Abstract
Non-alcoholic fatty liver disease (NAFLD) is the leading cause of chronic liver disease and considered a liver manifestation of metabolic syndrome. It is in close relationship with insulin resistance, obesity, diabetes mellitus, all of which increase risk of cardiovascular disease (CVD). Besides, many studies point out that NAFLD independently contributes to the development of atherosclerosis and CHD. On the other hand, CVDs are the leading cause of death in NAFLD patients. Many pathophysiological changes and molecular mechanisms play an important role in NAFLD for CVD formation. Atherosclerosis is common in NAFLD, which also mainly contributes to the CVD formation and CHD. Many studies linking atherosclerotic CHD and NAFLD are present in the literature. Subclinical CHD, mainly detected by coronary computed tomography views, have been detected more common in NAFLD patients. Presence of NAFLD has been found to be more common in patients with severe CHD and in stable CHD, NAFLD has been found to be associated with more diffuse disease. In acute coronary syndromes, especially in acute myocardial infarction, patients with NAFLD have been found to have poor prognosis when compared with NAFLD free patients. In this review, our aim is to evaluate the relationship between NAFLD and CHD in detail and go over the pathophysiological mechanisms underlying this relationship.
Keywords: Fatty liver, Hepatosteatosis, Coronary heart disease, Atherosclerosis, Cardiovascular, Artery
Core Tip: Non-alcoholic fatty liver disease (NAFLD) is the leading cause of liver disease and coronary heart disease (CHD) is the leading cause of death in the world. Both of these diseases are significantly associated with each other and NAFLD has been found to be an independent risk factor for CHD in many studies. In this review, our aim is to evaluate the relationship between NAFLD and CHD in detail and go over the pathophysiological mechanisms underlying this relationship.
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) has an increasing prevalence in concordance with the increasing prevalence of diabetes mellitus and obesity and cardiovascular disease (CVD) is an important cause of mortality in NAFLD[1]. NAFLD is commonly considered as the hepatic expression of metabolic syndrome (MS) manifested by insulin resistance and endothelial dysfunction, both of the latter take place in the pathogenesis and complications due to diabetes mellitus and obesity. NAFLD has been also found to be associated with hypertension and dyslipidemia, which are counted as two of the five components of MS[2].
NAFLD as a systemic disorder is associated with other chronic diseases including diabetes mellitus and chronic renal disease, but the most important association is between NAFLD and CVDs, especially CHD, because the most common cause of death in this disorder is due to cardiovascular diseases. NAFLD may also be considered as an individual risk factor for CVDs because in several clinical studies, it has been found to be independently associated with CVDs after the effects other traditional risk factors and components of MS have been eliminated.
In this review, we have overviewed the molecular mechanisms and clinical conditions linking NAFLD with CHD.
NAFLD DEFINITION AND EPIDEMIOLOGY
NAFLD is a general term including different degrees of liver damage, i.e. simple steatosis to cirrhosis. The pathological picture is similar to alcohol-induced liver damage and hepatic accumulation of triglyceride (TG) in combination with steatohepatitis characterizes the findings in NAFLD. NAFLD is known as the most common chronic liver disease with a prevalence of 10%-24% in different series. Its prevalence increases with age and is higher in males than females[2,3].
The diagnosis of NAFLD relies on 4 criteria: (1) Hepatic steatosis should be found with different imaging modalities or directly with tissue biopsy; (2) Alcohol consumption should not be present in significant amounts; (3) Other etiologic factors causing NAFLD should be absent; and (4) Other accompanying causes of chronic liver disease should not be found. NAFLD severity is designated by degree of fibrosis and existence of steatohepatitis; diagnosis of which requires at least 5% hepatic steatosis with hepatocyte injury and inflammation. Categorization of fibrosis can be made from stage 1 with limited hepatic involvement to stage 4 known as cirrhosis. Although gold standard method for diagnosis of NAFLD is liver biopsy, non-invasive imaging methods like computed tomography, ultrasonography, and magnetic resonance imaging can be used safer and less costly, with good sensitivity and specificity[2,4]
Increasing evidence suggests that NAFLD is not only a potentially progressive liver disease, but also has systemic consequences. A sedentary lifestyle and poor eating habits are the main reason for the increase in prevalence, along with many metabolic diseases. The prevalence in obese people rises to 57.5%-74%[5]. The prevalence in patients with type-2 diabetes mellitus varies between 50%-75%[6]. In addition, NAFLD is considered the hepatic manifestation of metabolic syndrome (MS)[7]. NAFLD is closely associated with all features of MS, abdominal obesity, atherogenic dyslipidemia, hypertension, insulin resistance and impaired glucose tolerance. At least one of the components of MS can be found in 90% of patients with NAFLD and about one third of NAFLD patients meet the complete diagnostic criteria for MS, therefore NAFLD may be counted as the hepatic representation of MS[7,8]. As a result, NAFLD is closely related to the multiple potential risk factors of cardiovascular diseases and thought to be an independent risk factor for CVD and events.
NAFLD AND CARDIOVASCULAR DISEASES
It should be noted that both NAFLD and CVD share similar risk factors such as diabetes mellitus, dyslipidemia, and obesity. Therefore, it may be thought that both diseases develop simultaneously and NAFLD may seem to be a by-stander during the development of CVD. However, the fact is different. Despite scarce number of studies in the literature, many studies point out that NAFLD independently contributes to the development of atherosclerosis and CHD.
Many studies show that NAFLD is associated with subclinical [increased carotid intima-media thickness, coronary calcium score (CAC), abdominal aortic calcification (AAC), endothelial dysfunction and arterial stiffness] and clinical cardiovascular diseases (coronary artery disease, acute coronary syndrome, ischemic stroke) most of which are associated with atherosclerosis[9]. Complex and heterogeneous mechanisms which have not been completely elucidated take place in the causal relationship between NAFLD and cardiovascular diseases and they include different functional interactions of many systems at the same time. Genetics, insulin resistance, adipose tissue dysfunction, dyslipidemia, oxidative stress, inflammation and endothelial dysfunction are the most important connection points between NAFLD and cardiovascular diseases. These links may explain the relationship between the liver disease and cardio-metabolic complications[4].
Many meta-analyzes which strengthen the relationship between NAFLD and CV events have been conducted. In a meta-analysis of 34043 individuals from 16 studies, NAFLD patients have been shown to be more affected by cardiovascular events. In addition, 6 studies showed a higher increase in CV event risk in patients with higher NAFLD severity[10]. In their meta-analysis, Mahfood Haddad et al[11] found that the risk of clinical cardiovascular events in NAFLD patients was significantly higher than those withoutin 25867 patients. In another meta-analysis of approximately 165000 participants from 34 studies, NAFLD has been found to be associated with an increased risk of cardiovascular events and CVDs. Severity of illness appears to be associated with an increase in cardiovascular events[12]. These studies show an association between increased cardiovascular events, especially for severe NAFLD patients. While it has been shown that NAFLD may be associated with increased cardiovascular mortality in meta-analyses showing the relationship with cardiovascular mortality[13,14], this relationship has not been shown in a few of them[15,16]. This relationship is less pronounced in these meta-analyses, especially since there are discordant studies used in these ones. Accordingly, it is recommended that early intervention is necessary in NAFLD patients to reduce the risk of progression of the disease and improve the cardiovascular outcomes due to increased cardiovascular risk.
