Abstract
BACKGROUND
High-flow nasal cannula (HFNC) therapy and morphine continuous subcutaneous infusion (CSI) have been used to ameliorate dyspnea in non-cancer patients with end-stage respiratory diseases, including chronic obstructive pulmonary disease and interstitial pneumonia, primarily in hospital settings. However, it is rare to perform home-based medical treatment using these. We observe a case to assess the feasibility of this treatment strategy.
CASE SUMMARY
Here, we report a case of a 75-year-old man who was diagnosed with interstitial pneumonia 11 years ago and was successfully nursed at home during his terminal phase for over 10 mo without hospitalization, by introducing domiciliary uses of HFNC and morphine CSI with a patient-controlled analgesia device.
CONCLUSION
Active utilization of HFNC and morphine CSI with patient-controlled analgesia device would substantiate successful end-of-life palliative home care of idiopathic interstitial pneumonia patients.
Keywords: High flow nasal cannula, Continuous subcutaneous infusion, Morphine, Patient controlled analgesia, Home care, Interstitial pneumonia, Case report
Core Tip: We propose that active utilization of high-flow nasal cannula and morphine continuous subcutaneous infusion with a patient-controlled analgesia device would substantiate successful palliative care of patients with idiopathic interstitial pneumonia at home, nearing their end-of-life.
INTRODUCTION
High-flow nasal cannula (HFNC) therapy has been widely used in hospital settings to treat various forms of respiratory disorders. These include acute hypoxemic respiratory failure of patients with congestive heart failure, exacerbation of chronic obstructive pulmonary disease (COPD), lung cancer, and interstitial pneumonia, postoperative respiratory support, post-extubation respiratory support[1]. Recently, the use of HFNC has been extended to the palliative care of critically ill patients[1]; however, there have been a limited number of reports of the domiciliary uses of HFNC for patients with COPD[2] and chronic respiratory tract infection after gastrectomy[3].
Morphine is commonly known to provide effective relief to dyspnea caused by malignant tumors[4]. Similarly, it has been shown to be an effective home-based treatment for dyspnea in patients with COPD, at the end of life[5]. In contrast, while both oral administration[6] and continuous subcutaneous injection (CSI)[7] have been used effectively in hospital settings to ease dyspnea in interstitial pneumonia, the domiciliary use of morphine in patients with terminal-stage interstitial pneumonia has not been reported.
Here, we report a case where a patient with severe interstitial pneumonia and frequent relapse of fatal type 1 respiratory failure spent his final 10 mo at home without hospitalization by utilizing HFNC and morphine CSI with a patient-controlled analgesia (PCA) device.
CASE PRESENTATION
Chief complaints
A 75-year-old man presented to our hospital with dyspnea.
History of present illness
He was diagnosed with interstitial pneumonia 11 years ago. He had been treated with prednisolone (5 mg/d) and home oxygen therapy (3 L/min) for 1 year. However, between November of the same year to January of the following year, his lung function deteriorated, leading to hospitalization for 10 times over the next 13 mo for acute type 1 respiratory failure. The maximum length of stay at home between hospitalization was 30 d. Before his final admission to our hospital (his eleventh admission), the patient was at home for 6 d. At that time, hospitalization until the very end of life was proposed to the patient.
History of past illness
The patient had type 2 diabetes.
Personal and family history
At home he lived with his wife, and while he could consume food independently, he required assistance in changing clothes. Furthermore, excretion was done on the floor, and bathing was not possible due to restricted movements caused by exertion dyspnea. His modified Medical Research Council grade was 4. He smoked 80 cigarettes a day between the ages of 20 and 50.
Physical examination
The patient’s was thin during the final admission. He was fully conscious, with a body temperature of 38.4 ºC and a respiratory rate of 57 breaths/min. His SpO2 was 60% at 15 L oxygen/min with a mask with reservoir and 94% with non-invasive positive pressure ventilation [inspiratory positive airway pressure, 8 cmH2O; expiratory positive airway pressure, 4 cmH2O, and fraction of inspired oxygen (FiO2) 100%]. Fine crackles were heard bilaterally on the chest.
Laboratory examinations
Blood tests showed increases in his white blood cell count [32100 cells/µL, (neutrophils: 48.2%)] and C-reactive protein level (9.12 mg/L); no abnormalities were detected in the patient’s urinalysis.
Imaging examinations
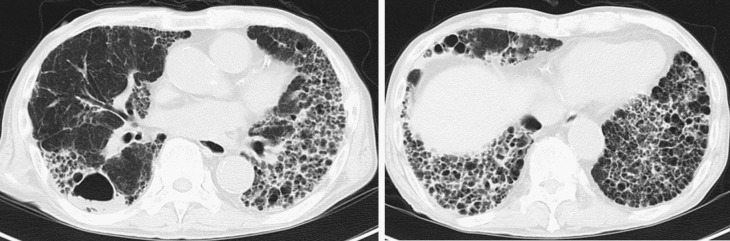
Chest X-rays showed reticular shadows on both sides of the lungs, and the computed tomography scan displayed a mixture of frosted glass shadows, reticular shadows, and tractive bronchodilation on both lungs (Figure 1).
