Abstract
The PEA3 subfamily is a subgroup of the E26 transformation-specific (ETS) family. Its members, ETV1, ETV4, and ETV5, have been found to be overexpressed in multiple cancers. The deregulation of ETV1, ETV4, and ETV5 induces cell growth, invasion, and migration in various tumor cells, leading to tumor progression, metastasis, and drug resistance. Therefore, exploring drugs or therapeutic targets that target the PEA3 subfamily may contribute to the clinical treatment of tumor patients. In this review, we introduce the structures and functions of the PEA3 subfamily members, systematically review their main roles in various tumor cells, analyze their prognostic and diagnostic value, and, finally, introduce several molecular targets and therapeutic drugs targeting ETV1, ETV4, and ETV5. We conclude that targeting a series of upstream regulators and downstream target genes of the PEA3 subfamily may be an effective strategy for the treatment of ETV1/ETV4/ETV5-overexpressing tumors.
Keywords: PEA3 subfamily, transcription factor, cancer, metastasis, resistance
Introduction
The E26 transformation-specific (ETS) family is one of the largest families of signal-dependent transcription factors, consisting of 28 protein-coding genes in the human genome [1,2]. A common feature of these ETS transcription factors is that they share an identical DNA binding domain (the ETS domain) that can bind to the core DNA sequence 5’-GGA(A/T)-3’ [3]. According to previous reports, ETS transcription factors participate in tumorigenesis and developmental processes by regulating a variety of biological processes, including cell proliferation, migration, apoptosis, senescence, angiogenesis, and stem cell development [4].
Based on their similarities, including high amino acid conservation in the ETS-domains and subgroup-specific amino acid sequences, ETS transcription factors have been classified into several different subfamilies [5]. The ERG and PEA3 subfamilies have received particular research attention owing to their important roles in cancer progression and metastasis. The ERG subfamily comprises ERG, FLI1, and FEV, which are generally overexpressed in prostate cancer and Ewing’s sarcoma owing to gene fusions. The PEA3 subfamily not only participates in tumorigenesis and tumor progression in prostate cancer and Ewing sarcoma, it also has complex and diverse roles in multiple types of tumors.
The PEA3 subfamily contains three transcription factors: PEA3/E1AF/ETV4, ER81/ETV1, and ERM/ETV5. Overexpression of ETV1, ETV4, and ETV5 is found in many tumors. High levels of these transcription factors usually lead to a more aggressive tumor phenotype and drug resistance [6]. ETV1 is often deregulated in prostate cancer [7,8] and has been shown to be specifically expressed in the majority of gastrointestinal stromal tumors (GISTs) [9]. The ETV4 transcription factor is frequently activated in gastric cancer [10], lung cancer [11], hepatocellular carcinoma (HCC) [12], and colorectal cancer (CRC) [13]. ETV5 is correlated with fertility and has been implicated in the progression of endometrial cancer [14] and ovarian cancer [15]. Owing to the extensive carcinogenesis associated with the PEA3 subfamily, ETV1, ETV4, and ETV5 have been proposed as prognostic markers in tumor patients.
However, oncogenic transcription factors are considered “undruggable” by conventional methods, hence it is necessary to better understand the protein structures, precise functions, and specific mechanisms of the PEA3 subfamily in cancer. Here, we review the genes and pathways upstream and downstream of the PEA3 subfamily. As oncogenic transcription factors, ETV1, ETV4, and ETV5 induce cancer progression by regulating multiple biological processes, including epithelial-mesenchymal transition (EMT), the cell cycle, apoptosis, cell migration, the maintenance of cancer stem cell (CSC) phenotype, and chemotherapy resistance. Therefore, targeting PEA3-related genes and pathways, or directly targeting the PEA3 subfamily, may improve cancer treatment and, more importantly, may provide options to overcome drug resistance.
Structures and functions of the PEA3 subfamily
ETV1, ETV4, and ETV5 are protein-coding genes with DNA-binding transcription factor activity. In humans, ETV1 is located on chromosome 7q22, whereas ETV4 and ETV5 are located on 17q21 and 3q27, respectively. The protein structures of ETV1, ETV4, and ETV5 are more than 95% identical within the DNA-binding domain [16]. All three transcription factors share two conserved domains: the ETS domain and the PEA3-type ETS transcription factor N-terminal domain (Figure 1). The N terminus of the PEA3 transcription factors is involved in transactivation and inhibition of DNA binding [16]. The N terminus contains conserved mitogen-activated protein kinase (MAPK) phosphorylation sites, and transactivation is enhanced by the MAPK signaling pathway [17]. Both N- and C-terminal inhibitory domains that repress DNA binding were identified and shown to mediate autoinhibition of DNA binding [18]. Post-translational modifications of ETV1 and ETV4 include phosphorylation and acetylation, whereas ETV5 contains post-translational phosphorylation and ubiquitination sites (Figure 1).
Figure 1.
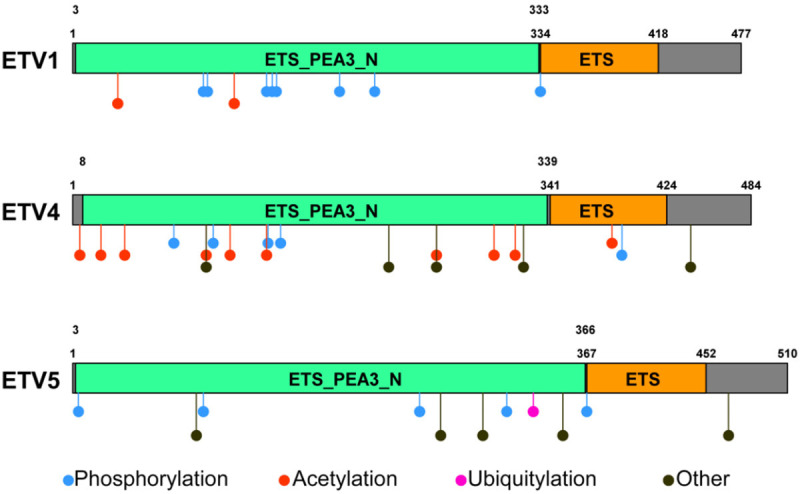
Conserved domains of the PEA3 subfamily. ETV1, ETV4, and ETV5 share an ETS domain and PEA3-type ETS transcription factor N-terminal domain. Post-translational modifications identified as phosphorylation sites, acetylation sites, and ubiquitination sites are highlighted.
Functionally, the PEA3 subfamily is correlated with motor coordination, axon guidance, neuron development, metabolism, hormonal regulation, fertility, and tumorigenesis. As the expression levels of ETV1, ETV4, and ETV5 may be different in specific tissues and organs, their functional tendencies may also differ.
ETV1 has been reported to be an important regulator in cardiac disease. For example, ETV1 expression is enriched in fast conduction tissues and was shown to be essential for rapid conduction in the heart, whereas ETV1 deficiency resulted in cardiac conduction defects and hypoplasia of the ventricular conduction system [19]. Besides, it was reported recently that ETV1 is upregulated in atrial biopsies from patients with atrial fibrillation and is responsible for arrhythmia; this highlights its regulatory role in atrial remodeling [20,21]. ETV1 is important for muscle organ development. For instance, ETV1 controls the innervation of facial muscles; therefore, its loss may induce facial synkinesis in humans [22]. ETV1 is also crucial for the development of the cerebellar circuit, functioning as a transcriptional determinant of the terminal program of cerebellar development by upregulating maturation genes and downregulating immaturation genes [23,24]. As a downstream gene of fibroblast growth factor signaling, ETV1 also has a role in coordinating the development of the Xenopus forebrain [25]. In addition, as a differentiation-related transcription factor, ETV1 is a critical gene in cementogenesis and periodontal ligament cell differentiation [26].
ETV4 and ETV5 are positively regulated by brain-derived neurotrophic factor (BDNF) in hippocampal neurons and are essential for hippocampal dendrite/synapse development [27]. Besides, overexpression of ETV4 and ETV5 was shown to promote BDNF-induced neurite outgrowth in dorsal root ganglion neurons [28]. These results indicate that ETV4 and ETV5 have key roles in normal neural axonal growth and development [29]. According to previous studies, ETV4 and ETV5 are downstream genes of RET (RET mutation usually leads to renal agenesis); therefore, they are both required for kidney development [30]. Mechanistically, ETV4 and ETV5 promote normal kidney development by mediating the formation of the ureteric bud tip domain and inducing directed cell movements in the ureteric bud tips [31,32].
It is well known that ETV4 and ETV5 are closely related to fertility in both males and females. Mechanistically, ETV5 is upregulated by sex-determining gene SRY-box9 (SOX9) and is essential for spermatogonial stem cell self-renewal. Therefore, ETV5 is indispensable for normal spermatogenesis [33,34]. ETV4 and ETV5 also have important roles in ovarian function. Jinwon et al. found that ETV4 and ETV5 were expressed in granulosa and cumulus; further studies indicated that they promote oocyte maturation and ovulation by upregulating cyclooxygenase-2 (PTGS2), a rate-limiting enzyme for prostaglandin synthesis [35]. As a target gene of Src family kinase, ETV5 was also found to promote self-renewal of female germline stem cells [36]. Besides, ETV5 mutation can lead to developmental abnormalities in mice, including postnatal growth restriction, renal asymmetry, and polydactyly [37]. ETV5 is also thought to be an obesity-related gene that is crucial for the regulation of energy balance and metabolism, for example, by regulating insulin secretion and circulating glucocorticoids [38,39].
In summary, the PEA3 subfamily is important for fertility, development, and metabolic processes. In addition, the PEA3 subfamily is actively involved in tumorigenesis, especially tumor metastasis. The present study focused on the role of the PEA3 subfamily in tumor progression, metastasis, and resistance.
Molecular mechanisms of PEA3 subfamily activation
The PEA3 subfamily can be activated by many factors. The RAS-RAF-MEK-ERK (MAPK) signaling pathway is its best known upstream positive regulator. Capicua (CIC) is a tumor suppressor downstream of the MAPK pathway. The loss of CIC usually increases the expression of PEA3 subfamily members by directly binding to their promoter regions. Some microRNAs (miRNAs) have been shown to inhibit PEA3 expression at the post-transcriptional level; alterations to these miRNAs usually lead to PEA3 subfamily dysregulation. Gene fusions of EWS/PEA3 and TMPRSS2/PEA3 are well known to increase ETV1/ETV4/ETV5 protein levels, subsequently inducing cell migration and invasion. Besides, several studies have reported that PI3K/Akt signaling could activate ETV1/ETV4/ETV5 expression. The molecular mechanisms of ETV1/ETV4/ETV5 activation are shown in Figure 2.
Figure 2.
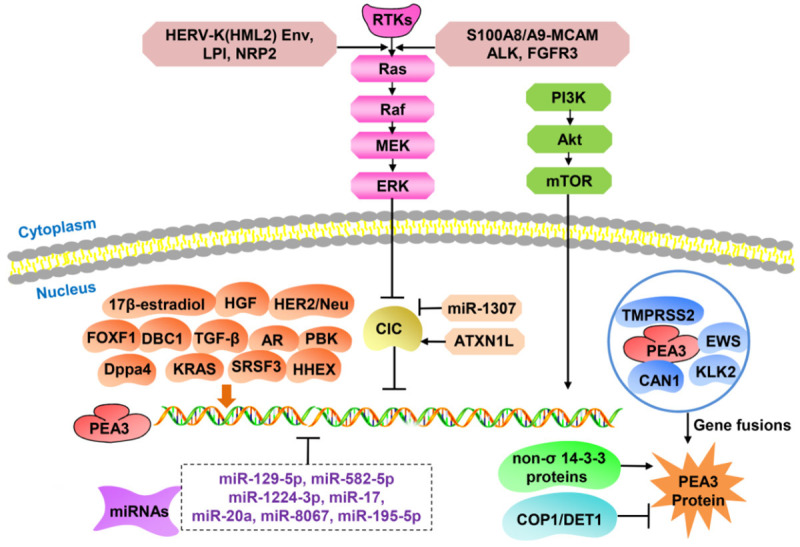
Multiple genes and signaling pathways regulate expression of the PEA3 subfamily. The PEA3 subfamily can be activated by a series of genes and pathways: activation of the MAPK and PI3K/Akt signaling pathways, loss of PEA3 repressors (CIC, COP1, and DET1), gene fusions, and miRNA-mediated post-transcriptional regulation.
Activation of the RAS-RAF-MEK-ERK pathway
The MAPK signaling pathway is often abnormally activated in tumors; therefore, many researchers have suggested targeting RAS-RAF-MEK-ERK signaling for cancer therapy [40]. Many factors can induce MAPK signaling activation; these include members of the receptor tyrosine kinase (RTK) family [41]. Here, we found that four RTKs (KIT, PDGFRA, Met, and EGFR) could upregulate the expression of the PEA3 subfamily by activating the MAPK signaling pathway.
GISTs are characterized and defined by an activating mutation of KIT or PDGFRA [42-44]. It has been reported that KIT and PDGFRA could synergistically activate the MAPK pathway and lead to significant overexpression of ETV1, a master regulator of GIST-specific transcription network [42], eventually causing liver metastasis of GIST [45]. ETV1 can in turn directly bind to the promoter region of KIT; therefore, KIT and ETV1 form a positive feedback circuit that promotes GIST development [44]. Crenolanib besylate, an inhibitor of PDGFRA, was found to partially reduce ETV1 expression by disrupting the MAPK pathway, thereby providing a therapeutic strategy for KIT-mutant GIST [46]. KIT has also been found to promote the expression of ETV4 by phosphorylating ERK and inducing migration of colorectal mucinous adenocarcinoma [47].
In gastric cancer and lung cancer, Met overexpression was shown to activate the RAS-RAF-MEK-ERK pathway; this subsequently increased ETV1/ETV4/ETV5 expression and eventually promoted cell migration and invasion by regulating matrix metalloproteinase 2 (MMP2) expression [48].
Bunda et al. recently identified a mechanism by which EGFR could increase the expression of ETV5 in two parallel ways [49]. On the one hand, EGFR activation led to ERK-mediated serine-phosphorylation, which reduced the levels of CIC, thereby disrupting the DNA combination of ETV5 and CIC [50]. On the other hand, EGFR activated c-Src kinase, resulting in tyrosine-phosphorylation of free CIC and promoting its nuclear export [49]. Therefore, combined inhibition of ERK and c-Src may be useful for the reduction of ETV5 expression in glioblastoma (GBM).
In addition to these RTKs, some other factors can activate the RAS-RAF-MEK-ERK signaling pathway. For instance, binding of melanoma cell adhesion molecule (MCAM) to an extracellular cytokine S100A8/A9 can accelerate the aggressiveness of breast cancer and melanoma. Mechanistically, S100A8/A9-MCAM binding activates ERK1/2 and mitogen-activated protein kinase kinase kinase 8 (MAP3K8), which further triggers downstream ETV4 expression, leading to tumor metastasis [50,51]. A human endogenous retrovirus-derived gene HERV-K (HML2) Env was also shown to activate the ERK1/2 pathway and increase the expression of ETV4 and ETV5, which contributed to breast oncogenesis [52]. Lysophosphatidylinositol increased ERK phosphorylation through coupling with Gq/11 and activating orphan receptor GPR55, which further activated ETV4 expression, thus driving malignant growth and metastasis of triple-negative breast cancer [53].
