Abstract
We investigated the clinicopathologic features, immunophenotype, (differential) diagnosis, pathogenesis, treatment, and follow-up of medullary thyroid carcinoma (MTC) combined with papillary thyroid carcinoma (PTC). A retrospective analysis of the clinical and pathologic features and immunophenotype was conducted in a patient with MTC and PTC. Relevant literature was also reviewed. Results of thyroid fine needle aspiration indicated malignant tumor in the right lobe of the thyroid, suggesting PTC; further analysis by biopsy confirmed this diagnosis. The left lobe exhibited MTC. Tumor metastases were absent from the lymph nodes of the left central area (0/2), and no tumor was present in the thymic tissue. In the right lobe and isthmus, PTC was observed, with a maximum infiltration diameter of 0.8 cm, and tumor metastases were absent from lymph nodes of the right central area (0/3). Immunohistochemistry of the left lobe was positive for calcitonin, CK, TTF-1, CD56, CgA, and Congo red, but negative for CK19, thyroglobulin, galectin-3, MC, and CEA, with a Ki-67 proliferation index of 1%. The right lobe was positive for CK19, galectin-3, and MC, but negative for CD56. The V600E mutation was detected in BRAF. MTC combined with PTC is a rare thyroid tumor. This condition is diagnosed mainly based on morphology, immunophenotyping, and molecular detection. It must be distinguished from other malignancies, such as thyroid follicular tumors, undifferentiated carcinoma, poorly differentiated carcinoma, transparent stellate tumor, and mixed PTC/MTC. Surgery and post-operative drug administration currently constitute the preferred treatments.
Keywords: Medullary thyroid carcinoma, papillary thyroid carcinoma, immunophenotype, differential diagnosis, pathogenesis
Introduction
Thyroid cancer is a common tumor. Its incidence rate has increased over the past years, with an annual increase of approximately 6.2% [1,2]. There are four common pathologic forms of thyroid cancer: papillary carcinoma (70% to 80%), follicular carcinoma (10.0% to 27.8%), medullary carcinoma (5% to 10%), and undifferentiated cancer (3% to 8%). Papillary thyroid carcinoma (PTC) is a malignant tumor derived from the thyroid follicular epithelium, whereas medullary thyroid carcinoma (MTC) is a neuroendocrine tumor derived from thyroid follicular C cells. Treatment and follow-up strategies for these conditions are not identical due to their different cellular origins. Concomitant MTC and PTC are rare, accounting for only 0.15% of thyroid tumors [3,4]. In the past 30 years, a total of only 18 cases have been reported in the literature. Here, we report a case of MTC combined with PTC, and provide a literature review to summarize the clinical and pathologic features, immunophenotypes, (differential) diagnosis, and pathogenesis associated with these tumors, as well as options for their treatment and follow-up.
Materials and methods
Clinical manifestation
A 53-year-old female patient was admitted to the hospital with a two-lobed thyroid nodule identified 12 years prior. The bilateral thyroid gland presented with a palpable mass measuring 1-2 cm in diameter, with good mobility and clear boundaries. Thyroid ultrasonography demonstrated a solid, mixed, echogenic nodule, mainly showing cystic features with clear boundaries, which was surrounded by a strip of blood flow signal measuring 22 × 11 mm in size (Figure 1A). Few hypoechoic nodules measuring 10 × 6 mm were observed in the right lobe (Figure 1B). The boundaries were clear, and the internal echo was uneven, with an internal punctate blood flow signal. Multiple solid hypoechoic flat nodules were found on both sides of the neck. The borders of the nodules were clear, as were the cortex and medulla. The nodule on the left side was larger than that on the right (13 × 5 mm vs. 9 × 6 mm).
Figure 1.