Figure 1.
Chest computed tomography at latest admission.
FINAL DIAGNOSIS
We considered acute exacerbations of interstitial pneumonia and bacterial pneumonia.
TREATMENT
Although his general condition improved with piperazine/tazobactam treatment and the use of non-invasive positive pressure ventilation, the effect of orally administered codeine phosphate and morphine hydrochloride to suppress dyspnea and bouts of coughing was limited. Accordingly, morphine CSI (7.2 mg/d) was introduced. HFNC (FiO2 35%, 35 L/min) was also used to successfully assist his respiration.
OUTCOME AND FOLLOW-UP
Adhering to the patient’s wish to stay home, we coordinated with home care support to establish a system at home to provide CSI service with PCA, as well as domiciliary HFNC with oxygen supplied through two oxygen concentrators (maximum productivity 7 L oxygen/min each); then, he was discharged. CSI used a CADD Legacy® (Smiths Medical, St. Paul, MN, United States) with a 50–100-mL disposable medication cassette reservoir, which was replaced weekly. Initially, HFNC was set to provide humidified air of FiO2 37% at the rate of 35 L/min. The patient developed pneumonia several times, which was treated with antimicrobials without hospitalization. He survived for 10 more mo within home care.
DISCUSSION
We present the case of a patient with advanced stage of interstitial pneumonia, who was treated at home for a long period of time through a combination of HFNC and CSI. To the best of our knowledge, there is no preceding report on the domiciliary use of HFNC for terminal care of patients with interstitial pneumonia, nor reports that successfully used CSI at home in such patients.
Compared to conventional oxygen therapy, HFNC provides more stable high-flow high-concentration oxygen[8]. It has a beneficial effect in flushing anatomical dead spaces in the airway[9] and improving airway health through heating and humidification[10,11]. This has broad implications for inpatients for both acute and terminal stages of lung diseases. The exacerbating symptoms of COPD patients, who are hospitalized, are alleviated by the use of HFNC[12]. The long-term high-flow oxygen administration facilitates home-based care for critically ill patients at their end of lives[2,13]. HFNC also improves respiration in idiopathic interstitial pneumonia[1]. Moreover, patients using HFNC can comfortably eat and converse while high-concentration oxygen is administrated, maintaining better quality of life. Nonetheless, there have been a limited number of reports of using HFNC to treat critically ill lung disease patients at home at the end of life, mainly due to issues associated with oxygen supplies and securing purified water for humidification. For example, in the current case, the patient needed approximately 10 L of purified distilled water per week, which was supplied through home visiting service by a community pharmacist. The maximum supply of oxygen from the two oxygen concentrators connected together was 14 L/min. We also discussed rescue plans with the local fire department for issues such as unscheduled power outage. Solving these problems was crucial for the success of home care.
Morphine has been reported to be useful for respiratory distress in malignant tumors[4]. In COPD, morphine is clearly effective in alleviating dyspnea symptoms in severely ill patients[14,15]. It has central and peripheral antitussive effects (i.e. alleviation of anxiety and perceptual changes in dyspnea in the center), while decreasing systemic oxygen consumption in the periphery[16]. The efficacy of CSI has been demonstrated for interstitial pneumonia[7], which we also confirmed in the current case. CSI of morphine has the advantages over oral or intravascular administration as follows: it does not require a blood vessel, patients’ movements are not restricted by infusion lines, and morphine can be administered at a stable rate[17].
The majority of idiopathic interstitial pneumonia cases are progressive and do not respond to therapy. For patients who do not respond to disease specific therapy and who are not candidates for lung transplantation, the focus needs to shift towards end-of-life care[18]. The symptoms of dyspnea, persistent cough, and general fatigue are difficult to manage. The prognosis of idiopathic interstitial pneumonia is variable and unpredictable[19]. As effective remedies are limited, early introduction of palliative care is important to alleviate symptoms[18]. However, this is not generally the case[20]. While many patients with interstitial pneumonia wish to spend their last days at home[21], only a small number are actually able to do so[22,23]. Patient-related factors such as their own and their families’ willingness, and the care environment they accept, are essential to facilitate spending their last days under home care[24]. On the other hand, it is necessary to have a factor that can provide medical services including a home-visit medical system and home-visit nursing system[24]. Here, we propose that active utilization of HFNC and morphine CSI with a PCA device would substantiate successful palliative care of patients with idiopathic interstitial pneumonia at home nearing their end of life.
