Abstract
The aim of the study was to evaluate the relationship between tumor mutational burden (TMB) and immune infiltration in ovarian cancer. We extracted somatic mutational data and gene expression profiles of ovarian cancer from The Cancer Genome Atlas (TCGA). The samples were separated into low and high TMB groups. Correlations between TMB and cancer prognosis were analyzed and immune cell infiltration in the high and low TMB subgroups was calculated using the CIBERSORT package software. High TMB was significantly related to an improved survival rate. We identified 4 TMB-related core genes that were significantly associated with prognosis. Furthermore, mutations in the 4 genes were associated with immune cell infiltration. We also found a high proportion of naive B cells and activated NK cells in the high TMB group, while increased proportions of memory B cells and plasma cells were found in the low TMB group. Overall, our study indicated that patients with a higher TMB level experienced a favorable survival outcome and this may influence immune infiltration in ovarian cancer. Furthermore, the 4 TMB-related core genes were highly correlated with prognosis and the level of immune cell infiltration.
Keywords: Tumor mutation burden, immune infiltration, CIBERSORT, ovarian cancer, prognosis, TCGA
Introduction
Ovarian cancer is one of the top 10 most common cancers in females. Globally, about 239,000 new cases are diagnosed and 152,000 patients die from this disease each year [1]. Due to the asymptomatic nature that is characteristic of early stage ovarian cancer and the lack of reliable cancer-specific screening biomarkers, most ovarian cancers are diagnosed at advanced stages [2]. Prognosis of ovarian cancer is poor, while the 5-year survival of early stage I cases is 70%, and the rate for late-stage III or IV cases is < 29% [3]. The therapeutic schedule for ovarian cancer consists of surgery, chemotherapy, radiotherapy, and targeted therapy. However, current treatment options available for ovarian cancer have been unsuccessful in achieving a long-term survival benefit [4].
Immunotherapy has been considered as a promising therapeutic option, and immune checkpoint inhibitor therapies play a vital role in treating certain solid malignant tumors, including lung and kidney cancer [5]. In early 2003, a study showed that the occurrence of CD3+ tumor-infiltrating lymphocytes was associated with higher overall survival of advanced stage ovarian cancer patients [6]. Furthermore, high expression levels of programmed death ligand 1 (PD-L1) or programmed cell death 1 ligand 2 (PD-L2) have been proven to be significantly related to worse overall survival in ovarian cancer [7]. The objective response rate is relatively poor using single agent immunotherapy in unselected ovarian cancer [8], while the immune microenvironment of tumors might influence the efficacy of immunotherapy. Therefore, it is necessary to explore the biologic mechanism behind immunotherapy responses.
Several studies have investigated the correlation between tumor mutation burden (TMB) and immunotherapy response [9,10]. Mutations can generate novel peptide sequences, which may affect the immune response [11]. In recent years, the development of bioinformatics approaches, such as CIBERSORT, has allowed researchers to investigate TMB and immune infiltration using transcriptomic data obtained from various cancers. Wang et al. implied that the prognosis of TMB and the association between TMB and immune infiltration varies between tumor types [12]. Nevertheless, only a few studies have investigated the association between TMB and immune infiltration in ovarian cancer. Therefore, we utilized bioinformatics to analyze the prognostic role of TMB and to explore the interaction between TMB and immune infiltration in ovarian cancer.
Materials and methods
Data collection and processing
The Cancer Genome Atlas (TCGA) is an open-access database that contains an abundance of cancer-related data (https://cancergenome.nih.gov/) [13]. Exome somatic mutational data and raw mRNA data were acquired from the TCGA database. Since raw single nucleotide polymorphisms (SNP) data is not available to the public, we acquired SNP-related data that had already been processed. Thus, genes with mutations were identified using SNP-related data. Overall, data on 379 ovarian cancer samples was collected. Mutation Annotation Format (MAF) files were analyzed using the VarScan method and visualized using the Maftools software package.
Evaluation of TMB and associations with prognosis
TMB refers to the total number of somatic genetic errors, base substitutions, gene insertions, and deletion errors found among a million bases. Using the Perl scripts established through the JAVA8 platform, mutation frequency was calculated using the amount of variants and the size of the exons (38 million) in each ovarian cancer sample. We further stratified the ovarian cancer samples into either a low or high TMB group based on the median TMB value. Using Kaplan-Meier analysis, we assessed the effect of low and high TMB on the survival of patients. In addition, we used the Kruskal-Wallis (K-W) test to further explore the relationship between the level of TMB and tumor stage.
