Abstract
Objective: To investigate the effects of dexmedetomidine on perioperative inflammation and lung protection in elderly patients undergoing thoracoscopic radical resection of lung cancer. Methods: A total of 116 elderly patients undergoing elective radical resection of lung cancer in the Second Affiliate Hospital of Soochow University were selected and divided into two groups by random number table method, 58 cases in each group. Observation group was given 1 μg/kg loading dose of dexmedetomidine by continuously intravenous pump for 10 min before anesthesia induction, which was maintained at a rate of 0.3 μg/(kg·h) until 20 min before the end of operation. Control group was given equal volume of normal saline. Heart rate, mean arterial pressure, and alveolar-arterial oxygen pressure difference (P(A-a)O2) were measured and recorded respectively at before anesthesia induction (T0), immediately after endotracheal intubation (T1), 1 h after one-lung ventilation (T2) and 10 min before the end of operation (T3). Results: Compared with control group, heart rate, P(A-a)O2, interleukin-6, interleukin-8 and malondialdehyde levels at T1-T3 in observation group were significantly lower; the superoxide dismutase level was significantly higher (all P<0.05), and alveolar damage index of quantitative assessment and apoptotic index at T3 in observation group were significantly lower (P<0.05). The incidence of postoperative pulmonary complications was 3.4% in observation group and 25.8% in control group (P<0.05). The postoperative awake and spontaneous breathing recovery time in the observation group was significantly shorter compared with control group (P<0.05). There was no significant difference in mean arterial pressure at each time point between the two groups (P>0.05). Conclusion: Dexmedetomidine can reduce inflammatory response and oxidative stress response in elderly patients undergoing radical resection of lung cancer, and reduce the occurrence of postoperative pulmonary complications, thus playing a role in lung protection.
Keywords: Dexmedetomidine, radical resection of lung cancer, lung protection, inflammatory response, oxidative stress response
Introduction
In recent years, video-assisted thoracoscopic surgery (VATS) has been widely used in the surgical treatment of lung cancer, in which one-lung ventilation (OLV) is required to artificially cause the lung collapse on the affected side, but it may cause different degrees of lung injury [1]. Dexmedetomidine reduces the degree of lung injury by inhibiting the expression of inflammatory factors such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-8 (IL-8) in lung tissues [2-4]. Dexmedetomidine can reduce the generation of reactive oxygen species and the release of cytochrome C in lung tissues, and weaken the apoptosis of alveolar epithelial cells [5,6]. Therefore, the effect of dexmedetomidine on lung injury in elderly patients with lung cancer undergoing thoracoscopic lobectomy has not been determined. The purpose of this study was to investigate the protective effect and mechanism of dexmedetomidine on lung injury in elderly patients undergoing thoracoscopic radical resection of lung cancer.
Materials and methods
General information
A total of 116 elderly patients who underwent radical resection of lung cancer in the Second Affiliate Hospital of Soochow University from January 2019 to March 2020 were selected, regardless of gender, aged 63-78 years, and weighed 50 kg-75 kg.
Inclusion criteria: (1) Patients with age >60 years, ASA II-III, cardiac function I-II; (2) Patients who underwent elective thoracoscopic radical resection of lung cancer; (3) Patients without important organ diseases such as obvious cardiovascular, lung, liver, and kidney diseases, without electrolyte disturbances and infections, and without immune and endocrine system diseases; (4) Patients who did not take non-steroid anti-inflammatory drugs (NSAIDs) and hormone drugs recently and did not receive other drug research one month before operation; (5) Patients with normal lung function after preoperative lung function training; (6) The estimated operation time was 2 h-3 h.
Exclusion criteria: (1) Patients with immune, endocrine, neurologic, and psychiatric diseases before operation; (2) Patients with severe heart, lung, liver, and kidney dysfunction; (3) Patients with unilateral or bilateral main bronchus stenosis or occlusion due to lung disease or other diseases, but unable to maintain normal blood oxygen intraoperatively despite good compensation; (4) Patients who took immunosuppressive drugs and NSAIDs before operation; (5) Patients with respiratory tract and pulmonary infection before operation; (6) Patients with forced expiratory volume in the first second (FEV1) <50% or FEV1/forced vital capacity (FVC) >0.70 in preoperative pulmonary function examination; (7) Patients with pulmonary artery pressure >30 mmHg; (8) Patients with intraoperative oxygen saturation consistently below 90% and airway pressure >40 cmH2O; (9) Patients with persistent hypotension or hypertension during operation, mean arterial pressure (MAP) lower than or higher than 20% of the basic value, and intraoperative blood loss >1,000 mL.
SPSS20.0 statistical software was used to generate random numbers, and the patients enrolled in radical resection of lung cancer were randomly divided into an observation group and control group (58 cases each). This study met the requirements of Ethics Committee of the Second Affiliate Hospital of Soochow University and the patients or their immediate family members signed the informed consent.