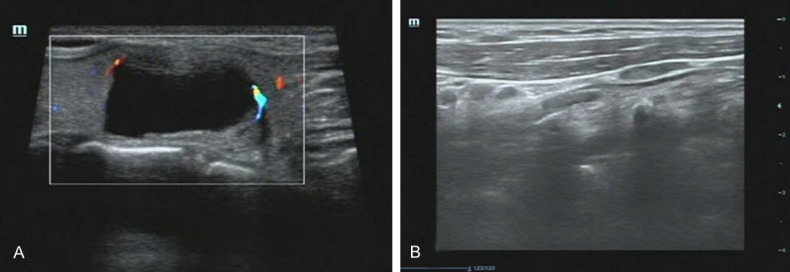
A. Thyroid ultrasonography demonstrated left thyroid cystic-solid mixed echo nodule; B. Thyroid ultrasonography demonstrated multiple hypoechoic nodules in right lobe.
Immunohistochemistry
Specimens were fixed in 10% neutral formalin, embedded in paraffin, and stained with hematoxylin and eosin (H&E). The streptavidin-peroxidase (SP) method was used for immunohistochemistry, and the antibodies used were purchased from Fuzhou Maixin Biotechnology Development Co.
Results
Macroscopic morphology
Thyroid fine needle aspiration was indicative of malignant tumor in the right lobe (according to the TBSRTC), suggesting PTC, and thus, further examination by biopsy was recommended to be done to confirm this diagnosis (Figure 2A, 2B).
Figure 2.
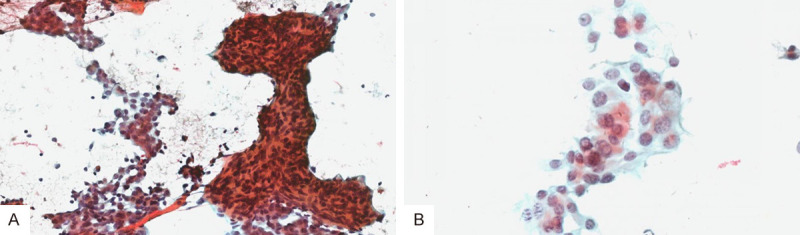
Thyroid fine needle aspiration results. A. Fine needle aspiration of right lobe showed medullary carcinoma with pasteurization (magnification × 200); B. Fine needle aspiration of the right lobe showed papillary carcinoma with pasteurization (magnification × 200).
Under visual inspection, the right half of the thyroid gland exhibited a reddish cut surface, and a soft texture with a volume of 3.5 × 2 × 0.5 cm3. Gray nodules with a diameter of 0.8 cm, a reddish-colored cut surface, and a moderate texture were observed. A cystic mass 1.5 cm in diameter was found in the left thyroid tangential section. The cyst was filled with fluid containing blood, and the inner wall (thickness, 0.3 cm) was smooth. The isthmus (1.5 × 1.5 × 0.5 cm3) exhibited a grayish-red, cut, smooth, unconvoluted surface, with distinct nodules and areas of hardening.
Microscopic morphology
The left lobe contained tumor cells, which were polygonal, round, or fusiform, and without clear boundaries, distributed in sheets, nests, or rows. The nuclei were irregular in shape and had thick chromatin structures. Nucleoli and mitoses were absent. Tumor nests were separated by uneven fibrous interstitial fibrosis with powdery amyloid deposits (Figure 3A). The tumor boundary was indistinct and showed invasive growth, with no obvious tumor capsule.
Figure 3.
