Abstract
Purpose
To keep and increase spermatogonial stem cell number (SSC) is the only available option for pediatric cancer survivors to maintain fertility. Leptin is secreted by the epididymal white adipose tissue and has receptors on stem/progenitor spermatogonia. The purpose of this study is to demonstrate dose- and time-dependent proliferative effect of leptin on stem/progenitor spermatogonia cultures from prepubertal mice testes.
Methods
CD90.2 (+) stem/progenitor spermatogonia were isolated from the C57BL/6 mouse testis on postnatal day 6 and placed in culture. The proliferative effect of leptin supplementation was assessed by colony formation (diameter and number), WST proliferation assays, and xCELLigence real-time cell analysis (RTCA) on days 3, 5, and 7 of culture. Expressions of p-ERK1/2, p-STAT3, total STAT3, and p-SHP2 levels were determined by western blot analysis.
Results
Leptin supplementation of 100 ng/ml increased the diameter (p = 0.001) and number (p = 0.01) of colonies in stem/progenitor spermatogonial cultures and caused higher proliferation by WST-1 (p = 0.009) compared with the control on day 7. The EC50 was calculated as 114 ng/ml for leptin by RTCA. Proliferative dose of leptin induced increased expression of p-ERK1/2 (p = 0.009) and p-STAT3 (p = 0.023) on stem/progenitor spermatogonia when compared with the untreated group.
Conclusion
The results indicated that leptin supplementation exhibited a dose- and time-dependent proliferative effect on stem/progenitor spermatogonia that was associated with increased expression of ERK1/2 and STAT3 pathways while maintaining their undifferentiated state. This output presents a new agent that may help to expand the stem/progenitor spermatogonia pool from the neonatal testis in order to autotransplant after cancer treatment.
Keywords: Stem/progenitor spermatogonia, Leptin, Proliferation, p-STAT3, p-ERK1/2
Introduction
Cancer treatments such as chemotherapy and/or radiation may induce permanent loss of germ cells [1]. While the survival rate of pediatric cancer patients has increased significantly, fertility preservation is a major concern of these patients [2]. Adult men can preserve their fertility by sperm cryopreservation before cancer treatments, but this is not applicable for prepubertal boys because spermatogenesis has not yet started. If the childhood cancer patients hope to have their biological children in the future, the only available option is to cryopreserve their testicular tissues (TT) before cancer treatments. The TT includes SSCs that have the capacity to regenerate spermatogenesis after cancer treatment [3]. The pool of SSCs in the testis is limited. Only 0.3 and 22% of total germ cells are undifferentiated spermatogonia in rodent and human, respectively [4]. Cryopreservation of TT is the first step to restore fertility, but those procedures may cause a decrease in the number of SSCs after thawing [5]. Thus the proliferation and maintenance of SSCs are critical steps to preserve fertility after cancer treatment in prepubertal cancer patients [6].
Intratubular supporting Sertoli and extra tubular Leydig cells, interstitial and peritubular macrophages, and testicular endothelial and peritubular myoid cells function as accessory cells of the SSC niche and support spermatogenesis by chemical and physical interactions [7–9]. Furthermore, adipocytes located within the epididymal white adipose tissues (EWAT) contribute to the regulation of spermatogenesis through locally secreted products [10] including leptin. The removal of EWAT disturbs maintenance of spermatogonia and Sertoli cells via different mechanisms [11] that cannot be restored by autologous EWAT transplantation to the subcutaneous dorsal fat pad [11]. Leptin, an adipocyte-derived polypeptide hormone, regulates energy balance and male reproductive functions [12] via its receptors located in the hypothalamus and testes [13, 14]. Mouse type A spermatogonia, human and mouse Leydig cells, and human spermatozoa express Ob-Rb receptors [15–17]. Leptin-deficient ob/ob mice exhibit low blood levels of GnRH, gonadotropins, and testosterone [18] and they are infertile due to increased germ cell apoptosis [19, 20]. Leptin treatment partially improves Leydig cell function, spermatogenesis, and fertility in ob/ob mice [21, 22]. On the other hand, long-term leptin administration reduces male fertility in adult mice by decreasing germ cell number [23] and leads to adverse effect on sperm counts and morphology [12].
Leptin causes proliferation of different types of stem cells [24–28] and differentiated [29–32] somatic cells via ERK and/or STAT3 pathways in vitro [24, 31–34]. Leptin secreting adipocytes from EWAT [10, 11, 35, 36] and the expression of leptin and its receptor on mouse type A spermatogonia [15, 16] suggest its potential proliferative paracrine and autocrine effects on SSCs. Up to now, no reports have been published regarding the effect of leptin on SSCs in neonatal mouse testis.
Methods to maintain and expand SSCs in vitro may be required to preserve and transplant these cells into the testes of men who are survivors of childhood cancer and suffering infertility in adulthood. We hypothesize that leptin supplementation may induce proliferation of neonatal mouse SSCs in vitro via ERK and/or STAT3 pathways. We tested this hypothesis in the current study using in vitro cultures of neonatal C57BL/6 mouse stem/progenitor spermatogonia.
Materials and method
Experimental design
A prospective randomized study with control and leptin-treated experimental SSC groups is designed in order to assess the proliferative potential of this agent. The Hacettepe University Animal Experimentations Local Ethics Board (#2016/59/1) approved the use of animal material. The testes were obtained from neonatal male C57BL/6 mice. First, the undifferentiated spermatogonia (including SSCs) were morphologically characterized in the testis tissues; then isolated, and separated by MACS. Stem/progenitor spermatogonia cultures were established from isolated cells, and cultured cells were treated with four different leptin (R&D Systems) concentrations (10, 50, 100, 200 ng/ml in 20 mM Tris-HCI) according to the literature [31, 37–39]. In this study, we assessed the dose-dependent colony forming and proliferative capacity of leptin using colony assay, WST-1 and xCELLigence RTCA assays on days 3, 5, and 7. Flow cytometry was carried out to characterize stem/progenitor spermatogonia after leptin treatment to demonstrate their undifferentiated state. Western blotting determined the molecular signaling mechanism of leptin.
Morphologic characterization of stem/progenitor spermatogonia in testis tissue
The presence of stem/progenitor spermatogonia has been reported in testes of 5–8-day-old male C57BL/6 mice [40–43]. For the efficient cell isolation and culture, detection of the stem/progenitor spermatogonia was undertaken by screening testicular sections obtained from the 6-day-old mice prior to experiments. According to the preliminary observations, experiments were proceeded using 6-day-old male C57BL/6 mice.
Bright-field microscopy
For paraffin-embedded tissues, testes were washed, processed in a tissue-processing machine (Leica TP 1020 Nussloch, Germany), and embedded in paraffin (Leica Eg1150H, Nussloch, Germany), after fixation with 10% neutral buffered formalin (NBF) in PBS. Sections were stained with hematoxylin eosin following deparaffinization. The sections were analyzed and photographed using a bright-field microscope attached with a digital camera (Leica DM 6000 Wetzlar, Germany).
Electron microscopy
For plastic-embedded tissues, after first fixation by 2.5% glutaraldehyde for 2 h, at room temperature (RT), post-fixation was performed by 1% osmium tetroxide in the dark for 1 h. Fixed testes were dehydrated and cleared in Leica EM TP tissue processor (Wetzlar, Germany) and embedded in plastic. The plastic-embedded blocks were cut into ultrathin sections and uranyl acetate and lead citrate staining was performed. The sections were analyzed and imaged using transmission electron microscope (JEOL-JEM 1400, Japan) attached with a CCD camera (Gatan Inc., Pleasanton, CA, USA).
Immunofluorescence microscopy
For frozen tissues, neonatal testes were fixed in chilled acetone at 4 °C for 15 min. After drying, permeabilization was performed by 0.1% Triton-X solution. The sections were stained with PE-Armenian hamster anti-mouse PLZF antibody (BioLegend) at RT for 1 h. After washing, DAPI staining for 2 min was performed. The sections were observed and photographed by fluorescence microscope (Leica DM 6000 Wetzlar, Germany).
