Abstract
BACKGROUND
Congenital tufting enteropathy (CTE) is a rare cause of diarrhea in children. However, it can result in early-onset of chronic diarrhea and failure to thrive. Children with this disease have to depend on total parenteral nutrition (TPN), and eventually small intestine transplantation. The epithelial cell adhesion molecule (EPCAM) gene was identified to be associated with CTE. Here, we present a case of an infant with CTE due to a mutation not reported in the literature before.
CASE SUMMARY
A 1-year and 7-mo infant boy exhibited intractable watery diarrhea and mushy stool within 1 wk after birth, for which he had required medical treatment and hospitalization several times. His sister presented similar symptoms and died at the age of two. On admission, his body weight was 5700 g (-4.8SDS) and measured 66 cm (-5.4SDS) in height. Meanwhile, he cannot speak or climb. He exhibited mild anemia, hypocalcemia, hypomagnesemia, and an infection in the upper respiratory tract. Microvilli sparse and vacuolar degeneration of epithelial cells were reported by small intestine biopsy. Whole-exome sequencing showed a novel homozygous splice mutation (c.657+1[IVS6] G>A) in the EPCAM gene. He was treated with TPN and recombinant human growth hormone. After 2 mo, his body weight was up to 8500 g and he has been waiting for small bowel transplantation.
CONCLUSION
CTE is rare but fatal. Patients with CTE require rapid diagnosis and therapy to improve their survival.
Keywords: Congenital tufting enteropathy, Congenital diarrhea, Failure to thrive, Children, Case report
Core Tip: Congenital tufting enteropathy (CTE) is a rare cause of congenital diarrheal disorders. Patients suffering from CTE are characterized by intractable watery diarrhea and severe malnutrition throughout their life. They depend on total parenteral nutrition to sustain their weight and eventually small intestine transplantation. We reported the case of an infant with severe watery diarrhea and failure to thrive who was subsequently found to have a new mutation in epithelial cell adhesion molecule gene (c.657+1[IVS6] G>A). Patients with CTE require rapid diagnosis and therapy to improve their survival.
INTRODUCTION
Diarrhea is a major cause of infant death in developing countries. Although most diarrheal diseases result from infection or inflammation, some conditions such as congenital diarrhea are problematic to cure. Congenital tufting enteropathy (CTE), an autosomal recessive disease, was first described by Reifen et al[1] in 1994. It has been estimated to occur in about 1/50000 to 100000 live births in Western Europe[2]. Higher incidence has been reported in the Arabian Peninsula due to the high degree of consanguinity[3]. Patients suffering from CTE are characterized by intractable watery diarrhea and severe malnutrition throughout their life. They depend on total parenteral nutrition (TPN) to sustain their weight[4]. Since Sivagnanam et al[5] identified epithelial cell adhesion molecule (EPCAM) as a gene for CTE, plenty of mutations of EPCAM have been found. In this case, our aim was to further characterize the clinical, histopathologic, and molecular features of CTE with a new mutation in a Chinese child.
CASE PRESENTATION
Chief complaints
A 1-year and 7-mo infant boy was admitted to our hospital with intractable watery diarrhea over 1 year.
History of present illness
The patient exhibited intractable watery diarrhea and mushy stool within 1 wk after birth, which cannot be cured by antibiotics or other treatments, and failed to thrive. From born to now, he just gained 2 kg or so in weight. Currently, he is unable to climb or speak even a word. He also suffered from a snotty nose and a slight cough.
History of past illness
The patient is allergic to contrast medium, milk, and protein. He was frequently hospitalized due to diarrhea, malnutrition, low calcium, low magnesium, and recurrent infection. He has been infused with suspended red blood cells and human immunoglobulin several times.
Personal and family history
The patient was born to a G4P2 mother at full-term gestation. His birth weight was 3200 g. His parents are Chinese and are healthy without consanguineous mating or genetic diseases. His sister presented similar symptoms and died at the age of two. He could raise his head at 3 mo, turn over at 6 mo, sit by himself at 8 mo, but to date he cannot climb or stand. Moreover, he was breastfed until 7 mo and lived off porridge mixed with extensively hydrolyzed infant formula powder.