Methods
The same monitoring method and anesthesia induction method were used in the two groups. Anesthesia induction was given intravenously midazolam injection (Jiangsu Enhua Pharmaceutical Co., Ltd., China) 0.05 mg/kg, sufentanil citrate injection (Hubei Yichang Renfu Pharmaceutical Co., Ltd., China) 0.4 μg/kg, cis-atracurium for injection (Jiangsu Dongying Pharmaceutical Co., Ltd., China) 0.2 mg/kg and propofol (Beijing Fresenius Kabi Co., Ltd., Germany) infusion plasma target concentration 3 μg/mL. The Bispectral Index Monitor (Bispectral Index VISTA type, Aspect Company, USA) showed 40-50, the muscle relaxation monitor (TOF Watch ®SX, Organon Company, Ireland) had train-of-four (TOF) stimulation value =0, and endobronchial intubation was performed. After successful docking with anesthesia machine (Datex Ohmeda, USA), pure oxygen ventilation, bilateral lung ventilation, tidal volume was 8-10 mL/kg, respiratory rate was 12-14 times/min, inhalation/exhalation was 1:2. Target controlled infusion aimed for a plasma concentration of remifentanil 0.2-0.4 ng/mL, and for propofol 2-3 μg/mL, and BIS was maintained at 45-50 levels. Central venous catheterization was performed by the right internal jugular vein, and radial artery catheterization was used to monitor blood pressure. During the operation, the patient took a lateral position, and the tidal volume was changed to 6 mL/kg and PEEP 3 cmH2O when performing OLV. The respiratory rate was adjusted according to the concentration of end-tidal carbon dioxide (EtCO2; 32-45 mmHg) and blood gas analysis. Observation group was given 1 μg/kg loading dose of dexmedetomidine hydrochloride (Jiangsu Yangzijiang Pharmaceutical Co., Ltd., China, 19012031) by continuous intravenous pump for 10 min before anesthesia induction, and then maintained at a rate of 0.3 μg/(kg·h) until 20 min before the end of operation. Then, 0.3 mg-0.5 mg of atropine (Tianjin Jinyao Pharmaceutical Co., Ltd., China) was intravenously injected when the intraoperative heart rate (HR) was lower than 50 times/min, and 5 mg-15 mg of ephedrine (Shenyang First Pharmaceutical Co., Ltd., China) was intravenously injected when the systolic blood pressure was lower than 90 mmHg. Control group was given equal volume of normal saline. Intraoperative BIS was maintained at 45-50 levels.
Outcome measures
Main outcome measures: (1) Detection of inflammatory factors and stress indicators: 3 mL of internal jugular vein blood was drawn at before anesthesia induction (T0), immediately after endotracheal intubation (T1), 1 h after OLV (T2) and 10 min before the end of operation (T3), respectively, and TNF-α, IL-6 and IL-8 levels were detected by enzyme-linked immunosorbent assay (ELISA); the activity of superoxide dismutase (SOD) in serum was measured by xanthine oxidase method; the content of malondialdehyde (MDA) was determined by thiobarbituric acid (TBA) method; (2) Detection of alveolar damage index of quantitative assessment (IQA): at T3, normal lung tissues around the excised lung lobes were taken, fixed with 4% formaldehyde solution, embedded in paraffin, and sectioned for HE staining. The 20 visual fields were observed under 200-fold light microscope continuously. The percentage of damaged alveoli with more than 2 red blood cells and white blood cells in the alveoli in the total number of alveoli, that is, IQA; (3) Detection of apoptosis: at T3, the normal lung tissues around the excised lung lobe were taken, and the apoptosis of lung tissue was detected by in situ nick end-labeling (TUNEL). Sections were randomly selected and 5 visual fields were counted under 200 times visual field. The percentage of positive cells in more than 200 pulmonary epithelial and endothelial cells was apoptotic index (AI) in each visual field; (4) Pulmonary complications including pulmonary edema, atelectasis, pulmonary infection and hypoxemia were recorded within 72 h after operation.
Secondary outcome measures: (1) Alveolar-arterial oxygen pressure difference (P(A-a)O2), oxygenation index (OI), and respiratory index (RI) were detected at each time point for collection of radial artery blood; (2) Intraoperative mean arterial pressure MAP and HR of patients in the two groups, postoperative awake and spontaneous breathing recovery time, and occurrence of restlessness during waking period were recorded; (3) Intraoperative fluid intake and blood loss were recorded in both groups.
Statistical analysis
SPSS20.0 software was used for data analysis. The measurement data were expressed as mean ± standard deviation (x̅ ± sd). Independent sample t test was used for comparison between groups. Repeated measurement analysis of variance was used for comparison at different time points within the group. If there was difference, Bonferroni method was further used for comparison between two data. The enumeration data were represented by cases or (%), using χ2 test or Fisher’s exact test. P<0.05 was considered significant.
Results
General information
There were no significant differences in terms of gender, age, body mass index, underlying disease, pathologic type, FEV1, FVC, operation time, OLV time, fluid intake, and blood loss between the two groups (P>0.05). See Table 1.
Table 1.