Identification of DEGs and functional enrichment analysis
Based on the level of TMB in the ovarian cancer samples, we classified transcriptomic data into either a low or a high TMB subgroup. The LIMMA software package was applied to screen for differentially expressed genes (DEGs) between both cohorts, with a p value of < 0.05 and a |log fold change (FC)| of > 1. The pheatmap software package was used to visualize differences. GeneMANIA is a flexible web platform that contains a large set of functional association data (http://www.genemania.org) [14]. The DEGs were uploaded to GeneMANIA to obtain the associated Gene Ontology (GO) terms and pathways.
Core genes and immune cell infiltration
Kaplan-Meier analysis was conducted to identify core TMB-related genes that significantly affected the prognosis of ovarian cancer. A P value of < 0.05 was considered significant.
CIBERSORT is a widely used method to evaluate diverse immune cell types in the cancer microenvironment. This method de-convolutes the expression matrix of immune cell subtypes based on the principle of linear support vector regression. It contains 547 biomarkers and distinguishes between 22 human hematopoietic cell phenotypes, including T cells, B cells, plasma cells, and myeloid cell subsets. RNA-seq data obtained from ovarian cancer patients in the low and high TMB groups were normalized using the LIMMA software package. We applied the CIBERSORT algorithm to analyze the RNA-seq data and further estimated the proportion of 22 tumor-infiltrating immune cells. The results were consistent with those of a prior report [15]. The violin software package was used to visualize differentially infiltrated immune cells between the two groups through the Wilcoxon test.
Results
Landscape of mutation profiles in the ovarian cancer samples
A total of 379 ovarian cancer samples were obtained from TCGA database. Missense mutation was the most frequent variant classification (Figure 1A), while single nucleotide polymorphism (SNP) was the most frequent variant (Figure 1B), and C > T was the most frequent single nucleotide variant (SNV) (Figure 1C). The top 10 frequently mutated genes were TP53 (90%), TTN (21%), TOP2A (6%), MUC16 (7%), NF1 (6%), CSMD3 (6%), RYR2 (5%), HMCN1 (5%), USH2A (6%), and FAT3 (5%) (Figure 1F).
Figure 1.
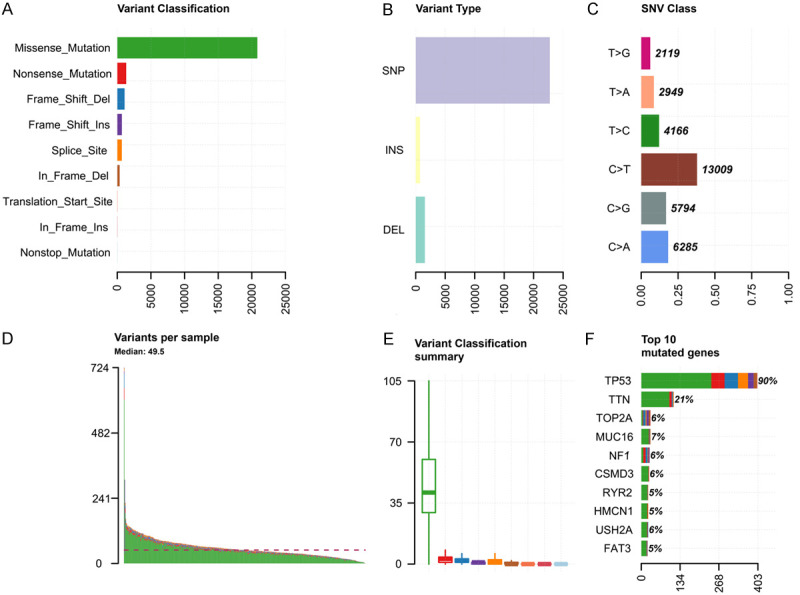
Primary genetic alterations in ovarian cancer samples. A. Classification of genetic variations in ovarian cancer. B. Types of genetic alteration in ovarian cancer. C. Classification of SNVs in ovarian cancer. D. Genetic alterations in each sample of ovarian cancer. E. Summary of genetic variant classification in ovarian cancer. F. Top 10 mutated genes in ovarian cancer. SNV, single nucleotide variants; TMB, tumor mutation burden.
TMB level is associated with survival prognosis and tumor grade
The TMB score was calculated based on the total number of mutational events in each sample. All ovarian cancer samples were divided into low and high TMB subgroups based on the median TMB score. Kaplan-Meier plots showed that an elevated level of TMB led to an improved survival rate (P = 0.033). Furthermore, elevated TMB levels were related to advanced tumor grades in ovarian cancer (P = 0.013), as shown in Figure 2.
Figure 2.