Anaplastic lymphoma kinase (ALK)-activating mutations upregulate ETV5 expression through the RAS/MAPK axis in neuroblastoma [54]. Activating mutations of fibroblast growth factor receptor 3 (FGFR3) induce a high level of ETV5 mediated by MAPK/ERK in bladder cancer [55]. Similarly, transmembrane and soluble neuropilin-2 (NRP2) was shown to induce ETV4 expression through the NRP2-ERK-MAPK-ETV4 axis in oesophageal squamous cell carcinoma [56].
Consequently, these RTKs and cytokines increase ERK phosphorylation and lead to the activation of MAPK signaling pathway. As an upstream regulator of ETV1/ETV4/ETV5, the MAPK pathway causes overexpression of ETV1/ETV4/ETV5 in different types of tumors.
Loss of ETV1/ETV4/ETV5 repressors
CIC is an acknowledged tumor suppressor and transcriptional repressor that is negatively regulated by RAS/MAPK signaling [57,58]. ETV1, ETV4, and ETV5 are the best known downstream targets of CIC. Thus, CIC loss usually induces overexpression of ETV1/ETV4/ETV5 [6,59].
Zhou et al. confirmed that CIC was negatively regulated by miR-1307, and identified a direct binding motif between miR-1307 and the 3’ untranslated region (3’-UTR) of CIC. This miR-1307/CIC axis further causes accumulation of ETV4 and ETV5 in ovarian cancer [60]. Likewise, ETV4 and ETV5 have been shown to be overexpressed in breast cancer [61] and multiple myeloma [62], whereas ETV1, ETV4, and ETV5 were all derepressed in pancreatic cancer owing to the loss of CIC [6].
Research in CRC showed that CIC expression was decreased in CRC tissues compared with paired normal tissues. Besides, a negative correlation was found between CIC and the PEA3 subfamily. ETV4 was the most upregulated transcription factor in the PEA3 subfamily in CRC [59]; similarly, ETV4 was the most significantly overexpressed member of the PEA3 subfamily in CIC-deficient HCC [63]. The inactivation of CIC in lung cancer also led to the derepression of ETV4 [64]. These outcomes suggest that ETV4 may play a more important role in CRC, HCC, and lung cancer.
Similar to CIC, E3 ubiquitin ligase constitutive photomorphogenetic 1 (COP1) protein is a repressor of the PEA3 subfamily [65]. Mechanistically, COP1 binds to the two motifs in conserved VP residues in the N terminus of PEA3 subfamily and leads to the ubiquitination degradation of ETV1/ETV4/ETV5 [65,66]. Therefore, the loss of COP1 blocks the binding of COP1 to ETV1/ETV4/ETV5, thereby enhancing the expression levels of ETV1/ETV4/ETV5.
In GIST and melanoma, COP1 and DET1 deficiency were shown to lead to the maintenance of ETV1 protein levels [67]. Overexpression of ETV1 induced by COP1 deficiency has also been reported in other tumor types, including renal cell carcinoma (RCC) [68], breast cancer [69], and prostate cancer [65]. As well as ETV1, ETV4 was also correlated with COP1 loss in GIST. As expected, a reduction in COP1 levels resulted in ETV4 accumulation [70].
Post-transcriptional regulation mediated by miRNAs
miRNAs are well known to mediate gene inhibition by binding to the 3’-UTRs of their target genes. Here, we found several miRNAs targeting ETV1 and ETV5. Gao et al. confirmed that ETV1 was a direct target of miR-129-5p, and that ETV1 was negatively regulated by miR-129-5p in prostate cancer [71]. This indicates that miR-129-5p could serve as a therapeutic target to inhibit prostate cancer progression and metastasis by decreasing the transcriptional activity of ETV1. A recent study found that the circ-ZNF609/miR-1224-3p/ETV1 axis was involved in lung adenocarcinoma (LAUD) progression: circ-ZNF609 acted as a sponge for miR-1224-3p to reduce miR-1224-3p expression, which subsequently increased ETV1 expression and finally led to LAUD progression [72]. Similarly, the circRNA_001160/miR-195-5p/ETV1 axis was identified as a potential therapeutic target to increase blood-tumor barrier permeability in glioma [73]. Besides, miR-582-5p was found to be a negative regulator of ETV1 in lung cancer [74]. In triple-negative breast cancer, miR-17-5p was found to inhibit cell proliferation and invasion by directly targeting ETV1 [75]. In GIST, miR-17 and miR-20a were found to reduce cell proliferation and accelerate cell apoptosis by inhibiting ETV1 transcription [76]. On the contrary, Cohen et al. found that miR-17 functioned as an oncogenic miRNA in melanoma; miR-17 promoted cell motility and cancer metastasis by inhibiting ETV1 expression, indicating that ETV1 could inhibit cell migration and motility in melanoma cells [77]. In contrast to ETV1, miRNAs targeting ETV4 and ETV5 have been rarely reported. Wang et al. found that ETV5 was negatively regulated by miR-8067 in GBM and recommended miR-8067 as a candidate therapeutic target for GBM [78].
In summary, the PEA3 subfamily is negatively regulated by several miRNAs. The dysregulation of miRNAs may alter the expression levels of ETV1, ETV4, and ETV5, leading to cancer progression and metastasis. Furthermore, these miRNAs could serve as therapeutic targets by regulating ETV1/ETV4/ETV5 expression in cancer treatment.
Gene fusions induced by chromosome rearrangement
Chromosome rearrangements related to the ETS family occur in 50-70% of prostate cancer cases [79], and represent the main cause of this cancer [2]. Such chromosome rearrangements occur when androgen-regulated gene promoters are fused to the ETS genes, leading to high levels of ETS oncoproteins [80]. Transmembrane serine protease 2 (TMPRSS2) is an androgen-regulated prostate-specific gene that is involved in the vast majority of gene fusions in prostate cancer [81]. Compared with fusion-negative patients, expression levels of PEA3 subfamily members were found to be elevated hundreds of folds in the fusion-positive patients [82].
Among the gene fusions between TMPRSS2 and the ETS family, the TMPRSS2-ERG fusion is the most frequent, occurring in approximately 50% of cases, followed by TMPRSS2-ETV1 (10%), TMPRSS2-ETV4 (< 1%), TMPRSS2-ETV5 (< 1%), and TMPRSS2-FLI1 (< 1%) [1,83-85]. Although the frequency of ETV1, ETV4, and ETV5 gene fusions are not as high as those of ERG, they are thought to be among the major driving forces of higher-grade tumors owing to the invasive potential they confer [86]. In support of this, Dedigama-Arachchige et al. observed that ETV4 expression was mainly expressed in high-grade prostate cancers [87]. Additional 5’ fusion partners of ETV1/ETV4/ETV5 have been reported; these gene fusions include SNRPN-ETV1, MALAT1-ETV1, OR51E2-ETV1, KLK2-DGKB-ETV1, UBTF-ETV4, SLC45A3-ETV4, HERVK17-ETV4, and EWS-ETV1/ETV4/ETV5 [85,88,89]. All of these gene fusions cause enrichment of ETV1/ETV4/ETV5, which contributes to the progression and metastasis of prostate cancer.
Compared with prostate cancer, chromosome rearrangement occurs more frequently in Ewing’s sarcoma (approximately 85% of cases) [90]. In contrast to the TMPRSS2-ETS gene fusions in prostate cancer, EWS is the most common fusion partner of the ETS family in Ewing’s sarcoma [91,92], with EWS-FLI1 accounting for 90% of all gene fusions, followed by ERG (5%), ETV1 (1%), ETV4 (1%), and FEV (1%) [93]. These EWS/ETS gene fusions can activate human telomerase activity through upregulating TERT (a target of EWS/ETS), causing the development of Ewing’s sarcoma and increasing its invasiveness [94].
Recently, a novel gene fusion, PTPRZ1-ETV1, was detected in gliomas. This may provide a new therapeutic target, given the carcinogenic potential of PTPRZ1 and ETV1 in other tumors [95].
Effects of PI3K/Akt signaling and other factors
In addition to the MAPK signaling pathway, PI3K/Akt signaling was reported to induce the overexpression of the members of PEA3 subfamily. In clear cell RCC, PI3K/Akt signaling activates ETV4 expression, then ETV4 promotes cell migration by directly binding to its downstream promoter FOSL1 [96]. In advanced prostate cancer, ETV4 (rather than ETV1 or ETV5) is overexpressed under the combinatorial activation of the PI3-kinase and RAS signaling pathways, indicating that ETV4 may represent an effective therapeutic target in metastatic prostate cancer [97].
Many other factors can activate PEA3 subfamily overexpression. In GIST, FOXF1 directly regulates the expression of ETV1 by binding to the ETV1 enhancer sites [98]. In GBM, ETV1-E7 inclusion can be induced by serine and arginine rich splicing factor 3 (SRSF3), a splicing factor responsible for tumorigenesis and tumor progression, resulting in increased stability of the ETV1 protein [99].
The KRAS oncogene has been reported to induce ETV4 expression, which suppressed PDCD4 expression and improved the invasiveness of pancreatic ductal adenocarcinoma cells [100]. The ETV4 transcription factor was also upregulated by stimulation with hepatocyte growth factor (HGF), a scatter factor involved in cell invasion [101], contributing to the malignancy potential of non-small-cell lung cancer (NSCLC) [102,103] and oral squamous cell carcinoma (OSCC) [104]. Depletion of acetyl-CoA carboxylase (ACC1) and ATP citrate lyase (ACLY) upregulated ETV4 levels through reduction of α-ketoglutarate, which further protected lung cancer cells from hypoxia-induced apoptosis [105]. PDZ-binding kinase (PBK) was found to induce HCC metastasis by enhancing the binding of ETV4 to its promoter uPAR [12]. In addition, ETV4 gene expression was significantly enhanced by 17β-estradiol, a potent estrogenic agent, inducing proliferation and invasiveness of cholangiocarcinoma [106]. In 3T3 fibroblasts, developmental pluripotency associated factor 4 (Dppa4) activated ETV4 expression and induced a tumor phenotype [107].
In additional sex combs-like 1 (ASXL1)-mutated myeloid leukemia, Hematopoietically Expressed Homeobox (HHEX) promoted myeloid leukemogenesis by directly upregulating ETV5 expression, indicating that ETV5 may be a critical target for ASXL1-mutated myeloid malignancies [108].
In prostate cancer, non-σ 14-3-3 proteins were also found to increase ETV1 protein levels by binding to ETV1 and protecting it from degradation [109]. Besides, androgen-activated androgen receptor could increase expression of ETV1, directly activating the Twist1 promoter to induce EMT and tumor metastasis [110].
In breast cancer, transforming growth factor β (TGF-β) could recruit ETV4 to open chromatin regions and induce EMT [111]. Deleted in breast cancer 1 (DBC1) acted as a coactivator of ETV4 to drive the progression of estrogen receptor-negative breast cancer [112]. In addition, ETV1 transcription factor could be activated by proto-oncoprotein HER2/Neu in breast cancer, endometrial cancer, and ovarian cancer, thereby promoting tumorigenesis and metastasis [113,114].
Various functions of the PEA3 subfamily in cancer
The most common function of the PEA3 subfamily is to promote cell migration and invasion, thereby contributing to tumor progression and metastasis. Here, we summarize the involvement of the PEA3 subfamily in multiple processes associated with tumor progression and metastasis. As shown in Figure 3, the PEA3 subfamily members regulate many genes related to EMT, the cell cycle, apoptosis, cell migration and invasion, the CSC phenotype, and chemotherapy resistance. Therefore, targeting the PEA3 subfamily and its downstream genes could provide effective treatments for cancer.
Figure 3.
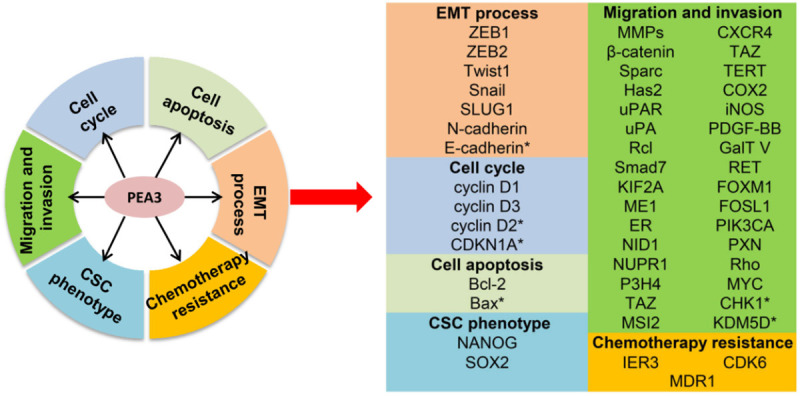
Roles of the PEA3 subfamily in cancer. The PEA3 subfamily influences cancer progression and metastasis by regulating the cell cycle, apoptosis, EMT, cell migration and invasion, development of the cancer stem cell phenotype, and chemotherapy resistance. *Genes downregulated by PEA3 subfamily.
PEA3 subfamily induces EMT
EMT is the transformation of epithelial cells to mesenchymal cells, which reduces their adhesion ability and enhances their mobility. Multiple studies have shown that EMT plays an important part in promoting migration and invasion of tumor cells [115-117]. The PEA3 subfamily can enhance the EMT process by directly or indirectly regulating EMT markers including ZEB1, ZEB2, Twist1, Snail, N-cadherin, and SLUG1.
In breast cancer, ETV4 was shown to activate the expression of ZEB1 and Snail by directly binding to the promoter regions of ZEB1 and Snail, leading to EMT and promoting metastasis [51,118]. In prostate cancer, ETV1 and ETV4 significantly increased the expression of several mesenchymal markers, including Twist1, SLUG1, ZEB1, ZEB2, and N-cadherin [110,119]. Similar results were found in CRC, where ETV4 enhanced the expression of EMT markers [13]. Besides, epithelial marker E-cadherin was reduced in oesophageal squamous cell carcinoma in response to ETV4 overexpression [56]. In gastric cancer, EMT and metastasis could be partly attributed to the overexpression of Snail induced by ETV1 [120].
The ETV5 transcription factor was shown to be closely related to papillary thyroid cancer cell growth by directly targeting Twist1 to trigger the EMT process [121]. In endometrial cancer, ETV5 promoted the invasive potential of tumor cells by upregulating ZEB1 and downregulating E-cadherin [122].
PEA3 subfamily promotes cell migration and invasion
It is well acknowledged that ETV1, ETV4, and ETV5 are involved in tumor progression and metastasis, but how they regulate cell migration and invasion has not been systematically reported. Here, we summarize some genes related to cell migration and invasion regulated by the PEA3 subfamily in multiple cancers.
