Abstract
To determine easy-to-use predictors of overall survival (OS), locoregional recurrence (LRR), and distant metastasis (DM) in breast invasive ductal carcinoma (IDC) patients receiving neoadjuvant chemotherapy (NACT) and total mastectomy (TM), we used the pathologic response (PR) of primary breast diseases (T stages), nodal diseases (N stages), and combined primary and nodal diseases (American Joint Committee on Cancer [AJCC] stages) based on existing clinical and pathologic reports as predictors. We enrolled patients with IDC who received NACT followed by TM. Cox regression analysis was used to calculate hazard ratios (HRs) and confidence intervals (CIs) of PR; other independent predictors were controlled for or stratified in the analysis. We analyzed 3654 IDC patients (1031, 1215, 1003, and 405 patients with clinical stages IIB, IIIA, IIIB, and IIIC, respectively) receiving NACT and TM. After multivariate Cox regression analyses, the adjusted HRs (aHRs) (95% CI) for all-cause mortality, LRR, and DM were noted to be 0.21 (0.13-0.34), 0.19 (0.08-0.48), and 0.33 (0.23-0.47), respectively, for pCR; 0.56 (0.48-0.65), 0.67 (0.51-0.89), and 0.61 (0.52-0.70), respectively, for AJCC downstaging; and 1.85 (1.56-2.18), 1.17 (0.84-1.62), and 1.61 (1.36-1.90), respectively, for AJCC upstaging. The PR parameters used in the study are easily applied because they are based on existing staging records, and they can strongly predict OS, LRR, and DM in IDC patients receiving NACT and TM, regardless of clinical stage. The results can be used to guide adjuvant treatment.
Keywords: Breast cancer, neoadjuvant chemotherapy, total mastectomy, pathologic response, survival
Introduction
Systemic therapy for nonmetastatic, invasive ductal carcinoma (IDC) is intended to reduce the risk of distant failure, whereas neoadjuvant chemotherapy (NACT) is administered to downstage the primary tumor (T stage) or lymph nodes (N stage) and provide information regarding treatment response after NACT. Downstaging the T or N stage may allow for less extensive surgery-breast-conserving surgery instead of mastectomy-thereby improving cosmetic outcomes, avoiding the risks associated with breast reconstruction or axillary lymph node dissection, and reducing postoperative complications such as lymphedema [1-5]. Evaluation of the response to NACT in IDC patients can be used to guide adjuvant treatment recommendations. The pathologic complete response (pCR) of residual invasive cancer after NACT is a strong prognostic factor of recurrence [6-12]. The achievement of pCR in the breast (T-pCR) and regional lymph nodes (N-pCR) after NACT correlates with improved survival [13]. The Collaborative Trials in Neoadjuvant Breast Cancer pooled analysis of neoadjuvant breast cancer trials with mature follow-up, with 11,955 patients, characterized the relationship between pCR and long-term outcomes [14]. Achieving pCR in the breast and the axilla (pCR of T stages and N stages [ypT0N0]) was associated with improved event-free survival (EFS) and overall survival (OS) compared with achieving only T-pCR. When a pCR of American Joint Committee on Cancer (AJCC) stages (ypT0N0) was achieved, the risk of death was reduced [14,15].
However, patients with hormone receptor (HoR)-positive breast cancers rarely achieve a pCR upon neoadjuvant endocrine therapy or NACT; therefore, the response is quantified using indexes such as the residual cancer burden (RCB) index, which provides a standardized approach for assessing the extents of residual invasive disease in the tumor bed and of residual nodal involvement after NACT [16]. The RCB index can predict relapse-free survival at 10 years, and patients with either pCR or minimal residual disease (RCB class I) have improved outcomes compared with the overall group of non-pCRs [16]. Other evaluations, such as assessing the presence and prevalence of tumor-infiltrating lymphocytes, have been proposed as prognostic tools [17], but validation in clinical trials is necessary. Moreover, measurement of the continuous RCB index depends on pathologic review by well-trained pathologists.
Therefore, in the current study, we used a simple predictive tool to estimate OS, locoregional recurrence (LRR), and distant metastasis (DM) for breast cancer patients using the pathologic response (PR) after NACT and total mastectomy (TM) of the T, N, and AJCC stages based on existing clinical and pathologic reports.
Patients and methods
In this study, we established an IDC cohort using data from the Taiwan Cancer Registry Database (TCRD) maintained by the Collaboration Center of Health Information Application. We enrolled patients with an IDC diagnosis between January 1, 2007, and December 31, 2015, who received NACT followed by TM. The follow-up duration was from the index date to December 31, 2016. The TCRD contains detailed cancer-related information of patients, including those on clinical stage (AJCC, seventh edition), treatment modalities, pathologic data (including pathologic stage), irradiation doses, HoR status, human epidermal growth factor receptor 2 (HER2) status, and chemotherapy regimens used [18-26]. Our protocols were approved by the Institutional Review Board of Taipei Medical University. Patient diagnoses were confirmed through their pathologic data, and patients who received a new diagnosis of IDC were confirmed to have no other cancer. Other inclusion criteria were age ≥ 20 years and AJCC stage IIB-IIIC. Patients with metastasis, missing sex data, age < 20 years, nonstandard postmastectomy radiation therapy (PMRT) (standard PMRT including irradiation to both the chest wall and regional nodes with a minimum of 50 Gy), unclear differentiation of tumor grade, unclear PR, missing HoR status, missing HER2 status, and unclear staging were excluded.
Furthermore, we excluded patients with unclear NACT regimen, < 4 cycles of NACT, ill-defined nodal surgery, and nonrecorded hospital type [27] (academic center or community hospital) in our cohort. PR were separated into upstaging (increase in the clinical stage to the advanced pathologic stage), equal stages (clinical stage equal to pathologic stage), downstaging (decrease in the clinical stage to earlier pathologic stages), and pCR (absence of residual invasive cancer). HoR positivity was defined as ≥ 1% of tumor cells demonstrating positive nuclear staining through immunohistochemistry [28], and HER2 positivity was defined as having immunohistochemistry score 3+ or fluorescence in situ hybridization ratio ≥ 2 [27,29].
Finally, we enrolled patients with IDC receiving NACT followed by TM and grouped them based on clinical AJCC stage. The index date was the date of breast cancer diagnosis. Comorbidities were assessed using the Charlson comorbidity index (CCI) [30,31]. Only comorbidities observed 6 months before the index date were included. Comorbidities were identified according to the main International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes; disease(s) existing on first admission or identified more than twice during outpatient visits were included as comorbidities.
After confounders were adjusted for, the time-dependent Cox proportional method was used to model the time from the index date to all-cause mortality, LRR, and DM among patients who received NACT followed by TM. In the multivariate analysis, hazard ratios (HRs) were adjusted for the PRs of the following: AJCC stages, initial clinical stages, age, diagnosis year, CCI scores, differentiation, NACT regimen, nodal surgery, adjuvant PMRT, HoR status, HER2 status, and academic hospital. Stratified analyses by initial clinical stage were performed to evaluate the predictors of all-cause mortality. Multivariate analyses of all-cause mortality, LRR, and DM for patients receiving NACT and TM stratified by different PR of T stages, N stages, or AJCC stages impact were conducted. All analyses were performed using SAS (version 9.3; SAS, Cary, NC, USA). Two-tailed P < 0.05 was considered statistically significant.
Results
The study cohort comprised 3654 patients (1031, 1215, 1003, and 405 with clinical stages IIB, IIIA, IIIB, and IIIC, respectively) (Table 1). No significant differences were noted in CCI scores, tumor differentiation, and nodal surgery between patients with different initial clinical stages (Table 1). Patients at advanced stages (IIIB-IIIC) were more likely to be older adults. Clinical stage IIIC patients were more likely to be diagnosed in 2011-2015. More IDC patients with advanced stages (IIIA-C) received a taxane-based regimen as NACT, adjuvant PMRT, or NACT followed by TM in a non-academic hospital setting. The rate of HoR positivity was higher among those at clinical stages IIB-IIIA, and that of HER2 positivity was higher among those at stages IIIB-IIIC. Patients at clinical stages IIIA-IIIC had greater downstaging or pCR of the AJCC, T, and N stages. The risks of all-cause mortality, LRR, and DM were significantly higher at advanced clinical stages (IIIA-IIIC) than at stage IIB (Table 1).
