Abstract
BACKGROUND
Endodontic-periodontal lesion is a commonly encountered dental condition. However, the prognosis of the condition varies from good to poor. Some cases are associated with a poor prognosis that requires tooth extraction. This report presents a case of an endodontic-periodontal lesion in a tooth that was successfully treated by root canal treatment.
CASE SUMMARY
A 51-year-old female patient with no medical history complained about persistent pain and discomfort in her left mandibular first molar. Clinical examination showed the left mandibular first molar with poor restoration. It was also associated with underlying necrotic pulp and periodontal involvement. Radiographic examination revealed visible bone defects in the apical and periodontal areas. Based on the findings, the patient was diagnosed with a primary endodontic lesion. A root canal treatment for the endodontic lesion was performed. The patient received a coronal all-ceramic endocrown restoration. A follow-up was arranged to check the prognosis. At the 3 mo follow-up, the clinical and radiography evaluations showed complete disappearance of signs and symptoms and an increase in the radiopacity of the root area.
CONCLUSION
Despite the poor prognosis associated with many endodontic lesions, this case report highlights that a good prognosis is still possible for an endodontic lesion with apical and periodontal bone loss. In this case, it was achieved via successful root canal treatment without the need for periodontal or surgical intervention.
Keywords: Apical bone defect, Endo-perio lesion, Primary endodontic lesion, Root canal treatment, Nonsurgical root canal treatment, Conservative, Case report
Core Tip: We present a case of an endodontic-periodontal lesion in a tooth that was successfully treated by root canal treatment. Sufficient knowledge about the diagnosis, treatment strategies, and intervals of treatment of the endodontic-periodontal lesion is essential to obtain the best outcomes in a short period with a conservative and minimally invasive procedure.
INTRODUCTION
Endodontic-periodontal disease refers to lesions that inflict both pulpal and periodontal tissues of a tooth. Due to the underlying anatomy, the pulpal and periodontal tissue structures are closely connected via three pathways. The main channels of communication between the pulpal and periodontal structures are dentinal tubules, lateral and accessory canals, and apical foramen[1]. The relationship between endodontic and periodontal diseases was first described by Simring and Goldberg[2] in 1964. Currently, there is a common consensus among clinicians that bacterial infections are the main etiology of endodontic-periodontal disease[3]. The bacteria can penetrate the periodontal tissue and the root canal system in different manners. The main access route between the pulpal and periodontal tissues for the microorganism is the root end foramen. Apart from that, other parts of the root canal system such as the abovementioned dentinal tubules, lateral canals, and accessory canals or foramen can also act as the medium of contamination for the bacterial byproducts[4,5]. The presence of pulp exposure, caries, and periodontitis may aggravate the development of bacterial infection. The failure to treat the lesions and achieve a completely disinfected and sealed root canal may enable the remaining bacteria to develop further endodontic-periodontal disease or endodontic reinfection[6-8]. Additionally, the presence of vertical root fractures or root cracks may create a communication channel that links the pulp system to the surrounding periodontal tissue. When this happens, previous periodontal inflammation may spread to the surrounding areas, subsequently resulting in pulp necrosis[9].
Based on the underlying pathological origin, an endodontic-periodontal lesion can be classified as a primary endodontic lesion, a primary periodontal lesion, a primary endodontic lesion with secondary periodontal involvement, a primary periodontal lesion with secondary endodontic involvement, or combined lesions[10]. It is important to achieve an optimal diagnosis via careful history-taking, intra-oral, and extra-oral examinations, and application of individual tests to ensure the treatment success of such lesions. In this case report, we described the comprehensive diagnosis and treatment planning for the management of an endodontic-periodontal lesion of endodontic origin.
CASE PRESENTATION
Chief complaints
A 51-year-old female patient with no medical history presented to the Endodontic Department of Jilin University Stomatological Hospital with a complaint of persistent painful swelling at the left mandibular posterior buccal area.