In prostate cancer, ETV1 is a negative regulator of checkpoint kinase 1 (CHK1). Inhibition of CHK1 by ETV1 overexpression results in accumulation of DNA damage and promotes invasive tumorigenesis [123]. MMPs are common genes related to cell migration and invasion. ETV1 can stabilize β-catenin and directly bind to MMP1/7, leading to increased accumulation of β-catenin and MMP1/7, and inducing migration and invasion of prostate cancer cells [7,109,124]. By binding to the promoter of TAZ [125] or uPA [126], ETV4 can significantly increase TAZ/uPA expression, which contributes to tumor metastasis. As an oncogenic transcription factor, ETV4 was also found to directly bind to the 5’ and 3’ MYC enhancers [127], indicating that ETV4 may regulate the expression of some key oncogenes.
In bladder cancer, the PEA3 subfamily has been shown to be responsible for tumor invasion and metastasis by directly binding to the promoter region of P3H4 [128] and TAZ [55]. In OSCC [104], oesophageal squamous cell carcinoma [56], and oesophageal adenocarcinoma [129], ETV4 overexpression dramatically increased MMP levels and drove metastatic progression. Besides, the PEA3 subfamily can regulate many genes in other types of tumors; these are listed in Table S1.
According to these studies, the PEA3 subfamily regulates the expression of various genes related to tumor migration and invasion, implying that PEA3 subfamily members and their downstream genes could be effective therapeutic targets for metastatic tumors.
PEA3 subfamily regulates cell cycle and apoptosis
Studies have shown that ETV1, ETV4, and ETV5 contribute to cell cycle progression [76,119,128,130] and protect cells from apoptosis [10,131]. Abnormal overexpression of cyclin D1 usually contributes to cell cycle progression and cyclin D1 expression has been found to be promoted by ETV4 activation in pancreatic cancer [132,133] and GIST [70]. As expected, TMPRSS2-ETV5 gene fusion also led to high levels of cyclin D1 in prostate cancer [134]. p21 is a cyclin-dependent kinase inhibitor that plays an important part in cancer proliferation by regulating cell cycle progression [135]. Cos et al. found that ETV4 could downregulate p21 protein levels through directly binding to the CDKN1A promoter and downregulating p53 protein in ETV4 transgenic mouse model, resulting in the development of mouse prostatic intraepithelial neoplasia [136]. In breast cancer, ETV4 promoted cell proliferation by negatively regulating its downstream target gene cyclin D2 [137] and positively regulating cyclin D3 [138].
In response to overexpression of ETV1, expression of anti-apoptotic protein Bcl-2 was increased and that of pro-apoptotic protein Bax was reduced in GIST, which prevented apoptosis of tumor cells [139]. Moreover, ETV1 was overexpressed in breast cancer cells, whereas knockdown of ETV1 significantly increased cell apoptosis rate and inhibited tumor growth in mice [130].
PEA3 subfamily maintains the cancer stem cell phenotype
CSCs represent an extremely small subset of cancer cells with unlimited self-renewal and differentiation potential, which results in the malignant proliferation of tumors [140]. CSCs have been reported to be responsible for the development of drug resistance, and for cancer metastasis and recurrence [141]. As mentioned, CIC is a negative regulator of the PEA3 subfamily and a known tumor suppressor. More importantly, CIC deficiency was found to induce CSC characteristics through the derepression of ETV4/ETV5 in breast cancer [61] and oligodendroglioma [142].
NANOG and SOX2 are stem cell transcription factors that contribute to the maintenance of embryonic cell pluripotency and cancer cell stemness [143,144]. Park et al. found that NANOG was activated by the ETV4 transcription factor in human embryonic carcinoma NCCIT cells through the ETV4-ETS binding site [145]. In addition, a positive correlation between ETV4 and SOX2 was found in squamous cell carcinomas [146], indicating that ETV4 may play an important part in the maintenance of CSC characteristics.
PEA3 subfamily mediates chemotherapy resistance
Based on the results discussed above, the accumulation of the PEA3 subfamily is primarily due to MAPK pathway activation stimulated by RTKs. CIC is a downstream effector of MAPK, which is negatively regulated by the MAPK pathway. Interestingly, many studies have reported that CIC loss was closely related to drug resistance independent of the MAPK signaling pathway.
In BRAF-mutated multiple myeloma, a combination of dabrafenib and trametinib was shown to effectively block the RAS-BRAF-MEK-ERK pathway, but a subset of patients could develop drug resistance due to CIC mutation [62]. Mechanistically, mutation or downregulation of CIC increases the expression of its downstream target genes ETV1, ETV4, and ETV5, which subsequently confers MEK-BRAF inhibitor resistance. Similar mechanisms have been found in several other tumors. For example, CIC inactivation drove the development of resistance to trametinib (MAPK inhibitor) in human T-cell lymphoblastic lymphoma [58] and pancreatic cancer [6] by restoring the expression of ETV1, ETV4, and ETV5, which are necessary for the full resistance mediated by CIC loss. Besides, the activation of ETV1 contributed to resistance to gefitinib (an EGFR inhibitor) mediated by CIC deficiency in NSCLC [147]. ETV5 was found to be a potential target to overcome resistance to cetuximab (a monoclonal antibody against EGFR) in CRC [148].
Zhou et al. found that miR-1307 was involved in chemoresistance in ovarian cancer, and identified a direct binding motif between miR-1307 and the 3’-UTR of CIC, indicating that CIC is a downstream target of miR-1307 [60]. Therefore, targeting the miR-1307-CIC-ETV1/ETV4/ETV5 axis may increase drug sensitivity.
In addition to CIC loss, COP1 and DET1 loss also resulted in ETV1/ETV4/ETV5 overexpression. In GIST and melanoma, loss of COP1 and DET1 led to maintenance of ETV1 protein levels, and resistance to MAPK inhibitors (including imatinib, trametinib, and vemurafenib) eventually developed in vitro and in vivo [67].
Recent studies also found that ETV4 and ETV5 could act as biomarkers for drug response [149]. ETV4 was correlated with clinical response to MEK inhibitors in melanoma; however, ETV4 depletion did not alter sensitivity to selumetinib or trametinib [150]. Copy number alterations of ETV5 in the 3q chromosomal arm are predictive biomarkers of immune checkpoint inhibitor response in advanced cutaneous squamous cell carcinoma [151]. PEA3 subfamily could also regulate the expression of genes related to drug resistance by binding to a variety of downstream targets. CHEN et al. showed that ETV4 overexpression protected HCC cells from apoptosis and promoted resistance to sorafenib and cisplatin by directly binding to the promoter region of IER3, an oncogene related to tumor progression and drug resistance [131]. In melanoma, CDK6 induced resistance to BRAF inhibitor vemurafenib by altering the cell cycle. Further analysis revealed that the JUN family and ETV5 are key regulators of CDK6 [152]. Therefore, ETV5 is involved in resistance to BRAF inhibition in melanoma. Multi-drug resistance protein 1 (MDR1) is known to induce chemotherapy resistance in multiple cancers. In gastric cancer, ETV4 was found to activate MDR1 expression, which conferred chemotherapy resistance [153].
In summary, the loss of PEA3 repressors CIC, COP1, and DET1 could maintain the expression of ETV1/ETV4/ETV5 independently of the MAPK signaling pathway, leading to MAPK inhibitor resistance. In addition, ETV1, ETV4, and ETV5 could regulate the expression of their downstream genes related to drug resistance, thereby also mediating drug resistance (Table 1). Combined targeting of the MAPK signaling and the PEA3 subfamily may increase the sensitivity of cancers to chemotherapy drugs.
Table 1.
PEA3 subfamily mediates chemotherapy resistance in cancer
| Major gene | Cancer type | PEA3 member | Drug | Mechanism |
|---|---|---|---|---|
| CIC | BRAF-mutated multiple myeloma | ETV4/5 | Dabrafenib, trametinib | Mediated by CIC inactivation, ETV4/5 contributes to dabrafenib and trametinib resistance in BRAF-mutated multiple myeloma cells. |
| CIC | T-ALL | ETV4 | Trametinib | CIC inactivation induces chemotherapy resistance to MAPK inhibition. ETV4 is the main downstream target of CIC in human T-ALL cells. |
| CIC | Pancreatic cancer | ETV1/4/5 | Trametinib | Deletion of ATXN1L induces chemotherapy resistance by reducing CIC protein levels and restoring expression of ETV1, ETV4, and ETV5. |
| CIC | NSCLC | ETV1 | Gefitinib | CIC suppresses the effects of EGFR inhibition by partially restoring the expression of ETV1. |
| CIC | Colorectal cancer | ETV5 | Cetuximab | ETV5 is a potential target to overcome cetuximab resistance. Knockdown of ETV5 increases cetuximab sensitivity in KRAS WT cells. |
| CIC | Ovarian cancer | ETV4/5 | Paclitaxel | ETV4 and ETV5 are upregulated by the miR-1307/CIC axis, which contributes to chemotherapy resistance. |
| COP1, DET1 | GIST | ETV1/4/5 | Imatinib, PD325901 | By stabilizing ETV1/ETV4/ETV5 protein, COP1 and DET1 loss results in chemotherapy resistance in GIST and melanoma. |
| Melanoma | ETV1/4/5 | Vemurafenib | ||
| IER3 | HCC | ETV4 | Sorafenib and cisplatin | ETV4 promotes sorafenib or cisplatin resistance in HCC by upregulating IER3, an oncogene related to chemotherapy resistance. |
| CDK6 | Melanoma | ETV5 | Vemurafenib | CDK6-mediated resistance to BRAF inhibition is collaboratively regulated by JUN and ETV5. |
| MDR1 | Gastric cancer | ETV4 | Vincristine | ETV4 upregulates MDR1 expression by binding to the promoter region of MDR1. |
T-ALL, T-cell lymphoblastic lymphoma; NSCLC, non-small-cell lung cancer; EGFR, epidermal growth factor receptor; GIST, gastrointestinal stromal tumor; HCC, hepatocellular carcinoma; WT, wild type; MDR1, multi-drug resistance protein 1.
Controversy regarding the PEA3 subfamily as carcinogenic transcription factors
HER2/Neu overexpression occurs frequently in breast cancer and usually leads to an aggressive tumor phenotype. As described above, ETV1 contributes to tumorigenesis and metastasis in breast and ovarian cancers via HER2/Neu stimulation. Besides, the PEA3 subfamily promotes breast cancer cell migration and invasion by regulating the expression of its downstream genes, including hTERT, Rcl, Smad13, CXCR4, and MMPs. However, a few studies have reported that the PEA3 subfamily could inhibit breast cancer development and progression.
Hu et al. [154] and Xing et al. [155] found that ETV4 suppressed tumor growth and invasiveness by directly binding to the HER2/Neu promoter in prostate cancer, breast cancer, and ovarian cancer. These two studies indicated that HER2/Neu was negatively regulated by ETV4, thereby providing a therapeutic strategy for HER2/Neu-overexpressing tumors. However, Span et al. did not find any association between HER2/Neu and ETV4 in a clinical study [156]. Therefore, whether ETV4 could act as a prognostic target or therapeutic agent for breast cancer, prostate cancer, and ovarian cancer requires further exploration.
The antitumor effect of the PEA3 subfamily was also found in several other tumors. ETV1 is considered to be the downstream target of miR-17, an oncogene associated with cell motility. Therefore, overexpression of miR-17 could enhance melanoma cell motility by inhibiting ETV1 expression [77]. ETV4 was also found to inhibit SiHa cervical cancer cell invasion: ETV4 could activate the promotor of squamous cell carcinoma antigen, a serine proteinase inhibitor that may suppress cancer invasiveness, thus inhibiting cancer metastasis [157]. In addition, ETV4 increased the expression of pro-apoptotic protein Bax and enhanced mithramycin A-induced Huh-7 cell apoptosis, supporting ETV4 as an adjunctive therapeutic agent for mithramycin A in HCC [158].
Owing to these controversies, whether the PEA3 subfamily inhibits or promotes cancer progression in these tumor cells needs further validation.
Clinical significance of the PEA3 subfamily
The PEA3 subfamily is significantly correlated with distant metastasis of tumors and is considered to be an independent adverse prognostic factor. Of the PEA3 subfamily members, the ETV1 transcription factor was the most frequently expressed in prostate cancer and high ETV1 levels are associated with shorter time to prostate-specific antigen recurrence [159]. ETV4 overexpression is correlated with depth of invasion, lymph node metastasis, and advanced pTNM stage in colorectal cancer, which indicated that ETV4 could act as a prognostic marker in CRC progression and metastasis [160,161]. As an oncogenic transcription factor, ETV5 is an independent predictor for the prognosis of HCC patients [162]. This was also shown to be the case for triple-negative breast cancer [163,164], gastric cancer [165], lung cancer [64], OSCC [166] and GBM [78], in which ETV1, ETV4 and ETV5 were positively associated with advanced stage, depth of invasion, lymph node metastasis and recurrence, resulting in shorter overall survival, disease-free survival, and relapse-free survival of patients. Consequently, all of these studies highlight the prognostic value of ETV1, ETV4 and ETV5.
In addition to its prognostic value, the PEA3 subfamily also has a role in the clinical diagnosis of several cancers. CIC-rearranged sarcomas (which usually refers to CIC-DUX4 gene fusion sarcomas) are a small subset of primitive round-cell sarcomas, which are difficult to diagnose and classify owing to the similarity between their characteristics and those of other small round cell sarcomas [167]. It has been reported that ETV1/ETV4/ETV5 triple-positivity could be helpful for the identification of CIC-rearranged sarcomas [168]. Interestingly, ETV1/ETV4/ETV5 overexpression was more reliable and sensitive than RNA sequencing and fluorescence in situ hybridization for diagnosing CIC-rearranged sarcomas [169]. In addition, compared with other PEA3 subfamily members, ETV4 seems to be more effective for diagnosis because of its high sensitivity and specificity [170]. Detecting gene fusions can also be beneficial for diagnosis of other cancers. For instance, the TMPRSS2-ERG (53.1%) and TMPRSS2-ETV1 (6.3%) gene fusions can be used as diagnostic tools for prostate cancer [171], whereas EWS-ETV4 gene fusion can be used to diagnose Ewing’s sarcoma [172].
Molecules and drugs targeting the PEA3 subfamily
Generally, inhibition of the MAPK signaling pathway can reduce the expression of the PEA3 subfamily to some extent. However, some factors (such as CIC mutation) allow the level of the PEA3 subfamily to be maintained independently of the MAPK pathway, which finally results in chemotherapy resistance. Thus, there is an urgent need to target the PEA3 subfamily.
As discussed above, several miRNAs (miR-129-5p, miR-1224-3p, miR-195-5p, miR-582-5p, miR-17-5p, miR-17/20a, and miR-8067) have been reported to directly target ETV1 and ETV5. These miRNAs are potential therapeutic targets in ETV1/ETV5-overexpressing tumors. However, no miRNA that targets ETV4 transcription factor has yet been reported. Therefore, identifying new miRNAs that target the PEA3 subfamily in different tumor types may help in the search for therapeutic targets.
Compounds that target the ETS proteins have been investigated as potential treatments for ETS-directed cancer [173]. YK-4-279, a small-molecule inhibitor of EWS-FLI1, ERG, and ETV1, has been found to significantly inhibit tumor growth and metastasis in ETV1 fusion-positive prostate cancer; this result was validated at both cell and animal levels [174,175]. Besides, a combination of YK-4-279 and low-dose docetaxel synergistically inhibited the proliferation and migration of prostate cancer cells. Importantly, this combination allowed the systemic toxicity caused by high doses of docetaxel to be avoided [176].
