senescence and aging, while caloric restriction delays this process [15, 16].
Human studies have also characterized abnormal embryologic patterns. One study identified a higher prevalence of oocyte spindle dysfunction after fertilization in severely obese women, while another identified altered embryo morphokinetics from overweight and obese women’s oocytes [17, 18]. While these studies inform potential mechanisms of diminished outcomes in obese patients after fertilization has occurred, the impact of obesity on human ovarian and testicular reserve and oocyte and sperm production requires investigation.
When investigating the effect of obesity on reproduction in human subjects, one of the challenges is choosing how to quantify obesity. While BMI has become the de facto method for measuring obesity, there are several ways this metric can be prone to error. Although meant to provide a risk estimation for comorbidities such as metabolic syndrome, BMI does not account for actual adiposity but is rather a surrogate marker [19]. Additionally, BMI is calculated by the same formula in both men and women; however, it has been demonstrated that women in general have higher percentages of adiposity compared with men at varying fitness levels without increased risk of metabolic disease [20]. In an effort to accurately isolate the impact of adiposity, bioelectric impedance analysis (BIA) represents an attractive option to measure percentage body fat (%BF), as it has been validated against BMI in the past for studying obesity [19].
To date, a number of studies investigated the impact of obesity on parameters of reproductive reserve and gonadal function [21–23]. However, most of these studies suffered from small sample size and retrospective design, and they focused on BMI alone. Therefore, a large-scale prospective investigation of the impact of obesity on gonadal parameters has not yet been done. The primary hypothesis of this prospective study was that obesity and increased adiposity would negatively impact quantitative and qualitative parameters of gamete production in men and women undergoing fertility treatment. The secondary aim of this study was to see if %BF may be a potentially superior method of evaluating obesity-related infertility risks compared with BMI.
Materials and methods
Patient population
All patients undergoing infertility treatment with in vitro fertilization (IVF) at the Reproductive Medicine Associates of New Jersey (RMANJ, Basking Ridge, NJ, USA) between June 15, 2016 and January 12, 2019 were offered the opportunity to participate in this prospective study. IRB approval was obtained to collect couples’ body composition and track IVF outcomes (IRB approved April 26, 2016: RMA1-16-189). The inclusion requirements were women aged 18 through 45 years old with a male partner providing spermatozoa. Exclusion criteria included use of gamete donation or a gestational carrier, presence of communicating hydrosalpinx, health contraindications to IVF, and planned use of preimplantation genetic testing for monogenic disease (PGT-M) or structural rearrangements (PGT-SR). The only compensations for participation were complementary reports of body composition generated by the InBody scale.
Determination of %BF and BMI
To determine percent body fat, all male and female participants were weighed on an InBody 770 Scale on the day of oocyte retrieval. The InBody scale measures adiposity through multifrequency BIA, quantifying direct impedance measurements from the user’s various body compartments [24]. This technology has been demonstrated as having as high as 99% correlation with dual-energy x-ray absorption (DEXA) in quantifying lean mass measurements and has also been found to have high accuracy in measuring body fat in a healthy population [24, 25]. Classification of %BF categories was used as previously determined by Zhu et al, which calculated mean %BF determined by BIA that most highly correlated with BMI [19]. In men, the %BF established as corresponding to established BMI cutoffs were as follows: < 17% representing underweight, 17.0–21.9% representing normal weight, 22.0–29.9% representing overweight, and ≥ 30% representing obese. In women, the %BF established as corresponding to established BMI cutoffs were as follows: < 25% representing underweight, 25.0–30.9% representing normal weight, 31.0–39.9% representing overweight, and ≥ 40% representing obese.
BMI data were collected on the same day and calculated as weight in kilograms divided by height in meters squared. Subjects were assigned to the BMI categories put forth by the Centers for Disease Control and World Health Organization: underweight (BMI < 18.5), normal weight (18.5–24.9), overweight (25.0–29.9), and obese (BMI ≥ 30) [26, 27]. These categories were the same for men and women.
Determination of ovarian reserve parameters
To quantify ovarian reserve, all participating female subjects had serum anti-Mullerian hormone level (AMH) and day 3 follicle stimulating hormone (FSH) levels measured as part of their initial infertility workup. An antral follicle count (AFC) was obtained on day 3 of the studied retrieval cycle.
Controlled ovarian stimulation and oocyte collection
Female patients presented to the office on day 3 of their menstrual cycle for bloodwork and an ultrasound to confirm they were ready to start gonadotropin medication for the purposes of an IVF cycle. These patients were stimulated according to a GnRH antagonist, GnRH agonist down-regulation, or GnRH agonist flare protocol at the discretion of their primary physician [28–30]. Gonadotropin dosing was also decided by each patient’s primary physician and titrated according to the results of serial monitoring of ultrasound, estradiol, and progesterone throughout stimulation. When optimal follicular recruitment was deemed to have occurred, patients self-administered an ovulation trigger with either urinary HCG, leuprolide acetate, or a combination of the two. Response to this trigger injection was measured by bloodwork and ultrasound the next morning. Vaginal oocyte retrieval was scheduled for 36 h after the time of initial ovulation trigger.
