Abstract
In pediatric Crohn’s disease (CD) patients, it is important to define the disease phenotype at diagnosis for stratifying risk. In this retrospective study, we aimed to assess the disease phenotype compared to EUROKIDS registry and analyze disease outcome of pediatric CD patients according to upper gastrointestinal (GI) tract involvement. A total of 312 patients were included. The median age at diagnosis was 13.7 years and 232 patients (74.4%) were identified to have upper GI involvement at diagnosis. In Korean pediatric CD patients, there were significant differences in male predominance (72.8% vs. 59.2, p < 0.001), proportion of upper GI involvement (74.4% vs. 46.2%, p < 0.001), and perianal disease (62.1% vs. 8.2%, p < 0.001) compared to data in the EUROKIDS registry. Younger age (OR 2.594, p = 0.0139) and ileal involvement (OR 2.293, p = 0.0176) at diagnosis were associated with upper GI involvement. There were no significant differences in disease outcomes between patients with and without upper GI tract involvement. This study revealed that upper GI involvement is more prevalent in Korean patients with pediatric Crohn’s disease than in European patients, and the disease outcome did not appear to differ according to upper GI tract involvement.
Subject terms: Gastroenterology, Medical research
Introduction
Crohn’s disease (CD) is a multifactorial idiopathic chronic disease of the gastrointestinal (GI) tract with complex pathophysiology including genomes and immune responses, and variable clinical manifestations and disease course1,2. Approximately 25% of the patients with CD are diagnosed during childhood and adolescence. The incidence in pediatric patients has increased significantly over the past decades and the age at diagnosis of CD is also getting younger3–6.
To select the optimal treatment and assess the prognosis of pediatric CD patients, it is important to clarify the factors influencing poor outcomes. It is known that deep colonic ulcers, extensive disease, growth retardation, B2 and/or B3 disease behavior at the onset, severe osteoporosis, and severe perianal disease are potentially predictive of poor outcomes7. In addition, the GETAID cohort study identified young age, upper GI tract and rectal involvement (but not colonic or ileal), and B2 and/or B3 disease behavior as prognostic factors for poor outcomes over 15 years of disease8. Therefore, in pediatric CD patients, it is important to define the disease phenotype at diagnosis for stratifying risk and prognostic factors.
In Korea, there are scarce data on the disease phenotype of the entire GI tract and the disease course according to disease phenotype in pediatric CD patients9,10. The primary aim of the present study was to assess the disease phenotype of pediatric CD patients and investigate the characteristics including upper GI tract involvement. The secondary aim of this study was to find the factors associated with upper GI tract involvement in pediatric CD patients and differences in the disease course according to upper GI tract involvement.
Methods
Patients and study design
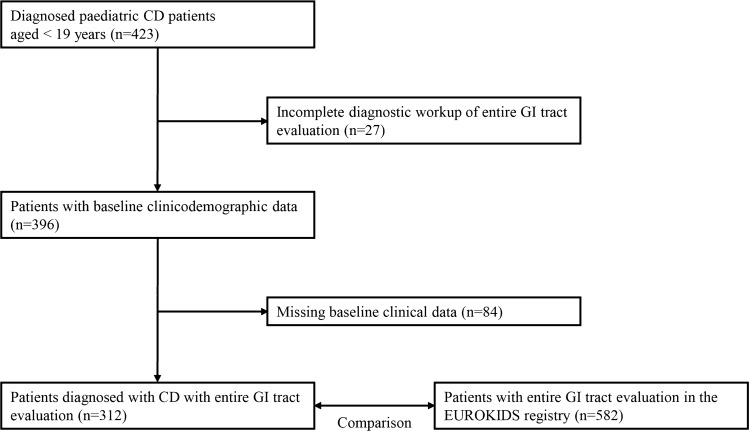
This study was a retrospective, observational cohort study conducted at the Department of Pediatrics of Samsung Medical Center between January 2004 and July 2019. The subjects were pediatric CD patients diagnosed at < 19 years of age with available data and evaluation of the entire GI tract. CD was diagnosed in accordance with the revised Porto criteria of the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition, which implies that small bowel evaluation, as well as esophagogastroduodenoscopy (EGD) and ileocolonoscopy, were performed in all subjects11. The entire GI tract evaluation consisted of EGD, ileocolonoscopy, and appropriate imaging of the small bowel including capsule endoscopy, and/or magnetic resonance enterography (MRE). Also, there were complete follow-up data for at least one year after diagnosis in all subjects. Patients diagnosed with ulcerative colitis, inflammatory bowel disease (IBD)-unspecified, diagnosed age at ≥ 19 years old, and those with missing baseline clinicodemographic data were excluded. Disease classification and behavior were based on the Paris classification12. The inclusion criteria for this study were the same as those for the EUROKIDS study to enable comparison of the disease phenotypes13.