Clinical findings
| Finding | Observation group (n=58) | Control group (n=58) | t/χ2 | P |
|---|---|---|---|---|
| Gender (male/female) | 39/19 | 37/21 | 0.153 | 0.696 |
| Age (year) | 67.4±2.5 | 68.1±2.7 | 0.863 | 0.383 |
| BMI (kg/m2) | 20.10±1.14 | 20.18±1.05 | 0.217 | 0.705 |
| Underlying disease (n, %) | ||||
| Hypertension | 9 (15.52) | 8 (13.79) | 0.069 | 0.794 |
| Diabetes | 7 (12.07) | 6 (10.34) | 0.087 | 0.769 |
| CHD | 5 (8.62) | 6 (10.34) | 0.100 | 0.751 |
| Tumor histology (n, %) | ||||
| Adenocarcinoma | 36 (62.07) | 34 (58.62) | 0.144 | 0.704 |
| SCC | 22 (37.93) | 24 (41.38) | 0.144 | 0.704 |
| Predicted value of FEV1 (%) | 59.71±4.82 | 60.34±5.11 | 0.652 | 0.516 |
| Predicted value of FVC (%) | 83.57±8.39 | 82.75±7.90 | 0.531 | 0.596 |
| Operation time (min) | 135±26.41 | 138±24.37 | 0.637 | 0.526 |
| OLV time (min) | 101±21.66 | 103±25.80 | 0.430 | 0.668 |
| Fluid intake (mL) | 790.16±80.47 | 798.44±78.62 | 1.163 | 0.247 |
| Urine volume (mL) | 263.74±105.63 | 270.83±110.44 | 0.852 | 0.396 |
| Blood loss (mL) | 112.52±45.32 | 116.46±50.21 | 0.394 | 0.694 |
Note: BMI: body mass index; CHD: coronary heart disease; SCC: squamous cell carcinoma; FEV1: forced expiratory volume in the first second; FVC: forced vital capacity; OLV: one-lung ventilation.
Comparison of hemodynamics
There was no significant difference in MAP within the same group at each time point (P>0.05), and no significant difference between the two groups at each time point (P>0.05). There was no significant difference in HR at T1-T3 compared with at T0 in control group (P>0.05); there was a significant difference in HR at T1-T3 compared with at T0 in observation group (P<0.05). Compared with control group, the HR values of observation group were slowed down at each time point at T1-T3 (P<0.05), the postoperative awake and spontaneous breathing recovery time in the observation group was significantly shorter, and the incidence of restlessness during waking period in the observation group was significantly lower (all P<0.05). See Tables 2, 3 and Figure 1.
Table 2.
Comparison of mean arterial pressure and heart rate at different time points (n=58, x̅ ± sd)
| Indicator | Group | T0 | T1 | T2 | T3 | F | P |
|---|---|---|---|---|---|---|---|
| MAP (mmHg) | Observation group (n=58) | 91.32±7.83 | 89.67±5.35 | 90.63±7.25 | 88.44±6.54 | 1.954 | 0.122 |
| Control group (n=58) | 90.20±6.41 | 91.93±6.72 | 91.81±3.64 | 90.17±4.36 | 1.858 | 0.138 | |
| t | 0.689 | 1.429 | 1.108 | 1.674 | |||
| P | 0.492 | 0.156 | 0.270 | 0.097 | |||
| HR (times/min) | Observation group (n=58) | 79.42±6.47 | 60.76±5.72 | 65.47±6.67 | 67.90±5.26 | 103.27 | 0.000 |
| Control group (n=58) | 77.93±6.63 | 74.03±5.34 | 76.52±5.28 | 79.71±5.21 | 2.361 | 0.081 | |
| t | 1.225 | 12.915 | 9.892 | 12.149 | |||
| P | 0.223 | 0.000 | 0.000 | 0.000 |
Note: T0: before anesthesia induction, T1: immediately after endotracheal intubation, T2: 1 h after one-lung ventilation; T3: 10 min before the end of operation. MAP: mean arterial pressure; HR: heart rate.
Table 3.
Comparison of postoperative recovery indicators
| Group | Postoperative awake time (min, x̅ ± sd) | Spontaneous breathing recovery time (min, x̅ ± sd) | Restlessness in waking period (n, %) |
|---|---|---|---|
| Observation group (n=58) | 13.52±2.40 | 15.61±2.63 | 2 (3.45) |
| Control group (n=58) | 24.55±2.74 | 26.50±2.86 | 10 (17.25) |
| Statistical value | t=23.191 | t=21.732 | χ2=5.948 |
| P | 0.000 | 0.000 | 0.015 |
Figure 1.
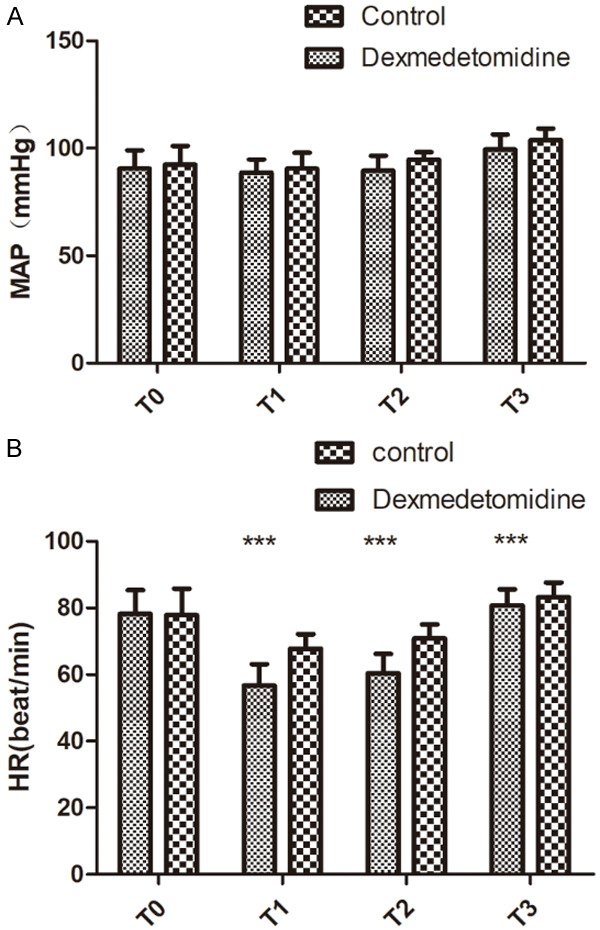
Comparison of mean arterial pressure and heart rate at different time points. A: Comparison of MAP at different time points; B: Comparison of HR at different time points. Compared with control group, ***P<0.001. T0: before anesthesia induction, T1: immediately after endotracheal intubation, T2: 1 h after one-lung ventilation; T3: 10 min before the end of operation. MAP: mean arterial pressure; HR: heart rate.