Vaginal oocyte retrieval took place under ultrasound guidance with needle aspiration of all follicles. The number of oocytes retrieved was recorded by an embryologist who counted the number of oocytes real time as oocytes were being mechanically separated from surrounding cumulus cells. Cumulus-oocyte complexes then underwent further mechanical denuding as well as chemical denuding with Origio SAGE media. The number of mature oocytes was then able to be quantified and was recorded.
Sperm analyses
In male subjects, the sperm concentration and total motile sperm count (TMSC) were calculated from the ejaculated sample produced for the purposes of IVF/ICSI. In the 95.6% of cases where a fresh ejaculated sample was used for IVF, male subjects were instructed to abstain from ejaculation 2 to 5 days prior to the day of their female partner’s retrieval. In the remainder of cases where a frozen ejaculated sample was used, male subjects were instructed to abstain from ejaculation 2 to 5 days prior to the day of collection. After recording the initial specimen volume, sperm quantification was performed by placing a 10 μL droplet of the ejaculated specimen on a Makler sperm counting chamber. A projected concentration and TMSC were then calculated according to the number of motile and total sperm on the Makler grid.
Statistical analysis
Demographic variables were calculated for female and male subjects, respectively. Ovarian reserve markers and response to gonadotropin stimulation were compared among the 4 adiposity groups: underweight, normal, overweight, and obese. Similarly, sperm concentration and TMSC were also compared among the 4 adiposity groups in men. It is important to note that the cut-offs for these %BF groups are sex specific as in Zhu et al.’s study [19].
Statistical analysis was performed using general linear models to assess the study variables and their association with changes in adiposity and BMI. The Kruskal-Wallis test was used to assess statistical significance among the groups, which is reflected in the unadjusted p values. The adjusted p values in the female subjects represent the overall effect of the weight variable on the generalized linear model while adjusting for age.
In comparing %BF with BMI as a metric of estimating obesity in women and men undergoing fertility treatment, it was important to identify patients where these two classifications were discordant. Two different “mismatch groups” were noted: the first were patients with a normal BMI but increased %BF, and the second were patients with overweight or obese BMI but normal %BF. To examine whether %BF captured the impact of obesity where BMI did not, outcome measures were compared between female patients deemed to be normal weight by both BMI and %BF versus mismatch patients called normal weight by BMI but overweight or obese by %BF (Figs. 1 and 2). In men, outcome measures were compared between those meeting overweight or obese criteria according to BMI and %BF versus subjects with an elevated BMI but normal %BF (as seen in men with high muscle content in Figs. 3 and 4). The Kruskal-Wallis test was used to assess statistical significance between the mismatch groups and the correctly matched groups.
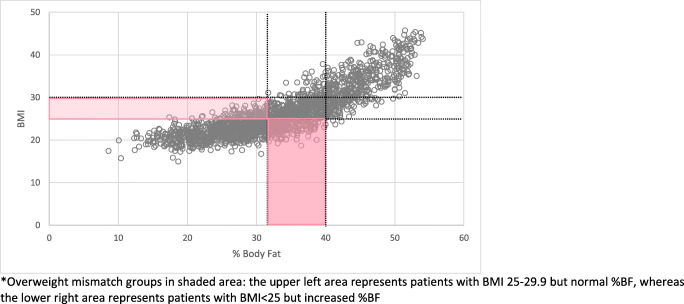
Fig. 1.
Overweight female percentage body fat vs. BMI. Overweight mismatch groups in shaded area: the upper left area represents patients with BMI 25–29.9 but normal %BF, whereas the lower right area represents patients with BMI
Fig. 2.
Obese female percentage body fat vs. BMI. Obese mismatch groups in shaded area: the upper left area represents patients with BMI ≥ 30 but normal %BF, whereas the lower right area represents patients with BMI
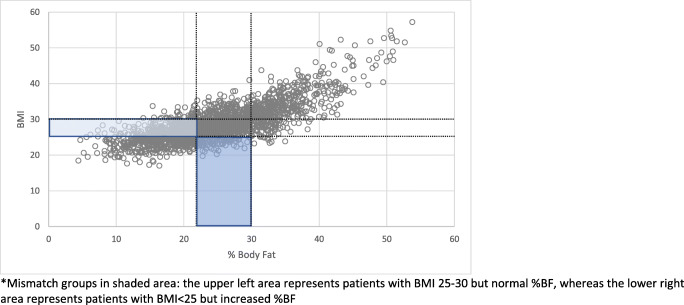
Fig. 3.
Overweight male percentage body fat vs. BMI. Mismatch groups in shaded area: the upper left area represents patients with BMI 25–30 but normal %BF, whereas the lower right area represents patients with BMI
Fig. 4.
Obese male percentage body fat vs. BMI. Obese mismatch groups in shaded area: the upper left area represents patients with BMI ≥ 30 but normal %BF, whereas the lower right area represents patients with BMI
Results
A total of 2013 infertile couples participated in this study. Mean female age was 35.4 years old (± 4.25) with female participants having a mean BMI of 26.2 (± 5.5) and mean %BF of 33.3% (± 8.8) (Table 1). In terms of markers of ovarian reserve: median AMH was 2.39 (IQR 1.2–4.3), median day 3 FSH was 7.3 (IQR 6.0–8.9), and median AFC was 14 (IQR 10–21). Mean male age was 36.9 years old (± 5.6) with male subjects having a mean BMI of 28.7 (± 5.2) and mean %BF of 25.0% (± 7.9) (Table 2). Median TMSC was 50.1 million sperm (IQR 17.8–105.7).