Demographic information and the clinical data at diagnosis, including sex, diagnosis age, age at symptom onset, duration of disease, disease phenotype including the entire GI tract, previous history of perianal or CD-related GI tract surgery before diagnosis, and a first-degree family history of IBD, were collected from the electronic medical records. The severity of the disease was evaluated through the Pediatric Crohn’s Disease Activity Index (PCDAI)14, laboratory results, and the simple endoscopic score for CD (SES-CD).
To evaluate disease outcomes according to upper GI tract involvement and small bowel (jejunal) involvement, the need for CD-related intestinal surgery and perianal fistula/abscess surgery was assessed.
Definitions
The diagnosis of CD was made by physicians in a single center based on clinical symptoms, laboratory results, endoscopic appearance, histologic findings, and small bowel imaging studies. The disease phenotype at diagnosis was categorized according to the Paris classification12 and compared with the EUROKIDS registry13 (Fig. 1).
Figure 1.
Study flow diagram.
The location of GI tract involvement in CD was determined by the presence of ulcers, erosions, cobblestoning, and/or luminal narrowing15–17. The GI tract from the esophagus to the second portion of the duodenum was evaluated by EGD, and from the terminal ileum to the rectum by ileocolonoscopy. In the remaining small bowel, disease involvement was determined by findings on small bowel imaging and/or capsule endoscopy. Upper GI tract involvement (L4) was defined as disease from the esophagus to the proximal 2/3 of the ileum and lower GI tract involvement was defined from the distal 1/3 of the ileum to the rectum according to the Paris classification system12. Upper GI disease was further divided into esophagoduodenal (L4a), jejunal/proximal ileal (L4b), or both (L4ab). We regarded nonspecific macroscopic findings, such as erythema, edema, and/or nodularity in the esophagogastroduodenum (L4a), as insufficient evidence of upper GI tract involvement. Endoscopic findings on the terminal ileum were considered more accurate than small bowel studies in the case of disagreement. Perianal disease was defined as a perianal abscess and/or fistula, and isolated skin tags, fissures, or hemorrhoids were excluded.
Statistical analysis
Continuous variables are presented as medians and the interquartile range (IQR) for non-normally distributed data and categorical variables are presented as number and proportion. For statistical comparison between the groups according to upper GI involvement and between Korean cohort and ERUOKIDS registry, the t-test or Wilcoxon rank-sum test was used for continuous variables and the chi-squared test or Fischer’s exact test was used for categorical variables. Univariate and multivariate logistic regression analyses were conducted to examine the association between upper GI involvement and the variables. Factors with p < 0.1 in the univariate analysis were included in the multivariate analysis using a stepwise selection procedure. The results are expressed as adjusted ORs with 95% confidence intervals (CIs). Kaplan–Meier survival analysis was used to evaluated surgery free survival stratified by upper GI tract involvement. All statistical analyses were carried out using Rex (Version 3.0.3, RexSoft Inc., Seoul, Korea).
Ethics declarations
This study was approved by the Institutional Review Board of the Samsung Medical Center (IRB file No. 2020-01-033). All patients and A parent and/or legal guardian of subjects who are under 18 provided written informed consent. We confirmed that all methods were performed in accordance with the approved guidelines and regulations. We reported and presented data according to the STROBE statement.
Results
Baseline characteristics of patients with and without upper gastrointestinal involvement
From January 2004 and July 2019, a total of 312 pediatric patients were diagnosed with CD in accordance with the revised Porto criteria and were eligible for analysis. The median age of the patients was 13.7 years (IQR 11.9–15.7, 89.7% was ≤ 16 years old), with a median diagnostic delay (time interval from the onset of the first symptoms to the establishment of a CD diagnosis) of 5.4 months (IQR 2.4–12.8). Of the patients, 227 (72.8%) were male and the median follow-up duration was 6.6 years (IQR 4.1–9.9 years). Eighteen patients (5.6%) had previous bowel resection history and 102 patients (32.7%) had previous anal fistula or perianal abscess surgery before diagnosis. Twenty patients (6.4%) had first-degree relatives with IBD. The other baseline characteristics are summarized in Table 1.
Table 1.
