Abstract
The SPARC/osteonectin, CWCV and Kazal-like domains proteoglycan 1 (SPOCK1) is a highly conserved, multi-domain proteoglycan that regulates the dynamic equilibrium of extracellular matrix (ECM). Besides, SPOCK1 is one of the key regulatory genes in the tumor ECM dynamic homeostasis process, which activates many molecular signaling pathways (such as EMT process, Wnt/β-catenin, PI3K/Akt, and mTOR/S6K signaling pathways). This activation leads to ECM remodeling and promotes cell proliferation and invasion, but inhibits cell apoptosis. Whereas there is immense information about SPOCK1’s roles in different biological settings, there is need for further studies that interrogate this protein as a potential therapeutic target in cancer.
Keywords: SPOCK1, extracellular matrix, proteoglycan, cancer, signaling pathway
Introduction
The extracellular matrix (ECM), a non-cellular component, presents in tissues and organs. The compositions of ECM are elastin, collagen, laminins, glycoproteins, fibronectin and proteoglycans. Each of the matrix components binds to each other via cell adhesion receptors to form a complex macromolecular network [1]. New evidence emerged that ECM heterogeneity is significant in tumor proliferation. The heterogeneity provides tumor cells with growth signals, helps cells evade growth inhibitors and resist cell death, as well as aid tumor angiogenesis, invasion and metastasis [2].
Matricellular proteins are composed of matrix-binding proteins and soluble cytokines that are either secreted or intracellular [3]. The SPARC/osteonectin, CWCV and Kazal-like domains proteoglycan 1 (SPOCK1), also referred as testican-1, is a member of the BM-40/SPARC/osteonectin secreted matricellular family of proteins [4]. As an ECM proteoglycan, SPOCK1 directly or indirectly regulates the ECM remodeling thus affecting tumor progression [5,6].
SPOCK1 in ECM remodeling
Structure of SPOCK1
SPOCK1 is expressed in the brain, prostate, testicle, heart, blood, and cartilage tissues [7-10]. It has been found to alter homeostasis of ECM and in turn trigger the occurrence of diseases. SPOCK1 is a multi-domain proteoglycan which consists of an N-terminus, a follistatin-like (FS) domain, an extracellular calcium-binding (EC) domain, and a C-terminal containing a thymidine (TY) domain and domain V. The domain V has two potential glycosaminoglycan (GAG) attachment sites; heparin sulfate, and chondroitin sulfate [11]. The first 17 amino acids of SPOCK1 are hydrophobic residues, which direct the protein into the extracellular space [8]. The extracellular calcium-binding domain is involved in cell adhesion (Figure 1) [12].
Figure 1.
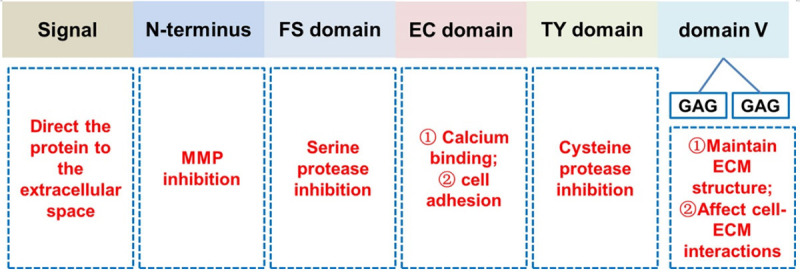
The structure of SPOCK1. SPOCK1 consists of N-terminus, a follistatin-like (FS) domain, an extracellular calcium binding (EC) domain and C-terminus containing a thyropin (TY) domain and domain V (including two potential glycosaminoglycan (GAG) attachment sites).
Belong to one of the following subgroups: (1) heparin sulfate, (2) keratan sulfate, (3) chondroitin/dermatan sulfate and (4) hyaluronic acid sulfate, GAGs are well-established regulators of tumor metastasis and are negatively charged polysaccharide structures [13]. Unlike heparin sulfate, the other GAGs are covalently attached to the proteoglycans’ core proteins. Heparin sulfate proteoglycan plays a wide range of roles from the maintenance and development of structures to the ECM organization and basement membrane via binding to matrix molecules (such as fibronectin, collagen IV, and laminin) [14]. Heparin sulfate especially acts as a co-receptor to regulate cell-cell interactions for different cell surface receptors, thus affecting cell-ECM interactions (Figure 1). Besides, the heparin sulfate proteoglycan regulates cell migration and adhesion, differentiation, proliferation, angiogenesis, and morphogenesis, as well as cytoskeletal organization and tissue repair [15]. However, data on the role of the GAGs in SPOCK1 is complex and remains scant.
Because of the modular structure, SPOCK 1 can be categorized as a regulator of the ECM and could interact with the cell surface. SPOCK1 might be stimulated by platelet-derived growth factor-BB (PDGF-BB) via the activation of PI3K/Akt/FoxM1 signaling pathway. The secreted SPOCK1 also could interact with integrin α5β1, and activate the PI3K/Akt signaling pathway which accelerates excessive deposition of ECM (Figure 2A) [16]. Another study showed that SPOCK1 expression is predominantly stromal in pancreatic cancer. Transforming growth factor-β (TGF-β) stimulates the expression of SPOCK1 which regulates the composition of the ECM [17]. In addition, proteoglycan is an important component of the ECM in brain tissues and is involved in the regulation of presynaptic and postsynaptic terminal structures during neuronal activity [18]. Aβ precursor protein (APP) modulates the SPOCK1 endocytic pathway, thus leading to the accumulation of Aβ40 and Aβ42 in the cerebrospinal fluid and brain tissue in patients with Alzheimer’s disease [19] (Figure 2B).
Figure 2.
