Abstract
Background: EPDR1 is widely expressed in cancer, especially colorectal cancer. However, the biologic function of EPDR1 in breast cancer is uncertain. Methods: The expression profile of EPDR1 was assessed by Gene Expression Profiling Interactive Analysis (GEPIA; gepia.cancer-pku.cn). We constructed EPDR1-overexpressing (EPDR1-Ov) plasmids that were transfected into breast cancer cells (MCF-7 and MDA-MB-453) to examine the EPDR1 effect on their malignant behavior. The EPDR1 overexpression and the critical components of the P53 signaling pathway were determined by western blot or RT-PCR. Cell proliferation, colony formation, invasive capacity, and cell apoptotic proportions were examined after transfection. Results: mRNA expression of EPDR1 was significantly lessened in breast cancer tissues when compared to the adjacent normal tissues by data analysis from GEPIA. There was an impairment in proliferative ability, viability, invasion, and anti-apoptotic effect in EPDR1 overexpressed breast cancer cells. Mechanistic studies showed that EPDR1 overexpression increased the p53, p21 and Bcl-2 expression while inhibiting Bax expression. Conclusion: EPDR1 inhibited malignant behaviors and promoted apoptosis in breast cancer cells by activation of the p53 signaling pathway.
Keywords: Breast cancer, EPDR1, invasive capacity, proliferation, viability, P53
Introduction
Epithelial tumor transformation of breast tissue leading to breast cancer ranks as one of the four most prevalent cancers globally. In the USA alone, this disease contributed to 279,100 newly diagnosed cases and 42,690 deaths in 2019 [1]. Considerable advancement has been made in screening techniques and targeted therapies, causing improvement of the 5-year relative survival rate [2-4]. However, genetic variants and mutations that are known to be associated with the carcinogenesis and metastasis of breast cancer lead to rapid deterioration of the patient [5,6]. Therefore, to improve its prognosis and decrease morbidity and mortality, it is necessary to understand the underlying etiological factors as well as pathological mechanisms of the cancer.
Ependymin related 1 (EPDR1), also known as EPDR, UCC1, MERP1; MERP-1 is a member of mammalian ependymin-related proteins (MERPs). The protein shares a conserved critical amino acid and primary structure with piscine pendymins, which is a type II transmembrane protein and opposes cell adhesion by intracellular and extracellular mechanisms [7,8]. EPDR1 is differentially expressed in a wide range of tissues [9] including various malignant tissues and cell lines. This suggests EPDR1 may play a vital role in the occurrence or progression of certain cancers [10]. In human colorectal cancer overexpression of EPDR1 can facilitate the viability and invasion of cancer cells and serve as a reliable prognostic marker [5,11,12]. Data mined from the TCGA database revealed that EPDR1 expression may be down-regulated in certain types of cancer such as uterine carcinosarcoma [13]. Due to the varied expression of EPDR1 in different cancers, it is thought to possess a complex function in carcinogenesis and metastasis.
In the present work, the mRNA level of EPDR1 in breast cancer tissue is compared with normal tissues, and the data were analyzed using the GEPIA database. EPDR1 was further overexpressed in breast cancer cells, namely, MCF-7 and MDA-MB-45, aiming to reveal the role of EPDR1 in cell proliferation, viability, invasion, and apoptosis.
Materials and methods
Cell culture, maintenance, and antibodies
Two breast cancer cell lines, MCF-7, and MDA-MB-453 were obtained from the Wuhan University Type Culture Collection (Wuhan, China). Both breast cancer cell lines were maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS). The cells were maintained in a 37ºC cell culture incubator. Anti-EPDR1 and anti-GAPDH antibodies were acquired from Proteintech Group, Inc., Wuhan, Hubei.
EPDR1 overexpression and transfection
For the overexpression of EPDR1, the primer sequence of the human EPDR1 gene was constructed as 5’-TAGGTTGGATGGAGTGTAGTGGTAT and 3’-ACTCCCATTCCCAATAAAAAATCTA. The EPDR1 cDNA was further cloned into a pAdEasy/CMV plasmid vector (named EPDR1-Ov). For every well of 12-well plate 1*104 MCF-7 and MDA-MB-45 cells at 70-80% confluence were transfected with 0.50 µg EPDR1-Ov or its corresponding empty vector (named NC) using Lipofectamine 2000 for 48 h. The overexpression of EPDR1 was finally validated by western blot.