Isolation, characterization, and culture of stem/progenitor spermatogonia
Cell isolation and MACS separation
The testes obtained from neonatal (6-day-old) C57BL/6 mice were collected in Hank’s balanced salt solution (HBSS) (Sigma-Aldrich) and the tunica albuginea was removed under a dissecting microscope. The testes were digested with 0.25% Trypsin-EDTA (4.5 ml) (Gibco) and 7 mg/ml DNase I (0.5 ml) (Sigma-Aldrich) at 37 °C, for 4–5 min in order to generate single-cell suspension. After the first incubation, tubules were digested for an additional 2–3 min at 37 °C and enzymatic digestion was stopped with fetal bovine serum (FBS) (Gibco). Filtration of suspension was performed through a 40-μm-pore nylon cell strainer (Thermo Fisher Scientific) and centrifuged at 1400 rpm for 7 min. The pellet was re-suspended in 10 ml of PBS (Calbiochem) supplemented with 1% FBS, 10 mM HEPES (Sigma- Aldrich) MO, USA), 1 mg/ml glucose (Sigma-Aldrich), 1 mM pyruvate (Sigma- Aldrich), and 1% penicillin and streptomycin (Hycolen). Enrichment of SSCs was done through a 30% Percoll (Sigma-Aldrich) gradient and collected using Thy-1.2 antibody-conjugated magnetic microbeads (Miltenyi Biotec, 130-049-101). In brief, after Percoll gradient, suspension was centrifuged at 1400 rpm for 7 min and pellet was re-suspended in 180 μl of PBS-MACS including 1 mg/ml glucose, 10 mM HEPES, 1% FBS, 1 mM pyruvate, and 1% penicillin and streptomycin, and 20 μl of CD 90.2 magnetic microbeads were added to 180 μl of cell suspension and the mixture was incubated for 1 h at 4 °C. The mixture was washed by 2 ml of PBS-MACS and centrifuged and re-suspended in serum-free medium (SFM). Then, the suspension was loaded onto a column to collect CD 90.2 (+) cells. CD 90.2 (+) cells were cultured on STO feeder cells.
Preparation of feeder layer
The mouse embryonic fibroblasts (STO cell line, ATCC CRL-1503) were maintained in Dulbecco’s modified Eagle’s medium (DMEM) with high glucose (Sigma-Aldrich) including 1% L-glutamine (Hyclone), 10% FBS, and 1% penicillin-streptomycin. Growth-arrested feeder layer was prepared using mitomycin-C (Sigma-Aldrich). Inactivated STO cells were cultured as 2 × 105 per well in 0.1% gelatin (Sigma-Aldrich) coated 12-well plate. Following isolation, stem/progenitor spermatogonia were co-cultured with STO feeder cells.
Culture of stem/progenitor spermatogonia
The number of CD90.2 positive stem/progenitor spermatogonia and the mitotically inactivated STO feeder fibroblastic cells per well of 12-well plate were designated as 7 × 104 and 2 × 105, respectively. Stem/progenitor spermatogonia were maintained in serum-free MEM-α medium (Gibco) containing 0.2% bovine serum albumin (BSA) (Sigma-Aldrich), 10 μg/ml holo-transferrin (Sigma-Aldrich), 3 × 10−8 M sodium selenite (Na2SeO3) (Sigma 21,448-5, St. Louis, MO, USA), 2 mM L-glutamine, 50 μM mercaptoethanol (Sigma-Aldrich), 5 μg/ml insulin (Sigma-Aldrich) MO, USA), 10 mM HEPES (Sigma-Aldrich), 1% MEM non-essential amino acid solution (Sigma-Aldrich), 60 μM putrescine dihydrochloride (Sigma-Aldrich), 20 ng/ml glial-derived neurotrophic factor (GDNF) (BioLegend), 150 ng/ml glial-derived neurotrophic factor family receptor alpha 1 (GFRα-1) (R&D Systems), and 1 ng/ml human basic fibroblast growth factor (bFGF) (BioLegend). Medium was replaced every other day.
Characterization of isolated and cultured stem/progenitor spermatogonia
Flow cytometry
The stem/progenitor spermatogonia have been characterized by flow cytometry (FCM) prior to and after MACS separation. Briefly, the sample was treated with FITC-conjugated rat anti-mouse CD90.2 (BioLegend) and APC-conjugated c-kit (BioLegend) at 4 °C for 20 min after washing in wash buffer and centrifugation. The fixation and permeabilization were carried out by True-Nuclear Transcription Factor Buffer Set (BioLegend). The sample was then labeled with PE-conjugated Armenian hamster anti-mouse PLZF at 4 °C for 20 min in the dark.
On day 7 of the culture, FCM was carried out by CD90.2 antibody to demonstrate whether stem/progenitor spermatogonia maintain their undifferentiated state after leptin treatment. In brief, stem/progenitor spermatogonia cell suspensions were labeled with FITC-conjugated CD90.2 antibody (BioLegend) for 30 min. All samples were washed with wash buffer and analyzed. Death cells were excluded by gating and analysis was carried out by BD FACS-Diva software version 6.1.2 with 100.000 list mode events recorded for each sample. Isotype controls were used for each antibody to rule out the background labeling.
Indirect immunofluorescence labeling for PLZF and leptin receptor in cultured stem/progenitor spermatogonia
Ten microliter of stem/progenitor spermatogonia suspensions (5 × 106/ml) was placed on (+) charged slides and fixation was carried out with cold methanol. After rinsing two times with PBS, slides were blocked with a blocking buffer including 3% BSA and 0.1% Triton-X 100 to eliminate nonspecific binding. Slides were incubated for 2 h at RT with PLZF (1:100, Santa Cruz) [41, 44–46] or leptin receptor (1:100, Novus Biologicals) primary antibody. Isotype-matched normal IgG (1:50, BD Biosciences) was used as negative control. After washing with PBS, slides were treated with anti-rabbit AlexaFluor-488 or AlexaFluor-568-conjugated secondary antibody (1:200, Invitrogen) for 45 min at RT. The slides were rinsed with PBS and covered by mounting medium containing DAPI. Immunofluorescence staining was observed with a fluorescence microscope (Nikon Eclipse E600).
Dose-dependent proliferative effect of leptin on stem/progenitor spermatogonia
Colony assay
The stem/progenitor spermatogonia and the STO feeder cells were plated onto 12-well plates as 7 × 104 and 2 × 105 per well, respectively. Totally 3 sets of experiment were performed with 4 different doses of leptin in each well (0, 10, 50, 100, 200 ng/ml). The colony numbers were calculated and the diameters of all SSC colonies were measured in the control and leptin-treated groups on days 3, 5, and 7 of culture by using an inverted microscope (Leica DM6B, Wetzlar, Germany) attached with a computerized digital camera (Leica DFC 480, Wetzlar, Germany). All colony micrographs were captured and analyzed and the largest diameter of each colony was measured in micrometer by using an image analyzing the Leica Las X software (Wetzlar, Germany) [43].
WST-1 assay
Feeder cells were plated at 2 × 104 cells/well in 96-well plate and they were treated with mitomycin-C for 4 h at 37 °C to inhibit cell proliferation after 2 days. Stem/progenitor spermatogonia were placed at a density of 4 × 103 cells in 200-μl serum-free MEM-α media into the wells of the 96-well plate and then the stem/progenitor spermatogonia were treated with 10–200 ng/ml leptin to analyze possible proliferative effect. For WST analysis, the stem/progenitor spermatogonia were treated with 10 μl/well cell proliferation reagent WST-1 (Roche) for 2 h at 37 °C on days 3, 5, and 7 of culture. Absorbance measurement was performed at 460 nm. This experiment was repeated four different times [42].
xCELLigence real-time cell analysis
To investigate dose- and time-dependent effect of leptin, proliferation of stem/progenitor spermatogonia after leptin treatment was observed via the xCELLigence RTCA DP instrument (Roche, Switzerland). At first, 2 × 104 STO feeder cells were added to 96-well e-plates (Roche, Basel, Switzerland). The STO cells were treated with mitomycin-C for 4 h to inactivate cell proliferation. Spermatogonial stem cells were seeded into the wells of the e-plate at a density of 4 × 103 cells and treated with medium containing 10–200 ng/ml leptin. Each treatment condition was measured in 6 wells and the medium was changed every other day. The impedance value of each control and leptin-treated well was monitored by xCELLigence every 1 h for totally 10 days. A 100-μl medium was added to e-plate 96 for the measurement of background values. The cell index values were normalized before addition of leptin doses. The RTCA Software v1.2.1 (OLS) was used to analyze data and calculate temporal dynamics of cellular attachment and half maximal effective concentration (EC50) [47–49].