History of present illness
Upon history-taking, she revealed that she underwent a tooth-filling procedure on the left mandibular posterior area 10 years ago. For the past 3 mo, she had been experiencing slight pain, discomfort, and swelling over the affected area. She took a type of anti-inflammatory drug, and the pain decreased. She was unsure of the type and dosage of the drug she took. However, for the past 1 mo, the pain and swelling recurred. It was worse in severity and not relieved by the same anti-inflammatory drug she took before.
History of past illness
She had no significant medical history.
Physical examination
The external examination of the face and palpation for lymphadenopathy did not show any significant abnormality. However, a composite resin restoration in the left mandibular first molar (tooth #36) was detected upon the intraoral examination. There was no response on the pulp vitality test, but the patient complained of obvious pain upon percussion. Furthermore, there was slight gingival congestion and swelling at the buccal area of tooth #36 with exudate extrusion upon the application of pressure. The periodontal examination showed a pocket of 6 mm depth in the mesiobuccal (MB) aspect of tooth #36, with a grade I tooth mobility. The intraoral examination also reveals a fair oral hygiene status with no signs of trauma or malocclusion.
Laboratory examinations
No laboratory tests.
Imaging examinations
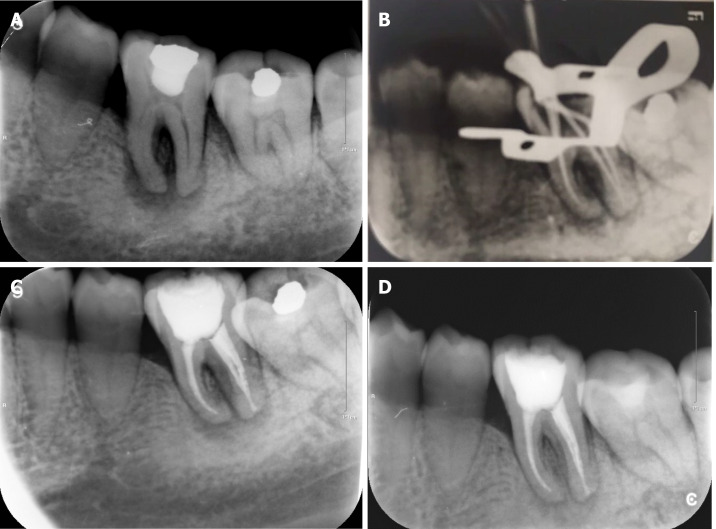
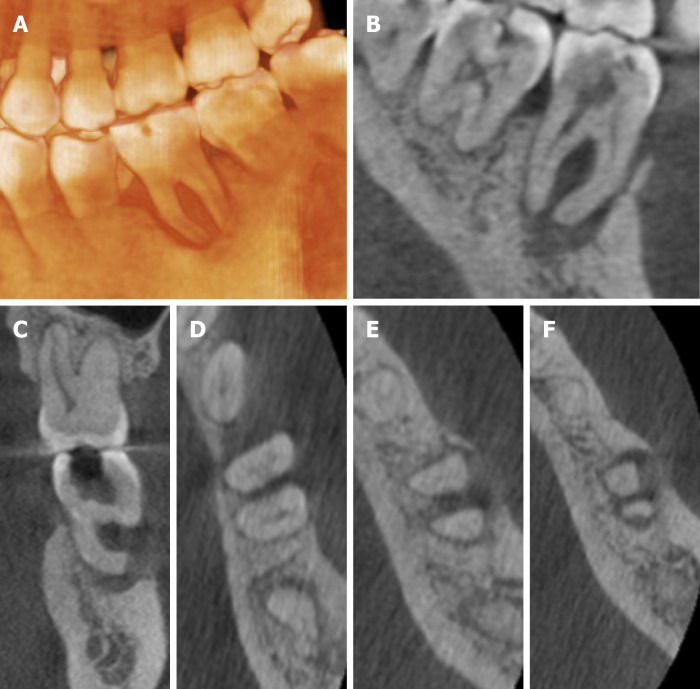
The preoperative radiograph (Figure 1A) revealed an occlusal filling close to the pulp cavity in tooth #36. A large area of radiolucency in the apical area was observed along the periodontal lining. It was associated with a bifurcation involvement. Cone beam computed tomography (CBCT) imaging showed that the apical, periodontal, and root bifurcation areas were all connected. Furthermore, a high level of resorption of the buccal bone plate in the tooth #36 region (Figure 2).