Baseline characteristics and disease phenotype of pediatric Crohn’s disease patients.
| Total | No Upper GI involvement | Upper GI involvement | p value | |
|---|---|---|---|---|
| Observational duration | 6.6 (4.1, 9.9) | |||
| Number of patients (%) | 312 (100) | 80 (25.6) | 232 (74.4) | |
| Sex (%) | ||||
| Male | 227 (72.8) | 56 (70) | 171 (73.7) | 0.6195 |
| Female | 85 (2.0) | 24 (30) | 61 (26.3) | |
| Age at diagnosis (years) | 13.7 (11.9, 15.7) | 14.8 (12.2, 16.5) | 13.4 (11.6, 15.4) | 0.0133 |
| Age at diagnosis | ||||
| 16 or less | 280 (89.7) | 66 (82.5) | 214 (92.2) | 0.0236 |
| More than 16 | 32 (10.3) | 14 (17.5) | 18 (7.8) | |
| Diagnostic delay (months) | 5.4 (2.4, 12.8) | 5.1 (2.5, 12.3) | 5.3 (2.4, 12.5) | 0.5094 |
| Body mass index (kg/m2) | 18.3 (16.2, 20.3) | 18.4 (16.1, 19.6) | 18.2 (16.3, 20.4) | 0.4649 |
| PCDAI | 32.5 (21.9, 40) | 32.5 (22.5, 40) | 32.5 (20, 40) | 0.8072 |
| SES-CD | 14 (7, 22) | 14.5 (7, 23.5) | 14.5 (7.75, 21) | 0.9186 |
| White blood cell count (/uL) | 8585 (6800, 10,730) | 8325 (6960, 10,730) | 8605 (6732.5, 10,667.5) | 0.8749 |
| Hemoglobin (g/dL) | 12.2 (10.9, 13.2) | 12.3 (10.9, 13.2) | 12.1 (10.97, 13.2) | 0.9656 |
| Platelet count (/uL) | 368.5 (298.75, 474.25) | 378.5 (304.75, 474.25) | 366.5 (297.5, 471.75) | 0.699 |
| Erythrocyte sedimentation rate (mm/hr) | 45 (20, 71) | 44 (19.5, 72.75) | 45 (21, 70.25) | 0.8914 |
| C-reactive protein (mg/dL) | 1.54 (0.39, 4.2) | 1.2 (0.36, 3.81) | 1.75 (0.41, 4.25) | 0.2218 |
| Lower GI (%)a | ||||
| No other location | 1 (0.32) | – | 1 (0.43) | 0.0815 |
| L1 | 36 (11.5) | 8 (10) | 28 (12.1) | |
| L2 | 41 (13.1) | 17 (21.3) | 24 (10.3) | |
| L3 | 234 (74.8) | 55 (68.8) | 179 (77.2) | |
| Behavior (%)a | ||||
| B1 | 255 (81.2) | 70 (87.5) | 185 (79.7) | 0.5302 |
| B2 | 37 (11.8) | 6 (7.5) | 31 (13.4) | |
| B3/B2B3 | 20 (6.4) | 4 (5.0) | 16 (6.9) | |
| Perianal symptom (%) | 170 (54.5) | 40 (50) | 130 (56.0) | 0.4211 |
| Perianal lesion on MRE (%) | 193 (62.1) | 49 (61.3) | 144 (62.3) | 0.9688 |
| Surgical history (%) | ||||
| Fistula/abscess surgery | 102 (32.7) | 24 (30) | 78 (33.6) | 0.6476 |
| Intestinal resection | 18 (5.6) | 5 (6.3) | 13 (5.6) | 0.786 |
| Family history of IBD (%) | 20 (6.4) | 2 (2.5) | 18 (7.8) | 0.1642 |
| Anti-TNF agents | 280 (89.7) | 67 (83.8) | 213 (91.8) | 0.0664 |
PCDAI Paediatric Crohn’s disease activity index, SES-CD simple endoscopic score for Crohn’s disease, Anti-TNF agents Anti-tumor necrosis factor agents.
aAccording to the Paris classificsation.
Among the patients, 232 patients (74.4%) had upper GI involvement at diagnosis. A comparison of the variables between the patients with and without upper GI involvement revealed only a significant difference in younger diagnosis age (13.4 vs. 14.8, p = 0.0133) with a higher proportion of A1a/A1b (92.2% vs. 82.5%, p = 0.0236). There were no significant differences in other disease phenotypes, disease behavior, disease severity, family history of IBD, surgical history, and anti-TNFα therapy during the study period, as described in Table 1.
Comparison of disease phenotype with the EUROKIDS registry
Compared to the EUROKIDS registry, there were statistically significant differences in male predominance (72.8% vs. 59.2, p < 0.001), first-degree family history of IBD (6.4% vs. 10.8%, p = 0.003129), ileal involvement (L1/L3, 86.3% vs. 69.1, p < 0.001), upper GI involvement (74.4% vs. 46.2%, p < 0.001), and perianal disease (62.1% vs. 8.2%, p < 0.001) in this study. The disease behavior according to the Paris classification was comparable between the two studies (p > 0.05) (Table 2). Regarding upper GI tract involvement, there were no remarkable differences in the upper GI macroscopic and microscopic findings between the two studies (Table 3).
Table 2.