Determination of signal pathways of leptin to proliferate stem/progenitor spermatogonia
Western blot analysis
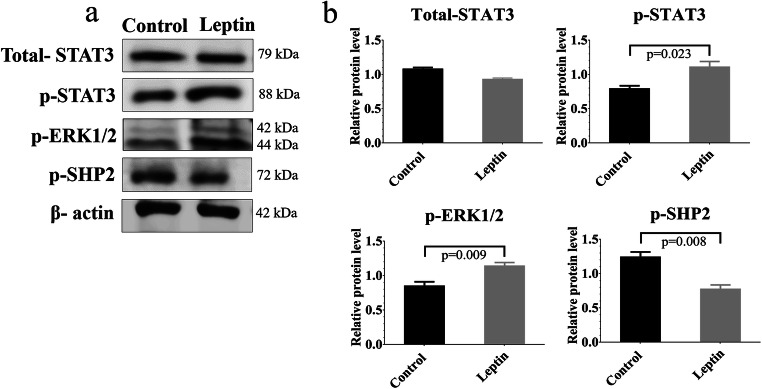
Protein levels of total STAT3, p-STAT3, p-SHP2, and p-ERK1/2 were ascertained according to the literature [31, 32]. Initially, stem/progenitor spermatogonia were treated 114 ng/ml leptin for 30 min. Proteins were extracted by lysis buffer containing protease and phosphatase inhibitors, and samples were centrifuged at 4 °C 10,000 × g for 15 min to obtain supernatant. The amount of protein was calculated by a Pierce BCA Protein Assay kit (Thermo Fisher Scientific) and 15 μg of protein was loaded into the gels. Samples were boiled at 95 °C, after samples were mixed with sample buffer. Samples were run on SDS-PAGE gel and transferred to PVDF membranes (Thermo Fisher Scientific). Next, in order to prevent unspecific binding, the blocking was performed by 5% non-fat milk powder in PBS for 1 h. Membranes were incubated overnight with specific primary antibodies such as p-SHP2 (1:2000) (Cell signaling 3751), total- STAT3 (1:2000) (Direct-Blot HRP anti STAT3, BioLegend), p-STAT3 (1:2000) (Direct-Blot HRP anti STAT3 Phospho (Tyr 705), BioLegend), and p-ERK1/2 (1:2000) (Direct-Blot HRP anti ERK1/2 Phospho (Thr202/Tyr 204), BioLegend) and secondary antibody (Goat Anti-Rabbit IgG, Secondary Antibody, HRP Conjugate (Boster) for 1 h. Immune detection was carried out by the Super Signal West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific). The membranes were striped for incubation with anti-β-actin (BioLegend) antibody. In order to quantify protein expressions, densitometry analysis was performed utilizing Image J. Three replicates were performed.
Statistical analysis
We evaluated the normality of the distribution of variables by Shapiro-Wilk test. The comparison between multiple groups was assessed by the repeated measure ANOVA with one fixed factor difference. The pair comparison was done by Sidak correction. Descriptive statistics were demonstrated as mean ± SEM. Statistical significant was evaluated by p < 0.05.
Results
Mouse stem/progenitor spermatogonia were characterized by PLZF labeling and morphologically in neonatal testis
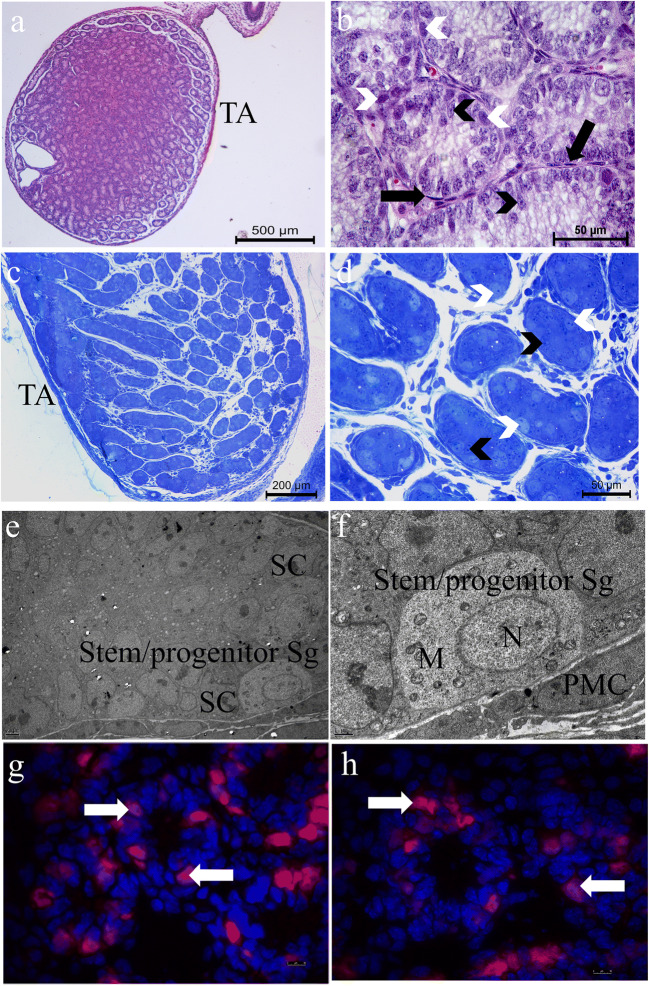
The 6-day-old mouse testes exhibited lobular parenchyma consisting of seminiferous cords and surrounding stroma with interstitial tissue and tunica albuginea under the light microscope (Fig. 1a and b). Sertoli cells with perpendicular orientation to the basement membrane and the basally located spermatogonia form the seminiferous cords in the lobules at the semi-thin section’s micrographs (Fig. 1c and d). The stem/progenitor spermatogonia located at the basal compartment present large spherical euchromatic nuclei with small clumps of heterochromatin at the ultrastructural level. Those cells have little amount of cytoplasm with numerous mitochondria (Fig. 1e and f). The 6-day-old mouse testes contain PLZF (+) stem/progenitor spermatogonia by IF (Fig. 1g and h).
Fig. 1.
Light (a–d) and electron (e, f) micrographs of a 6-day-old C57/BL-6 mouse testis The low a and b high power micrographs of paraffin sections exhibit testicular lobules consisting of seminiferous cords and the tunica albuginea (TA) that surrounds the testis. Seminiferous cords are surrounded by monolayer of peritubular myoid cells (black arrows) and the spermatogonia (white arrowheads) are located in the cords. c and d are the semi-thin plastic section micrographs clearly showing the presence of the basally located large, bright spermatogonia (white arrowheads) and Sertoli cells (black arrowheads) arranged perpendicularly to the basement membrane inside the seminiferous cords. e and f present the Sertoli cells (SC) and the stem/progenitor spermatogonia at the ultrastructural level within the cords. Note the high amount of mitochondria (M) in the cytoplasm and small heterochromatin clumps inside the nuclei (N) of the stem/progenitor spermatogonia and peritubular myoid cell (PMC) at high magnification. g and h are the frozen sections showing the PLZF (+) stem/progenitor spermatogonia (white arrowheads) in the seminiferous cords by IF. a, b Hematoxylin eosin × 100 and × 600. c, d Methylene blue azure II × 100 and × 400. e, f Uranyl acetate–lead citrate × 4000 and × 20000. g, h IF × 1000
Isolated stem/progenitor spermatogonia were characterized by FCM and expression of PLZF and leptin receptor in cultured stem/progenitor spermatogonia were confirmed by IF
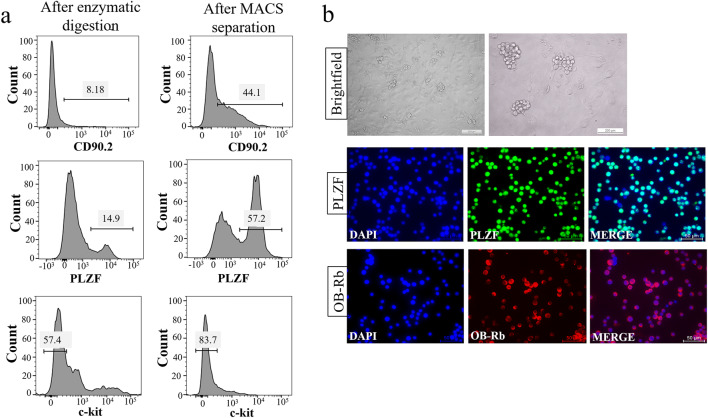
Whole testis cells were successfully obtained from 6-day-old prepubertal mice by enzymatic digestion. The total testis cell population comprises 8.18% CD90. 2 (+), 14.9% PLZF (+), and 57.4% c-kit (−) cells by FCM. MACS selection revealed the presence of CD 90.2 and PLZF immune-labeled spermatogonia as 44.1 CD90.2 (+), 57.2% PLZF (+), and 83.7% c-kit (−) by FCM (Fig. 2a). Therefore, the MACS separation protocol with CD90.2 microbeads provided the effective enrichment of spermatogonia expressing stem/progenitor spermatogonia markers for all work packages. The cultured stem/progenitor spermatogonia exhibited intense immune labeling for PLZF and Ob-Rb receptor (Fig. 2b) by IF.
Fig. 2.