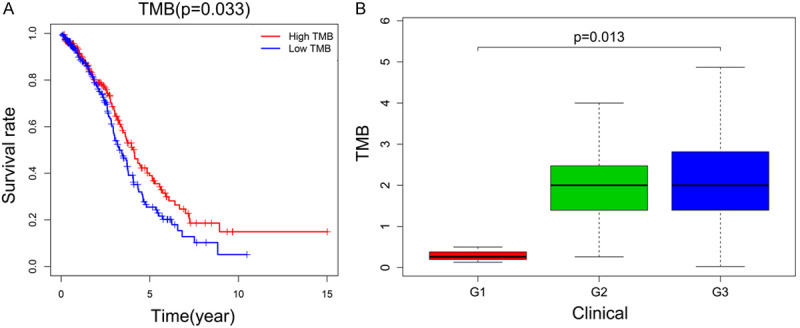
Association between TMB and prognosis, as well as the tumor grade of ovarian cancer patients. A. High TMB expression was correlated with favorable survival outcomes. B. High TMB expression was associated with advanced tumor grade. TMB, tumor mutation burden.
Functional enrichment and survival analysis of the DEGs
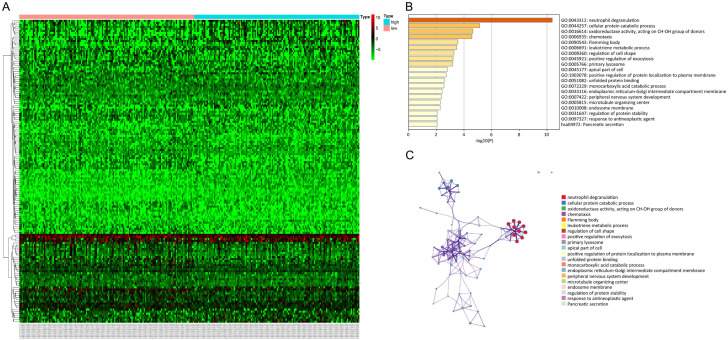
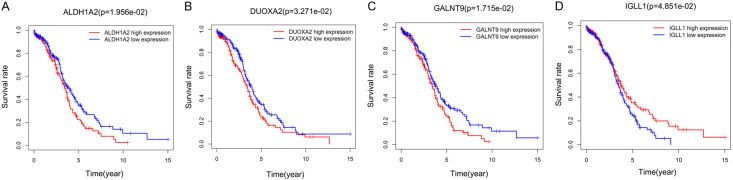
Differential genetic analysis of the low and high TMB groups were conducted using the LIMMA software package, with a cut-off values of |log FC| > 1 and P < 0.05. Overall, 150 DEGs were identified. The results from the enrichment analysis suggested that the DEGs were substantially enriched in the GO terms neutrophil degranulation, cellular protein catabolic process, oxidoreductase activity, and targeting of CH-OH group of donors, as shown in Figure 3. Furthermore, 4 core TMB-related genes that were significantly correlated with prognosis were identified. The increased expression of aldehyde dehydrogenase 1 family member A2 (ALDH1A2), dual oxidase maturation factor 2 (DUOXA2) and polypeptide N-acetylgalactosaminyltransferase 9 (GALNT9) were associated with a worse survival rate, while a higher expression level of immunoglobulin lambda like polypeptide 1 (IGLL1) was associated with a better survival rate in ovarian cancer patients (Figure 4).
Figure 3.
Comparison of the gene expression profiles between the low and high TMB group. A. Using cut-off values of |log FC| > 1 and P < 0.05, a total of 150 DEGs were identified. The Heatmap depicts the expression of the DEGs in the two groups. Each column indicates the expression level of a specific DEG, while each row indicates a sample. B, C. Functional enrichment analysis of the 150 DEGs using the Genemania platform. The top 20 GO terms and pathways are shown. DEGs, differentially expressed genes; TMB, tumor mutation burden; GO, Gene Ontology.
Figure 4.
Survival curves of the 4 core TMB-related genes. A-D. ALDH1A2, DUOXA2, and GALNT9 were negatively associated with survival rate, while IGLL1 was positively associated with survival rate. ALDH1A2, aldehyde dehydrogenase 1 family member A2; DUOXA2, dual oxidase maturation factor 2; GALNT9, polypeptide N-acetylgalactosaminyltransferase 9; IGLL1, immunoglobulin lambda like polypeptide 1.