Table 1.
Female participant demographic info
| Mean age (years) | 35.4 ± 4.25* |
|---|---|
| Mean BMI (kg/m2) | 26.2 ± 5.5* |
| Mean % body fat | 33.3 ± 8.8* |
| Median AMH | 2.39 [IQR 1.2–4.3] |
| Median FSH | 7.3 [IQR 6–8.9 ] |
| Median AFC | 14 [IQR 10–21] |
*Represent standard deviations
Table 2.
Male participant demographic info
| Mean age (years) | 36.9 ± 5.6* |
|---|---|
| Mean BMI (kg/m2) | 28.7 ± 5.2* |
| Mean % body fat | 25.0 ± 7.9* |
| Median TMSC | 50.1 [IQR 17.8–105.7] |
*Represent standard deviations
Ovarian reserve and response to controlled ovarian hyperstimulation according to %BF are outlined in Table 3. When all patients (n = 2013) were included in the analysis, higher %BF was associated with a lower mean FSH. A lower serum AMH was also found to be associated with higher %BF. AFC was equivalent across all %BF groups after adjusting for age.
Table 3.
Ovarian response according to %BF category*
| All patients (n = 2013) | ||||||
|---|---|---|---|---|---|---|
| Metric | Underweight %BF < 25 N = 363 |
Normal %BF 25.0–30.9 N = 461 |
Overweight %BF 31.0–39.9 N = 715 |
Obese %BF ≥ 40 N = 474 |
p value unadjusted |
p value adjusted§ |
| AMH | 2.5 [1.3–4.4] | 2.3 [1.2–4.5] | 2.5 [1.3–4.4] | 2.3 [1.1–4.0] | 0.105 | 0.024† |
| FSH | 7.6 [6.3–9.2] | 7.6 [6.2–9.2] | 7.2 [6.0–8.9] | 7.0 [5.7–8.4] | < 0.001 | 0.048‡ |
| AFC | 15 [10.3–22] | 14 [10–20] | 14 [10–21] | 15 [10–21] | 0.418 | 0.202 |
| # oocytes retrieved | 12 [7–20] | 13 [8–21] | 13 [8–20] | 14 [8–20] | 0.589 | 0.619 |
| #M2 | 9 [5–15] | 9.5 [6–16] | 10 [6–16] | 10 [5–15] | 0.471 | 0.349 |
| PCOS patients excluded (n = 1706) | ||||||
| Metric |
Underweight %BF < 25 N = 322 |
Normal %BF 25.0–30.9 N = 412 |
Overweight %BF 31.0–39.9 N = 590 |
Obese %BF ≥ 40 N = 382 |
p value unadjusted |
p value adjusted§ |
| AMH | 2.2 [1.3–4.4] | 2.2 [1.2–3.8] | 1.9 [1.1–3.3] | 1.8 [0.8–3.5] | 0.002 | 0.014 |
| FSH | 8.18 [7.3–8.6] | 7.7 [6.4–9.5] | 7.6 [6.2–9.3] | 7.2 [5.9–8.5] | 0.001 | 0.080 |
| AFC | 13.5 [10–19] | 13 [10–19] | 13 [9–18] | 13 [9–183.] | 0.387 | 0.481 |
| # oocytes retrieved | 10 [6–21] | 11 [7–18] | 11 [7–19] | 12 [7–18] | 0.999 | 0.688 |
| #M2 | 8.5 [4–17] | 9 [5–14] | 9 [5–15] | 9 [5–14] | 0.959 | 0.734 |
*Categories derived from Zhu et al
†AMH difference (age adjusted) is between ≥ 40 and < 25% and between ≥ 40 and 31–39.9%
‡FSH difference (age adjusted) is between ≥ 40 and < 25% and between ≥ 40 and 25–30.9%
§p value represents comparison between 4 adiposity groups
When examining these same metrics according to BMI category (Table 4), FSH was again noted to be significantly decreased according to increasing BMI, while AMH was found to be similar between the groups. When analysis was based on BMI categories, AFC was noted to be higher in obese patients after adjusting for age.
Table 4.