Comparison of pediatric Crohn’s disease phenotype at diagnosis between Korean cohort and the EUROKIDS registry.
| Korea | EUROKIDS | p value | |
|---|---|---|---|
| n = 312 | n = 1221 | ||
| Sex (%) | < 0.001 | ||
| Male | 227 (72.8) | 723 (59.2) | |
| Female | 85 (27.2) | 498 (40.8) | |
| Ethnicity (%) | < 0.001 | ||
| Asia | 312 (100) | 50 (4.1) | |
| Caucasian | 0 | 1049 (86.7) | |
| Arabian | 0 | 43 (3.6) | |
| African-Caribbean | 0 | 18 (1.5) | |
| Others | 0 | 50 (4.1) | |
| 1st-degree family history of IBD | 20 (6.4) | 129 (10.8) | 0.003129 |
| n = 312 | n = 582 | p value | |
|---|---|---|---|
| Age at diagnosis (%)a | < 0.001 | ||
| A1a | 24 (7.7) | 114 (19.6) | |
| A1b/A2 | 288 (92.3) | 468 (80.4) | |
| Lower gastrointestinal tract location (%)a | < 0.001 | ||
| No other location | 1 (0.6) | 21 (3.6) | |
| L1 | 36 (11.5) | 95 (16.3) | |
| L2 | 41 (13.1) | 159 (27.3) | |
| L3 | 234 (74.8) | 307 (52.8) | |
| Upper gastrointestinal tract location (%)a | < 0.001 | ||
| No other location | 80 (25.6) | 313 (53.8) | |
| L4a | 82 (26.3) | 129 (22.1) | |
| L4b | 76 (24.4) | 97 (16.7) | |
| L4ab | 74 (23.7) | 43 (7.4) | |
| Behavior (%)a | 0.434286 | ||
| B1 | 255 (81.7) | 477 (82) | |
| B2 | 37 (11.9) | 77 (13.2) | |
| B3/B2B3 | 20 (6.4) | 28 (4.8) | |
| Perianal disease (%)a | 193 (62.1) | 48 (8.2) | < 0.001 |
aAccording to the Paris classification.
Table 3.
Comparison of macroscopic and microscopic findings in the upper gastrointestinal tract of pediatric Crohn’s disease patients between Korean cohort and the EUROKIDS registry.
| Korea | EUROKIDS | p value | |
|---|---|---|---|
| Number of patients | 312 | 582 | |
| Upper gastrointestinal macroscopic findings (%) | 0.546652 | ||
| Esophageal involvement | 14 (4.5) | 34 (5.8) | |
| Ulceration | 4 | 12 | |
| Erosion | 11 | 23 | |
| Gastric involvement | 62 (19.9) | 102 (17.5) | |
| Ulceration | 26 | 36 | |
| Erosion | 45 | 71 | |
| Cobble stone | 0 | 1 | |
| Duodenal involvement | 48 (15.4) | 100 (17.2) | |
| Ulceration | 18 | 31 | |
| Erosion | 41 | 72 | |
| Cobble stone | 0 | 2 | |
| Luminal narrowing | 2 | 2 | |
| Number of patients | 312 | 427 | |
| Upper gastrointestinal microscopic findings-granuloma (%) | 0.121849 | ||
| Esophagus | 3 (1.0) | 20 (4.7) | |
| Stomach | 24 (7.7) | 49 (11.5) | |
| Duodenum | 2 (0.6) | 14 (3.3) |
Factors associated with upper gastrointestinal tract involvement at diagnosis
According to the univariate logistic regression analysis, the age at diagnosis and the initial location in the lower GI tract were associated with upper GI tract involvement. These factors as well as sex were included in the multivariate logistic regression analysis by stepwise selection and revealed that the age at diagnosis (16 years or less vs. more than 16, OR 2.594, 95% CI 1.214–5.541, p = 0.0139) and the initial location in the lower GI tract (ileal vs. non-ileal involvement, OR 2.293, 95% CI 1.156–4.441, p = 0.0176) were positively associated with the presence of upper GI tract involvement in pediatric CD patients (Table 4). In addition, during the study period, anti-TNFα therapy was associated with upper GI tract involvement. (OR = 2.292, p = 0.0365).
Table 4.
