Abstract
Direct oral anticoagulants (DOACs) may be good alternatives to low molecular weight heparin (LMWH) or vitamin K antagonists (VKA) for treatment of cancer associated thrombosis (CAT). We conducted a meta-analysis of ten randomized clinical trials to evaluate the efficacy and safety of DOACs in patients with CAT. All had study populations composed in entirety or in part of patients with CAT. The primary outcome (efficacy) was recurrent VTE and the secondary outcomes (safety outcomes) included major bleeding, clinically relevant non-major bleeding (CRNMB), and all bleeding (major bleeding + CRNMB). Participants treated with DOACs had lower risk of recurrent VTE, overall (RR 0.63; 95% CI 0.51–0.79; p < 0.0001), compared to LMWH (RR 0.57; 95% CI 0.40–0.83; p = 0.003), but not compared to VKA (RR 0.69; 95% CI 0.44–1.06; p = 0.09). Compared to LMWH, DOACs showed no difference in major bleeding risk (RR 1.31; 95% CI 0.78–2.18; p = 0.31), though had higher risk of CRNMB (RR 1.60; 95% CI 1.13–2.26; p = 0.008) and all bleeding (RR 1.49; 95% CI 1.10–2.01; p = 0.010). These results indicate that DOACs are more effective than LMWH for prevention of recurrent VTE with CAT though carry an increased risk for non-major bleeding compared to standard of care, LMWH.
Subject terms: Cancer, Medical research, Oncology
Introduction
Active malignancy is a well described prothrombotic state1 and cancer patients carry a four to seven fold higher increased risk for venous thromboembolism (VTE) including pulmonary embolism (PE) and deep vein thrombosis (DVT) compared to the general population2,3. Cancer associated thrombosis (CAT) is consequential, resulting in significant morbidity and increased mortality4–6. Even with anticoagulation, the reported incidence of recurrent VTE can be as high as 6%, or 3.5 times that of the general population with VTE7,8.
For the past decade, the standard of care for treatment of CAT has been low molecular weight heparin (LMWH) following the landmark CLOT study demonstrating superiority of dalteparin over vitamin K antagonist (VKA) for prevention of recurrent VTE9. However, in clinical practice, providing optimal treatment with LMWH is challenging. Reports show that only half of all patients are fully adherent and many discontinue treatment prematurely10. Poor compliance with LMWH occurs for a variety of reasons including financial burden, inconvenience of administration, or development of painful hematoma and scarring at the injection site. Furthermore, adequate dosing of LMWH in elderly patients, patients with impaired renal function, and obese patients is difficult to achieve due to variable absorption, metabolism, and clearance11.
Direct oral anticoagulants (DOACs) including dabigatran, rivaroxaban, apixaban, and edoxaban, have less variable pharmacokinetics with rapid onset of action, uniform peak levels, and clearance less affected by extrinsic factors12. Given these reasons and ease of administration, DOACs are a desirable alternative to LMWH for patients with active malignancy who may need prolonged anticoagulation. DOACs have demonstrated comparable efficacy and safety to VKA in unselected cancer subpopulations from major RCT13–18. Separately, rivaroxaban, apixaban, and edoxaban were shown to be non-inferior to LMWH for secondary VTE prevention in patients with CAT19–21. However, clinical guidelines, including those of the National Comprehensive Cancer Network (NCCN) continue to recommend LMWH as the first line treatment for CAT and only recently adopted edoxaban, rivaroxaban and apixaban as treatment options for CAT. Other DOACs are listed as secondary treatment options in patients with compelling reasons to avoid LMWH22. Here, we present a meta-analysis to evaluate the clinical usefulness of all DOACs for the treatment of CAT, considering the efficacy and safety of this category of anticoagulants.
Methods
The methods for this meta-analysis are in accordance with “Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA)” (Fig. 1).
Figure 1.
Prisma flow diagram of selection of studies.
Search strategy
We conducted a systemic literature search of MEDLINE, EMBASE, and Cochrane Central Register of Controlled Trials (CENTRAL) data bases from June 1, 2014-April 31, 2020. We hand searched the American Society for Clinical Oncology (ASCO) and American Society of Hematology (ASH) annual meeting guidelines from 2017–2019. We identified all randomized clinical trials (RCT) which enrolled patients with active malignancy for inclusion in the study for further review. Detailed search strategy is shown in Fig. 1.
Study selection
Two authors (GK and NT) independently identified studies eligible for inclusion in the systematic review based on screen of titles and abstracts. Discrepancies were resolved by consensus. At all stages of screening, number of studies identified and reasons for inclusion and exclusion were documented. Full papers were included for review if they met inclusion criteria for the meta-analysis. Inclusion criteria were: RCT with study population consisted in whole or in part of adult (age > 18 years) patients with active malignancy and CAT with intervention consisting of DOAC (dabigatran, rivaroxaban, apixaban, or edoxaban) compared to LMWH or VKA.
Data extraction and quality assessment
Two authors (GK and NT) extracted data independently in duplicate. The primary outcome (efficacy outcome) of interest was incidence of recurrent VTE. The secondary outcomes (safety outcome) of interest was incidence of major bleeding (MB), clinically relevant non-major bleeding (CRNMB), and all bleeding events (composite MB and CRNMB). Outcomes were defined according to criteria used in the included studies though most studies noted bleeding outcomes per ISTH criteria23. Data regarding methods, conduct, and design of studies were extracted for assessing risk of bias. We employed the Cochrane Collaboration’s risk of bias tool to assess risk for bias24. The authors independently judged quality domains using a two point scale: low risk of bias, plausible bias is unlikely to seriously alter results; high risk of bias, plausible bias that may seriously weaken confidence in results. The GRADE approach was applied to assess quality of evidence for each outcome25. We used the GRADE-Pro software to create an evidence profile26.