Figure 1.
Periapical radiograph. A: Initial preoperative periapical radiograph; B: Gutta-Percha cone fitting and working length confirmation with periapical radiograph; C: Post-obturation periapical radiograph; D: Periapical radiograph at the 3 mo follow-up.
Figure 2.
Images of cone beam computed tomography showing buccal, bifurcation, and apical bone resorption (A-F).
FINAL DIAGNOSIS
Based on the clinical and imaging examination, a final pupal diagnosis of pulp necrosis and chronic apical periodontitis of tooth #36 was made, while the periodontal status was apical, bifurcation and buccal bone resorption at the #36 tooth area associated with 6 mm mesiobuccal depth pocket. The case was classified according to endodontic-periodontal lesions classification, as a primary endodontic lesion. The periodontal involvement was secondary to the endodontic one, and may also cure after the root canal treatment success. In view of the periodontal involvement on top of the endodontic lesion, the patient was counseled for root canal treatment.
TREATMENT
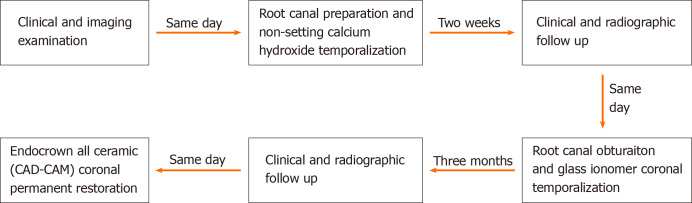
A non-surgical root canal treatment was proposed. The treatment plan was discussed with the patient and based on her condition, the treatment plan was developed and is shown in Figure 3. The patient provided written informed consent in accordance with the bioethical guidelines of the hospital. Before the procedure, local single tooth anesthesia (STA) of 2% lidocaine with 1:100000 epinephrine was applied using the electronic STA Wand System (Milestone Scientific, Livingston, NJ, United States). After rubber dam isolation, the old restoration and decayed tooth tissue were removed, and an appropriate access cavity was refined using a Diamond stone (NTI flex; Kavo Kerr Dent, Berlin, Germany) adapted on high-speed dental handpiece (Sirona T3; Sirona Dental Systems, Bensheim Germany). The coronal pulp tissue was excavated. Using the DG16 endodontic explorer (Hu-Freddy, Chicago, IL, United States) under the dental operating microscope (Leica M320 F12; Lecia Microsystems, Singapore), the detection of four root canal orifices were performed (MB, mesiolingual [ML], distobuccal [DB], distolingual [DL]). The working length was determined using an electronic apex locator (Propex II; Dentsply Maillefer, Ballaigues, Switzerland). The canals were initially instrumented using the ISO 10 K hand file (Dentsply Maillefer). A glide path preparation was done using rotary path file instruments (Pro-Glider; Dentsply Maillefer) adapted on the endodontic micromotor (X-SMART Plus; Dentsply Maillefer). The final instrumentation of the canals was performed using Nickel-titanium rotary instruments (Protaper Next, Dentsply Maillefer) up to size 25 in the MB/ML canals, and up to size 30 in the DB/DL canals. The irrigation was done using 30-gauge needle (NaviTip; Ultradent, South Jordan, UT, United States) and 5.25% sodium hypochlorite (Beyond Technology Corp., Beijing, China). Passive ultrasonic irrigation activation of the 5.25% sodium hypochlorite was applied using ultrasonic tip (Irrisafe; Acteon, Merignac Cedex, France) that was adopted on a piezoelectric ultrasonic unit (P5 Newtron XS; Acteon, Merignac Cedex, France). It was followed by 17% EDTA (Canal Pro, Coltene/Whaledent, Cuyahoga Falls, OH, United States) irrigation and activation and 0.9% sodium chloride (China Otsuka Pharmaceutical Co., Ltd., Tianjin, China) as the final rinse. Canals were dried with absorbent paper points (Dentsply Maillefer) and temporized with non-setting calcium hydroxide (Pulpdent Corp., Watertown, MA, United States) and the coronal cavity was temporized with glass ionomer filling (Ketac Fil plus; 3M Espe, Seefeld, Germany) to be observed during follow-up. The patient was given a prescription for anti-inflammatory drugs with instructions to take for pain only if needed and to inform the doctor immediately in case of discomfort.