NAFLD AND ATHEROSCLEROSIS
Atherosclerosis (AS), known as the most common etiologic factor for CVD, is defined as a pathological plaque formation within blood vessels that initiates intimal thickening the earliest lesion in the arterial wall[17], hardening of the arteries, and narrowing of the lumen. According to the modified classification of the American heart association, many phenotypes of AS disease have been demonstrated[18,19]. Classification includes intimal xanthoma, pathological intimal thickening, fibrocalcific plate, fine-fiber valve atheroma, and fibroadenoma. The best pathophysiology of AS is hypertension, dyslipidemia, etc. It is defined by the "endothelial damage theory", which shows that components of AS induce vascular intimal damage. In addition, AS is associated with many inflammatory cells and a systematic chronic inflammation[20].
NAFLD is closely related to many metabolic conditions that expose an increased risk of CV disease. Studies have also shown that an independent relationship between NAFLD and CVD is found when other metabolic risk factors for CVD are also counted in[21]. Therefore, NAFLD may be thought to contribute actively to the pathogenesis of AS besides its role as a marker for CVD[9]. Therefore, NAFLD is closely associated with AS and appears to be an early risk factor for AS. The high prevalence of NAFLD in patients with AS have made physicians get interested in the possible pathophysiologic role of the fatty liver in AS development.
Insulin resistance, defined as "first-hit" to the liver, disrupts cellular energy metabolism and damages peripheral tissues. The liver interferes with the reuptake and synthesis of fatty acid, causing its accumulation in liver, leading to insulin resistance, as the endogenous liver glucose production is not suppressed[22]. NAFLD patients develop hyperglycemia, hyperinsulinemia, hyperlipidemia and damage to vascular endothelial cells due to insulin resistance. It can also cause the proliferation of smooth muscle cells (SMCs). Therefore, insulin resistance contributes to the development of both NAFLD and AS.
Dilation and dysfunction of adipose tissue have an important place in cardiovascular complications. When overeating and excessive calorie intake exceed the expansion and storage capacity of adipose tissue, serum levels of free fatty acids (FFA) increase, and ectopic fat deposits form in the liver and other organs. NAFLD is associated with dyslipidemia, given that lipid intake, synthesis and regulation of metabolism are impaired in the liver of NAFLD patients due to enlarged fat mass. In NAFLD, low-density lipoprotein (LDL) receptors are down-regulated resulting in cholesterol uptake and very low-density lipoprotein synthesis in liver, causing an increase in TG[23]. Elevated TG levels may further impair the lipid profile by decreasing high-density lipoprotein cholesterol and increasing small and dense LDL particles and oxidized LDL[24]. In addition, as blood FFA levels increase due to increased energy intake and decreased FFA oxidation, it can be morphologically affected by inter-endothelial cell dilatation[25]. These changes directly contribute to AS and accelerate local atherosclerotic development.
As a "second hit" to the liver in NAFLD development, lipid peroxidation and oxidative stress may indicate the relationship between NAFLD and AS. Oxidative stress is a condition in which the body's reactive oxygen type (ROS) production is above ROS detoxification and causes tissue damage[26]. Increasing asymmetric dimethyl arginine levels in NAFLD, as an endogenous antagonist of nitric oxide synthase, results in a decrease in NO level. In addition, the formation of an inflammatory environment increases NO consumption and inhibits endothelium-dependent vasodilation, decreases the blood vessels elasticity, destroys the apoptosis of endothelial cells, promotes hyperplasia of vascular smooth muscle cells, contributes macrophages apoptosis in the atherosclerotic plaque and induces oxidized LDL through lipid peroxidation[19].
As a result of inflammation in NAFLD patients, levels of tumor necrosis factor-α, interleukin (IL)-6, monocyte chemoattractant protein-1 and C-reactive protein which were all known to be associated with AS, increase. Elevated expression of IL-6 in liver cells and elevated IL-6 in blood may increase partial liver damage and AS[27]. The insulin resistance that occurs with this systemic inflammation contributes to the development of AS. In addition, an elevation of matrix metalloproteinase levels has been also found in NAFLD[28], and this elevation may play a critical role in AS process by promoting sensitive atherosclerotic plaque formation.
The adipocytes secrete a molecule called adiponectin which increases insulin sensitivity in liver and other tissues to decrease serum fatty acid levels and increase fatty acid oxidation in muscle[29]. Adiponectin can reduce TG, total cholesterol and LDL-C concentrations[30], and it can stimulate vascular endothelial nitric oxide synthase and gradually reduce atherosclerotic lesions. Adiponectin levels were found to be low in NAFLD patients[31]. Produced mostly by adipose tissue, leptin plays a critical role in energy consumption and food intake. It is closely related to insulin resistance and shows a synergistic effect. Leptin was found to be higher in NAFLD patients in relation to disease severity[32]. Visfatin, a new adipocytokin expressed by visceral adipose tissue activity, is related with certain risk factors of AS including endothelial dysfunction, vascular endothelial proliferation, inflammation, and atherosclerotic plaque formation. Visfatin is thought to be related to the atherosclerotic process in NAFLD since it is closely related to the degree of NAFLD and increases in the foamy macrophages of AS plaques[19]. Resistin promotes SMC proliferation, enhances the migration capability of SMCs and induces inflammatory reaction under oxidative stress and dyslipidemia[33]. Therefore, resistin is also associated with NAFLD and functions as an indicator of AS severity.
The intestinal microbiota has been shown to be a risk factor contributing to the development of NAFLD[34]. The levels of lipopolysaccharide derived from the intestinal microbiota are increased in NAFLD patients and it shows endotoxic effects. When plasma endotoxin concentrations increase in the portal vein, this causes systemic endotoxemia and mild chronic inflammation[35]. Obese individuals develop an increased endotoxin load in the portal vein. Therefore, intestinal microorganisms may contribute to AS by indirect ways in patients with NAFLD.
Genetic factors associated with NAFLD's cardiovascular events are still undefined. PNPLA3 and transmembrane 6 superfamily 2 genes are related to lipid metabolism in the liver and atherogenic dyslipidemia has been associated in NAFLD patients[36,37]. These genes may also contribute to the formation of AS.