Statistical analysis
For each outcome, data was pooled using the Mantel–Haenszel method and random effects model was applied to report risk ratio (RR) and 95% confidence interval (CI). In cases where the study authors did not separately report number of MB and CRNMB, bleeding data was applied to all bleeding events. The Cochran χ2 test and I2 statistic were used to test for heterogeneity between studies. We deemed I2 > 50% as substantial heterogeneity. A p-value < 0.05 was considered statistically significant24. Two separate subgroup meta-analysis of studies that evaluated DOAC compared to LMWH and DOAC compared to VKA were performed. Overall met-analysis incorporating all studies was also conducted. The meta-analysis was conducted using the Review Manger (RevMan), version 5.3 software27.
Results
Results of search
The data base search identified 238 citations. 204 citations were excluded by title and abstract alone. Thirty-four studies were evaluated in full text review. Of these, 24 were excluded based on the following reasons: 15 did not include any active cancer patients, five were post-hoc analyses of already included studies, two studies were duplicates, two were not RCT with one study being a risk benefit analysis of an already included study and one study was an economic analysis. We included ten RCTs in this systematic review and meta-analysis (Fig. 1).
Included studies
We included ten RCTs comparing DOAC to VKA or LMWH for treatment of CAT in patients with active malignancy. One RCT evaluated apixaban compared to enoxaparin followed by warfarin (AMPLIFY)13; two RCTs evaluated rivaroxaban compared to enoxaparin followed by warfarin or acenocoumarin (EINSTEIN DVT, EINSTEIN PE)14,15 with CAT subset data reported in pooled analysis (EINSTEIN DVT/PE)28; two RCTs reported effects of dabigatran compared to LMWH followed by warfarin (RE-COVER I, RE-COVER II)17,18 with CAT subset data reported in pooled analysis (RE-COVER I&II)29; one RCT reported the effects of edoxaban compared to warfarin (Hokusai 2013)15; one RCT reported the effects of edoxaban compared to dalteparin (Hokusai 2018)19; one RCT reported the effects of rivaroxaban compared to dalteparin (SELECT-D)20; two RCTs reported the effects of apixaban compared to dalteparin (ADAM VTE and Caravaggio)21,30. Characteristics of RCT and participants are described in Table 1. Inclusion criteria for individual studies are noted in Fig. 1.
Table 1.
Description of study and participants. NR, not reported. *type of cancer diagnosis was only reported for 114 patients.
| Clinical trial | % cancer patients in study | RCT design | Follow up | Primary outcome | Study participants | ||
|---|---|---|---|---|---|---|---|
| Characteristic | DOAC | Comparator | |||||
| ADAM VTE | 100% | Open label, superiority | 6 months | Major bleeding |
Drug Number patients Age, gender Most common cancer Metastatic cancer Anticancer therapy |
Apixaban 150 64, 48% male Lung 22% (32/150) 65% (96/150) 73% (108/150) |
Dalteparin 150 64, 49% male Pancreatic 16% (24/150) 66% (97/150) 74% (110/150) |
| Hokusai 2018 | 100% | Open label, non-inferiority | 12 months | VTE recurrence |
Drug Number patients Age, gender Most common cancer Metastatic cancer Anticancer therapy |
Edoxaban 522 64, 53% male Colorectal (16%) 53% (274/522) 72% (374/522) |
Dalteparin 524 64, 50% male Colorectal (15%) 53% (280/524) 73% (383/524) |
| SELECT-D | 100% | Open label, pilot trial | 6 months | VTE recurrence |
Drug Number patients Age, gender Most common cancer Metastatic cancer Anticancer therapy |
Rivaroxaban 203 67, 57% male Colorectal 27% (55/203) 58% (118/203) 69% (140/203) |
Dalteparin 203 67, 48% male Colorectal 23% (47/203) 58% (118/203) 70% (142/203) |
| Caravaggio | 100% | Open label, non-inferiority | 6 months | VTE recurrence |
Drug Number patients Age, gender Most common cancer Metastatic cancer Anticancer therapy |
Apixaban 576 67, 51% male Colorectal 21% (121/576) 68% (389/576) 61% (350/576) |
Dalteparin 579 67, 48% male Colorectal 19% (113/579) 69% (396/579) 63% (350/579) |
| Amplify | 2.5% | Double- blind, non-inferiority | 6 months | VTE recurrence |
Drug Number patients Age, gender Most common cancer Metastatic cancer Anticancer therapy |
Apixaban 88 66, 57% male Prostate %NR “approximately 1/3” NR |
Warfarin 81 65, 61% male Prostate %NR “approximately 1/3” NR |
| Hokusai 2013 | 2.5% | Double-blind, non-inferiority | 12 months | VTE recurrence |
Drug Number patients Age, gender Most common cancer Metastatic cancer Anticancer therapy |
Edoxaban 109 NR NR NR NR |
Warfarin 99 NR NR NR NR |
| RE-COVER I & II | 7.0% | Double-blind, double-dummy, non-inferiority | 6 months | VTE recurrence |
Drug Number patients Age, gender Most common cancer Metastatic cancer Anticancer therapy |
Dabigatran 173 63, 64% male Prostate 20% (23/114)* 9% (11/114)* NR |
Warfarin 162 65, 62% male Prostate 21% (22/107)* 16% (17/107)* NR |
| EINSTEIN DVT/PE | 7.2% | Open label, non-inferiority | 3–12 months | VTE recurrence |
Drug Number patients Age, gender Most common cancer Metastatic cancer Anticancer therapy |
Rivaroxaban 316 NR NR NR NR |
Warfarin or acenocoumerol 281 NR NR NR NR |
Risk of bias
Overall, included RCTs were free from any major risk of bias (Fig. 2).
Figure 2.

Cochrane Collaboration risk of bias summary.
Blinding
Four of ten RCTs were randomized, double blinded clinical trials (Amplify, Re-cover I, Re-cover II, Hokusai 2013). Six of ten RCTs were randomized, open label clinical trials (Einstein DVT, Einstein PE, Hokusai 2018, Select-D, Adam VTE, Caravaggio). All RCTs reported detailed blinding procedures for an independent adjudication process for outcomes assessment. Therefore, all ten RCTs were determined to have low risk for performance and selection bias.