Figure 3.
Flow chart timeline of the treatment plan.
During the 2 wk follow-up, the patient reported that all the symptoms had resolved. On examination, the gingival tissue showed proper healing and a normal response to percussion, and the root canals did not show any exudation. Recapitulation and irrigation of the canals were done. Gutta-Percha cones (Dentsply Maillefer) fitting and confirmation of the working length were performed with a periapical radiograph (Figure 1B). By applying absorbent paper points that matched the final root canal preparation size, the canals were dried. Continuous-wave compaction (Gapadent, Tianjin, China) with AH Plus sealer (Dentsply Maillefer) was used for canal obturation. The access cavity was temporized with glass ionomer filling. Finally, a post obturation periapical radiograph was taken for obturation confirmation (Figure 1C).
OUTCOME AND FOLLOW-UP
After 3 mo of observation, the patient remained asymptomatic. A repeated periapical radiograph showed bone reposition in the apical and root bifurcation areas (Figure 1D). The periodontal examination showed decreased probing depth in the mesiobuccal aspect to 4 mm, with absence of tooth mobility. Next, Endocrown, an all-ceramic crown, was performed for the final coronal restoration (IPS e-max CAD; Ivoclar Vivadent, Schaan, Liechtenstein) using the chairside CAD-CAM technology (CEREC; Dentsply Sirona, York, PA, United States). Following the final restoration, her discomfort was alleviated, and she was satisfied by the final result.
DISCUSSION
The endodontic-periodontal lesion is a complicated disease entity that can be challenging for clinicians to diagnose and treat. The lesion might be difficult to diagnose. Often, the prognosis of the affected tooth is poor if not properly managed. According to Kim et al[11], even by using an operating dental microscope during the treatment, endodontic-periodontal lesions often have a lower success rate than isolated endodontic lesion. In endodontic-periodontal lesions, the pulpal and periodontal tissues are mainly connected through the apical foramen. Thus, any pulpal inflammation may extend from the apical foramen into the periapical tissue. This typically results in local periapical inflammation that is associated with bone resorption[12]. Other studies have found that the accessory canals in the furcation area of the multirooted tooth can act as communication channels[1,13]. Nevertheless, the effects of periodontal diseases on the pulpal tissues have not been clearly delineated. In a previous study, there were no changes to the pulpal tissues before the periodontal inflammation spread the root apex[14]. Similarly, other studies have reported no severe effects in the pulp in most periodontal diseases unless there were communication channels created from the recession and exposure of the lateral accessory canals[15,16].