Ovarian response according to BMI category
| All patients (n = 2013) | ||||||
|---|---|---|---|---|---|---|
| Metric | Underweight BMI < 18.5 N = 28 |
Normal BMI 18.5–24.9 N = 989 |
Overweight BMI 25.0–29.9 N = 582 |
Obese BMI ≥ 30 N = 414 |
p value unadjusted |
p value adjusted§ |
| AMH | 2.4 [1.5–5.6] | 2.5 [1.3–4.4] | 2.4 [1.2–4.1] | 2.3 [1.1–4.3] | 0.278 | 0.527 |
| FSH | 8.14 [7.1–8.6] | 7.6 [6.2–9.2] | 7.3 [5.9–8.9] | 6.9 [5.6–8.2] | < 0.001* | 0.001* |
| AFC | 14 [10.5–21] | 14 [10–20] | 14 [10–21] | 16 [10–22] | 0.205 | 0.016† |
| # oocytes retrieved | 11 [6–22] | 13 [7–20] | 13.5 [8–21] | 14 [8-20] | 0.411 | 0.041‡ |
| #M2 | 10 [4.5–18] | 9 [5–15] | 10 [6–16] | 10 [5–15] | 0.623 | 0.083 |
| PCOS patients excluded (n = 1706) | ||||||
| Metric |
Underweight BMI < 18.5 N = 25 |
Normal BMI 18.5–24.9 N = 870 |
Overweight BMI 25.0–29.9 N = 487 |
Obese BMI ≥ 30 N = 324 |
p value unadjusted |
p value adjusted§ |
| AMH | 2.2 [1.2–3.9] | 2.1 [1.1–3.7] | 2.1 [1.1–3.6] | 1.9 [0.84–3.3] | 0.004 | 0.002 |
| FSH | 7.6 [6.4–9.4] | 7.7 [6.4–9.5] | 7.6 [6.3–9.3] | 7.3 [6.3–9.3] | 0.007 | 0.242 |
| AFC | 14 [10–20] | 13 [10–18] | 13 [9–18] | 13 [9–18] | 0.015 | 0.027 |
| # oocytes retrieved | 10 [7–19] | 12 [7–19] | 11 [7–18] | 12 [7–18] | 0.438 | 0.886 |
| #M2 | 8 [5–14] | 9 [5–15] | 9 [5–14] | 9 [5–13] | 0.630 | 0.910 |
*FSH difference is between the obese and normal BMI categories even after adjusting for age
†AFC difference (age adjusted) is between obese and normal BMI and between obese and overweight BMI
‡Oocytes retrieved difference (age adjusted) is between normal and overweight BMI and between normal and obese BMI
§For age-adjusted analysis, variables log transformed and used in a generalized linear model with the p value shown representing the overall model effect of the adiposity variable
Upon limiting the analysis to patients who were not diagnosed with PCOS based on the Rotterdam criteria [31], serum FSH was no longer associated with %BF or BMI, while AMH was found to be lower in obese patients (Tables 3 and 4).
When assessed according to %BF, the number of oocytes retrieved and number of mature oocytes were equivalent across all groups, irrespective of PCOS diagnosis (Table 3). Comparing BMI categories for the same parameters (Table 4) when all patients were included in the analysis, the number of oocytes retrieved increased with increasing BMI, but the number of mature oocytes was unchanged, similar to the observation with %BF. When the analysis was limited to only those without PCOS, the number of oocytes retrieved and number of oocytes that were mature were equivalent across all groups (Table 4).
Mean spermatozoa concentration and mean TMSC according to %BF are outlined in Table 5. While concentration across all male subjects was equivalent, obese patients had significantly lower TMSC. Interestingly, when these data were classified according to BMI, this finding was no longer seen, and both spermatozoa concentration and TMSC were equivalent across all groups (Table 6).
Table 5.
Male semen analysis parameters according to %BF category*
| Metric | Underweight %BF < 17.0 N = 313 |
Normal %BF 17.0–21.9 N = 450 |
Overweight %BF 22.0–29.9 N = 757 |
Obese %BF ≥ 30 N = 494 |
p value† |
|---|---|---|---|---|---|
| Concentration | 49 [23–80.5] | 47.5 [25–72] | 51 [27–80] | 47 [23–77] | 0.352 |
| TMSC | 52.8 [17.7–110.8] | 52.4 [18.6–104.7] | 52.3 [20.2–108.6] | 42 [13.2–93.1] | 0.026 |
*Categories derived from Zhu et al.
†p value represents comparison between 4 adiposity groups
Table 6.
Male semen analysis parameters according to BMI
| Metric | Underweight BMI < 18.5 N = 7 |
Normal BMI 18.5–24.9 N = 441 |
Overweight BMI 25.0–29.9 N = 892 |
Obese BMI ≥ 30.0 N = 674 |
p value* |
|---|---|---|---|---|---|
| Concentration | 40.5 [29.5–64] | 50.5 [26–82] | 46 [24–72] | 50 [26–80] | 0.123 |
| TMSC | 42.5 [6.8–80.4] | 51.9 [18.5–109] | 48.6 [17.8–103.3] | 51.3 [16–106] | 0.749 |
*p value represents comparison between 4 adiposity groups
In evaluating the different mismatch groups between men and women, women were more likely to have an underestimated risk of overweight or obesity according to BMI (Figs. 1 and 2), whereas men were more likely to have an overestimated risk (Figs. 3 and 4). Among females, 83 subjects had a BMI ≥ 25 with %BF < 31% and 283 subjects had a BMI < 25 but %BF ≥ 31%. In men, 418 had a BMI ≥ 25 with %BF < 22% and 100 subjects had a BMI < 25 but %BF ≥ 22%. While these mismatch groups represent the minority in both sexes, they expose the potential for patients to be inaccurately classified based on BMI alone. To address the potential impact of this misclassification, a comparison of the larger mismatch groups was made to correctly classify subjects. In women, comparison of all outcome measures between subjects with BMI < 25 and %BF < 31 and those with BMI < 25 and %BF ≥ 31 was not found to be different. Similarly, the men with BMI ≥ 25 and %BF < 22 had equivalent results to men with BMI ≥ 25 and %BF ≥ 22 (Tables 7 and 8).