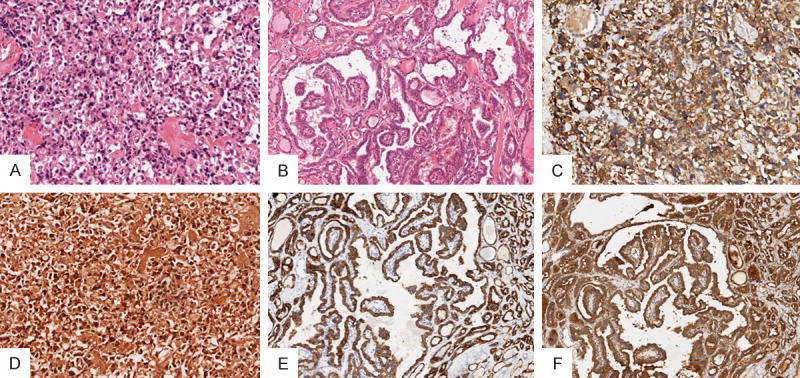
Histologic examination findings. A. Left thyroid tumor morphology is medullary thyroid carcinoma (H&E staining magnification × 200); B. Right thyroid tumor morphology is papillary thyroid carcinoma (H&E staining magnification × 200); C. Calcitonin-positive expression in medullary thyroid carcinoma (magnification × 200); D. Positive expression of Congo red in medullary thyroid carcinoma (Congo red staining magnification × 200); E. Positive expression of CK19 in papillary thyroid carcinoma (magnification × 200); F. Positive expression of Galectin-3 in papillary thyroid carcinoma (magnification × 200).
Tumor cells in the right lobe exhibited complex branched papillary structures with an avascular axis and were lined by a monolayer of stratified cuboid cells. The cells were round or oval with a dense arrangement. Nuclei were enlarged, rounded, elongated, and overlapping, showing ground-glass-like changes. Pseudo-inclusions and grooves in the nucleus were clearly visible. The nucleolus was not clearly distinguishable, and mitoses were absent (Figure 3B).
Immunohistochemistry
Immunohistochemical analysis revealed that the left lobe stained positive for calcitonin (Figure 3C), CK, TTF-1, CD56, CgA, and Congo red (Figure 3D), but negative for CK19, thyroglobulin (TG), galectin-3, MC, and CEA. The Ki-67 proliferation index was 1%. The right lobe stained positive for CK19 (Figure 3E), galectin-3 (Figure 3F), and MC, but negative for CD56. The v-raf murine sarcoma viral oncogene homolog B1 (BRAF) V600E mutation was identified by gene detection analysis.
Pathologic diagnosis
In the case of MTC (left lobe), no tumor metastasis was found in the central region of the left lobe or the lymph nodes (0/2), and no tumors were found in the thymic tissue. In the PTC, maximum infiltration (with a diameter of 0.8 cm) was found in the right lobe and isthmus, with no tumor metastasis in the right central area or lymph nodes (0/3).
Discussion
The case presented here was analyzed in the context of 18 cases reported in previous literature (Table 1). These 19 cases presenting with both MTC and PTC comprised 13 females and 6 males (male to female ratio of around 2:1). The age at cancer onset ranged from 27 to 70 years, with the median age of females and males being 52 and 56 years, respectively. Patients often sought medical advice after the discovery of neck or thyroid masses upon physical examination. The masses in these cases ranged from 0.2 to 6 cm in diameter, while a mixture of clear or indistinct boundaries with the surrounding tissue was reported. The cut surface was gray or grayish-red, with a medium or hard texture. Some cases were accompanied by hemorrhage, and some were cystic. Pre-operative laboratory examinations showed that the serum CEA and calcitonin levels were increased in five cases and normal in three cases, while the levels of these components in the remaining eleven cases were not examined. There were eight cases of lymph node metastasis, including three cases of ipsilateral side occurrence, five cases of different side occurrence, three cases of ipsilateral and different side tumors with lymph node metastasis of two types of cancer, and one case of ipsilateral and different side PTC with lymph node metastasis. There was no distant metastasis in the case presented here, or in the other 18 cases reported in the literature.
Table 1.