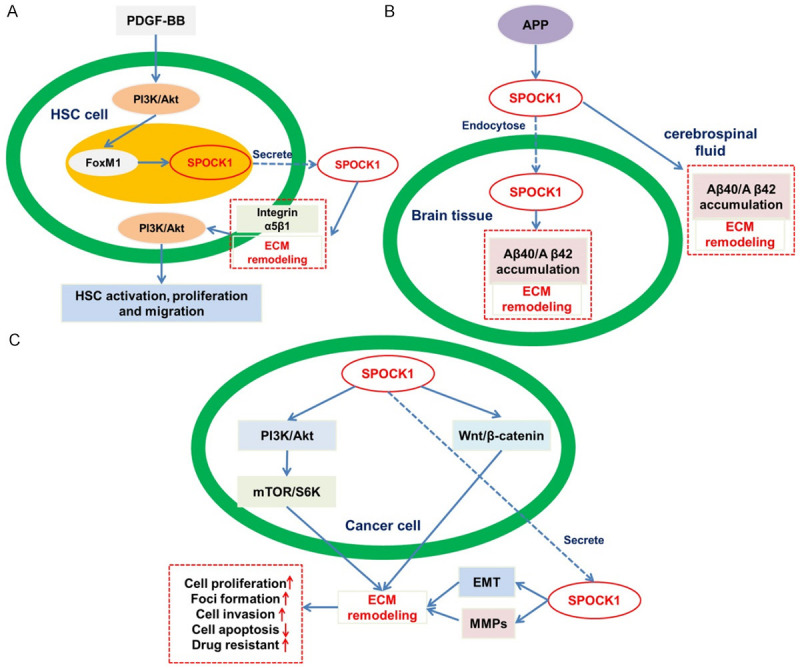
SPOCK1 and its substrate in the ECM remodeling. SPOCK1 directly or indirectly regulates several targets that play key roles in various biological processes. A. SPOCK1 promotion of liver fibrosis. B. SPOCK1 modulation of Aβ deposition. C. SPOCK1 in cancer development.
MMPs in ECM remodeling
Matrix metalloproteinases (MMPs) are a family of endopeptidases that degrade ECM. Recent studies found that ECM and basement membrane degradation plays a pivotal role in tumor metastasis and invasion [20]. The study also found that the levels of MMPs were positively correlated with the invasive ability of glioma cells [21]. Overexpression of SPOCK1 could stimulate the MMP-2 and MMP-9 expression and activity, which might degrade ECM and promote the invasion of glioma cells [22]. Degradation of the matrix facilitates the movement of inflammatory cells to infected tissue sites, thus mounting an effective inflammatory/immune response. In addition, SPOCK1 could regulate the expression and activity of MMP-2 after induction of bacterial keratitis [23]. Besides, SPOCK1 is a component of joint and growth plate cartilage. Given its role as an inhibitor of MT1-MMP-mediated pro-MMP-2 activation, it was hypothesized that it could regulate matrix turnover in cartilage [10]. SPOCK1 inhibits cell adhesion by binding to the substratum instead of the cell. Another study demonstrated that, SPOCK1 had a high density of negatively charged amino acids near its carboxyl terminus, and might block positively charged substrates in the ECM. This positively charged matrix sites might be important for cell attachment and neuron formation, as it has been shown that treatment of culture dishes with high-density cations such as polylysine enhances these cellular processes [24].
SPOCK1 in cancer development
High levels of SPOCK1 in cancer development
Studies have implicated in SPOCK1 in the regulation of ECM of tumor tissues [25]. Besides, SPOCK1 mRNA levels are upregulated in multiple cancer types, such as prostate [26,27], lung [28-30], colorectal [12,31], liver [32,33], breast [34,35], esophagus [36,37], pancreatic [17,38], gallbladder [39], head and neck [40], urothelial [41] and gastric cancers [42] as well as glioblastoma [43], osteosarcoma [44] and glioma [22] (Table 1). High levels of SPOCK1 expression are usually associated with worse prognosis, enhance metastasis, and reduce the survival rate of cancer patients [12,17,25-29,32,34,36-48]. SPOCK1 knockout mice showed a reduction in tumor growth and metastasis (Table 1).
Table 1.
Roles of SPOCK1 in cancer development
| Cancer types | SPOCK1 expression level | Overexpression of SPOCK1 | Knockdown of SPOCK1 | Studies | Ref. |
|---|---|---|---|---|---|
| Prostate cancer | High | Cell proliferation↑, Colony formation↑, Tumor growth↑ | Cell cycle arrest↑, Cell invasion↓, Cell apoptosis↑, Tumor metastasis↓ | In vitro, in vivo | [26,27] |
| Non-small cell lung cancer | High | / | Cell proliferation↓, Colony formation↓, Cell invasion↓, Osimertinib-resistant cell proliferation↓ | In vitro | [28-30] |
| Colorectal cancer | High | / | Cell proliferation↓, Colony formation↓, Cell apoptosis↑, Cell cycle arrest↑, Cell invasion↓, Tumor growth↓ | In vitro, in vivo | [12,31] |
| Hepatocellular carcinoma | High | Cell proliferation↑, Foci formation↑, Colony formation↑, Tumor growth↑, Cell apoptosis ↓ | Cell proliferation↓, Foci formation↓, Colony formation↓, Tumor growth↓, Cell apoptosis↑ | In vitro, in vivo | [32,33] |
| Breast cancer | High | Cell invasion↑ | Tumor growth↓, Tumor metastasis ↓ | In vitro, in vivo | [34,35] |
| Esophageal squamous cell carcinoma | High | Cell invasion↑ | Cell proliferation↓, Cell invasion↓ | In vitro | [36,37] |
| Pancreatic ductal adenocarcinoma | High | / | Cell proliferation↓, Cell invasion↓, Cell apoptosis↑, Cell cycle arrest↑, Tumor growth↓ | In vitro, in vivo | [17,38] |
| Gallbladder cancer | High | Cell proliferation↑, DNA replication↑, Colony formation↑, Cell invasion↑, Tumor growth↑, Tumor metastasis↑ | Cell apoptosis↑ | In vitro, in vivo | [39] |
| Glioblastoma | High | / | Cell invasion↓ | In vitro | [43] |
| Osteosarcoma | High | / | Cell proliferation↓, Tumor growth↓, Cell apoptosis↑, Cell cycle arrest↑ | In vitro, in vivo | [44] |
| Glioma | High | / | Cell proliferation↓, Colony formation↓, Cell invasion↓, Cell apoptosis↑, Cell cycle arrest↑ | In vitro | [22] |
| Head and neck squamous cell carcinoma | High | / | Cell proliferation↓, Cell invasion↓ | In vitro | [40] |
| Gastric cancer | High | Cell invasion↑, Tumor metastasis↑ | Cell invasion↓, Tumor metastasis↓ | In vitro, in vivo | [42] |
ECM remodeling
ECM maintains highly dynamic and remodels constantly to keep the differentiation and balance of tissue [49]. The process is mediated by different kinds of peptidases which degrade the macromolecules found in the ECM [50]. Moreover, given that peptidases participate in other biological functions, including receptor activation, apoptosis, morphogenesis, and angiogenesis, they show critical potential for the development of cancer [51]. During tumorigenesis, abnormalities in cellular behavior and tumor phenotype result from altered peptidases and disturbance of the balance between ECM degradation and synthesis [52]. Therefore, the direct or indirect regulation of the dynamic equilibrium of ECM by SPOCK1 reflects its role in cancer development (Figure 2C).