Univariate and multivariate logistic regression model for the association of upper gastrointestinal tract involvement at the time of Crohn’s disease diagnosis.
| Upper GI involvement at diagnosis | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR [95% CI] | p value | OR [95% CI] | p value | |
| Sex [female vs. male] | 0.711 [0.383–1.320] | 0.2795 | ||
| Age at diagnosis [16 years or less vs. more than 16] | 2.522 [1.190–5.344] | 0.0158 | 2.594 [1.214–5.541] | 0.0139 |
| Initial location [ileal vs. non-ileal involvement] | 2.234 [1.135–4.400] | 0.0201 | 2.293 [1.156–4.441] | 0.0176 |
| Behavior [complicated vs. non-complicated] | 1.778 [0.852–3.712] | 0.1252 | ||
| 1st-degree family history of IBD | 3.280 [0.744–14.462] | 0.1165 | ||
| Previous intestinal surgery | 0.890 [0.307–2.581] | 0.831 | ||
| Previous fistula/abscess surgery | 1.182 [0.682–2.049] | 0.5519 | ||
| Diagnostic delay | 0.992 [0.982–1.002] | 0.1165 | ||
| Body mass index | 1.057 [0.981–1.140] | 0.1441 | ||
| Paediatric Crohn’s disease activity index | 0.997 [0.979–1.015] | 0.441 | ||
| Simple endoscopic score for Crohn’s disease | 0.994 [0.9678–1.021] | 0.6733 | ||
| White blood cell count | 1.000 [0.999–1.000] | 0.6186 | ||
| Haemoglobin | 1.021 [0.892–1.170] | 0.7595 | ||
| Platelet count | 1.000 [ | 0.9916 | ||
| Erythrocyte sedimentation rate | 1.000 | 0.9751 | ||
| C-reactive protein | 1.066 | 0.1282 | ||
| Anti-tumor necrosis factor agents therapy | 2.175 [1.020–4.637] | 0.0442 | 2.292 [1.053–4.988] | 0.0365 |
Impact of upper gastrointestinal tract involvement on CD-related complications in pediatric patients
To investigate the impact of upper GI tract involvement on disease course, a follow-up analysis of all patients with upper GI tract involvement and especially jejunal involvement at the time of CD diagnosis was conducted and compared to patients without such involvement. During a median follow-up of 6.6 years (IQR 4.1–9.9), we identified 51 cases (16.3%) with the occurrence of severe complications, in which 27 cases of perianal fistula required fistulectomy and 24 cases of intestinal stenosis or perforation required intestinal resection surgery.
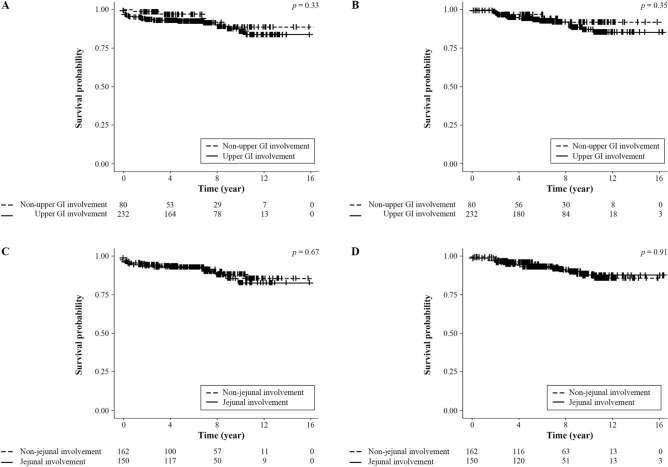
Forty-two cases were detected in the group of patients with upper GI tract involvement at the time of CD diagnosis (82.4%), and nine cases were observed in the subjects without upper GI tract involvement at diagnosis (17.6%). According to the Kaplan–Meier analysis for complication-free survival, there were no significant differences in severe complications requiring surgery such as fistulectomy or intestinal resection between the patients with upper GI tract involvement and the patients without such involvement (Fig. 2A,B), p > 0.05). In addition, the subgroup analysis of patients with and without jejunal involvement revealed no statistically significant differences between the groups (Fig. 2C,D), p > 0.05).
Figure 2.
Impact of upper gastrointestinal tract and jejunal involvement on CD-related complications in pediatric CD patients. Kaplan–Meier analysis for perianal surgery (A) and intestinal resection (B) according to upper gastroinetstinal involvement. Kaplan–Meier analysis for perianal surgery (C) and intestinal resection (D) according to jejunal involvement.
Discussion
To date, a few studies analysing disease phenotypes in pediatric CD in Korea have been conducted9,10,18. In particular, there was only one study that evaluated the entire GI tract according to the Paris classification10. This is the first study in Korea to clearly compare the disease phenotypes of pediatric CD patients in Korea with those of a European cohort and investigate the impact of upper GI tract involvement on the disease course.
This study revealed that Korean pediatric patients had the distinct features of male predominance, less family history of IBD, more perianal disease, less isolated colonic involvement, and more upper GI tract involvement (74.4%) compared to those in the EUROKIDS registry13, consistent with other known studies19,20. As far as we know, this is the first study in Korea to assess disease phenotypes, investigate the factors associated with upper GI tract involvement, and compare the long-term disease outcomes according to upper GI tract involvement in pediatric CD patients.
