Abstract
Objective: To explore the clinicopathologic features and differential diagnosis of breast primary mucinous cystadenocarcinoma (MCA). Methods: Pathological characteristics and immunophenotype of one case of MCA were analyzed. Literature was reviewed. Results: Grossly, the area of the tumor cut surface was gelationous. Microscopcally, the tumor was composed of variably sized cystic spaces lined by mucus-rich tumor cells with single columnar, stratified appearance and papillary formation. The degree of cytologic atypia varied from region to region. The tumor cells were positive for CK7, GATA3, negative for CK20, ER, PR and HER2. Most peripheral myoepithelial cells were negative for P63 and SMMHC. Conclusions: MCA is a rare primary breast cancer and strikingly similar to ovarian, pancreatic and gastrointestinal counterparts. The diagnosis cannot be made until the metastatic lesion is ruled out. On the other hand, the biologic behavior of MCA is reportedly favorable despite a high proliferation index and triple negative biomarker status. Therefore, the role of adjuvant chemotherapy or radiation is questionable.
Keywords: Breast, primary, mucinous cystadenocarcinoma, immunohistochemistry
Introduction
Primary mucinous cystadenocarcinoma (MCA) is a rare malignant tumor of the breast. In the third edition “Pathology and Genetics of Breast and Female Reproductive Organs” (2003 edition, WHO) [1], it was defined as a malignant tumor formed by intracellular mucin-rich columnar cells lining the wall of the cyst, similar to the mucinous cystadenocarcinoma of ovary or pancreas. At present, less than 30 cases have been reported and most of them are case reports. We reviewed the clinicopathologic features, histomorphology, immunophenotype, and other data of breast primary MCA, combined with literature review and discussion, aiming to improve the judgment of the features of this type of tumor, and provide a theoretical basis for the choice of treatment.
Materials and methods
Clinical materials
By collecting the external inspection and consultation pathologic archives from January 2009 to June 2019 in The Department of Pathology, Affiliated Hospital of Qingdao University, we obtained 1 case of MCA and reviewed all the sections.
Methods
We use EnVision two-step approach and special stain methods of PAS and mucicarmine staining, then observed by light microscopy. The primary antibodies we used include CK7, CK20, GATA3, Mammaglobin, P63, SMMHC, ER, PR, HER2, Ki-67, E-cad, CDX-2, CA19-9, TTF-1, WT-1 and CA125. The results of immunohistochemical staining were determined by diaminobenzidine, and the positive expression was brownish yellow. GATA3, P63, ER, PR, Ki-67, TTF-1 and WT-1 were located in the nucleus, and others were located in the cytoplasm. The results of HER2 staining were referred to the “Guidelines for Detection of HER2 in Breast Cancer (2019 Edition)” [2].
Results
Clinical materials: A 66-year-old post-menopausal women was admitted to the hospital after her right breast lump was found two months later. In the outpatient examination, a 35×25 mm lump with hard texture and unclear boundaries was touched in the upper inner quadrant of the right breast. Ultrasonography demonstrated a 12-point hypoechoic mass in the right breast with multiple hypoechoic nodules around it, and the border was unclear (Figure 1A). BI-RAD classification is 5. The mammography showed a high-density shadow in the upper quadrant of the right breast, and the edge was rough, measuring about 32 mm×23 mm in dimension (MLO position) (Figure 1B). Gynecological ultrasonography showed no abnormalities in the uterus and bilateral attachments. Malignant tumor of breast was diagnosed based on the intraoperative frozen section. Then radical mastectomy was performed. After 13 months of follow-up, the patient was still alive.
Figure 1.
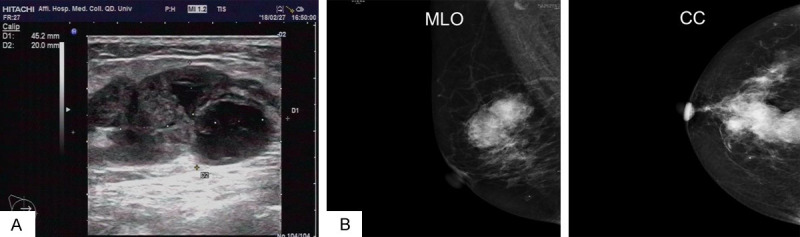
A. Ultrasonography demonstrated a hypoechoic mass in the right breast with multiple hypoechoic nodules around it, and the border was unclear. B. The mammography showed a high-density shadow in the upper quadrant of the right breast, and the edge was rough, measuring about 32 mm×23 mm in dimension (MLO position).