Distribution of immune cells
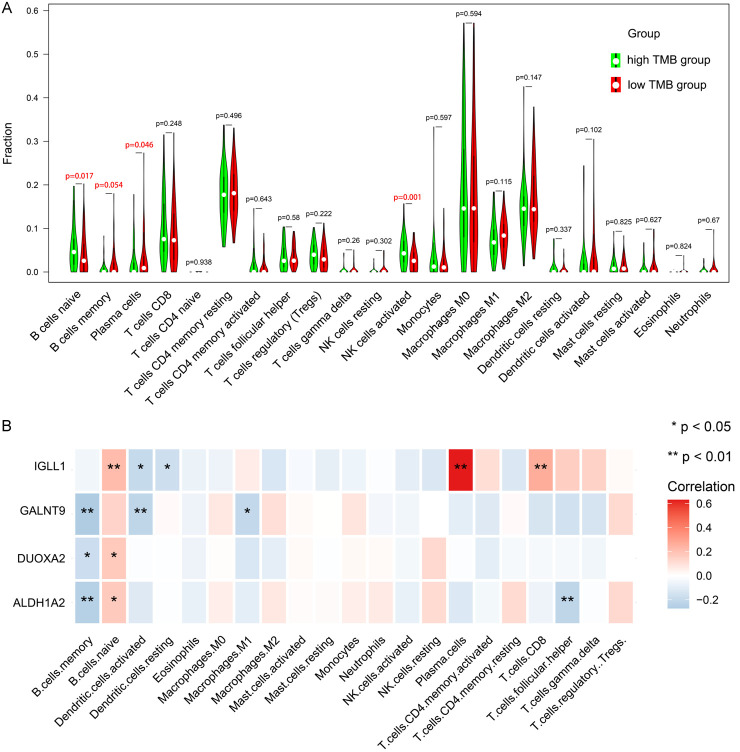
By applying the CIBERSORT algorithm, we estimated the differential variation of immune cell infiltration in the low and high TMB groups of ovarian cancer. The bar plot and heat map showed that 22 immune cell subtypes infiltrated both groups (Figure S1). The correlation matrix showed that T regulatory (Tregs) and CD8 + T cells showed the strongest association (Pearson correlation = 0.48), while macrophages M0 and CD8+ T cells displayed the strongest negative association (Pearson correlation = -0.50) (Figure S2). Wilcoxon rank-sum test indicated that naive B cell (P = 0.017) and activated NK cell (P = 0.001) levels were elevated in the high TMB group. Contrastingly, the infiltration levels of memory B cells (P = 0.054) and plasma cells (P = 0.046) were lower in the high TMB group. Additionally, high expression levels of GALNT9, DUOXA2, and ALDH1A2 were negatively correlated with Memory B cell infiltration, while high expression levels of IGLL1, DUOXA2, and ALDH1A2 were positively correlated with naive B cell infiltration, as shown in Figure 5.
Figure 5.
TMB level and gene expression were correlated with the infiltration of immune cells in ovarian cancer. A. Differentially infiltrated immune cells between the low and high TMB groups. B. Differentially infiltrated immune cells among the 4 core TMB-related DEGs. DEGs, differentially expressed genes; TMB, tumor mutation burden.
Discussion
Over the last decade, the occurrence of ovarian cancer has increased by 30% and mortality has increased by 18% in China [16]. A family history of breast or ovarian cancer is the most predictable risk factor for the development of ovarian cancer, as approximately 25% of all ovarian cancer patients have been found to show a heritable genetic risk [17]. Therefore, elucidating the mechanism by which genetic alterations in ovarian cancer may contribute to individual diagnostic and therapeutic strategies has important clinical implications for their family members [18].
In this study, we found that the mutational frequency of the TP53 gene was high, which is similar to that of prior reports. Ross et al. showed that a mutation of the TP53 gene occurred in 79% of cases with ovarian cancer, with a higher frequency in papillary serous carcinoma, compared with that of non-papillary serous tumors (83% vs. 50%) [19]. Zhang et al. also found that the most common genomic alteration in ovarian cancer patients was of the TP53 gene [20].
Recently, tumor immunotherapy has gained much attention. Compared with conventional tumor treatment, immunotherapy can eliminate tumor cells by improving the immune function of the human body [21]. Nevertheless, only a small number of patients are able to take advantage of immunotherapy and even fewer patients achieve a favorable response [22]. It has been reported that TMB can be used to predict cancer behavior and immunotherapy response in several tumors [23]. However, it remains unknown whether TMB levels can influence survival outcomes and immune infiltration in ovarian cancer.
In this analysis, higher TMB levels were found to be associated with an advanced tumor grade and better survival rate. Wang et al. found that TMB levels were substantially higher in advanced stage gynecological cancers [24]. Birkbak et al. showed that ovarian cancer patients with a high mutational burden showed better postoperative chemotherapy outcomes and increased overall survival [25]. Furthermore, high TMB patients could acquire a favorable prognosis in cutaneous melanoma [26]. Tumor mutations are consider to be a vital process in the development of anti-cancer immunity, and it is well demonstrated that heavily mutated tumors harbor more neoantigens, which allow them to become a target of stimulated immune cells [10].