Outcomes data
Five of ten RCTs reported the intention to treat (ITT) method to evaluate the benefit outcome of recurrent VTE (AMPLIFY, EINSTEN DVT, Einstein PE, SELECT-D, Caravaggio) and utilized per-protocol method to analyze the secondary safety outcomes (bleeding). Accordingly, these four RCTs were assessed to have low risk of attrition bias. Five studies used a modified ITT (mITT) analysis of benefit outcome of recurrent VTE. The RE-COVER I, RE-COVER II, Hokusai 2013, Hokusai 2018, and ADAM VTE defined mITT as population of patients who were randomized and received at least one dose of study drug. The safety outcomes in these RCTs were not analyzed per protocol prespecified rules, instead they were analyzed using mITT population. However, overall difference in analyzed participants between treatment arms were minimal in these studies. Hence these RCTs were also assessed to have low risk of attrition bias.
Other potential bias
All ten RCTs reported methods of randomization, allocation concealment, and consistently reported the outcomes states before the trial. Pre-specified alpha and beta errors (elements for calculating risk of random error) and sample sizes were reported for all RCTs. All RCTs were therefore assessed as having low risk of selection bias.
Effects of interventions
Ten RCTs were evaluated for overall effect. Four RCTs (ADAM VTE, Hokusai 2018, SELECT-D, Caravaggio) were included for subgroup analysis comparing DOACs to LMWH and six RCTs (AMPLIFY, EINSTEIN DVT/PE, RE-COVER I & II, and Hokusai 2013) were included for subgroup analysis comparing DOACs to VKA. The Hokusai 2013 study did not report separate safety outcomes for MB or CRNMB and thus was excluded from subgroup analysis of these outcomes.
Efficacy of DOACs (recurrent VTE)
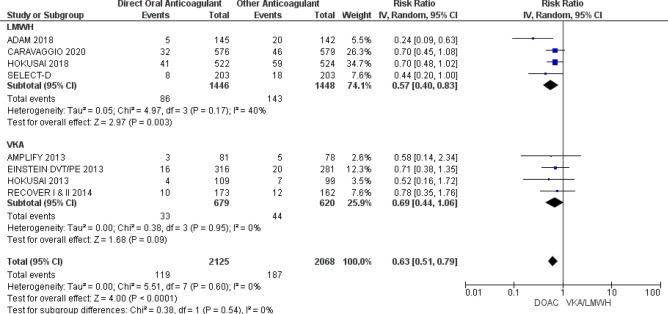
Data from 4193 participants was pooled for evaluation of recurrent VTE. Participants treated DOACs had lower risk of recurrent VTE compared to participants treated with either VKA or LMWH (RR 0.63; 95% CI 0.51–0.79; p < 0.0001; I2 = 0%). In subgroup analysis comparing DOAC to LMWH reduced risk for recurrent VTE was noted with use of DOACs (RR 0.57; 95% CI 0.40–0.83; p = 0.003; I2 = 40%). Subgroup analysis comparing DOAC to VKA showed no difference in recurrent VTE risk (RR 0.69; 95% CI 0.44–1.06; p = 0.09; I2 = 0%) (Fig. 3). Per GRADE criteria, the quality of evidence was judged to be high for VTE recurrence (Table 2).
Figure 3.
Efficacy (VTE recurrence) of DOAC. Forest plots show risk ratio (RR) of VTE recurrence of pooled data from all studies and subgroup analyses of studies evaluating DOAC compared to LMWH and DOAC compared VKA. Boxes superimposing RR estimates are proportional to the weight of the included study. Heterogeneity between RCT is assessed by the I2 statistic.
Table 2.
GRADE analysis for quality of evidence.
| Outcome | Certainty Assessment | Patients | Anticipated effects | Certainty | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other | DOAC | Other AC | Relative risk (95% CI) | Absolute risk reduction with DOAC (95% CI) | ||
| DOAC compared to VKA/LMWH | ||||||||||||
| VTE | 10 | RCT | Not serious | Not serious | Not serious | Not serious | None | 5.6% (119/2125) | 9.0% (187/2068) | 0.63 (0.51–0.79) | 33 fewer per 1000 (44 fewer to 19 fewer) |
⊕ ⊕ ⊕ ⊕ High |
| MB | 9 | RCT | Not serious | Not serious | Not serious | Not serious | None | 4.3% (86/2008) | 4.0% (78/1958) | 1.01 (0.65–1.56) | 0 fewer per 1000 (14 fewer to 22 more) |