BRD32048 is another inhibitor of ETV1 screened by small-molecule microarray, which has become a new therapeutic drug for ETV1-positive cancers. For example, BRD32048 inhibited the transcriptional activity of ETV1 and cell invasion by directly binding to ETV1 in ETV1-dependent prostate cancer cells [177]. Confusingly, research in neuroblastoma found that YK-4-279 significantly reduced cell growth and promoted cell apoptosis, whereas BRD32048 did not [178]. It was further shown that YK-4-279 triggered cell apoptosis through inhibiting mitotic progression instead of regulating ETV1 transcription activity, indicating that the anticancer effects of YK-4-279 are independent of the RAS-MEK/ERK-ETS(ETV1) axis in neuroblastoma cells [178].
Tamoxifen, a selective estrogen receptor modifier, was found to decrease ETV4 and ETV5 mRNA expression in benign breast tissues, suggesting that it is an effective agent to reduce breast cancer risk [179].
A recent study of GIST identified several possible ETV1 targeting drugs, among which trifluoperazine and thioridazine were considered to have strong anticancer effects, especially when combined with a MEK inhibitor [180].
Compared with synthetic drugs, phytochemicals have low toxicity and fewer side effects. Therefore, Nath et al. characterized p-anisidine, a plant compound inhibiting ETV1 expression, which shows promiscuous anticancer activity in human cervical carcinoma HeLa cells [181].
In general, YK-4-279, BRD3208, tamoxifen, trifluoperazine, thioridazine and p-anisidine are potential drugs targeting the PEA3 family, but their lack of specificity could cause off-target effects in patients [182]. In addition, oncogenic transcription factors are often considered to be undruggable; therefore, developing new drugs that target the PEA3 subfamily remains challenging [177]. The miRNAs and drugs that target the PEA3 subfamily are shown in Table S2.
Conclusions and future perspectives
Overexpression of the PEA3 subfamily has been reported in many different cancer types and is significantly correlated with the malignant potential of tumors. However, some studies have found that members of the PEA3 subfamily could inhibit cell growth and promote apoptosis, indicating that they could also act as tumor suppressors. This discrepancy may be attributed to the fact that different cell lines were employed in these studies. In addition, overexpression of PEA3 subfamily members may be accompanied by an increase in the expression of other ETS genes, some of which are tumor suppressors, such as ELF1.
By systematically analyzing the activation mechanisms and biological functions of the PEA3 subfamily, we concluded that it could be activated by a series of genes and pathways: activation of the MAPK signaling pathway and the PI3K/Akt signaling pathway; loss of PEA3 repressors (CIC, COP1, and DET1); gene fusions induced by chromosome rearrangement; and miRNA-mediated post-transcriptional regulation. Many investigations have shown that the PEA3 subfamily contributes to cancer progression and metastasis by regulating several biological processes, including cell growth, apoptosis, EMT, cell migration and invasion, cell stemness, and chemotherapy resistance.
Chemotherapy resistance is the main cause of cancer recurrence and treatment failure. We note that ETV1, ETV4, and ETV5 are located downstream of MAPK and PI3K/Akt signaling; thus, MAPK inhibitors and PI3K/Akt inhibitors are available for the treatment of ETV1/ETV4/ETV5-overexpressing tumors. However, inhibition of these two pathways alone is not enough to keep expression of the PEA3 subfamily at a low level owing to CIC/COP1/DET1 deficiency-induced PEA3 subfamily recovery, which leads to drug resistance against the MAPK inhibitor and the PI3K/Akt inhibitor. In order to reduce drug resistance, combined targeting of the MAPK signaling pathway and the PEA3 subfamily may be considered in the future. However, directly targeting transcription factors is challenging. Up to now, only YK-4-279, BRD3208, tamoxifen, trifluoperazine, thioridazine, and p-anisidine have been reported to target the PEA3 subfamily. Therefore, developing new drugs that target the PEA3 subfamily may greatly improve cancer therapy in the future.
CSCs generally correlate with tumor metastasis, resistance, and recurrence owing to their strong tumorigenic ability. Several studies have linked the PEA3 subfamily to CSC characteristics, indicating that the PEA3 subfamily may contribute to the maintenance of CSC characteristics. However, the mechanisms by which the PEA3 subfamily promotes stem cell properties is unclear, and there has been very limited research on the PEA3 subfamily and CSC. Thus, there is a need for in vitro and in vivo experiments in specific tumor types to further investigate whether the PEA3 subfamily promotes CSC phenotypes and how it regulates CSC characteristics. CSCs are now an urgent topic in cancer research, targeting these cells seems to represent an effective therapy for patients with metastatic tumors.
Several miRNAs can directly bind to the 3’-UTR of ETV1 and ETV5 to inhibit ETV1/ETV5 expression and reduce cell growth. However, no miRNA that targets ETV4 has yet been reported. ETV4 is closely related to a more aggressive tumor phenotype and is repeatedly activated in advanced and metastatic tumors. Besides, ETV4 is known to be an independent and unfavorable prognostic indicator in cancer patients. Therefore, exploring miRNAs that directly target ETV4 for use in cancer therapy should be a priority in future research.
Cis-regulatory elements (CREs), including enhancers, usually have strong regulatory effects on tumors. Recently, a mutant CRE was found to interact with the ETV1 promoter to induce overexpression of ETV1, leading to poor prognosis in CRC patients [183]. As an oncogenic transcription factor, ETV4 directly binds the 5’ and 3’ MYC enhancers and increases MYC expression in prostate cancer. Besides, ETV4 and transcriptional cofactor mediator subunit 25 (MED25) could occupy enhancers to promote the expression of ETV4 target genes in prostate cancer cells [184]. These results indicate that the PEA3 subfamily may occupy enhancer sites to regulate the expression of genes closely related to cancer progression. Oncogenic transcription factors are usually enriched in the enhancer region, especially the super-enhancer region, to regulate gene expression. Therefore, whether ETV1, ETV4, and ETV5 transcription factors occupy super-enhancer sites to mediate key oncogene expression is worth exploring.
In summary, deregulation of the PEA3 subfamily usually promotes tumor growth, progression, resistance, and metastasis by inducing EMT, regulating the expression of invasion/migration-related genes, and maintaining CSC characteristics. Therefore, targeting ETV1/ETV4/ETV5-related genes or pathways may provide effective therapeutic regimens for cancer in the future. Besides, as oncogenic transcription factors, ETV1, ETV4, and ETV5 may serve as useful biological markers for tumor diagnosis and prognosis.
Acknowledgements
The study was supported by the grant of the Hunan Provincial Department of Finance (No. 2019-93, No. 2018-92).
Disclosure of conflict of interest
None.
Abbreviations
- ACC1
Acetyl-CoA carboxylase
- ACLY
ATP citrate lyase
- ALK
Anaplastic lymphoma kinase
- ASXL1
Additional sex combs-like 1
- BDNF
Brain-derived neurotrophic factor
- CHK1
Checkpoint kinase 1
- CIC
Capicua
- COP1
Constitutive photomorphogenetic 1
- CRC
Colorectal cancer
- CRE
Cis-regulatory element
- CSC
Cancer stem cell
- DBC1
Deleted in Breast Cancer 1
- Dppa4
Developmental pluripotency associated factor 4
- EMT
Epithelial-mesenchymal transition
- ETS
E26 transformation-specific
- FGFR3
Fibroblast growth factor receptor 3
- GBM
Glioblastoma
- GIST
Gastrointestinal stromal tumor
- HCC
Hepatocellular carcinoma
- HGF
Hepatocyte growth factor
- HHEX
Hematopoietically Expressed Homeobox
- LAUD
Lung adenocarcinoma
- MAP3K8
Mitogen-activated protein kinase kinase kinase 8
- MAPK
Mitogen-activated protein kinase
- MCAM
Melanoma cell adhesion molecule
- MDR1
Multi-drug resistance protein 1
- MED25
Mediator subunit 25
- miRNA
microRNAs
- MMP
Matrix metalloproteinase
- NRP2
Neuropilin 2
- NSCLC
Non-small-cell lung cancer
- OSCC
Oral squamous cell carcinoma
- PBK
PDZ-binding kinase
- PTGS2
Cyclooxygenase-2
- RCC
Renal cell carcinoma
- RTK
Receptor tyrosine kinase
- SOX9
SRY-box9
- SRSF3
Serine and arginine rich splicing factor 3
- TGF-β
Transforming growth factor β
- TMPRSS2
Transmembrane Serine Protease 2
- UTR
Untranslated region
Supporting Information
References
- 1.Oh S, Shin S, Janknecht R. ETV1, 4 and 5: an oncogenic subfamily of ETS transcription factors. Biochim Biophys Acta. 2012;1826:1–12. doi: 10.1016/j.bbcan.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicholas TR, Strittmatter BG, Hollenhorst PC. Oncogenic ETS factors in prostate cancer. Adv Exp Med Biol. 2019;1210:409–436. doi: 10.1007/978-3-030-32656-2_18. [DOI] [PubMed] [Google Scholar]
- 3.Clark JP, Cooper CS. ETS gene fusions in prostate cancer. Nat Rev Urol. 2009;6:429–439. doi: 10.1038/nrurol.2009.127. [DOI] [PubMed] [Google Scholar]
- 4.Sementchenko VI, Watson DK. Ets target genes: past, present and future. Oncogene. 2000;19:6533–6548. doi: 10.1038/sj.onc.1204034. [DOI] [PubMed] [Google Scholar]
- 5.de Launoit Y, Chotteau-Lelievre A, Beaudoin C, Coutte L, Netzer S, Brenner C, Huvent I, Baert JL. The PEA3 group of ETS-related transcription factors. Role in breast cancer metastasis. Adv Exp Med Biol. 2000;480:107–116. doi: 10.1007/0-306-46832-8_13. [DOI] [PubMed] [Google Scholar]
- 6.Wang B, Krall EB, Aguirre AJ, Kim M, Widlund HR, Doshi MB, Sicinska E, Sulahian R, Goodale A, Cowley GS, Piccioni F, Doench JG, Root DE, Hahn WC. ATXN1L, CIC, and ETS transcription factors modulate sensitivity to mapk pathway inhibition. Cell Rep. 2017;18:1543–1557. doi: 10.1016/j.celrep.2017.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morsalin S, Yang C, Fang J, Reddy S, Kayarthodi S, Childs E, Matthews R, Rao VN, Reddy ESP. Molecular mechanism of β-Catenin signaling pathway inactivation in ETV1-Positive prostate cancers. J Pharm Sci Pharmacol. 2015;2:208–216. doi: 10.1166/jpsp.2015.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eid W, Abdel-Rehim W. Genome-wide analysis of ETV1 targets: insights into the role of ETV1 in tumor progression. J Cell Biochem. 2019;120:8983–8991. doi: 10.1002/jcb.28169. [DOI] [PubMed] [Google Scholar]
- 9.Jang BG, Lee HE, Kim WH. ETV1 mRNA is specifically expressed in gastrointestinal stromal tumors. Virchows Arch. 2015;467:393–403. doi: 10.1007/s00428-015-1813-9. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Wang Y, Liu X, Zhao A, Yang Z, Kong F, Sun L, Yu Y, Jiang L. KIF2A promotes the progression via AKT signaling pathway and is upregulated by transcription factor ETV4 in human gastric cancer. Biomed Pharmacother. 2020;125:109840. doi: 10.1016/j.biopha.2020.109840. [DOI] [PubMed] [Google Scholar]
- 11.Cheng T, Zhang Z, Cheng Y, Zhang J, Tang J, Tan Z, Liang Z, Chen T, Liu Z, Li J, Zhao J, Zhou R. ETV4 promotes proliferation and invasion of lung adenocarcinoma by transcriptionally upregulating MSI2. Biochem Biophys Res Commun. 2019;516:278–284. doi: 10.1016/j.bbrc.2019.06.115. [DOI] [PubMed] [Google Scholar]
- 12.Yang QX, Zhong S, He L, Jia XJ, Tang H, Cheng ST, Ren JH, Yu HB, Zhou L, Zhou HZ, Ren F, Hu ZW, Gong R, Huang AL, Chen J. PBK overexpression promotes metastasis of hepatocellular carcinoma via activating ETV4-uPAR signaling pathway. Cancer Lett. 2019;452:90–102. doi: 10.1016/j.canlet.2019.03.028. [DOI] [PubMed] [Google Scholar]
- 13.Mesci A, Taeb S, Huang X, Jairath R, Sivaloganathan D, Liu SK. Pea3 expression promotes the invasive and metastatic potential of colorectal carcinoma. World J Gastroenterol. 2014;20:17376–17387. doi: 10.3748/wjg.v20.i46.17376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pedrola N, Devis L, Llauradó M, Campoy I, Martinez-Garcia E, Garcia M, Muinelo-Romay L, Alonso-Alconada L, Abal M, Alameda F, Mancebo G, Carreras R, Castellví J, Cabrera S, Gil-Moreno A, Matias-Guiu X, Iovanna JL, Colas E, Reventós J, Ruiz A. Nidogen 1 and nuclear protein 1: novel targets of ETV5 transcription factor involved in endometrial cancer invasion. Clin Exp Metastasis. 2015;32:467–478. doi: 10.1007/s10585-015-9720-7. [DOI] [PubMed] [Google Scholar]
- 15.Llauradó M, Majem B, Castellví J, Cabrera S, Gil-Moreno A, Reventós J, Ruiz A. Analysis of gene expression regulated by the ETV5 transcription factor in OV90 ovarian cancer cells identifies FOXM1 overexpression in ovarian cancer. Mol Cancer Res. 2012;10:914–924. doi: 10.1158/1541-7786.MCR-11-0449. [DOI] [PubMed] [Google Scholar]
- 16.de Launoit Y, Baert JL, Chotteau A, Monte D, Defossez PA, Coutte L, Pelczar H, Leenders F. Structure-function relationships of the PEA3 group of Ets-related transcription factors. Biochem Mol Med. 1997;61:127–135. doi: 10.1006/bmme.1997.2605. [DOI] [PubMed] [Google Scholar]