In the management of endodontic-periodontal disease, proper diagnosis is critical to ensure treatment success and favorable long-term prognosis. Accurate classification of the lesion is the first step in helping the clinicians to design the most appropriate treatment strategy. To achieve an optimal diagnosis, proper history taking must be performed to determine the most accurate diagnosis based on the disease characteristics. The diagnosis of endodontic-periodontal lesions should be based on a combination of history taking from the patient, clinical evaluation, and radiographic examination. In addition, special tests such as vitality tests, percussion, mobility, and periodontal probing should be performed on the infected tooth after removal of the defective restorations. These are essential diagnostic tests aimed at differentiating between pulpal and periodontal diseases[17].
The first step of clinical evaluation in endodontic-periodontal disease involves the examination of the infected tooth vitality. While the pulp vitality status can be estimated by direct viewing, the actual histological status of the pulp cannot be determined completely in this way[18]. For this patient, the presence of defective occlusal restoration and the absence vitality of the affected tooth suggested an endodontic involvement. Periodontal examination of tooth #36 displayed a narrow pocket of 6 mm depth mesiobuccally. This pocket acted as a sinus tract for the drainage of the pulp exudates. Otherwise, the periodontal examination did not show any other significant abnormality. There was also no premature contact of the occlusal surface with the infected tooth, thus indicating that the lesion was of an endodontic origin. Such lesions usually have a better prognosis following the root canal treatment. Furthermore, a previous study reported that the sinus tract extending through the gingival sulcus or the furcation area might disappear in the early stage after disinfection, shaping, and obturation of the root canals[19].
Following clinical evaluation, a radiographic examination should be performed. A two-dimensional periapical technique can show the extension of the radiolucency area around the roots of the tooth. Nevertheless, the sole use of the periapical radiograph cannot determine whether the lesion is of an endodontic or periodontal origin. It must be combined with other clinical modalities to confirm the diagnosis. For example, the CBCT radiographic imaging is often used to obtain an accurate diagnosis before deciding on the best treatment plan[20]. In this case, the CBCT imaging showed extensive bone destruction at the buccal aspect of the affected tooth, indicating a poor prognosis. However, it is possible for lesions with endodontic origin to resolve completely after the removal of the infection source. During the treatment for a lesion with an endodontic origin, the application of intracanal non-setting calcium hydroxide medication was recommended. Calcium hydroxide has good antibacterial activity, and biocompatibility[21] and its application after the root canal procedure could inactivate any exotoxins by hindering the increase in cytokine chemical inflammatory mediators. It could also inhibit any post-penetration preapical inflammation[22-24]. In this patient, the root canal preparation was done with irrigation of sodium hypochlorite and EDTA, followed by a non-setting calcium hydroxide temporization.
During the follow-up, the outcome of the procedure was favorable. There was an acceptable healing process. The patient’s initial complaints had resolved. The clinical examination showed improvement in the gingival status and a decrease in the secretion of sinus tract exudates. Therefore, the obturation procedure of the root canals could be done safely by performing a successful root canal disinfection and preparation. During the patient’s post-obturation follow-up, significant healing could be detected in the gingival area. The follow-up radiographs showed bone deposition in the affected area, as evidenced by the reduced area of radiolucency. Based on this, it was decided that a final coronal restoration would be sufficient, and no further surgical or periodontal interventions would be needed.
CONCLUSION
In practice, to improve the treatment success of endodontic-periodontal disease, the clinicians must have sufficient knowledge concerning the etiology, classification, diagnosis, and treatment strategies of the condition. The complexity of such lesions highlighted the importance of an accurate diagnosis to ensure the best treatment plan and outcomes. Therefore, detailed extraoral and intraoral examination, periapical and CBCT radiographic imaging, periodontal analysis, and the application of additional tests to check for the tooth vitality should be performed. The detailed investigation can guide the clinicians towards a proper diagnosis before deciding on the final treatment plan. In this case report, the treatment outcomes suggested that primary endodontic lesions can be managed by root canal treatment alone without surgical interventions, provided that the clinician has the sufficient knowledge about the diagnosis, treatment strategies, and intervals of treatment. In short, immediate and optimum management of such lesions can prevent further complications such as tooth loss.