⊕ ⊕ ⊕ ⊕ High |
| CRNMB | 9 | RCT | Not serious | Not serious | Not serious | Not serious | None | 11.7% (235/2008) | 8.9% (174/1958) | 1.28 (0.95–1.73) | 25 more per 1000 (4 fewer to 65 more) |
⊕ ⊕ ⊕ ⊕ High |
| All bleed | 10 | RCT | Not serious | Not serious | Not serious | Not serious | None | 16.1% (341/2117) | 13.5% (277/2057) | 1.11 (0.84–1.46) | 15 more per 1000 (22 fewer to 62 more) |
⊕ ⊕ ⊕ ⊕ High |
| DOAC compared to LMWH | ||||||||||||
| VTE | 4 | RCT | Not serious | Not serious | Not serious | Not serious | None | 5.9% (86/1446) | 9.9% (143/1448) | 0.57 (0.40–0.83) | 42 fewer per 1000 (59 fewer to 17 fewer) |
⊕ ⊕ ⊕ ⊕ High |
| MB | 4 | RCT | Not serious | Not serious | Not serious | Not serious | None | 4.8% (69/1446) | 3.7% (53/1448) | 1.31 (0.78–2.18) | 11 more per 1000 (8 fewer to 43 more) |
⊕ ⊕ ⊕ ⊕ High |
| CRNMB | 4 | RCT | Not serious | Not serious | Not serious | Not serious | None | 11.2% (162/1446) | 7.3% (106/1448) | 1.6 (1.13–2.26) | 44 more per 1000 (10 more to 92 more) |
⊕ ⊕ ⊕ ⊕ High |
| All bleed | 4 | RCT | Not serious | Not serious | Not serious | Not serious | None | 16.0% (231/1446) | 11.0% (159/1448) | 1.49 (1.1–2.01) | 54 more per 1000 (11 more to 111 more) |
⊕ ⊕ ⊕ ⊕ High |
| DOAC compared to VKA | ||||||||||||
| VTE | 6 | RCT | Not serious | Not serious | Not serious | Not serious | None | 4.9% (33/679) | 7.1% (44/620) | 0.69 (0.44– 1.06) | 22 fewer per 1000 (40 fewer to 4 more) |
⊕ ⊕ ⊕ ⊕ High |
| MB | 5 | RCT | Not serious | Not serious | Not serious | Not serious | None | 3.0% (17/562) | 4.9% (25/510) | 0.62 (0.34– 1.14) | 19 fewer per 1000 (32 fewer to 7 more) |
⊕ ⊕ ⊕ ⊕ High |
| CRNMB | 5 | RCT | Not serious | Not serious | Not serious | Not serious | None | 13.0% (73/562) | 13.3% (68/510) | 0.95 (0.63– 1.41) | 7 fewer per 1000 (49 fewer to 55 more) |
⊕ ⊕ ⊕ ⊕ High |
| All bleed | 6 | RCT | Not serious | Not serious | Not serious | Not serious | None | 16.4% (110/671) | 19.4% (118/609) | 0.84 (0.65– 1.08) | 31 fewer per 1000 (68 fewer to 16 more) |
⊕ ⊕ ⊕ ⊕ High |
VTE (recurrent) venous thromboembolism, MB major bleeding, CRNMB clinically relevant non major bleeding, all bleeding MB + CRNMB, DOAC direct oral anticoagulant, other AC other anticoagulant (VKA or LMWH).
Safety outcomes (bleeding)
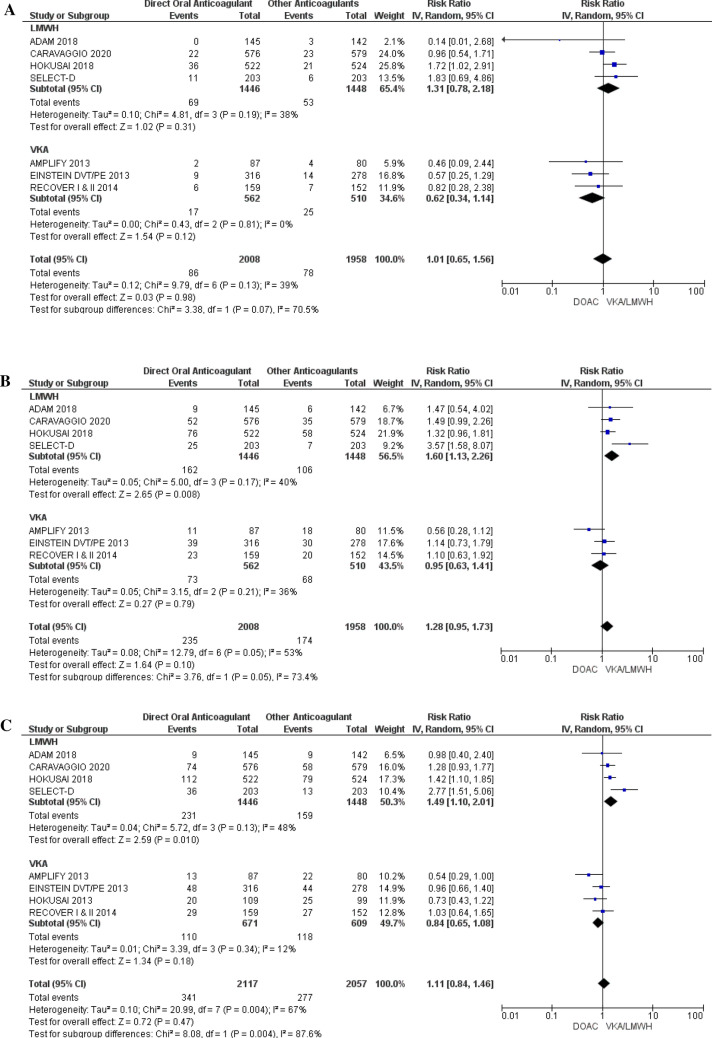
Data for MB and CRNMB were extracted from nine RCTs (n = 3966 participants) and data for all bleeding (major bleeding + CRNMB) were extracted from ten RCTs (n = 4147 participants). Overall, no difference in risk of MB (RR 1.01; 95% CI 0.65–1.56; p = 0.98; I2 = 39%), CRNMB (RR 1.28; 95% CI 0.95–1.73; p = 0.10; I2 = 53%), or all bleeding (RR 1.1; 95% CI 0.84–1.46; p = 0.47; I2 = 67%), was observed in patients treated with DOACs versus comparators. Compared to LMWH, DOACS had no difference in MB risk (RR 1.31; 95% CI 0.78–2.18; p = 0.31; I2 = 38%) was observed but had increased risk for CRNMB (RR 1.60; 95% CI 1.13–2.26; p = 0.008; I2 = 40%) and all bleeding (RR 1.49; 95% CI 1.10–2.01; p = 0.010; I2 = 48%). Compared to VKA, DOACS showed no difference in MB (RR 0.62; 95% CI 0.34–1.14; p = 0.12; I2 = 0%), CRNMB (RR 0.95; 95% CI 0.63–1.41; p = 0.78; I2 = 36%), and all bleeding (RR 0.84; 95% CI 0.81–3.01; p = 0.18; I2 = 27%) risks (Fig. 4). Per GRADE criteria, the quality of evidence was judged to be high for safety outcomes (Table 2).
Figure 4.