- 17.Chotteau-Lelièvre A, Desbiens X, Pelczar H, Defossez PA, de Launoit Y. Differential expression patterns of the PEA3 group transcription factors through murine embryonic development. Oncogene. 1997;15:937–952. doi: 10.1038/sj.onc.1201261. [DOI] [PubMed] [Google Scholar]
- 18.Currie SL, Lau DKW, Doane JJ, Whitby FG, Okon M, McIntosh LP, Graves BJ. Structured and disordered regions cooperatively mediate DNA-binding autoinhibition of ETS factors ETV1, ETV4 and ETV5. Nucleic Acids Res. 2017;45:2223–2241. doi: 10.1093/nar/gkx068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shekhar A, Lin X, Liu FY, Zhang J, Mo H, Bastarache L, Denny JC, Cox NJ, Delmar M, Roden DM, Fishman GI, Park DS. Transcription factor ETV1 is essential for rapid conduction in the heart. J Clin Invest. 2016;126:4444–4459. doi: 10.1172/JCI87968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rommel C, Rösner S, Lother A, Barg M, Schwaderer M, Gilsbach R, Bömicke T, Schnick T, Mayer S, Doll S, Hesse M, Kretz O, Stiller B, Neumann FJ, Mann M, Krane M, Fleischmann BK, Ravens U, Hein L. The transcription factor ETV1 induces atrial remodeling and arrhythmia. Circ Res. 2018;123:550–563. doi: 10.1161/CIRCRESAHA.118.313036. [DOI] [PubMed] [Google Scholar]
- 21.Fatkin D. ETV1: a new player in atrial remodeling. Circ Res. 2018;123:515–517. doi: 10.1161/CIRCRESAHA.118.313606. [DOI] [PubMed] [Google Scholar]
- 22.Tenney AP, Livet J, Belton T, Prochazkova M, Pearson EM, Whitman MC, Kulkarni AB, Engle EC, Henderson CE. Etv1 controls the establishment of non-overlapping motor innervation of neighboring facial muscles during development. Cell Rep. 2019;29:437–452. e434. doi: 10.1016/j.celrep.2019.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abe H, Okazawa M, Nakanishi S. Gene regulation via excitation and BDNF is mediated by induction and phosphorylation of the Etv1 transcription factor in cerebellar granule cells. Proc Natl Acad Sci U S A. 2012;109:8734–8739. doi: 10.1073/pnas.1206418109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okazawa M, Abe H, Nakanishi S. The Etv1 transcription factor activity-dependently downregulates a set of genes controlling cell growth and differentiation in maturing cerebellar granule cells. Biochem Biophys Res Commun. 2016;473:1071–1077. doi: 10.1016/j.bbrc.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 25.Yang JJ, Bertolesi GE, Hehr CL, McFarlane S. Lhx2/9 and Etv1 transcription factors have complementary roles in regulating the expression of guidance genes slit1 and sema3a. Neuroscience. 2020;434:66–82. doi: 10.1016/j.neuroscience.2020.03.021. [DOI] [PubMed] [Google Scholar]
- 26.Iwata T, Mizuno N, Nagahara T, Kaneda-Ikeda E, Kajiya M, Kitagawa M, Takeda K, Yoshioka M, Yagi R, Takata T, Kurihara H. Identification of regulatory mRNA and microRNA for differentiation into cementoblasts and periodontal ligament cells. J Periodontal Res. 2020 doi: 10.1111/jre.12794. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Fontanet PA, Ríos AS, Alsina FC, Paratcha G, Ledda F. Pea3 transcription factors, Etv4 and Etv5, are required for proper hippocampal dendrite development and plasticity. Cereb Cortex. 2018;28:236–249. doi: 10.1093/cercor/bhw372. [DOI] [PubMed] [Google Scholar]
- 28.Liu D, Liu Z, Liu H, Li H, Pan X, Li Z. Brain-derived neurotrophic factor promotes vesicular glutamate transporter 3 expression and neurite outgrowth of dorsal root ganglion neurons through the activation of the transcription factors Etv4 and Etv5. Brain Res Bull. 2016;121:215–226. doi: 10.1016/j.brainresbull.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 29.Fontanet P, Irala D, Alsina FC, Paratcha G, Ledda F. Pea3 transcription factor family members Etv4 and Etv5 mediate retrograde signaling and axonal growth of DRG sensory neurons in response to NGF. J Neurosci. 2013;33:15940–15951. doi: 10.1523/JNEUROSCI.0928-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu BC, Cebrian C, Chi X, Kuure S, Kuo R, Bates CM, Arber S, Hassell J, MacNeil L, Hoshi M, Jain S, Asai N, Takahashi M, Schmidt-Ott KM, Barasch J, D’Agati V, Costantini F. Etv4 and Etv5 are required downstream of GDNF and Ret for kidney branching morphogenesis. Nat Genet. 2009;41:1295–1302. doi: 10.1038/ng.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuure S, Chi X, Lu B, Costantini F. The transcription factors Etv4 and Etv5 mediate formation of the ureteric bud tip domain during kidney development. Development. 2010;137:1975–1979. doi: 10.1242/dev.051656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riccio P, Cebrian C, Zong H, Hippenmeyer S, Costantini F. Ret and Etv4 Promote directed movements of progenitor cells during renal branching morphogenesis. PLoS Biol. 2016;14:e1002382. doi: 10.1371/journal.pbio.1002382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alankarage D, Lavery R, Svingen T, Kelly S, Ludbrook L, Bagheri-Fam S, Koopman P, Harley V. SOX9 regulates expression of the male fertility gene Ets variant factor 5 (ETV5) during mammalian sex development. Int J Biochem Cell Biol. 2016;79:41–51. doi: 10.1016/j.biocel.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 34.Morrow CM, Hostetler CE, Griswold MD, Hofmann MC, Murphy KM, Cooke PS, Hess RA. ETV5 is required for continuous spermatogenesis in adult mice and may mediate blood testes barrier function and testicular immune privilege. Ann N Y Acad Sci. 2007;1120:144–151. doi: 10.1196/annals.1411.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eo J, Han K, M Murphy K, Song H, Lim HJ. Etv5, an ETS transcription factor, is expressed in granulosa and cumulus cells and serves as a transcriptional regulator of the cyclooxygenase-2. J Endocrinol. 2008;198:281–290. doi: 10.1677/JOE-08-0142. [DOI] [PubMed] [Google Scholar]
- 36.Zhang X, Wei R, Sun Y, Xia Q, Xie W, Song H, Wang W, Zou K. AKT3 is a pivotal molecule of Cadherin-22 and GDNF Family receptor-α1 signal pathways regulating self-renewal in female germline stem cells. Stem Cells. 2019;37:1095–1107. doi: 10.1002/stem.3030. [DOI] [PubMed] [Google Scholar]
- 37.Jamsai D, Clark BJ, Smith SJ, Whittle B, Goodnow CC, Ormandy CJ, O’Bryan MK. A missense mutation in the transcription factor ETV5 leads to sterility, increased embryonic and perinatal death, postnatal growth restriction, renal asymmetry and polydactyly in the mouse. PLoS One. 2013;8:e77311. doi: 10.1371/journal.pone.0077311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gutierrez-Aguilar R, Thompson A, Marchand N, Dumont P, Woods SC, de Launoit Y, Seeley RJ, Ulrich-Lai YM. The obesity-associated transcription factor ETV5 modulates circulating glucocorticoids. Physiol Behav. 2015;150:38–42. doi: 10.1016/j.physbeh.2015.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gutierrez-Aguilar R, Kim DH, Casimir M, Dai XQ, Pfluger PT, Park J, Haller A, Donelan E, Park J, D’Alessio D, Woods SC, MacDonald PE, Seeley RJ. The role of the transcription factor ETV5 in insulin exocytosis. Diabetologia. 2014;57:383–391. doi: 10.1007/s00125-013-3096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Degirmenci U, Wang M, Hu J. Targeting aberrant RAS/RAF/MEK/ERK signaling for cancer therapy. Cells. 2020;9:198. doi: 10.3390/cells9010198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bunda S, Heir P, Li ASC, Mamatjan Y, Zadeh G, Aldape K. c-Src phosphorylates and inhibits the function of the cic tumor suppressor protein. Mol Cancer Res. 2020;18:774–786. doi: 10.1158/1541-7786.MCR-18-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chi P, Chen Y, Zhang L, Guo X, Wongvipat J, Shamu T, Fletcher JA, Dewell S, Maki RG, Zheng D, Antonescu CR, Allis CD, Sawyers CL. ETV1 is a lineage survival factor that cooperates with KIT in gastrointestinal stromal tumours. Nature. 2010;467:849–853. doi: 10.1038/nature09409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ran L, Sirota I, Cao Z, Murphy D, Chen Y, Shukla S, Xie Y, Kaufmann MC, Gao D, Zhu S, Rossi F, Wongvipat J, Taguchi T, Tap WD, Mellinghoff IK, Besmer P, Antonescu CR, Chen Y, Chi P. Combined inhibition of MAP kinase and KIT signaling synergistically destabilizes ETV1 and suppresses GIST tumor growth. Cancer Discov. 2015;5:304–315. doi: 10.1158/2159-8290.CD-14-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duensing A. Targeting ETV1 in gastrointestinal stromal tumors: tripping the circuit breaker in GIST? Cancer Discov. 2015;5:231–233. doi: 10.1158/2159-8290.CD-15-0116. [DOI] [PubMed] [Google Scholar]
- 45.Wang HC, Li TY, Chao YJ, Hou YC, Hsueh YS, Hsu KH, Shan YS. KIT exon 11 codons 557-558 deletion mutation promotes liver metastasis through the CXCL12/CXCR4 axis in gastrointestinal stromal tumors. Clin Cancer Res. 2016;22:3477–3487. doi: 10.1158/1078-0432.CCR-15-2748. [DOI] [PubMed] [Google Scholar]
- 46.Hayashi Y, Bardsley MR, Toyomasu Y, Milosavljevic S, Gajdos GB, Choi KM, Reid-Lombardo KM, Kendrick ML, Bingener-Casey J, Tang CM, Sicklick JK, Gibbons SJ, Farrugia G, Taguchi T, Gupta A, Rubin BP, Fletcher JA, Ramachandran A, Ordog T. Platelet-derived growth factor receptor-α regulates proliferation of gastrointestinal stromal tumor cells with mutations in KIT by stabilizing ETV1. Gastroenterology. 2015;149:420–432. e416. doi: 10.1053/j.gastro.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan J, Yang S, Shen P, Sun H, Xiao J, Wang Y, Wu B, Ji F, Yan J, Xue H, Zhou D. C-kit signaling promotes proliferation and invasion of colorectal mucinous adenocarcinoma in a murine model. Oncotarget. 2015;6:27037–27048. doi: 10.18632/oncotarget.4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kherrouche Z, Monte D, Werkmeister E, Stoven L, De Launoit Y, Cortot AB, Tulasne D, Chotteau-Lelievre A. PEA3 transcription factors are downstream effectors of Met signaling involved in migration and invasiveness of Met-addicted tumor cells. Mol Oncol. 2015;9:1852–1867. doi: 10.1016/j.molonc.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bunda S, Heir P, Metcalf J, Li ASC, Agnihotri S, Pusch S, Yasin M, Li M, Burrell K, Mansouri S, Singh O, Wilson M, Alamsahebpour A, Nejad R, Choi B, Kim D, von Deimling A, Zadeh G, Aldape K. CIC protein instability contributes to tumorigenesis in glioblastoma. Nat Commun. 2019;10:661. doi: 10.1038/s41467-018-08087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen Y, Sumardika IW, Tomonobu N, Winarsa Ruma IM, Kinoshita R, Kondo E, Inoue Y, Sato H, Yamauchi A, Murata H, Yamamoto KI, Tomida S, Shien K, Yamamoto H, Soh J, Liu M, Futami J, Sasai K, Katayama H, Kubo M, Putranto EW, Hibino T, Sun B, Nishibori M, Toyooka S, Sakaguchi M. Melanoma cell adhesion molecule is the driving force behind the dissemination of melanoma upon S100A8/A9 binding in the original skin lesion. Cancer Lett. 2019;452:178–190. doi: 10.1016/j.canlet.2019.03.023. [DOI] [PubMed] [Google Scholar]
- 51.Chen Y, Sumardika IW, Tomonobu N, Kinoshita R, Inoue Y, Iioka H, Mitsui Y, Saito K, Ruma IMW, Sato H, Yamauchi A, Murata H, Yamamoto KI, Tomida S, Shien K, Yamamoto H, Soh J, Futami J, Kubo M, Putranto EW, Murakami T, Liu M, Hibino T, Nishibori M, Kondo E, Toyooka S, Sakaguchi M. Critical role of the MCAM-ETV4 axis triggered by extracellular S100A8/A9 in breast cancer aggressiveness. Neoplasia. 2019;21:627–640. doi: 10.1016/j.neo.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lemaître C, Tsang J, Bireau C, Heidmann T, Dewannieux M. A human endogenous retrovirus-derived gene that can contribute to oncogenesis by activating the ERK pathway and inducing migration and invasion. PLoS Pathog. 2017;13:e1006451. doi: 10.1371/journal.ppat.1006451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Andradas C, Blasco-Benito S, Castillo-Lluva S, Dillenburg-Pilla P, Diez-Alarcia R, Juanes-García A, García-Taboada E, Hernando-Llorente R, Soriano J, Hamann S, Wenners A, Alkatout I, Klapper W, Rocken C, Bauer M, Arnold N, Quintanilla M, Megías D, Vicente-Manzanares M, Urigüen L, Gutkind JS, Guzmán M, Pérez-Gómez E, Sánchez C. Activation of the orphan receptor GPR55 by lysophosphatidylinositol promotes metastasis in triple-negative breast cancer. Oncotarget. 2016;7:47565–47575. doi: 10.18632/oncotarget.10206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mus LM, Lambertz I, Claeys S, Kumps C, Van Loocke W, Van Neste C, Umapathy G, Vaapil M, Bartenhagen C, Laureys G, De Wever O, Bexell D, Fischer M, Hallberg B, Schulte J, De Wilde B, Durinck K, Denecker G, De Preter K, Speleman F. The ETS transcription factor ETV5 is a target of activated ALK in neuroblastoma contributing to increased tumour aggressiveness. Sci Rep. 2020;10:218. doi: 10.1038/s41598-019-57076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.di Martino E, Alder O, Hurst CD, Knowles MA. ETV5 links the FGFR3 and Hippo signalling pathways in bladder cancer. Sci Rep. 2019;9:5740. doi: 10.1038/s41598-018-36456-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fung TM, Ng KY, Tong M, Chen JN, Chai S, Chan KT, Law S, Lee NP, Choi MY, Li B, Cheung AL, Tsao SW, Qin YR, Guan XY, Chan KW, Ma S. Neuropilin-2 promotes tumourigenicity and metastasis in oesophageal squamous cell carcinoma through ERK-MAPK-ETV4-MMP-E-cadherin deregulation. J Pathol. 2016;239:309–319. doi: 10.1002/path.4728. [DOI] [PubMed] [Google Scholar]