A number of studies have provided mechanistic insight into the relationship between SPOCK1 and ECM remodeling, and the ultimate effect on tumorigenesis [53]. MMPs-mediated degradation of the ECM and basement membrane is an important proteolytic event in cancer metastasis, especially in the process of tumor cell invasion into surrounding tissues, vascular infiltration and extravasation [53,54]. MMP-9 and MMP-2 are the primary MMPs whose expression levels are enhanced in some tumors [55,56]. Increased expression of SPOCK1 at the tumor margin may trigger ECM remodeling by stimulating the MMP-3, MMP-9, and MMP-2 causing targeted migration of tumor cells away from the tumor mass margin (Figure 2C) [22,26,32].
To find the upstream regulator of SPOCK1, Veenstra VL et al. observed that TGF-βpromoted the expression of SPOCK1 in pancreatic ductal adenocarcinoma (PDAC) cells. Functional assessment in co-cultures suggested that SPOCK1 strongly influenced the composition of the extracellular collagen matrix, consequently influencing the growth of invasive tumor cells in PDAC [17]. In addition, Li Y et al. found that chromodomain helicase/adenosine triphosphatase DNA binding protein 1-like (CHD1L) could bind to the 5’ upstream region of SPOCK1 and subsequently activate the SPOCK1 transcription [32]. Via the promotion of SPOCK1 expression, both the TGF-β and CHD1L were found to enhance ECM degradation, and tumor metastasis in various cancer types [17,29,32].
Cell cycle arrest, apoptosis and metastasis
In addition to the regulation of ECM dynamic homeostasis, SPOCK1 modulates other cancer-related cellular features including apoptosis, cell cycle progression, invasion and metastatic potential. In their studies, Zhang J et al. observed that SPOCK1 knockdown led to the downregulation of Cdc25C, Cyclin B1, and Cyclin D1 in colorectal cancer cells. Besides, SPOCK1 depletion resulted into the suppression of Bcl-2 expression, otherwise cleavages of caspase-3, Bax, and cleavages of PARP levels [12]. Li Y et al. found that SPOCK1 contributed to the anti-apoptotic effect by activating the Akt pathway followed with inhibition of the Cyt C-caspase-9-caspase-3 pathway [32]. Different from the control group, the Akt (Ser473) and phosphorylated PI3K (Tyr607) levels in SPOCK1 transfected gallbladder cancer cells were reduced, but the total protein levels were not affected. The inactivated Akt affected the Bcl-2 protein family. Therefore, compared with control cells, the subsequent cleavage of PARP, caspase-3, and caspase-9 in SPOCK1 knockout cells increased [39]. In addition, immunoblotting analysis of apoptosis-related proteins (including Bcl-xl, Bad, and Bcl-2) showed that the pro-apoptotic factor Bad in RWPE-1 cells and PC3 cells was negatively regulated by SPOCK1, while SPOCK1 positively regulated the anti-apoptotic factor Bcl-2 and Bcl-xl [26]. Knockdown of SPOCK1 in glioma cells promoted the expression of cleaved PARP, Bax, and cleaved caspase-3, while decreasing the expression of Bcl-2 [22]. Li J et al. observed that, SPOCK1 knockdown PDAC cells increased the cleavage of caspase-3, caspase-9 and PARP, compared to the control cells [38]. Interestingly, many of the processes are associated with the dynamic balance of ECM [17,29,32]. The downregulation of SPOCK1, to some extent, was related to cell cycle arrest in G0/G1 phase, lowering cell invasion and migration, as well as aggravation of cell apoptosis. On the other hand, other studies demonstrated that overexpression of SPOCK1 promotes proliferation, colony formation and tumorigenicity of multiple cancer types (Table 1).
EMT process
Epithelial-mesenchymal transition (EMT) is an important process in cancer development. The EMT leads to weaken the connection between cells and to lose cell polarity and epithelial characteristics that enhanced motor ability and invasiveness [57]. EMT plays an important role in the carcinogenic processes such as tumor invasion, intravascular infiltration and formation of micro-metastasis [58]. The EMT process is complex as it is regulated by many mediators including growth factors and hormones [59]. Briefly, EMT is initiated by EMT-inducing transcriptional factors (EMT-TFs), such as the zinc finger proteins Snail, Slug (Snail2), Twist, Zeb2, and Zeb1 [60]. Moreover, several transcription factors and signaling pathways such as epithelial markers (E-cadherin) and interstitial markers (vimentin) were shown to affect EMT [60]. Among the signaling pathways, the Wnt, TGF-β, and Notch are considered to be the key inducers of the EMT phenotype [61]. At the transcription level, a series of transcription factors, for example, Slug and Snail, can be activated by TGF-β, which subsequently affects the EMT [34]. Moreover, TGF-β interacts with the Wnt/β-catenin signaling pathway and the Notch signaling pathway to promote the EMT process. On the other hand, Wnt communicates with other signaling pathways, such as RTKs and its downstream regulators PI3K, MAPK, and PKB, to promote the progress of EMT [37]. Wnt/β-catenin pathway is associated with the expression of Snail, GSK3β, c-myc, and cyclin D1 [22]. It has been demonstrated that the Notch pathway is supportive and synergizes other oncogenic pathways to cause cell transformation and induce EMT processes. Some of the Notch target genes include mTOR, Akt, NF-κB, c-myc, VEGF, and cyclin D1 [62,63]. Other studies have shown that SPOCK1 promotes the EMT progression in multiple cancer types by upregulating the N-cadherin, Snail, Vimentin and Slug but downregulates the E-cadherin and ZO-1 [26,32-34,36-37]. Besides, the EMT process can regulate the expression of ECM remodeling-related genes [64]. Importantly, the EMT process correlates with the SPOCK1-mediated ECM remodeling (Figure 2C).