Safety (bleeding risk) of DOAC. (a) Major bleeding (MB), (b) clinically relevant non major bleeding (CRNMB), (c) all bleeding (composite MB and CRNMB). Forest plots show risk ratio (RR) of VTE recurrence of pooled data from all studies and subgroup analyses of studies evaluating DOAC compared to LMWH and DOAC compared to VKA. Boxes superimposing RR estimates are proportional to the weight of the included study. Heterogeneity between RCT is assessed by the I2 statistic.
Discussion
The purpose of our meta-analysis is to evaluate the efficacy and safety of DOACs for the treatment of CAT. Incorporating 4193 participant data our meta-analysis is an update on prior studies on the same subject.
The efficacy results of our meta-analysis indicate that DOACs are superior to VKA or LMWH for secondary prevention of VTE in patients with CAT. The improved efficacy with DOACs is demonstrated in comparison to LMWH, the current standard of care for CAT, but is not demonstrated compared to VKA. However, it should be noted that while statistically important, the benefit of DOACs is small, with absolute risk reduction of 3.3% overall and 4.2% compared to LMWH. The safety results of our meta-analysis indicate no difference in MB, CRNMB, or all bleeding risk with DOACs overall, but note risk of CNRMB and all bleeding without increased MB risk compared to LMWH. However, absolute risk increases with DOACs compared to LMWH is small for both CRNMB (4.4%) and (5.4%). No difference in bleeding risk with DOACs compared to VKA is noted. These results indicate that DOACs are efficacious drugs for treatment of CAT though have an increased risk for non-major bleeding compared to LMWH.
Previous, smaller studies on the same topic have noted variable efficacy and bleeding risks with DOACs. In one meta-analysis of 2151 participants including the Hokusai 2018 study, one study reported reduced risk for VTE recurrence in patients treated with DOACs compared to LMWH or VKA (RR 0.64; 95% CI 0.46–0.88) but no difference in bleeding outcomes31. Another meta-analysis of 1952 participants from nine RCTs with unselected cancer subpopulations noted similarly reduced VTE recurrence risk with DOACs compared to LMWH or VKA though there was no difference in VTE recurrence rate and bleeding risk in subgroup analysis of two RCTs comparing DOACs to LMWH32. A meta-analysis including 1132 participants’ from six RCTs with unselected cancer subpopulations comparing DOACs to VKA showed no difference in risk for VTE recurrence, MB, or CRNMB with DOACs33. A meta-analysis of two RCT comparing DOACs to LMWH (Hokusai 2018 and SELECT-D) no difference in VTE recurrence risk was observed but an increased risk for MB was noted (RR 1.74; 95% CI 1.05–2.88) with DOACs34. Most recently, Brunetti et al. showed superiority of DOACs over LMWH in meta-analyses including ADAM VTE, Hokusai 2018, and SELECT-D studies (RR 0.56; 95% CI 0.40–0.79)35. With the addition of the Caravaggio trial data, the results of our meta-analysis are only the second to show superiority for secondary VTE prevention with DOACs and the first to note no increase in MB with DOACs overall or compared to LMWH.
One explanation for the favorable efficacy with DOACs compared to LMWH is compliance with treatment. In general, compliance and patient desire to take drug is higher with DOACs, leading to longer on treatment time, than with LMWH. In the Hokusai 2018 study, premature discontinuation of study drug due to “patient decision” occurred in 15% of patients treated with dalteparin compared to 4% treated with edoxaban19. In the SELECT-D study, 10% of patients treated with dalteparin discontinued treatment due to either “patient decision” or “withdrawal of consent by the patient” compared to 6% in the rivaroxaban group20. In the ADAM VTE study, 15% of patients in the dalteparin group refused further treatment compared to 4% in the apixaban group30. Though compliance with DOAC and LMWH was roughly the same, the Caravaggio study reported withdrawal of consent as cause for permanent study drug discontinuation in 16% of patients in the dalteparin group, compared to 5.8% of the apixaban group21. Increased adherence and on treatment time with DOACs has also been noted in real world data. In an insurance claims database study, longer average on-treatment time with rivaroxaban than LMWH (3 months vs. 1 month) was noted, suggesting noncompliance and/or suboptimal treatment is prevalent with LMWH in clinical practice36.
The results of our meta-analysis that depicts comparable efficacy of DOACs and VKA for secondary prevention of CAT has several limitations. Firstly, large confidence intervals are noted on individual studies and in the pooled results of our meta-analysis, suggestive of underlying uncertainty in precision of risk ratio. This is likely reflective of small sample size of patients with active malignancy within each RCT populations (3–7% of trial population). In addition, there is notable exclusion of patients with aggressive cancers (patients with end organ dysfunction, reduced life expectancy, low percentage of metastatic cancers, and low percentage of patients on chemotherapy) which may have limited representation of patients at highest risk for VTE and recurrence of VTE. In fact, VTE recurrence with VKA in our meta-analysis showed a pooled rate ~ 7% (44/620) which is lower than the rate of VTE with VKA (10–15%) in the CATCH and CLOT trials which evaluated VKA for CAT treatment in a population of cancer patients9,37. Nevertheless, though statistically not different, we note numerically fewer VTE recurrences in patients treated with DOACs. Differences in efficacy between DOACs and VKA for treatment of CAT could be better evaluated in a dedicated randomized study with cancer patients, but this type of study is of low clinical value as LMWH has been clearly shown to be superior to VKA for treatment of CAT9,37,38.
Interpretation of bleeding risks associated with DOACs is complex. Statistically, no significant heterogeneity exists between studies in subgroups comparing DOACs to VKA or DOACs to LMWH, lending reassurance about cross trial comparison in subgroup analysis. However, significant heterogeneity does exist with regards to the overall meta-analysis and is likely a result of significant differences in trials, including study definitions of bleeding, active malignancy, participant inclusion and exclusion criteria, and sample selection. Thus, interpretation of overall bleeding results is limited and likely not clinically useful and bleeding risk conclusions may best be characterized in subgroup analysis.