Pathological findings: Gross inspection revealed an unclear boundary tumor with gray and crisp cut surface, measuring approximately 25×25×20 mm. Some areas are jelly-like and shiny (Figure 2). Microscopically, the tumor is composed of variably sized cystic cavities lined by columnar cells and filled with hemorrhagic mucin (Figure 3A). Some cavities are lined by monolayer high mild columner cells with basally placed nuclei and abundant intracytoplasmic mucus (Figure 3B). In some areas, multi-layered cells display complex, branching, papillary processes, protruding into the cystic cavity (Figure 3C). Some tumor cells have reduced intracellular mucus, increased atypia, cytoplasmic eosinophilia (Figure 3D) and pathologic mitosis (Figure 3E). A small amount of mucus overflows into the stroma, forming a mucus lake with the nested or papillary cell clusters floating there (Figure 3F). Common ductal carcinoma in situ (DCIS) were observed around the main lesion. No cancer metastasis was found in the ipsilateral axillary lymph nodes (0/17).
Figure 2.
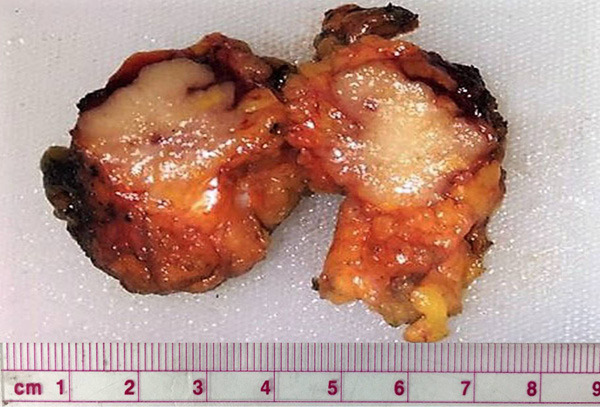
Gross inspection revealed an unclear boundary tumor with gray and crisp cut surface, measuring approximately 25×25×20 mm. Some areas are jelly-like and shiny.
Figure 3.
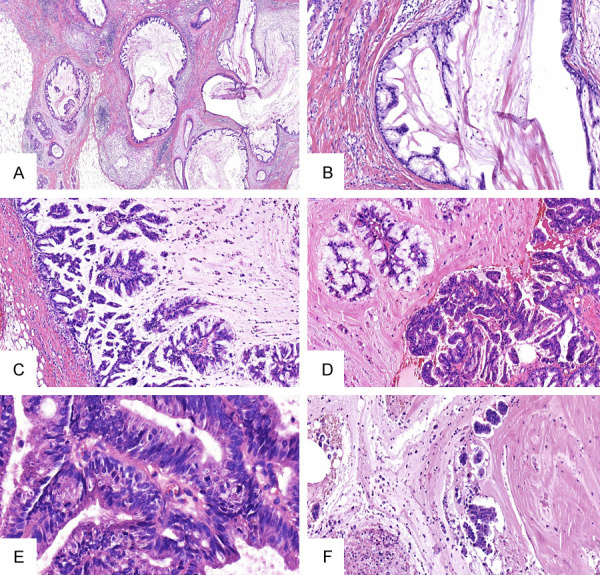
A. Microscopically, the tumor showed variably sized cystic cavities lined by columnar cells and filled with hemorrhagic mucin (HE ×40). B. Some cavities are lined by monolayer high mild columnar cells with basally placed nuclei and abundant intracytoplasmic mucus (HE ×100). C. The cells in some regions were hyperplastic and protruded into the capsule cavity, forming a complex branching nipple (HE ×200). D. Some tumor cells have reduced mucus, increased atypia, and cytoplasmic eosinophilia (HE ×200). E. Pathological mitosis can be seen (HE ×400). F. A small amount of mucus overflows into the stroma, forming a mucus lake with the nested or papillary cell clusters floating there (HE ×100).