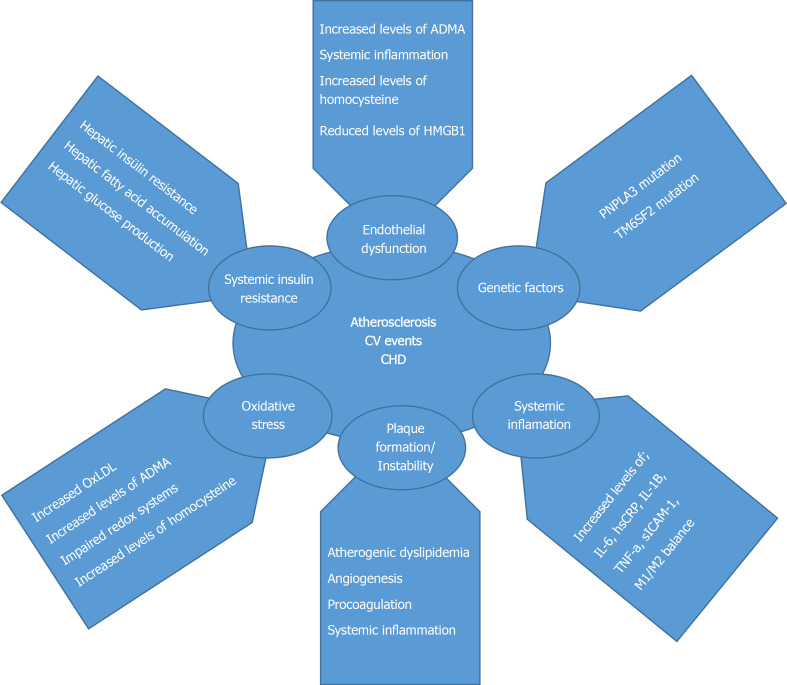
As a result, NAFLD is highly associated with cardio-metabolic syndromes and it is also highly related to AS. Pathophysiological changes occur in NAFLD patients, which predispose to AS formation. Clinical studies have shown the relationship between atherosclerotic cardiovascular diseases and NAFLD. NAFLD is closely related to carotid intima-media thickening, coronary artery atherosclerosis, and increased arterial stiffness[38]. Potential pathophysiological mechanisms responsible for increased CVD in NAFLD are shown in Figure 1.
Figure 1.
Potential pathophysiological mechanisms responsible for increased cardiovascular disease in non-alcoholic fatty liver disease. CHD: Coronary heart disease; TNF: Tumor necrosis factor; IL: Interleukin; CRP: C-reactive protein.
NAFLD AND CORONARY HEART DISEASE
It is increasingly accepted that NAFLD and CHD are closely related, because presence of NAFLD may be an important risk factor for CHD development. NAFLD is closely related to CAC and the formation of risky coronary artery plaques. Cross-sectional studies examining the relationship between NAFLD and the presence and severity of CHD are shown in Table 1.
Table 1.
Clinical studies examining the relationship between non-alcoholic fatty liver disease and the presence and severity of clinical coronary heart disease
|
Ref.
|
Study characteristics
|
NAFLD diagnosis
|
CHD diagnosis
|
Main findings
|
| NAFLD and cardiac CT studies | ||||
| Santos et al[43], 2007 | Data were analyzed from a Brazil occupational cohort of 505 people | US | Cardiac CT, CAC score | Hepatic steatosis associated with CAC |
| Sung et al[41], 2012 | Data were analyzed from a South Korean occupational cohort of 10153 people | US | Cardiac CT, CAC score | NAFLD associated with a CAC score > 0 |
| Kim et al[44], 2012 | Out of 5648 subjects who visited one of our health screening centers between 2003 and 2008, 4023 subjects | CT | Cardiac CT, CAC score | Patients with NAFLD are at increased risk for coronary atherosclerosis independent of classical coronary risk factors |
| Vanwagner et al[45], 2014 | Participants from the Coronary Artery Risk Development in Young Adults study (n = 2424) | CT | Cardiac CT, CAC and AAC score | NAFLD associated with a subclinic atherosclerosis (CAC and AAC score > 0) |
| Rifai et al[46], 2015 | Participants from the Multi-Ethnic Study of Atherosclerosis (MESA) (n = 3976) | CT | Cardiac CT, CAC score | NAFLD is associated with increased inflammation and CAC |
| Mellinger et al[42], 2015 | Analysis of the Framingham Heart Study, 3529 participants | CT | Cardiac CT, CAC and AAC score | NAFLD associated with a subclinic atherosclerosis (CAC and AAC score) |
| Park et al[47], 2016 | 1732 subjects who underwent serial CAC evaluation | US | Cardiac CT, CAC score | NAFLD plays a role in the early development of CAC |
| Ishiba et al[48], 2016 | 698 patients with chest pain or ECG abnormalities who underwent coronary computed tomography | CT | Cardiac CT, CAC score | The progression of arteriosclerosis and that of liver fibrosis may be associated in NAFLD patients |
| Kim et al[49], 2017 | Participants in a health screening program (n = 1575) | CT | Cardiac CT, CAC score | The concomitant presence of NAFLD and systemic inflammation as assessed by hs-CRP increases the risk of CAC |
| Sinn et al[50], 2017 | This retrospective cohort study included 4731 adult men and women with no history of cardiovascular disease (CVD) | US | Cardiac CT, CAC score | NAFLD was significantly associated with the development of CAC |
| Wu et al[51], 2017 | A total of 2345 participants aged ≥ 40 (1035 men and 1310 women) | US | Cardiac CT, CAC score | NAFLD was significantly associated with the development of CAC |
| NAFLD and stable CHD | ||||
| Lin et al[58], 2005 | 2088 male worker undergoing health surveillence | US | Patient history, ECG | NAFLD associated with higher prevalence of CHD, independently of obesity and other traditional CVD risk factors |
| Arslan et al[55], 2007 | Turkish patients admitted with stable CHD (n = 92) | US | CAG (elective) | NAFLD was an independent predictor of CHD (> 50% stenosis of ≥ 1 major coronary artery) |
| Mirbagheri et al[59], 2007 | Iranian patients admitted with ACS and CHD (n = 317) | US | CAG (elective) | NAFLD was an independent predictor of clinically relevant CHD (> 30% stenosis of ≥ 1 major coronary artery) |
| Akabame et al[60], 2008 | Japanese patients with suspected CHD (n = 298) | CT | CT (elective) | NAFLD was independently associatedwith lipid core ofcoronary plaques |
| Alper et al[56], 2008 | Turkish patients with suspected CHD (n = 80) | US | CAG (elective) | NAFLD is associated with more severe CAD |
| Açikel et al[61] (2009) | Turkish patients admitted for stable CHD and ACS (n = 355) | US | CAG (elective and acute) | NAFLD was an independent predictor of CHD (> 50% stenosis of ≥ 1 major or CHD suspicion |
| Targher et al[62], 2010 | 250 consecutive type 1 diabetes mellitus patients | US | Patient history, ECG | NAFLD was associated with higher prevalence of CHD |
| Sun et al[63], 2011 | Hospitalized Chinese patients with high suspicion of CHD (n = 542) | CT | CAG (elective) | NAFLD was associated with severity of CHD |
| Wong et al[54], 2011 | Chinese patients with suspicion of CHD (n = 612) | US | CAG (elective) | NAFLD was associated with CHD, independently of established CVD risk factors |
| Agac et al[64], 2013 | Turkish patients with ACS (n = 80) | US | CAG (elective) | NAFLD was independently associated with a greater severity of CHD (syntax score) |
| Puchner et al[65], 2014 | 445 patients randomized to coronary CT angiography arm in ROMICAT II study | CT | Coronary CT angiography | NAFLD is associated with advanced high-risk coronary plaque, independent of traditional CV risk factors and the extent and severity of CHD |
| NAFLD and ACS | ||||
| Boddi et al[57], 2013 | Nondiabetic Italian patients admitted for ACS (n = 95) | US | CAG (acute) | NAFLD was independently associated with higher risk of multi-vessel CHD |
| Emre et al[69], 2015 | 186 consecutive nondiabetic patients who underwent primary PCI for STEMI | US | CAG (acute) | High rates of TIMI 3 after primary PCI, patients with Fatty liver disease score ≥ 3 are more likely to have impaired myocardial perfusion which may contribute to adverse in hospital outcome |
| Wong et al[70], 2016 | 612 prospective patients undergoing CAG for stable CHD and ACS | US | CAG (acute, elective) | In patients with clinical indications for coronary angiogram, the presence of NAFLD is associated with coronary artery stenosis and need for coronary intervention |
| Perera et al[67], 2016 | The study group including patients with non-fatal ACS (n = 120) | US | CAG (acute, elective) | Patients with NAFLD have a higher predicted mortality from ACS and thus require aggressive treatment of CAD |
| Keskin et al[68], 2017 | The study group consisted of 360 patients with STEMI | US | CAG (acute) | In STEMI patients, presence of NAFLD is associated with unfavorable clinical outcomes. Grade 3 NAFLD had the highest mortality rates |
ACS: Acute coronary syndrome; AAC: Abdominal aortic calcification; CAC: Coronary artery calcification; CAG: Coronary angiography; CHD: Coronary heart disease; CT: Computed tomography; DM: Diabetes mellitus; ECG: Electrocardiogram; NAFLD: Non-alcoholic fatty liver disease; PCI: Percutane coronary investigation STEMI: ST segment elevation miyocardial infarction; TIMI: Thrombolysis In myocardial infarction; US: Ultrasonography.