Comparison of inflammatory factors and oxidative stress indicators
TNF-α, IL-6, IL-8, and MDA levels in both groups were significantly increased at T1 and T2, and were significantly decreased at T3 (all P<0.05). Compared with the control group, TNF-α, IL-6, IL-8 and MDA levels at T1-T3 in observation group were significantly lower (P<0.05). SOD level in both groups showed a significant downward trend, while it showed a significant upward trend at T3. Compared with control group, the SOD levels at T1-T3 in observation group were significantly higher (P<0.05). See Table 4 and Figure 2.
Table 4.
Changes in TNF-α, IL-6, IL-8, SOD, and MDA levels at different time points (n=58, x̅ ± sd)
| Indicator | Group | T0 | T1 | T2 | T3 | F | P |
|---|---|---|---|---|---|---|---|
| TNF-α (pg/mL) | Observation group | 34.20±7.48 | 44.20±8.46 | 55.14±12.60 | 37.50±9.10 | 4.164 | 0.047 |
| Control group | 35.00±7.52 | 46.30±8.70 | 72.10±14.86 | 49.00±7.54 | 4.838 | 0.040 | |
| t | 0.391 | 0.635 | 5.896 | 4.318 | |||
| P | 0.530 | 0.436 | 0.034 | 0.044 | |||
| IL-6 (pg/mL) | Observation group | 13.28±4.10 | 19.50±6.44 | 28.46±9.30 | 17.20±6.18 | 5.682 | 0.036 |
| Control group | 13.35±4.06 | 20.10±6.58 | 39.20±7.48 | 25.11±5.70 | 6.175 | 0.024 | |
| t | 0.281 | 1.112 | 5.835 | 5.984 | |||
| P | 0.712 | 0.134 | 0.033 | 0.030 | |||
| IL-8 (pg/mL) | Observation group | 9.41±1.42 | 12.80±2.91 | 16.71±2.81 | 13.60±4.31 | 4.211 | 0.042 |
| Control group | 8.92±1.23 | 13.32±1.71 | 20.93±3.40 | 16.54±3.42 | 6.456 | 0.033 | |
| t | 1.001 | 0.728 | 4.825 | 4.118 | |||
| P | 0.148 | 0.479 | 0.041 | 0.048 | |||
| SOD (U/mL) | Observation group | 439.12±27.24 | 402.43±18.23 | 346.08±17.41 | 389.35±15.32 | 5.425 | 0.037 |
| Control group | 441.23±25.06 | 389.45±16.61 | 326.25±14.35 | 361.71±16.28 | 6.634 | 0.021 | |
| t | 0.414 | 4.112 | 6.927 | 9.732 | |||
| P | 0.668 | 0.000 | 0.000 | 0.000 | |||
| MDA (mmol/L) | Observation group | 4.85±0.16 | 5.77±0.34 | 6.59±0.32 | 5.21±0.17 | 4.009 | 0.046 |
| Control group | 4.89±0.20 | 6.15±0.25 | 7.25±0.26 | 6.18±0.32 | 6.082 | 0.035 | |
| t | 1.189 | 6.857 | 12.191 | 20.387 | |||
| P | 0.237 | 0.000 | 0.000 | 0.000 |
Note: T0: before anesthesia induction, T1: immediately after endotracheal intubation, T2: 1 h after one-lung ventilation; T3: 10 min before the end of operation. TNF-α: tumor necrosis factor-α; IL-6: interleukin-6; IL-8: interleukin-8; SOD: superoxide dismutase; MDA: malondialdehyde.
Figure 2.
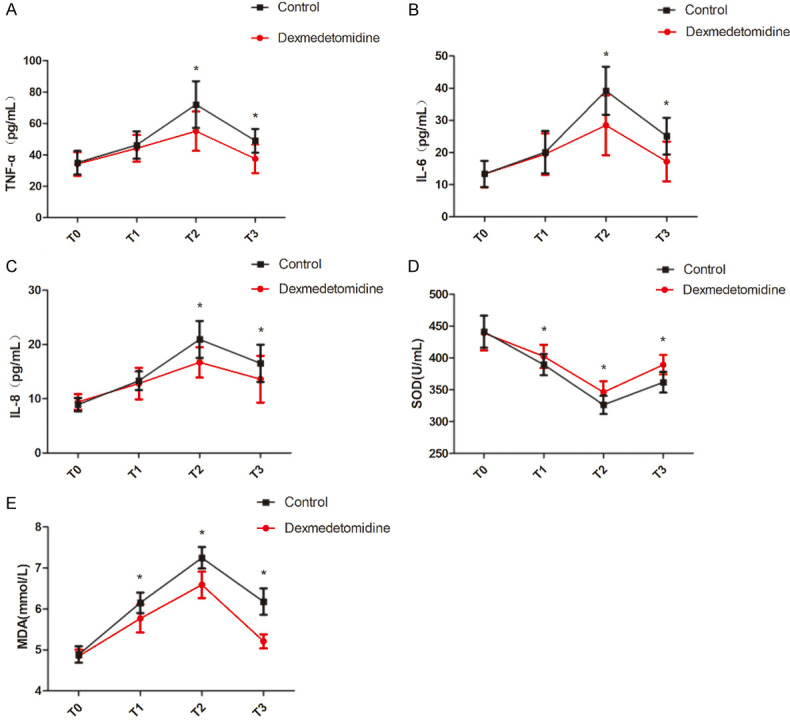
Comparison of TNF-α, IL-6, IL-8, SOD, and MDA levels at different time points. A: Comparison of TNF-α at different time points; B: Comparison of IL-6 at different time points; C: Comparison of IL-8 at different time points; D: Comparison of SOD at different time points; E: Comparison of MDA at different time points. Compared with control group, *P<0.05. T0: before anesthesia induction, T1: immediately after endotracheal intubation, T2: 1 h after one-lung ventilation; T3: 10 min before the end of operation. TNF-α: tumor necrosis factor-α; IL-6: interleukin-6; IL-8: interleukin-8; SOD: superoxide dismutase; MDA: malondialdehyde.