Isolation and characterization of stem/progenitor spermatogonia. a Flow cytometric analysis of stem/progenitor spermatogonia for CD90.2, PLZF, and c-kit antibodies from whole testis after enzymatic digestion and after MACS separation. b Bright-field phase contrast micrographs (low × 100 and high × 200 magnification) and IF labeling of cultured stem/progenitor spermatogonia for PLZF and Ob-Rb leptin receptor. DAPI; nucleus. × 600
Leptin promoted stem/progenitor spermatogonia proliferation
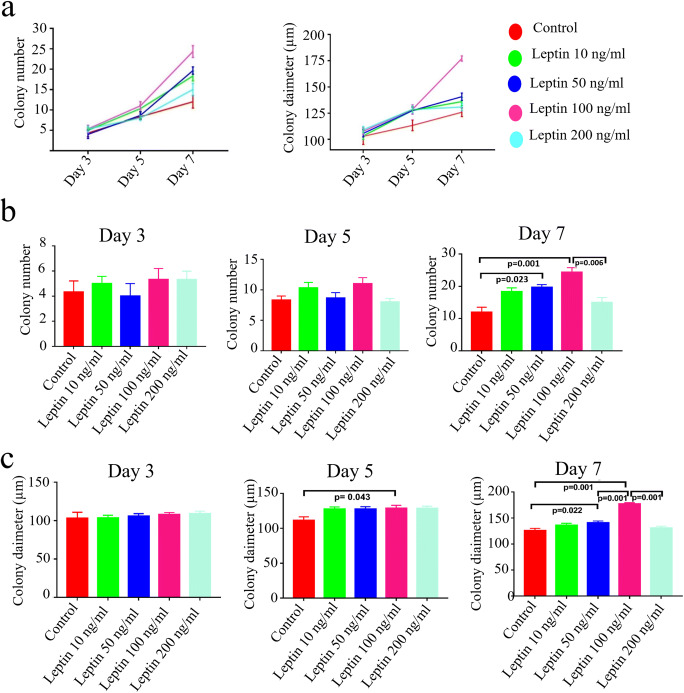
The number and the diameter of the colonies increased significantly from day 3 to day 7 in both the control and all leptin-treated groups (p < 0.05, Fig. 3a). No statistical difference was noted in the mean diameter and the mean number of colonies among the control and leptin groups on day 3 of the culture. The colony number did not change between groups; however, the mean colony diameter was significantly higher in 100 ng/ml leptin-treated SSCs, when compared with control on day 5 of the culture (p = 0.043, Fig. 3b and c). The mean diameter and the number of colonies were significantly higher in 50 and 100 ng/ml leptin-treated groups when compared with control on day 7 (p = 0.022 and p = 0.001 for the diameter and p = 0.023 and p = 0.001 for number of colonies, respectively, Fig. 3b and c). A 100 ng/ml of leptin treatment provided the largest colony diameter rate when compared with other doses of leptin (p = 0.001, each), and it initiated a better colony number compared with 200 ng/ml leptin treatment on day 7 of culture (p = 0.006) (Fig. 3b and c).
Fig. 3.
Dose- and time-dependent proliferative effect of leptin on stem/progenitor spermatogonia. a Graphs present an increase in time for colony number and the diameter for leptin-treated and control groups from day 3 to day 7. b and c The mean colony number and diameter in control and leptin-treated groups are compared at each time point, Mean ± SEM
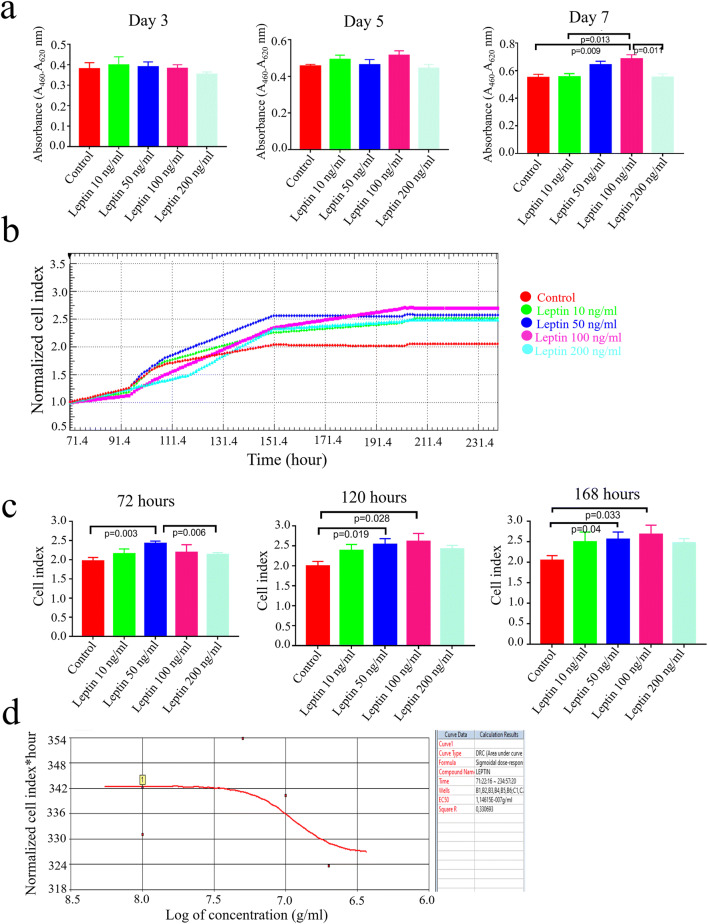
The WST-1 assay and xCELLigence RTCA indicated dose- and time-dependent proliferative effect of leptin on SSC cultures (Fig. 4a, b, c and d). The addition of 100 ng/ml leptin promoted proliferation on day 7 of culture compared with the control in WST-1 assay (p = 0.009, Fig. 4a). Leptin treatment of 50 ng/ml (p = 0.003, p = 0.019, and p = 0.04 on days 3, 5, and 7, respectively) and 100 ng/ml (p = 0.0028 and p = 0.0033 on days 5 and 7, respectively) significantly increased the cell growth curve from day 3 to 7 compared with the control by xCELLigence RTCA. Leptin treatment of 10 and 200 ng/ml did not significantly promote the growth curve in any of the time points compared with the control by xCELLigence RTCA. Leptin’s proliferative effect sustained on SSCs, at the concentration of 100 ng/ml, from 104 to 135 h when compared with the control and other experimental groups (Fig. 4b and c). The growth curves remained unchanged from 135 h in all groups. The real-time analysis revealed the EC50 dose for leptin as 114 ng/ml for 7 days of SSC culture (Fig. 4d).
Fig. 4.
The proliferation analysis of leptin on stem/progenitor spermatogonia with WST-1 assay and xCELLigence RTCA. a The WST-1 assay data presents the proliferative effect of leptin on stem/progenitor spermatogonia in a dose- and time-dependent manner. Note that the maximum proliferative effect is observed in 100 ng/ml leptin treatment group on day 7 of culture, Mean ± SEM, n = 12. b The growth curve shows the dose-dependent proliferative effect of leptin on stem/progenitor spermatogonia compared with the control, in time, by xCELLigence RTCA. Analysis was performed real time for totally 7 days and results were expressed as a cell index (CI) value. c Statistical analysis of CI calculated from six repeated experiments, Mean ± SEM. d The EC50 of leptin was calculated approximately as 114 ng/ml based on the dose-response curves of cell index during 7 days by the RTCA software 1.2.1 (ACEA Bioscience)
Leptin treatment did not change undifferentiated state of stem/progenitor spermatogonia
The total number of CD90.2 (+) spermatogonia was calculated as 75.1, 77.9, 80.8, and 71% following 10, 50, 100, and 200 ng/ml of leptin treatment, respectively (Fig. 5). The highest rate of CD90.2 positivity on stem/progenitor spermatogonia were detected in 100 ng/ml leptin group, insignificantly. The results showed that nearly of all of the stem/progenitor spermatogonia express CD90.2 after leptin treatment independent of the leptin concentration.
Fig. 5.