CAC which can be detected by multi-slice computed tomography (CT) is an important marker of subclinical CHD[39]. CAC represents the atherosclerotic load on the arterial wall, and there is a significant correlation between the presence of CHD and increased CV poor results[40]. In the study conducted by Sung et al[41], the CAC score measured with CT was independently associated with NAFLD. In a study of 3529 patients undergoing CT imaging showed that NAFLD was found to be related with subclinical markers of atherosclerosis, including calcium deposits in coronary arteries, independent from other metabolic risk factors[42]. In Table 1, other studies which investigate the relationship between CAC and NAFLD are summarized[43-51].
NAFLD has been shown to be associated with higher CHD prevalence regardless of other traditional risk factors[52]. In a prospective cohort study of 2088 adults, NAFLD was found to be significantly associated with CHD prevalence independent of other risk factors[53]. There is a strong correlation between CHD detected by continuously developing imaging methods and increasing NAFLD prevalence[40]. Both NAFLD and CHD share common risk factors and considering the increasing prevalence of obesity, metabolic syndrome and diabetes mellitus, it is possible that the prevalence of both diseases increase in parallel to each other, but, as we have already mentioned, many studies disclosed that NAFLD might be an independent risk factor the development of CHD.
NAFLD patients have an accelerated atherosclerotic process. Genetic susceptibility, insulin resistance, atherogenic dyslipidemia, oxidative stress, systemic inflammation, decreased adiponectin level, and procoagulant factors contribute to the acceleration of the atherosclerotic process. In their study, Wong et al[54] found more NAFLD in patients who underwent coronary angiography and who had significant coronary artery stenosis. Assy et al[53] found higher CHD in NAFLD patients compared to the control group in their study. Arslan et al[55] found the existence of NAFLD to be independently related to the presence of CHD in their study. Those studies indicate that NAFLD patients have an increased risk for atherosclerotic CHD.
NAFLD may also increase CHD severity. In a prospective study, it has been found that NAFLD is an independent risk factor affecting CHD severity[56]. In the study conducted by Boddi et al[57], it has been shown that the risk of moderate to severe NAFLD is closely associated with development of multi-vessel atherosclerosis. In their study, Wong et al[54] showed that NAFLD is associated with greater severity of angiographic CHD, independent of other CVD risk factors. Uncontrolled risk factors, insulin resistance and accelerated atherogenesis, already present in NAFLD patients, may all contribute to widespread plaque formation in coronary arteries and to more serious coronary artery stenosis. In Table 1, studies investigating the relationship between stable CHD and NAFLD have been summarized[54-65].
Another aspect of CHD is acute coronary syndromes (ACS). Several studies have been performed in the literature which investigated NAFLD in ACS[57,66-70] especially in acute myocardial infarction (AMI). In a study, AMI has been associated with significant cardiovascular mortality in NAFLD patients[66]. Perera et al[67] showed that NAFLD patients had higher mortality when they experienced AMI. In their study, Keskin et al[68] demonstrated that the presence of NAFLD among ST segment elevation myocardial infarction patients had an independent effect on CHD severity and in-hospital and long-term mortality. Emre et al[69] showed in their study that the presence of NAFLD in non-diabetic ST segment elevation myocardial infarction patients may have abnormal myocardial perfusion and increase in-hospital major adverse cardiac events rates. These studies demonstrate the relationship between NAFLD and ACS development and poor outcomes in this patient group. Table 1 summarizes the studies in which NAFLD and ACS have been studied together. Disruption of microvascular circulation, increased inflammatory endothelial dysfunction, oxidative stress and prothrombotic environment in NAFLD patients may explain the poor clinical outcomes in AMI patients.
Coronary collateral circulation is an important protective mechanism for myocardial cells in case of an occlusion in any coronary artery such as in acute MI. Collateral development may be affected from several situations. Diabetes mellitus is known to be associated with poor collateral development, whereas severity and duration of CHD is positively correlated with collateralization. Poor collateral development has been also observed in patients with insulin resistance. Arslan et al[71] found that the presence of NAFLD in non-diabetic patients with significant CHD was also independently associated with poor coronary collateralization. This result has been thought to be due to inflammatory cytokine increase in the presence of NAFLD, prothrombotic environment, and endothelial dysfunction resulting from accelerated atherogenesis. Poor collateral circulation in NAFLD may explain the poor prognosis of acute MI patients with this disease.
The significant relationship between NAFLD and CHD has been shown in many studies some of which are discussed in the before-mentioned paragraphs. Many scoring systems are used to predict CVD risk in the general population. The Framingham risk score is a simple and commonly used tool to assess CAD risk level over 10 years. Furthermore, the Framingham risk score has been validated as a predictor of CVD in NAFLD. Since NAFLD patients have an increased risk of CHD, presence of NAFLD may be added to this scoring system for the prediction of CHD.