Comparison of IQA and AI in lung tissues at T3
The pathologic results of patients in observation group showed that a large number of alveoli were intact, only a few red blood cells could be seen in a few alveoli, and the morphology of red blood cells was intact (Figure 3A). In the control group, partial alveolar wall thickening, disordered alveolar structure, exudation of interstitial fluid, and a small amount of profiloid red blood cells and inflammatory cells, filling several alveolar cavities were observed (Figure 3B). There were significant differences in IQA and AI at T3 between the two groups (both P<0.05). See Table 5 and Figure 3.
Figure 3.
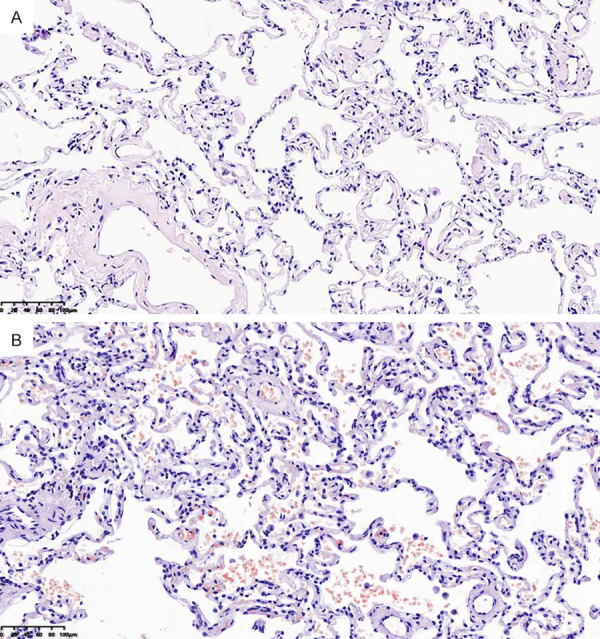
Histology of lung cancer patients (200×). A: Patient in observation group; B: Patient in control group.
Table 5.
Comparison of IQA and AI (n=58, %, x̅ ± sd)
| Group | IQA (%, x̅ ± sd) | AI (%, x̅ ± sd) |
|---|---|---|
| Observation group | 12.25±3.47 | 13.26±1.77 |
| Control group | 29.65±5.03 | 30.55±4.86 |
| t | 21.691 | 25.463 |
| P | 0.000 | 0.000 |
Note: IQA: alveolar damage index of quantitative assessment; AI: apoptotic index.
Changes of OI, RI and P(A-a)O2 levels at different time points
OI, RI and P(A-a)O2 levels in the two groups fluctuated to different degrees at each time point (P<0.05). Compared with the control group, the RI values at T2 and T3 in observation group were lower, the OI values were higher (P<0.05), and the P(A-a)O2 at all time points at T1-T3 in observation group were lower (P<0.05). See Table 6 and Figure 4.
Table 6.
Changes of oxygenation index, respiratory index, and P(A-a)O2 levels at different time points (n=58, x̅ ± sd)
| Indicator | Group | T0 | T1 | T2 | T3 | F | P |
|---|---|---|---|---|---|---|---|
| OI (mmHg) | Observation group | 384.51±20.00 | 354.64±18.50 | 342.18±21.10 | 318.14±22.54 | 4.052 | 0.048 |
| Control group | 386.30±21.65 | 349.23±19.10 | 296.50±13.40 | 265.62±18.20 | 4.673 | 0.041 | |
| t | 0.216 | 0.512 | 13.918 | 13.806 | |||
| P | 0.703 | 0.508 | 0.000 | 0.000 | |||
| RI | Observation group | 0.12±0.03 | 0.58±0.10 | 1.07±0.18 | 0.33±0.06 | 4.185 | 0.046 |
| Control group | 0.11±0.04 | 0.61±0.11 | 1.76±0.30 | 0.74±0.14 | 5.184 | 0.038 | |
| t | 0.169 | 1.113 | 5.947 | 4.618 | |||
| P | 0.774 | 0.162 | 0.031 | 0.041 | |||
| P(A-a)O2 (mmHg) | Observation group | 28.54±5.65 | 251.72±75.90 | 261.31±85.27 | 41.31±9.18 | 361.64 | 0.000 |
| Control group | 27.91±6.23 | 284.34±74.31 | 296.83±63.14 | 48.17±8.30 | 374.22 | 0.000 | |
| t | 0.517 | 2.189 | 2.450 | 2.697 | |||
| P | 0.606 | 0.031 | 0.015 | 0.008 |
Note: T0: before anesthesia induction, T1: immediately after endotracheal intubation, T2: 1 h after one-lung ventilation; T3: 10 min before the end of operation. OI: oxygenation index; RI: respiratory index; P(A-a)O2: alveolar-arterial oxygen pressure difference.