The TMB-related DEGs were found to be enriched in several GO terms including neutrophil degranulation, chemotaxis, and leukotriene metabolic process. Neutrophil degranulation can lead to differential cell-surface protein expression and changes in cell function. A study showed that neutrophil degranulation may be involved in cancer advancement and metastasis. Therefore, neutrophils may be targeted by anti-cancer therapy [27]. Chemotaxis can cause the upregulation of EMT markers and matrix metalloproteinases, which are associated with the development of ovarian cancer [28]. Leukotriene B4 receptor-2 contributed to ovarian cancer invasion and metastasis by activating STAT3 and elevating MMP2 [29].
Additionally, the 4 core DEGs were significantly correlated with prognosis and immune cell infiltration. ALDH1A2, DUOXA2 and GALNT9 were negatively associated with survival rate, while IGLL1 was positively associated with survival rate. ALDH1A2, DUOXA2 and GALNT9 were negatively associated with memory B cells infiltration, while ALDH1A2, DUOXA2, and IGLL1 were positively associated with naïve B cell infiltration. ALDH1A2 is a widespread marker of stem cells that converts retinaldehyde into retinoic acid. A high expression level of ALDH1A2 results in a poor survival outcome in liver cancer and ovarian cancer [30-32], while in prostate cancer and head and neck squamous cell carcinoma, low ALDH1A2 expression leads to an unfavorable survival outcome prognosis [33,34]. DUOXA2 is a type of ROS-generating enzyme that is involved in the development of cancer, which regulates mucin, reactive oxygen species (ROS) and IL-8 expression [35]. Previous studies have shown that GALNT9 is overexpressed in ovarian cancer. IGLL1 belongs to the immunoglobulin gene superfamily [36,37]. Moreover, IGLL1 is considered to be associated with B cell-mediated immunodeficiency, and mutation of IGLL1 can lead to B cell deficiency, with few antibodies being generated [38].
On the other hand, high TMB levels were significantly correlated with a higher fraction of naive B cells and activated NK cells. In the low TMB subgroup, the proportion of memory B cells and plasma cells were elevated. Many cancers are characterized by immune cell infiltration [39]. Other studies have also found that TMB levels have an impact on immune cell infiltration signatures of bladder cancer and clear cell renal cell carcinoma [40,41]. These findings reveal that TMB may affect the tumor microenvironment and that TMB-associated genes may lead to variations in the tumor microenvironment.
Both memory B cell and naive B cell infiltration have been linked with the expressions of the core DEGs and TMB level in our study. Previous studies have demonstrated that the infiltration of B cells plays an important role in tumor immunotherapy [42-44]. A report by Beth et al. investigated the response rate of melanoma patients who received immune checkpoint blockade treatment, and the results of single cell sequencing and flow cytometry analysis showed that the proportions of memory B cells in the tumors of responders were significantly higher than non-responders [42]. For patients with solid malignancies who have received chemotherapy, the proportion of memory B cells was significantly decreased after chemotherapy [45]. In the study by Wei et al. naive B cells were observed to suppress the antitumor adaptive immune response in ovarian cancer patients [46]. Ren et al. proposed that naive B cells could be considered as a readily available and effective source of antigen-presenting cells in clinical research on tumor immunotherapy [47]. Taken together, the expression of the 4 core TMB-related genes and TMB level may affect immune cell infiltrates and ultimately affect the prognosis of ovarian cancer patients.
To date, this is the first study to comprehensively investigate the interaction between TMB and immune infiltration in ovarian cancer. However, there are some limitations in this study. First, the data were obtained from a single database, rather than multiple databases. Therefore, these findings require verification through large-scale clinical studies and basic experiments. Despite the limitations in the study, our results can still provide a reasonable basis for further studies of immunity in ovarian cancer and can be expanded to study other types of cancer. Finally, we identified several core genes, which could provide new clues for the further study of ovarian cancer.
In summary, high TMB was correlated with a promising prognosis in ovarian cancer, and several TMB-related genes, which might affect the immune cell infiltration, were identified. This study indicated that a deeper understanding of interactions among TMB and immune cells in ovarian cancer would be useful to further investigate the role of TMB in ovarian cancer.