NAFLD AND CORONARY HEART DISEASE TREATMENT
Lifestyle change consisting of diet, exercise and weight loss plays an important role in the treatment of NAFLD and CAD. In addition to the Mediterranean diet, which is thought to have a beneficial effect on cardio-metabolic risk factors in terms of NAFLD and CAD, a low calorie (1200-1600 kcal/d), low fat (less than 10% of saturated fatty acid), low carbohydrate diet (total kcal of < 50%) is recommended[72]. Aerobic and resistance exercises should be done as physical activity. In particular, European Association for the Study of the Liver[73], National Institute for Health and Care Excellence[74] and American Association for the Study of Liver Diseases[2] guidelines on diet and physical activity propose programs targeting lifestyle changes. According to these guidelines, the goal of most lifestyle changes is a weight loss of 7%-10%.
Insulin sensitizers are pharmacologically important for both NAFLD and CAD treatment. Metformin used in the treatment of type 2 DM has been proven to reduce the risk of hepatocellular carcinoma, but does not improve the histologic changes in liver of NAFLD patients. Currently, metformin is not recommended in the new guidelines specifically for liver disease in NAFLD patients[75]. Pioglitazone, a peroxisome proliferator-activated receptor gamma agonist with insulin sensitizing effects, improves insulin sensitivity, inflammation and steatosis in NAFLD patients and type 2 DM. Since heart failure may accompany in CAD, patients with NAFLD may be considered for type 2 DM treatment after heart failure is ruled out[76]. Glucagon-like peptide-1 receptor agonists (exenatide, liraglutide, lixisenatide, and semaglutide) may reduce body weight by reducing appetite due to their hypoglycemic effects[77]. Sodium-glucose transporter 2 inhibitors (empagliposine, dapaglifosine, remoglyphosine etabonate) act with increased glycosuria by blocking renal glucose reabsorption. It has been shown that long-acting Glucagon-like peptide-1 receptor agonists (liraglutide and semaglutide) decrease major adverse cardiovascular events in patients with type 2 DM[78].
Statins are the most commonly used and effective lipid-lowering drugs for primary and secondary prevention of CVD and their use in NAFLD patients has proven safe despite the concerns about them for liver enzyme elevations. In studies, statins have been shown to improve liver enzymes, steatosis, and necroinflammation[79]. Ezetimibe, another lipid-lowering agent that reduces the intestinal uptake of dietary cholesterol, has been also shown to improve the histological findings in NAFLD. The benefits of the statin ezetimibe combination for CVD have been demonstrated[80]. Fibrates, by activating peroxisome proliferator-activated receptor-α, decrease serum TG levels while improving high-density lipoprotein-cholesterol levels simultaneously. However, fibrates have been shown to reduce CVD morbidity and mortality only in atherogenic dyslipidemia. Although fibrates are not demonstrated to improve histologic findings, they offer an effective and safe treatment for atherogenic dyslipidemia in NAFLD patients, particularly MetS and / or Type 2 DM patients[81].
Omega-3 polyunsaturated fatty acids may be also considered to treat hypertriglyceridemia in NAFLD patients. Given the joint role of oxidative stress in the pathogenesis of both NAFLD and atherosclerosis, antioxidants and specific vitamins (Vitamin E) may improve liver histology and reduce the risk of CVD, but their role in treatment of NAFLD and CHD has not been sufficiently proved yet.
CONCLUSION
The prevalence of NAFLD increases with cardiometabolic diseases. Insulin resistance, adipose tissue dysfunction, atherogenic dyslipidemia, oxidative stress, inflammation and endothelial dysfunction in NAFLD are the most important pathophysiological mechanisms in the formation of atherosclerotic CVD. Besides, NAFLD patients have an increased risk of CHD, independent of other traditional cardiovascular diseases. Subclinical and clinical CHDs, either in the acute or chronic settings, have been associated with NAFLD in several studies in the literature. Acute coronary syndromes can result in worse outcome in NAFLD patients. In addition, weaker coronary collateral circulation may be observed in NAFLD patients. Therefore, NAFLD patients should be followed closely for the development of CVDs, especially CHD. Whether medical and non-medical treatment of NAFLD prevents the development of CVD is a debate of question and should be investigated in randomized, prospective, long-term follow-up studies.
Footnotes
Conflict-of-interest statement: The authors declare no conflict of interest.
Manuscript source: Invited manuscript
Peer-review started: June 11, 2020
First decision: September 12, 2020
Article in press: September 26, 2020
Specialty type: Medicine, research and experimental
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Armellini E, Han T, Matowicka-Karna J S-Editor: Zhang L L-Editor: A P-Editor: Li JH
Contributor Information
Ugur Arslan, Department of Cardiology, University of Health Sciences Samsun Training and Research Hospital, Samsun 55400, Turkey. ugurarslan5@yahoo.com.
Mustafa Yenerçağ, Department of Cardiology, University of Health Sciences Samsun Training and Research Hospital, Samsun 55400, Turkey.
References
- 1.Ballestri S, Lonardo A, Bonapace S, Byrne CD, Loria P, Targher G. Risk of cardiovascular, cardiac and arrhythmic complications in patients with non-alcoholic fatty liver disease. World J Gastroenterol. 2014;20:1724–1745. doi: 10.3748/wjg.v20.i7.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 3.Lazo M, Hernaez R, Eberhardt MS, Bonekamp S, Kamel I, Guallar E, Koteish A, Brancati FL, Clark JM. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988-1994. Am J Epidemiol. 2013;178:38–45. doi: 10.1093/aje/kws448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stahl EP, Dhindsa DS, Lee SK, Sandesara PB, Chalasani NP, Sperling LS. Nonalcoholic Fatty Liver Disease and the Heart: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73:948–963. doi: 10.1016/j.jacc.2018.11.050. [DOI] [PubMed] [Google Scholar]
- 5.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 6.Bedogni G, Miglioli L, Masutti F, Castiglione A, Crocè LS, Tiribelli C, Bellentani S. Incidence and natural course of fatty liver in the general population: the Dionysos study. Hepatology. 2007;46:1387–1391. doi: 10.1002/hep.21827. [DOI] [PubMed] [Google Scholar]