CONCLUSION
We facilitated and examined a case where HFNC and morphine CSI with PCA device were introduced at home to a patient with interstitial pneumonia who had recurrent type 1 respiratory failure; this enabled long-term treatment and home care. Our observations supported the option of having end-of-life medical care at home even in patients with interstitial pneumonia.
Footnotes
Informed consent statement: Written informed consent was obtained from the patient’s wife for the publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Conflict-of-interest statement: The authors declare that they have no competing interests.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Manuscript source: Unsolicited manuscript
Peer-review started: August 24, 2020
First decision: September 13, 2020
Article in press: September 25, 2020
Specialty type: Medicine, research and experimental
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chan SM S-Editor: Zhang L L-Editor: A P-Editor: Zhang YL
Contributor Information
Ken Goda, Department of Internal Medicine, Hyogo Prefectural Tamba Medical Center, Tamba 669-3495, Japan; Division of Community Medicine and Career Development, Kobe University Graduate School of Medicine, Kobe 652-0032, Japan; Department of Medicine, Tamba City mirune Clinic, Tamba 669-3464, Japan.
Tsuneaki Kenzaka, Department of Internal Medicine, Hyogo Prefectural Tamba Medical Center, Tamba 669-3495, Japan; Division of Community Medicine and Career Development, Kobe University Graduate School of Medicine, Kobe 652-0032, Japan; Department of Medicine, Tamba City mirune Clinic, Tamba 669-3464, Japan. smile.kenzaka@jichi.ac.jp.
Kyosuke Kuriyama, Clinical Engineer, Nursing Department, Hyogo Prefectural Tamba Medical Center, Tamba 669-3495, Japan.
Masahiko Hoshijima, Department of Internal Medicine, Hyogo Prefectural Tamba Medical Center, Tamba 669-3495, Japan.
Hozuka Akita, Department of Internal Medicine, Hyogo Prefectural Tamba Medical Center, Tamba 669-3495, Japan.
References
- 1.Ito J, Nagata K, Sato S, Shiraki A, Nishimura N, Izumi S, Tachikawa R, Morimoto T, Tomii K. The clinical practice of high-flow nasal cannula oxygen therapy in adults: A Japanese cross-sectional multicenter survey. Respir Investig. 2018;56:249–257. doi: 10.1016/j.resinv.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Nagata K, Kikuchi T, Horie T, Shiraki A, Kitajima T, Kadowaki T, Tokioka F, Chohnabayashi N, Watanabe A, Sato S, Tomii K. Domiciliary High-Flow Nasal Cannula Oxygen Therapy for Patients with Stable Hypercapnic Chronic Obstructive Pulmonary Disease. Ann Am Thorac Soc. 2018;15:432–439. doi: 10.1513/AnnalsATS.201706-425OC. [DOI] [PubMed] [Google Scholar]
- 3.Hamada S, Tsukino M. The clinical utility of domiciliary nocturnal high-flow nasal cannula in a post-gastrectomy patient with chronic lower respiratory tract infection. Pulmonology. 2018;24:312–313. doi: 10.1016/j.pulmoe.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Bruera E, MacEachern T, Ripamonti C, Hanson J. Subcutaneous morphine for dyspnea in cancer patients. Ann Intern Med. 1993;119:906–907. doi: 10.7326/0003-4819-119-9-199311010-00007. [DOI] [PubMed] [Google Scholar]
- 5.Faes K, Cohen J, Annemans L. Resource Use during the Last Six Months of Life among COPD Patients: A Population-Level Study. J Pain Symptom Manage. 2018;56:318–326.e7. doi: 10.1016/j.jpainsymman.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Allen S, Raut S, Woollard J, Vassallo M. Low dose diamorphine reduces breathlessness without causing a fall in oxygen saturation in elderly patients with end-stage idiopathic pulmonary fibrosis. Palliat Med. 2005;19:128–130. doi: 10.1191/0269216305pm998oa. [DOI] [PubMed] [Google Scholar]
- 7.Matsuda Y, Maeda I, Tachibana K, Nakao K, Sasaki Y, Sugimoto C, Arai T, Tokoro A, Akira M, Inoue Y. Low-Dose Morphine for Dyspnea in Terminally Ill Patients with Idiopathic Interstitial Pneumonias. J Palliat Med. 2017;20:879–883. doi: 10.1089/jpm.2016.0432. [DOI] [PubMed] [Google Scholar]