Acknowledgements
This study was supported by the Basic Ability Enhancement Project of Young Teachers in Guangxi Zhuang Autonomous Region (No. 2018KY0134), Guangxi Science and Technology Cooperation and Exchange Project (GKH 159905-2-11), Central Guided Local Science and Technology Development Project (GKZY18076006), Guangxi Science and Technology Program Project (GK AD17129013).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Lheureux S, Braunstein M, Oza AM. Epithelial ovarian cancer: evolution of management in the era of precision medicine. CA Cancer J Clin. 2019;69:280–304. doi: 10.3322/caac.21559. [DOI] [PubMed] [Google Scholar]
- 2.Feng W, Dean DC, Hornicek FJ, Shi H, Duan Z. Exosomes promote pre-metastatic niche formation in ovarian cancer. Mol Cancer. 2019;18:124. doi: 10.1186/s12943-019-1049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 4.Zheng M, Hu Y, Gou R, Liu O, Nie X, Li X, Liu Q, Hao Y, Liu J, Lin B. Identification of immune-enhanced molecular subtype associated with BRCA1 mutations, immune checkpoints and clinical outcome in ovarian carcinoma. J Cell Mol Med. 2020;24:2819–2831. doi: 10.1111/jcmm.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drake CG, Lipson EJ, Brahmer JR. Breathing new life into immunotherapy: review of melanoma, lung and kidney cancer. Nat Rev Clin Oncol. 2014;11:24–37. doi: 10.1038/nrclinonc.2013.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–13. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 7.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, Honjo T, Fujii S. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:3360–5. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tian W, Shan B, Zhang Y, Ren Y, Liang S, Zhao J, Zhao Z, Wang G, Zhao X, Peng D, Bi R, Cai S, Bai Y, Wang H. Association between DNA damage repair gene somatic mutations and immune-related gene expression in ovarian cancer. Cancer Med. 2020;9:2190–2200. doi: 10.1002/cam4.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359:1361–5. doi: 10.1126/science.aar6711. [DOI] [PubMed] [Google Scholar]
- 10.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, Miller ML, Rekhtman N, Moreira AL, Ibrahim F, Bruggeman C, Gasmi B, Zappasodi R, Maeda Y, Sander C, Garon EB, Merghoub T, Wolchok JD, Schumacher TN, Chan TA. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–8. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, Schrock A, Campbell B, Shlien A, Chmielecki J, Huang F, He Y, Sun J, Tabori U, Kennedy M, Lieber DS, Roels S, White J, Otto GA, Ross JS, Garraway L, Miller VA, Stephens PJ, Frampton GM. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9:34. doi: 10.1186/s13073-017-0424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Li M. Correlate tumor mutation burden with immune signatures in human cancers. BMC Immunol. 2019;20:4. doi: 10.1186/s12865-018-0285-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomczak K, Czerwińska P, Wiznerowicz M. The Cancer Genome Atlas (TCGA): an immeasurable source of knowledge. Contemp Oncol (Pozn) 2015;19:A68–77. doi: 10.5114/wo.2014.47136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franz M, Rodriguez H, Lopes C, Zuberi K, Montojo J, Bader GD, Morris Q. GeneMANIA update 2018. Nucleic Acids Res. 2018;46:W60–4. doi: 10.1093/nar/gky311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiong Y, Wang K, Zhou H, Peng L, You W, Fu Z. Profiles of immune infiltration in colorectal cancer and their clinical significant: a gene expression-based study. Cancer Med. 2018;7:4496–508. doi: 10.1002/cam4.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 17.Walsh T, Casadei S, Lee MK, Pennil CC, Nord AS, Thornton AM, Roeb W, Agnew KJ, Stray SM, Wickramanayake A, Norquist B, Pennington KP, Garcia RL, King MC, Swisher EM. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc Natl Acad Sci U S A. 2011;108:18032–7. doi: 10.1073/pnas.1115052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Konstantinopoulos PA, Lacchetti C, Annunziata CM. Germline and somatic tumor testing in epithelial ovarian cancer: ASCO guideline summary. JCO Oncol Pract. 2020;16:e835–e838. doi: 10.1200/JOP.19.00773. [DOI] [PubMed] [Google Scholar]