The etiology of favorable MB profile, increased CRNMB, and all bleeding risk described with DOACs compared to LMWH is unclear, though increased bleeding with DOACs seems to be related to mucosal bleeding. Factors influencing bleeding include malignancy type and DOAC type with higher risk observed in upper gastrointestinal malignancies and with use of edoxaban and rivaroxaban. In the Hokusai 2018 study, a significant difference in MB was noted. However, when stratified by cancer type, only gastrointestinal malignancies showed increased MB rates with edoxaban compared to dalteparin (13.2% vs. 2.4%)19. Similarly, in the SELECT-D study, patients with esophageal or gastroesophageal junction cancers were excluded from enrollment after interim analysis showed three-fold increase in major bleeding with rivaroxaban in these cancer types. CRNMB was statistically higher in patients treated with rivaroxaban in the SELECT-D study, with most prevalent bleeding cancers being bladder and colorectal and most prevalent bleeding sites being genitourinary and gastrointestinal20. The risk for bleeding in patients with gastrointestinal cancers was also noted in a large retrospective cohort study with a MB rate of 16.7% with rivaroxaban compared to 7.2% with LMWH39. Conversely, the ADAM VTE trial, which was powered to detect bleeding differences and included patients with both upper and lower gastrointestinal malignancies, did not show increased risk for MB or CRNMB in patients treated with apixaban. However, these data are difficult to interpret as the study did not meet its prespecified primary outcome due to lower than anticipated bleeding rates for both treatment arms. The Caravaggio study, which included patients with different malignancies including gastrointestinal cancers in similar proportions to the Hokusai 2018 study, did not show differences in MB or CRNMB between apixaban and LMWH treated patients. Bleeding data from the Caravaggio study was not stratified by cancer type. No difference in bleeding site including gastrointestinal bleeding was noted for MB in the Caravaggio study. Interestingly, the Caravaggio study did note greater numbers of CRNMB with apixaban compared to dalteparin (52/576 vs. 34/579) with most prevalent sites of bleeding being genitourinary, upper airway, and gastrointestinal, which suggests possible systemic mucosal bleeding risk instead of localized bleeding risk from the tumor itself.
Ultimately, many of the observed patterns of bleeding with DOACs may be related to intrinsic properties of DOACs themselves. Several mechanisms have been conjectured to explain gastrointestinal bleeding with specific DOACS including topical anticoagulant effects (dabigatran, rivaroxaban, edoxaban), direct caustic effect (tartaric acid in dabigatran), and pharmacodynamic differences (higher peak concentrations with rivaroxaban and edoxaban)40. Currently, there are no head-to-head comparison studies evaluating bleeding risks with different DOACs. However in one meta-analysis of 43 trials utilizing DOACs for any indication including VTE (but excluding CAT), increased gastrointestinal and CRNMB risks were noted in patients treated with dabigatran and rivaroxaban but not with apixaban41. In another meta-analysis of 28 observational database studies of DOACs use in atrial fibrillation, dabigatran and rivaroxaban had increased risk and apixaban had lower risk for gastrointestinal bleeding and major hemorrhage compared to LMWH or VKA42. There is not enough data to perform a subgroup meta-analysis of bleeding risk, stratified by cancer type and DOAC type to confirm these observations; hence, these results warrant further study with a clinical trial.
Though our results show equivalence of bleeding risk with DOACs and VKA, the interpretation of these results is limited by uncertainty of precision with small sample sizes and exclusion of high bleeding risk cancer types. Thus, our results are likely not truly reflective of risks of bleeding with DOACs compared to VKA. We presume VKA has similar bleeding risk as LMWH in treatment of CAT, as previously noted in RCT incorporating patients with high risk for bleeding and in meta-analyses9,37,38,43. The incidence of bleeding with VKA in our study is approximately four times lower than LMWH in our meta-analysis, suggesting exclusion of high bleeding risk cancer patients in the AMPLIFY, RE-COVER I & II, EINSTEIN DVT/PE, and Hokusai 2013 studies.
A strength of our meta-analysis is the inclusion of all current high quality RCTs evaluating currently available DOACs for treatment of CAT, thus providing strength to outcomes findings, as evidenced by the GRADE analysis showing high certainty for outcomes. In addition, the results of our meta-analysis are generally similar to real-world observational studies showing benefit of DOACs in CAT treatment without major increase in bleeding36,44,45. These studies generally incorporate more variability in patient population, including presence of high VTE risk cancer subtypes, cancer severity (stage), access to care, duration of treatment, and patient comorbidities, and thus lend generalizability and clinical relevance to our findings.
There are limitations to our meta-analysis and the results should be included in the context of the DOACs included. Our results are heavily influenced by the outcomes of the Caravaggio and Hokusai 2018 studies which enrolled more than half of all participants and more than three fourths of participants in subgroup analysis comparing DOACs to LMWH. Thus, edoxaban and apixaban are over-represented as DOAC types in this meta-analysis and are primarily the drivers of outcomes. Additionally, no direct comparison of dabigatran to LMWH for treatment of CAT exists and < 10% of patients in the study received dabigatran overall. Therefore, it remains unclear whether the results of this meta-analysis can be extrapolated to dabigatran. Furthermore, while data comparing DOACs to LMWH are clinically relevant, the findings of equivalence of DOACs to VKA is not clinically useful, as LMWH has been previously shown to be superior to VKA for treatment of CAT38,43. Few patients with hematologic malignancies or hematopoietic stem cell transplant were included in the studies and it remains unclear whether the results noted here can be applied to such patients. Lastly, the duration of anticoagulation in patients with CAT is not clear. Most studies in this meta-analysis provided anticoagulation for 6–12 months, but many patients have ongoing risk factors for thrombosis for an extended duration of time.
The CANVAS study (NCT 02744092), evaluating the role of DOAC (rivaroxaban, apixaban, edoxaban, or dabigatran), is currently ongoing and should help clarify the role of DOACs and specifically provide more data regarding use of dabigatran, in the management of CAT. The EVE Trial (NCT 030808), evaluating extended duration apixaban in CAT is currently ongoing and will provide more evidence about the duration and dosing of anticoagulation in patients with CAT.