- 7.Pacifico L, Nobili V, Anania C, Verdecchia P, Chiesa C. Pediatric nonalcoholic fatty liver disease, metabolic syndrome and cardiovascular risk. World J Gastroenterol. 2011;17:3082–3091. doi: 10.3748/wjg.v17.i26.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaudio E, Nobili V, Franchitto A, Onori P, Carpino G. Nonalcoholic fatty liver disease and atherosclerosis. Intern Emerg Med. 2012;7 Suppl 3:S297–S305. doi: 10.1007/s11739-012-0826-5. [DOI] [PubMed] [Google Scholar]
- 9.Targher G, Bertolini L, Padovani R, Rodella S, Tessari R, Zenari L, Day C, Arcaro G. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30:1212–1218. doi: 10.2337/dc06-2247. [DOI] [PubMed] [Google Scholar]
- 10.Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J Hepatol. 2016;65:589–600. doi: 10.1016/j.jhep.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 11.Mahfood Haddad T, Hamdeh S, Kanmanthareddy A, Alla VM. Nonalcoholic fatty liver disease and the risk of clinical cardiovascular events: A systematic review and meta-analysis. Diabetes Metab Syndr. 2017;11 Suppl 1:S209–S216. doi: 10.1016/j.dsx.2016.12.033. [DOI] [PubMed] [Google Scholar]
- 12.Wu S, Wu F, Ding Y, Hou J, Bi J, Zhang Z. Association of non-alcoholic fatty liver disease with major adverse cardiovascular events: A systematic review and meta-analysis. Sci Rep. 2016;6:33386. doi: 10.1038/srep33386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 14.Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol. 2008;49:608–612. doi: 10.1016/j.jhep.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 15.Lazo M, Hernaez R, Bonekamp S, Kamel IR, Brancati FL, Guallar E, Clark JM. Non-alcoholic fatty liver disease and mortality among US adults: prospective cohort study. BMJ. 2011;343:d6891. doi: 10.1136/bmj.d6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013;57:1357–1365. doi: 10.1002/hep.26156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaudio E, Carpino G, Grassi M, Musca A. [Morphological aspects of atherosclerosis lesion: past and present] Clin Ter. 2006;157:135–142. [PubMed] [Google Scholar]
- 18.Bhanvadia VM, Desai NJ, Agarwal NM. Study of coronary atherosclerosis by modified american heart association classification of atherosclerosis-an autopsy study. J Clin Diagn Res. 2013;7:2494–2497. doi: 10.7860/JCDR/2013/6828.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu X, Lu L, Dong Q, Li X, Zhang N, Xin Y, Xuan S. Research advances in the relationship between nonalcoholic fatty liver disease and atherosclerosis. Lipids Health Dis. 2015;14:158. doi: 10.1186/s12944-015-0141-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 21.Targher G, Arcaro G. Non-alcoholic fatty liver disease and increased risk of cardiovascular disease. Atherosclerosis. 2007;191:235–240. doi: 10.1016/j.atherosclerosis.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 22.Severova MM, Saginova EA, Galliamov MG, Ermakov NV, Rodina AV, Fomin VV, Mukhin NA. [Clinicopathogenetic characteristics of cardiorenal syndrome in non-alcoholic fatty liver disease] Ter Arkh. 2012;84:15–20. [PubMed] [Google Scholar]
- 23.Nakamuta M, Fujino T, Yada R, Yada M, Yasutake K, Yoshimoto T, Harada N, Higuchi N, Kato M, Kohjima M, Taketomi A, Maehara Y, Nakashima M, Kotoh K, Enjoji M. Impact of cholesterol metabolism and the LXRalpha-SREBP-1c pathway on nonalcoholic fatty liver disease. Int J Mol Med. 2009;23:603–608. doi: 10.3892/ijmm_00000170. [DOI] [PubMed] [Google Scholar]
- 24.Czyzewska M, Wolska A, Cwiklińska A, Kortas-Stempak B, Wróblewska M. [Disturbances of lipoprotein metabolism in metabolic syndrome] Postepy Hig Med Dosw (Online) 2010;64:1–10. [PubMed] [Google Scholar]
- 25.Boden G. Obesity and free fatty acids. Endocrinol Metab Clin North Am 2008; 37: 635-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delbosc S, Paizanis E, Magous R, Araiz C, Dimo T, Cristol JP, Cros G, Azay J. Involvement of oxidative stress and NADPH oxidase activation in the development of cardiovascular complications in a model of insulin resistance, the fructose-fed rat. Atherosclerosis. 2005;179:43–49. doi: 10.1016/j.atherosclerosis.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 27.Kaneto H, Katakami N, Matsuhisa M, Matsuoka TA. Role of reactive oxygen species in the progression of type 2 diabetes and atherosclerosis. Mediators Inflamm. 2010;2010:453892. doi: 10.1155/2010/453892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ando W, Yokomori H, Tsutsui N, Yamanouchi E, Suzuki Y, Oda M, Inagaki Y, Otori K, Okazaki I. Serum matrix metalloproteinase-1 level represents disease activity as opposed to fibrosis in patients with histologically proven nonalcoholic steatohepatitis. Clin Mol Hepatol. 2018;24:61–76. doi: 10.3350/cmh.2017.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polyzos SA, Kountouras J, Zavos C, Tsiaousi E. The role of adiponectin in the pathogenesis and treatment of non-alcoholic fatty liver disease. Diabetes Obes Metab. 2010;12:365–383. doi: 10.1111/j.1463-1326.2009.01176.x. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Pu H, Ma C, Jiang T, Wei Q, Zhang C, Duan M, Shou X, Su L, Zhang J, Yang Y. Adiponectin abates atherosclerosis by reducing oxidative stress. Med Sci Monit. 2014;20:1792–1800. doi: 10.12659/MSM.892299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haukeland JW, Damås JK, Konopski Z, Løberg EM, Haaland T, Goverud I, Torjesen PA, Birkeland K, Bjøro K, Aukrust P. Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. J Hepatol . 2006; 44: :1167–1174. doi: 10.1016/j.jhep.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 32.Fitzpatrick E, Mitry RR, Quaglia A, Hussain MJ, DeBruyne R, Dhawan A. Serum levels of CK18 M30 and leptin are useful predictors of steatohepatitis and fibrosis in paediatric NAFLD. J Pediatr Gastroenterol Nutr. 2010;51:500–506. doi: 10.1097/MPG.0b013e3181e376be. [DOI] [PubMed] [Google Scholar]
- 33.Jung HS, Park KH, Cho YM, Chung SS, Cho HJ, Cho SY, Kim SJ, Kim SY, Lee HK, Park KS. Resistin is secreted from macrophages in atheromas and promotes atherosclerosis. Cardiovasc Res. 2006;69:76–85. doi: 10.1016/j.cardiores.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 34.Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ, Camporez JP, Shulman GI, Gordon JI, Hoffman HM, Flavell RA. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology. 2009;50:638–644. doi: 10.1002/hep.23009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lauridsen BK, Stender S, Kristensen TS, Kofoed KF, Køber L, Nordestgaard BG, Tybjærg-Hansen A. Liver fat content, non-alcoholic fatty liver disease, and ischaemic heart disease: Mendelian randomization and meta-analysis of 279 013 individuals. Eur Heart J. 2018;39:385–393. doi: 10.1093/eurheartj/ehx662. [DOI] [PubMed] [Google Scholar]
