Abstract
Sclerosing pneumocytoma (SP) is a rare and benign tumor predominantly occurring in Asian women, easily misdiagnosed by imaging and pathologic frozen diagnosis during surgery because of its diverse histomorphology (4 structures, 2 types of cells). It may form multiple tumors. When SP is combined with carcinoid, adenoma, or other tumors (although rare), diagnosis is more complicated. SP mixed with carcinoid tumor is rare. At present, only 4 cases have been reported in English literature. Here, we report a case of sclerosing pneumocytoma combined not only with carcinoid, but also with clear cell adenoma of the lung. The patient was a 52-year-old female and CT found a nodule in the middle lobe of the right lung. SP was not excluded by intraoperative frozen section diagnosis. The above 3 components formed a 1.4 cm nodule. The related literature is reviewed to strengthen the understanding of SP, and aid clinicopathological diagnosis.
Keywords: Sclerosing pneumocytoma (SP), adenoma, carcinoid, TTF-1
Introduction
Sclerosing pneumocytoma (SP) used to be called sclerosing hemangioma of the lung (SHL), first reported by Liebow et al. [1] in 1956. SP occurs mostly in the lung parenchyma, and is more common in middle-aged women. Clinical symptoms are generally absent [2], and the incidence of the tumor in Asia is higher than that in Europe and the United States. Most of them are solid lesions on CT, with various pathologic forms. Frozen sections are easily misdiagnosed as lung adenocarcinoma or carcinoid. There are many theories about its origin [3-8]. At present, it is believed to originate from primitive lung epithelial cells. Therefore, in the 2015 edition of WHO, pulmonary sclerosing hemangioma originally belonged to “mixed tumors,” was classified as “pulmonary adenoma” and was renamed sclerosing pneumocytoma. In the past, SP was considered a rare pulmonary benign tumor. However, recent literature reported that SP may be associated with carcinoid, and even recurrence and lymph node metastasis [9-12]. Therefore, it has been suggested that SP is a tumor with uncertain malignant potential, which complicates the preoperative and intraoperative diagnosis of SP. A case of sclerosing pneumocytoma mixed with clear cell adenoma and carcinoid is reported. Preoperative imaging and pathologic diagnosis during and after operation are detailed.
Clinical information
The patient was a 52-year-old woman admitted to the hospital for 9 days after finding lung space-occupying lesions on physical examination. She had no clinical symptoms. CT showed a nodular high-density shadow in the right middle lobe, with clear edges and lobulated shape, about 1.4 × 1.0 cm. There were multiple plaque-like high-density shadows under the pleura of both lungs. Stripes of high density shadow were seen in both lungs. The cystic low density shadow was seen near the right inferior pulmonary vein, about 1.1 × 1.5 cm. A cystic gas density shadow was seen at the right rear aspect of the trachea. By imaging diagnosis, the nature of the middle lobe nodules of the right lung was uncertain, and follow-up and re-examination was recommended (Figure 1). Multiple patches under the pleura of both lungs were considered as benign lesions. With cystic low density near the right inferior pulmonary vein, pericardial cyst or tracheal diverticulum were considered.
Figure 1.
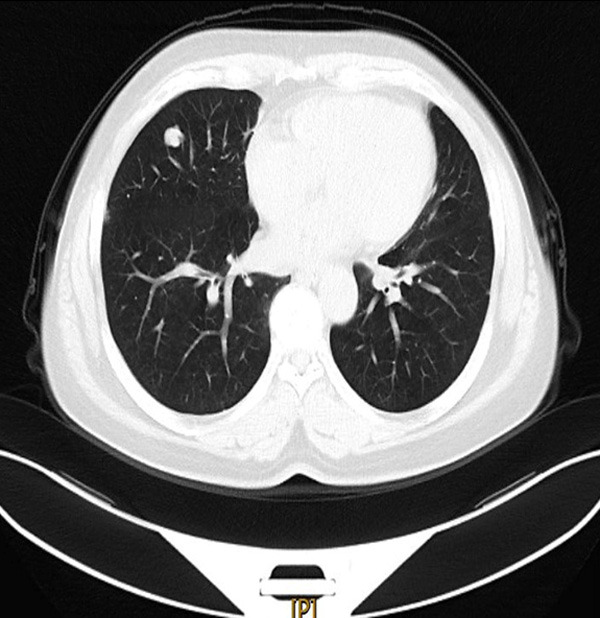
Chest CT (lung window): a nodular high-density shadow in the right middle lobe, with clear edges and lobulated shape.
Relevant examinations were completed (no abnormalities in the laboratory examination) in the hospital, and wedge resection of right middle lobe of lung was performed under thoracoscopy. During operation, it was found that the tumors were located in the lateral segment of the middle lobe of the right lung, about 1 × 1 × 1 cm in size, tough and active.