Conclusion
This meta-analysis shows that DOACs are more effective at preventing VTE recurrence though carry a small risk for increased non-major bleeding. In the appropriately selected patient, DOACs are safe for the treatment of CAT. The results warrant consideration for changing the paradigm for treatment of CAT.
Author contributions
This study was conceived by M.J., R.M, N.V., Data was collected and analyzed by G.K. N.T., R.D., and R.M. The first draft of the manuscript was written by R.D. Figures and tables authored by G.K., N.T., R.M. All authors reviewed the manuscript.
Funding
This project was supported by grants from the Swiss Cancer League # BIL KLS-3352-02-0214; from the DeBartolo Family Personalized Medicine institute; and in part by the Biostatics Core Facility at the Moffitt Cancer Center & Research Institute, a National Cancer Institute designated Comprehensive Cancer Center (P30CA076292-16).
Competing interests
Ruchi Desai No conflict of interest to disclose. Gautam Krishna Koipallil No conflict of interest to disclose. Nelson Thomas No conflict of interest to disclose. Rahul Mhaskar No conflict of interest to disclose. Nathan Visweshwar No conflict of interest to disclose. Damian Laber No conflict of interest to disclose. Ankita Patel No conflict of interest to disclose. Michael Jaglal advisory board Novartis.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Gautam Krishna Koipallil and Nelson Thomas.
References
- 1.Lip GY, Chin BS, Blann AD. Cancer and the prothrombotic state. Lancet Oncol. 2002;3:27–34. doi: 10.1016/s1470-2045(01)00619-2. [DOI] [PubMed] [Google Scholar]
- 2.Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293:715–722. doi: 10.1001/jama.293.6.715. [DOI] [PubMed] [Google Scholar]
- 3.Cronin-Fenton DP, et al. Hospitalisation for venous thromboembolism in cancer patients and the general population: A population-based cohort study in Denmark, 1997–2006. Br. J. Cancer. 2010;103:947–953. doi: 10.1038/sj.bjc.6605883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chew HK, Wun T, Harvey D, Zhou H, White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch. Intern. Med. 2006;166:458–464. doi: 10.1001/archinte.166.4.458. [DOI] [PubMed] [Google Scholar]
- 5.Deng A, Galanis T, Graham MG. Venous thromboembolism in cancer patients. Hosp. Pract. 2014;1995(42):24–33. doi: 10.3810/hp.2014.12.1156. [DOI] [PubMed] [Google Scholar]
- 6.Lee AY, Levine MN. Venous thromboembolism and cancer: Risks and outcomes. Circulation. 2003;107:I17–21. doi: 10.1161/01.Cir.0000078466.72504.Ac. [DOI] [PubMed] [Google Scholar]
- 7.Kraaijpoel N, et al. Treatment and long-term clinical outcomes of incidental pulmonary embolism in patients with cancer: An international prospective cohort study. J. Clin. Oncol. 2019;37:1713–1720. doi: 10.1200/jco.18.01977. [DOI] [PubMed] [Google Scholar]
- 8.Prandoni P. Cancer and thromboembolic disease: How important is the risk of thrombosis? Cancer Treat. Rev. 2002;28:133–136. doi: 10.1016/s0305-7372(02)00041-5. [DOI] [PubMed] [Google Scholar]
- 9.Lee AY, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N. Engl. J. Med. 2003;349:146–153. doi: 10.1056/NEJMoa025313. [DOI] [PubMed] [Google Scholar]
- 10.10Mohammady, M., Janani, L. & Akbari Sari, A. Slow versus fast subcutaneous heparin injections for prevention of bruising and site pain intensity. Cochrane Database Syst. Rev.11, CD008077, 10.1002/14651858.CD008077.pub5 (2017). [DOI] [PMC free article] [PubMed]
- 11.Clark NP. Low-molecular-weight heparin use in the obese, elderly, and in renal insufficiency. Thromb. Res. 2008;123(Suppl 1):S58–61. doi: 10.1016/j.thromres.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Padrini R. Clinical pharmacokinetics and pharmacodynamics of direct oral anticoagulants in patients with renal failure. Eur. J. Drug Metab. Pharmacokinet. 2019;44:1–12. doi: 10.1007/s13318-018-0501-y. [DOI] [PubMed] [Google Scholar]
- 13.Agnelli G, et al. Oral apixaban for the treatment of venous thromboembolism in cancer patients: Results from the AMPLIFY trial. J. Thromb. Haemost. (JTH) 2015;13:2187–2191. doi: 10.1111/jth.13153. [DOI] [PubMed] [Google Scholar]
- 14.Bauersachs R, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N. Engl. J. Med. 2010;363:2499–2510. doi: 10.1056/NEJMoa1007903. [DOI] [PubMed] [Google Scholar]
- 15.Buller HR, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N. Engl. J. Med. 2013;369:1406–1415. doi: 10.1056/NEJMoa1306638. [DOI] [PubMed] [Google Scholar]
- 16.Buller HR, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N. Engl. J. Med. 2012;366:1287–1297. doi: 10.1056/NEJMoa1113572. [DOI] [PubMed] [Google Scholar]
- 17.Schulman S, et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation. 2014;129:764–772. doi: 10.1161/circulationaha.113.004450. [DOI] [PubMed] [Google Scholar]
- 18.Schulman S, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N. Engl. J. Med. 2009;361:2342–2352. doi: 10.1056/NEJMoa0906598. [DOI] [PubMed] [Google Scholar]
- 19.Raskob GE, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N. Engl. J. Med. 2018;378:615–624. doi: 10.1056/NEJMoa1711948. [DOI] [PubMed] [Google Scholar]
- 20.Young AM, et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: Results of a randomized trial (SELECT-D) J. Clin. Oncol. 2018;36:2017–2023. doi: 10.1200/jco.2018.78.8034. [DOI] [PubMed] [Google Scholar]
- 21.Agnelli G, et al. Apixaban for the treatment of venous thromboembolism associated with cancer. N. Engl. J. Med. 2020;382:1599–1607. doi: 10.1056/NEJMoa1915103. [DOI] [PubMed] [Google Scholar]
- 22.Cancer Associated Venous Thromboembolic Disease (Version 1.2020). https://www.nccn.org/professionals/physician_gls/default.aspx#supportive (2020). [DOI] [PubMed]
- 23.Kaatz, S., Ahmad, D., Spyropoulos, A. C., Schulman, S. & Anticoagulation, t. S. o. C. o. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: Communication from the SSC of the ISTH. J. Thromb. Haemost.13, 2119–2126, 10.1111/jth.13140 (2015). [DOI] [PubMed]
- 24.Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019) (2019).