Physical examination
The patient had stable vital signs with dot long tooth. His body weight was 5700 g (-4.8 SDS) and measured 66 cm (-5.4 SDS) in height. His anterior fontanelle was unclosed (0.5 cm × 0.2 cm). The head circumference was 43 cm, similar to his chest circumference. The patient had abdominal distention short of the subcutaneous varicose vein in the abdominal wall.
Laboratory examinations
He exhibited mild anemia (hemoglobin 86 g/L, normal range 110-160 g/L), hypocalcemia (total calcium 1.65 mmol/L, normal range 2.25-2.75 mmol/L), hypomagnesemia (magnesium 0.46 mmol/L, normal range 0.87-1.12 mmol/L), and an infection in the upper respiratory tract. Also, he had mild functional abnormalities in the liver. However, his 25-OH vitamin D3, vitamin B12, folic acid, trace elements, parathormone level, immunoglobulin level, and stool tests showed normal results.
Imaging examinations
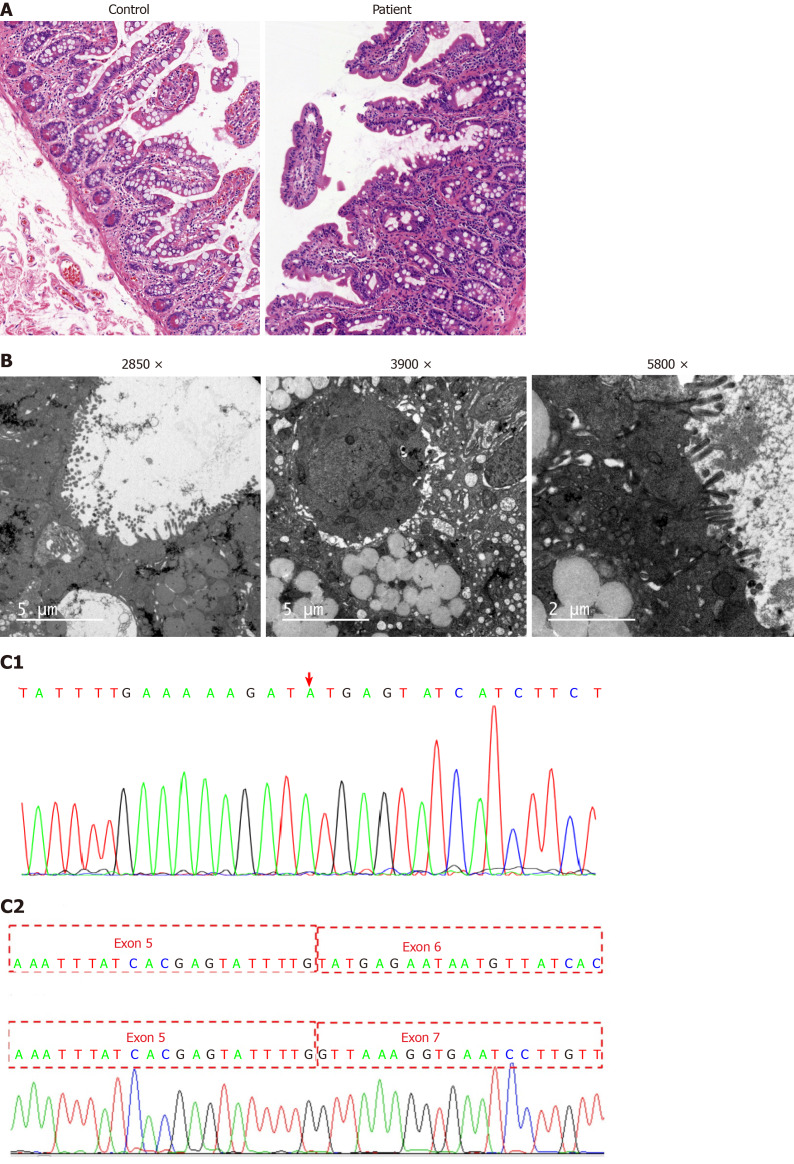
Doppler echocardiography showed congenital heart disease (atrial septal defect) and mild tricuspid regurgitation. The ultrasound reads for liver, bile, pancreas, spleen, and blood vessels were normal. Enteroscopy showed roughly normal small intestine mucosa. Histopathological findings of jejunal biopsies using light microscopy showed enterocytes with focal resembling tufts when stained with hematoxylin and eosin (Figure 1A). Microvilli sparse and vacuolar degeneration of epithelial cells were reported by small intestine biopsy (Figure 1B). Whole-exome sequencing showed a novel homozygous splice mutation (c.657+1[IVS6] G>A) in the EPCAM gene from the patient. By contrast, both parents had heterozygous mutations. The patient’s RNA was extracted and subjected to reverse transcription polymerase chain reaction (PCR) amplification. This was followed by direct sequencing of the EPCAM target site in the patient’s cDNA. The sequence analysis results showed that exon 6 was lost through mutation (Figure 1C1 and C2).