Table 7.
Female mismatch comparison*
| Metric | Matched N = 767 |
Mismatched N = 283 |
p value unadjusted† |
|---|---|---|---|
| Mean age | 35.1 years old | 35 years old | 0.844 |
| AMH | 2.4 [1.3–4.5] | 2.6 [1.3–4.4] | 0.521 |
| FSH | 7.6 [6.2–9.2] | 7.5 [6.3–9.2] | 0.787 |
| AFC | 14 [10–21] | 14 [10–20] | 0.187 |
| # oocytes retrieved | 12 [7–20] | 14 [7–20] | 0.469 |
| #M2 | 9 [5–15] | 11 [6–16] | 0.245 |
*Comparison made between females BMI < 25 and %BF < 31 versus BMI < 25 and %BF ≥ 31
†No age adjustment made as mean age of 2 groups was equivalent
Table 8.
Male mismatch comparison*
| Metric | Matched N = 1171 |
Mismatched N = 418 |
p value unadjusted |
|---|---|---|---|
| Conc | 49 [25–77] | 47 [24–75] | 0.394 |
| TMSC | 49 [18–104] | 52 [17–105] | 0.499 |
*Comparison made between males BMI ≥ 25 and %BF ≥ 22 versus BMI ≥ 25 and %BF < 22
Discussion
In this study where 2013 couples were prospectively evaluated, we investigated the association between %BF and BMI and female and male reproductive markers. When all patients were included in the analyses, a lower FSH was found to be associated with obesity diagnosed by %BF or BMI. However, in women without PCOS, high %BF or BMI in the range of obesity was associated with decreased AMH. Importantly, with or without the inclusion of women with PCOS, a number of mature oocytes retrieved were similar between all %BF and BMI categories. In addition, TMSC was inversely associated with increased adiposity in men.
As obesity becomes increasingly prevalent, its implications on baseline fecundability as well as infertility treatment are important to elucidate. To this end, it is essential that obesity be correctly calculated in order to correctly assign risk to the appropriate patients. Although BMI is easy to calculate and widely accepted as the standard metric, it may not be the most accurate representation of an individual’s obesity. In fact, one meta-analysis investigating BMI’s ability to assess adiposity found that standard BMI cutoffs possess high specificity (97%) but low sensitivity (42%), thus failing to identify approximately half of patients with excess %BF [32]. A study that identified %BF cutoffs equivalent to BMI in terms of metabolic risk additionally found that men and women have differing %BF criteria at which risk of metabolic syndrome becomes significant [19]. In this analysis, we made use of the sex-specific criteria identified in Zhu et al.’s study, with the goal of accurately assessing the impact of adiposity on male and female outcomes, respectively.
Obesity has previously been recognized to impact ovulatory function, ovarian response, and oocyte quality [33]. Prior work investigating obesity’s impact on fertility has primarily relied on BMI classification. One study integrated the measurement of waist circumference in assessing the relationship between ovulatory function and BMI and found more subcutaneous abdominal fat in anovulatory women compared with ovulatory women of similar BMI [21]. This earlier study had the benefit of being performed prospectively but had a relatively small sample size of 57 women. Moreover, it is important to draw the distinction between this prior study and the current analysis which, by evaluating ovarian reserve, examines reproductive potential as opposed to ovulatory function. While multiple studies have identified decreased fecundability in obese women, there has not yet been conclusive evidence of this being attributed to ovarian reserve [22, 34].
The literature has been mixed with regard to the relationship between obesity and markers of ovarian reserve with some studies identifying no relationship and others reporting an inverse relationship [35, 36]. These mixed results may stem from the retrospective nature of these analyses. When all female patients in the current study were classified according to %BF, both FSH and AMH were negatively correlated with obesity after adjusting for age. Although the absolute difference was very small, observing a lower AMH in obese patients seemed paradoxical. When further analysis was performed by excluding women with PCOS, a higher %BF was associated with decreasing serum AMH. The same observation was made in non-PCOS women with higher BMI. Our finding are consistent with a meta-analysis by Moslehi et al, which proposed that the lower AMH may be due to obesity-induced apoptosis in granulosa cells, resulting in decreased AMH production on a per follicle basis [35]. It is also possible that increased adiposity and associated metabolic dysfunction may lead to oocyte and follicle loss, similar to that observed in animal studies using oocyte-targeted deletion of genes that regulate mitochondrial function [13, 14, 12].
In terms of ovarian response across all patients, the number of oocytes retrieved trended higher but was ultimately not significant when classifying patients according to %BF; the number retrieved was significantly higher when classifying obese patients according to BMI after adjusting for age. Under both categorizations, however, the number of mature oocytes was equivalent among all patients. The subsequently reduced proportion of mature oocytes in obese patients may be explained by diminished oocyte quality in obese patients with PCOS [37]. Lower maturity rates in obese patients have been attributed to mitochondrial dysfunction in mouse models, specifically abnormal mitochondrial morphology as well as abnormal activity [11, 38]. Fortunately, the obese patients in this analysis had a small diminution in maturity and ultimately responded to gonadotropin stimulation as well as normal-weight counterparts. Similarly, when women with PCOS were removed from the analyses, a number of oocytes retrieved and mature oocytes were similar between all BMI and %BF categories.