Clinical history data of 18 cases of MTC combined with PTC
| Case | Sex | Age | MTC location and size (cm) | PTC location and size (cm) | Case history | Preoperative CEA | Preoperative calcitonin | Lymph node metastasis | Distant metastasis | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 70 | Right lobe, 6 × 5 × 3.5 | Right lobe, Not measured | Right neck mass for 2 days | Not examined | Not examined | No | No | No |
| 2 | Female | 35 | Right lobe, 5.5 × 4 | Right lobe, Not measured | Found right neck mass 2 years previously | Not examined | Not examined | Yes | No | No |
| 3 | Female | 60 | Right lobe, 2 × 2 | Right lobe, Not measured | Neck mass 2 years previously; increased after 1 month | Not examined | Not examined | No | No | 6 |
| 4 | Male | 48 | Left lobe, 5 × 3 × 2 | Isthmus, 2 × 1.2 × 0.8 | Right neck mass for 3 months | Normal | Normal | No | No | No |
| 5 | Female | 36 | Left lobe, 0.8 × 0.6 | Left lobe, Not measured | Found a neck mass more than 1 month previously | Not examined | Not examined | No | No | No |
| 6 | Female | 60 | Right lobe, 0.5 | Right lobe, 0.25 | Found neck mass 3 months previously, significantly increased after 1 month | Not examined | Not examined | No | No | 15 |
| 7 | Female | 60 | Right lobe, 2.5 | Left lobe, 0.8 | Found thyroid nodules 3 months previously via physical examination | Not examined | Not examined | No | No | 6 |
| 8 | Male | 41 | Right lobe, 0.6 | Left lobe, 0.2 | Found thyroid enlargement via physical examination | 6.89 ng/ml | 188 ng/ml | Yes | No | 15 |
| 9 | Female | 57 | Right lobe, 0.2 | Left lobe, 0.5 | Found bilateral thyroid nodules 2 days previously | Not examined | Not examined | No | No | No |
| 10 | Male | 56 | Right lobe, 4 × 2.5 × 2.5 | Left lobe, 0.6 | Right thyroid mass for 2 months | Normal | Normal | Yes | No | No |
| 11 | Male | 66 | Left lobe, 1.6 × 1.3 × 1.0 | Right lobe, 1.3 × 0.9 × 0.8 | Thyroid nodules for more than 1 year | Not examined | Not examined | Yes | No | No |
| 12 | Female | 52 | Right lobe, 1 × 0.5 | Left lobe, 0.5 × 0.5 | Found thyroid nodules via ultrasonic examination | Not examined | Not examined | No | No | 60 |
| 13 | Female | 68 | Left lobe, 4.2 × 3.4 × 2.3 | Left lobe, Not measured | Found thyroid occupying for 1 week previously via physical examination | Not examined | Not examined | No | No | No |
| 14 | Male | 56 | Right lobe, 4 × 4 × 2.5 | Right lobe, 0.6 | Found right neck mass 3 months previously | 326.2 ng/ml | 1051 pg/ml | Yes | No | 50 |
| 15 | Female | 66 | Left lobe, 0.8 | Right lobe, 0.5 | Found left lobe mass in thyroid via physical examination | Not examined | Not examined | No | No | 12 |
| 16 | Female | 27 | Left lobe, 4 × 2.5 × 2 | Left lobe, 0.4 | Due to the left neck mass found 1.5 months previously | Normal | Normal | Yes | No | 24 |
| 17 | Female | 44 | Right lobe, 2.7 × 2.1 × 2.4 | Left lobe, 0.8 × 0.8 × 0.7 | Found thyroid mass more than 3 years previously | 29.6 mg/l | 8.5 pmol/l | Yes | No | No |
| 18 | Female | 69 | Left lobe, 1 × 0.8 | Right lobe, 2.5 × 1.8 | Found neck mass more than 3 months previously | 9.3 ng/ml | 17 ng/l | Yes | No | 12 |
Under microscopic examination, PTC tumor cells were arranged in a complex branched papillary structure, with typical PTC nuclear features. MTC tumor cells were distributed in sheets, nests, or rows, and were polygonal, round, or fusiform in shape. The tumor stroma contained amyloid deposits. However, there were some differences with regard to staining for immunohistochemical markers: the MTC tissue stained positive for calcitonin, CK, CD56, Syn, CgA, and Congo red, while the PTC tissue stained positive for CK19, TTF-1, HBME-1, galectin-3, and TG; gene detection analyses revealed BRAF V600E mutations.