Footnotes
Informed consent statement: Informed written consent was obtained from the patient for publication of this report and any accompanying images.
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Manuscript source: Unsolicited manuscript
Peer-review started: May 29, 2020
First decision: July 25, 2020
Article in press: August 29, 2020
Specialty type: Dentistry, oral surgery and medicine
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Paredes-Vieyra JP S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Li JH
Contributor Information
Hamed Alshawwa, Department of Endodontics, Hospital of Stomatology, Jilin University, Changchun 130021, Jilin Province, China.
Jia-Feng Wang, Department of Endodontics, Hospital of Stomatology, Jilin University, Changchun 130021, Jilin Province, China.
Min Liu, Department of Periodontics, Hospital of Stomatology, Jilin University, Changchun 130021, Jilin Province, China.
Shu-Fen Sun, Department of Endodontics, Hospital of Stomatology, Jilin University, Changchun 130021, Jilin Province, China. gzwssf@163.com.
References
- 1.Gutmann JL. Prevalence, location, and patency of accessory canals in the furcation region of permanent molars. J Periodontol. 1978;49:21–26. doi: 10.1902/jop.1978.49.1.21. [DOI] [PubMed] [Google Scholar]
- 2.Simring M, Goldberg M. The pulpal pocket approach: retrograde periodontitis. J Periodontol. 1964;35:22–48. [Google Scholar]
- 3.Das AC, Sahoo SK, Parihar AS, Bhardwaj SS, Babaji P, Varghese JG. Evaluation of role of periodontal pathogens in endodontic periodontal diseases. J Family Med Prim Care. 2020;9:239–242. doi: 10.4103/jfmpc.jfmpc_725_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adriaens PA, De Boever JA, Loesche WJ. Bacterial invasion in root cementum and radicular dentin of periodontally diseased teeth in humans. A reservoir of periodontopathic bacteria. J Periodontol. 1988;59:222–230. doi: 10.1902/jop.1988.59.4.222. [DOI] [PubMed] [Google Scholar]
- 5.Adriaens PA, Edwards CA, De Boever JA, Loesche WJ. Ultrastructural observations on bacterial invasion in cementum and radicular dentin of periodontally diseased human teeth. J Periodontol. 1988;59:493–503. doi: 10.1902/jop.1988.59.8.493. [DOI] [PubMed] [Google Scholar]
- 6.Meirinhos J, Martins JNR, Pereira B, Baruwa A, Gouveia J, Quaresma SA, Monroe A, Ginjeira A. Prevalence of apical periodontitis and its association with previous root canal treatment, root canal filling length and type of coronal restoration - a cross-sectional study. Int Endod J. 2020;53:573–584. doi: 10.1111/iej.13256. [DOI] [PubMed] [Google Scholar]
- 7.Thampibul P, Jantarat J, Arayasantiparb R. Post-treatment apical periodontitis related to the technical quality of root fillings and restorations in Thai population. Aust Endod J. 2019;45:163–170. doi: 10.1111/aej.12302. [DOI] [PubMed] [Google Scholar]
- 8.Sritharan A. Discuss that the coronal seal is more important than the apical seal for endodontic success. Aust Endod J. 2002;28:112–115. doi: 10.1111/j.1747-4477.2002.tb00404.x. [DOI] [PubMed] [Google Scholar]
- 9.Andreasen JO, Andreasen FM, Skeie A, Hjørting-Hansen E, Schwartz O. Effect of treatment delay upon pulp and periodontal healing of traumatic dental injuries -- a review article. Dent Traumatol. 2002;18:116–128. doi: 10.1034/j.1600-9657.2002.00079.x. [DOI] [PubMed] [Google Scholar]