- 57.Wong D, Yip S. Making heads or tails - the emergence of capicua (CIC) as an important multifunctional tumour suppressor. J Pathol. 2020;250:532–540. doi: 10.1002/path.5400. [DOI] [PubMed] [Google Scholar]
- 58.Simón-Carrasco L, Graña O, Salmón M, Jacob HKC, Gutierrez A, Jiménez G, Drosten M, Barbacid M. Inactivation of capicua in adult mice causes T-cell lymphoblastic lymphoma. Genes Dev. 2017;31:1456–1468. doi: 10.1101/gad.300244.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee JS, Kim E, Lee J, Kim D, Kim H, Kim CJ, Kim S, Jeong D, Lee Y. Capicua suppresses colorectal cancer progression via repression of ETV4 expression. Cancer Cell Int. 2020;20:42. doi: 10.1186/s12935-020-1111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou Y, Wang M, Shuang T, Liu Y, Zhang Y, Shi C. MiR-1307 influences the chemotherapeutic sensitivity in ovarian cancer cells through the regulation of the CIC transcriptional repressor. Pathol Res Pract. 2019;215:152606. doi: 10.1016/j.prp.2019.152606. [DOI] [PubMed] [Google Scholar]
- 61.Yoe J, Kim D, Kim S, Lee Y. Capicua restricts cancer stem cell-like properties in breast cancer cells. Oncogene. 2020;39:3489–3506. doi: 10.1038/s41388-020-1230-7. [DOI] [PubMed] [Google Scholar]
- 62.Da Vià MC, Solimando AG, Garitano-Trojaola A, Barrio S, Munawar U, Strifler S, Haertle L, Rhodes N, Teufel E, Vogt C, Lapa C, Beilhack A, Rasche L, Einsele H, Kortüm KM. CIC mutation as a molecular mechanism of acquired resistance to combined BRAF-MEK inhibition in extramedullary multiple myeloma with central nervous system involvement. Oncologist. 2020;25:112–118. doi: 10.1634/theoncologist.2019-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim E, Kim D, Lee JS, Yoe J, Park J, Kim CJ, Jeong D, Kim S, Lee Y. Capicua suppresses hepatocellular carcinoma progression by controlling the ETV4-MMP1 axis. Hepatology. 2018;67:2287–2301. doi: 10.1002/hep.29738. [DOI] [PubMed] [Google Scholar]
- 64.Okimoto RA, Breitenbuecher F, Olivas VR, Wu W, Gini B, Hofree M, Asthana S, Hrustanovic G, Flanagan J, Tulpule A, Blakely CM, Haringsma HJ, Simmons AD, Gowen K, Suh J, Miller VA, Ali S, Schuler M, Bivona TG. Inactivation of Capicua drives cancer metastasis. Nat Genet. 2017;49:87–96. doi: 10.1038/ng.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vitari AC, Leong KG, Newton K, Yee C, O’Rourke K, Liu J, Phu L, Vij R, Ferrando R, Couto SS, Mohan S, Pandita A, Hongo JA, Arnott D, Wertz IE, Gao WQ, French DM, Dixit VM. COP1 is a tumour suppressor that causes degradation of ETS transcription factors. Nature. 2011;474:403–406. doi: 10.1038/nature10005. [DOI] [PubMed] [Google Scholar]
- 66.Baert JL, Monte D, Verreman K, Degerny C, Coutte L, de Launoit Y. The E3 ubiquitin ligase complex component COP1 regulates PEA3 group member stability and transcriptional activity. Oncogene. 2010;29:1810–1820. doi: 10.1038/onc.2009.471. [DOI] [PubMed] [Google Scholar]
- 67.Xie Y, Cao Z, Wong EW, Guan Y, Ma W, Zhang JQ, Walczak EG, Murphy D, Ran L, Sirota I, Wang S, Shukla S, Gao D, Knott SR, Chang K, Leu J, Wongvipat J, Antonescu CR, Hannon G, Chi P, Chen Y. COP1/DET1/ETS axis regulates ERK transcriptome and sensitivity to MAPK inhibitors. J Clin Invest. 2018;128:1442–1457. doi: 10.1172/JCI94840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ta L, Xuan C, Xing N, Zhu X. COP1 is downregulated in renal cell carcinoma (RCC) and inhibits the migration of RCC ACHN cells in vitro. Mol Med Rep. 2016;14:1371–1378. doi: 10.3892/mmr.2016.5373. [DOI] [PubMed] [Google Scholar]
- 69.Ouyang M, Wang H, Ma J, Lü W, Li J, Yao C, Chang G, Bi J, Wang S, Wang W. COP1, the negative regulator of ETV1, influences prognosis in triple-negative breast cancer. BMC Cancer. 2015;15:132. doi: 10.1186/s12885-015-1151-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zeng S, Seifert AM, Zhang JQ, Kim TS, Bowler TG, Cavnar MJ, Medina BD, Vitiello GA, Rossi F, Loo JK, Param NJ, DeMatteo RP. ETV4 collaborates with Wnt/β-catenin signaling to alter cell cycle activity and promote tumor aggressiveness in gastrointestinal stromal tumor. Oncotarget. 2017;8:114195–114209. doi: 10.18632/oncotarget.23173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gao G, Xiu D, Yang B, Sun D, Wei X, Ding Y, Ma Y, Wang Z. miR-129-5p inhibits prostate cancer proliferation via targeting ETV1. Onco Targets Ther. 2019;12:3531–3544. doi: 10.2147/OTT.S183435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zuo Y, Shen W, Wang C, Niu N, Pu J. Circular RNA Circ-ZNF609 promotes lung adenocarcinoma proliferation by modulating miR-1224-3p/ETV1 signaling. Cancer Manag Res. 2020;12:2471–2479. doi: 10.2147/CMAR.S232260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li H, Shen S, Ruan X, Liu X, Zheng J, Liu Y, Yang C, Wang D, Liu L, Ma J, Ma T, Wang P, Cai H, Li Z, Zhao L, Xue Y. Biosynthetic CircRNA_001160 induced by PTBP1 regulates the permeability of BTB via the CircRNA_001160/miR-195-5p/ETV1 axis. Cell Death Dis. 2019;10:960. doi: 10.1038/s41419-019-2191-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jin X, Guan Y, Sheng H, Liu Y. Crosstalk in competing endogenous RNA network reveals the complex molecular mechanism underlying lung cancer. Oncotarget. 2017;8:91270–91280. doi: 10.18632/oncotarget.20441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li J, Lai Y, Ma J, Liu Y, Bi J, Zhang L, Chen L, Yao C, Lv W, Chang G, Wang S, Ouyang M, Wang W. miR-17-5p suppresses cell proliferation and invasion by targeting ETV1 in triple-negative breast cancer. BMC Cancer. 2017;17:745. doi: 10.1186/s12885-017-3674-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gits CM, van Kuijk PF, Jonkers MB, Boersma AW, van Ijcken WF, Wozniak A, Sciot R, Rutkowski P, Schöffski P, Taguchi T, Mathijssen RH, Verweij J, Sleijfer S, Debiec-Rychter M, Wiemer EA. MiR-17-92 and miR-221/222 cluster members target KIT and ETV1 in human gastrointestinal stromal tumours. Br J Cancer. 2013;109:1625–1635. doi: 10.1038/bjc.2013.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cohen R, Greenberg E, Nemlich Y, Schachter J, Markel G. miR-17 regulates melanoma cell motility by inhibiting the translation of ETV1. Oncotarget. 2015;6:19006–19016. doi: 10.18632/oncotarget.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang H, Zhang H, Zeng J, Tan Y. ceRNA network analysis reveals prognostic markers for glioblastoma. Oncol Lett. 2019;17:5545–5557. doi: 10.3892/ol.2019.10275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rahim S, Uren A. Emergence of ETS transcription factors as diagnostic tools and therapeutic targets in prostate cancer. Am J Transl Res. 2013;5:254–268. [PMC free article] [PubMed] [Google Scholar]
- 80.Gasi Tandefelt D, Boormans J, Hermans K, Trapman J. ETS fusion genes in prostate cancer. Endocr Relat Cancer. 2014;21:R143–152. doi: 10.1530/ERC-13-0390. [DOI] [PubMed] [Google Scholar]
- 81.Kumar-Sinha C, Tomlins SA, Chinnaiyan AM. Recurrent gene fusions in prostate cancer. Nat Rev Cancer. 2008;8:497–511. doi: 10.1038/nrc2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yun JW, Yang L, Park HY, Lee CW, Cha H, Shin HT, Noh KW, Choi YL, Park WY, Park PJ. Dysregulation of cancer genes by recurrent intergenic fusions. Genome Biol. 2020;21:166. doi: 10.1186/s13059-020-02076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Torres A, Alshalalfa M, Tomlins SA, Erho N, Gibb EA, Chelliserry J, Lim L, Lam LLC, Faraj SF, Bezerra SM, Davicioni E, Yousefi K, Ross AE, Netto GJ, Schaeffer EM, Lotan TL. Comprehensive determination of prostate tumor ETS gene status in clinical samples using the CLIA decipher assay. J Mol Diagn. 2017;19:475–484. doi: 10.1016/j.jmoldx.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Linn DE, Bronson RT, Li Z. Genetic interaction between Tmprss2-ERG gene fusion and Nkx3.1-loss does not enhance prostate tumorigenesis in mouse models. PLoS One. 2015;10:e0120628. doi: 10.1371/journal.pone.0120628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barros-Silva JD, Paulo P, Bakken AC, Cerveira N, Løvf M, Henrique R, Jerónimo C, Lothe RA, Skotheim RI, Teixeira MR. Novel 5’ fusion partners of ETV1 and ETV4 in prostate cancer. Neoplasia. 2013;15:720–726. doi: 10.1593/neo.13232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lu Z, Williamson SR, Carskadon S, Arachchige PD, Dhamdhere G, Schultz DS, Stricker H, Peabody JO, Jeong W, Chitale DA, Bismar TA, Rogers CG, Menon M, Gupta NS, Palanisamy N. Clonal evaluation of early onset prostate cancer by expression profiling of ERG, SPINK1, ETV1, and ETV4 on whole-mount radical prostatectomy tissue. Prostate. 2020;80:38–50. doi: 10.1002/pros.23914. [DOI] [PubMed] [Google Scholar]
- 87.Dedigama-Arachchige P, Carskadon S, Li J, Loveless I, Alhamar M, Peabody JO, Stricker H, Chitale DA, Rogers CG, Menon M, Gupta NS, Bismar TA, Williamson SR, Palanisamy N. Clonal evaluation of prostate cancer molecular heterogeneity in biopsy samples by dual immunohistochemistry and dual RNA in situ hybridization. Mod Pathol. 2020;33:1791–1801. doi: 10.1038/s41379-020-0525-0. [DOI] [PubMed] [Google Scholar]
- 88.Qu X, Yeung C, Coleman I, Nelson PS, Fang M. Comparison of four next generation sequencing platforms for fusion detection: oncomine by ThermoFisher, AmpliSeq by illumina, FusionPlex by ArcherDX, and QIAseq by QIAGEN. Cancer Genet. 2020;243:11–18. doi: 10.1016/j.cancergen.2020.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang J, Chen Y, Lu J, Wang X, Wang L, Liang J, Sun ZS. Identification and characterization of novel fusion genes in prostate cancer by targeted RNA capture and next-generation sequencing. Acta Biochim Biophys Sin (Shanghai) 2018;50:1166–1172. doi: 10.1093/abbs/gmy112. [DOI] [PubMed] [Google Scholar]
- 90.Delattre O. Ewing’s tumours, genetic and cellular aspects. Pathol Biol (Paris) 2008;56:257–259. doi: 10.1016/j.patbio.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 91.Specht K, Hartmann W. Ewing sarcomas and Ewing-like sarcomas : new aspects. Pathologe. 2018;39:154–163. doi: 10.1007/s00292-018-0421-2. [DOI] [PubMed] [Google Scholar]
- 92.Tsuda Y, Zhang L, Meyers P, Tap WD, Healey JH, Antonescu CR. The clinical heterogeneity of round cell sarcomas with EWSR1/FUS gene fusions: impact of gene fusion type on clinical features and outcome. Genes Chromosomes Cancer. 2020;59:525–534. doi: 10.1002/gcc.22857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shulman SC, Katzenstein H, Bridge J, Bannister LL, Qayed M, Oskouei S, Shehata BM. Ewing sarcoma with 7;22 translocation: three new cases and clinicopathological characterization. Fetal Pediatr Pathol. 2012;31:341–348. doi: 10.3109/15513815.2012.659397. [DOI] [PubMed] [Google Scholar]
- 94.Takahashi A, Higashino F, Aoyagi M, Yoshida K, Itoh M, Kyo S, Ohno T, Taira T, Ariga H, Nakajima K, Hatta M, Kobayashi M, Sano H, Kohgo T, Shindoh M. EWS/ETS fusions activate telomerase in Ewing’s tumors. Cancer Res. 2003;63:8338–8344. [PubMed] [Google Scholar]
- 95.Matjašič A, Zupan A, Boštjančič E, Pižem J, Popović M, Kolenc D. A novel PTPRZ1-ETV1 fusion in gliomas. Brain Pathol. 2020;30:226–234. doi: 10.1111/bpa.12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xu L, Hu H, Zheng LS, Wang MY, Mei Y, Peng LX, Qiang YY, Li CZ, Meng DF, Wang MD, Liu ZJ, Li XJ, Huang BJ, Qian CN. ETV4 is a theranostic target in clear cell renal cell carcinoma that promotes metastasis by activating the pro-metastatic gene FOSL1 in a PI3K-AKT dependent manner. Cancer Lett. 2020;482:74–89. doi: 10.1016/j.canlet.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 97.Aytes A, Mitrofanova A, Kinkade CW, Lefebvre C, Lei M, Phelan V, LeKaye HC, Koutcher JA, Cardiff RD, Califano A, Shen MM, Abate-Shen C. ETV4 promotes metastasis in response to activation of PI3-kinase and Ras signaling in a mouse model of advanced prostate cancer. Proc Natl Acad Sci U S A. 2013;110:E3506–3515. doi: 10.1073/pnas.1303558110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ran L, Chen Y, Sher J, Wong EWP, Murphy D, Zhang JQ, Li D, Deniz K, Sirota I, Cao Z, Wang S, Guan Y, Shukla S, Li KY, Chramiec A, Xie Y, Zheng D, Koche RP, Antonescu CR, Chen Y, Chi P. FOXF1 defines the core-regulatory circuitry in gastrointestinal stromal tumor. Cancer Discov. 2018;8:234–251. doi: 10.1158/2159-8290.CD-17-0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Song X, Wan X, Huang T, Zeng C, Sastry N, Wu B, James CD, Horbinski C, Nakano I, Zhang W, Hu B, Cheng SY. SRSF3-regulated RNA alternative splicing promotes glioblastoma tumorigenicity by affecting multiple cellular processes. Cancer Res. 2019;79:5288–5301. doi: 10.1158/0008-5472.CAN-19-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hashimoto S, Furukawa S, Hashimoto A, Tsutaho A, Fukao A, Sakamura Y, Parajuli G, Onodera Y, Otsuka Y, Handa H, Oikawa T, Hata S, Nishikawa Y, Mizukami Y, Kodama Y, Murakami M, Fujiwara T, Hirano S, Sabe H. ARF6 and AMAP1 are major targets of KRAS and TP53 mutations to promote invasion, PD-L1 dynamics, and immune evasion of pancreatic cancer. Proc Natl Acad Sci U S A. 2019;116:17450–17459. doi: 10.1073/pnas.1901765116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jiang Y, Xu W, Lu J, He F, Yang X. Invasiveness of hepatocellular carcinoma cell lines: contribution of hepatocyte growth factor, c-met, and transcription factor Ets-1. Biochem Biophys Res Commun. 2001;286:1123–1130. doi: 10.1006/bbrc.2001.5521. [DOI] [PubMed] [Google Scholar]