- 19.Ross JS, Ali SM, Wang K, Palmer G, Yelensky R, Lipson D, Miller VA, Zajchowski D, Shawver LK, Stephens PJ. Comprehensive genomic profiling of epithelial ovarian cancer by next generation sequencing-based diagnostic assay reveals new routes to targeted therapies. Gynecol Oncol. 2013;130:554–9. doi: 10.1016/j.ygyno.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Luo M, Yang H, Zhu S, Cheng X, Qing C. Next-generation sequencing-based genomic profiling analysis reveals novel mutations for clinical diagnosis in Chinese primary epithelial ovarian cancer patients. J Ovarian Res. 2019;12:19. doi: 10.1186/s13048-019-0494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Li F, Jiang F, Lv X, Zhang R, Lu A, Zhang G. A mini-review for cancer immunotherapy: molecular understanding of PD-1/PD-L1 pathway & translational blockade of immune checkpoints. Int J Mol Sci. 2016;17:1151. doi: 10.3390/ijms17071151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coukos G, Tanyi J, Kandalaft LE. Opportunities in immunotherapy of ovarian cancer. Ann Oncol. 2016;27(Suppl 1):i11–i15. doi: 10.1093/annonc/mdw084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodman AM, Kato S, Bazhenova L, Patel SP, Frampton GM, Miller V, Stephens PJ, Daniels GA, Kurzrock R. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther. 2017;16:2598–608. doi: 10.1158/1535-7163.MCT-17-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang M, Fan W, Ye M, Tian C, Zhao L, Wang J, Han W, Yang W, Gu C, Li M, Zhang Z, Wang Y, Zhang H, Meng Y. Molecular profiles and tumor mutational burden analysis in Chinese patients with gynecologic cancers. Sci Rep. 2018;8:8990. doi: 10.1038/s41598-018-25583-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Birkbak NJ, Kochupurakkal B, Izarzugaza JM, Eklund AC, Li Y, Liu J, Szallasi Z, Matulonis UA, Richardson AL, Iglehart JD, Wang ZC. Tumor mutation burden forecasts outcome in ovarian cancer with BRCA1 or BRCA2 mutations. PLoS One. 2013;8:e80023. doi: 10.1371/journal.pone.0080023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta S, Artomov M, Goggins W, Daly M, Tsao H. Gender disparity and mutation burden in metastatic melanoma. J Natl Cancer Inst. 2015;107:djv221. doi: 10.1093/jnci/djv221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mollinedo F. Neutrophil degranulation, plasticity, and cancer metastasis. Trends Immunol. 2019;40:228–42. doi: 10.1016/j.it.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Singh SK, Mishra MK, Singh R. Hypoxia-inducible factor-1α induces CX3CR1 expression and promotes the epithelial to mesenchymal transition (EMT) in ovarian cancer cells. J Ovarian Res. 2019;12:42. doi: 10.1186/s13048-019-0517-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seo JM, Park S, Kim JH. Leukotriene B4 receptor-2 promotes invasiveness and metastasis of ovarian cancer cells through signal transducer and activator of transcription 3 (STAT3)-dependent up-regulation of matrix metalloproteinase 2. J Biol Chem. 2012;287:13840–9. doi: 10.1074/jbc.M111.317131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu X, Gao Y, Zhao B, Li X, Lu Y, Zhang J, Li D, Li L, Yin F. Discovery of microarray-identified genes associated with ovarian cancer progression. Int J Oncol. 2015;46:2467–78. doi: 10.3892/ijo.2015.2971. [DOI] [PubMed] [Google Scholar]
- 31.Ma YM, Zhao S. Prognostic values of aldehyde dehydrogenase 1 isoenzymes in ovarian cancer. Onco Targets Ther. 2016;9:1981–8. doi: 10.2147/OTT.S101063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zou RC, Xiao SF, Shi ZT, Ke Y, Tang HR, Wu TG, Guo ZT, Ni F, Li WX, Wang L. Identification of metabolism-associated pathways and genes involved in male and female liver cancer patients. J Theor Biol. 2019;480:218–28. doi: 10.1016/j.jtbi.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 33.Kim H, Lapointe J, Kaygusuz G, Ong DE, Li C, van de Rijn M, Brooks JD, Pollack JR. The retinoic acid synthesis gene ALDH1a2 is a candidate tumor suppressor in prostate cancer. Cancer Res. 2005;65:8118–24. doi: 10.1158/0008-5472.CAN-04-4562. [DOI] [PubMed] [Google Scholar]
- 34.Seidensaal K, Nollert A, Feige AH, Muller M, Fleming T, Gunkel N, Zaoui K, Grabe N, Weichert W, Weber KJ, Plinkert P, Simon C, Hess J. Impaired aldehyde dehydrogenase 1 subfamily member 2A-dependent retinoic acid signaling is related with a mesenchymal-like phenotype and an unfavorable prognosis of head and neck squamous cell carcinoma. Mol Cancer. 2015;14:204. doi: 10.1186/s12943-015-0476-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang J, Wang X, Xu L, Zhang Z, Wang F, Tang X. Investigation of potential genetic biomarkers and molecular mechanism of ulcerative colitis utilizing bioinformatics analysis. Biomed Res Int. 2020;2020:4921387. doi: 10.1155/2020/4921387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheta R, Bachvarova M, Plante M, Gregoire J, Renaud MC, Sebastianelli A, Popa I, Bachvarov D. Altered expression of different GalNAc transferases is associated with disease progression and poor prognosis in women with high-grade serous ovarian cancer. Int J Oncol. 2017;51:1887–97. doi: 10.3892/ijo.2017.4147. [DOI] [PubMed] [Google Scholar]