Figure 1.
Histology and whole-exome sequencing and analysis. A: Histopathological findings by light microscopy: Hematoxylin and eosin staining revealed enterocytes with focal resembling tufts in the patient’s jejunal biopsies (healthy mother was used as a control); B: Histopathological findings by electron microscope: Electric mirror reports microvilli sparse and vacuolar degeneration of epithelial cells; C1: Whole-exome sequencing showing a novel homozygous splice mutation (c.657+1[IVS6]G>A) in the epithelial cell adhesion molecule gene in the patient; C2: Sequence analysis results showing the loss of exon 6 through mutation.
FINAL DIAGNOSIS
Congenital tufting enteropathy ([OMIM] 613217).
TREATMENT
From the first day, the patient received TPN with electrolyte and trace elements through peripherally inserted central venous catheters. To accelerate weight gain, recombinant human growth hormone (0.05 mg/[kg·d]) was prescribed. Moreover, the patient was administered with symptomatic treatments for vomiting, tetany and an infection in the upper respiratory tract.
OUTCOME AND FOLLOW-UP
After 2 mo in the pediatric department, the patient gained up to 2800 g in weight (total body weight was 8500 g) and was fit for the small intestine transplantation.
DISCUSSION
Patients with CTE usually present with onset of intractable diarrhea during early infantile period, severe malnutrition, and TPN dependence which leads to inevitable complications such as abnormal liver function, vascular complications, and infections. Small intestine transplantation is the only available treatment[4]. Currently, the minimum age for small intestine transplantation for CTE infants is 2.5 years old[3].
The current diagnostic criteria for CTE include total or partial villus atrophy, crypt hyperplasia and epithelial tufts composed of enterocytes in the small intestine. Evidence of inflammation and infection are not visible during diagnosis[6]. The histopathological findings of our patient were typical, but these features are nonspecific and can be seen in other enteropathies.
Proper CTE diagnosis requires a differential diagnosis for other congenital diarrheal disorders (CDDs, [OMIM] 251850) including microvillus inclusion disease (MID, [OMIM] 251850), chloride diarrhea, sodium diarrhea, enteroendocrine cell dysgenesis, abetalipoproteinemia among others. CDD can be classified into four categories based on alteration in absorption and transport of nutrients and electrolytes, enterocyte differentiation and polarization, enteroendocrine cell differentiation, and intestinal immune response modulation[7]. MID shows a similar onset and blunted villi as that of the CTE and both of them are related to the differentiation and polarization in enterocyte. However, the presence of apical inclusion bodies as opposed to surface apical tufts in enterocytes distinguishes MID from CTE at the pathological level[8]. Meanwhile, they have different virulence genes. Children with MID have mutations in MYO5B, STX3, and STXBP2 while have changes in EPCAM with CTE[8].
EPCAM, also known as TACSTD1 or CD326, is a 40 kDa transmembrane glycoprotein consisting of the extracellular domain, single transmembrane domain, and intracellular domain. It plays an important role in cell signaling, proliferation, differentiation, and formation and maintenance of organ morphology[9,10]. The EPCAM gene was identified to be associated with CTE by Sivagnanam et al[5]. Since then, more than 40 EPCAM mutations have been discovered[5,6,11]. There are four types of EPCAM knockout mice globally, suggesting that the phenotype is similar to the symptoms in human beings[12,13].