Table 1.
Characteristics of patients receiving neoadjuvant chemotherapy and total mastectomy stratified by AJCC stage
| Variable | AJCC clinical stage | p | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| IIB (N = 1031) | IIIA (N = 1215) | IIIB (N = 1003) | IIIC (N = 405) | |||
| Age | Mean (SD) | 50.1 (10.1) | 50.6 (10.4) | 54.3 (10.6) | 53.4 (11.1) | 0.0426 |
| Median (Q1, Q3) | 50 (43, 57) | 50 (44, 58) | 54 (47, 61) | 53 (46, 61) | ||
| 20-49 | 514 (49.9%) | 587 (48.3%) | 330 (32.9%) | 149 (36.8%) | < 0.0001 | |
| 50+ | 517 (50.1%) | 628 (51.7%) | 673 (67.1%) | 256 (63.2%) | ||
| Diagnosis year | 2007-2010 | 344 (33.4%) | 434 (35.7%) | 380 (37.9%) | 103 (25.4%) | < 0.0001 |
| 2011-2015 | 687 (66.6%) | 781 (64.3%) | 623 (62.1%) | 302 (74.6%) | ||
| CCI scores | 0 | 828 (80.3%) | 1000 (82.3%) | 835 (83.3%) | 339 (83.7%) | 0.6067 |
| 1 | 144 (14.0%) | 147 (12.1%) | 114 (11.4%) | 47 (11.6%) | ||
| 2+ | 59 (5.7%) | 68 (5.6%) | 54 (5.4%) | 19 (4.7%) | ||
| Differentiation | I | 65 (6.3%) | 76 (6.3%) | 63 (6.3%) | 16 (4.0%) | 0.4723 |
| II | 549 (53.2%) | 603 (49.6%) | 525 (52.3%) | 184 (45.4%) | ||
| III | 417 (40.5%) | 536 (44.1%) | 415 (41.4%) | 205 (50.6%) | ||
| NACT regimen | Taxanes | 376 (36.5%) | 477 (39.3%) | 346 (34.5%) | 191 (47.2%) | < 0.0001 |
| Anthracycline | 331 (32.1%) | 303 (24.9%) | 290 (28.9%) | 100 (24.7%) | ||
| Both | 246 (23.9%) | 366 (30.1%) | 320 (31.9%) | 106 (26.2%) | ||
| Neither | 78 (7.6%) | 69 (5.7%) | 47 (4.7%) | 8 (2.0%) | ||
| Nodal surgery | ALND | 837 (81.2%) | 1010 (83.1%) | 827 (82.5%) | 355 (87.7%) | 0.0559 |
| SLNB | 194 (18.8%) | 205 (16.9%) | 176 (17.5%) | 50 (12.3%) | ||
| Adjuvant PMRT | 657 (63.7%) | 954 (78.5%) | 739 (73.7%) | 346 (85.4%) | < 0.0001 | |
| Hormone receptor positive | 557 (54.0%) | 638 (52.5%) | 467 (46.6%) | 158 (39.0%) | < 0.0001 | |
| HER2 positive | 328 (31.8%) | 465 (38.3%) | 368 (36.7%) | 186 (45.9%) | < 0.0001 | |
| Academic hospital | Yes | 663 (64.3%) | 679 (55.9%) | 583 (58.1%) | 210 (51.9%) | < 0.0001 |
| No | 368 (35.7%) | 536 (44.1%) | 420 (41.9%) | 195 (48.1%) | ||
| Response of AJCC-stages ratio | pCR | 70 (6.8%) | 76 (6.3%) | 58 (5.8%) | 27 (6.7%) | < 0.0001 |
| Downstages | 416 (40.3%) | 529 (43.5%) | 543 (54.1%) | 251 (62.0%) | ||
| Equal-stages | 207 (20.1%) | 359 (29.5%) | 248 (24.7%) | 127 (31.4%) | ||
| Upstages | 338 (32.8%) | 251 (20.7%) | 154 (15.4%) | 0 (0%) | ||
| Response of T-stages ratio | T-pCR | 78 (7.6%) | 91 (7.5%) | 61 (6.1%) | 41 (10.1%) | < 0.0001 |
| Downstages | 424 (41.1%) | 738 (60.7%) | 635 (63.3%) | 234 (57.8%) | ||
| Equal-stages | 444 (43.1%) | 343 (28.2%) | 307 (30.6%) | 122 (30.1%) | ||
| Upstages | 85 (8.2%) | 43 (3.5%) | 0 (0%) | 8 (2.0%) | ||
| Response of N-stages ratio | N-pCR | 281 (27.3%) | 336 (27.7%) | 255 (25.4%) | 100 (24.7%) | < 0.0001 |
| Downstages | 0 (0%) | 119 (9.8%) | 74 (7.4%) | 178 (43.9%) | ||
| Equal-stages | 411 (39.9%) | 382 (31.4%) | 352 (35.1%) | 127 (31.4%) | ||
| Upstages | 339 (32.9%) | 378 (31.1%) | 322 (32.1%) | 0 (0%) | ||
| Mean follow-up time, months (SD) | 63.6 (31.0) | 60.6 (30.4) | 57.6 (30.5) | 52.7 (28.6) | < 0.0001 | |
| Death | 190 (18.4%) | 327 (26.9%) | 339 (33.8%) | 148 (36.5%) | < 0.0001 | |
| Locoregional recurrence | 70 (6.8%) | 89 (7.3%) | 88 (8.8%) | 38 (9.4%) | < 0.0001 | |
| Distant metastasis | 228 (22.1%) | 341 (28.1%) | 326 (32.5%) | 138 (34.1%) | < 0.0001 | |
PMRT, postmastectomy radiation therapy; T, tumor; N, nodal; NACT, neoadjuvant chemotherapy; TM, total mastectomy; HR, hormone receptor; HER2, human epidermal growth factor receptor 2; pCR, pathologic complete response; ALND, axillary lymph node dissection; SNLB, sentinel lymph node biopsy; SD, standard deviation; CCI, Charlson comorbidity index; AJCC, American Joint Committee on Cancer; ypT, postchemotherapy pathologic tumor stages; ypN, postchemotherapy pathologic nodal stages; IQR, interquartile range; T-pCR, pathologic complete response in the breast; N-pCR, pathologic complete response in regional lymph nodes.
According to the multivariate Cox regression analysis, the PR of AJCC stages were significant independent predictors of all-cause mortality, LRR, and DM (Table 2). pCR and downstaging strongly predicted a decrease, and upstaging strongly predicted an increase, in the risks of all-cause mortality, LRR, and DM. pCR of AJCC stages (ypT0N0) was the strongest predictor for decreasing all-cause mortality, LRR, and DM. The adjusted HRs (aHRs) (95% confidence interval [CI]) of all-cause mortality, LRR, and DM were 0.21 (0.13-0.34), 0.19 (0.08-0.48), and 0.33 (0.23-0.47), respectively, for pCR; 0.56 (0.48-0.65), 0.67 (0.51-0.89), and 0.61 (0.52-0.70), respectively, for downstaging; and 1.85 (1.56-2.18), 1.17 (0.84-1.62), and 1.61 (1.36-1.90), respectively, for upstaging (Table 2). Multivariate Cox regression analyses indicated that upstaging, non-PMRT, CCI ≥ 2, poor differentiation, initial AJCC clinical stages IIIA-IIIC, and HoR positivity were poor prognostic factors for OS (Table 2). Well-differentiated tumor grade, pCR, downstaging, and HoR positivity were independent and good prognostic factors for OS. The poor prognostic factors for LRR were non-PMRT, AJCC clinical stages IIIA-IIIC, AJCC upstaging after NACT, and HER2-positive status (Table 2). Moreover, AJCC clinical stages IIIA-IIIC, AJCC upstaging after NACT, poor differentiation, and HER2-positive status were independent and poor prognostic factors for DM. Table 3 presents the independent predictors of all-cause mortality for patients receiving NACT and TM, as stratified by clinical stage after multivariate analysis. The aHRs (95% CI) of pCR of AJCC stages were 0.18 (0.05-0.58), 0.17 (0.07-0.42), 0.30 (0.15-0.63), and 0.13 (0.03-0.53) for all-cause mortality among initial clinical stages IIB, IIIA, IIIB, and IIIC, respectively; those of AJCC downstaging were 0.64 (0.42-0.96), 0.50 (0.37-0.66), 0.57 (0.44-0.75), and 0.59 (0.42-0.83), respectively; and those of AJCC upstaging were 1.51 (1.03-2.21), 1.82 (1.39-2.37), and 2.17 (1.64-2.89), respectively. The set of independent risk factors for all-cause mortality comprised CCI ≥ 2, poor differentiation, and HoR negativity in patients with stage IIB-IIIC tumors; only adjuvant PMRT in patients with stage IIIA-IIIC tumors; and no factors in patients at stage IIB (Table 3).