- 37.Keita M, Wang ZQ, Pelletier JF, Bachvarova M, Plante M, Gregoire J, Renaud MC, Mes-Masson AM, Paquet ÉR, Bachvarov D. Global methylation profiling in serous ovarian cancer is indicative for distinct aberrant DNA methylation signatures associated with tumor aggressiveness and disease progression. Gynecol Oncol. 2013;128:356–63. doi: 10.1016/j.ygyno.2012.11.036. [DOI] [PubMed] [Google Scholar]
- 38.Hu K, Chen F. Identification of significant pathways in gastric cancer based on protein-protein interaction networks and cluster analysis. Genet Mol Biol. 2012;35:701–8. doi: 10.1590/S1415-47572012005000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jin Y, Qin X. Profiles of immune cell infiltration and their clinical significance in head and neck squamous cell carcinoma. Int Immunopharmacol. 2020;82:106364. doi: 10.1016/j.intimp.2020.106364. [DOI] [PubMed] [Google Scholar]
- 40.Wu Z, Wang M, Liu Q, Liu Y, Zhu K, Chen L, Guo H, Li Y, Shi B. Identification of gene expression profiles and immune cell infiltration signatures between low and high tumor mutation burden groups in bladder cancer. Int J Med Sci. 2020;17:89–96. doi: 10.7150/ijms.39056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang C, Li Z, Qi F, Hu X, Luo J. Exploration of the relationships between tumor mutation burden with immune infiltrates in clear cell renal cell carcinoma. Ann Transl Med. 2019;7:648. doi: 10.21037/atm.2019.10.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, Yizhak K, Sade-Feldman M, Blando J, Han G, Gopalakrishnan V, Xi Y, Zhao H, Amaria RN, Tawbi HA, Cogdill AP, Liu W, LeBleu VS, Kugeratski FG, Patel S, Davies MA, Hwu P, Lee JE, Gershenwald JE, Lucci A, Arora R, Woodman S, Keung EZ, Gaudreau PO, Reuben A, Spencer CN, Burton EM, Haydu LE, Lazar AJ, Zapassodi R, Hudgens CW, Ledesma DA, Ong S, Bailey M, Warren S, Rao D, Krijgsman O, Rozeman EA, Peeper D, Blank CU, Schumacher TN, Butterfield LH, Zelazowska MA, McBride KM, Kalluri R, Allison J, Petitprez F, Fridman WH, Sautès-Fridman C, Hacohen N, Rezvani K, Sharma P, Tetzlaff MT, Wang L, Wargo JA. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020;577:549–55. doi: 10.1038/s41586-019-1922-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petitprez F, de Reyniès A, Keung EZ, Chen TW, Sun CM, Calderaro J, Jeng YM, Hsiao LP, Lacroix L, Bougoüin A, Moreira M, Lacroix G, Natario I, Adam J, Lucchesi C, Laizet YH, Toulmonde M, Burgess MA, Bolejack V, Reinke D, Wani KM, Wang WL, Lazar AJ, Roland CL, Wargo JA, Italiano A, Sautès-Fridman C, Tawbi HA, Fridman WH. B cells are associated with survival and immunotherapy response in sarcoma. Nature. 2020;577:556–60. doi: 10.1038/s41586-019-1906-8. [DOI] [PubMed] [Google Scholar]
- 44.Cabrita R, Lauss M, Sanna A, Donia M, Skaarup Larsen M, Mitra S, Johansson I, Phung B, Harbst K, Vallon-Christersson J, van Schoiack A, Lövgren K, Warren S, Jirström K, Olsson H, Pietras K, Ingvar C, Isaksson K, Schadendorf D, Schmidt H, Bastholt L, Carneiro A, Wargo JA, Svane IM, Jönsson G. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. 2020;577:561–5. doi: 10.1038/s41586-019-1914-8. [DOI] [PubMed] [Google Scholar]
- 45.Waidhauser J, Schuh A, Trepel M, Schmälter AK, Rank A. Chemotherapy markedly reduces B cells but not T cells and NK cells in patients with cancer. Cancer Immunol Immunother. 2020;69:147–57. doi: 10.1007/s00262-019-02449-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei X, Jin Y, Tian Y, Zhang H, Wu J, Lu W, Lu X. Regulatory B cells contribute to the impaired antitumor immunity in ovarian cancer patients. Tumour Biol. 2016;37:6581–8. doi: 10.1007/s13277-015-4538-0. [DOI] [PubMed] [Google Scholar]
- 47.Ren H, Zhao S, Li W, Dong H, Zhou M, Cao M, Hu HM, Wang LX. Therapeutic antitumor efficacy of B cells loaded with tumor-derived autophagasomes vaccine (DRibbles) J Immunother. 2014;37:383–93. doi: 10.1097/CJI.0000000000000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.