Cell proliferation assay
The cell viability of MCF-7 and MDA-MB-453 cells was elucidated by the Cell Counting Kit-8 (CCK-8) following the manufacturer’s protocol. Briefly, 1×103 cells were incubated in a 96-well plate for 4 h. Further, 100 mL of culture medium containing 10% CCK-8 reagent was added to each well at a specific time interval (0th day, 1st day, 2nd day, and 3rd day) followed by continuous culture for 2 h. The absorbance was measured at 450 nm by a Multiskan.
Colony formation assay
The transfected MCF-7 and MDA-MB-453 cells were cultured, maintaining a density of 1000 cells/well in 10 cm plates for two weeks. Cell colonies over 0.5 mm diameter were taken into consideration and counted. Furthermore, the cells were fixed, stained with 0.5% crystal violet, and photographed.
Cell invasion assay
For quantification of the motility of transfected cells, a transwell insert was added to the Matrigel layer on the 24-well plate. The transfected cells (1×105) were seeded onto the upper compartment, which was placed in the lower compartment containing 2.6 mL of DMEM. The transwell plate was incubated for 2.5 h. The migrated cells were fixed by the addition of 5% glutaraldehyde. Crystal violet was subsequently added for staining the cells which were quantified under the microscope.
Flow cytometric analysis
The overexpressed-EPDR1 and the control of breast cancer cells were maintained in the 6-well plates for two days. The harvested cells were then trypsinized and washed. Acridine orange (AO) and propidium iodide (PI) was used for staining the cells for 15 min. Flow cytometry was employed to monitor the apoptotic cells.
Western blot assay
Cells were harvested, and to them, 30 µL ice-cold radioimmunoprecipitation assay (RIPA) buffer was added for cell lysis. The protein concentration of cell lysate was quantified following Bicinchoninic Acid (BCA) protein assay. Protein lysate of 25 µg was run on 12% SDS-PAGE. The proteins were transferred to blotting membrane by electroblotting technique. Blocking buffer was added onto the blot and incubated to prevent any unspecific binding. Subsequently, the primary antibody was added against EPDR1, P53, or GAPDH followed by treatment with the secondary antibody. The blots were developed following the instructions provided with the kit. GAPDH used as endogenous control.
Gene expression analysis
RNA from the cells was isolated with TRIzol reagent followed by RNase-free DNase digestion. iScript cDNA synthesis kit (Bio-Rad USA) was utilized for cDNA synthesis. TaqMan Gene Expression Assay protocol was followed for the quantification of mRNA expression. Transcript level analysis of EPDR1, p53, p21, Bax, and Bcl2 was carried out by the 2-ΔΔCt method.
Bioinformatic analysis and statistical analysis
The EPDR1 mRNA level in breast cancer was investigated by the GEPIA database (http://gepia.cancer-pku.cn). The unpaired Student’s t-test was adopted to analyze the EPDR1 mRNA difference between 1081 invasive breast carcinoma (BRCA) tissues and 291 normal tissues from TCGA gene expression data. |Log2FC| Cutoff and p-value Cutoff were set as 1 and 0.01, respectively.
The data collected were compared between two groups with the help of a nonparametric test using Prism GraphPad 8. Differences were considered significant when P < 0.05.
Results
Reduced expression of EPDR1 mRNA in breast cancer tissues in TCGA
EPDR1 mRNA expression was investigated in invasive breast cancer (BRCA) compared with normal controls following the instruction of GEPIA website creator. The expression of EPDR1 was downregulated in breast cancer tissues (Figure 1).
Figure 1.

Expression of EPDR1 in breast cancer patients. Down-regulated expression of EPDR1 in breast cancer patients when compared with adjacent normal tissues by an open database, namely, GEPIA database.
Overexpression of EPDR1 inhibits proliferation and promotes apoptosis of breast cancer cells
To further investigate the EPDR1 in breast cancer, EPDR1-overexpressing plasmid was constructed and infected into MCF-7 and MDA-MB-453 cells. RT-PCR analysis and western blotting technique were used to validate the efficacy in the overexpressed cells. The exogenous EPDR1 expression was found to be significantly enhanced when compared with the cells infected with plasmids without constructs (Figure 2A and 2B). The CCK8 and colony formation assays were carried out to assess whether the overexpression of EPDR1 influences the viability of breast cancer cells. As shown in Figure 2C and 2D, the proliferative rate of cells overexpressing EPDR1 cells decreased in comparison to that of the blank control cells. Similarly, the colony formation capacity of EPDR1-overexpressing cells was attenuated when compared to the control group (Figure 2E-H). Apoptosis plot obtained from flow cytometry revealed that EPDR1 promoted apoptosis of breast cancer cells (Figure 3). Thus, ectopic expression of EPDR1 might suppress the proliferation and viability, and significantly promote apoptosis of breast cancer cells.