Figure 4.
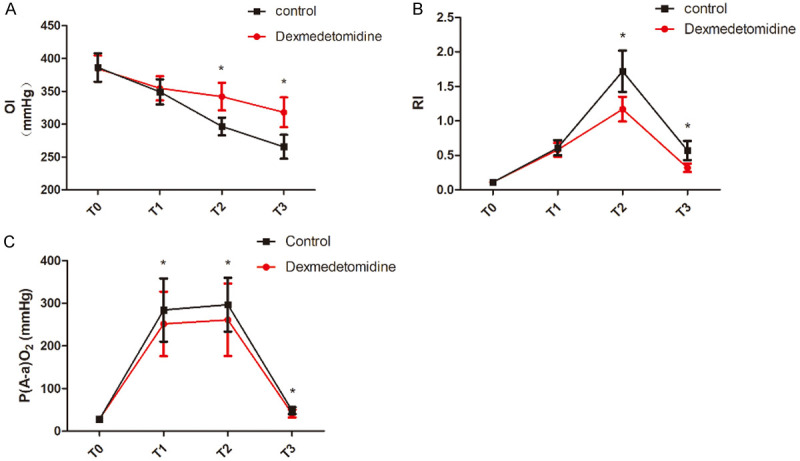
Comparison of oxygenation index, respiratory index, and P(A-a)O2 levels at different time points. A: Comparison of OI at different time points; B: Comparison of RI at different time points; C: Comparison of P(A-a)O2 at different time points. Compared with control group, *P<0.05. T0: before anesthesia induction, T1: immediately after endotracheal intubation, T2: 1 h after one-lung ventilation; T3: 10 min before the end of operation. OI: oxygenation index; RI: respiratory index; P(A-a)O2: alveolar-arterial oxygen pressure difference.
Comparison of pulmonary complications
The incidence of postoperative pulmonary complications in the observation group was 3.4%, and that in the control group was 25.8%. Observation group was significantly lower compared with the control group (P<0.05, Table 7).
Table 7.
Comparison of pulmonary complications (n=58, n, %)
| Group | Pulmonary edema | Atelectasis | Pulmonary infection | Hypoxemia | Total |
|---|---|---|---|---|---|
| Observation group | 0 | 0 | 1 (1.72) | 1 (1.72) | 2 (3.45) |
| Control group | 5 (8.62) | 2 (3.45) | 4 (6.90) | 4 (6.90) | 15 (25.86) |
| χ2 | 0.836 | 0.836 | 11.648 | ||
| P | 0.057 | 0.495 | 0.360 | 0.360 | 0.000 |
Discussion
One-lung ventilation (OLV) can provide VATS operators with a very clear surgical field of view, which is convenient for surgical operation, helps shorten the operation time, and prevents cross infection between the two lungs. However, since OLV is a non-physiologic ventilation, various pathophysiologic changes may occur during this process, and various pro-inflammatory mediators may be released, thus forming a cascade effect, leading to the release of a large number of inflammatory factors, even a systemic inflammatory response syndrome, and lung injury [7]. Dexmedetomidine hydrochloride is a new type of high-potency α2 adrenergic receptor agonist. It is mediated by α2 receptor subtypes and terminates the conduction of pain signals when acts on the presynaptic membrane; it produces the analgesia, sedation, and anti-anxiety effects when acts on the spinal cord; and it causes blood pressure drop and HR slowdown when it acts on the postsynaptic membrane. The protective mechanisms of dexmedetomidine on the lungs may be diverse and continuous, including inhibition of oxidative stress, anti-sympathetic nerve, inhibition of PMN activation and aggregation, and anti-inflammatory response. Over the years, most studies have focused on protective lung ventilation strategies, which have made a great contribution to the advancement of clinical OLV technology. However, there is a lack of research on protective drugs for lung injury caused by OLV. The protective effect of dexmedetomidine on lung injury will promote research on dexmedetomidine, so as to improve its clinical application value. This provides a favorable choice for the protection of patients’ lungs in clinical practice.
This study found that patients in the observation group had more stable hemodynamic indicators, faster postoperative awakening, and spontaneous breathing recovery time, and lower incidence of restlessness, suggesting that dexmedetomidine can reduce sympathetic nerve activity by activating α2 receptors, suppress the stress response, stabilize hemodynamics, and reduce the incidence of delirium and restlessness during the recovery period of anesthesia [8]. Compared with the control group, TNF-α, IL-6, and IL-8 levels at T1-T3 in the observation group were significantly lower, consistent with the results of a previous study [9]. The possible mechanism is that dexmedetomidine can not only inhibit the proliferation and differentiation of immune response related cells, cellular immunity, and the expression of pro-inflammatory factors, but also promote the expression of anti-inflammatory factors, regulate the balance of pro-inflammatory and anti-inflammatory factors, reduce the inflammatory response, and reduce the incidence of restlessness during the waking period [10-12]. SOD is an important antioxidant in the human body, and MDA is an important indicator reflecting the level and activity of oxygen free radicals in the body, both of which play an important role in oxidative stress [13]. This study found that compared with a control group at T1-T3, the MDA level in observation group was significantly lower, and SOD level was significantly higher. This may be related to the fact that dexmedetomidine can stimulate the α2 receptor, inhibit the release of norepinephrine in the lung tissues of patients, reduce the level of plasma catecholamine, reduce the ischemic and hypoxic part of the lung tissues after OLV, and weaken the oxidative stress response [14].