Intraoperative frozen section showed that the tumors consisted of classical SP areas (papillary structure, sclerosing area, hemangioma-like structure) and epithelioid cell nest areas with light staining of cytoplasm. There was no necrosis or mitosis. However, the epithelioid cell nest areas with light staining of cytoplasm were unstable and could not be classified as benign or malignant. (If only such epithelioid cell nests existed, lung adenocarcinoma may be considered).
After communicating with the clinical doctor during the operation, the report was given: Tumor lesions in the middle lobe of the right lung were of various shapes and clear borders. Sclerosing pneumocytoma was not excluded. The diagnosis needed to be confirmed by paraffin and immunohistochemistry. (Intraoperative frozen section images are shown in Figure 2A-C).
Figure 2.
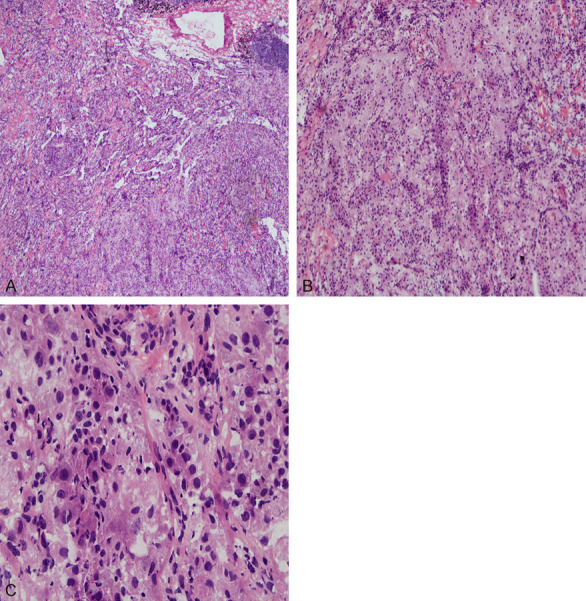
A. Intraoperative frozen section (HE × 50) the tumor consisted of the classic SP area (papillary structure, sclerosing area, hemangioma-like structure) and cytoplasmic epithelioid cell nest area. B. Intraoperative frozen section (HE × 100) the tumor with cytoplasmic epithelioid cell nest area. C. Intraoperative frozen section (HE × 400) the cytoplasmic epithelioid cell nest area of the tumor.
Because the cystic dilatation was seen behind the right lower pulmonary vein, the boundary between the lower pulmonary vein and the lower pulmonary vein was unclear, and there was a risk of bleeding, the surgeon did not perform a right middle lobectomy. The patient did not receive radiotherapy and chemotherapy after surgery, and no tumor recurrence or metastasis was observed during the follow-up period of 8 months.
Immunohistochemistry
Tumor tissues were fixed in 10% neutral formalin, embedded in paraffin, sectioned (thickness 4 μm), routinely stained with H&E and immunohistochemical staining. CK, EMA, Vimentin, Synaptophysin, P53, and S-100 antibodies were purchased from Fuzhou Maixin Co.; TTF-1, CK8/18, CgA, and HMB45 were purchased from Beijing Zhongshan Company; ER, PR, and Ki67 were purchased from Roche. SP method was used to carry out the immunohistochemistry according to the instructions of kit operation, and the positive and negative controls were set up at the same time.
Histomorphology and immunohistochemical findings
Macroscopic description
The right lung mid-lobe tumor was a wedge-shaped resection of lung tissue, 7.5 × 4.0 × 1.5 cm. A gray-yellow mass was found 1 cm away from the incision margin, 0.7 cm under the capsule, with the size of 1.3 × 0.9 × 0.8 cm, hard and with clear boundaries.
Microscopic description
The tumor had a clear boundary and a complex tissue structure. It consisted of three parts: in addition to the classical sclerosing hemangioma-like area (3A, 0.8 × 0.3 cm under the microscope) (such as the sclerotic, the papillary, the hemangioma-like, and the solid small round cell regions), columnar clear cell nests (3B, 0.4 × 0.4 cm under the microscope) and short spindle solid cell nests (3C, 0.6 × 0.5 cm under the microscope) were also seen (see Figure 3).
Figure 3.