Pediatric CD involving the upper GI tract has become increasingly recognized by the introduction of routine EGD and biopsies for all patients being evaluated for CD11,17,21. It is generally known that upper GI involvement is more prevalent in pediatric CD patients than in adult CD patients22. The frequency of upper GI tract involvement has been reported to vary widely23,24 and the definition of upper GI tract involvement in CD is still controversial. Some suggest that a wide spectrum of macroscopic and microscopic lesions should be considered proximal CD. Others recommend stricter classifications in terms of the mucosal alterations compatible with proximal CD. Our study chose the strict definition of upper GI tract involvement in CD, which was the same inclusion criterion as the EUROKIDS registry, and Korean patients showed significantly higher proportions of upper GI tract involvement compared to those in the EUROKIDS study (74.4% vs. 46.2%, p < 0.001). One possible explanation for this result is that the genetic differences between ethnicities may play a role in the expression of different phenotypes in pediatric CD. The other explanation is differences in the modalities of small bowel evaluation that were included in the upper GI tract evaluations between the two studies. Since this study was conducted between 2004 and 2019, more accurate modalities for small bowel evaluation (i.e., MRE or capsule endoscopy) in almost all the patients were used. However, since the EUROKIDS study was conducted between 2004 to 2009, less than half of the patients received advanced modalities for small bowel evaluation.
The comparison of the groups with and without upper GI tract involvement confirmed that the patients with upper GI tract involvement had a younger median age at diagnosis (13.4 vs. 14.8; p = 0.0133), as already suggested in the literature25. In addition, in the multivariate logistic regression model in this study, younger age at diagnosis (16 years or less, OR 2.594, p = 0.0139) was associated with more upper GI tract involvement, consistent with other published studies13,26–28. Moreover, previous studies reported that patients with upper GI tract involvement had more extensive disease at diagnosis compared to patients without such involvement, supporting our results that ileal involvement was associated with upper GI tract involvement (OR 2.293, p = 0.0176)26,29–31. However, compared to the EUROKIDS registry, the proportion of patients with upper GI involvement was higher in this study despite the older age at diagnosis, which can be explained by ethnic differences.
In this study, the disease course of pediatric patients with upper GI tract involvement at CD diagnosis did not appear to be significantly different from that of patients with non-upper GI tract involvement, inconsistent with the findings of previous studies. Also, when analysing disease outcomes according to jejunal involvement, there was no significant difference between the two groups, which is also different from previous reports30. Upper GI tract involvement was associated with poor prognosis such as prolonged hospitalization, increased need for surgery, and relapse in CD patients26,31,32. There are two possible explanations for our results. First, the subjects in this study had received more aggressive top-down scheduled anti-TNFα therapy without steroids, which resulted in favourable outcomes in patients with upper GI tract involvement. Anti-TNFα therapy was a factor significantly associated with upper GI tract involvement in this study (OR 2.292, p = 0.0365) (Table 4) and this was consistent with one study suggesting that patients with upper GI tract involvement required more aggressive therapeutic approaches33. Second, the increased detection of patients with upper GI tract involvement at the time of diagnosis, due to the increased use of EGD and small bowel imaging over time, may have resulted in the overdiagnosis of such involvement, considering even minor and particularly asymptomatic involvement as significant.
The strengths of this study are strict and uniform diagnostic criteria and classification of CD, standardized enrolment, and conduct at a single-center, meaning that all subjects received unified treatment strategies, minimizing the drawbacks of retrospective data analysis. Also, we analysed a relatively large number of patients (n = 312) and long-term outcomes (median follow-up of 6.6 years) compared to other pediatric studies. The limitations of our study are its retrospective nature and, consequently, unstructured patient follow-up. In addition, selection bias may have been introduced by excluding patients who had not undergone a complete GI evaluation.
In conclusion, in our study, 74.4% of the pediatric CD patients had upper GI tract involvement. A young age of ≤ 16 years and ileal involvement were identified as factors associated with upper GI tract involvement at the time of CD diagnosis. The long-term disease course did not appear different between the patients with and without upper GI tract involvement. However, patients with upper GI disease might require more aggressive therapeutic approaches.
Author contributions
E.S.K. contributed in the conception and design of the study, acquisition, analysis and interpretation of data, drafting of the initial manuscript and critical revision for important intellectual content. Y.K. contributed in the acquisition, analysis and interpretation of data. Y.H.C. contributed in the conception and design of the study, analysis and interpretation of data and critical revision for important intellectual content. M.J.K. contributed in the conception and design of the study, analysis and interpretation of data and critical revision for important intellectual content. All authors approved the final version of the article. Guarantor of the article: Eun Sil Kim.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2020R1A2C2007192).