Table 2.
Multivariable analysis of all-cause mortality, locoregional recurrence, and distant metastasis for patients receiving neoadjuvant chemotherapy and total mastectomy
| All-Cause Mortality | LRR | DM | |||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | ||
| Response of AJCC-stages ratio | Equal-stages | ref | < 0.0001 | ref | < 0.0001 | ref | < 0.0001 |
| pCR | 0.21 (0.13-0.34) | 0.19 (0.08-0.48) | 0.33 (0.23-0.47) | ||||
| Downstages | 0.56 (0.48-0.65) | 0.67 (0.51-0.89) | 0.61 (0.52-0.70) | ||||
| Upstages | 1.85 (1.56-2.18) | 1.17 (0.84-1.62) | 1.61 (1.36-1.90) | ||||
| AJCC stage | IIB | ref | < 0.0001 | ref | 0.0017 | ref | < 0.0001 |
| IIIA | 1.85 (1.54-2.22) | 1.15 (1.03-1.59) | 1.61 (1.36-1.92) | ||||
| IIIB | 2.66 (2.21-3.21) | 1.60 (1.15-2.22) | 2.24 (1.88-2.67) | ||||
| IIIC | 3.76 (2.97-4.77) | 2.06 (1.34-3.16) | 2.91 (2.31-3.66) | ||||
| Age | 20-49 | ref | 0.9544 | ref | 0.2095 | ref | 0.1458 |
| 50+ | 1.00 (0.88-1.15) | 1.02 (0.86-1.09) | 1.07 (0.76-1.10) | ||||
| Diagnosis year | 2007-2010 | ref | 0.7865 | ref | 0.4417 | ref | 0.3807 |
| 2011-2015 | 0.98 (0.86-1.13) | 0.90 (0.70-1.17) | 1.06 (0.93-1.21) | ||||
| CCI scores | 0 | ref | < 0.0001 | ref | 0.1208 | ref | 0.3412 |
| 1 | 0.89 (0.73-1.10) | 1.05 (0.72-1.53) | 1.08 (0.89-1.31) | ||||
| 2+ | 1.69 (1.34-2.14) | 1.03 (0.99-1.18) | 1.20 (0.92-1.57) | ||||
| Differentiation | III | ref | < 0.0001 | ref | 0.4035 | ref | 0.0418 |
| II | 0.76 (0.66-0.88) | 0.96 (0.81-1.13) | 0.94 (0.81-1.09) | ||||
| I | 0.42 (0.28-0.62) | 0.80 (0.64-1.29) | 0.63 (0.45-0.89) | ||||
| NACT regimen | Anthracycline | ref | 0.4389 | ref | 0.6519 | ref | 0.1084 |
| Taxanes | 1.07 (0.91-1.26) | 0.91 (0.67-1.24) | 1.09 (0.91-1.52) | ||||
| Both | 1.07 (0.91-1.26) | 1.11 (0.82-1.49) | 1.16 (0.99-1.37) | ||||
| Neither | 1.24 (0.95-1.63) | 1.01 (0.60-1.71) | 1.26 (0.93-1.79) | ||||
| Nodal surgery | ALND | ref | 0.8862 | ref | 0.1204 | ref | 0.4326 |
| SLNB | 0.93 (0.61-1.42) | 0.92 (0.24-1.09) | 0.79 (0.54-1.16) | ||||
| Adjuvant PMRT | 0.75 (0.65-0.87) | 0.0001 | 0.43 (0.37-0.49) | < 0.0001 | 0.84 (0.64-1.10) | 0.1991 | |
| Hormone receptor positive | 0.68 (0.59-0.77) | < 0.0001 | 1.15 (0.89-1.49) | 0.2737 | 0.95 (0.80-1.31) | 0.2451 | |
| HER2 positive | 0.99 (0.87-1.13) | 0.9283 | 1.92 (1.51-2.45) | < 0.0001 | 1.51 (1.33-1.72) | < 0.0001 | |
| Academic hospital | Yes | ref | 0.2981 | ref | 0.2759 | ref | 0.3640 |
| No | 0.93 (0.82-1.06) | 0.87 (0.68-1.12) | 1.06 (0.93-1.21) | ||||
HR, hazard ratio; CI, confidence interval; PMRT, postmastectomy radiation therapy; NACT, neoadjuvant chemotherapy; HER2, human epidermal growth factor receptor 2; ALND, axillary lymph node dissection; SLNB, sentinel lymph node biopsy; CCI, Charlson comorbidity index; AJCC, American Joint Committee on Cancer; LRR, locoregional recurrence; DM, distant metastasis.
Table 3.