Special staining and immunohistochemistry: Special staining showed that the mucus inside and outside the tumor cells was positive for PAS and mucicarmine (Figure 4A). Immunohistochemistry showed that tumor cells were positive for CK7, GATA3 (Figure 4B, 4C), Mammaglobin, and negative for CK20 (Figure 4D). The lateral myoepithelial cells of the tumor wall were positive for a small part of the small wall of P63 and SMMHC, and most of the wall was negative (Figure 4E, 4F). ER, PR, HER2, CA19-9, CDX-2, TTF-1, CA125, WT-1 were negative in tumor cells. The positive rate of Ki-67 was about 60%. Including areas with mild cell morphology, the Ki-67 positive rate was also higher.
Figure 4.
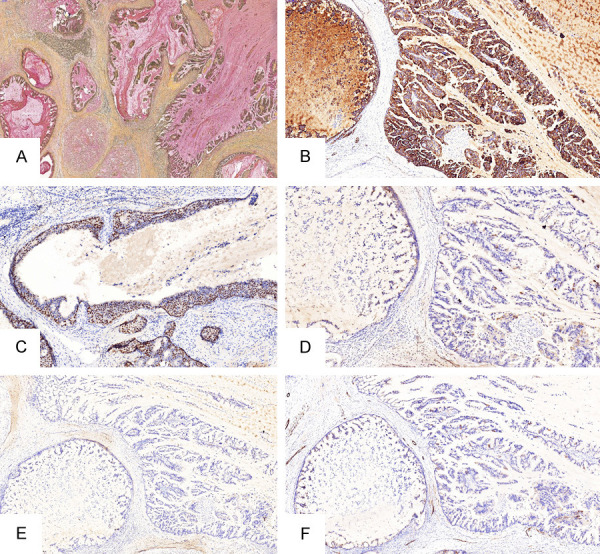
A. Special staining showed positive mucus inside and outside tumor cells (Mucicarmine staining ×100). B, C. CK7 and GATA3 was positive in tumor cells (EnVision ×100). D. CK20 was negative in tumor cells (EnVision ×100). E, F. Most of the lateral myoepithelial cells of cystic cavities were negative for P63 and SMMHC (EnVision ×100).
Discussion
Breast primary MCA is a rare malignant tumor that bears a striking resemblance to MCAs of the ovarian, pancreatic and/or gastrointestinal mucinous cystadenocarcinoma. In 1998, Koening and Tavassoli reported four cases of breast primary MCA firstly. Since then, 22 cases of histologic morphology and immunohistochemical features of primary mammary MCA were reported [3-22]. In 2003, the third edition of WHO “Pathology and Genetics of Breast and Female Reproductive Organ Tumors” classified it as a carcinoma of the breast that can produce mucus, including mucinous carcinoma (colloidal carcinoma), columnar cell mucinous carcinoma, and signet ring cell carcinoma. Due to the limited number of MCA cases, the fourth edition “Pathology and Genetics of Breast and Female Reproductive Organs (2012 edition, WHO)” did not classify it by name, and only reserved the cancer of mucus and the cancer with differentiation of signet cells.
At present, there are only 26 cases of breast primary MCA have been published from 1998, and the clinicopathologic characteristics are summarized in Table 1. They ranged in age from 49-96 years old. Almost all of them are postmenopausal women. The first reported of four breast primary MCAs are morphologically expressed as cystic cavities with unequal size and unevenly distribution [3]. The cystic cavities were lined with mild columnar mucous cells lacking of myoepithelial markers. Koening and Tavassoli described it as the subtype of low-grade invasive ductal carcinoma. In some areas, the cells proliferate and stratify forming cell clusters into the cystic cavity or a papillary structure. Cells can express different degrees of atypia. Some cells are rich in mucus with the nucleus located in the basal part. Some cells have reduced mucus, enhanced cytosolic eosinophilic, and increased nuclear atypia. In addition, 3 of these 4 cases showed local squamous cell carcinoma differentiation, 1 case spilled mucin substance into the stroma, 2 cases contained common DCIS components, and 1 case contained high-grade sarcomatoid components.