Thyroid follicular tumors are follicular or beam-shaped, with cubic-, columnar-, or polygonal-shaped tumor cells that are occasionally enlarged. The tumor cells exhibit deeply stained nuclei with focal papillary structures. However, hyperplastic papillae often had no fibrovascular axis, and few papillae branches were present, as well as a single layer of cuboid epithelium with a small nucleus. Papillary structures were often located in dilated follicular or cystic regions, and there were no papillary nuclear features, such as ground-glass nuclei and nuclear overlap. Immunohistochemical analysis of CK19 and RET expression may be helpful in the differential diagnosis of thyroid follicular tumors and PTC, since papillary carcinoma is likely to be diffusely positive for these markers.
A typical case of PTC shows a complex system of branched papillae with a fibrovascular axis. The cells are crowded and overlap with a thickened nuclear membrane, and a frosted glass-like nucleus. The nuclear sulcus and pseudo-envelope may be apparent [5,6]. However, in some cases, the nuclear features of papillary carcinoma are not obvious, and vitreous lesions may appear in the interstitium. In particular, vitreous lesions need to be differentiated from MTC. Characteristic nuclear changes are the major focus of differentiation, together with the expression of TG. Furthermore, most cases are characterized by the expression of CK19 and galectin-3, but do not feature the expression of calcitonin and the neuroendocrine markers Syn and CgA and stain Congo red negative.
The tumor cells of poorly differentiated thyroid cancer and undifferentiated carcinoma are arranged in an island or row shape with a solid structure and may be mixed with papillae and/or small follicles rich in blood vessels. The tumor cells are heterogeneous, and may even exhibit a spindle cell sarcoma-like appearance with a large nucleus. Nuclear division is common in coagulative necrosis. The tumors generally stain positive for TG, TTF-1, and p53 in immunohistochemical studies. MTC displays relatively low cell heterogeneity, with fewer mitotic figures and necrotic foci. There is often amyloid deposition in the interstitial region, and tumors are negative for TG in immunochemical analyses. Positive staining for calcitonin and Congo red is helpful for discrimination.
Hyalinizing trabecular tumor (HTT) is a rare follicular tumor that is classified by the WHO as an independent tumor type based on its clinical features. These include clear boundaries, which can have thin fibrous capsules, and beam- or acinar-like growth patterns. Tumor cells are polygonal, short, and fusiform, with a distinguishable nuclear sulcus, and feature pseudo-inclusions in the nucleus. Evidence of interstitial tissue with obvious hyaline degeneration needs to be analyzed with respect to histologic morphology, and requires careful observation of possible infiltration and vascular involvement. According to immunohistochemical analyses, HTT expresses TG, TTF-1, and Ki-67 in the cell membrane, and it stains positive for PAS (indicating interstitial hyaline degeneration), but negative for calcitonin and cholesterol. If necessary, RET/PTC gene detection can be used to assist diagnosis.
In this mixed type of thyroid cancer, PTC usually accounts for a small proportion (< 25%) of the tumor, in which the subdominant MTC predominates. Therefore, PTC/MTC has a prognosis similar to that of MTC. The PTC component of the tumor is generally TG-positive and calcitonin-negative, while the MTC component exhibits the opposite pattern in the immunohistochemical analysis. In the case examined here, the two cancerous lesions were located on different sides of the thyroid, and existed independently, with no mixed component.