- 25.The GRADE Working Group. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. Updated 2013. (2013).
- 26.26Evidence Prime, I. GRADEpro GDT: GRADEpro Guideline Development Tool. (2015).
- 27.27Review Manager v. 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, 2014).
- 28.Prins MH, et al. Oral rivaroxaban versus standard therapy for the treatment of symptomatic venous thromboembolism: A pooled analysis of the EINSTEIN-DVT and PE randomized studies. Thromb. J. 2013;11:21. doi: 10.1186/1477-9560-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schulman S, et al. Treatment with dabigatran or warfarin in patients with venous thromboembolism and cancer. Thromb. Haemost. 2015;114:150–157. doi: 10.1160/th14-11-0977. [DOI] [PubMed] [Google Scholar]
- 30.McBane RD, 2nd, et al. Apixaban and dalteparin in active malignancy-associated venous thromboembolism: The ADAM VTE trial. J. Thromb. Haemost. (JTH) 2020;18:411–421. doi: 10.1111/jth.14662. [DOI] [PubMed] [Google Scholar]
- 31.Al Yami, M. S. et al. Direct oral anticoagulants for the treatment of venous thromboembolism in patients with active malignancy: A systematic review and meta-analysis. J. Thromb. Thromb.46, 145–153, 10.1007/s11239-018-1696-0 (2018). [DOI] [PubMed]
- 32.Brunetti ND, et al. Direct oral anti-coagulants compared with vitamin-K inhibitors and low-molecular-weight-heparin for the prevention of venous thromboembolism in patients with cancer: A meta-analysis study. Int. J. Cardiol. 2017;230:214–221. doi: 10.1016/j.ijcard.2016.12.168. [DOI] [PubMed] [Google Scholar]
- 33.Vedovati MC, Germini F, Agnelli G, Becattini C. Direct oral anticoagulants in patients with VTE and cancer: A systematic review and meta-analysis. Chest. 2015;147:475–483. doi: 10.1378/chest.14-0402. [DOI] [PubMed] [Google Scholar]
- 34.Li A, Garcia DA, Lyman GH, Carrier M. Direct oral anticoagulant (DOAC) versus low-molecular-weight heparin (LMWH) for treatment of cancer associated thrombosis (CAT): A systematic review and meta-analysis. Thromb. Res. 2018 doi: 10.1016/j.thromres.2018.02.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brunetti ND, et al. Direct oral anticoagulants more effective than low-molecular-weight heparin for venous thrombo-embolism in cancer: An updated meta-analysis of randomized trials. J. Thromb. Thromb. 2019 doi: 10.1007/s11239-019-01974-y. [DOI] [PubMed] [Google Scholar]
- 36.Streiff MB, et al. Effectiveness and safety of anticoagulants for the treatment of venous thromboembolism in patients with cancer. Am. J. Hematol. 2018;93:664–671. doi: 10.1002/ajh.25059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee AYY, et al. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: A randomized clinical trial. JAMA. 2015;314:677–686. doi: 10.1001/jama.2015.9243. [DOI] [PubMed] [Google Scholar]
- 38.Sobieraj DM, et al. Anticoagulation for the treatment of cancer-associated thrombosis: A systematic review and network meta-analysis of randomized trials. Clin. Appl. Thromb. Hemost. 2018;24:182s–187s. doi: 10.1177/1076029618800792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seo, S. et al. Oral rivaroxaban versus subcutaneous low molecular weight heparin treatment for venous thromboembolism in patients with upper gastrointestinal, hepatobiliary and pancreatic cancer. Ann. Oncol.27, 10.1093/annonc/mdw371.87 (2016).
- 40.Cheung KS, Leung WK. Gastrointestinal bleeding in patients on novel oral anticoagulants: Risk, prevention and management. World J. Gastroenterol. 2017;23:1954–1963. doi: 10.3748/wjg.v23.i11.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holster IL, Valkhoff VE, Kuipers EJ, Tjwa ETTL. New oral anticoagulants increase risk for gastrointestinal bleeding: A systematic review and meta-analysis. Gastroenterology. 2013;145:105–112.e115. doi: 10.1053/j.gastro.2013.02.041. [DOI] [PubMed] [Google Scholar]
- 42.Ntaios G, et al. Real-world setting comparison of nonvitamin-K antagonist oral anticoagulants versus vitamin-K antagonists for stroke prevention in atrial fibrillation: A systematic review and meta-analysis. Stroke. 2017;48:2494–2503. doi: 10.1161/strokeaha.117.017549. [DOI] [PubMed] [Google Scholar]
- 43.Rossel A, et al. Anticoagulant therapy for acute venous thrombo-embolism in cancer patients: A systematic review and network meta-analysis. PLoS ONE. 2019;14:e0213940. doi: 10.1371/journal.pone.0213940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Phelps MK, et al. A single center retrospective cohort study comparing low-molecular-weight heparins to direct oral anticoagulants for the treatment of venous thromboembolism in patients with cancer—A real world experience. J. Oncol. Pharm. Pract. 2019;25:793–800. doi: 10.1177/1078155218757856. [DOI] [PubMed] [Google Scholar]
- 45.Simmons B, et al. Efficacy and safety of rivaroxaban compared to enoxaparin in treatment of cancer-associated venous thromboembolism. Eur. J. Haematol. 2018 doi: 10.1111/ejh.13074. [DOI] [PubMed] [Google Scholar]