Table 1.
Clinicopathologic analysis of breast primary mucinous cystadenocarcinoma
| Case | Age (years) | Size (mm) | DCIS | MCA in situ | Mucus spillover into stroma | lymphatic metastasis | ER | PR | HER2 | CK7 | CK20 | Follow-up | Relapse |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 79 | 60 | + | + | + | - | - | - | - | + | - | DOD, 9 years | - |
| 2 | 54 | 190 | - | - | - | + | - | - | - | + | - | ANED, 24 months | - |
| 3 | 67 | 23 | + | - | + | - | - | - | - | + | - | ANED, 22 months | - |
| 4 | 49 | 85 | + | + | - | - | - | - | - | + | - | ANED, 11 months | - |
| 5 | 61 | 8 | - | - | - | - | - | - | - | + | - | NA | NA |
| 6 | 74 | 100 | + | - | + | - | - | - | - | + | - | ANED, 24 months | - |
| 7 | 96 | 20 | - | - | - | + | - | - | - | + | - | DOD, 46 months | - |
| 8 | 65 | 30 | + | + | - | - | - | - | - | + | + | ANED, 8 months | - |
| 9 | 51 | 40 | - | - | - | - | - | - | NA | + | - | NA | NA |
| 10 | 52 | 100 | - | - | + | - | + | - | - | - | - | ANED, 24 months | - |
| 11 | 55 | 25 | + | - | - | - | - | - | - | + | - | ANED, 6 months | - |
| 12 | 61 | 30 | - | - | - | - | - | - | - | + | - | ANED, 6 months | - |
| 13 | 73 | 45 | + | - | + | - | - | - | + | + | - | NA | NA |
| 14 | 55 | 30 | + | - | + | - | - | - | - | + | - | NA | NA |
| 15 | 65 | 30 | + | + | + | + | - | - | - | + | - | ANED, 6 months | - |
| 16 | 41 | 70 | + | + | - | - | - | - | - | + | - | ANED, 24 months | - |
| 17 | 52 | 65 | - | - | - | - | - | - | - | + | - | ANED, 12 months | - |
| 18 | 59 | 9 | + | - | - | - | - | - | - | + | - | ANED, 3 months | - |
| 19 | 62 | 56 | - | - | - | - | - | - | - | + | - | ANED, 5 months | - |
| 20 | 55 | 20 | + | - | - | - | - | - | + | + | - | ANED, 10 months | - |
| 21 | 59 | 20 | - | - | - | - | - | - | + | NA | NA | NA | NA |
| 22 | 50 | 22 | - | - | - | - | - | - | - | NA | NA | NA | NA |
| 23 | 91 | 75 | + | - | - | - | - | - | - | + | - | DOD, 14 months | - |
| 24 | 63 | 16 | - | - | - | + | - | - | - | + | - | ANED, 48 months | - |
| 25 | 68 | 62 | + | - | + | - | - | - | - | + | - | ANED, 3 months | - |
| 26 | 51 | 20 | + | - | + | ITC | - | - | - | + | - | ANED, 8 years | + |
| 27 | 66 | 25 | + | + | + | - | - | - | - | + | - | ANED, 10 months | - |
MCA in situ, mucinous cystadenocarcinoma in situ; DOD, died of other diseases; ANED, alive with no evidence of disease; NA, not available; ITC, isolated tumor cells.
The morphological characteristics of breast MCA in subsequent reports are similar to those reported by Koening and Tavassoli [4-22], which are characterized by abundant intracellular mucus and extracellular mucus in the center of the cyst. In some cases (11 cases), mucoid secretion spill into the surrounding stroma forming a mucus lake in which there were single or small clusters of tumor cells floating, similar to breast mucinous carcinoma (gelatinous carcinoma). Common DCIS (with or without mucus) was seen in some cases (14 cases), and 6 of them showed cystic dilatation catheters lined by a monolayer or stratified high columnar mucous cells in varying degrees of cell dysplasia. These capsules may be accompanied by or without myoepithelium. The case in this paper also has the above morphologic and immunophenotypic characteristics. Chen et al believed that these DCIS originated from the mucinous metaplasia of epithelial cells of common DCIS [7], and it was named as the in situ carcinoma form of MCA.