PI3K/Akt signaling pathway
Tumor can resist cell death by activating survival signal or destroying apoptosis process. The PI3K/Akt signaling pathway plays a critical role in the survival signals activation [65]. Activated Akt can phosphorylate several substrate proteins such as Bax, and the phosphorylation may suppress its activation. Inactivation of Bax is crucial in maintaining the mitochondrial membrane integrity, while its activation may activate caspase-9, caspase-3, and PARP [66]. Depletion of SPOCK1 in colorectal cancer (CRC) caused the PI3K/Akt signaling pathway inactivation. On the other hand, knockdown of SPOCK1 also induced the downregulation of Bcl-2 and upregulation of Bax, as well as cleavages of PARP and caspase-3 levels [12]. In other studies, it has been demonstrated that the PI3K/Akt signaling pathway activation might accelerate the excessive ECM deposition [16]. Li Z et al. found that melatonin potentially inhibited human nucleus pulposus cell proliferation by downregulating the expression of the ECM-degrading enzymes MMP-3/9 and upregulating the expression of ECM components such as lectin and COL2A1 to positively regulate ECM remodeling. These cascades of events were mediated by the melatonin membrane receptor MT1/2 and its downstream PI3K-Akt signaling pathway [67]. Thus, SPOCK1 might be regulating the ECM remodeling through the PI3K/Akt signaling pathway (Figure 2C).
mTOR-S6K signaling pathway
The mTOR-S6K pathway is well-conserved and participates in cancer development. Several studies revealed that mTOR, a downstream gene of Akt, plays a crucial role in promoting cell proliferation [68]. mTOR promotes the cancer cell survival through the phosphorylation of S6K. The mTOR-S6K pathway can promote the transcription of oncogenes, inhibit cell apoptosis, and enhance angiogenesis [69]. mTOR pathway dysregulation affects several cancer types [70,71]. One study showed that phosphorylated mTOR (p-mTOR) and phosphorylated S6K (p-S6K) protein were downregulated in osteosarcoma cells after the depletion of SPOCK1 [44]. Another study supported a neoteric role for glial adhesion molecules in cellular regulation by selectively activating the mTOR/S6K signaling pathway [72]. Goc A et al. observed that TGF-β stimulates ECM protein synthesis in a time- and dose-dependent manner. Besides, TGF-β treatment increased phosphorylation of Akt, mTOR and its downstream signaling partners S6K, 4E-BP1, and ribosomal S6 protein, leading to its activation. The effect of TGF-β on ECM synthesis was blunted by pretreatment with the mTOR inhibitor rapamycin. In addition, mTOR was responsible for some of the transcriptional regulation of the adhesion molecules, ECM proteins, and matrix metalloproteinases (MMPs). Under the action of TGF-β, fibroblasts synthesized main ECM proteins, for example, fibrin and collagen (type I, type II, and type V). The synthesis process was regulated by mTOR at the translation level [73]. The observations suggested that SPOCK1 might regulate the ECM remodeling through the mTOR/S6K signaling pathway (Figure 2C).
Wnt/β-catenin signaling pathway
Wnt/βnt/β-cat signaling pathway is another pathway that plays an important role in tumor biology. One study showed that miR-497-5p attenuated IL-1β-induced chondrocyte cartilage matrix degradation via the Wnt/β-catenin signaling pathway, hence it might be a potential therapeutic target for the treatment of osteoarthritis [74]. C-myc and cyclin D1 might be one of the target genes of Wnt/β-catenin signaling pathway [75,76]. In glioma, SPOCK1 knockdown significantly suppresses the Wnt and β-catenin expression. Furthermore, depletion of SPOCK1 could decrease the c-myc and cyclin D1 expression levels [22]. Lin H et al. showed that secreted Frizzled-related protein 2 (sFRP2) activates cardiac fibroblasts (CFs) partly through the classical Wnt/β-catenin signaling pathway. In parallel with these phenotypic changes, CFs upregulated MMP-1 and MMP-13 gene expression and the enzymatic activity of MMP-9 and MMP-2, thereby accelerating ECM remodeling. The results of non-CF cell types analysis revealed that the multifaceted effects of sFRP2 on glucose metabolism, growth control, and ECM regulation were mainly confined to CFs and were highly sensitive to the Wnt/β-catenin signaling pathway [77]. Therefore, SPOCK1 might regulate the dynamic homeostasis of the ECM through the Wnt/β-catenin pathway and in turn influence tumor development (Figure 2C).
Resistance to chemotherapeutic agents
Notably, SPOCK1 has also been shown to affect the resistance to chemotherapeutic agents. Data from CCK-8 assay indicated that the depletion of SPOCK1 significantly inhibited the proliferation of Osimertinib-resistant NSCLC cell lines [47]. Integrin-mediated attachment to ECM proteins has emerged as an important cue event for the transformational phenotype of cancer cells. The cross-talk between integrin and growth factor receptors plays a central role in motility, adhesion, and cell growth. This functional interaction has been linked with the integrin and tyrosine kinase inhibitor (TKI) resistance in epidermal growth factor receptors (EGFRs) [78].
The potential therapeutic role in cancer development
Given the important roles of SPOCK1 in tumorigenesis, it might be a potential therapeutic target. Apigenin, 4’,5,7-trihydroxyflavone, suppresses cancer cell invasion and migration through the downregulation of SPOCK1 [46]. On the other hand, nitroglycerin is effective in the treatment of coronary ischemic diseases, and exposure to nitroglycerin (100 μM, 48 h) downregulates the expression and activity of SPOCK1 which potentially alters atherosclerotic plaque stability [79]. In addition to exogenous inhibitors, several endogenous inhibitors have been studied. Some microRNAs (miR-193a-5p, miR-139-5p, miR-627-5p, miR-150-5p, miR-150-3p, miR-129-5p, miR-130a-3p, and miR-940) have been shown to block tumor development by inhibiting SPOCK1 expression [30,33,36,40,80]. Taken together, these results demonstrate that SPOCK1 may be a potential therapeutic target for cancer treatment.