- 37.Kozlitina J, Smagris E, Stender S, Nordestgaard BG, Zhou HH, Tybjærg-Hansen A, Vogt TF, Hobbs HH, Cohen JC. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2014;46:352–356. doi: 10.1038/ng.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niikura T, Imajo K, Ozaki A, Kobayashi T, Iwaki M, Honda Y, Kessoku T, Ogawa Y, Yoneda M, Kirikoshi H, Saito S, Nakajima A. Coronary Artery Disease is More Severe in Patients with Non-Alcoholic Steatohepatitis than Fatty Liver. Diagnostics (Basel) 2020;10 doi: 10.3390/diagnostics10030129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ampuero J, Gallego-Durán R, Romero-Gómez M. Association of NAFLD with subclinical atherosclerosis and coronary-artery disease: meta-analysis. Rev Esp Enferm Dig. 2015;107:10–16. [PubMed] [Google Scholar]
- 40.Patil R, Sood GK. Non-alcoholic fatty liver disease and cardiovascular risk. World J Gastrointest Pathophysiol. 2017;8:51–58. doi: 10.4291/wjgp.v8.i2.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sung KC, Wild SH, Kwag HJ, Byrne CD. Fatty liver, insulin resistance, and features of metabolic syndrome: relationships with coronary artery calcium in 10,153 people. Diabetes Care. 2012;35:2359–2364. doi: 10.2337/dc12-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mellinger JL, Pencina KM, Massaro JM, Hoffmann U, Seshadri S, Fox CS, O'Donnell CJ, Speliotes EK. Hepatic steatosis and cardiovascular disease outcomes: An analysis of the Framingham Heart Study. J Hepatol. 2015;63:470–476. doi: 10.1016/j.jhep.2015.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santos RD, Nasir K, Conceição RD, Sarwar A, Carvalho JA, Blumenthal RS. Hepatic steatosis is associated with a greater prevalence of coronary artery calcification in asymptomatic men. Atherosclerosis. 2007;194:517–519. doi: 10.1016/j.atherosclerosis.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 44.Kim D, Choi SY, Park EH, Lee W, Kang JH, Kim W, Kim YJ, Yoon JH, Jeong SH, Lee DH, Lee HS, Larson J, Therneau TM, Kim WR. Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology. 2012;56:605–613. doi: 10.1002/hep.25593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.VanWagner LB, Ning H, Lewis CE, Shay CM, Wilkins J, Carr JJ, Terry JG, Lloyd-Jones DM, Jacobs DR Jr, Carnethon MR. Associations between nonalcoholic fatty liver disease and subclinical atherosclerosis in middle-aged adults: the Coronary Artery Risk Development in Young Adults Study. Atherosclerosis. 2014;235:599–605. doi: 10.1016/j.atherosclerosis.2014.05.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Al Rifai M, Silverman MG, Nasir K, Budoff MJ, Blankstein R, Szklo M, Katz R, Blumenthal RS, Blaha MJ. The association of nonalcoholic fatty liver disease, obesity, and metabolic syndrome, with systemic inflammation and subclinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2015;239:629–633. doi: 10.1016/j.atherosclerosis.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park HE, Kwak MS, Kim D, Kim MK, Cha MJ, Choi SY. Nonalcoholic Fatty Liver Disease Is Associated With Coronary Artery Calcification Development: A Longitudinal Study. J Clin Endocrinol Metab. 2016;101:3134–3143. doi: 10.1210/jc.2016-1525. [DOI] [PubMed] [Google Scholar]
- 48.Ishiba H, Sumida Y, Kataoka S, Kuroda M, Akabame S, Tomiyasu K, Tanaka M, Arai M, Taketani H, Seko Y, Okajima A, Hara T, Umemura A, Nishikawa T, Yamaguchi K, Moriguchi M, Mitsuyoshi H, Yasui K, Itoh Y. Association of coronary artery calcification with liver fibrosis in Japanese patients with non-alcoholic fatty liver disease. Hepatol Res. 2016;46:1107–1117. doi: 10.1111/hepr.12665. [DOI] [PubMed] [Google Scholar]
- 49.Kim J, Lee DY, Park SE, Park CY, Lee WY, Oh KW, Park SW, Rhee EJ. Increased risk for development of coronary artery calcification in subjects with non-alcoholic fatty liver disease and systemic inflammation. PLoS One. 2017;12:e0180118. doi: 10.1371/journal.pone.0180118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sinn DH, Kang D, Chang Y, Ryu S, Gu S, Kim H, Seong D, Cho SJ, Yi BK, Park HD, Paik SW, Song YB, Lazo M, Lima JA, Guallar E, Cho J, Gwak GY. Non-alcoholic fatty liver disease and progression of coronary artery calcium score: a retrospective cohort study. Gut. 2017;66:323–329. doi: 10.1136/gutjnl-2016-311854. [DOI] [PubMed] [Google Scholar]
- 51.Wu R, Hou F, Wang X, Zhou Y, Sun K, Wang Y, Liu H, Wu J, Zhao R, Hu J. Nonalcoholic Fatty Liver Disease and Coronary Artery Calcification in a Northern Chinese Population: a Cross Sectional Study. Sci Rep. 2017;7:9933. doi: 10.1038/s41598-017-09851-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tana C, Ballestri S, Ricci F, Di Vincenzo A, Ticinesi A, Gallina S, Giamberardino MA, Cipollone F, Sutton R, Vettor R, Fedorowski A, Meschi T. Cardiovascular Risk in Non-Alcoholic Fatty Liver Disease: Mechanisms and Therapeutic Implications. Int J Environ Res Public Health. 2019;16 doi: 10.3390/ijerph16173104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Assy N, Djibre A, Farah R, Grosovski M, Marmor A. Presence of coronary plaques in patients with nonalcoholic fatty liver disease. Radiology. 2010;254:393–400. doi: 10.1148/radiol.09090769. [DOI] [PubMed] [Google Scholar]
- 54.Wong VW, Wong GL, Yip GW, Lo AO, Limquiaco J, Chu WC, Chim AM, Yu CM, Yu J, Chan FK, Sung JJ, Chan HL. Coronary artery disease and cardiovascular outcomes in patients with non-alcoholic fatty liver disease. Gut. 2011;60:1721–1727. doi: 10.1136/gut.2011.242016. [DOI] [PubMed] [Google Scholar]
- 55.Arslan U, Türkoğlu S, Balcioğlu S, Tavil Y, Karakan T, Cengel A. Association between nonalcoholic fatty liver disease and coronary artery disease. Coron Artery Dis. 2007;18:433–436. doi: 10.1097/MCA.0b013e3282583c0d. [DOI] [PubMed] [Google Scholar]
- 56.Alper AT, Hasdemir H, Sahin S, Ontürk E, Akyol A, Nurkalem Z, Cakmak N, Erdinler I, Gürkan K. The relationship between nonalcoholic fatty liver disease and the severity of coronary artery disease in patients with metabolic syndrome. Turk Kardiyol Dern Ars. 2008;36:376–381. [PubMed] [Google Scholar]
- 57.Boddi M, Tarquini R, Chiostri M, Marra F, Valente S, Giglioli C, Gensini GF, Abbate R. Nonalcoholic fatty liver in nondiabetic patients with acute coronary syndromes. Eur J Clin Invest. 2013;43:429–438. doi: 10.1111/eci.12065. [DOI] [PubMed] [Google Scholar]