Multivariable analysis of all-cause mortality for patients receiving neoadjuvant chemotherapy and total mastectomy stratified by clinical stage
| Clinical Stage IIB | Clinical Stage IIIA | Clinical Stage IIIB | Clinical Stage IIIC | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | ||
| Response of AJCC-stages ratio | Equal-stages | ref | < 0.0001 | ref | < 0.0001 | ref | < 0.0001 | ref | 0.0006 |
| ypCR | 0.18 (0.05-0.58) | 0.17 (0.07-0.42) | 0.30 (0.15-0.63) | 0.13 (0.03-0.53) | |||||
| Downstages | 0.64 (0.42-0.96) | 0.50 (0.37-0.66) | 0.57 (0.44-0.75) | 0.59 (0.42-0.83) | |||||
| Upstages | 1.51 (1.03-2.21) | 1.82 (1.39-2.37) | 2.17 (1.64-2.89) | - | |||||
| Age | 20-49 | ref | 0.7656 | ref | 0.4405 | ref | 0.3858 | ref | 0.6774 |
| 50+ | 1.05 (0.77-1.43) | 0.91 (0.72-1.15) | 1.12 (0.87-1.43) | 0.92 (0.64-1.34) | |||||
| diagnosis year | 2007-2010 | ref | 0.7036 | ref | 0.5551 | ref | 0.4552 | ref | 0.5093 |
| 2011-2015 | 1.06 (0.77-1.47) | 0.93 (0.73-1.18) | 0.91 (0.72-1.16) | 1.14 (0.77-1.69) | |||||
| CCI Scores | 0 | ref | 0.0389 | ref | 0.0036 | ref | 0.0430 | ref | 0.0388 |
| 1 | 0.98 (0.63-1.54) | 0.71 (0.49-1.05) | 1.11 (0.79-1.57) | 0.79 (0.44-1.41) | |||||
| 2+ | 1.11 (1.02-1.57) | 1.74 (1.16-2.62) | 1.68 (1.12-2.52) | 1.07 (1.01-1.76) | |||||
| Differentiation | III | ref | 0.0408 | ref | 0.0004 | ref | 0.0340 | ref | 0.0296 |
| II | 0.78 (0.56-0.99) | 0.72 (0.56-0.93) | 0.76 (0.59-0.97) | 0.83 (0.56-0.92) | |||||
| I | 0.66 (0.31-0.94) | 0.19 (0.08-0.46) | 0.58 (0.32-0.94) | 0.49 (0.33-0.98) | |||||
| NACT regimen | Anthracycline | ref | 0.1138 | ref | 0.9713 | ref | 0.4014 | ref | 0.6776 |
| Taxanes | 1.39 (0.97-1.98) | 1.08 (0.80-1.45) | 0.95 (0.71-1.28) | 0.96 (0.63-1.47) | |||||
| Both | 0.92 (0.62-1.38) | 1.04 (0.78-1.39) | 1.14 (0.87-1.49) | 1.15 (0.72-1.86) | |||||
| Neither | 1.40 (0.83-2.35) | 1.05 (0.66-1.65) | 1.37 (0.82-2.28) | 1.53 (0.58-4.01) | |||||
| Nodal surgery | ALND | ref | 0.9488 | ref | 0.5288 | ref | 0.6781 | ref | 0.4133 |
| SLNB | 1.09 (0.53-2.24) | 0.88 (0.41-1.88) | 0.65 (0.23-1.80) | 1.11 (0.32-1.83) | |||||
| Adjuvant PMRT | No | ref | 0.8612 | ref | < 0.0001 | ref | 0.0136 | ref | 0.0227 |
| Yes | 0.97 (0.70-1.35) | 0.57 (0.44-0.74) | 0.82 (0.65-0.93) | 0.67 (0.43-0.95) | |||||
| Hormone receptors positive | No | ref | 0.0459 | ref | < 0.0001 | ref | 0.0128 | ref | 0.0010 |
| Yes | 0.75 (0.55-0.92) | 0.61 (0.48-0.77) | 0.74 (0.59-0.94) | 0.54 (0.37-0.78) | |||||
| HER2 positive | No | ref | 0.6962 | ref | 0.4107 | ref | 0.8489 | ref | 0.1361 |
| Yes | 1.07 (0.77-1.47) | 1.10 (0.88-1.38) | 1.02 (0.81-1.28) | 1.02 (0.49-1.08) | |||||
| Academic Hospitals | Yes | ref | 0.6800 | ref | 0.0714 | ref | 0.2342 | ref | 0.2775 |
| No | 1.07 (0.78-1.46) | 0.80 (0.64-1.02) | 0.87 (0.69-1.10) | 1.21 (0.86-1.71) | |||||
HR, hazard ratio; CI, confidence interval; PMRT, postmastectomy radiation therapy; NACT, neoadjuvant chemotherapy; HER2, human epidermal growth factor receptor 2; ALND, axillary lymph node dissection; SLNB, sentinel lymph node biopsy; CCI, Charlson comorbidity index; AJCC, American Joint Committee on Cancer.
Because the PR of AJCC stages were significant predictors for all-cause mortality, LRR, and DM, we estimated the effect of PR of T, N, and AJCC stages on all-cause mortality, LRR, and DM. Table 4 reveals that the PR of T and AJCC stages were significant predictors of all-cause mortality, LRR, and DM-with the good, significant prognostic factors being pCR, T downstaging, and AJCC downstaging, and the poor, significant prognostic factors being T upstaging, and AJCC upstaging. However, first, pCR or N upstaging were significant predictors of all-cause mortality and DM but not LRR and second, N downstaging was not a statistically significant predictor of all-cause mortality, LRR, or DM in breast cancer patients receiving NACT and TM (Table 4).
Table 4.
Multivariable analysis of all-cause mortality, locoregional recurrence, and distant metastasis for patients receiving neoadjuvant chemotherapy and total mastectomy stratified by pathologic response ratio of T, N, or AJCC stage
| All-cause mortality | LRR | DM | |||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | ||
| Response of T-stages ratio | Equal-stage | ref | < 0.0001 | ref | < 0.0001 | ref | < 0.0001 |
| T pCR | 0.37 (0.24-0.55) | 0.27 (0.13-0.57) | 0.49 (0.35-0.70) | ||||
| Downstages | 0.68 (0.60-0.78) | 0.58 (0.45-0.75) | 0.82 (0.72-0.94) | ||||
| Upstages | 1.53 (1.13-2.07) | 1.08 (1.02-1.91) | 1.49 (1.09-2.03) | ||||
| Response of N-stages ratio | Equal-stage | ref | < 0.0001 | ref | 0.2379 | ref | < 0.0001 |
| N pCR | 0.60 (0.49-0.73) | 0.80 (0.56-1.14) | 0.65 (0.54-0.78) | ||||
| Downstages | 0.86 (0.69-1.08) | 0.93 (0.61-1.43) | 0.85 (0.68-1.07) | ||||
| Upstages | 1.53 (1.32-1.78) | 1.17 (0.88-1.56) | 1.63 (1.40-1.90) | ||||
| Response of AJCC-stages ratio | Equal-stage | ref | < 0.0001 | ref | < 0.0001 | ref | < 0.0001 |
| pCR | 0.21 (0.13-0.34) | 0.19 (0.08-0.48) | 0.33 (0.23-0.47) | ||||
| Downstages | 0.56 (0.48-0.65) | 0.67 (0.51-0.89) | 0.61 (0.52-0.70) | ||||
| Upstages | 1.85 (1.56-2.18) | 1.17 (0.84-1.62) | 1.61 (1.36-1.90) | ||||
HR, hazard ratio; CI, confidence interval; AJCC, American Joint Committee on Cancer; LRR, locoregional recurrence; DM, distant metastasis; T-stages, primary breast diseases; N-stages, nodal diseases; T, tumor; N, nodal. All variables presented in Tables 1 and 2 were used in this multivariate analysis.
Figure 1A-D present the Kaplan-Meier survival curves for all-cause mortality, LRR-free survival, DM-free survival, and disease-free survival (DFS) for IDC patients receiving NACT and TM who had different PR-such as equal stages, upstaging, downstaging, or pCR of AJCC stages. The areas under the survival curves for pCR and downstaging were higher compared with equal stages and upstages, regardless of all-cause mortality, LRR-free survival, DM-free survival, and DFS (Figure 1A-D, all P < 0.001).
Figure 1.
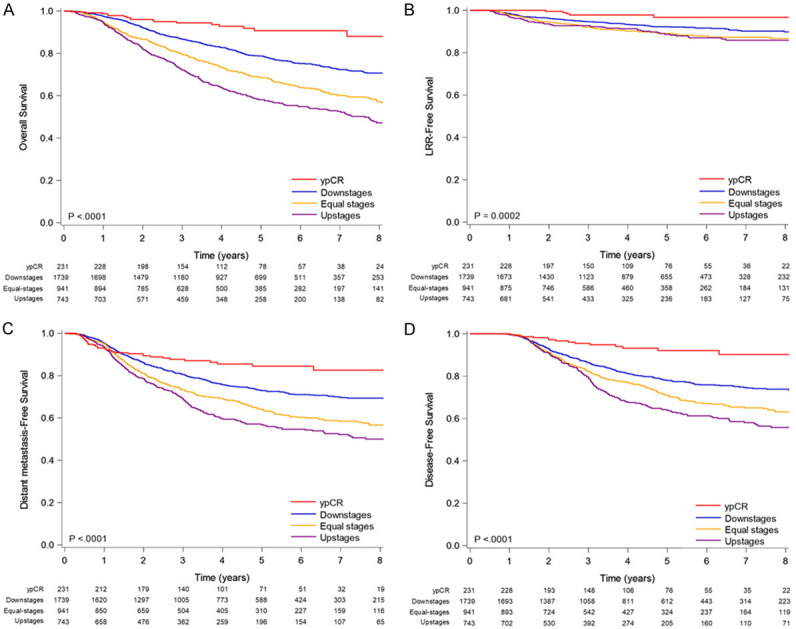
Kaplan-Meier survival curves of (A) overall survival (B) locoregional recurrence-free survival, (C) distant metastasis-free survival, and (D) disease-free survival in patients receiving neoadjuvant chemotherapy and total mastectomy stratified by pathologic response of AJCC stages.