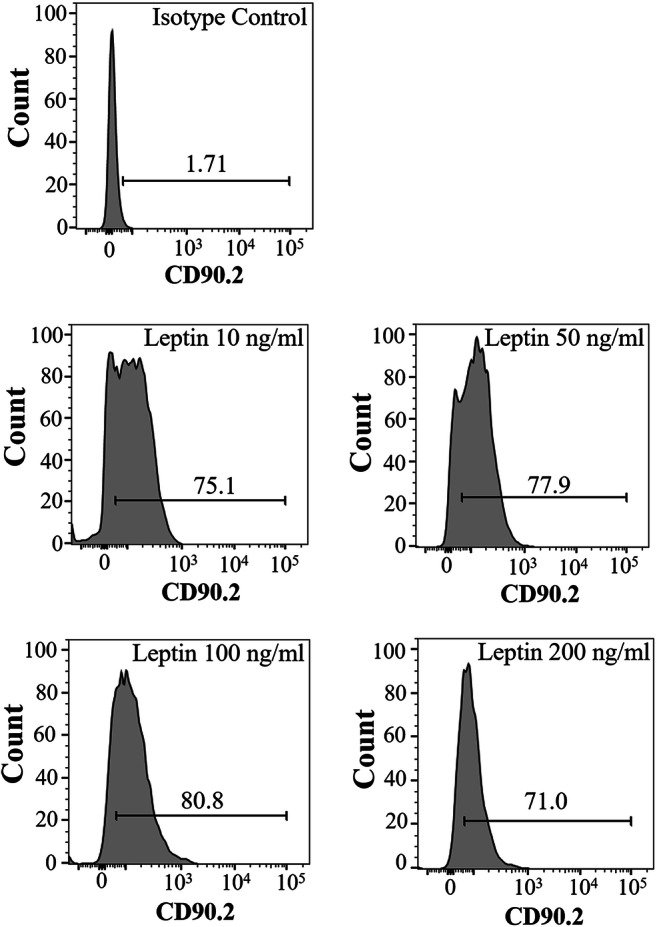
The percentage of CD90.2 (+) stem/progenitor spermatogonia after leptin treatment. Note that 100 ng/ml of leptin provides the highest percentage of CD90.2 positivity
Leptin proliferated stem/progenitor spermatogonia via STAT3 and ERK1/2 signaling pathways
Densitometric analysis of the bands demonstrated that 114 ng/ml leptin treatment increased the level of p-ERK1/2 (p = 0.009) and p-STAT3 (p = 0.023) and decreased p-SHP2 level (p = 0.008) when compared with the control. Leptin treatment did not change the total STAT3 protein level (Fig. 6a and b).
Fig. 6.
Expression of p-STAT3, total-STAT3, p-ERK1/2, and p-SHP2 protein levels in the control and leptin-treated groups by western blot analysis. The stem/progenitor spermatogonia were treated with 114 ng/ml leptin for 30 min. The expression level of proteins was normalized to β-actin. a Representative images for total STAT3, p-STAT3, p-ERK1/2, and p-SHP2 expression. b Statistical analysis of p-SHP2, p-ERK1/2, total STAT3, and p-STAT3 protein levels, Mean ± SEM, n = 3
Discussion
In this study, the neonatal stem/progenitor spermatogonia were isolated successfully and the proliferative effect of leptin in a dose- and time-dependent manner on cultured stem/progenitor spermatogonia was evaluated. Our findings indicated that leptin hormone at 100 ng/ml concentration promoted the proliferation of stem/progenitor spermatogonia on days 5 and 7 of culture compared with the control by means of colony number, colony diameter, WST-1 assay, and xCELLigence RTCA. Moreover, 114 ng/ml of leptin was determined as EC50 to proliferate stem/progenitor spermatogonia for 7 days. Flow cytometry results showed that the 100 ng/ml leptin group provided the highest percentage of CD90.2 positive cells and that highlighted the maintenance of stem and progenitor spermatogonia at undifferentiated state after leptin treatment as well. Our findings also suggested that STAT3 and ERK1/2 signaling pathways were activated by leptin treatment of stem/progenitor spermatogonia cultures, suggesting that these pathways may mediate the proliferative effects of leptin.
We initially mapped the neonatal stem/progenitor spermatogonia within the testes of 6-day-old male C57BL/6 mice by IF labeling and histologic and ultrastructural morphologic assessments and then obtained SSC-enriched testicular cells by using 30% Percoll and MACS technique by CD90.2 microbeads to eliminate other cell fractions, after enzymatic digestion of tubules [40, 50, 51]. Several approaches such as extracellular matrix selection [44, 52], differential plating [42, 43, 53], fluorescent-activated cell sorting (FACS) [54–56], and MACS [40, 50, 51, 56–58] have been used to enrich SSC population and maintain their viability and functionality. The percentage of PLZF (+), CD90.2 (+), and c-kit (−) cells was checked with FCM following MACS separation. Additionally, the cultured stem/progenitor spermatogonia were evaluated for PLZF by IF labeling. These findings clearly indicated that the MACS separation method was an effective and appropriate technique to enrich undifferentiated stem and progenitor spermatogonia prior to in vitro culture. Our results related to fractionation by Percoll and MACS separation by CD90.2 were consistent with previous research groups using similar methods to enrich SSCs [40, 50, 51]. Indeed, the true SSC subpopulation may be further labeled with specific SSC markers such as Id4 and Eomes.
Here we report the existence of the functional leptin receptor (Ob-Rb) on cultured stem/progenitor spermatogonia by IF. The Ob-Rb of leptin presented membranous labeling pattern within the stem/progenitor spermatogonia in our study. El-Hefnawy et al. [59] and Herrid et al [15] indicated the presence of leptin and its receptors in neonatal testis tissue of mice using IHC and RT-PCR. Their IHC assessment revealed membranous labeling pattern for Ob-Rb, and our data is consistent with both studies [15, 59]. Leptin is endogenously produced by EWAT [11] and it is capable of crossing blood-testis barrier to act in paracrine manner on SSCs [60]. Taken together, the Ob-Rb labeling suggests exogenous leptin might target the stem/progenitor spermatogonia in the neonatal testis to exert its potential proliferating effect.
Our proliferation assay results indicated that leptin hormone at 100 ng/ml concentration promoted proliferation in stem/progenitor spermatogonia cultures on days 5 and 7 compared with the control by colony number, colony diameter, WST-1 assay, and xCELLigence RTCA. The proliferative effect of exogenous leptin terminates and reaches the control values when used at a dose of 200 ng/ml. The proliferating effect of exogenous leptin on several somatic stem [24–28] and differentiated [29–32] cells has been reported at a range of 10–500 ng/ml at various time intervals in vitro. Supplementation of 10–100 ng/ml leptin induces derivation of mouse ESCs from blastocysts in embryo culture media [28]. In the same study, leptin-treated, embryo-derived ESCs exhibited Oct-4, Nanog, SSEA-1 pluripotency markers, and positive staining for alkaline phosphatase [28]. Leptin stimulated porcine skeletal myoblast proliferation by MTT assay at a dose range of 2–20 ng/ml for 48 h [26]. It induced and inhibited pre-adipocyte and stromal vascular cell proliferation by 3H-thymidine incorporation assay at a dose range of 50–100 and 250–500 ng/ml, respectively [25]. Those results suggest dose-dependent specific effect of leptin on cellular proliferation of pre-adipocytes. Exogenous 100 ng/ml leptin treatment increased human breast stem cell [27] and mouse embryonic mesenchymal stem cell number [24] by secondary mammosphere formation and thymidine uptake respectively, and it did not change phenotypic features of those cells [24]. Leptin proliferated the human umbilical vein and microvascular endothelial cells at a dose of 10 nmol/l for 48 h by 3 H-thymidine incorporation [29]. Rat smooth muscle cells proliferated with exogenous leptin at a dose range of 10–200 ng/ml when supplemented into the culture [32]. In our study, we report the proliferative dose window for leptin on stem/progenitor spermatogonia as 50–100 ng/ml in terms of colony number, colony diameter and proliferation rate by WST assay from day 5 to 7. Furthermore, we determined for the first time the accurate real-time EC50 dose as 114 ng/ml for 7 days by xCELLigence RTCA. Notably the EC50 is in the proliferative dose range of leptin determined by WST. Leptin was able to proliferate stem/progenitor spermatogonia at undifferentiated state in culture. Even though our results are consistent with proliferative effect of leptin on stem and mature somatic cells shown in previous studies in vitro, controversial observations were reported in in vivo animal models with high leptin doses. Long-term (2 to 6 weeks) systemic leptin administration in a dose range from 3 μg/kg to 3 mg/kg caused alteration in spermatogenesis by reduced sperm count, disruption of seminiferous tubules morphology, and decreased germ cell apoptosis in adult male mice [23, 61] and adult rats [12, 62]. Leptin at 0.1 and 0.5 mg/kg of dose led to no alteration in sperm parameters and number of apoptotic germ cells when applied daily for 2 weeks in adult mice [61]. On the other hand, exogenous systemic leptins’ effects were reported to be reversible on day 56 following 60 μg/kg treatment in adult rats [12]. In vivo studies reveal some negative systemic effects of high doses of leptin. Leptin has not been investigated for its dose-dependent cellular effect on spermatogonial cells. Here we report for the first time the real-time dose-dependent effects of leptin at the cellular level by RTCA. xCELLigence real-time analysis has been reported as a reliable method frequently used to investigate the effective and safe application dose window of several mediators including testosterone, on stem cells [47] or somatic cells [48, 49] so far.