- 8.Dysart K, Miller TL, Wolfson MR, Shaffer TH. Research in high flow therapy: mechanisms of action. Respir Med. 2009;103:1400–1405. doi: 10.1016/j.rmed.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Kernick J, Magarey J. What is the evidence for the use of high flow nasal cannula oxygen in adult patients admitted to critical care units? Aust Crit Care. 2010;23:53–70. doi: 10.1016/j.aucc.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Hasani A, Chapman TH, McCool D, Smith RE, Dilworth JP, Agnew JE. Domiciliary humidification improves lung mucociliary clearance in patients with bronchiectasis. Chron Respir Dis. 2008;5:81–86. doi: 10.1177/1479972307087190. [DOI] [PubMed] [Google Scholar]
- 11.Tomii K. Indications and limitations of nasal high-flow therapy. J Care Rehabil . 2015;25:53–57. [Google Scholar]
- 12.Storgaard LH, Hockey HU, Laursen BS, Weinreich UM. Long-term effects of oxygen-enriched high-flow nasal cannula treatment in COPD patients with chronic hypoxemic respiratory failure. Int J Chron Obstruct Pulmon Dis. 2018;13:1195–1205. doi: 10.2147/COPD.S159666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dolidon S, Dupuis J, Molano Valencia LC, Salaün M, Thiberville L, Muir JF, Cuvelier A, Patout M. Characteristics and outcome of patients set up on high-flow oxygen therapy at home. Ther Adv Respir Dis. 2019;13:1753466619879794. doi: 10.1177/1753466619879794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh D, Agusti A, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, Criner GJ, Frith P, Halpin DMG, Han M, López Varela MV, Martinez F, Montes de Oca M, Papi A, Pavord ID, Roche N, Sin DD, Stockley R, Vestbo J, Wedzicha JA, Vogelmeier C. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: the GOLD science committee report 2019. Eur Respir J. 2019;53 doi: 10.1183/13993003.00164-2019. [DOI] [PubMed] [Google Scholar]
- 15.Rocker GM, Sinuff T, Horton R, Hernandez P. Advanced chronic obstructive pulmonary disease: innovative approaches to palliation. J Palliat Med. 2007;10:783–797. doi: 10.1089/jpm.2007.9951. [DOI] [PubMed] [Google Scholar]
- 16.Kin SC. Palliative medicine in malignant respiratory diseases. 4th ed. Hawks G, Cherhy NI, Christakis NA, editors. Oxford textbook of palliative medicine. Oxford: Oxford University Press, 2009: 1129-1130. [Google Scholar]
- 17.Koshi A, Masaru H. Both switching from a winged needle to a small plastic intravenous catheter and adding dexamethasone to continuous subcutaneous infusion (CSCI) successfully treated inflammatory skin changes caused by CSCI. Palliat Care Res. 2012;7:112–120. [Google Scholar]
- 18.Garibaldi BT, Danoff SK. Symptom-based management of the idiopathic interstitial pneumonia. Respirology. 2016;21:1357–1365. doi: 10.1111/resp.12649. [DOI] [PubMed] [Google Scholar]
- 19.Wijsenbeek MS, Holland AE, Swigris JJ, Renzoni EA. Comprehensive Supportive Care for Patients with Fibrosing Interstitial Lung Disease. Am J Respir Crit Care Med. 2019;200:152–159. doi: 10.1164/rccm.201903-0614PP. [DOI] [PubMed] [Google Scholar]
- 20.Ahmadi Z, Wysham NG, Lundström S, Janson C, Currow DC, Ekström M. End-of-life care in oxygen-dependent ILD compared with lung cancer: a national population-based study. Thorax. 2016;71:510–516. doi: 10.1136/thoraxjnl-2015-207439. [DOI] [PubMed] [Google Scholar]
- 21.Skorstengaard MH, Neergaard MA, Andreassen P, Brogaard T, Bendstrup E, Løkke A, Aagaard S, Wiggers H, Bech P, Jensen AB. Preferred Place of Care and Death in Terminally Ill Patients with Lung and Heart Disease Compared to Cancer Patients. J Palliat Med. 2017;20:1217–1224. doi: 10.1089/jpm.2017.0082. [DOI] [PubMed] [Google Scholar]
- 22.Rajala K, Lehto JT, Saarinen M, Sutinen E, Saarto T, Myllärniemi M. End-of-life care of patients with idiopathic pulmonary fibrosis. BMC Palliat Care. 2016;15:85. doi: 10.1186/s12904-016-0158-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindell KO, Liang Z, Hoffman LA, Rosenzweig MQ, Saul MI, Pilewski JM, Gibson KF, Kaminski N. Palliative care and location of death in decedents with idiopathic pulmonary fibrosis. Chest. 2015;147:423–429. doi: 10.1378/chest.14-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hattori A, Uemura K, Masuda Y, Mogi N, Naito M, Iguchi A. [Factors contributing to dying at home in elderly patients who received home care service] Nihon Ronen Igakkai Zasshi. 2001;38:399–404. doi: 10.3143/geriatrics.38.399. [DOI] [PubMed] [Google Scholar]