Data availability
Please contact the corresponding author for data requests.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hyams JS. Inflammatory bowel disease. Pediatr Rev. 2005;26(9):314–320. doi: 10.1542/pir.26-9-314. [DOI] [PubMed] [Google Scholar]
- 2.Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369(9573):1641–1657. doi: 10.1016/S0140-6736(07)60751-X. [DOI] [PubMed] [Google Scholar]
- 3.Benchimol EI, Fortinsky KJ, Gozdyra P, Van den Heuvel M, Van Limbergen J, Griffiths AM. Epidemiology of pediatric inflammatory bowel disease: a systematic review of international trends. Inflamm. Bowel Dis. 2011;17(1):423–439. doi: 10.1002/ibd.21349. [DOI] [PubMed] [Google Scholar]
- 4.Ghersin I, Khteeb N, Katz LH, Daher S, Shamir R, Assa A. Trends in the epidemiology of inflammatory bowel disease among Jewish Israeli adolescents: a population-based study. Aliment Pharmacol. Ther. 2019;49(5):556–563. doi: 10.1111/apt.15160. [DOI] [PubMed] [Google Scholar]
- 5.Ashton JJ, Cullen M, Afzal NA, Coelho T, Batra A, Beattie RM. Is the incidence of paediatric inflammatory bowel disease still increasing? Arch. Dis. Child. 2018;103(11):1093–1094. doi: 10.1136/archdischild-2018-315038. [DOI] [PubMed] [Google Scholar]
- 6.Sýkora J, Pomahačová R, Kreslová M, Cvalínová D, Štych P, Schwarz J. Current global trends in the incidence of pediatric-onset inflammatory bowel disease. World J. Gastroenterol. 2018;24(25):2741–2763. doi: 10.3748/wjg.v24.i25.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruemmele FM, Veres G, Kolho KL, et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn's disease. J. Crohns Colitis. 2014;8(10):1179–1207. doi: 10.1016/j.crohns.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Cosnes J, Bourrier A, Nion-Larmurier I, Sokol H, Beaugerie L, Seksik P. Factors affecting outcomes in Crohn's disease over 15 years. Gut. 2012;61(8):1140–1145. doi: 10.1136/gutjnl-2011-301971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee HA, Suk JY, Choi SY, et al. Characteristics of pediatric inflammatory bowel disease in Korea: comparison with EUROKIDS data. Gut Liver. 2015;9(6):756–760. doi: 10.5009/gnl14338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang B, Kim JE, Jung JH, et al. Korean children and adolescents with Crohn's disease are more likely to present with perianal fistulizing disease at diagnosis compared to their European counterparts. Pediatr. Gastroenterol. Hepatol. Nutr. 2020;23(1):49–62. doi: 10.5223/pghn.2020.23.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levine A, Koletzko S, Turner D, et al. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J. Pediatr. Gastroenterol. Nutr. 2014;58:795–806. doi: 10.1097/MPG.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 12.Levine A, Griffiths A, Markowitz J, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm. Bowel Dis. 2011;17(6):1314–1321. doi: 10.1002/ibd.21493. [DOI] [PubMed] [Google Scholar]
- 13.de Bie CI, Paerregaard A, Kolacek S, et al. Disease phenotype at diagnosis in pediatric Crohn's disease: 5-year analyses of the EUROKIDS Registry. Inflamm. Bowel Dis. 2013;19(2):378–385. doi: 10.1002/ibd.23008. [DOI] [PubMed] [Google Scholar]
- 14.Hyams JS, Ferry GD, Mandel FS, et al. Development and validation of a pediatric Crohn's disease activity index. J. Pediatr. Gastroenterol. Nutr. 1991;12(4):439–447. doi: 10.1097/00005176-199105000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Abuquteish D, Putra J. Upper gastrointestinal tract involvement of pediatric inflammatory bowel disease: a pathological review. World J. Gastroenterol. 2019;25(16):1928–1935. doi: 10.3748/wjg.v25.i16.1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartzberg DM, Brandstetter S, Grucela AL. Crohn's disease of the esophagus, duodenum, and stomach. Clin. Colon Rectal Surg. 2019;32(4):231–242. doi: 10.1055/s-0039-1683850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ingle SB, Adgaonkar BD, Jamadar NP, Siddiqui S, Hinge CR. Crohn's disease with gastroduodenal involvement: diagnostic approach. World J. Clin. Cases. 2015;3(6):479–483. doi: 10.12998/wjcc.v3.i6.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim HJ, Oh SH, Kim DY, et al. Clinical characteristics and long-term outcomes of paediatric Crohn's disease: a single-centre experience. J. Crohns Colitis. 2017;11(2):157–164. doi: 10.1093/ecco-jcc/jjw146. [DOI] [PubMed] [Google Scholar]