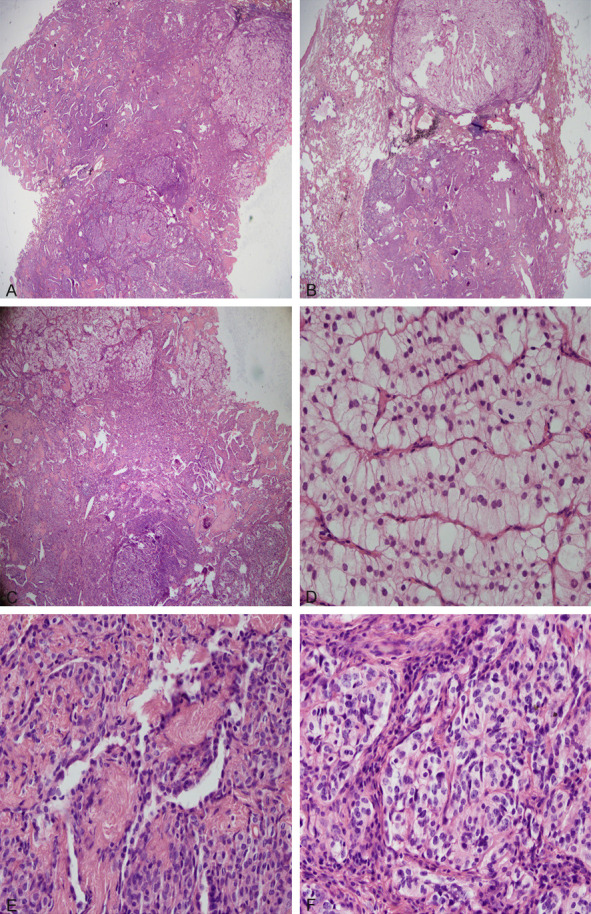
A. The mass consisted of 3 parts (HE × 20). B. Another HE slice showed a lobulated mass (HE × 20). C. The mass consisted of 3 parts (HE × 40). D. The columnar clear cells area of the tumour (HE × 400). E. The classical sclerosing hemangioma-like area of the tumour (HE × 400). F. The short spindle solid cell nests area of the tumour (HE × 400).
The papillary area was composed of typically surface-covered cubic cells, interstitial round or polygonal cells. The surface cells were similar to type II pneumocytes, and the stromal cells had central bland nuclei with fine chromatin and absent nucleoli, with well-defined borders. TTF-1 and EMA were expressed in both cell types by immunohistochemistry; CK and CK8/18 are expressed in cuboid cells, VIM is expressed in interstitial cells, but CK, CK8/18, syn and CgA were not expressed. The immunophenotypes of the cells in the solid small round cell area were consistent with the stromal round cells in the papillary area. Fibrous tissue with hyaline degeneration and coarsely sclerosed collagen were seen in the sclerotic zone, and TTF-1 positive cells were found between collagen. In the hemangioma-like area, erythrocyte filling was found between the papillary fissures, and TTF-1 positive cubic or flat cells were covered on the surface of the papilla.
The conventional staining corresponding to the epithelioid cell nests with light cytoplasm staining on the frozen section was the columnar clear cell area (as shown in Figure 3D). Tumor cells were large, columnar, with clear borders, abundant cytoplasm, transparent or very light pink, round or oval nuclei, moving up or away from the basement membrane, with small nucleoli. Cells were arranged in trabecular shape, with little stroma, abundant squeezed blood vessels, and no obvious necrosis and nuclear division. These tumor cells were positive for TTF-1, CK, and CK8/18 by immunohistochemistry, with EMA weakly positive and close to the luminal margin. Cells were negative for Vimentin, S-100, HMB45, Synaptophysin, CgA, CEA and CD68. Adenocarcinoma could not be excluded in frozen sections and it was difficult to classify them as adenoma or adenocarcinoma listed by WHO (2015) in conventional paraffin sections.
Another special feature was the appearance of short spindle solid cell nests on paraffin sections (Figure 3E, 3F), which was not apparent on frozen sections. The tumour cells grew in an organoid nesting arrangement, with fine vascular fibrous stroma. The cytoplasm of the cells was eosinophilic or transparent, the nucleus was oval, and the nuclear chromatin was finely granular, separated by fiber tissue, distributed in the form of nest, no necrosis and mitosis were found, with the strong expression of syn, CgA, and weak expression of TTF-1, CK and CK8/18, and did not express EMA, Vim, S-100, or HMB45. The Ki67 index of the entire tumor was not high, less than 2%. The immunohistochemistry of the tumor was shown in Figure 4A-G, Table 1.
Figure 4.