Immunohistochemical results showed that most of the cases (including the current case) were negative for ER, PR and HER2 [3,5,7-11,13-17,19-22], and only 3 cases was positive (FISH was also positive) in HER2 [12,18,20], and 1 case (case 10) was positive for ER [10]. Interestingly, the positive rate of Ki-67 in most tumor cells including areas with mild cells was higher, ranging from 20.5% to 90% [7,9]. It is about 60% in this case. This suggests that most of the tumor cells of MCA are in a high proliferative state, regardless of the degree of atypia of the cells.
Although Ki-67 showed a high index of proliferation, and its tumor cells showed a triple negative expression in ER, PR, HER2. But the prognosis of MCA was better. All cases were followed up from 6 months to 9 years (Table 1), of which only case 1 (9 years followup), case 7 (46 months follow-up), and case 23 (14 months follow-up) died of other diseases [5,6,19]. Case 26 (8 years of follow-up) patients with local recurrence, suggested that this kind of tumors may be at risk of long-term local recurrence [22]. The risk of lymph node metastasis was low with only 4 cases of ipsilateral axillary lymph node metastasis [3,5,14,21] and 1 case of isolated tumor cells (ITC) were seen in the lymph nodes [22]. All of the above results suggest that the prognosis of primary breast MCA is better, but further long-term follow-up studies of more cases are still needed.
Breast MCA is rare with similar morphology to ovarian, pancreatic, and/or gastrointestinal counterparts. But ovarian MCA is also a malignant tumor in women of this age [23]. Therefore, in the diagnosis of primary breast MCA, the ovary, pancreas and gastrointestinal tract should be excluded first by combing with medical history, clinical manifestations and imaging examination. Since the breast is not an early metastatic site of mucinous cystadenocarcinoma in ovary, the patient should have clinical manifestations of the primary lesion and other metastatic sites before the metastatic lesion appears in the breast. In addition, CK7 and CK20 immunohistochemical detection can assist diagnosis [24]: most ovarian, pancreatic mucinous carcinoma is positive for CK7, CK20; gastrointestinal tumors are negative for CK7 and positive for CK20. No evidence of ovarian cancer was found in all patients in Table 1, except a small number of tumor cells were positive for CK20 in case 8 [7], and the remaining tumor cells were CK7-positive and CK20-negative, so was the case in this article. At the same time, in most cases (including this case), ductal carcinoma in situ (general DCIS) is present around the MCA lesion, and the case of MCA in situ is seen in this case. This supports that the tumor is primary to the mammary gland.
Secondly, in some cases, mucus overflows into the interstitial and forms a mucus lake. When there is a lack of floating cell clusters, it is still necessary to distinguish from mucinous cyst-like lesions. The mucinous cyst-like lesions are lined with flat, cuboid or low columnar epithelium, with a mild morphology and no mucus in the cytoplasm, while the epithelium of the MCA cyst wall is a high columnar cell rich in mucus. In addition, some cases in Table 1 and the case in this article can be seen the cluster of clustered or micropapillary cancer cells floating in the spilled mucus lake, the cytoplasmic mucus is reduced, Similar to primary mucinous carcinoma (gelatinous cancer) of the breast [4,6,11-13,16,22]. However, the latter is more common in the breast, and the ER and PR of tumor cells are mostly positive.
Finally, MCA still needs to be differentiated from cystic hypersecretory cancer of the breast. Both of them contain cystic cavities with high cystic expansion, but both of MCA cells and cysts contain mucus that is positive for PSA and mucicarmine, while the capsule high-secretion cancer has no intracellular mucus, and the cystic cavity contains red-stained, homogeneous secretions, similar to glial thyroid follicles, and the colloid-like secretions do not contain cancer cells, or only contain a small number of macrophages.