Discussion
Sensitivity to NACT can be an indicator of survival because pCR is a predictor of favorable long-term outcomes and lower risk of recurrence [2,13-16,32]. Nevertheless, no study has analyzed the associations that PR of AJCC, T, or N stages has with LRR, DM, and all-cause mortality. One study reviewed pathologic slides and reports, revealing that the RCB index is a predictor of survival [33]. RCB was calculated as a continuous index combining pathologic measurements of primary tumor (size and cellularity) and nodal metastases (number and size) for predicting DM-free survival [33]. The index score is derived from first, the largest area and cellularity of residual invasive primary cancer and second, the number of involved lymph nodes and size of largest metastasis [33]. pCR (ypT0N0) indicates no residual disease (RCB = 0), and RCB > 0 is divided into minimal (RCB-I), moderate (RCB-II), or extensive (RCB-III) residual disease based on the predefined thresholds of 1.36 and 3.28 [33]. In addition, RCB was validated as a prognostic factor for long-term DFS after NACT [16]. However, RCB calculation is complicated and highly dependent on the judgment of well-trained pathologists [33], precluding its widespread use in the assessment of post-NACT PR in breast cancer patients. Clinical and pathologic AJCC, T, or N stages have long been used to stage breast cancer. The AJCC stages recorded in TCRD were richly descriptive and of high quality. Consequently, it is more convenient to use the commonly used staging systems-such as clinical and pathologic AJCC, T, or N stages-to predict the outcomes of OS, LRR, or DM. Moreover, RCB is a predictor of DFS but not OS, LRR, or DM [16,33]. In the current study, we used the staging reports of 3654 IDC patients receiving NACT and TM based on the 7th edition of the AJCC stages, and we separated the initial clinical and pathologic stages into different PRs: upstaging, no change, downstaging, and pCR based on T, N, and AJCC stages. The PRs were estimated to predict OS, LRR, or DM.
Clinical stages IIB-IIIC demonstrated different characteristic distributions (Table 1). The T-pCR, N-pCR, and AJCC pCR were 7.42%, 26.60%, and 6.32%, respectively. Our study is the largest and the first to demonstrate the effect of PR on survival outcomes in IDC patients receiving NACT and TM. The patients in advanced clinical stages (IIIB-IIIC) had better PR (pCR and downstaging) than did those with the disease at stages IIB-IIIA (Table 1). Advanced clinical stage with better PR (pCR and downstaging) predicted good OS, but advanced clinical stage itself predicted poor OS (Table 2). To resolve this contradiction, clinical stages and PR were stratified to clarify the effect of PR on OS in IDC patients (Table 3).
The independent predictors for all-cause mortality were PR, clinical stages, tumor differentiation, CCI ≥ 2, adjuvant PMRT, and HoR positivity; those for LRR were PR, clinical stages, adjuvant PMRT, and HER2 positivity; and those for DM were PR, tumor differentiation, and HER2 positivity (Table 2). Previous studies have also found that adjuvant PMRT is an independent and good prognostic factor for LRR [6-12]. Poor differentiation, CCI ≥ 2, and HoR negativity were found to be poor prognostic factors for OS. No study has demonstrated that poor differentiation, CCI ≥ 2, and HoR negativity are poor prognostic factors for breast cancer after NACT and TM, but studies have noted high CCI score [34], HoR negativity [35], and poor tumor differentiation [36-38] to be poor prognostic factors for OS, DM, or LRR in patients with breast cancer who received various treatments. In addition, our data indicated that HER2 positivity was a poor prognostic factor for LRR and that poor differentiation and HER2 positivity were high risk factors for DM-similar to previous studies using different treatments for breast cancer [36,37,39,40]. Thus, poor differentiation was a poor prognostic factor for OS and DM; HER2 positivity was a poor prognostic factor for LRR and DM; and CCI ≥ 2 and HoR negativity were poor prognostic factors for OS. In addition to PR, poor differentiation, CCI ≥ 2, and HER2 positivity were poor prognostic factors for survival. In our study, anthracycline- or taxane-based NACT regimens was not as a significant predictor of survival, similar to the results of a meta-analysis involving 1695 patients in nine trials [41]. Clinical stages before NACT and PR, such as pCR, were independent predictors of survival, consistent with previous studies [42-44]. For patients receiving NACT, pCR is associated with increased DFS [42-44]. In preliminary results of a patient-level meta-analysis of 52 studies including 28,000 patients treated with NACT for breast cancer, achieving pCR was associated with better EFS and OS [45]. Nevertheless, no studies have reported on the association of the degree of PR (pCR, downstaging, no change, and upstaging) among OS, LRR, and DM in IDC patients receiving NACT and TM. Our study is the first to use a large cohort to estimate survival and outcome parameters by using existing, widely used clinical and pathologic staging records in the TCRD on IDC patients receiving NACT and TM.
We stratified patients by clinical stage, and found that PR was still a strong predictor of OS in all initial clinical stages (IIB, IIIA, IIIB, and IIIC). pCR and AJCC downstaging were good prognostic factors for OS; and AJCC upstaging was a poor prognostic factor for OS irrespective of the clinical stage. Our outcomes indicate that administering precision medicine with customized NACT regimens to specific breast patients is critical [46,47]. For example, in patients with higher-risk triple-negative breast cancer, the addition of carboplatin to the weekly paclitaxel component of standard NACT substantially increases the pCR rate [46,47]. The PR effectively predicts survival outcomes for breast cancer patients receiving NACT and TM regardless of clinical stage. Moderate to well-differentiated tumors, CCI ≥ 2, and HoR positivity were also significant predictors of OS at different clinical stages. Consistent with this result, in previous studies, high CCI score [34], HoR negativity [35], and poor tumor differentiation [36-38] were poor prognostic factors of OS in patients with breast cancer. Adjuvant PMRT was an independent, significant, and good prognostic factor for OS at clinical stages IIIA-IIIC but not IIB. Our outcomes are compatible with those of studies demonstrating that PMRT first, significantly benefits breast cancer patients who present with the disease at clinical stage III and second, does not affect the 10-year rates of LRR in patients with the disease at clinical stage I or II after NACT [6,11]. In the absence of prospective data to guide our approach to patients with changes in AJCC stages after NACT, physicians should treat patients presenting with the disease at clinical stage III with PMRT (regardless of PR) or even pCR [6,8-11]. For patients presenting with stage II disease, we evaluated pretreatment risk factors (HoR status, HER2 status, and tumor differentiation) and the patient’s PR to NACT; per our findings, we recommend that PMRT may be omitted in some patients who have pCR or AJCC downstaging, whereas we recommend adjuvant PMRT for patients with stage III disease.
Physicians treated patients with any degree of residual nodal (without N-pCR) disease after NACT with PMRT based on retrospective evidence, which suggested a higher rate of recurrence in such patients [48]. Physicians also offer adjuvant PMRT to patients with RCB (without T-pCR), although the threshold to omit adjuvant PMRT in such patients without T-pCR is lower than that for patients without N-pCR [49]. The survival effects of residual primary breast and residual nodal status after NACT and TM are different [48]. Therefore, we estimated the effect of the PRs of primary breast diseases (T stages), nodal diseases (N stages), or combined primary and nodal disease (AJCC stages) on OS, LRR, and DM in breast cancer patients receiving NACT and TM (Table 4). We found that first, changes in both AJCC and T stages were strong predictors for OS, LRR, and DM and second,changes in N stages were not significant for LRR. pCR or N upstaging were strong predictors for OS and DM but not LRR (Table 4). These findings may be reasonable because nodal stages in breast cancer were more associated with the risk of DM than of LRR [50]. Our study is the first to reveal that the predictive value of N downstaging was nonsignificant for OS, LRR, or DM.