- 19.Shi HY, Levy AN, Trivedi HD, Chan FKL, Ng SC, Ananthakrishnan AN. Ethnicity influences phenotype and outcomes in inflammatory bowel disease: a systematic review and meta-analysis of population-based studies. Clin. Gastroenterol. Hepatol. 2018;16(2):190–197.e11. doi: 10.1016/j.cgh.2017.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng SC, Kaplan GG, Tang W, et al. Population density and risk of inflammatory bowel disease: a prospective population-based study in 13 countries or regions in Asia-Pacific. Am. J. Gastroenterol. 2019;114(1):107–115. doi: 10.1038/s41395-018-0233-2. [DOI] [PubMed] [Google Scholar]
- 21.van Hogezand RA, Witte AM, Veenendaal RA, Wagtmans MJ, Lamers CB. Proximal Crohn's disease: review of the clinicopathologic features and therapy. Inflamm. Bowel Dis. 2001;7(4):328–337. doi: 10.1097/00054725-200111000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Horjus Talabur Horje CS, Meijer J, Rovers L, van Lochem EG, Groenen MJ, Wahab PJ. Prevalence of upper gastrointestinal lesions at primary diagnosis in adults with inflammatory bowel disease. Inflamm. Bowel Dis. 2016;22(8):1896–1901. doi: 10.1097/MIB.0000000000000786. [DOI] [PubMed] [Google Scholar]
- 23.Laube R, Liu K, Schifter M, Yang JL, Suen MK, Leong RW. Oral and upper gastrointestinal Crohn's disease. J. Gastroenterol. Hepatol. 2018;33(2):355–364. doi: 10.1111/jgh.13866. [DOI] [PubMed] [Google Scholar]
- 24.Tobin JM, Sinha B, Ramani P, Saleh AR, Murphy MS. Upper gastrointestinal mucosal disease in pediatric Crohn disease and ulcerative colitis: a blinded, controlled study. J. Pediatr. Gastroenterol. Nutr. 2001;32(4):443–448. doi: 10.1097/00005176-200104000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Greuter T, Piller A, Fournier N, et al. Upper gastrointestinal tract involvement in Crohn's disease: frequency, risk factors, and disease course. J. Crohns Colitis. 2018;12(12):1399–1409. doi: 10.1093/ecco-jcc/jjy121. [DOI] [PubMed] [Google Scholar]
- 26.Sun XW, Wei J, Yang Z, et al. Clinical features and prognosis of Crohn's disease with upper gastrointestinal tract phenotype in Chinese patients. Dig. Dis. Sci. 2019;64(11):3291–3299. doi: 10.1007/s10620-019-05651-1. [DOI] [PubMed] [Google Scholar]
- 27.Cuffari C, Darbari A. Inflammatory bowel disease in the pediatric and adolescent patient. Gastroenterol. Clin. North Am. 2002;31(1):275–291. doi: 10.1016/S0889-8553(01)00017-6. [DOI] [PubMed] [Google Scholar]
- 28.Aloi M, Lionetti P, Barabino A, et al. Phenotype and disease course of early-onset pediatric inflammatory bowel disease. Inflamm. Bowel Dis. 2014;20(4):597–605. doi: 10.1097/01.MIB.0000442921.77945.09. [DOI] [PubMed] [Google Scholar]
- 29.Kuriyama M, Kato J, Morimoto N, Fujimoto T, Okada H, Yamamoto K. Specific gastroduodenoscopic findings in Crohn's disease: comparison with findings in patients with ulcerative colitis and gastroesophageal reflux disease. Dig. Liver Dis. 2008;40(6):468–475. doi: 10.1016/j.dld.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Mao R, Tang RH, Qiu Y, et al. Different clinical outcomes in Crohn's disease patients with esophagogastroduodenal, jejunal, and proximal ileal disease involvement: is L4 truly a single phenotype? Ther. Adv. Gastroenterol. 2018;11:1756284818777938. doi: 10.1177/1756284818777938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chow DK, Sung JJ, Wu JC, Tsoi KK, Leong RW, Chan FK. Upper gastrointestinal tract phenotype of Crohn's disease is associated with early surgery and further hospitalization. Inflamm. Bowel Dis. 2009;15(4):551–557. doi: 10.1002/ibd.20804. [DOI] [PubMed] [Google Scholar]
- 32.Kim OZ, Han DS, Park CH, et al. The clinical characteristics and prognosis of Crohn's disease in Korean patients showing proximal small bowel involvement: results from the CONNECT Study. Gut Liver. 2018;12(1):67–72. doi: 10.5009/gnl16500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crocco S, Martelossi S, Giurici N, Villanacci V, Ventura A. Upper gastrointestinal involvement in paediatric onset Crohn's disease: prevalence and clinical implications. J. Crohns Colitis. 2012;6(1):51–55. doi: 10.1016/j.crohns.2011.06.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Please contact the corresponding author for data requests.