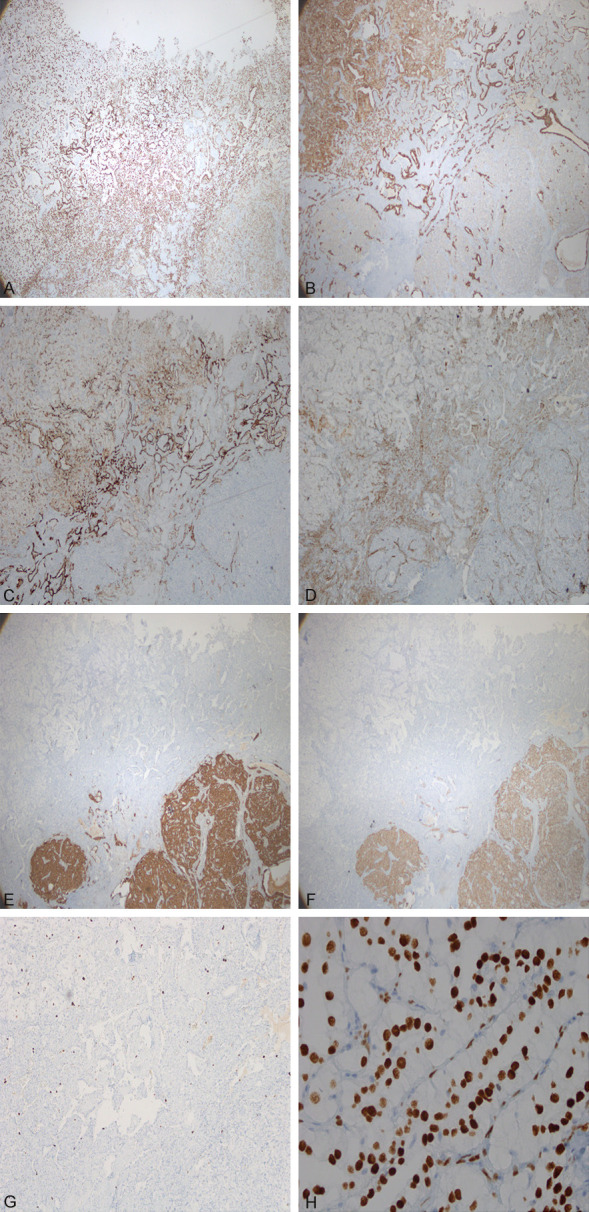
A. TTF-1 was positive in each part of the tumor (× 40). B. Expression of CK in various parts of tumor (× 40). C. Expression of EMA in various parts of tumor (× 40). D. Expression of vimentin in various parts of tumor (× 40). E. Expression of CgA in various parts of tumor (× 40). F. Expression of Syn in various parts of tumor (× 40). G. Low expression of Ki67 in various parts of tumor (× 100). H. Expression of TTF-1 in the columnar clear cell area of tumor (× 400).
Table 1.
Expression of immunohistochemistry in various regions of the tumor
| Classic SP | Columnar clear cell area | Short spindle solid cell nests | ||
|---|---|---|---|---|
|
| ||||
| Cuboidal surface cells | Stromal small round cells | |||
| TTF-1 | +++ | ++ | +++ | + |
| CK | +++ | - | +++ | + |
| CK8/18 | +++ | - | +++ | + |
| Vimentin | - | ++ | - | - |
| EMA | ++ | + | + | - |
| CEA | - | - | - | - |
| CgA | - | - | - | +++ |
| Syn | - | - | - | +++ |
| CD68 | - | - | - | - |
| S-100 | - | - | - | - |
| HMB45 | - | - | - | - |
| ER | Partially + | Partially + | Scattered + | Scattered + |
| PR | Scattered + | Partially + | - | Partially + |
| P53 | Scattered + | Scattered + | Scattered + | Scattered + |
| TFE3 | - | - | - | - |
| Ki67 index | <1% | <1% | <2% | <1% |
Histochemical staining
PAS (-) (see Figure 5).
Figure 5.
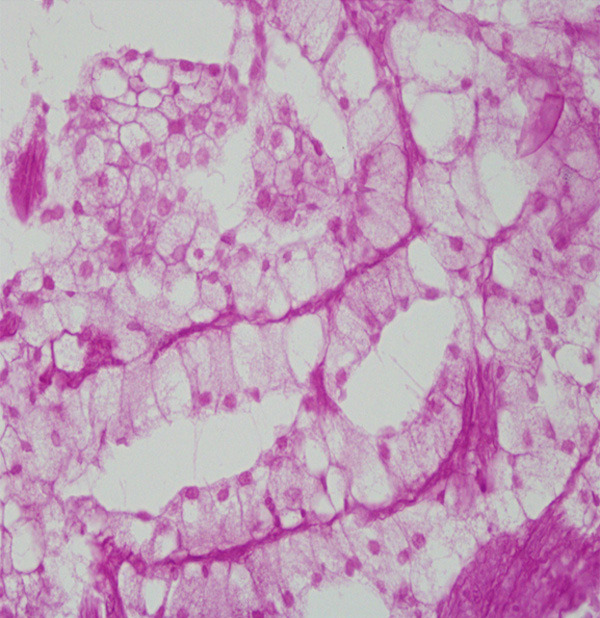
PAS was negtive in the cytoplasm of the columnar clear cell area (× 400).
Discussion
Combining clinical information, histomorphology, immunohistochemistry and special staining results, the final diagnosis was the sclerosing pneumocytoma mixed with a columnar clear cell adenoma and a typical carcinoid.