Because this type of tumor is extremally rare, its tissue occurrence and biologic behavior are still uncertain. Some scholars have proposed the hypothesis of catheter epithelial mucus metaplasia. Lee and Chaung believe that the accumulation of mucus in the intraductal papillary carcinoma with mucous epithelial metaplasia and extracellular mucus leads to cystic expansion of the lumen, loss of myoepithelium and adjacent interstitial infiltration, leading to MCA eventually [9]. However, this hypothesis does not explain the biologic behavior of MCA, which has a basal-like molecular phenotype but a good prognosis. Similarly, Chen et al proposed the theory of mucinous metaplasia of conventional DCIS cancer cells and mucinous cyst-like lesions with epithelial malignant transformation does not receive completely approvement [7].
The limited number of cases limits the recognition of MCA and the choices of treatment strategies. Most of the previously reported cases underwent partial or radical mammectomy, and receive chemotherapy and radiotherapy [21]. However, follow-up results indicate that the prognosis of patients with MCA is good. So, the significance of neoadjuvant chemotherapy or radiotherapy remains questionable. Moreover, the triple-negative immunophenotype and high proliferation index of MCA may lead to false perceptions or overtreatment with unnecessary chemotherapy and radiotherapy in some patients.
In conclusion, MCA is an extremely rare variant of the primary breast carcinomas. It is similar to ovary and pancreas counterparts in morphology. In diagnosis, it is necessary to combine clinical and imaging examinations to exclude the abnormalities in other organs firstly. In addition, MCA’s high proliferative index and triple-negative immunophenotype are not fully consistent with its better prognostic biologic behavior. There are still doubts about the choice of treatment strategies such as neoadjuvant chemotherapy or radiotherapy. To solve this problem, more cases need to be accumulated for further investigation and discussion.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (Nos. 81672606).
Disclosure of conflict of interest
None.
References
- 1.Tavassoli FA, Devilee P. Pathology and genetics of tumours of the breast and female genital organs. Lyon, France: IARC Press; 2003. World Health Organization Classification of Tumours; pp. 30–1. [Google Scholar]
- 2.HER2 Gftdobc. Guidelines for the detection of breast cancer HER 2 (2019) Chinese Journal of Pathology. 2019;48:169–75. doi: 10.3760/cma.j.issn.0529-5807.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Koenig C, Tavassoli FA. Mucinous cystadenocarcinoma of the breast. Am J Surg Pathol. 1998;22:698–703. doi: 10.1097/00000478-199806000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Domoto H, Terahata S, Yamazaki T, Sato K, Takeo H, Tamai S. Mucinous cystadenocarcinoma of the breast showing sulfomucin production. Histopathology. 2000;36:567–9. doi: 10.1046/j.1365-2559.2000.00918-3.x. [DOI] [PubMed] [Google Scholar]
- 5.Honma N, Sakamoto G, Ikenaga M, Kuroiwa K, Younes M, Takubo K. Mucinous cystadenocarcinoma of the breast: a case report and review of the literature. Arch Pathol Lab Med. 2003;127:1031–3. doi: 10.5858/2003-127-1031-MCOTBA. [DOI] [PubMed] [Google Scholar]
- 6.Rosen PP, Scott M. Cystic hypersecretory duct carcinoma of the breast. Am J Surg Pathol. 1984;8:31–41. doi: 10.1097/00000478-198401000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Chen WY, Chen CS, Chen HC, Hung YJ, Chu JS. Mucinous cystadenocarcinoma of the breast coexisting with infiltrating ductal carcinoma. Pathol Int. 2004;54:781–6. doi: 10.1111/j.1440-1827.2004.01755.x. [DOI] [PubMed] [Google Scholar]
- 8.Coyne JD, Irion L. Mammary mucinous cystadenocarcinoma. Histopathology. 2006;49:659–60. doi: 10.1111/j.1365-2559.2006.02471.x. [DOI] [PubMed] [Google Scholar]