Cui et al. found that there was a positive correlation between the degree of apoptosis in lung tissue and the degree of lung injury [15]. The index of quantitative assessment (IQA) is a key indicator reflecting the degree of lung histopathology and ultrastructure damage [16]. This study showed that the IQA at T3 in observation group was significantly lower than that in control group, suggesting that the ultrastructural damage of lung tissue in the observation group was lower than that in control group, consistent with a previous study [17]. The mechanism is that dexmedetomidine may inhibit the activation of the protein kinase R-like endoplasmic reticulum kinase pathway, inhibit the expression of activating transcription factor 4, inhibit its binding to the promoter amino acid reaction element site, and inhibit the activation of CHOP, thereby inhibiting cell apoptosis and alleviating lung injury [18].
OI (PaO2/FiO2) can reflect pulmonary vascular and alveolar injury, and the lower the OI, the more severe the lung injury [19]. During OLV, under the interaction of inflammatory factors and chemokines, neutrophils in the blood adhere, activate, and migrate into the alveoli and release a large amount of oxygen free radicals and proteolytic enzymes. This destroys the alveolar-capillary barrier function, resulting in the enhancement of alveolar membrane permeability, edema in alveoliand interstitium, which results in a decreased PaO2 and OI. This study showed that at T1-T3, OI values of both groups were significantly decreased compared with before operation, indicating that OLV can cause lung injury. Compared with the control group, the OI values at T2 and T3 in observation group were significantly higher, suggesting that dexmedetomidine can reduce the inflammatory response of lung tissue during OLV, improve oxygenation, and protect the lungs.
Respiratory index (RI) is an indicator that reflects the lung diffusion function, and can more accurately reflect the degree of lung injury. The higher the RI, the worse the lung diffusion function, and the more serious the lung injury [20]. This study showed that the RI values of both groups at T2 were significantly higher than those at T0 and T1, and both groups were maintained at a high level during the operation. Compared with control group, the RI values at T2 and T3 in observation group were lower, and the difference was statistically significant.
This study showed that the incidence of complications such as pulmonary edema, atelectasis, lung infection, and hypoxemia was 3.4% in the observation group and 25.8% in control group, and observation group was significantly lower compared to control group (P<0.05). The main reason was that dexmedetomidine could reduce the content of inflammatory factors in serum of patients with OLV and improve oxygenation and respiratory mechanics [21]. Some scholars also believe that dexmedetomidine can reduce the inhibitory effect of inhaled anesthetics on human papillomavirus by dilating pulmonary vascular effect, which is consistent with the previous studies [22,23].
There were some limitations in this study. First of all, our study was limited to preoperative, intraoperative and postoperative time points, and the dynamic observation of inflammatory factors may be missed at certain time points. Secondly, we observed the effect of dexmedetomidine only on inflammatory factors. Although the change of inflammatory factors was statistically significant, it is not clear whether it has clinical significance. In the future, we need to further observe whether dexmedetomidine can reduce the occurrence of early postoperative cardiovascular events by reducing the level of inflammatory factors in a large sample population. Finally, this study observed only the effect of 1 μg/kg loading dose of dexmedetomidine on inflammatory factors, and 0.3 μg/(kg·h) maintenance dose was used. Whether changing the loading dose and maintenance dose will change the anti-inflammatory effect remains to be discussed.
In conclusion, dexmedetomidine can reduce the perioperative inflammatory response of lung tissue in elderly patients undergoing radical resection of lung cancer, reduce oxidative stress response, improve pulmonary oxygenation function, and produce lung protection.
Acknowledgements
This work was supported by the Suzhou Applied and Basic Research Program for Medical Science and Technology relating to the People’s Livelihood in Health Care (SYS201772).
Disclosure of conflict of interest
None.