In this current study, western blotting results showed that the EC50 of leptin (114 ng/ml) increased p-ERK1/2 and p-STAT3 levels after 30 min of administration, when compared with those of the untreated control, while p-SHP2 protein level was lower with leptin compared with that of control. Thirty minutes has been reported to be adequate for the induction of p-ERK1/2 pathway in the literature [31, 32] and our results confirmed the induction at that time interval. Leptin of 10 to 100 ng/ml induced ERK1/2 phosphorylation in rat vascular smooth muscle cells [31, 32]. This dose range is almost parallel to ours. Phosphorylation of Tyr985 via JAK2 provides a binding site for SHP2 [16, 63, 64]. Leptin exerts its effect via SHP2- ERK pathway or ERK pathway can be activated independent of SHP2 [63]. The SHP2 exists in neonatal SSCs [65]. Thus, leptin might show its proliferative effect on SSCs through SHP2/ERK signal pathway. In our study, we showed that ERK1/2 phosphorylation increased and the p-SHP2 protein decreased after leptin treatment in stem/progenitor spermatogonia cultures. Several findings reported that SHP2 might be important for survival and proliferation SSCs and to maintain blood-testis barrier [65, 66]. SHP2 also acts on bFGF- and GDNF-mediated activation of ERK signal pathway [65]. Our findings suggest that SHP2 may not have an effective role in the activation of ERK1/2 pathway to proliferate SSCs, and we clearly showed the activation of STAT3 pathway after leptin treatment. Previous studies showed that the activated SHP2 can downregulate STAT3 [67]. While STAT3 pathway promotes differentiation in mouse SSCs [51], this pathway is an effective pathway for stem cell renewal and the maintenance of pluripotency in human and mouse ESCs [68–70] and in the Drosophila male germline [71, 72]. Leptin serves as a potent mitogen for somatic cells by phosphorylation of Ob-Rb on Tyr1138, which is the essential site for STAT3 binding and activation [30]. Leptin increased the renal and endometrial cancer cell proliferation by enhancing the expression of both p-ERK and p-STAT3 [33, 73]. Our findings are in line with those previous results that highlight the Ob-Rb mediated effect of leptin via both SHP2-independent ERK and STAT3 pathway activation.
The study results are limited to in vitro conditions within the mouse model. They should be tested in vitro human SSC cultures. Leptin- proliferated SSCs need to be further transplanted into infertile mouse recipients to evaluate functional capability of these cells in vivo. Those limitations however do not restrict future in vivo and clinical studies as statistical accuracy was validated for our work. Furthermore, the xCELLigence RTCA has been reported as one of the most reliable in vitro techniques that provide assessment of the potential future personalized treatments before clinic. Although SSCs are the only option to regenerate spermatogenesis in pediatric cancer survivors, techniques to remove malignant contamination from testicular tissue should be optimized to eliminate the risk of transplanting cancer cells back into pediatric cancer survivors. Ultimately leptin provides a stepping stone in vitro to proliferate stem/progenitor spermatogonia and maintain their undifferentiated state before human clinical applications.
Conclusion
A large SSC pool is crucial to regenerate spermatogenesis in pediatric cancer survivors. In this current study, we clearly showed that leptin promotes proliferation in neonatal mouse stem/progenitor spermatogonia cultures. Cells in leptin-treated cultures maintained undifferentiated state and exhibited increased activation of the ERK1/2 and STAT3 pathways in vitro. The neonatal mouse testes are developmental similar to the testes of prepubertal or peripubertal boys, suggesting that these results may have implications for the use of testicular tissues that have been preserved from young cancer patients. This output may allow the expansion of SSC pool, in vitro, prior to autotransplantation to regenerate spermatogenesis in cancer survivors.
Authors’ contributions
Nilgun Yersal generated the hypothesis, established the rationale and designed the study together with her mentor Petek Korkusuz, and wrote the manuscript. Nilgun Yersal performed the experimental study, cultured the stem/progenitor spermatogonia, and performed histological examination of the testes. Utku Horzum analyzed and interpreted the flow cytometry and western blotting data. Sevil Kose supervised the MACS isolation and interpreted the xCELLigence data. Sinan Ozkavukcu contributed to the analysis of the cell data. Dr Kyle E Orwig, as the co-advisor of Nilgun Yersal, supervised the Ob receptor immune labeling; Petek Korkusuz and Kyle E Orwig edited the manuscript. All authors read and approved the final manuscript
Funding information
The Research Coordination Unit of Hacettepe University funded this work (THD-2017-13430). Professor Kyle E Orwig was also supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development grant HD092084.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
The Hacettepe University Animal Experimentations Local Ethics Board (#2016/59/1) approved the use of animal material.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nilgün Yersal, Email: nilgunyersal@gmail.com.
Sevil Köse, Email: sevilarslan@hotmail.com.
Utku Horzum, Email: utkuhorzum@gmail.com.
Sinan Özkavukcu, Email: sinozk@gmail.com.
Kyle E. Orwig, Email: korwig@mwri.magee.edu
Petek Korkusuz, Email: petek@hacettepe.edu.tr.
References
- 1.Gholami M, Hemadi M, Saki G, Zendedel A, Khodadadi A, Mohammadi-Asl J. Does prepubertal testicular tissue vitrification influence spermatogonial stem cells (SSCs) viability? J Assist Reprod Genet. 2013;30(10):1271–1277. doi: 10.1007/s10815-013-0050-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Casteren NJ, van der Linden GH, Hakvoort-Cammel FG, Hahlen K, Dohle GR, van den Heuvel-Eibrink MM. Effect of childhood cancer treatment on fertility markers in adult male long-term survivors. Pediatr Blood Cancer. 2009;52(1):108–112. doi: 10.1002/pbc.21780. [DOI] [PubMed] [Google Scholar]
- 3.Mei XX, Wang J, Wu J. Extrinsic and intrinsic factors controlling spermatogonial stem cell self-renewal and differentiation. Asian J Androl. 2015;17(3):347–354. doi: 10.4103/1008-682X.148080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.von Kopylow K, Schulze W, Salzbrunn A, Spiess AN. Isolation and gene expression analysis of single potential human spermatogonial stem cells. Mol Hum Reprod. 2016;22(4):229–239. doi: 10.1093/molehr/gaw006. [DOI] [PubMed] [Google Scholar]
- 5.Wyns C, Van Langendonckt A, Wese FX, Donnez J, Curaba M. Long-term spermatogonial survival in cryopreserved and xenografted immature human testicular tissue. Hum Reprod. 2008;23(11):2402–2414. doi: 10.1093/humrep/den272. [DOI] [PubMed] [Google Scholar]
- 6.Galuppo AG. Spermatogonial stem cells as a therapeutic alternative for fertility preservation of prepubertal boys. Einstein (Sao Paulo) 2015;13(4):637–639. doi: 10.1590/S1679-45082015RB3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen SR, Liu YX. Regulation of spermatogonial stem cell self-renewal and spermatocyte meiosis by Sertoli cell signaling. Reproduction. 2015;149(4):R159–R167. doi: 10.1530/REP-14-0481. [DOI] [PubMed] [Google Scholar]
- 8.Potter SJ, DeFalco T. Role of the testis interstitial compartment in spermatogonial stem cell function. Reproduction. 2017;153(4):R151–RR62. doi: 10.1530/REP-16-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kose S, Yersal N, Onen S, Korkusuz P. Comparison of hematopoietic and spermatogonial stem cell niches from the regenerative medicine aspect. Adv Exp Med Biol. 2018;1107:15–40. doi: 10.1007/5584_2018_217. [DOI] [PubMed] [Google Scholar]
- 10.Jalali AS. Epididymal white adipose tissue: endocrine backbone of spermatogonial stem cells maintenance. J Stem Cell Biol Transplant. 2017;01(03):17