SP is a rare lung tumor, which occurs mostly in the lung parenchyma, and a few can occur in the bronchial cavity, the hilar [13-15] and so on. It is most commonly seen in middle-aged women, and may be related to sex hormones. Patients are typically asymptomatic [2]. The incidence in Asia is significantly higher than that in Europe and the United States. Single lesions are more common, and multiple lesions or the lesions in double lungs are rare [16-18]. There are many theories about the origin of histology: vascular endothelial cells [3,4], mesothelial cells [5], neuroendocrine cells [6], primitive undifferentiated respiratory epithelium [7,8]. Devouassoux-Shisheboran M et al. [7] observed that both cubic cells and polygonal cells strongly expressed TTF-1. TTF-1 is specifically expressed in mature type II alveolar epithelium cells, Clara cells, and embryonic alveolar epithelium cells. These findings suggested that SP originated from the original respiratory epithelium, and some scholars believed that the two cells are different stages of primitive respiratory epithelial differentiation.
SP was considered to be a rare benign tumor of lung in the past, but recently there have been reports in the literature that a small number of SP can be combined with carcinoid [19], or canceration [20-22], and even recurrence [9], mediastinal and local lymph node metastasis [10-12], and a small number of SP can even be transferred to the stomach and bones [23,24]. Therefore, it has been suggested that SP may be a tumor with undetermined malignant potential. P53 mutation [25] was found in some cases, and AKT1 activation was associated with multiple lung diseases [26], which suggested that SP may have malignant potential and requires long-term follow-up. However, these behaviors have no significant effect on the prognosis of patients, so the current view is that SP is clinically benign, and there is no literature report on the need for adjuvant chemotherapy after SP resection. Only one patient underwent postoperative radiotherapy [27].
Typical SP can be seen in four structures (papillary area, sclerotic area, solid area and haemorrhagic area), two kinds of cells (cubic cells and round cells). Due to its diverse composition or multiple tumors, it is often misdiagnosed during imaging or intraoperative freezing. Especially when there is a shallow lobulation in imaging, it is easier to be misdiagnosed [28,29]. Histologically, the papillary area needs to be differentiated from lepidic lung adenocarcinoma. The solid area needs to be differentiated from carcinoid (primary or metastatic), glomus tumors and paraganglioma, while the cytoplasm of cubic cells can be transparent, so it should be differentiated from clear cell tumors (primary or metastatic), and these identifications should be combined with immunohistochemical staining and histochemical staining.
When the SP merges with other tumors, the diagnosis becomes more complicated. In this case, the imaging CT showed that the tumor was so small that the lobulation appeared. Therefore, the imaging doctor favored lung cancer during the preoperative evaluation, but no clear diagnosis was given. It has also been confirmed under the microscope (Figure 3B), different sections showed the lobulation of the tumor, one end of the lobulated leaf was a clear cell adenoma, the other end was a carcinoid, and the middle was a classic sclerosing pneumocytoma. The three parts of the area were not completely separated, but were transition and interspersed with each other, and it seems to be impossible to explain this with “collision tumors”. Immunohistochemistry showed that TTF-1 was expressed in all three parts of the lung. Although the intensity of TTF-1 was different, it suggested that they might originate from primitive alveolar epithelium. So it seemed to be more reasonable to use one tumor to differentiate in different directions to explain the characteristics of this case, which also supported the view of Devouassoux-Shisheboran M [7]. In this case, it can be roughly distinguished the classic sclerosing pneumocytoma from the wide cytoplasmic epithelial nest on the frozen section during the operation. The carcinoid area was not obvious. If only the wide cytoplasmic epithelial nest was concerned, or only the material of which was taken, then the frozen report of adenocarcinoma might be sent out. Fortunately, the SP component was taken at the time, and the overall boundary was clear, the possibility of SP was considered first. However, we also communicated with the surgeon at that time, because of the blood vessels, the patient’s lung lobe was not removed.
SP is more common in East Asia such as Japan, South Korea, India, and China than in Europe and the United States. Hundreds of cases can be found in pubmed, but the cases of SP combined with carcinoid were rare, and only 4 cases were currently retrieved in English literature [19,30-32], and one case in Chinese literature [33]. Typical carcinoid (TC) is relatively rare in the lung and is a low-grade neuroendocrine tumor. Because there has been similar pathological features between TC and the solid region of SP (such as cell morphology, abundant blood vessels, nesting tumor cells tend to appear under the glandular epithelium), it is easy to misdiagnose TC as the small round, polygonal cells of SP. Therefore, TC needs to be differentiated from SP. However, there may be scattered or focal hyperplasia of neuroendocrine cells in the SP, and even aggregated into carcinoid tumorlets with diameters more than 2 mm, less than 5 mm. When the maximum diameter of nested hyperplasia of neuroendocrine cells exceeds 5 mm, it is necessary to diagnose TC. It has been suggested that SP originated from neuroendocrine cells, so SP can be seen in scattered or small focal distribution of neuroendocrine cells (as seen in SP and carcinoid transition areas in this case), but in the present case, neuroendocrine cells are distributed in a sheet-like manner and exceed 5 mm, which met the diagnostic criteria for carcinoid. There is a constant debate in the literature about whether the two tumors are collisions or an origin [19,30-33].