References
- 1.Zhu YL, Jiang YO, Xiao HB, Liu MJ, Du HJ, Sun JM. Effects of dexmedetomidine on arterial blood gas and inflammatory factors in patients with one lung ventilation. J Clin Anesthesiol. 2017;33:1070–1073. [Google Scholar]
- 2.Yang Z, Xin R, Lv R. Effects of dexmedetomidine pretreatment on lung injury for patients after liver surgery. J Reg Anat and Oper Surg. 2015;24:553–556. [Google Scholar]
- 3.Obara S. Dexmedetomidine as an adjuvant during general anesthesia. J Anesth. 2018;32:313–315. doi: 10.1007/s00540-018-2509-5. [DOI] [PubMed] [Google Scholar]
- 4.Geng MQ, Chen X, Lu L, Li ZG, He QY. Effects of different anesthesia methods on immune function, oxidative stress and related cytokine levels in elderly patients undergoing radical resection of lung cancer. J Hainan Med Univ. 2018;24:2001–2006. [Google Scholar]
- 5.Zhang H, Fang B, Zhou W. The efficacy of dexmedetomidine-remifentanil versus dexmedetomidine-propofol in children undergoing flexible bronchoscopy: a retrospective trial. Medicine (Baltimore) 2017;96:e5815. doi: 10.1097/MD.0000000000005815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma HJ, Huang SX, Liu B, Wu WJ, Zeng N. Study on serum acute phase reaction protein in patients infected by radical resection of lung cancer under thoracoscopic guidance. Chin J Nosocomiol. 2019;29:1677–1681. [Google Scholar]
- 7.Thongrong C, Sirikannarat P, Kasemsiri P, Duangthongphon P. Comparison of dexmedetomidine and fentanyl to prevent haemodynamic response to skull pin application in neurosurgery: double blind randomized controlled trial. Anaesthesiol Intensive Ther. 2017;49:268–273. doi: 10.5603/AIT.a2017.0051. [DOI] [PubMed] [Google Scholar]
- 8.Pasin L, Landoni G, Nardelli P, Belletti A, Di Prima AL, Taddeo D, Isella F, Zangrillo A. Dexmedetomidine reduces the risk of delirium, agitation and confusion in critically Ill patients: a meta-analysis of randomized controlled trials. J Cardiothorac Vasc Anesth. 2014;28:1459–1466. doi: 10.1053/j.jvca.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Wu XD, Wang PE, Zhang JB, Jin XH, Xu GB, Zhu JQ. Effect of dexmedetomidine sedation on inflammatory factors in patients with mechanical ventilation sepsis. Chin J Gen Pract. 2018;16:675–677. [Google Scholar]
- 10.Li YQ, Wang ZG, Gong BJ, Li D, Li S. Effects of different doses of dexmedetomidine on lung injury in pediatric patients undergoing open heart surgery under cardiopulmonary bypass. Chin J Anesthesiol. 2014;34:529–532. [Google Scholar]
- 11.Şentürk M, Slinger P, Cohen E. Intraoperative mechanical ventilation strategies for one-lung ventilation. Best Pract Res Clin Anaesthesiol. 2015;29:357–369. doi: 10.1016/j.bpa.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Xiang H, Hu B, Li Z, Li J. Dexmedetomidine controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Inflammation. 2014;37:1763–1770. doi: 10.1007/s10753-014-9906-1. [DOI] [PubMed] [Google Scholar]
- 13.Xu XH. Carvacrol inhibits acute lung injury in rats by inhibiting oxidative stress reaction. J Clin Exp Med. 2017;16:320–322. [Google Scholar]
- 14.Xiang BQ, Luo ZY, Gao H, Dai YY, Wang WT. Dexmedetomidine-induced cardioprotection in a mouse model of lung ischemia-reperfusion: the relationship with endoplasmic reticulum stress. Chin J Anesthesiol. 2017;37:61–65. [Google Scholar]
- 15.Cui J, Li CS, He XH, Song YG. Protective effects of penehyclidine hydrochloride on acute lung injury caused by severe dichlorvos poisoning in swine. Chin Med J (Engl) 2013;126:4764–4770. [PubMed] [Google Scholar]
- 16.Xiang BQ, Gao H, Chen JH, Lou GQ, Zhou ZL, Wu YL, Zhang JJ, Xu YX, Wang WT. Regulation of retinoid X receptor-mediated-autophagy pathway in rat pulmonary ischemia/reperfusion injury. Chin J Pathophysiol. 2018;34:2054–2061. [Google Scholar]
- 17.Lu TH, Su CC, Tang FC, Chen CH, Yen CC, Fang KM, Lee KI, Huang DZ, Chen YW. Chloroacetic acid triggers apoptosis in neuronal cells via a reactive oxygen species-induced endoplasmic reticulum stress signaling pathway. Chem Biol Interact. 2015;225:1–12. doi: 10.1016/j.cbi.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 18.Liu CL, Li X, Li RJ, He YY, He KL, Wang LL. PERK signal pathway is involved in hypoxia-induced endoplasmic reticulum stress and apoptosis in cultured cardiac myocytes. Chin J Pathophys. 2012;28:1392–1398. [Google Scholar]
- 19.Xiao RH, Zhang Z, Mi ZH, Shu GS. The role of PI3K/A kt signaling pathway in the protection of dexmedetomidine against limb ischemia/reperfusion (I/R) -induced acute lung injury in rats. J North Pharm. 2017;14:140–141. 137. [Google Scholar]
- 20.Lee SH, Kim N, Lee CY, Ban MG, Oh YJ. Effects of dexmedetomidine on oxygenation and lung mechanics in patients with moderate chronic obstructive pulmonary disease undergoing lung cancer surgery: a randomised double-blinded trial. Eur J Anaesthesiol. 2016;33:275–282. doi: 10.1097/EJA.0000000000000405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu YT, Liu YG, Wei L. Effect of dexmedetomidine in patients with radical resection of one-lung ventilation on serum inflammatory response of esophageal cancer. J Clin Anesthesiol. 2016;32:1053–1056. [Google Scholar]
- 22.Luo JJ, Liang X, Pi LH, Huang Y, Xie BY, Yang FR. Effects of dexmedetomidine combined with sevoflurane inhalation on lung injury in patients with one-lung ventilation. Chongqing Med. 2019;48:1844–1847. [Google Scholar]
- 23.Yao D, Qian YH, Wan YH, Luo WB, Wu GD. Lung protective effect and mechanism of combined application of dexmedetomidine and remote ischemic preconditioning on thoracoscopic lung surgery. J Hebei Med Univ. 2017;38:897–900. [Google Scholar]