Prior studies have evaluated the impact of increased BMI and waist circumference on male infertility and have noted obesity to negatively impact both endocrinologic parameters and semen analysis parameters including volume, concentration, and TMSC [39, 23]. These studies have proposed that the hyperestrogenic hypogonadal milieu as well as increased scrotal and testicular temperature negatively impact spermatogenesis. While the male patients in this analysis had equivalent concentrations in all adiposity groups, TMSC was significantly decreased in men with obese %BF, as seen in earlier studies [39, 40]. While the approximately 20% TMSC decrease in obese males may play a role in a couple’s subfertility, the average obese male TMSC would still not qualify for a diagnosis of oligospermia [41].
Aside from evaluating obesity, the use of BIA in this study was also able to detect patients with significantly decreased %BF. It may be misleading to call these patients underweight, as their categorization is a function of lean muscle mass as opposed to an absolute weight as in BMI. Historically, patients with hypothalamic hypogonadism due to the “Female Athlete Triad,” experience infertility due to disruption of the HPO axis, in part due to disrupted GnRH pulsatility from decreased leptin levels [42]. This analysis did not investigate nutritional or exercise habits of study subjects, but patients who meet the “Female Athlete Triad” criteria would not necessarily be expected to have infertility caused by decreased ovarian reserve. Importantly, in animal models where caloric restriction has led to reduced adiposity, there has been evidence that this periodic fasting increases insulin sensitivity and also has positive effects on longevity [43]. It is unclear as to whether this may play a mechanism in slowing reproductive aging in human females, but our findings suggest that in women without PCOS, the lowest %BF category is associated with a higher AFC compared with the normal or obese categories. When this observation is combined with the finding of higher AMH associated with lower %BF and BMI, a potential protective effect is plausible. Further investigation is needed, possibly using more extreme phenotypes such as patients with anorexia nervosa, to determine whether calorie restriction is associated with an increased ovarian reserve. In men, the TMSC was slightly higher in lower adiposity patients than in normal weight subjects. Data on male athletes is limited; however, vigorous exercise has been associated with suppressed hormonal activity at the hypothalamus and testes, causing suppression of both GnRH and serum testosterone, which could affect spermatogenesis [42].
The secondary goal of this analysis was to evaluate BIA’s ability in identifying patients at risk of being miscategorized by the standard evaluation of BMI. In evaluating outcomes between patients who had differing classifications according to BMI class and %BF class, it was found that outcomes were not different between those who had agreement between BMI and %BF classification and those who did not. Because there were very few differences in outcome across the entire study population, it is difficult to conclude whether the similarities between mismatched subjects and BMI/%BF-concordant subjects are truly due to a lack of difference among subjects or whether the studied outcome measures are not significantly impacted by obesity in general.
This study has several limitations including the limited number of parameters collected regarding male infertility, as well as lack of information regarding nutritional and exercise regimens of all study subjects. Additionally, the outcomes presented in this study are quantitative and thus do not inform the quality of assessed gametes or how they may perform clinically. This study’s strengths lie in its prospective evaluation of a large number of male and female patients. Additionally, this analysis demonstrates a novel use of BIA to evaluate obesity in the infertile population; to our knowledge, this is the first study to do so.
In conclusion, while obesity may impact fecundability and result in lower ovarian reserve in obese women without PCOS, this study highlights that ovarian response to COH is relatively equivalent in obese females. This may represent the capability of current methods employed to collect male and female gametes in the context of IVF to bypass the mechanisms that reduce female fertility. Similarly, while obese male patients may have lower TMSC compared with normal weight counterparts, the average decrease in TMSC would not be likely to limit the success of assisted reproductive technologies. While %BF and BMI ultimately seemed to identify similar trends in female patients, %BF did present itself as more useful in identifying male patients who had a significantly decreased TMSC. Further research is needed in terms of validating %BF’s utility in comparing embryologic and pregnancy outcomes in the IVF population.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.NCD Risk Factor Collaboration. NRFC Trends in adult body-mass in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387:9945. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray G, Kim K, Wilding J, Foundation WO Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev. 2017;18(7):715–723. doi: 10.1111/obr.12551. [DOI] [PubMed] [Google Scholar]
- 3.Bellver J, Pellicer A, García-Velasco JA, Ballesteros A, Remohí J, Meseguer M. Obesity reduces uterine receptivity: clinical experience from 9,587 first cycles of ovum donation with normal weight donors. Fertil Steril. 2013;100(4):1050–1058. doi: 10.1016/j.fertnstert.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Koning AMH, Kuchenbecker WKH, Groen H, Hoek A, Land JA, Khan KS, et al. Economic consequences of overweight and obesity in infertility: a framework for evaluating the costs and outcomes of fertility care. Hum Reprod Update. 2010;16(3):246–254. doi: 10.1093/humupd/dmp053. [DOI] [PubMed] [Google Scholar]
- 5.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. Hyattsville, MD: National Center for Health Statistics.2020.