The composition of MTC is highly diverse. Tumor cells are often arranged in sheets, nests, or rows, and can also exhibit a papillary, bundle-like, sinus-like, adenoid, or carcinoid-like arrangement. Papillary structures are considered to be a subtype or variant of medullary carcinoma, which is rare, but is associated with good prognosis. The papilla, the surface of which is covered with a single or multi-layered columnar epithelium, is composed of a fibrovascular axis. The cells exhibit a disordered arrangement, and the cytoplasm is rich and powdery.
Thyroid paraganglioma tumor cells are lobular or nested (Zellballen arrangement) and separated by fibrovascular connective tissue. Tumor tissue is composed of a mixture of primary and supporting cells. The morphology of the main cells is polygonal, with a fine granular amphophilic cytoplasm. The nucleus is round or oval, and the chromatin is finely granulated with small nucleoli. The morphology of the supporting cells is fusiform, and the cytoplasm is eosinophilic, with most cells distributed around the nest of tumor cells or interspersed between the main cells. Immunohistochemical analysis revealed the expression of NSE, Syn, chromogranin, and S-100, while these cells are CK-, EMA-, TG-, and calcitonin-negative. MTC is characterized by the expression of CK, calcitonin, and CEA, and positive interstitial amyloid Congo red staining can be helpful for its identification.
Pathogenesis
The current consensus supports six theories for the pathogenesis of MTC combined with PTC.
Hijacking theory
Normal follicular cells deteriorate into PTC after being encapsulated in MTC. This theory mainly explains mixed MTC/PTC cases.
Local effect theory
Genetic and environmental factors, as well as radiation, have been shown to induce or modulate thyroid cancer formation; therefore, it can be hypothesized that these factors can act simultaneously to cause tumorigenesis from two different sources [7].
Stem cell theory
Studies have shown that common stem cells can differentiate into follicular epithelial cells and parafollicular C cells, which form different pathologic types of thyroid cancer [8]. However, more recently, Alavi and Azarpira proposed and confirmed that the two tumor types are not derived from the same type of stem cell.
Common proto-oncogene theory
Since follicular cells and parafollicular C cells are derived from the post-embryonic corpus callosum, it is believed that PTC and MTC share a common proto-oncogene, which is transformed in different directions under the action of cancer-promoting factors. Both cancers are familial, especially MTC.
Collision theory
Some experts believe that MTC complicated bywith PTC may be caused incidentally by the increased incidence of MTC, i.e., the two tumors occur coincidentally [9].
Environmental factor theory
Studies have shown that the occurrence of PTC in MTC patients may be related to the abnormal uptake of iodine, which is a risk factor for BRAF gene mutation in PTC patients. It has been reported that the BRAF gene mutation is a molecular genetic event that occurs with high incidence in thyroid cancer. The BRAF V600E mutation is the most representative type of mutation in PTC. Furthermore, BRAF gene mutations generally activate the mitogen-activated protein kinase (MAPK) signaling pathway, leading to the malignant transformation of the thyroid follicular epithelium [10]. Recent studies have confirmed that RET proto-oncogenes cause tyrosine kinase activation by point mutations or gene rearrangements, promoting tumorigenesis in MTC and PTC [11]. The occurrence of MTC is often caused by a point mutation in the RET proto-oncogene, which occurs in an autosomal dominant manner, but with a different penetrance [12]. However, abnormal expression of the RET gene is not limited to MTC, and approximately 20% to 40% of PTCs exhibit abnormal RET gene expression [13]. Therefore, RET gene mutations also have the potential to induce PTC, while causing hereditary MTC [14]. Furthermore, RET protein tyrosine kinase dysregulation can lead to the expression of fusion proteins, such as RET/PTC, which is a common genetic change in PTC, accounting for 10% to 40% of cases [15]. These findings indicate that RET gene mutations cause MTC to be associated with PTC. Although BRAF mutations and RET/PTC gene rearrangements are common in PTC, they do not overlap. Studies have shown that BRAF is a necessary factor for the RET/PTC-mediated activation of the MAPK pathway. It can also be speculated that mutations of both BRAF and RET lead to the simultaneous onset of MTC and PTC [16].