- 102.Hakuma N, Kinoshita I, Shimizu Y, Yamazaki K, Yoshida K, Nishimura M, Dosaka-Akita H. E1AF/PEA3 activates the Rho/Rho-associated kinase pathway to increase the malignancy potential of non-small-cell lung cancer cells. Cancer Res. 2005;65:10776–10782. doi: 10.1158/0008-5472.CAN-05-0060. [DOI] [PubMed] [Google Scholar]
- 103.Hiroumi H, Dosaka-Akita H, Yoshida K, Shindoh M, Ohbuchi T, Fujinaga K, Nishimura M. Expression of E1AF/PEA3, an Ets-related transcription factor in human non-small-cell lung cancers: its relevance in cell motility and invasion. Int J Cancer. 2001;93:786–791. doi: 10.1002/ijc.1410. [DOI] [PubMed] [Google Scholar]
- 104.Hanzawa M, Shindoh M, Higashino F, Yasuda M, Inoue N, Hida K, Ono M, Kohgo T, Nakamura M, Notani K, Fukuda H, Totsuka Y, Yoshida K, Fujinaga K. Hepatocyte growth factor upregulates E1AF that induces oral squamous cell carcinoma cell invasion by activating matrix metalloproteinase genes. Carcinogenesis. 2000;21:1079–1085. [PubMed] [Google Scholar]
- 105.Keenan MM, Liu B, Tang X, Wu J, Cyr D, Stevens RD, Ilkayeva O, Huang Z, Tollini LA, Murphy SK, Lucas J, Muoio DM, Kim SY, Chi JT. ACLY and ACC1 regulate hypoxia-induced apoptosis by modulating ETV4 via α-ketoglutarate. PLoS Genet. 2015;11:e1005599. doi: 10.1371/journal.pgen.1005599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Singsuksawat E, Thuwajit C, Charngkaew K, Thuwajit P. Increased ETV4 expression correlates with estrogen-enhanced proliferation and invasiveness of cholangiocarcinoma cells. Cancer Cell Int. 2018;18:25. doi: 10.1186/s12935-018-0525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Klein RH, Tung PY, Somanath P, Fehling HJ, Knoepfler PS. Genomic functions of developmental pluripotency associated factor 4 (Dppa4) in pluripotent stem cells and cancer. Stem Cell Res. 2018;31:83–94. doi: 10.1016/j.scr.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Takeda R, Asada S, Park SJ, Yokoyama A, Becker HJ, Kanai A, Visconte V, Hershberger CE, Hayashi Y, Yonezawa T, Tamura M, Fukushima T, Tanaka Y, Fukuyama T, Matsumoto A, Yamasaki S, Nakai K, Yamazaki S, Inaba T, Shibata T, Inoue D, Honda H, Goyama S, Maciejewski JP, Kitamura T. HHEX promotes myeloid transformation in cooperation with mutant ASXL1. Blood. 2020;136:1670–1684. doi: 10.1182/blood.2019004613. [DOI] [PubMed] [Google Scholar]
- 109.Oh S, Shin S, Lightfoot SA, Janknecht R. 14-3-3 proteins modulate the ETS transcription factor ETV1 in prostate cancer. Cancer Res. 2013;73:5110–5119. doi: 10.1158/0008-5472.CAN-13-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Khatiwada P, Kannan A, Malla M, Dreier M, Shemshedini L. Androgen up-regulation of Twist1 gene expression is mediated by ETV1. PeerJ. 2020;8:e8921. doi: 10.7717/peerj.8921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Arase M, Tamura Y, Kawasaki N, Isogaya K, Nakaki R, Mizutani A, Tsutsumi S, Aburatani H, Miyazono K, Koinuma D. Dynamics of chromatin accessibility during TGF-β-induced EMT of Ras-transformed mammary gland epithelial cells. Sci Rep. 2017;7:1166. doi: 10.1038/s41598-017-00973-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kim HJ, Kim SH, Yu EJ, Seo WY, Kim JH. A positive role of DBC1 in PEA3-mediated progression of estrogen receptor-negative breast cancer. Oncogene. 2015;34:4500–4508. doi: 10.1038/onc.2014.381. [DOI] [PubMed] [Google Scholar]
- 113.Dowdy SC, Mariani A, Janknecht R. HER2/Neu- and TAK1-mediated up-regulation of the transforming growth factor beta inhibitor Smad7 via the ETS protein ER81. J Biol Chem. 2003;278:44377–44384. doi: 10.1074/jbc.M307202200. [DOI] [PubMed] [Google Scholar]
- 114.Shin S, Bosc DG, Ingle JN, Spelsberg TC, Janknecht R. Rcl is a novel ETV1/ER81 target gene upregulated in breast tumors. J Cell Biochem. 2008;105:866–874. doi: 10.1002/jcb.21884. [DOI] [PubMed] [Google Scholar]
- 115.Jørgensen CLT, Forsare C, Bendahl PO, Falck AK, Fernö M, Lövgren K, Aaltonen K, Rydén L. Expression of epithelial-mesenchymal transition-related markers and phenotypes during breast cancer progression. Breast Cancer Res Treat. 2020;181:369–381. doi: 10.1007/s10549-020-05627-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nader JS, Guillon J, Petit C, Boissard A, Franconi F, Blandin S, Lambot S, Grégoire M, Verrièle V, Nawrocki-Raby B, Birembaut P, Coqueret O, Guette C, Pouliquen DL. S100A4 is a biomarker of tumorigenesis, EMT, invasion, and colonization of host organs in experimental malignant mesothelioma. Cancers (Basel) 2020;12:939. doi: 10.3390/cancers12040939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wu QQ, Zhao M, Huang GZ, Zheng ZN, Chen Y, Zeng WS, Lv XZ. Fibroblast Activation Protein (FAP) overexpression induces Epithelial-Mesenchymal Transition (EMT) in oral squamous cell carcinoma by Down-Regulating Dipeptidyl Peptidase 9 (DPP9) Onco Targets Ther. 2020;13:2599–2611. doi: 10.2147/OTT.S243417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yuen HF, Chan YK, Grills C, McCrudden CM, Gunasekharan V, Shi Z, Wong AS, Lappin TR, Chan KW, Fennell DA, Khoo US, Johnston PG, El-Tanani M. Polyomavirus enhancer activator 3 protein promotes breast cancer metastatic progression through snail-induced epithelial-mesenchymal transition. J Pathol. 2011;224:78–89. doi: 10.1002/path.2859. [DOI] [PubMed] [Google Scholar]
- 119.Pellecchia A, Pescucci C, De Lorenzo E, Luceri C, Passaro N, Sica M, Notaro R, De Angioletti M. Overexpression of ETV4 is oncogenic in prostate cells through promotion of both cell proliferation and epithelial to mesenchymal transition. Oncogenesis. 2012;1:e20. doi: 10.1038/oncsis.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li Z, Zhang L, Ma Z, Yang M, Tang J, Fu Y, Mao Y, Hong X, Zhang Y. ETV1 induces epithelial to mesenchymal transition in human gastric cancer cells through the upregulation of Snail expression. Oncol Rep. 2013;30:2859–2863. doi: 10.3892/or.2013.2776. [DOI] [PubMed] [Google Scholar]
- 121.Puli OR, Danysh BP, McBeath E, Sinha DK, Hoang NM, Powell RT, Danysh HE, Cabanillas ME, Cote GJ, Hofmann MC. The transcription factor ETV5 mediates BRAFV600E-induced proliferation and TWIST1 expression in papillary thyroid cancer cells. Neoplasia. 2018;20:1121–1134. doi: 10.1016/j.neo.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Alonso-Alconada L, Eritja N, Muinelo-Romay L, Barbazan J, Lopez-Lopez R, Matias-Guiu X, Gil-Moreno A, Dolcet X, Abal M. ETV5 transcription program links BDNF and promotion of EMT at invasive front of endometrial carcinomas. Carcinogenesis. 2014;35:2679–2686. doi: 10.1093/carcin/bgu198. [DOI] [PubMed] [Google Scholar]
- 123.Lunardi A, Varmeh S, Chen M, Taulli R, Guarnerio J, Ala U, Seitzer N, Ishikawa T, Carver BS, Hobbs RM, Quarantotti V, Ng C, Berger AH, Nardella C, Poliseno L, Montironi R, Castillo-Martin M, Cordon-Cardo C, Signoretti S, Pandolfi PP. Suppression of CHK1 by ETS family members promotes DNA damage response bypass and tumorigenesis. Cancer Discov. 2015;5:550–563. doi: 10.1158/2159-8290.CD-13-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shin S, Oh S, An S, Janknecht R. ETS variant 1 regulates matrix metalloproteinase-7 transcription in LNCaP prostate cancer cells. Oncol Rep. 2013;29:306–314. doi: 10.3892/or.2012.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu CY, Yu T, Huang Y, Cui L, Hong W. ETS (E26 transformation-specific) up-regulation of the transcriptional co-activator TAZ promotes cell migration and metastasis in prostate cancer. J Biol Chem. 2017;292:9420–9430. doi: 10.1074/jbc.M117.783787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Qi M, Liu Z, Shen C, Wang L, Zeng J, Wang C, Li C, Fu W, Sun Y, Han B. Overexpression of ETV4 is associated with poor prognosis in prostate cancer: involvement of uPA/uPAR and MMPs. Tumour Biol. 2015;36:3565–3572. doi: 10.1007/s13277-014-2993-7. [DOI] [PubMed] [Google Scholar]
- 127.Hollenhorst PC, Paul L, Ferris MW, Graves BJ. The ETS gene ETV4 is required for anchorage-independent growth and a cell proliferation gene expression program in PC3 prostate cells. Genes Cancer. 2011;1:1044–1052. doi: 10.1177/1947601910395578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hao L, Pang K, Pang H, Zhang J, Zhang Z, He H, Zhou R, Shi Z, Han C. Knockdown of P3H4 inhibits proliferation and invasion of bladder cancer. Aging (Albany NY) 2020;12:2156–2168. doi: 10.18632/aging.102732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Keld R, Guo B, Downey P, Gulmann C, Ang YS, Sharrocks AD. The ERK MAP kinase-PEA3/ETV4-MMP-1 axis is operative in oesophageal adenocarcinoma. Mol Cancer. 2010;9:313. doi: 10.1186/1476-4598-9-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen Y, Zou H, Yang LY, Li Y, Wang L, Hao Y, Yang JL. ER81-shRNA inhibits growth of triple-negative human breast cancer cell line MDA-MB-231 in vivo and in vitro. Asian Pac J Cancer Prev. 2012;13:2385–2392. doi: 10.7314/apjcp.2012.13.5.2385. [DOI] [PubMed] [Google Scholar]
- 131.Xiaohui C, Xin LI, Dehua WU. E26 transformation-specific variant 4 promotes sorafenib and cisplatin resistance in hepatocellular carcinoma cells in vitro. Nan Fang Yi Ke Da Xue Xue Bao. 2019;39:875–882. doi: 10.12122/j.issn.1673-4254.2019.08.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Deshmukh SK, Singh AP, Singh S. ETV4: an emerging target in pancreatic cancer. Oncoscience. 2018;5:260–261. doi: 10.18632/oncoscience.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tyagi N, Deshmukh SK, Srivastava SK, Azim S, Ahmad A, Al-Ghadhban A, Singh AP, Carter JE, Wang B, Singh S. ETV4 facilitates cell-cycle progression in pancreatic cells through transcriptional regulation of cyclin D1. Mol Cancer Res. 2018;16:187–196. doi: 10.1158/1541-7786.MCR-17-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kim H, Datta A, Talwar S, Saleem SN, Mondal D, Abdel-Mageed AB. Estradiol-ERβ2 signaling axis confers growth and migration of CRPC cells through TMPRSS2-ETV5 gene fusion. Oncotarget. 2017;8:62820–62833. doi: 10.18632/oncotarget.11355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xiao BD, Zhao YJ, Jia XY, Wu J, Wang YG, Huang F. Multifaceted p21 in carcinogenesis, stemness of tumor and tumor therapy. World J Stem Cells. 2020;12:481–487. doi: 10.4252/wjsc.v12.i6.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cosi I, Pellecchia A, De Lorenzo E, Torre E, Sica M, Nesi G, Notaro R, De Angioletti M. ETV4 promotes late development of prostatic intraepithelial neoplasia and cell proliferation through direct and p53-mediated downregulation of p21. J Hematol Oncol. 2020;13:112. doi: 10.1186/s13045-020-00943-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ladam F, Damour I, Dumont P, Kherrouche Z, de Launoit Y, Tulasne D, Chotteau-Lelievre A. Loss of a negative feedback loop involving pea3 and cyclin d2 is required for pea3-induced migration in transformed mammary epithelial cells. Mol Cancer Res. 2013;11:1412–1424. doi: 10.1158/1541-7786.MCR-13-0229. [DOI] [PubMed] [Google Scholar]
- 138.Jiang J, Wei Y, Liu D, Zhou J, Shen J, Chen X, Zhang S, Kong X, Gu J. E1AF promotes breast cancer cell cycle progression via upregulation of Cyclin D3 transcription. Biochem Biophys Res Commun. 2007;358:53–58. doi: 10.1016/j.bbrc.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 139.Zhang Y, Gu ML, Zhou XX, Ma H, Yao HP, Ji F. Altered expression of ETV1 and its contribution to tumorigenic phenotypes in gastrointestinal stromal tumors. Oncol Rep. 2014;32:927–934. doi: 10.3892/or.2014.3281. [DOI] [PubMed] [Google Scholar]
- 140.Yang L, Shi P, Zhao G, Xu J, Peng W, Zhang J, Zhang G, Wang X, Dong Z, Chen F, Cui H. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct Target Ther. 2020;5:8. doi: 10.1038/s41392-020-0110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chivu-Economescu M, Necula LG, Matei L, Dragu DL, Neagu AI, Alexiu I, Bleotu C, Diaconu CC. Gastrointestinal cancer stem cells as targets for innovative immunotherapy. World J Gastroenterol. 2020;26:1580–1593. doi: 10.3748/wjg.v26.i14.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ahmad ST, Rogers AD, Chen MJ, Dixit R, Adnani L, Frankiw LS, Lawn SO, Blough MD, Alshehri M, Wu W, Marra MA, Robbins SM, Cairncross JG, Schuurmans C, Chan JA. Capicua regulates neural stem cell proliferation and lineage specification through control of Ets factors. Nat Commun. 2019;10:2000. doi: 10.1038/s41467-019-09949-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Menendez ST, Rey V, Martinez-Cruzado L, Gonzalez MV, Morales-Molina A, Santos L, Blanco V, Alvarez C, Estupiñan O, Allonca E, Rodrigo JP, García-Castro J, Garcia-Pedrero JM, Rodriguez R. SOX2 expression and transcriptional activity identifies a subpopulation of cancer stem cells in sarcoma with prognostic implications. Cancers (Basel) 2020;12:964. doi: 10.3390/cancers12040964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Siddiqui Z, Srivastava AN, Sankhwar SN, Dalela D, Singh V, Zaidi N, Fatima N, Bano I, Anjum S. Synergic effects of cancer stem cells markers, CD44 and embryonic stem cell transcription factor Nanog, on bladder cancer prognosis. Br J Biomed Sci. 2020;77:69–75. doi: 10.1080/09674845.2019.1692761. [DOI] [PubMed] [Google Scholar]