Conclusion and future perspectives
Recent studies have elucidated multiple biological functions of SPOCK1 in tumors. SPOCK1 is one of the key regulatory genes in the tumor ECM dynamic homeostasis process, which further activates many molecular signaling pathways (such as EMT process, Wnt/β-catenin, PI3K/Akt, and mTOR/S6K signaling pathways). These cascades of events lead to ECM remodeling, which ultimately promote cell proliferation, cell invasion, but inhibit cell apoptosis. The process of how SPOCK1 contributes to cancer development, as well as other diseases, can be preferably understand that it is timely to provide new insights into the therapeutic potential harbored by this proteoglycan.
It has been demonstrated that genes in tumorigenesis are associated with specific genetic backgrounds, tumor types, tissue or organ microenvironments, as well as external stimuli and stages of tumor development. Therefore, there is need for further studies that interrogate the role of SPOCK1 and the exact mechanisms involved in the relationship between ECM dynamic homeostasis and cancer development under different pharmacological and pathophysiological conditions.
Acknowledgements
This study was supported by Joint Fund of Zhejiang Provincial Natural Science Foundation, China (Grant number LYY18H310002) and Funding of Zhejiang Pharmaceutical Association (Grant number ZYYZL01).
Disclosure of conflict of interest
None.
References
- 1.Wang M, Zhao J, Zhang L, Wei F, Lian Y, Wu Y, Gong Z, Zhang S, Zhou J, Cao K, Li X, Xiong W, Li G, Zeng Z, Guo C. Role of tumor microenvironment in tumorigenesis. J Cancer. 2017;8:761–773. doi: 10.7150/jca.17648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clause KC, Barker TH. Extracellular matrix signaling in morphogenesis and repair. Curr Opin Biotechnol. 2013;24:830–833. doi: 10.1016/j.copbio.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy-Ullrich JE, Sage EH. Revisiting the matricellular concept. Matrix Biol. 2014;37:1–14. doi: 10.1016/j.matbio.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradshaw AD, Sage EH. SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J Clin Invest. 2001;107:1049–1054. doi: 10.1172/JCI12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu T, Ouyang G. Matricellular proteins: multifaceted extracellular regulators in tumor dormancy. Protein Cell. 2014;5:249–252. doi: 10.1007/s13238-014-0023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradshaw AD. Diverse biological functions of the SPARC family of proteins. Int J Biochem Cell Biol. 2012;44:480–488. doi: 10.1016/j.biocel.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marr HS, Basalamah MA, Edgell CJ. Endothelial cell expression of testican mRNA. Endothelium. 1997;5:209–219. doi: 10.3109/10623329709053399. [DOI] [PubMed] [Google Scholar]
- 8.BaSalamah MA, Marr HS, Duncan AW, Edgell CJ. Testican in human blood. Biochem Biophys Res Commun. 2001;283:1083–1090. doi: 10.1006/bbrc.2001.4900. [DOI] [PubMed] [Google Scholar]
- 9.Edgell CJ, BaSalamah MA, Marr HS. Testican-1: a differentially expressed proteoglycan with protease inhibiting activities. Int Rev Cytol. 2004;236:101–122. doi: 10.1016/S0074-7696(04)36003-1. [DOI] [PubMed] [Google Scholar]
- 10.Hausser HJ, Decking R, Brenner RE. Testican-1, an inhibitor of pro-MMP-2 activation, is expressed in cartilage. Osteoarthritis Cartilage. 2004;12:870–877. doi: 10.1016/j.joca.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Bocock JP, Edgell CJ, Marr HS, Erickson AH. Human proteoglycan testican-1 inhibits the lysosomal cysteine protease cathepsin L. Eur J Biochem. 2003;270:4008–4015. doi: 10.1046/j.1432-1033.2003.03789.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, Zhi X, Shi S, Tao R, Chen P, Sun S, Bian L, Xu Z, Ma L. SPOCK1 is up-regulated and promotes tumor growth via the PI3K/AKT signaling pathway in colorectal cancer. Biochem Biophys Res Commun. 2017;482:870–876. doi: 10.1016/j.bbrc.2016.11.126. [DOI] [PubMed] [Google Scholar]
- 13.Knelson EH, Nee JC, Blobe GC. Heparan sulfate signaling in cancer. Trends Biochem Sci. 2014;39:277–288. doi: 10.1016/j.tibs.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitelock JM, Iozzo RV. Heparan sulfate: a complex polymer charged with biological activity. Chem Rev. 2005;105:2745–2764. doi: 10.1021/cr010213m. [DOI] [PubMed] [Google Scholar]
- 15.Bishop JR, Schuksz M, Esko JD. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature. 2007;446:1030–1037. doi: 10.1038/nature05817. [DOI] [PubMed] [Google Scholar]
- 16.Du Z, Lin Z, Wang Z, Liu D, Tian D, Xia L. SPOCK1 overexpression induced by platelet-derived growth factor-BB promotes hepatic stellate cell activation and liver fibrosis through the integrin alpha5beta1/PI3K/Akt signaling pathway. Lab Invest. 2020;100:1042–1056. doi: 10.1038/s41374-020-0425-4. [DOI] [PubMed] [Google Scholar]
- 17.Veenstra VL, Damhofer H, Waasdorp C, Steins A, Kocher HM, Medema JP, van Laarhoven HW, Bijlsma MF. Stromal SPOCK1 supports invasive pancreatic cancer growth. Mol Oncol. 2017;11:1050–1064. doi: 10.1002/1878-0261.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fawcett JW. The extracellular matrix in plasticity and regeneration after CNS injury and neurodegenerative disease. Prog Brain Res. 2015;218:213–226. doi: 10.1016/bs.pbr.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Barrera-Ocampo A, Arlt S, Matschke J, Hartmann U, Puig B, Ferrer I, Zurbig P, Glatzel M, Sepulveda-Falla D, Jahn H. Amyloid-beta precursor protein modulates the sorting of testican-1 and contributes to its accumulation in brain tissue and cerebrospinal fluid from patients with alzheimer disease. J Neuropathol Exp Neurol. 2016;75:903–916. doi: 10.1093/jnen/nlw065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown GT, Murray GI. Current mechanistic insights into the roles of matrix metalloproteinases in tumour invasion and metastasis. J Pathol. 2015;237:273–281. doi: 10.1002/path.4586. [DOI] [PubMed] [Google Scholar]