- 6.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 7.Craig JR, Jenkins TG, Carrell DT, Hotaling JM. Obesity, male infertility, and the sperm epigenome. Fertil Steril. 2017;107(4):848–859. doi: 10.1016/j.fertnstert.2017.02.115. [DOI] [PubMed] [Google Scholar]
- 8.Kahn BE, Brannigan RE. Obesity and male infertility. Curr Opin Urol. 2017;27(5):441–445. doi: 10.1097/MOU.0000000000000417. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann A, Manjowk G-M, Wagner IV, Klöting N, Ebert T, Jessnitzer B, et al. Leptin within the subphysiological to physiological range dose dependently improves male reproductive function in an obesity mouse model. Endocrinology. 2016;157(6):2461–2468. doi: 10.1210/en.2015-1966. [DOI] [PubMed] [Google Scholar]
- 10.Bazzano MV, Paza DA, Elia EM. Obesity alters the ovarian glucidic homeostasis disrupting the reproductive outcome of female rats. J Nutr Biochem. 2017;42(Apr):194–202. doi: 10.1016/j.jnutbio.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Grindler NM, Moley KH. Maternal obesity, infertility and mitochondrial dysfunction: potential mechanisms emerging from mouse model systems. Mol Hum Reprod. 2013;19(8):486–494. doi: 10.1093/molehr/gat026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang T, Babayev E, Jiang Z, Li G, Zhang M, Esencan E, et al. Mitochondrial unfolded protein response gene Clpp is required to maintain ovarian follicular reserve during aging, for oocyte competence, and development of pre-implantation embryos. Aging Cell. 2018;17(4):e12784. doi: 10.1111/acel.12784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang M, Bener MB, Jiang Z, Wang T, Esencan E, Scott R, III, et al. Mitofusin 1 is required for female fertility and to maintain ovarian follicular reserve. Cell Death Dis. 2019;10(8):560. doi: 10.1038/s41419-019-1799-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang M, Bener MB, Jiang Z, Wang T, Esencan E, Scott R, et al. Mitofusin 2 plays a role in oocyte and follicle development, and is required to maintain ovarian follicular reserve during reproductive aging. Aging. 2019;11(12):3919–3938. doi: 10.18632/aging.102024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vitale G, Pellegrino G, Vollery M, Hofland LJ. Role of IGF-1 system in the modulation of longevity: controversies and new insights from a centenarians’ perspective. Front Endocrinol. 2019;10:27. doi: 10.3389/fendo.2019.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang Y, Liu C, Lu M, Dong Q, Wang Z, Wang Z, et al. Calorie restriction is the most reasonable anti-ageing intervention: a meta-analysis of survival curves. Sci Rep. 2018;8(1):5779. doi: 10.1038/s41598-018-24146-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Machtinger R, Combelles CMH, Missmer SA, Correia KF, Fox JH, Racowsky C. The association between severe obesity and characteristics of failed fertilized oocytes. Hum Reprod. 2012;27(11):3198–3207. doi: 10.1093/humrep/des308. [DOI] [PubMed] [Google Scholar]
- 18.Leary C, Leese HJ, Sturmey RG. Human embryos from overweight and obese women display phenotypic and metabolic abnormalities. Hum Reprod. 2015;30(1):122–132. doi: 10.1093/humrep/deu276. [DOI] [PubMed] [Google Scholar]
- 19.Zhu S, Wang Z, Shen W, Heymsfield SB, Heshka S. Percentage body fat ranges associated with metabolic syndrome risk: results based on the third National Health and Nutrition Examination Survey (1988–1994) Am J Clin Nutr. 2003;78:228–235. doi: 10.1093/ajcn/78.2.228. [DOI] [PubMed] [Google Scholar]
- 20.Dong B, Peng Y, Wang Z, Adegbija O, Hu J, Ma J, et al. Joint association between body fat and its distribution with all-cause mortality: a data linkage cohort study based on NHANES (1988- 2011) PLoS One. 2018;13(2):e0193368. doi: 10.1371/journal.pone.0193368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuchenbecker WKH, Groen H, Zijlstra TM, Bolster JHT, Slart RHJ, Jagt EJ, et al. The subcutaneous abdominal fat and not the intraabdominal fat compartment is associated with anovulation in women with obesity and infertility. J Clin Endocrinol Metab. 2010;95(5):2107–2112. doi: 10.1210/jc.2009-1915. [DOI] [PubMed] [Google Scholar]
- 22.Yilmaz N, Kilic S, Kanat-Pektas M, Gulerman C, Mollamahmutoglu L. The relationship between obesity and fecundity. J Women's Health. 2009;2009(18):5. doi: 10.1089/jwh.2008.1057. [DOI] [PubMed] [Google Scholar]
- 23.Hammiche F, Laven JSE, Twigt JM, Boellaard WPA, Steegers EAP, Steegers-Theunissen RP. Body mass index and central adiposity are associated with sperm quality in men of subfertile couples. Hum Reprod. 2012;27(8):2365–2372. doi: 10.1093/humrep/des177. [DOI] [PubMed] [Google Scholar]
- 24.Ling CH, deCraen AJ, Slagboom PE, Gunn DA, Stokkel MP, Westendorp RG, et al. Accuracy of direct segmental multi-frequency bioimpedance analysis in the assessment of total body and segmental body composition in middle-aged adult population. Clin Nutr. 2011;30(5):610–615. doi: 10.1016/j.clnu.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Demura S, Sato S, Kitabayashi T. Percentage of total body fat as estimated by three automatic bioelectrical impedance analyzers. J Physiol Anthropol Appl Hum Sci. 2004;23(3):93–99. doi: 10.2114/jpa.23.93. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention Assessing Your Weight: Adult Body Mass Index or BMI. 2020. https://www.cdc.gov/healthyweight/assessing/index.html. Accessed May 8, 2020.