Treatment and follow-up
The patient was discharged 8 days after the operation, and returned to the hospital for re-examination at 1, 2, 3, 4, 8, 10, and 13 months post-operation. The patient was asymptomatic, with no abnormalities in cardiac function, and had no signs of recurrence of the primary tumor or metastasis upon re-examination (Table 2).
Table 2.
Follow-up of current patient
| time | Serum calcitonin (CT) (< 6.4 pg/ml) | Thyroglobulin (Tg) (3.5-77 ng/ml) | Alpha fetoprotein (CEA) (Smoking< 5.1, Non-smoking< 3 ug/l) |
|---|---|---|---|
| 1 month after operation | 2.69 | 0.497 | 6.44 |
| 2 month after operation | 0.84 | 0.062 | Not provided |
| 3 month after operation | 0.55 | < 0.04 | Not provided |
| 4 month after operation | 0.5 | < 0.04 | Not provided |
| 8 month after operation | < 0.5 | 0.11 | Not provided |
| 10 month after operation | Not provided | Not provided | 1.4 |
| 13 month after operation | < 0.5 | < 0.04 | 2 |
At present, the American Thyroid Association (ATA) recommends that the basic treatment for MTC is total thyroidectomy and bilateral central lymph node dissection [17], and that thyroid hormone suppression should be continued after PTC surgery [18]. Numerous studies have shown that thyroid cancer cells produce cytokines and chemokines capable of inducing a variety of tumor-promoting effects, and targeted therapy and/or reduced concentrations of cytokines and chemokines in the tumor microenvironment will yield therapeutic benefits [18]. The recent use of cancer immunotherapies, including the use of CTLA-4, PD-1, and PD-L1 antagonists, was shown to positively impact the overall survival rates of cancer patients [19]. These patients should be evaluated in accordance with the follow-up principles for MTC and PTC, relying mainly on the detection of serum calcitonin, serum CEA, and TG, as well as a review of thyroid function, and cervical ultrasound imaging. If necessary, patients should undergo additional procedures, such as neck CT, and PET-CT imaging to assess the existence of recurrence or metastasis [20].
Disclosure of conflict of interest
None.
References
- 1.Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol. 2013;2013:965212. doi: 10.1155/2013/965212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roman BR, Morris LG, Davies L. The thyroid cancer epidemic, 2017 perspective. Curr Opin Endocrinol Diabetes Obes. 2017;24:332–336. doi: 10.1097/MED.0000000000000359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baloch ZW, LiVolsi VA. Special types of thyroid carcinoma. Histopathology. 2018;72:40–52. doi: 10.1111/his.13348. [DOI] [PubMed] [Google Scholar]
- 4.Tang PY, Khor LY, Takano A. Synchronous papillary thyroid carcinoma and medullary thyroid carcinoma - a pitfall waiting to happen. Malays J Pathol. 2017;39:171–174. [PubMed] [Google Scholar]
- 5.Liu MJ, Liu ZF, Hou YY, Men YM, Zhang YX, Gao LY, Liu H. Ultrasonographic characteristics of medullary thyroid carcinoma: a comparison with papillary thyroid carcinoma. Oncotarget. 2017;8:27520–27528. doi: 10.18632/oncotarget.15897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weng YC, Zeng DQ, Gu DS, Chen YW. One case in which papillary thyroid carcinoma and papillary carcinoma occur on the same side. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2016;30:499–500. doi: 10.13201/j.issn.1001-1781.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 7.Gurkan E, Gurbuz Y, Tarkun I, Canturk Z, Cetinarslan B. Mixed medullary-papillary carcinoma of the thyroid: report of two cases and review of the literature. Indian J Pathol Microbiol. 2014;57:598–602. doi: 10.4103/0377-4929.142684. [DOI] [PubMed] [Google Scholar]