- 21.Sawaya RE, Yamamoto M, Gokaslan ZL, Wang SW, Mohanam S, Fuller GN, McCutcheon IE, Stetler-Stevenson WG, Nicolson GL, Rao JS. Expression and localization of 72 kDa type IV collagenase (MMP-2) in human malignant gliomas in vivo. Clin Exp Metastasis. 1996;14:35–42. doi: 10.1007/BF00157684. [DOI] [PubMed] [Google Scholar]
- 22.Yang J, Yang Q, Yu J, Li X, Yu S, Zhang X. SPOCK1 promotes the proliferation, migration and invasion of glioma cells through PI3K/AKT and Wnt/beta-catenin signaling pathways. Oncol Rep. 2016;35:3566–3576. doi: 10.3892/or.2016.4757. [DOI] [PubMed] [Google Scholar]
- 23.Berger EA, McClellan SA, Barrett RP, Hazlett LD. Testican-1 promotes resistance against Pseudomonas aeruginosa-induced keratitis through regulation of MMP-2 expression and activation. Invest Ophthalmol Vis Sci. 2011;52:5339–5346. doi: 10.1167/iovs.10-6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marr HS, Edgell CJ. Testican-1 inhibits attachment of Neuro-2a cells. Matrix Biol. 2003;22:259–266. doi: 10.1016/s0945-053x(03)00036-2. [DOI] [PubMed] [Google Scholar]
- 25.Yang C, Fischer-Keso R, Schlechter T, Strobel P, Marx A, Hofmann I. Plakophilin 1-deficient cells upregulate SPOCK1: implications for prostate cancer progression. Tumour Biol. 2015;36:9567–9577. doi: 10.1007/s13277-015-3628-3. [DOI] [PubMed] [Google Scholar]
- 26.Chen Q, Yao YT, Xu H, Chen YB, Gu M, Cai ZK, Wang Z. SPOCK1 promotes tumor growth and metastasis in human prostate cancer. Drug Des Devel Ther. 2016;10:2311–2321. doi: 10.2147/DDDT.S91321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okato A, Arai T, Kojima S, Koshizuka K, Osako Y, Idichi T, Kurozumi A, Goto Y, Kato M, Naya Y, Ichikawa T, Seki N. Dual strands of pre-miR150 (miR1505p and miR1503p) act as antitumor miRNAs targeting SPOCK1 in naive and castration-resistant prostate cancer. Int J Oncol. 2017;51:245–256. doi: 10.3892/ijo.2017.4008. [DOI] [PubMed] [Google Scholar]
- 28.Wang T, Liu X, Tian Q, Liang T, Chang P. Reduced SPOCK1 expression inhibits non-small cell lung cancer cell proliferation and migration through Wnt/beta-catenin signaling. Eur Rev Med Pharmacol Sci. 2018;22:637–644. doi: 10.26355/eurrev_201802_14288. [DOI] [PubMed] [Google Scholar]
- 29.Miao L, Wang Y, Xia H, Yao C, Cai H, Song Y. SPOCK1 is a novel transforming growth factor-beta target gene that regulates lung cancer cell epithelial-mesenchymal transition. Biochem Biophys Res Commun. 2013;440:792–797. doi: 10.1016/j.bbrc.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 30.Yu XF, Wang J, OUYang N, Guo S, Sun H, Tong J, Chen T, Li J. The role of miR-130a-3p and SPOCK1 in tobacco exposed bronchial epithelial BEAS-2B transformed cells: comparison to A549 and H1299 lung cancer cell lines. J Toxicol Environ Health A. 2019;82:862–869. doi: 10.1080/15287394.2019.1664479. [DOI] [PubMed] [Google Scholar]
- 31.Zhao P, Guan HT, Dai ZJ, Ma YG, Liu XX, Wang XJ. Knockdown of SPOCK1 inhibits the proliferation and invasion in colorectal cancer cells by suppressing the PI3K/Akt pathway. Oncol Res. 2016;24:437–445. doi: 10.3727/096504016X14685034103554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y, Chen L, Chan TH, Liu M, Kong KL, Qiu JL, Li Y, Yuan YF, Guan XY. SPOCK1 is regulated by CHD1L and blocks apoptosis and promotes HCC cell invasiveness and metastasis in mice. Gastroenterology. 2013;144:179–191. e174. doi: 10.1053/j.gastro.2012.09.042. [DOI] [PubMed] [Google Scholar]
- 33.Li P, Xiao Z, Luo J, Zhang Y, Lin L. MiR-139-5p, miR-940 and miR-193a-5p inhibit the growth of hepatocellular carcinoma by targeting SPOCK1. J Cell Mol Med. 2019;23:2475–2488. doi: 10.1111/jcmm.14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan LC, Jeng YM, Lu YT, Lien HC. SPOCK1 is a novel transforming growth factor-beta-induced myoepithelial marker that enhances invasion and correlates with poor prognosis in breast cancer. PLoS One. 2016;11:e0162933. doi: 10.1371/journal.pone.0162933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perurena N, Zandueta C, Martinez-Canarias S, Moreno H, Vicent S, Almeida AS, Guruceaga E, Gomis RR, Santisteban M, Egeblad M, Hermida J, Lecanda F. EPCR promotes breast cancer progression by altering SPOCK1/testican 1-mediated 3D growth. J Hematol Oncol. 2017;10:23. doi: 10.1186/s13045-017-0399-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Osako Y, Seki N, Koshizuka K, Okato A, Idichi T, Arai T, Omoto I, Sasaki K, Uchikado Y, Kita Y, Kurahara H, Maemura K, Natsugoe S. Regulation of SPOCK1 by dual strands of pre-miR-150 inhibit cancer cell migration and invasion in esophageal squamous cell carcinoma. J Hum Genet. 2017;62:935–944. doi: 10.1038/jhg.2017.69. [DOI] [PubMed] [Google Scholar]
- 37.Song X, Han P, Liu J, Wang Y, Li D, He J, Gong J, Li M, Tu W, Yan W, Liu M, Huang H, Tian D, Liao J. Up-regulation of SPOCK1 induces epithelial-mesenchymal transition and promotes migration and invasion in esophageal squamous cell carcinoma. J Mol Histol. 2015;46:347–356. doi: 10.1007/s10735-015-9627-2. [DOI] [PubMed] [Google Scholar]