- 27.World Health Organization body mass index - BMI. In: Health Topics: Nutrition. World Health Organization. 2020. http://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi. Accessed 4/22/2020
- 28.Olivennes F, Cunha-Filho J, Fanchin R, Bouchard P, Frydman R. The use of GnRH antagonists in ovarian stimulation. Hum Reprod. 2002;8(3):279–290. doi: 10.1093/humupd/8.3.279. [DOI] [PubMed] [Google Scholar]
- 29.Padilla S, Dugan K, Maruschak V, Shalika S, Smith R. Use of the flare-up protocol with high dose follicle stimulating hormone and human menopausal gonadotropins for in vitro fertilization in poor responders. Fertil Steril. 1996;65(4):796–799. doi: 10.1016/S0015-0282(16)58216-8. [DOI] [PubMed] [Google Scholar]
- 30.Hughes E, Fedorkow D, Daya S, Sagle M, Koppel PV, Collins J. The routine use of gonadotropin-releasing hormone agonists prior to in vitro fertilization and gamete intrafallopian transfer: a meta-analysis of randomized controlled trials. Fertil Steril. 1992;58(5):888–896. doi: 10.1016/S0015-0282(16)55430-2. [DOI] [PubMed] [Google Scholar]
- 31.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 32.Okorodudu DO, Jumean MF, Montori VM, Romero-Corral A, Somers VK, Erwin PJ, et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int J Obes. 2010;34(5):791–799. doi: 10.1038/ijo.2010.5. [DOI] [PubMed] [Google Scholar]
- 33.Practice Committee of the American Society for Reproductive Medicine Obesity and reproduction: a committee opinion. Fertil Steril. 2015;104(5):1116–1126. doi: 10.1016/j.fertnstert.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 34.Gesink-Law DC, Maclehose RF, Longnecker MP. Obesity and time to pregnancy. Hum Reprod. 2007;22(2):414–420. doi: 10.1093/humrep/del400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moslehi N, Shab-Bidar S, Tehrani FR, Mirmiran P, Azizi F. Is ovarian reserve associated with body mass index and obesity in reproductive aged women? A Meta-Analysis. Menopause. 2018;25(9):1046–1055. doi: 10.1097/GME.0000000000001116. [DOI] [PubMed] [Google Scholar]
- 36.Sahmay S, Guralp O, Senturk LM, Imamoglu M, Kucuk M, Irez T. Serum anti-müllerian hormone concentrations in reproductive age women with and without polycystic ovary syndrome: the influence of body mass index. Reprod Med Biol. 2011;12(2):113–120. doi: 10.1007/s12522-011-0078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snider AP, Wood JR. Obesity induces ovarian inflammation and reduces oocyte quality. Reproduction. 2019;158(3):R79–R90. doi: 10.1530/REP-18-0583. [DOI] [PubMed] [Google Scholar]
- 38.Igosheva N, Abramov AY, Poston L, Eckert JJ, Fleming TP, Duchen MR, et al. Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PLoS One. 2010;5(4):e10074. doi: 10.1371/journal.pone.0010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bieniek JM, Kashanian JA, Deibert CM, Grober ED, Lo KC, Brannigan RE, et al. Influence of increasing body mass index on semen and reproductive hormonal parameters in a multi-institutional cohort of subfertile men. Fertil Steril. 2016;106(5):1070–1075. doi: 10.1016/j.fertnstert.2016.06.041. [DOI] [PubMed] [Google Scholar]
- 40.Ma J, Wu L, Zhou Y, Zhang H, Xiong C, Peng Z, et al. Association between BMI and semen quality: an observational study of 3966 sperm donors. Hum Reprod. 2019;34(1):155–162. doi: 10.1093/humrep/dey328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cooper TG, Noonan E, Eckardstein S, Auger J, Baker HWG, Behre HM, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16(3):231–245. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 42.Boutaria C, Pappas PD, Mintziori G, Nigdelis MP, Athanasiadis L, Goulis DG et al. The effect of underweight on female and male reproduction. Metabolism. 2020;107(June) [DOI] [PubMed]
- 43.Velingkaar N, Mezhnina V, Poe A, Makwana K, Tulsian R, Kondratov RV. Reduced caloric intake and periodic fasting independently contribute to metabolic effects of caloric restriction. Aging Cell. 2020;19(4):e13138. doi: 10.1111/acel.13138. [DOI] [PMC free article] [PubMed] [Google Scholar]