- 8.Sadat Alavi M, Azarpira N. Medullary and papillary carcinoma of the thyroid gland occurring as a collision tumor with lymph node metastasis: a case report. J Med Case Rep. 2011;5:590. doi: 10.1186/1752-1947-5-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dikbas O, Duman AA, Guvendi GF. Medullary thyroid carcinoma and papillary thyroid carcinoma in the same patient as a collision tumour. Case Rep Endocrinol. 2019;2019:1–4. doi: 10.1155/2019/4038628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hedayati M, Zarif Yeganeh M, Sheikholeslami S, Afsari F. Diversity of mutations in the RET proto-oncogene and its oncogenic mechanism in medullary thyroid cancer. Crit Rev Clin Lab Sci. 2016;53:217–227. doi: 10.3109/10408363.2015.1129529. [DOI] [PubMed] [Google Scholar]
- 11.Lian EY, Maritan SM, Cockburn JG, Kasaian K, Crupi MJ, Hurlbut D, Jones SJ, Wiseman SM, Mulligan LM. Differential roles of RET isoforms in medullary and papillary thyroid carcinomas. Endocr Relat Cancer. 2017;24:53–69. doi: 10.1530/ERC-16-0393. [DOI] [PubMed] [Google Scholar]
- 12.Machens A, Dralle H. Simultaneous medullary and papillary thyroid cancer: a novel entity? Ann Surg Oncol. 2011;19:37–44. doi: 10.1245/s10434-011-1795-z. [DOI] [PubMed] [Google Scholar]
- 13.Samulski TD, LiVolsi VA, Montone K, Baloch Z. The variable pathologic presentations of medullary and micro-medullary thyroid carcinoma: an institutional experience. Patho Res Pract. 2014;210:182–185. doi: 10.1016/j.prp.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Guilmette J, Nosé V. Hereditary and familial thyroid tumours. Histopathology. 2018;72:70–81. doi: 10.1111/his.13373. [DOI] [PubMed] [Google Scholar]
- 15.Hedayati M, Zarif Yeganeh M, Sheikholeslami S, Afsari F. Diversity of mutations in the RET proto-oncogene and its oncogenic mechanism in medullary thyroid cancer. Crit Rev Clin Lab Sci. 2016;53:217–227. doi: 10.3109/10408363.2015.1129529. [DOI] [PubMed] [Google Scholar]
- 16.Romei C, Ciampi R, Elisei R. A comprehensive overview of the role of the RET proto-oncogene in thyroid carcinoma. Nat Rev Endocrinol. 2016;12:192–202. doi: 10.1038/nrendo.2016.11. [DOI] [PubMed] [Google Scholar]
- 17.Kloos RT, Eng C, Evans DB, Francis GL, Gagel RF, Gharib H, Moley JF, Pacini F, Ringel MD, Schlumberger M, Wells SA. Medullary thyroid cancer: management guidelines of the american thyroid association. Thyroid. 2009;19:565–612. doi: 10.1089/thy.2008.0403. [DOI] [PubMed] [Google Scholar]
- 18.Raue F, Frank-Raue K. Thyroid cancer: risk-stratified management and individualized therapy. Clin Cancer Res. 2016;22:5012–5021. doi: 10.1158/1078-0432.CCR-16-0484. [DOI] [PubMed] [Google Scholar]
- 19.Antonelli A, Ferrari SM, Fallahi P. Current and future immunotherapies for thyroid cancer. Expert Rev Anticancer Ther. 2018;18:149–159. doi: 10.1080/14737140.2018.1417845. [DOI] [PubMed] [Google Scholar]
- 20.Fagin JA, Wells SA Jr. Biologic and clinical perspectives on thyroid cancer. N Engl J Med. 2016;375:1054–1067. doi: 10.1056/NEJMra1501993. [DOI] [PMC free article] [PubMed] [Google Scholar]