The strength of our study is that it is the first study, using a large cohort, to estimate the effect of changes in AJCC, T, and N stages on OS, LRR, and DM in IDC patients receiving NACT and TM. The treatment of regimens of NACT was relatively homogenous in our study. No study has estimated the influence of PR on treatment outcomes in breast cancer patients receiving NACT and TM, and all predictors in our study, including clinical stages, were stratified. In our cohort, the poor prognostic factors of OS were no PMRT, advanced clinical stages IIIA-IIIC before NACT, poorly differentiated tumors, CCI ≥ 2, HoR negativity, and HER2 positivity. Our simple tool using existing AJCC clinical and pathologic staging records as PR is more convenient than using the RCB index, which requires well-trained pathologists [33]. Our findings can thus be easily used to guide adjuvant treatment recommendations. pCR, downstage, equal stages, and AJCC upstaging were significant predictors of all-cause mortality (P < 0.001), LRR-free survival (P = 0.0002), DM-free survival (P < 0.001), and DFS (P < 0.001). Future studies should determine the optimal NACT regimens to maximize the PR in breast cancer patients [46,47]. For AJCC upstaging in IDC patients receiving NACT and TM, adjuvant chemotherapy is strongly suggested because of the high risk of death, LRR, and DM.
Our study has some limitations. First, our cohort was derived from an Asian population. Thus, extrapolation of our results to non-Asian populations should be done with caution. However, evidence has not indicated any difference in PR between Asian and non-Asian breast cancer patients receiving NACT and TM. Second, the diagnoses of all comorbid conditions were based on ICD-9-CM codes. Nevertheless, the Taiwan Cancer Registry Administration randomly reviews charts and interviews patients to verify the diagnoses’ accuracy, and hospitals with outlier charges or practices may be audited and, if malpractice or discrepancies are identified, heavily penalized. Third, to prevent the creation of too many subgroups, we did not stratify patients by NACT regimen. Thus, the effects of different NACT treatments remain unclear. However, each patient received four cycles of NACT. Accordingly, to obtain crucial information on population specificity and disease occurrence, a large-scale randomized trial comparing carefully selected patients who are undergoing suitable treatments across ethnicities is essential. Finally, the TCRD does not contain information regarding dietary habits, socioeconomic status, or body mass index, all of which may be risk factors for mortality. However, considering the magnitude and statistical significance of the observed effects in this study, these limitations are unlikely to affect the conclusions.
Conclusions
PR (changes in T, N, and AJCC stage) can be easily used as a predictive tool for OS, LRR, and DM in IDC patients receiving NACT and TM, regardless of the clinical stage.
Acknowledgements
Lo-Hsu Medical Foundation, Lotung Poh-Ai Hospital, supports Szu-Yuan Wu’s work (Funding Number: 10908 and 10909). Lo-Hsu Medical Foundation, Lotung Poh-Ai Hospital, supports Szu-Yuan Wu’s work (Funding Number: 10908 and 10909). Lo-Hsu Medical Foundation, Lotung Poh-Ai Hospital, supports Szu-Yuan Wu’s work (Funding Number: 10908 and 10909). Our protocols were reviewed and approved by the Institutional Review Board of Taipei Medical University (TMU-JIRB No. 201712019).
Disclosure of conflict of interest
None.
Abbreviations
- PR
pathologic response
- PMRT
postmastectomy radiation therapy
- T
tumor
- N
nodal
- OS
overall survival
- LRR
locoregional recurrence
- DM
distant metastasis
- DFS
disease-free survival
- NACT
neoadjuvant chemotherapy
- TM
total mastectomy
- HRs
hazard ratios
- CI
confidence interval
- IDC
invasive ductal carcinoma
- TCRD
Taiwan Cancer Registry Database
- AJCC
American Joint Committee on Cancer
- HoR
hormone receptor
- HER2
human epidermal growth factor receptor 2
- CCI
Charlson comorbidity index
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- pCR
pathological complete response
- T stages
primary breast diseases
- N stages
nodal diseases
- ALND
axillary lymph nodes dissection
- BCS
breast-conserving surgery
- T-pCR
pathologic complete response in the breast
- N-pCR
pathologic complete response in regional lymph nodes
- EFS
event-free survival
- RCB
residual cancer burden
References
- 1.Gralow JR, Burstein HJ, Wood W, Hortobagyi GN, Gianni L, von Minckwitz G, Buzdar AU, Smith IE, Symmans WF, Singh B, Winer EP. Preoperative therapy in invasive breast cancer: pathologic assessment and systemic therapy issues in operable disease. J. Clin. Oncol. 2008;26:814–819. doi: 10.1200/JCO.2007.15.3510. [DOI] [PubMed] [Google Scholar]
- 2.Kaufmann M, Hortobagyi GN, Goldhirsch A, Scholl S, Makris A, Valagussa P, Blohmer JU, Eiermann W, Jackesz R, Jonat W, Lebeau A, Loibl S, Miller W, Seeber S, Semiglazov V, Smith R, Souchon R, Stearns V, Untch M, von Minckwitz G. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: an update. J. Clin. Oncol. 2006;24:1940–1949. doi: 10.1200/JCO.2005.02.6187. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz GF, Hortobagyi GN. Proceedings of the consensus conference on neoadjuvant chemotherapy in carcinoma of the breast, April 26-28, 2003, Philadelphia, Pennsylvania. Cancer. 2004;100:2512–2532. doi: 10.1002/cncr.20298. [DOI] [PubMed] [Google Scholar]
- 4.Shannon C, Smith I. Is there still a role for neoadjuvant therapy in breast cancer? Crit Rev Oncol Hematol. 2003;45:77–90. doi: 10.1016/s1040-8428(02)00091-4. [DOI] [PubMed] [Google Scholar]
- 5.Mamtani A, Barrio AV, King TA, Van Zee KJ, Plitas G, Pilewskie M, El-Tamer M, Gemignani ML, Heerdt AS, Sclafani LM, Sacchini V, Cody HS 3rd, Patil S, Morrow M. How often does neoadjuvant chemotherapy avoid axillary dissection in patients with histologically confirmed nodal metastases? Results of a prospective study. Ann Surg Oncol. 2016;23:3467–3474. doi: 10.1245/s10434-016-5246-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang EH, Tucker SL, Strom EA, McNeese MD, Kuerer HM, Buzdar AU, Valero V, Perkins GH, Schechter NR, Hunt KK, Sahin AA, Hortobagyi GN, Buchholz TA. Postmastectomy radiation improves local-regional control and survival for selected patients with locally advanced breast cancer treated with neoadjuvant chemotherapy and mastectomy. J. Clin. Oncol. 2004;22:4691–4699. doi: 10.1200/JCO.2004.11.129. [DOI] [PubMed] [Google Scholar]
- 7.Abdel-Wahab M, Wolfson A, Raub W, Mies C, Brandon A, Morrell L, Lee Y, Ling S, Markoe A. The importance of postoperative radiation therapy in multimodality management of locally advanced breast cancer: a phase II trial of neoadjuvant MVAC, surgery, and radiation. Int J Radiat Oncol Biol Phys. 1998;40:875–880. doi: 10.1016/s0360-3016(97)00897-3. [DOI] [PubMed] [Google Scholar]
- 8.Buchholz TA, Tucker SL, Masullo L, Kuerer HM, Erwin J, Salas J, Frye D, Strom EA, McNeese MD, Perkins G, Katz A, Singletary SE, Hunt KK, Buzdar AU, Hortobagyi GN. Predictors of local-regional recurrence after neoadjuvant chemotherapy and mastectomy without radiation. J. Clin. Oncol. 2002;20:17–23. doi: 10.1200/JCO.2002.20.1.17. [DOI] [PubMed] [Google Scholar]