- 11.Chu Y, Huddleston GG, Clancy AN, Harris RB, Bartness TJ. Epididymal fat is necessary for spermatogenesis, but not testosterone production or copulatory behavior. Endocrinology. 2010;151(12):5669–5679. doi: 10.1210/en.2010-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Almabhouh FA, Osman K, Siti Fatimah I, Sergey G, Gnanou J, Singh HJ. Effects of leptin on sperm count and morphology in Sprague-Dawley rats and their reversibility following a 6-week recovery period. Andrologia. 2015;47(7):751–758. doi: 10.1111/and.12325. [DOI] [PubMed] [Google Scholar]
- 13.Hart RA, Dobos RC, Agnew LL, Smart NA, McFarlane JR. Leptin pharmacokinetics in male mice. Endocr Connect. 2017;6(1):20–26. doi: 10.1530/EC-16-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, Gong M. Review of the role of leptin in the regulation of male reproductive function. Andrologia. 2018;50(4) [DOI] [PubMed]
- 15.Herrid M, O'Shea T, McFarlane JR. Ontogeny of leptin and its receptor expression in mouse testis during the postnatal period. Mol Reprod Dev. 2008;75(5):874–880. doi: 10.1002/mrd.20796. [DOI] [PubMed] [Google Scholar]
- 16.Landry D, Cloutier F, Martin LJ. Implications of leptin in neuroendocrine regulation of male reproduction. Reprod Biol. 2013;13(1):1–14. doi: 10.1016/j.repbio.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Ishikawa T, Fujioka H, Ishimura T, Takenaka A, Fujisawa M. Expression of leptin and leptin receptor in the testis of fertile and infertile patients. Andrologia. 2007;39(1):22–27. doi: 10.1111/j.1439-0272.2006.00754.x. [DOI] [PubMed] [Google Scholar]
- 18.Roumaud P, Martin LJ. Roles of leptin, adiponectin and resistin in the transcriptional regulation of steroidogenic genes contributing to decreased Leydig cells function in obesity. Horm Mol Biol Clin Invest. 2015;24(1):25–45. doi: 10.1515/hmbci-2015-0046. [DOI] [PubMed] [Google Scholar]
- 19.Bhat GK, Sea TL, Olatinwo MO, Simorangkir D, Ford GD, Ford BD, Mann DR. Influence of a leptin deficiency on testicular morphology, germ cell apoptosis, and expression levels of apoptosis-related genes in the mouse. J Androl. 2006;27(2):302–310. doi: 10.2164/jandrol.05133. [DOI] [PubMed] [Google Scholar]
- 20.Martins FF, Aguila MB, Mandarim-de-Lacerda CA. Impaired steroidogenesis in the testis of leptin-deficient mice (ob/ob -/-) Acta Histochem. 2017;119(5):508–515. doi: 10.1016/j.acthis.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann A, Manjowk GM, Wagner IV, Kloting N, Ebert T, Jessnitzer B, et al. Leptin within the subphysiological to physiological range dose dependently improves male reproductive function in an obesity mouse model. Endocrinology. 2016;157(6):2461–2468. doi: 10.1210/en.2015-1966. [DOI] [PubMed] [Google Scholar]
- 22.Schoeller EL, Chi M, Drury A, Bertschinger A, Esakky P, Moley KH. Leptin monotherapy rescues spermatogenesis in male Akita type 1 diabetic mice. Endocrinology. 2014;155(8):2781–2786. doi: 10.1210/en.2014-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Esmaili-Nejad MR, Babaei H, Kheirandish R. The effects of long-term leptin administration on morphometrical changes of mice testicular tissue. Iran J Basic Med Sci. 2015;18(12):1176–1182. [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi Y, Okimura Y, Mizuno I, Iida K, Takahashi T, Kaji H, Abe H, Chihara K. Leptin induces mitogen-activated protein kinase-dependent proliferation of C3H10T1/2 cells. J Biol Chem. 1997;272(20):12897–12900. doi: 10.1074/jbc.272.20.12897. [DOI] [PubMed] [Google Scholar]
- 25.Wagoner B, Hausman DB, Harris RB. Direct and indirect effects of leptin on preadipocyte proliferation and differentiation. Am J Phys Regul Integr Comp Phys. 2006;290(6):R1557–R1564. doi: 10.1152/ajpregu.00860.2005. [DOI] [PubMed] [Google Scholar]
- 26.Yu T, Luo G, Zhang L, Wu J, Zhang H, Yang G. Leptin promotes proliferation and inhibits differentiation in porcine skeletal myoblasts. Biosci Biotechnol Biochem. 2008;72(1):13–21. doi: 10.1271/bbb.70244. [DOI] [PubMed] [Google Scholar]
- 27.Esper RM, Dame M, McClintock S, Holt PR, Dannenberg AJ, Wicha MS, Brenner DE. Leptin and adiponectin modulate the self-renewal of normal human breast epithelial stem cells. Cancer Prev Res (Phila) 2015;8(12):1174–1183. doi: 10.1158/1940-6207.CAPR-14-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taskin AC, Kocabay A, Ebrahimi A, Karahuseyinoglu S, Sahin GN, Ozcimen B, Ruacan A, onder TT. Leptin treatment of in vitro cultured embryos increases outgrowth rate of inner cell mass during embryonic stem cell derivation. In Vitro Cell Dev Biol Anim. 2019;55(7):473–481. doi: 10.1007/s11626-019-00367-y. [DOI] [PubMed] [Google Scholar]
- 29.Artwohl M, Roden M, Holzenbein T, Freudenthaler A, Waldhausl W, Baumgartner-Parzer SM. Modulation by leptin of proliferation and apoptosis in vascular endothelial cells. Int J Obes Relat Metab Disord. 2002;26(4):577–580. doi: 10.1038/sj.ijo.0801947. [DOI] [PubMed] [Google Scholar]
- 30.Goren I, Pfeilschifter J, Frank S. Determination of leptin signaling pathways in human and murine keratinocytes. Biochem Biophys Res Commun. 2003;303(4):1080–1085. doi: 10.1016/s0006-291x(03)00480-7. [DOI] [PubMed] [Google Scholar]
- 31.Huang F, Xiong X, Wang H, You S, Zeng H. Leptin-induced vascular smooth muscle cell proliferation via regulating cell cycle, activating ERK1/2 and NF-kappa B. Acta Biochim Biophys Sin Shanghai. 2010;42(5):325–331. doi: 10.1093/abbs/gmq025. [DOI] [PubMed] [Google Scholar]
- 32.Tsai YC, Lee YM, Hsu CH, Leu SY, Chiang HY, Yen MH, Cheng PY. The effect of ferulic acid ethyl ester on leptin-induced proliferation and migration of aortic smooth muscle cells. Exp Mol Med. 2015;47:e180. doi: 10.1038/emm.2015.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li L, Gao Y, Zhang LL, He DL. Concomitant activation of the JAK/STAT3 and ERK1/2 signaling is involved in leptin-mediated proliferation of renal cell carcinoma Caki-2 cells. Cancer Biol Ther. 2008;7(11):1787–1792. doi: 10.4161/cbt.7.11.6837. [DOI] [PubMed] [Google Scholar]
- 34.Yoon KW, Park SY, Kim JY, Lee SM, Park CH, Cho SB, et al. Leptin-induced adhesion and invasion in colorectal cancer cell lines. Oncol Rep. 2014;31(6):2493–2498. doi: 10.3892/or.2014.3128. [DOI] [PubMed] [Google Scholar]
- 35.Oliver P, Pico C, Palou A. Ontogenesis of leptin expression in different adipose tissue depots in the rat. Pflugers Arch. 2001;442(3):383–390. doi: 10.1007/s004240100540. [DOI] [PubMed] [Google Scholar]
- 36.Hansel W. The essentiality of the epididymal fat pad for spermatogenesis. Endocrinology. 2010;151(12):5565–5567. doi: 10.1210/en.2010-1146. [DOI] [PubMed] [Google Scholar]
- 37.Sharma D, Saxena NK, Vertino PM, Anania FA. Leptin promotes the proliferative response and invasiveness in human endometrial cancer cells by activating multiple signal-transduction pathways. Endocr Relat Cancer. 2006;13(2):629–640. doi: 10.1677/erc.1.01169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Magarinos MP, Sanchez-Margalet V, Kotler M, Calvo JC, Varone CL. Leptin promotes cell proliferation and survival of trophoblastic cells. Biol Reprod. 2007;76(2):203–210. doi: 10.1095/biolreprod.106.051391. [DOI] [PubMed] [Google Scholar]
- 39.Fontoura P, Mello MD, Gallo-Sa P, Erthal-Martins MC, Cardoso MC, Ramos C. Leptin improves sperm cryopreservation via antioxidant defense. J Reprod Infertil. 2017;18(1):172–178. [PMC free article] [PubMed] [Google Scholar]
- 40.Niu Z, Goodyear SM, Avarbock MR, Brinster RL. Chemokine (C-X-C) Ligand 12 facilitates trafficking of donor spermatogonial stem cells. Stem Cells Int. 2016;2016:5796305. doi: 10.1155/2016/5796305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Azizi H, Skutella T, Shahverdi A. Generation of mouse spermatogonial stem-cell-colonies in a non-adherent culture. Cell J. 2017;19(2):238–249. doi: 10.22074/cellj.2016.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang P, Suo LJ, Wang YF, Shang H, Li GX, Hu JH, Li QW. Effects of GDNF and LIF on mouse spermatogonial stem cells proliferation in vitro. Cytotechnology. 2014;66(2):309–316. doi: 10.1007/s10616-013-9574-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang P, Zheng Y, Li Y, Shang H, Li GX, Hu JH, Li QW. Effects of testicular interstitial fluid on the proliferation of the mouse spermatogonial stem cells in vitro. Zygote. 2014;22(3):395–403. doi: 10.1017/S0967199413000142. [DOI] [PubMed] [Google Scholar]