- 38.Li J, Ke J, Fang J, Chen JP. A potential prognostic marker and therapeutic target: SPOCK1 promotes the proliferation, metastasis, and apoptosis of pancreatic ductal adenocarcinoma cells. J Cell Biochem. 2020;121:743–754. doi: 10.1002/jcb.29320. [DOI] [PubMed] [Google Scholar]
- 39.Shu YJ, Weng H, Ye YY, Hu YP, Bao RF, Cao Y, Wang XA, Zhang F, Xiang SS, Li HF, Wu XS, Li ML, Jiang L, Lu W, Han BS, Jie ZG, Liu YB. SPOCK1 as a potential cancer prognostic marker promotes the proliferation and metastasis of gallbladder cancer cells by activating the PI3K/AKT pathway. Mol Cancer. 2015;14:12. doi: 10.1186/s12943-014-0276-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koshizuka K, Hanazawa T, Kikkawa N, Katada K, Okato A, Arai T, Idichi T, Osako Y, Okamoto Y, Seki N. Antitumor miR-150-5p and miR-150-3p inhibit cancer cell aggressiveness by targeting SPOCK1 in head and neck squamous cell carcinoma. Auris Nasus Larynx. 2018;45:854–865. doi: 10.1016/j.anl.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 41.Ma LJ, Wu WJ, Wang YH, Wu TF, Liang PI, Chang IW, He HL, Li CF. SPOCK1 overexpression confers a poor prognosis in urothelial carcinoma. J Cancer. 2016;7:467–476. doi: 10.7150/jca.13625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen D, Zhou H, Liu G, Zhao Y, Cao G, Liu Q. SPOCK1 promotes the invasion and metastasis of gastric cancer through Slug-induced epithelial-mesenchymal transition. J Cell Mol Med. 2018;22:797–807. doi: 10.1111/jcmm.13357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu F, Li G, Gao J, Sun Y, Liu P, Gao H, Li P, Lei T, Chen Y, Cheng Y, Zhai X, Sayari AJ, Huang H, Mu Q. SPOCK1 is upregulated in recurrent glioblastoma and contributes to metastasis and Temozolomide resistance. Cell Prolif. 2016;49:195–206. doi: 10.1111/cpr.12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Wang W, Qiu E. SPOCK1 promotes the growth of Osteosarcoma cells through mTOR-S6K signaling pathway. Biomed Pharmacother. 2017;95:564–570. doi: 10.1016/j.biopha.2017.08.116. [DOI] [PubMed] [Google Scholar]
- 45.Chen ML, Ho CJ, Yeh CM, Chen SL, Sung WW, Wang SC, Chen CJ. High SPOCK1 expression is associated with advanced stage, T value, and gleason grade in prostate cancer. Medicina (Kaunas) 2019;55:343. doi: 10.3390/medicina55070343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chien MH, Lin YW, Wen YC, Yang YC, Hsiao M, Chang JL, Huang HC, Lee WJ. Targeting the SPOCK1-snail/slug axis-mediated epithelial-to-mesenchymal transition by apigenin contributes to repression of prostate cancer metastasis. J Exp Clin Cancer Res. 2019;38:246. doi: 10.1186/s13046-019-1247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao Y, Yu M, Ma M, Zhuang Y, Qiu X, Zhao Q, Dai J, Cai H, Yan X. SPOCK1 contributes to the third-generation EGFR tyrosine kinase inhibitors resistance in lung cancer. J Cell Biochem. 2019;120:12566–12573. doi: 10.1002/jcb.28523. [DOI] [PubMed] [Google Scholar]
- 48.Yan L, Sun K, Liu Y, Liang J, Cai K, Gui J. MiR-129-5p influences the progression of gastric cancer cells through interacting with SPOCK1. Tumour Biol. 2017;39:1010428317706916. doi: 10.1177/1010428317706916. [DOI] [PubMed] [Google Scholar]
- 49.Egeblad M, Rasch MG, Weaver VM. Dynamic interplay between the collagen scaffold and tumor evolution. Curr Opin Cell Biol. 2010;22:697–706. doi: 10.1016/j.ceb.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol. 2004;16:558–564. doi: 10.1016/j.ceb.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bode W, Huber R. Proteinase-protein inhibitor interaction. Biomed Biochim Acta. 1991;50:437–446. [PubMed] [Google Scholar]
- 52.Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123:4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 54.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 55.Rao JS, Steck PA, Mohanam S, Stetler-Stevenson WG, Liotta LA, Sawaya R. Elevated levels of M(r) 92,000 type IV collagenase in human brain tumors. Cancer Res. 1993;53:2208–2211. [PubMed] [Google Scholar]
- 56.Forsyth PA, Wong H, Laing TD, Rewcastle NB, Morris DG, Muzik H, Leco KJ, Johnston RN, Brasher PM, Sutherland G, Edwards DR. Gelatinase-A (MMP-2), gelatinase-B (MMP-9) and membrane type matrix metalloproteinase-1 (MT1-MMP) are involved in different aspects of the pathophysiology of malignant gliomas. Br J Cancer. 1999;79:1828–1835. doi: 10.1038/sj.bjc.6990291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim HP, Han SW, Song SH, Jeong EG, Lee MY, Hwang D, Im SA, Bang YJ, Kim TY. Testican-1-mediated epithelial-mesenchymal transition signaling confers acquired resistance to lapatinib in HER2-positive gastric cancer. Oncogene. 2014;33:3334–3341. doi: 10.1038/onc.2013.285. [DOI] [PubMed] [Google Scholar]
- 58.Thompson EW, Newgreen DF, Tarin D. Carcinoma invasion and metastasis: a role for epithelial-mesenchymal transition? Cancer Res. 2005;65:5991–5995. doi: 10.1158/0008-5472.CAN-05-0616. discussion 5995. [DOI] [PubMed] [Google Scholar]