- 9.Ring A, Webb A, Ashley S, Allum WH, Ebbs S, Gui G, Sacks NP, Walsh G, Smith IE. Is surgery necessary after complete clinical remission following neoadjuvant chemotherapy for early breast cancer? J. Clin. Oncol. 2003;21:4540–4545. doi: 10.1200/JCO.2003.05.208. [DOI] [PubMed] [Google Scholar]
- 10.Panades M, Olivotto IA, Speers CH, Shenkier T, Olivotto TA, Weir L, Allan SJ, Truong PT. Evolving treatment strategies for inflammatory breast cancer: a population-based survival analysis. J. Clin. Oncol. 2005;23:1941–1950. doi: 10.1200/JCO.2005.06.233. [DOI] [PubMed] [Google Scholar]
- 11.McGuire SE, Gonzalez-Angulo AM, Huang EH, Tucker SL, Kau SW, Yu TK, Strom EA, Oh JL, Woodward WA, Tereffe W, Hunt KK, Kuerer HM, Sahin AA, Hortobagyi GN, Buchholz TA. Postmastectomy radiation improves the outcome of patients with locally advanced breast cancer who achieve a pathologic complete response to neoadjuvant chemotherapy. Int J Radiat Oncol Biol Phys. 2007;68:1004–1009. doi: 10.1016/j.ijrobp.2007.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pierce LJ, Lippman M, Ben-Baruch N, Swain S, O’Shaughnessy J, Bader JL, Danforth D, Venzon D, Cowan KH. The effect of systemic therapy on local-regional control in locally advanced breast cancer. Int J Radiat Oncol Biol Phys. 1992;23:949–960. doi: 10.1016/0360-3016(92)90899-s. [DOI] [PubMed] [Google Scholar]
- 13.Fayanju OM, Ren Y, Thomas SM, Greenup RA, Plichta JK, Rosenberger LH, Tamirisa N, Force J, Boughey JC, Hyslop T, Hwang ES. The clinical significance of breast-only and node-only Pathologic Complete Response (pCR) After Neoadjuvant Chemotherapy (NACT): a review of 20,000 breast cancer patients in the National Cancer Data Base (NCDB) Ann Surg. 2018;268:591–601. doi: 10.1097/SLA.0000000000002953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P, Swain SM, Prowell T, Loibl S, Wickerham DL, Bogaerts J, Baselga J, Perou C, Blumenthal G, Blohmer J, Mamounas EP, Bergh J, Semiglazov V, Justice R, Eidtmann H, Paik S, Piccart M, Sridhara R, Fasching PA, Slaets L, Tang S, Gerber B, Geyer CE Jr, Pazdur R, Ditsch N, Rastogi P, Eiermann W, von Minckwitz G. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 15.Cortazar P, Geyer CE Jr. Pathological complete response in neoadjuvant treatment of breast cancer. Ann Surg Oncol. 2015;22:1441–1446. doi: 10.1245/s10434-015-4404-8. [DOI] [PubMed] [Google Scholar]
- 16.Symmans WF, Wei C, Gould R, Yu X, Zhang Y, Liu M, Walls A, Bousamra A, Ramineni M, Sinn B, Hunt K, Buchholz TA, Valero V, Buzdar AU, Yang W, Brewster AM, Moulder S, Pusztai L, Hatzis C, Hortobagyi GN. Long-term prognostic risk after neoadjuvant chemotherapy associated with residual cancer burden and breast cancer subtype. J. Clin. Oncol. 2017;35:1049–1060. doi: 10.1200/JCO.2015.63.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luen SJ, Salgado R, Dieci MV, Vingiani A, Curigliano G, Gould RE, Castaneda C, D’Alfonso T, Sanchez J, Cheng E, Andreopoulou E, Castillo M, Adams S, Demaria S, Symmans WF, Michiels S, Loi S. Prognostic implications of residual disease tumor-infiltrating lymphocytes and residual cancer burden in triple-negative breast cancer patients after neoadjuvant chemotherapy. Ann Oncol. 2019;30:236–242. doi: 10.1093/annonc/mdy547. [DOI] [PubMed] [Google Scholar]
- 18.Chang CL, Tsai HC, Lin WC, Chang JH, Hsu HL, Chow JM, Yuan KS, Wu ATH, Wu SY. Dose escalation intensity-modulated radiotherapy-based concurrent chemoradiotherapy is effective for advanced-stage thoracic esophageal squamous cell carcinoma. Radiother Oncol. 2017;125:73–79. doi: 10.1016/j.radonc.2017.08.025. [DOI] [PubMed] [Google Scholar]
- 19.Chang WW, Hsiao PK, Qin L, Chang CL, Chow JM, Wu SY. Treatment outcomes for unresectable intrahepatic cholangiocarcinoma: nationwide, population-based, cohort study based on propensity score matching with the Mahalanobis metric. Radiother Oncol. 2018;129:284–292. doi: 10.1016/j.radonc.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Chen TM, Lin KC, Yuan KS, Chang CL, Chow JM, Wu SY. Treatment of advanced nasopharyngeal cancer using low- or high-dose concurrent chemoradiotherapy with intensity-modulated radiotherapy: a propensity score-matched, nationwide, population-based cohort study. Radiother Oncol. 2018;129:23–29. doi: 10.1016/j.radonc.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Lin YK, Hsieh MC, Chang CL, Chow JM, Yuan KS, Wu ATH, Wu SY. Intensity-modulated radiotherapy with systemic chemotherapy improves survival in patients with nonmetastatic unresectable pancreatic adenocarcinoma: a propensity score-matched, nationwide, population-based cohort study. Radiother Oncol. 2018;129:326–332. doi: 10.1016/j.radonc.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Lin YK, Hsieh MC, Wang WW, Lin YC, Chang WW, Chang CL, Cheng YF, Wu SY. Outcomes of adjuvant treatments for resectable intrahepatic cholangiocarcinoma: chemotherapy alone, sequential chemoradiotherapy, or concurrent chemoradiotherapy. Radiother Oncol. 2018;128:575–583. doi: 10.1016/j.radonc.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 23.Yen YC, Hsu HL, Chang JH, Lin WC, Chang YC, Chang CL, Chow JM, Yuan KS, Wu ATH, Wu SY. Efficacy of thoracic radiotherapy in patients with stage IIIB-IV epidermal growth factor receptor-mutant lung adenocarcinomas who received and responded to tyrosine kinase inhibitor treatment. Radiother Oncol. 2018;129:52–60. doi: 10.1016/j.radonc.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 24.Lin WC, Ding YF, Hsu HL, Chang JH, Yuan KS, Wu ATH, Chow JM, Chang CL, Chen SU, Wu SY. Value and application of trimodality therapy or definitive concurrent chemoradiotherapy in thoracic esophageal squamous cell carcinoma. Cancer. 2017;123:3904–3915. doi: 10.1002/cncr.30823. [DOI] [PubMed] [Google Scholar]
- 25.Yen YC, Chang JH, Lin WC, Chiou JF, Chang YC, Chang CL, Hsu HL, Chow JM, Yuan KS, Wu ATH, Wu SY. Effectiveness of esophagectomy in patients with thoracic esophageal squamous cell carcinoma receiving definitive radiotherapy or concurrent chemoradiotherapy through intensity-modulated radiation therapy techniques. Cancer. 2017;123:2043–2053. doi: 10.1002/cncr.30565. [DOI] [PubMed] [Google Scholar]
- 26.Wu SY, Fang SC, Shih HJ, Wen YC, Shao YJ. Mortality associated with statins in men with advanced prostate cancer treated with androgen deprivation therapy. Eur J Cancer. 2019;112:109–117. doi: 10.1016/j.ejca.2018.11.032. [DOI] [PubMed] [Google Scholar]
- 27.Bahreini F, Soltanian AR, Mehdipour P. A meta-analysis on concordance between immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) to detect HER2 gene overexpression in breast cancer. Breast Cancer. 2015;22:615–625. doi: 10.1007/s12282-014-0528-0. [DOI] [PubMed] [Google Scholar]