- 58.Lin YC, Lo HM, Chen JD. Sonographic fatty liver, overweight and ischemic heart disease. World J Gastroenterol. 2005;11:4838–4842. doi: 10.3748/wjg.v11.i31.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mirbagheri SA, Rashidi A, Abdi S, Saedi D, Abouzari M. Liver: an alarm for the heart? Liver Int. 2007;27:891–894. doi: 10.1111/j.1478-3231.2007.01531.x. [DOI] [PubMed] [Google Scholar]
- 60.Akabame S, Hamaguchi M, Tomiyasu K, Tanaka M, Kobayashi-Takenaka Y, Nakano K, Oda Y, Yoshikawa T. Evaluation of vulnerable coronary plaques and non-alcoholic fatty liver disease (NAFLD) by 64-detector multislice computed tomography (MSCT) Circ J. 2008;72:618–625. doi: 10.1253/circj.72.618. [DOI] [PubMed] [Google Scholar]
- 61.Açikel M, Sunay S, Koplay M, Gündoğdu F, Karakelleoğlu S. Evaluation of ultrasonographic fatty liver and severity of coronary atherosclerosis, and obesity in patients undergoing coronary angiography. Anadolu Kardiyol Derg. 2009;9:273–279. [PubMed] [Google Scholar]
- 62.Targher G, Bertolini L, Padovani R, Rodella S, Zoppini G, Pichiri I, Sorgato C, Zenari L, Bonora E. Prevalence of non-alcoholic fatty liver disease and its association with cardiovascular disease in patients with type 1 diabetes. J Hepatol. 2010;53:713–718. doi: 10.1016/j.jhep.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 63.Sun L, Lü SZ. Association between non-alcoholic fatty liver disease and coronary artery disease severity. Chin Med J (Engl) 2011;124:867–872. [PubMed] [Google Scholar]
- 64.Agaç MT, Korkmaz L, Cavusoglu G, Karadeniz AG, Agaç S, Bektas H, Erkan H, Varol MO, Vatan MB, Acar Z, Mentese U, Celik S. Association between nonalcoholic fatty liver disease and coronary artery disease complexity in patients with acute coronary syndrome: a pilot study. Angiology. 2013;64:604–608. doi: 10.1177/0003319713479155. [DOI] [PubMed] [Google Scholar]
- 65.Puchner SB, Lu MT, Mayrhofer T, Liu T, Pursnani A, Ghoshhajra BB, Truong QA, Wiviott SD, Fleg JL, Hoffmann U, Ferencik M. High-risk coronary plaque at coronary CT angiography is associated with nonalcoholic fatty liver disease, independent of coronary plaque and stenosis burden: results from the ROMICAT II trial. Radiology. 2015;274:693–701. doi: 10.1148/radiol.14140933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hamaguchi M, Kojima T, Takeda N, Nagata C, Takeda J, Sarui H, Kawahito Y, Yoshida N, Suetsugu A, Kato T, Okuda J, Ida K, Yoshikawa T. Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J Gastroenterol. 2007;13:1579–1584. doi: 10.3748/wjg.v13.i10.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Perera N, Indrakumar J, Abeysinghe WV, Fernando V, Samaraweera WM, Lawrence JS. Non alcoholic fatty liver disease increases the mortality from acute coronary syndrome: an observational study from Sri Lanka. BMC Cardiovasc Disord. 2016;16:37. doi: 10.1186/s12872-016-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Keskin M, Hayıroğlu Mİ, Uzun AO, Güvenç TS, Şahin S, Kozan Ö. Effect of Nonalcoholic Fatty Liver Disease on In-Hospital and Long-Term Outcomes in Patients With ST-Segment Elevation Myocardial Infarction. Am J Cardiol. 2017;120:1720–1726. doi: 10.1016/j.amjcard.2017.07.107. [DOI] [PubMed] [Google Scholar]
- 69.Emre A, Terzi S, Celiker E, Sahin S, Yazıcı S, Erdem A, Ceylan US, Asik M, Yesilcimen K. Impact of Nonalcoholic Fatty Liver Disease on Myocardial Perfusion in Nondiabetic Patients Undergoing Primary Percutaneous Coronary Intervention for ST-Segment Elevation Myocardial Infarction. Am J Cardiol. 2015;116:1810–1814. doi: 10.1016/j.amjcard.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 70.Wong VW, Wong GL, Yeung JC, Fung CY, Chan JK, Chang ZH, Kwan CT, Lam HW, Limquiaco J, Chim AM, Yu CM, Chan HL. Long-term clinical outcomes after fatty liver screening in patients undergoing coronary angiogram: A prospective cohort study. Hepatology. 2016;63:754–763. doi: 10.1002/hep.28253. [DOI] [PubMed] [Google Scholar]
- 71.Arslan U, Kocaoğlu I, Balcı M, Duyuler S, Korkmaz A. The association between impaired collateral circulation and non-alcoholic fatty liver in patients with severe coronary artery disease. J Cardiol. 2012;60:210–214. doi: 10.1016/j.jjcc.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 72.Italian Association for the Study of the Liver (AISF) AISF position paper on nonalcoholic fatty liver disease (NAFLD): Updates and future directions. Dig Liver Dis. 2017;49:471–483. doi: 10.1016/j.dld.2017.01.147. [DOI] [PubMed] [Google Scholar]
- 73.European Association for the Study of the Liver (EASL) European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 74.National Guideline Centre (UK) Non-Alcoholic Fatty Liver Disease: Assessment and Management. London: National Institute for Health and Care Excellence (UK); 2016. [PubMed] [Google Scholar]
- 75.Ma S, Zheng Y, Xiao Y, Zhou P, Tan H. Meta-analysis of studies using metformin as a reducer for liver cancer risk in diabetic patients. Medicine (Baltimore) 2017;96:e6888. doi: 10.1097/MD.0000000000006888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Musso G, Cassader M, Paschetta E, Gambino R. Thiazolidinediones and Advanced Liver Fibrosis in Nonalcoholic Steatohepatitis: A Meta-analysis. JAMA Intern Med. 2017;177:633–640. doi: 10.1001/jamainternmed.2016.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, Hazlehurst JM, Guo K LEAN trial team; Abouda G; Aldersley MA; Stocken D; Gough SC; Tomlinson JW; Brown RM; Hübscher SG; Newsome PN. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679–690. doi: 10.1016/S0140-6736(15)00803-X. [DOI] [PubMed] [Google Scholar]
- 78.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE EMPA-REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 79.Athyros VG, Boutari C, Stavropoulos K, Anagnostis P, Imprialos KP, Doumas M, Karagiannis A. Statins: An Under-Appreciated Asset for the Prevention and the Treatment of NAFLD or NASH and the Related Cardiovascular Risk. Curr Vasc Pharmacol. 2018;16:246–253. doi: 10.2174/1570161115666170621082910. [DOI] [PubMed] [Google Scholar]
- 80.Nakade Y, Murotani K, Inoue T, Kobayashi Y, Yamamoto T, Ishii N, Ohashi T, Ito K, Fukuzawa Y, Yoneda M. Ezetimibe for the treatment of non-alcoholic fatty liver disease: A meta-analysis. Hepatol Res. 2017;47:1417–1428. doi: 10.1111/hepr.12887. [DOI] [PubMed] [Google Scholar]
- 81.Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Ž, Riccardi G, Taskinen MR, Tokgozoglu L, Verschuren WMM, Vlachopoulos C, Wood DA, Zamorano JL, Cooney MT ESC Scientific Document Group. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur Heart J. 2016;37:2999–3058. doi: 10.1093/eurheartj/ehw272. [DOI] [PubMed] [Google Scholar]