- 59.Stefania D, Vergara D. The many-faced program of epithelial-mesenchymal transition: a system biology-based view. Front Oncol. 2017;7:274. doi: 10.3389/fonc.2017.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simeone P, Trerotola M, Franck J, Cardon T, Marchisio M, Fournier I, Salzet M, Maffia M, Vergara D. The multiverse nature of epithelial to mesenchymal transition. Semin Cancer Biol. 2019;58:1–10. doi: 10.1016/j.semcancer.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 61.Holle AW, Young JL, Spatz JP. In vitro cancer cell-ECM interactions inform in vivo cancer treatment. Adv Drug Deliv Rev. 2016;97:270–279. doi: 10.1016/j.addr.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 62.Miele L, Osborne B. Arbiter of differentiation and death: notch signaling meets apoptosis. J Cell Physiol. 1999;181:393–409. doi: 10.1002/(SICI)1097-4652(199912)181:3<393::AID-JCP3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 63.Miele L, Golde T, Osborne B. Notch signaling in cancer. Curr Mol Med. 2006;6:905–918. doi: 10.2174/156652406779010830. [DOI] [PubMed] [Google Scholar]
- 64.Peixoto P, Etcheverry A, Aubry M, Missey A, Lachat C, Perrard J, Hendrick E, Delage-Mourroux R, Mosser J, Borg C, Feugeas JP, Herfs M, Boyer-Guittaut M, Hervouet E. EMT is associated with an epigenetic signature of ECM remodeling genes. Cell Death Dis. 2019;10:205. doi: 10.1038/s41419-019-1397-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 66.Igney FH, Krammer PH. Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer. 2002;2:277–288. doi: 10.1038/nrc776. [DOI] [PubMed] [Google Scholar]
- 67.Li Z, Li X, Chen C, Chan MTV, Wu WKK, Shen J. Melatonin inhibits nucleus pulposus (NP) cell proliferation and extracellular matrix (ECM) remodeling via the melatonin membrane receptors mediated PI3K-Akt pathway. J Pineal Res. 2017;63 doi: 10.1111/jpi.12435. [DOI] [PubMed] [Google Scholar]
- 68.Vahidnezhad H, Youssefian L, Uitto J. Molecular genetics of the PI3K-AKT-mTOR pathway in genodermatoses: diagnostic implications and treatment opportunities. J Invest Dermatol. 2016;136:15–23. doi: 10.1038/JID.2015.331. [DOI] [PubMed] [Google Scholar]
- 69.Wada S, Neinast M, Jang C, Ibrahim YH, Lee G, Babu A, Li J, Hoshino A, Rowe GC, Rhee J, Martina JA, Puertollano R, Blenis J, Morley M, Baur JA, Seale P, Arany Z. The tumor suppressor FLCN mediates an alternate mTOR pathway to regulate browning of adipose tissue. Genes Dev. 2016;30:2551–2564. doi: 10.1101/gad.287953.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lou K, Yang M, Duan E, Zhao J, Yu C, Zhang R, Zhang L, Zhang M, Xiao Z, Hu W, He Z. Rosmarinic acid stimulates liver regeneration through the mTOR pathway. Phytomedicine. 2016;23:1574–1582. doi: 10.1016/j.phymed.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 71.Riedl A, Schlederer M, Pudelko K, Stadler M, Walter S, Unterleuthner D, Unger C, Kramer N, Hengstschlager M, Kenner L, Pfeiffer D, Krupitza G, Dolznig H. Comparison of cancer cells in 2D vs 3D culture reveals differences in AKT-mTOR-S6K signaling and drug responses. J Cell Sci. 2017;130:203–218. doi: 10.1242/jcs.188102. [DOI] [PubMed] [Google Scholar]
- 72.Scheidenhelm DK, Cresswell J, Haipek CA, Fleming TP, Mercer RW, Gutmann DH. Akt-dependent cell size regulation by the adhesion molecule on glia occurs independently of phosphatidylinositol 3-kinase and Rheb signaling. Mol Cell Biol. 2005;25:3151–3162. doi: 10.1128/MCB.25.8.3151-3162.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goc A, Choudhary M, Byzova TV, Somanath PR. TGFbeta- and bleomycin-induced extracellular matrix synthesis is mediated through Akt and mammalian target of rapamycin (mTOR) J Cell Physiol. 2011;226:3004–3013. doi: 10.1002/jcp.22648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hou L, Shi H, Wang M, Liu J, Liu G. MicroRNA-497-5p attenuates IL-1beta-induced cartilage matrix degradation in chondrocytes via Wnt/beta-catenin signal pathway. Int J Clin Exp Pathol. 2019;12:3108–3118. [PMC free article] [PubMed] [Google Scholar]
- 75.Brabletz T, Herrmann K, Jung A, Faller G, Kirchner T. Expression of nuclear beta-catenin and c-myc is correlated with tumor size but not with proliferative activity of colorectal adenomas. Am J Pathol. 2000;156:865–870. doi: 10.1016/s0002-9440(10)64955-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang N, Wang Y, Hui L, Li X, Jiang X. SOX 1, contrary to SOX 2, suppresses proliferation, migration, and invasion in human laryngeal squamous cell carcinoma by inhibiting the Wnt/beta-catenin pathway. Tumour Biol. 2015;36:8625–8635. doi: 10.1007/s13277-015-3389-z. [DOI] [PubMed] [Google Scholar]
- 77.Lin H, Angeli M, Chung KJ, Ejimadu C, Rosa AR, Lee T. sFRP2 activates Wnt/beta-catenin signaling in cardiac fibroblasts: differential roles in cell growth, energy metabolism, and extracellular matrix remodeling. Am J Physiol Cell Physiol. 2016;311:C710–C719. doi: 10.1152/ajpcell.00137.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Javadi S, Zhiani M, Mousavi MA, Fathi M. Crosstalk between Epidermal Growth Factor Receptors (EGFR) and integrins in resistance to EGFR tyrosine kinase inhibitors (TKIs) in solid tumors. Eur J Cell Biol. 2020;99:151083. doi: 10.1016/j.ejcb.2020.151083. [DOI] [PubMed] [Google Scholar]
- 79.Krishnatry AS, Brazeau DA, Fung HL. Broad regulation of matrix and adhesion molecules in THP-1 human macrophages by nitroglycerin. Nitric Oxide. 2010;22:11–17. doi: 10.1016/j.niox.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fan Z, Zheng J, Xue Y, Liu X, Wang D, Yang C, Ma J, Liu L, Ruan X, Wang Z, Liu Y. NR2C2-uORF targeting UCA1-miR-627-5p-NR2C2 feedback loop to regulate the malignant behaviors of glioma cells. Cell Death Dis. 2018;9:1165. doi: 10.1038/s41419-018-1149-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