- 28.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fehrenbacher L, Cecchini RS, Geyer CE Jr, Rastogi P, Costantino JP, Atkins JN, Crown JP, Polikoff J, Boileau JF, Provencher L, Stokoe C, Moore TD, Robidoux A, Flynn PJ, Borges VF, Albain KS, Swain SM, Paik S, Mamounas EP, Wolmark N. NSABP B-47/NRG oncology phase III randomized trial comparing adjuvant chemotherapy with or without trastuzumab in high-risk invasive breast cancer negative for HER2 by FISH and with IHC 1+ or 2. J. Clin. Oncol. 2020;38:444–453. doi: 10.1200/JCO.19.01455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 31.Chen JH, Yen YC, Yang HC, Liu SH, Yuan SP, Wu LL, Lee FP, Lin KC, Lai MT, Wu CC, Chen TM, Chang CL, Chow JM, Ding YF, Wu SY. Curative-intent aggressive treatment improves survival in elderly patients with locally advanced head and neck squamous cell carcinoma and high comorbidity index. Medicine (Baltimore) 2016;95:e3268. doi: 10.1097/MD.0000000000003268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Uden DJP, van Maaren MC, Bult P, Strobbe LJA, van der Hoeven JJM, Blanken-Peeters CFJM, Siesling S, de Wilt JHW. Pathologic complete response and overall survival in breast cancer subtypes in stage III inflammatory breast cancer. Breast Cancer Res Treat. 2019;176:217–226. doi: 10.1007/s10549-019-05219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, Assad L, Poniecka A, Hennessy B, Green M, Buzdar AU, Singletary SE, Hortobagyi GN, Pusztai L. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J. Clin. Oncol. 2007;25:4414–4422. doi: 10.1200/JCO.2007.10.6823. [DOI] [PubMed] [Google Scholar]
- 34.Land LH, Dalton SO, Jensen MB, Ewertz M. Impact of comorbidity on mortality: a cohort study of 62,591 Danish women diagnosed with early breast cancer, 1990-2008. Breast Cancer Res Treat. 2012;131:1013–1020. doi: 10.1007/s10549-011-1819-1. [DOI] [PubMed] [Google Scholar]
- 35.Onitilo AA, Engel JM, Greenlee RT, Mukesh BN. Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin Med Res. 2009;7:4–13. doi: 10.3121/cmr.2009.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Medina-Franco H, Vasconez LO, Fix RJ, Heslin MJ, Beenken SW, Bland KI, Urist MM. Factors associated with local recurrence after skin-sparing mastectomy and immediate breast reconstruction for invasive breast cancer. Ann Surg. 2002;235:814–819. doi: 10.1097/00000658-200206000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schoppmann SF, Bayer G, Aumayr K, Taucher S, Geleff S, Rudas M, Kubista E, Hausmaninger H, Samonigg H, Gnant M, Jakesz R, Horvat R Austrian Breast and Colorectal Cancer Study Group. Prognostic value of lymphangiogenesis and lymphovascular invasion in invasive breast cancer. Ann Surg. 2004;240:306–312. doi: 10.1097/01.sla.0000133355.48672.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elston CW. The assessment of histological differentiation in breast cancer. Aust N Z J Surg. 1984;54:11–15. doi: 10.1111/j.1445-2197.1984.tb06677.x. [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez-Angulo AM, Litton JK, Broglio KR, Meric-Bernstam F, Rakkhit R, Cardoso F, Peintinger F, Hanrahan EO, Sahin A, Guray M, Larsimont D, Feoli F, Stranzl H, Buchholz TA, Valero V, Theriault R, Piccart-Gebhart M, Ravdin PM, Berry DA, Hortobagyi GN. High risk of recurrence for patients with breast cancer who have human epidermal growth factor receptor 2-positive, node-negative tumors 1 cm or smaller. J. Clin. Oncol. 2009;27:5700–5706. doi: 10.1200/JCO.2009.23.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duchnowska R, Dziadziuszko R, Czartoryska-Arlukowicz B, Radecka B, Szostakiewicz B, Sosinska-Mielcarek K, Karpinska A, Staroslawska E, Kubiatowski T, Szczylik C. Risk factors for brain relapse in HER2-positive metastatic breast cancer patients. Breast Cancer Res Treat. 2009;117:297–303. doi: 10.1007/s10549-008-0275-z. [DOI] [PubMed] [Google Scholar]
- 41.Zaheed M, Wilcken N, Willson ML, O’Connell DL, Goodwin A. Sequencing of anthracyclines and taxanes in neoadjuvant and adjuvant therapy for early breast cancer. Cochrane Database Syst Rev. 2019;2:CD012873. doi: 10.1002/14651858.CD012873.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pilewskie M, Morrow M. Axillary nodal management following neoadjuvant chemotherapy: a review. JAMA Oncol. 2017;3:549–555. doi: 10.1001/jamaoncol.2016.4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liedtke C, Mazouni C, Hess KR, Andre F, Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B, Green M, Cristofanilli M, Hortobagyi GN, Pusztai L. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J. Clin. Oncol. 2008;26:1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 44.von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, Gerber B, Eiermann W, Hilfrich J, Huober J, Jackisch C, Kaufmann M, Konecny GE, Denkert C, Nekljudova V, Mehta K, Loibl S. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J. Clin. Oncol. 2012;30:1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 45.Spring LM, Fell G, Arfe A, Sharma C, Greenup R, Reynolds KL, Smith BL, Alexander B, Moy B, Isakoff SJ, Parmigiani G, Trippa L, Bardia A. Pathologic complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: a comprehensive meta-analysis. Clin Cancer Res. 2020;26:2838–2848. doi: 10.1158/1078-0432.CCR-19-3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hahnen E, Lederer B, Hauke J, Loibl S, Krober S, Schneeweiss A, Denkert C, Fasching PA, Blohmer JU, Jackisch C, Paepke S, Gerber B, Kummel S, Schem C, Neidhardt G, Huober J, Rhiem K, Costa S, Altmuller J, Hanusch C, Thiele H, Muller V, Nurnberg P, Karn T, Nekljudova V, Untch M, von Minckwitz G, Schmutzler RK. Germline mutation status, pathological complete response, and disease-free survival in triple-negative breast cancer: secondary analysis of the GeparSixto randomized clinical trial. JAMA Oncol. 2017;3:1378–1385. doi: 10.1001/jamaoncol.2017.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loibl S, Weber KE, Timms KM, Elkin EP, Hahnen E, Fasching PA, Lederer B, Denkert C, Schneeweiss A, Braun S, Salat CT, Rezai M, Blohmer JU, Zahm DM, Jackisch C, Gerber B, Klare P, Kummel S, Schem C, Paepke S, Schmutzler R, Rhiem K, Penn S, Reid J, Nekljudova V, Hartman AR, von Minckwitz G, Untch M. Survival analysis of carboplatin added to an anthracycline/taxane-based neoadjuvant chemotherapy and HRD score as predictor of response-final results from GeparSixto. Ann Oncol. 2018;29:2341–2347. doi: 10.1093/annonc/mdy460. [DOI] [PubMed] [Google Scholar]
- 48.Liu J, Mao K, Jiang S, Jiang W, Chen K, Kim BY, Liu Q, Jacobs LK. The role of postmastectomy radiotherapy in clinically node-positive, stage II-III breast cancer patients with pathological negative nodes after neoadjuvant chemotherapy: an analysis from the NCDB. Oncotarget. 2016;7:24848–24859. doi: 10.18632/oncotarget.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rusthoven CG, Rabinovitch RA, Jones BL, Koshy M, Amini A, Yeh N, Jackson MW, Fisher CM. The impact of postmastectomy and regional nodal radiation after neoadjuvant chemotherapy for clinically lymph node-positive breast cancer: a National Cancer Database (NCDB) analysis. Ann Oncol. 2016;27:818–827. doi: 10.1093/annonc/mdw046. [DOI] [PubMed] [Google Scholar]
- 50.Colzani E, Johansson AL, Liljegren A, Foukakis T, Clements M, Adolfsson J, Hall P, Czene K. Time-dependent risk of developing distant metastasis in breast cancer patients according to treatment, age and tumour characteristics. Br J Cancer. 2014;110:1378–1384. doi: 10.1038/bjc.2014.5. [DOI] [PMC free article] [PubMed] [Google Scholar]