- 9.Lee SH, Chaung CR. Mucinous metaplasia of breast carcinoma with macrocystic transformation resembling ovarian mucinous cystadenocarcinoma in a case of synchronous bilateral infiltrating ductal carcinoma. Pathol Int. 2008;58:601–5. doi: 10.1111/j.1440-1827.2008.02278.x. [DOI] [PubMed] [Google Scholar]
- 10.Rakıcı S, Gönüllü G, Gürsel SB, Yıldız L, Bayrak IK, Yücel I. Mucinous cystadenocarcinoma of the breast with estrogen receptor expression: a case report and review of the literature. Case Rep Oncol. 2009;2:210–6. doi: 10.1159/000253866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gulwani H, Bhalla S. Mucinous cystadenocarcinoma: a rare primary malignant tumor of the breast. Indian J Pathol Microbiol. 2010;53:200–2. doi: 10.4103/0377-4929.59242. [DOI] [PubMed] [Google Scholar]
- 12.Petersson F, Pang B, Thamboo TP, Putti TC. Mucinous cystadenocarcinoma of the breast with amplification of the HER2-gene confirmed by FISH: the first case reported. Hum Pathol. 2010;41:910–3. doi: 10.1016/j.humpath.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 13.Sentani K, Tashiro T, Uraoka N, Aosaki Y, Yano S, Takaeko F, Yasui W. Primary mammary mucinous cystadenocarcinoma: cytological and histological findings. Diagn Cytopathol. 2012;40:624–8. doi: 10.1002/dc.21638. [DOI] [PubMed] [Google Scholar]
- 14.Deng Y, Xue D, Wang X, Xu S, Ao Q, Hu Z, Wang G. Mucinous cystadenocarcinoma of the breast with a basal-like immunophenotype. Pathol Int. 2012;62:429–32. doi: 10.1111/j.1440-1827.2012.02810.x. [DOI] [PubMed] [Google Scholar]
- 15.Li XY, Peng J, Zhang ZX, Zhang YM. Mammary mucinous cystadenocarcinoma. Breast J. 2012;18:282–3. doi: 10.1111/j.1524-4741.2012.01238.x. [DOI] [PubMed] [Google Scholar]
- 16.Kim SE, Park JH, Hong S, Koo JS, Jeong J, Jung WH. Primary mucinous cystadenocarcinoma of the breast: cytologic finding and expression of MUC5 are different from mucinous carcinoma. Korean J Pathol. 2012;46:611–6. doi: 10.4132/KoreanJPathol.2012.46.6.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin DL, Hu JL, Shao SH, Sun DM, Wang JG. Primary mucinous cystadenocarcinoma of the breast with endocervical-like mucinous epithelium. Breast Care (Basel) 2013;8:445–7. doi: 10.1159/000357657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kucukzeybek BB, Yigit S, Sari AA, Rezanko T, Durak E, Sadullahoglu C. Primary mucinous cystadenocarcinoma of the breast with amplification of the HER2 gene confirmed by FISH - case report and review of the literature. Pol J Pathol. 2014;65:70–3. doi: 10.5114/pjp.2014.42673. [DOI] [PubMed] [Google Scholar]
- 19.Witherspoon LE, Oxenhandler RW. A rare tumor: mucinous cystadenocarcinoma of the breast. Am Surg. 2015;81:E106–8. [PubMed] [Google Scholar]
- 20.Seong M, Ko EY, Han BK, Cho SY, Cho EY, Lee SK, Lee JE. Radiologic findings of primary mucinous cystadenocarcinoma of the breast: a report of two cases and a literature review. J Breast Cancer. 2016;19:330–3. doi: 10.4048/jbc.2016.19.3.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koufopoulos N, Goudeli C, Syrios J, Filopoulos E, Khaldi L. Mucinous cystadenocarcinoma of the breast: the challenge of diagnosing a rare entity. Rare Tumors. 2017;9:7016. doi: 10.4081/rt.2017.7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nayak A, Bleiweiss IJ, Dumoff K, Bhuiya TA. Mucinous cystadenocarcinoma of the breast: report of 2 cases including one with long-term local recurrence. Int J Surg Pathol. 2018;26:749–57. doi: 10.1177/1066896918775810. [DOI] [PubMed] [Google Scholar]
- 23.Yamasaki H, Saw D, Zdanowitz J, Faltz LL. Ovarian carcinoma metastasis to the breast case report and review of the literature. Am J Surg Pathol. 1993;17:193–7. doi: 10.1097/00000478-199302000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Lee AH. The histological diagnosis of metastases to the breast from extramammary malignancies. J Clin Pathol. 2007;60:1333–41. doi: 10.1136/jcp.2006.046078. [DOI] [PMC free article] [PubMed] [Google Scholar]