The virulence gene for CTE in EPCAM was classified into four parts: Chromosomal deletion, non-coding/splicing, frameshift/truncation, and missense mutation[6]. Among them, the mutations c.491+1G>A, c.492-2A>G, c.556-14A>G, c.498insC, and c.499dupC appeared most frequently, manifesting in more than five people. All CTE patients presented with severe persistent diarrhea in the first months after birth. They exhibited stunted growth. Some patients had complications such as cardiomyopathy, arthritis, facial deformity, choanal rectal, and or esophageal atresia, which were not associated with EPCAM mutations[2,14-16]. These complications may be associated with other gene mutations or arise as the disease progresses. A study by Al-Mayouf et al[16] reported that CTE patients are prone to chronic arthritis. The patient's clinical manifestation and auxiliary examination accord with the CTE diagnosis. Genetic studies revealed a homozygous mutation c.657+1G>A in the EPCAM gene, which was inherited from the father and mother, respectively. This mutation is a novel splice mutation that resulted in the loss of the exon 6. It was speculated that his sister may have died at the age of two years with similar symptoms caused by the same mutation. To rapidly achieve the required bodyweight for small intestinal transplantation, the patient was treated with TPN combined with human growth hormone.
CONCLUSION
Congenital tufting enteropathy is a rare disease in Chinese families having reported one case in recent past[17]. This research reports a new case bringing the total cases in the country to two. Patients with CTE require rapid diagnosis and therapy to improve their survival. Advancement in diagnostic techniques such as histological investigation, whole-exome sequencing will enhance the early prognosis of the disease. Besides, progress in small intestine transplantation implies that CTE patients are bound to live longer.
Footnotes
Informed consent statement: The patient’s legal guardian has provided informed written consent prior to study enrollment.
Conflict-of-interest statement: The author(s) declared no conflicts of interests with regard to the research, authorship, and/or publication of this article.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Manuscript source: Unsolicited manuscript
Peer-review started: June 12, 2020
First decision: August 21, 2020
Article in press: September 10, 2020
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Islek A S-Editor: Yan JP L-Editor: Filipodia P-Editor: Li JH
Contributor Information
Yan-Qiong Zhou, Department of Pediatrics, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, Zhejiang Province, China.
Guo-Sheng Wu, Department of Colorectal and Anal Surgery, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, Zhejiang Province, China.
Yuan-Mei Kong, Department of Pediatrics, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, Zhejiang Province, China.
Xiao-Yuan Zhang, Department of Pediatrics, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, Zhejiang Province, China.
Chun-Lin Wang, Department of Pediatrics, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, Zhejiang Province, China. hzwangcl@zju.edu.cn.
References
- 1.Reifen RM, Cutz E, Griffiths AM, Ngan BY, Sherman PM. Tufting enteropathy: a newly recognized clinicopathological entity associated with refractory diarrhea in infants. J Pediatr Gastroenterol Nutr. 1994;18:379–385. [PubMed] [Google Scholar]
- 2.Goulet O, Salomon J, Ruemmele F, de Serres NP, Brousse N. Intestinal epithelial dysplasia (tufting enteropathy) Orphanet J Rare Dis. 2007;2:20. doi: 10.1186/1750-1172-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.AlMahamed S, Hammo A. New mutations of EpCAM gene for tufting enteropathy in Saudi Arabia. Saudi J Gastroenterol. 2017;23:123–126. doi: 10.4103/1319-3767.203359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gambarara M, Diamanti A, Ferretti F, Papadatou B, Knafelz D, Pietrobattista A, Castro M. Intractable diarrhea of infancy with congenital intestinal mucosa abnormalities: outcome of four cases. Transplant Proc. 2003;35:3052–3053. doi: 10.1016/j.transproceed.2003.10.053. [DOI] [PubMed] [Google Scholar]