As can be seen from Table 2, there were 6 cases of SP combined with carcinoid, which had no gender difference and occurred in the middle and lower lobe of the right lung. It can be single or multiple, the tumor size was 0.3-2.6 cm, and the wedge or lobectomy has been performed. The longest follow-up period was 36 months, and no recurrence or metastasis was observed. Most of the tumor components were mixed, and there was only one case where the tumor components were completely separated, which supported the original alveolar epithelium instead of collision.
Table 2.
Summary of cases of SP combined with carcinoid in the literature (English and Chinese)
| Case number | Sex | Age | Clinical symptoms | Location | Number of tumors | Maximum diameter of the tumor (cm) | Growth pattern | Accompanying disease | Surgical approach | Follow-up (month) | Nationality | Issuing time |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 52 | Asymptomatic | Right middle lobe | Single | 1.3 | Mixed | Columnar clear cell adenoma | Wedge resection | 8 | China | |
| 2 | Female | 55 | Asymptomatic | Right middle lobe | Single | 1.6 | Mixed so well that appeared as a single neoplasm | NA | Lung lobectomy | 6 | China | 2019 19 |
| 3 | Male | 62 | Asymptomatic | Left lower lobe | Single | 1.3 | Mixed Clear borders between the SP and TC | Adenocarcinoma in the upper right lobe | Wedge resection + lobectomy | 17 | Korea | 2017 30 |
| 4 | Female | 50 | Repeated dry cough, chest pain, dyspnea | Right middle lobe | Multiple | 0.3-2.2 | Mixed and mosaic pattern, carcinoid tumorlets | NA | Lung lobectomy | 14 | China | 2014 31 |
| 5 | Male | 52 | Asymptomatic | Right middle and lower lobe | Multiple | 0.5-2.6 | Separated | pulmonarytuberculosis in childhood | Lung lobectomy | 36 | Korea | 2013 32 |
| 6 | Male | 41 | Paroxysmal dizziness, excessive drinking, polyuria, increased nocturnal urine for 6 months, low back pain after bending and loading for 2 months | Right lower lobe | Single | 2.0 | Mixed Overlapped at the borderline | Ectopic ACTH syndrome | Lung lobectomy | 8 | China | 2009 33 |
However, the most puzzling is the transparent cell nest component in this case. It has been reported in the literature that SP can be combined with papillary adenoma [14], atypical alveolar hyperplasia (AAH) [20], which can be cancerous [20-22], or associated with adenocarcinoma [30,34], but no cases with transparent cell nests have been retrieved. Of course, we have also made differential diagnosis related to clear cell tumors, including clear cell adenocarcinoma, clear cell carcinoid, acinar cell tumor, paraganglioma, PEComas, histiocytic tumor, metastatic renal clear cell carcinoma, etc, all of which were excluded by immunohistochemistry staining, histochemical staining and PET-CT. So, can this part of the tumor tissue be called a columnar clear cell adenoma? Because the tumor cell atypia was not obvious, the ki67 proliferation index was low, there was insufficient evidence to diagnose cancer. However, the corresponding names of these tumors can not be found in the new WHO classification in 2015, nor can they be found in the literature. We were confused. Since SP can recur and metastasize, and carcinoid is a low-grade neuroendocrine tumor, when columnar clear cells combined with SP and carcinoid, it is more necessary to follow up and review. At the current follow-up of 8 months, no new tumors or metastases have appeared in the patients.
To sum up, SP is a rare tumor, which is easy to be misdiagnosed because of its diverse or multiple lesions in imaging and intraoperative freezing. SP mixed with carcinoid and adenoma is rare, and its diagnosis should be combined with clinical information (age, sex, location), imaging data, histomorphology, immunohistochemistry and special staining. Because of the recurrence and metastasis of SP, it may be a tumor with undetermined malignant potential, and patients need to be informed of long-term follow-up. The molecular mechanism for the development of SP also needs to be further studied.
Disclosure of conflict of interest
None.