- 145.Park SW, Do HJ, Choi W, Song H, Chung HJ, Kim JH. NANOG gene expression is regulated by the ETS transcription factor ETV4 in human embryonic carcinoma NCCIT cells. Biochem Biophys Res Commun. 2017;487:532–538. doi: 10.1016/j.bbrc.2017.04.059. [DOI] [PubMed] [Google Scholar]
- 146.Watanabe H, Ma Q, Peng S, Adelmant G, Swain D, Song W, Fox C, Francis JM, Pedamallu CS, DeLuca DS, Brooks AN, Wang S, Que J, Rustgi AK, Wong KK, Ligon KL, Liu XS, Marto JA, Meyerson M, Bass AJ. SOX2 and p63 colocalize at genetic loci in squamous cell carcinomas. J Clin Invest. 2014;124:1636–1645. doi: 10.1172/JCI71545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Liao S, Davoli T, Leng Y, Li MZ, Xu Q, Elledge SJ. A genetic interaction analysis identifies cancer drivers that modify EGFR dependency. Genes Dev. 2017;31:184–196. doi: 10.1101/gad.291948.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Park SM, Hwang CY, Cho SH, Lee D, Gong JR, Lee S, Nam S, Cho KH. Systems analysis identifies potential target genes to overcome cetuximab resistance in colorectal cancer cells. FEBS J. 2019;286:1305–1318. doi: 10.1111/febs.14773. [DOI] [PubMed] [Google Scholar]
- 149.Uitdehaag JCM, Kooijman JJ, de Roos J, Prinsen MBW, Dylus J, Willemsen-Seegers N, Kawase Y, Sawa M, de Man J, van Gerwen SJC, Buijsman RC, Zaman GJR. Combined cellular and biochemical profiling to identify predictive drug response biomarkers for kinase inhibitors approved for clinical use between 2013 and 2017. Mol Cancer Ther. 2019;18:470–481. doi: 10.1158/1535-7163.MCT-18-0877. [DOI] [PubMed] [Google Scholar]
- 150.Gupta A, Towers C, Willenbrock F, Brant R, Hodgson DR, Sharpe A, Smith P, Cutts A, Schuh A, Asher R, Myers K, Love S, Collins L, Wise A, Middleton MR, Macaulay VM. Dual-specificity protein phosphatase DUSP4 regulates response to MEK inhibition in BRAF wild-type melanoma. Br J Cancer. 2020;122:506–516. doi: 10.1038/s41416-019-0673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kacew AJ, Harris EJ, Lorch JH, Haddad RI, Chau NG, Rabinowits G, LeBoeuf NR, Schmults CD, Thakuria M, MacConaill LE, Hanna GJ. Chromosome 3q arm gain linked to immunotherapy response in advanced cutaneous squamous cell carcinoma. Eur J Cancer. 2019;113:1–9. doi: 10.1016/j.ejca.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 152.Li Z, Wang B, Gu S, Jiang P, Sahu A, Chen CH, Han T, Shi S, Wang X, Traugh N, Liu H, Liu Y, Wu Q, Brown M, Xiao T, Boland GM, Shirley Liu X. CRISPR screens identify essential cell growth mediators in BRAF inhibitor-resistant melanoma. Genomics Proteomics Bioinformatics. 2020;18:26–40. doi: 10.1016/j.gpb.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhao Y, Liu J, Hong Q, Yang C, Chen L, Chen Y, Wang Q, Zhao K, Jin W. Involvement of MyoD and PEA3 in regulation of transcription activity of MDR1 gene. Acta Biochim Biophys Sin (Shanghai) 2010;42:900–907. doi: 10.1093/abbs/gmq094. [DOI] [PubMed] [Google Scholar]
- 154.Hu J, Zhu W, Wei B, Wen H, Mao S, Xu H, Hu M, Yang T, Jiang H. Antitumoral action of icaritin in LNCaP prostate cancer cells by regulating PEA3/HER2/AR signaling. Anticancer Drugs. 2016;27:944–952. doi: 10.1097/CAD.0000000000000420. [DOI] [PubMed] [Google Scholar]
- 155.Xing X, Wang SC, Xia W, Zou Y, Shao R, Kwong KY, Yu Z, Zhang S, Miller S, Huang L, Hung MC. The ets protein PEA3 suppresses HER-2/neu overexpression and inhibits tumorigenesis. Nat Med. 2000;6:189–195. doi: 10.1038/72294. [DOI] [PubMed] [Google Scholar]
- 156.Span PN, Manders P, Heuvel JJ, Beex LV, Sweep CG. E1AF expression levels are not associated with prognosis in human breast cancer. Breast Cancer Res Treat. 2003;79:129–131. doi: 10.1023/a:1023338424677. [DOI] [PubMed] [Google Scholar]
- 157.Iwasaki M, Nishikawa A, Akutagawa N, Fujimoto T, Teramoto M, Sakaguchi Y, Kato H, Ito M, Yoshida K, Kudo R. E1AF/PEA3 reduces the invasiveness of SiHa cervical cancer cells by activating serine proteinase inhibitor squamous cell carcinoma antigen. Exp Cell Res. 2004;299:525–532. doi: 10.1016/j.yexcr.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 158.Liu D, Wei Y, Zhou F, Ge Y, Xu J, Chen H, Zhang W, Yun X, Jiang J. E1AF promotes mithramycin A-induced Huh-7 cell apoptosis depending on its DNA-binding domain. Arch Biochem Biophys. 2008;477:20–26. doi: 10.1016/j.abb.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 159.Segalés L, Juanpere N, Lorenzo M, Albero-González R, Fumadó L, Cecchini L, Bellmunt J, Lloreta-Trull J, Hernández-Llodrà S. Strong cytoplasmic ETV1 expression has a negative impact on prostate cancer outcome. Virchows Arch. 2019;475:457–466. doi: 10.1007/s00428-019-02573-1. [DOI] [PubMed] [Google Scholar]
- 160.Eskandari E, Mahjoubi F, Motalebzadeh J. An integrated study on TFs and miRNAs in colorectal cancer metastasis and evaluation of three co-regulated candidate genes as prognostic markers. Gene. 2018;679:150–159. doi: 10.1016/j.gene.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 161.Liu HY, Zhou B, Wang L, Li Y, Zhou ZG, Sun XF, Xu B, Zeng YJ, Song JM, Luo HZ, Yang L. Association of E1AF mRNA expression with tumor progression and matrilysin in human rectal cancer. Oncology. 2007;73:384–388. doi: 10.1159/000136158. [DOI] [PubMed] [Google Scholar]
- 162.Wan G, Gao F, Chen J, Li Y, Geng M, Sun L, Liu Y, Liu H, Yang X, Wang R, Feng Y, Wang X. Nomogram prediction of individual prognosis of patients with hepatocellular carcinoma. BMC Cancer. 2017;17:91. doi: 10.1186/s12885-017-3062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chotteau-Lelièvre A, Révillion F, Lhotellier V, Hornez L, Desbiens X, Cabaret V, de Launoit Y, Peyrat JP. Prognostic value of ERM gene expression in human primary breast cancers. Clin Cancer Res. 2004;10:7297–7303. doi: 10.1158/1078-0432.CCR-04-0593. [DOI] [PubMed] [Google Scholar]
- 164.Yuan ZY, Dai T, Wang SS, Peng RJ, Li XH, Qin T, Song LB, Wang X. Overexpression of ETV4 protein in triple-negative breast cancer is associated with a higher risk of distant metastasis. Onco Targets Ther. 2014;7:1733–1742. doi: 10.2147/OTT.S66692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yamamoto H, Horiuchi S, Adachi Y, Taniguchi H, Nosho K, Min Y, Imai K. Expression of ets-related transcriptional factor E1AF is associated with tumor progression and over-expression of matrilysin in human gastric cancer. Carcinogenesis. 2004;25:325–332. doi: 10.1093/carcin/bgh011. [DOI] [PubMed] [Google Scholar]
- 166.Hida K, Shindoh M, Yoshida K, Kudoh A, Furaoka K, Kohgo T, Fujinaga K, Totsuka Y. Expression of E1AF, an ets-family transcription factor, is correlated with the invasive phenotype of oral squamous cell carcinoma. Oral Oncol. 1997;33:426–430. doi: 10.1016/s0964-1955(97)00047-x. [DOI] [PubMed] [Google Scholar]
- 167.Hung YP, Fletcher CD, Hornick JL. Evaluation of ETV4 and WT1 expression in CIC-rearranged sarcomas and histologic mimics. Mod Pathol. 2016;29:1324–1334. doi: 10.1038/modpathol.2016.140. [DOI] [PubMed] [Google Scholar]
- 168.Smith SC, Palanisamy N, Martin E, Almenara J, McHugh JB, Choi EK, Lucas DR, Betz BL, Thomas D, Patel RM. The utility of ETV1, ETV4 and ETV5 RNA in-situ hybridization in the diagnosis of CIC-DUX sarcomas. Histopathology. 2017;70:657–663. doi: 10.1111/his.13112. [DOI] [PubMed] [Google Scholar]
- 169.Kao YC, Sung YS, Chen CL, Zhang L, Dickson BC, Swanson D, Vaiyapuri S, Latif F, Alholle A, Huang SC, Hornick JL, Antonescu CR. ETV transcriptional upregulation is more reliable than RNA sequencing algorithms and FISH in diagnosing round cell sarcomas with CIC gene rearrangements. Genes Chromosomes Cancer. 2017;56:501–510. doi: 10.1002/gcc.22454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Le Guellec S, Velasco V, Pérot G, Watson S, Tirode F, Coindre JM. ETV4 is a useful marker for the diagnosis of CIC-rearranged undifferentiated round-cell sarcomas: a study of 127 cases including mimicking lesions. Mod Pathol. 2016;29:1523–1531. doi: 10.1038/modpathol.2016.155. [DOI] [PubMed] [Google Scholar]
- 171.Tu JJ, Rohan S, Kao J, Kitabayashi N, Mathew S, Chen YT. Gene fusions between TMPRSS2 and ETS family genes in prostate cancer: frequency and transcript variant analysis by RT-PCR and FISH on paraffin-embedded tissues. Mod Pathol. 2007;20:921–928. doi: 10.1038/modpathol.3800903. [DOI] [PubMed] [Google Scholar]
- 172.Urano F, Umezawa A, Yabe H, Hong W, Yoshida K, Fujinaga K, Hata J. Molecular analysis of Ewing’s sarcoma: another fusion gene, EWS-E1AF, available for diagnosis. Jpn J Cancer Res. 1998;89:703–711. doi: 10.1111/j.1349-7006.1998.tb03274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hsing M, Wang Y, Rennie PS, Cox ME, Cherkasov A. ETS transcription factors as emerging drug targets in cancer. Med Res Rev. 2020;40:413–430. doi: 10.1002/med.21575. [DOI] [PubMed] [Google Scholar]
- 174.Rahim S, Beauchamp EM, Kong Y, Brown ML, Toretsky JA, Üren A. YK-4-279 inhibits ERG and ETV1 mediated prostate cancer cell invasion. PLoS One. 2011;6:e19343. doi: 10.1371/journal.pone.0019343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rahim S, Minas T, Hong SH, Justvig S, Çelik H, Kont YS, Han J, Kallarakal AT, Kong Y, Rudek MA, Brown ML, Kallakury B, Toretsky JA, Üren A. A small molecule inhibitor of ETV1, YK-4-279, prevents prostate cancer growth and metastasis in a mouse xenograft model. PLoS One. 2014;9:e114260. doi: 10.1371/journal.pone.0114260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yu L, Wu X, Chen M, Huang H, He Y, Wang H, Li D, Du Z, Zhang K, Goodin S, Zheng X. The effects and mechanism of YK-4-279 in combination with docetaxel on prostate cancer. Int J Med Sci. 2017;14:356–366. doi: 10.7150/ijms.18382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Pop MS, Stransky N, Garvie CW, Theurillat JP, Hartman EC, Lewis TA, Zhong C, Culyba EK, Lin F, Daniels DS, Pagliarini R, Ronco L, Koehler AN, Garraway LA. A small molecule that binds and inhibits the ETV1 transcription factor oncoprotein. Mol Cancer Ther. 2014;13:1492–1502. doi: 10.1158/1535-7163.MCT-13-0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kollareddy M, Sherrard A, Park JH, Szemes M, Gallacher K, Melegh Z, Oltean S, Michaelis M, Cinatl J Jr, Kaidi A, Malik K. The small molecule inhibitor YK-4-279 disrupts mitotic progression of neuroblastoma cells, overcomes drug resistance and synergizes with inhibitors of mitosis. Cancer Lett. 2017;403:74–85. doi: 10.1016/j.canlet.2017.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Euhus D, Bu D, Xie XJ, Sarode V, Ashfaq R, Hunt K, Xia W, O’Shaughnessy J, Grant M, Arun B, Dooley W, Miller A, Flockhart D, Lewis C. Tamoxifen downregulates ets oncogene family members ETV4 and ETV5 in benign breast tissue: implications for durable risk reduction. Cancer Prev Res (Phila) 2011;4:1852–1862. doi: 10.1158/1940-6207.CAPR-11-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yen CC, Chen LT, Li CF, Chen SC, Chua WY, Lin YC, Yen CH, Chen YC, Yang MH, Chao Y, Fletcher JA. Identification of phenothiazine as an ETV1-targeting agent in gastrointestinal stromal tumors using the connectivity map. Int J Oncol. 2019;55:536–546. doi: 10.3892/ijo.2019.4829. [DOI] [PubMed] [Google Scholar]
- 181.Nath AI, Nair AS. Fingerprint-based similarity search identified p-anisidine as an anticancer agent in HeLa and a prospective phytochemical ETV1 transcription factor inhibitor. J Biomol Struct Dyn. 2020:1–8. doi: 10.1080/07391102.2020.1783364. [DOI] [PubMed] [Google Scholar]
- 182.Cooper CD, Newman JA, Aitkenhead H, Allerston CK, Gileadi O. Structures of the ets protein DNA-binding domains of transcription factors Etv1, Etv4, Etv5, and Fev: determinants of DNA binding and redox regulation by disulfide bond formation. J Biol Chem. 2015;290:13692–13709. doi: 10.1074/jbc.M115.646737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Orlando G, Law PJ, Cornish AJ, Dobbins SE, Chubb D, Broderick P, Litchfield K, Hariri F, Pastinen T, Osborne CS, Taipale J, Houlston RS. Promoter capture Hi-C-based identification of recurrent noncoding mutations in colorectal cancer. Nat Genet. 2018;50:1375–1380. doi: 10.1038/s41588-018-0211-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Currie SL, Doane JJ, Evans KS, Bhachech N, Madison BJ, Lau DKW, McIntosh LP, Skalicky JJ, Clark KA, Graves BJ. ETV4 and AP1 transcription factors form multivalent interactions with three sites on the MED25 activator-interacting domain. J Mol Biol. 2017;429:2975–2995. doi: 10.1016/j.jmb.2017.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