- 5.Sivagnanam M, Mueller JL, Lee H, Chen Z, Nelson SF, Turner D, Zlotkin SH, Pencharz PB, Ngan BY, Libiger O, Schork NJ, Lavine JE, Taylor S, Newbury RO, Kolodner RD, Hoffman HM. Identification of EpCAM as the gene for congenital tufting enteropathy. Gastroenterology. 2008;135:429–437. doi: 10.1053/j.gastro.2008.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pathak SJ, Mueller JL, Okamoto K, Das B, Hertecant J, Greenhalgh L, Cole T, Pinsk V, Yerushalmi B, Gurkan OE, Yourshaw M, Hernandez E, Oesterreicher S, Naik S, Sanderson IR, Axelsson I, Agardh D, Boland CR, Martin MG, Putnam CD, Sivagnanam M. EPCAM mutation update: Variants associated with congenital tufting enteropathy and Lynch syndrome. Hum Mutat. 2019;40:142–161. doi: 10.1002/humu.23688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berni Canani R, Terrin G, Cardillo G, Tomaiuolo R, Castaldo G. Congenital diarrheal disorders: improved understanding of gene defects is leading to advances in intestinal physiology and clinical management. J Pediatr Gastroenterol Nutr. 2010;50:360–366. doi: 10.1097/MPG.0b013e3181d135ef. [DOI] [PubMed] [Google Scholar]
- 8.Jayawardena D, Alrefai WA, Dudeja PK, Gill RK. Recent advances in understanding and managing malabsorption: focus on microvillus inclusion disease. F1000Res. 2019;8 (F1000 Faculty Rev):2061. doi: 10.12688/f1000research.20762.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang L, Yang Y, Yang F, Liu S, Zhu Z, Lei Z, Guo J. Functions of EpCAM in physiological processes and diseases (Review) Int J Mol Med. 2018;42:1771–1785. doi: 10.3892/ijmm.2018.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schnell U, Cirulli V, Giepmans BN. EpCAM: structure and function in health and disease. Biochim Biophys Acta. 2013;1828:1989–2001. doi: 10.1016/j.bbamem.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 11.Salomon J, Goulet O, Canioni D, Brousse N, Lemale J, Tounian P, Coulomb A, Marinier E, Hugot JP, Ruemmele F, Dufier JL, Roche O, Bodemer C, Colomb V, Talbotec C, Lacaille F, Campeotto F, Cerf-Bensussan N, Janecke AR, Mueller T, Koletzko S, Bonnefont JP, Lyonnet S, Munnich A, Poirier F, Smahi A. Genetic characterization of congenital tufting enteropathy: epcam associated phenotype and involvement of SPINT2 in the syndromic form. Hum Genet. 2014;133:299–310. doi: 10.1007/s00439-013-1380-6. [DOI] [PubMed] [Google Scholar]
- 12.Guerra E, Lattanzio R, La Sorda R, Dini F, Tiboni GM, Piantelli M, Alberti S. mTrop1/Epcam knockout mice develop congenital tufting enteropathy through dysregulation of intestinal E-cadherin/β-catenin. PLoS One. 2012;7:e49302. doi: 10.1371/journal.pone.0049302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mueller JL, McGeough MD, Peña CA, Sivagnanam M. Functional consequences of EpCam mutation in mice and men. Am J Physiol Gastrointest Liver Physiol. 2014;306:G278–G288. doi: 10.1152/ajpgi.00286.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ko JS, Seo JK, Shim JO, Hwang SH, Park HS, Kang GH. Tufting Enteropathy with EpCAM Mutations in Two Siblings. Gut Liver. 2010;4:407–410. doi: 10.5009/gnl.2010.4.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bodian DL, Vilboux T, Hourigan SK, Jenevein CL, Mani H, Kent KC, Khromykh A, Solomon BD, Hauser NS. Genomic analysis of an infant with intractable diarrhea and dilated cardiomyopathy. Cold Spring Harb Mol Case Stud. 2017;3:a002055. doi: 10.1101/mcs.a002055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Mayouf SM, Alswaied N, Alkuraya FS, Almehaidib A, Faqih M. Tufting enteropathy and chronic arthritis: a newly recognized association with a novel EpCAM gene mutation. J Pediatr Gastroenterol Nutr. 2009;49:642–644. doi: 10.1097/MPG.0b013e3181acaeae. [DOI] [PubMed] [Google Scholar]
- 17.Tang W, Huang T, Xu Z, Huang Y. Novel Mutations in EPCAM Cause Congenital Tufting Enteropathy. J Clin Gastroenterol. 2018;52:e1–e6. doi: 10.1097/MCG.0000000000000739. [DOI] [PubMed] [Google Scholar]