References
- 1.Liebow AA, Hubbell DS. Sclerosing hemangioma (histiocytoma, xanthoma) of the lung. Cancer. 1956;9:53–75. doi: 10.1002/1097-0142(195601/02)9:1<53::aid-cncr2820090104>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 2.Yuan ZQ, Wang Q, Bao M. Symptomatic pulmonary sclerosing hemangioma: a rare case of a solitary pulmonary nodule in a woman of advanced age. J Int Med Res. 2019;47:2302–2308. doi: 10.1177/0300060519840898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haas JE, Yunis EJ, Totten RS. Ultrastructure of a sclerosing hemangioma of the lung. Cancer. 1972;30:512–8. doi: 10.1002/1097-0142(197208)30:2<512::aid-cncr2820300231>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 4.Kay S, Still WJ, Borochovitz D. Sclerosing hemangioma of the lung: an endothelial or epithelial neoplasm. Hum Pathol. 1977;8:468–74. doi: 10.1016/s0046-8177(77)80012-9. [DOI] [PubMed] [Google Scholar]
- 5.Katzenstein AL, Weise DL, Fulling K, Battifora H. So-called sclerosing hemangioma of the lung. Evidence for mesothelial origin. Am J Surg Pathol. 1983;7:3–14. doi: 10.1097/00000478-198301000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Xu HM, Li WH, Hou N, Zhang SG, Li HF, Wang SQ, Yu ZY, Li ZJ, Zeng MY, Zhu GM. Neuroendocrine differentiation in 32 cases of so-called sclerosing hemangioma of the lung: identified by immunohistochemical and ultrastructural study. Am J Surg Pathol. 1997;21:1013–22. doi: 10.1097/00000478-199709000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Devouassoux-Shisheboran M, Hayashi T, Linnoila RI, Koss MN, Travis WD. A clinicopathologic study of 100 cases of pulmonary sclerosing hemangioma with immunohistochemical studies: TTF-1 is expressed in both round and surface cells, suggesting an origin from primitive respiratory epithelium. Am J Surg Pathol. 2000;24:906–16. doi: 10.1097/00000478-200007000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Einsfelder BM, Müller KM. “Pneumocytoma” or “sclerosing hemangioma”: histogenetic aspects of a rare tumor of the lung. Pathologe. 2005;26:367–77. doi: 10.1007/s00292-005-0751-8. [DOI] [PubMed] [Google Scholar]
- 9.Wei S, Tian J, Song X, Chen Y. Recurrence of pulmonary sclerosing hemangioma. Thorac Cardiovasc Surg. 2008;56:120–2. doi: 10.1055/s-2007-989280. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Zhang L, Wang Y, Jia X, Wang J, Zhang H. Sclerosing pneumocytoma with metastasis to the mediastinal and regional lymph nodes. Indian J Pathol Microbiol. 2018;61:407–409. doi: 10.4103/IJPM.IJPM_98_17. [DOI] [PubMed] [Google Scholar]
- 11.Pokharel S, Dhillon SS, Ylagan L, George S, Yendamuri S. Sclerosing pneumocytoma with lymph node metastasis. J Thorac Oncol. 2016;11:1802–4. doi: 10.1016/j.jtho.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Xu HM, Zhang G. A rare case of pulmonary sclerosing hemagioma with lymph node metastasis and review of the literature. Int J Clin Exp Pathol. 2015;8:8619–23. [PMC free article] [PubMed] [Google Scholar]
- 13.Khanna A, Alshabani K, Mukhopadhyay S, Lam L, Ghosh S. Sclerosing pneumocytoma: Case report of a rare endobronchial presentation. Medicine (Baltimore) 2019;98:e15038. doi: 10.1097/MD.0000000000015038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitawaki Y, Fujishima F, Taniuchi S, Saito R, Nakamura Y, Sato R, Aoyama Y, Onodera Y, Inoshita N, Matsuda Y, Watanabe M, Sasano H. Coexistence of glandular papilloma and sclerosing pneumocytoma in the bronchiole. Pathol Int. 2018;68:425–430. doi: 10.1111/pin.12677. [DOI] [PubMed] [Google Scholar]
- 15.Ikeda M, Okada Y, Hagiwara K, Murata Y, Kanayama T, Hara A, Fujinaga T. A case of pulmonary sclerosing pneumocytoma in the hilar lesion. Gen Thorac Cardiovasc Surg. 2018:28. doi: 10.1007/s11748-018-1043-6. [DOI] [PubMed] [Google Scholar]
- 16.Fan X, Lin L, Wang J, Wang Y, Feng A, Nie L, Wu H, Meng F, Xu H. Genome profile in a extremely rare case of pulmonary sclerosing pneumocytoma presenting with diffusely-scattered nodules in the right lung. Cancer Biol Ther. 2018;19:13–19. doi: 10.1080/15384047.2017.1360443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maeda R, Isowa N, Miura H, Tokuyasu H, Kawasaki Y, Yamamoto K. Bilateral multiple sclerosing hemangiomas of the lung. Gen Thorac Cardiovasc Surg. 2009;57:667–70. doi: 10.1007/s11748-009-0452-y. [DOI] [PubMed] [Google Scholar]