- 10.Simon JH, Glick DH, Frank AL. The relationship of endodontic-periodontic lesions. J Endod. 2013;39:e41–e46. doi: 10.1016/j.joen.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Kim E, Song JS, Jung IY, Lee SJ, Kim S. Prospective clinical study evaluating endodontic microsurgery outcomes for cases with lesions of endodontic origin compared with cases with lesions of combined periodontal-endodontic origin. J Endod. 2008;34:546–551. doi: 10.1016/j.joen.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 12.Jakovljevic A, Miletic M, Nikolic N, Beljic-Ivanovic K, Andric M, Milasin J. Notch signaling pathway mediates alveolar bone resorption in apical periodontitis. Med Hypotheses. 2019;124:87–90. doi: 10.1016/j.mehy.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 13.Wolf TG, Wentaschek S, Wierichs RJ, Briseño-Marroquín B. Interradicular Root Canals in Mandibular First Molars: A Literature Review and Ex Vivo Study. J Endod. 2019;45:129–135. doi: 10.1016/j.joen.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 14.Czarnecki RT, Schilder H. A histological evaluation of the human pulp in teeth with varying degrees of periodontal disease. J Endod. 1979;5:242–253. doi: 10.1016/S0099-2399(79)80018-7. [DOI] [PubMed] [Google Scholar]
- 15.Wan L, Lu HB, Xuan DY, Yan YX, Zhang JC. Histological changes within dental pulps in teeth with moderate-to-severe chronic periodontitis. Int Endod J. 2015;48:95–102. doi: 10.1111/iej.12282. [DOI] [PubMed] [Google Scholar]
- 16.Gautam S, Galgali SR, Sheethal HS, Priya NS. Pulpal changes associated with advanced periodontal disease: A histopathological study. J Oral Maxillofac Pathol. 2017;21:58–63. doi: 10.4103/0973-029X.203795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abbott PV, Salgado JC. Strategies for the endodontic management of concurrent endodontic and periodontal diseases. Aust Dent J. 2009;54 Suppl 1:S70–S85. doi: 10.1111/j.1834-7819.2009.01145.x. [DOI] [PubMed] [Google Scholar]
- 18.Aksel H, Serper A. A case series associated with different kinds of endo-perio lesions. J Clin Exp Dent. 2014;6:e91–e95. doi: 10.4317/jced.51219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rotstein I, Simon JH. The endo-perio lesion: a critical appraisal of the disease condition. Endod Top . 2006;13:34–56. [Google Scholar]
- 20.Patel S, Brown J, Pimentel T, Kelly RD, Abella F, Durack C. Cone beam computed tomography in Endodontics - a review of the literature. Int Endod J. 2019;52:1138–1152. doi: 10.1111/iej.13115. [DOI] [PubMed] [Google Scholar]
- 21.Mohammadi Z, Dummer PM. Properties and applications of calcium hydroxide in endodontics and dental traumatology. Int Endod J. 2011;44:697–730. doi: 10.1111/j.1365-2591.2011.01886.x. [DOI] [PubMed] [Google Scholar]
- 22.Marinho AC, Polay AR, Gomes BP. Accuracy of Turbidimetric Limulus Amebocyte Lysate Assay for the Recovery of Endotoxin Interacted with Commonly Used Antimicrobial Agents of Endodontic Therapy. J Endod. 2015;41:1653–1659. doi: 10.1016/j.joen.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 23.Pereira MS, Rossi MA, Cardoso CR, da Silva JS, Bezerra da Silva LA, Kuga MC, Faria G. Cellular and molecular tissue response to triple antibiotic intracanal dressing. J Endod. 2014;40:499–504. doi: 10.1016/j.joen.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 24.Tavares WL, de Brito LC, Henriques LC, Teles FR, Teles RP, Vieira LQ, Ribeiro Sobrinho AP. Effects of calcium hydroxide on cytokine expression in endodontic infections. J Endod. 2012;38:1368–1371. doi: 10.1016/j.joen.2012.06.036. [DOI] [PubMed] [Google Scholar]