- 44.Mohamadi SM, Movahedin M, Koruji SM, Jafarabadi MA, Makoolati Z. Comparison of colony formation in adult mouse spermatogonial stem cells developed in Sertoli and STO coculture systems. Andrologia. 2012;44(Suppl 1):431–437. doi: 10.1111/j.1439-0272.2011.01201.x. [DOI] [PubMed] [Google Scholar]
- 45.Navid S, Rastegar T, Baazm M, Alizadeh R, Talebi A, Gholami K, Khosravi-Farsani S, Koruji M, Abbasi M. In vitro effects of melatonin on colonization of neonate mouse spermatogonial stem cells. Syst Biol Reprod Med. 2017;63(6):370–381. doi: 10.1080/19396368.2017.1358774. [DOI] [PubMed] [Google Scholar]
- 46.Bai Y, Feng M, Liu S, Wei H, Li L, Zhang X, Shen C, Zhang S, Ma N. Differential gene expression in mouse spermatogonial stem cells and embryonic stem cells. Int J Mol Med. 2016;38(2):423–432. doi: 10.3892/ijmm.2016.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Corotchi MC, Popa MA, Simionescu M. Testosterone stimulates proliferation and preserves stemness of human adult mesenchymal stem cells and endothelial progenitor cells. Romanian J Morphol Embryol. 2016;57(1):75–80. [PubMed] [Google Scholar]
- 48.Mendi A, Yagci BG, Kiziloglu M, Sarac N, Yilmaz D, Ugur A, et al. Effects of Syzygium aromaticum, Cinnamomum zeylanicum, and Salvia triloba extracts on proliferation and differentiation of dental pulp stem cells. J Appl Oral Sci. 2017;25(5):515–522. doi: 10.1590/1678-7757-2016-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pakdemirli A, Toksoz F, Karadag A, Misirlioglu HK, Baspinar Y, Ellidokuz H, et al. Role of mesenchymal stem cell-derived soluble factors and folic acid in wound healing. Turk J Med Sci. 2019;49(3):914–921 [DOI] [PMC free article] [PubMed]
- 50.Kubota H, Brinster RL. Culture of rodent spermatogonial stem cells, male germline stem cells of the postnatal animal. Methods Cell Biol. 2008;86:59–84. doi: 10.1016/S0091-679X(08)00004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oatley JM, Kaucher AV, Avarbock MR, Brinster RL. Regulation of mouse spermatogonial stem cell differentiation by STAT3 signaling. Biol Reprod. 2010;83(3):427–433. doi: 10.1095/biolreprod.109.083352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shakeri M, Kohram H, Shahverdi A, Shahneh AZ, Tavakolifar F, Pirouz M, Shahrebabak HM, Koruji M, Baharvand H. Behavior of mouse spermatogonial stem-like cells on an electrospun nanofibrillar matrix. J Assist Reprod Genet. 2013;30(3):325–332. doi: 10.1007/s10815-012-9916-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koruji M, Movahedin M, Mowla SJ, Gourabi H, Arfaee AJ. Efficiency of adult mouse spermatogonial stem cell colony formation under several culture conditions. In Vitro Cell Dev Biol Anim. 2009;45(5-6):281–289. doi: 10.1007/s11626-008-9169-y. [DOI] [PubMed] [Google Scholar]
- 54.Shinohara T, Orwig KE, Avarbock MR, Brinster RL. Spermatogonial stem cell enrichment by multiparameter selection of mouse testis cells. Proc Natl Acad Sci U S A. 2000;97(15):8346–8351. doi: 10.1073/pnas.97.15.8346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kubota H, Avarbock MR, Brinster RL. Spermatogonial stem cells share some, but not all, phenotypic and functional characteristics with other stem cells. Proc Natl Acad Sci U S A. 2003;100(11):6487–6492. doi: 10.1073/pnas.0631767100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kubota H, Avarbock MR, Brinster RL. Culture conditions and single growth factors affect fate determination of mouse spermatogonial stem cells. Biol Reprod. 2004;71(3):722–731. doi: 10.1095/biolreprod.104.029207. [DOI] [PubMed] [Google Scholar]
- 57.Buageaw A, Sukhwani M, Ben-Yehudah A, Ehmcke J, Rawe VY, Pholpramool C, Orwig KE, Schlatt S. GDNF family receptor alpha1 phenotype of spermatogonial stem cells in immature mouse testes. Biol Reprod. 2005;73(5):1011–1016. doi: 10.1095/biolreprod.105.043810. [DOI] [PubMed] [Google Scholar]
- 58.Oatley JM, Oatley MJ, Avarbock MR, Tobias JW, Brinster RL. Colony stimulating factor 1 is an extrinsic stimulator of mouse spermatogonial stem cell self-renewal. Development. 2009;136(7):1191–1199. doi: 10.1242/dev.032243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.El-Hefnawy T, Ioffe S, Dym M. Expression of the leptin receptor during germ cell development in the mouse testis. Endocrinology. 2000;141(7):2624–2630. doi: 10.1210/endo.141.7.7542. [DOI] [PubMed] [Google Scholar]
- 60.Banks WA, McLay RN, Kastin AJ, Sarmiento U, Scully S. Passage of leptin across the blood-testis barrier. Am J Phys. 1999;276(6):E1099–E1104. doi: 10.1152/ajpendo.1999.276.6.E1099. [DOI] [PubMed] [Google Scholar]
- 61.Wang X, Zhang X, Hu L, Li H. Exogenous leptin affects sperm parameters and impairs blood testis barrier integrity in adult male mice. Reprod Biol Endocrinol. 2018;16(1):55. doi: 10.1186/s12958-018-0368-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haron MN, D'Souza UJ, Jaafar H, Zakaria R, Singh HJ. Exogenous leptin administration decreases sperm count and increases the fraction of abnormal sperm in adult rats. Fertil Steril. 2010;93(1):322–324. doi: 10.1016/j.fertnstert.2009.07.995. [DOI] [PubMed] [Google Scholar]
- 63.Fruhbeck G. Intracellular signalling pathways activated by leptin. Biochem J. 2006;393(Pt 1):7–20. doi: 10.1042/BJ20051578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moreira BP, Monteiro MP, Sousa M, Oliveira PF, Alves MG. Insights into leptin signaling and male reproductive health: the missing link between overweight and subfertility? Biochem J. 2018;475(22):3535–3560. doi: 10.1042/BCJ20180631. [DOI] [PubMed] [Google Scholar]
- 65.Puri P, Phillips BT, Suzuki H, Orwig KE, Rajkovic A, Lapinski PE, King PD, Feng GS, Walker WH. The transition from stem cell to progenitor spermatogonia and male fertility requires the SHP2 protein tyrosine phosphatase. Stem Cells. 2014;32(3):741–753. doi: 10.1002/stem.1572. [DOI] [PubMed] [Google Scholar]
- 66.Puri P, Walker WH. The regulation of male fertility by the PTPN11 tyrosine phosphatase. Semin Cell Dev Biol. 2016;59:27–34. doi: 10.1016/j.semcdb.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 67.Zhang J, Zhang F, Niu R. Functions of Shp2 in cancer. J Cell Mol Med. 2015;19(9):2075–2083. doi: 10.1111/jcmm.12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Niwa H, Burdon T, Chambers I, Smith A. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev. 1998;12(13):2048–2060. doi: 10.1101/gad.12.13.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Raz R, Lee CK, Cannizzaro LA, d'Eustachio P, Levy DE. Essential role of STAT3 for embryonic stem cell pluripotency. Proc Natl Acad Sci U S A. 1999;96(6):2846–2851. doi: 10.1073/pnas.96.6.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Y, Wang D, Xu J, Wang Y, Ma F, Li Z, Liu N. Stat3 activation is critical for pluripotency maintenance. J Cell Physiol. 2019;234(2):1044–1051. doi: 10.1002/jcp.27241. [DOI] [PubMed] [Google Scholar]
- 71.Kiger AA, Jones DL, Schulz C, Rogers MB, Fuller MT. Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science. 2001;294(5551):2542–2545. doi: 10.1126/science.1066707. [DOI] [PubMed] [Google Scholar]
- 72.Tulina N, Matunis E. Control of stem cell self-renewal in Drosophila spermatogenesis by JAK-STAT signaling. Science. 2001;294(5551):2546–2549. doi: 10.1126/science.1066700. [DOI] [PubMed] [Google Scholar]
- 73.Liu Y, Lv L, Xiao W, Gong C, Yin J, Wang D, Sheng H. Leptin activates STAT3 and ERK1/2 pathways and induces endometrial cancer cell proliferation. J Huazhong Univ Sci Technolog Med Sci. 2011;31(3):365–370. doi: 10.1007/s11596-011-0382-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.