- 18.Komatsu T, Fukuse T, Wada H, Sakurai T. Pulmonary sclerosing hemangioma with pulmonary metastasis. Thorac Cardiovasc Surg. 2006;54:348–9. doi: 10.1055/s-2005-872976. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z, Yang MQ, Huang WJ, Zhang D, Xu HT. Sclerosing pneumocytoma mixed with a typical carcinoid tumor: a case report and review of literature. Medicine (Baltimore) 2019;98 doi: 10.1097/MD.0000000000014315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim GY, Kim J, Choi YS, Kim HJ, Ahn G, Han J. Sixteen cases of sclerosing hemangioma of the lung including unusual presentations. J Korean Med Sci. 2004;19:352–8. doi: 10.3346/jkms.2004.19.3.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun Y, Li HF, Zu JC, Dong SHY, Li TT. A case of pulmonary sclerosing hemangioma carcinogenesis. China Journal of Modern Medicine. 2017;27:140–141. [Google Scholar]
- 22.Liu W, Tian XY, Li Y, Zhao Y, Li B, Li Z. Coexistence of pulmonary sclerosing hemangioma and primary adenocarcinoma in the same nodule of lung. Diagn Pathol. 2011;6:41. doi: 10.1186/1746-1596-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bae YS, Ro JY, Shim HS, Hong SW, Yoon SO. Pulmonary sclerosing haemangioma with metastatic spread to stomach. Histopathology. 2012;60:1162–4. doi: 10.1111/j.1365-2559.2012.04213.x. [DOI] [PubMed] [Google Scholar]
- 24.Kim MK, Jang SJ, Kim YH, Kim SW. Bone metastasis in pulmonary sclerosing hemangioma. Korean J Intern Med. 2015;30:928–30. doi: 10.3904/kjim.2015.30.6.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Dai SD, Qi FJ, Xu HT, Wang EH. p53 protein expression and genetic mutation in two primary cell types in pulmonary sclerosing haemangioma. J Clin Pathol. 2008;61:192–6. doi: 10.1136/jcp.2007.050401. [DOI] [PubMed] [Google Scholar]
- 26.Fan X, Lin L, Wang J, Wang Y, Feng A, Nie L, Wu H, Meng F, Xu H. Genome profile in a extremely rare case of pulmonary sclerosing pneumocytoma presenting with diffusely-scattered nodules in the right lung. Cancer Biol Ther. 2018;19:13–19. doi: 10.1080/15384047.2017.1360443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fayers RW, Lim TS, Havlat MF. Pulmonary sclerosing pneumocytoma (sclerosing haemangioma): radical radiation therapy. J Med Imaging Radiat Oncol. 2016;60:693–695. doi: 10.1111/1754-9485.12477. [DOI] [PubMed] [Google Scholar]
- 28.Shin SY, Kim MY, Oh SY, Lee HJ, Hong SA, Jang SJ, Kim SS. Pulmonary sclerosing pneumocytoma of the lung: CT characteristics in a large series of a tertiary referral center. Medicine (Baltimore) 2015;94:e498. doi: 10.1097/MD.0000000000000498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu J, Huang J, Yang L. Unusual CT features of pulmonary sclerosing hemangioma. Chin J Med Imaging Technol. 2010;26:272–274. [Google Scholar]
- 30.Cho HJ, Lee JH, Lee GK, Hong EK, Kim HY. Case of sclerosing pneumocytoma combined with a typical carcinoid and pulmonary adenocarcinoma in different lobes. Thorac Cancer. 2017;8:372–375. doi: 10.1111/1759-7714.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, He Q, Shi W, Wang J, Ji H. A mixture of carcinoid tumors, extensive neuroendocrine proliferation, and multiple pulmonary sclerosing hemangiomas. World J Surg Oncol. 2014;12:209. doi: 10.1186/1477-7819-12-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim Y, Choi YD, Kim BJ, Oh IJ, Song SY, Nam JH, Park CS. Multiple peripheral typical carcinoid tumors of the lung: associated with sclerosing hemangiomas. Diagn Pathol. 2013;8:97. doi: 10.1186/1746-1596-8-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi J, Yang D, Meng ZL. Carcinoid in pulmonary sclerosing hemangioma: a case report and review of literature. Chinese Journal of Diagnostic Pathology. 2009;16:273–276. [Google Scholar]
- 34.Liang FH, Liu ZHJ, Gao YM, Zhang CH, Lin FW. A case of sclerosing pneumocytoma combined with pulmonary adenocarcinoma and review of the literature. Chinese Journal of Laboratory Diagnosis. 2017;21:2021–2023. [Google Scholar]