Figure 2.
Overexpressing EPDR1 inhibited cell proliferation, and colony formation efficiency. (A and B) RT-PCR analysis to estimate the EPDR1 mRNA in both cell types. Western blot results corroborated the efficiency of EPDR1 overexpression in both cell types. (C and D) CCK8 assay indicating the proliferation of overexpressing-EPDR1 cells. (E-H) Demonstration of the colony formation capacity of overexpressing-EPDR1 cells by colony formation assay. ****P < 0.0001 vs. control; ***P < 0.001 vs. control.
Figure 3.
Flow cytometry analysis was done to monitor the apoptotic cells after overexpressing-EPDR1 MCF-7 and MDA-MB-453 cells. A and C. An apoptosis assay revealed the overexpression of EPDR1 resulted in cell apoptosis. In the dot plots, top left quadrant: dead cells; bottom left quadrant: living cells; bottom right quadrant: cells in early apoptosis; and top right quadrant: cells in late apoptosis. B and D. Apoptosis rate was assessed in EPDR1-overexpressing cells and normal controls. ****P < 0.0001 vs. control.
Overexpression of EPDR1 inhibits the migration of breast cancer cells
The transwell migration assay was performed to examine further the effect of EPDR1 on tumorigenicity of breast cancer cells. The results revealed that wild-type MCF-7 and MDA-MB-453 cells had higher migration ability compared with the EPDR1-overexpressing cells (Figure 4). This study suggests EPDR1 may suppress the migration of breast cancer cells.
Figure 4.
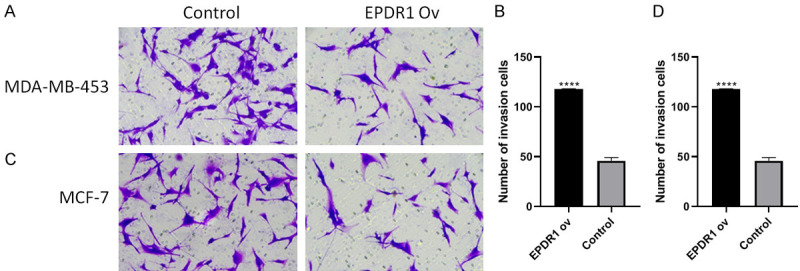
The invasive capacities after overexpressing-EPDR1 in MCF-7 and MDA-MB-453 cells. **P < 0.01 vs. control; ****P < 0.0001 vs. control.
Overexpression of EPDR1 activates P53 signaling pathway
Since P53 signaling pathway is transcriptionally or translationally alternated in cancer progression [14], we examined the P53 expression and its critical downstream effectors (P21, bcl-2, Bax) in EPDR1-Ov MCF-7 and MDA-MB-453 cells. The results showed that P53 was enhanced in EPDR1-ov breast cancer cells compared to the corresponding NC cells. Subsequent RT-PCR analysis also revealed that EPDR1 overexpression dysregulated the P53 mRNA level. In addition, we also identified increased expression level of P21 and Bax as well as reduced expression level of Bcl-2 in breast cancer cells (Figure 5).
Figure 5.

Enforced expression of EPDR1 activated p53 signaling pathway. The p53 and its crucial effectors p21 and Bax showed a considerably upregulated expression while bcl-2 was attenuated in MCF-7 (A) and MDA-MB-453 (B) cells. ***P < 0.0001 vs. its NC.
Discussion
We have reported the first account of the biologic role of EPDR1 in malignant behavior of breast cancer cells and its underlying mechanisms. EPDR1 has a possible anti-tumor role and a pro-apoptotic effect in MCF-7 and MDA-MB-453 cells, at least partially by activating P53 signaling pathway.
The overexpression of EPDR1 was observed first in fish, and later it was found to be widely distributed in human tissues as well as in certain cancer cells [8,12,15]. Recently, a study done by Gimeno-Valiente et al. exhibited that expression of EPDR1 is elevated in human colorectal cancer tissues and it serves as an adverse diagnostic marker [13]. In the present study, downregulated EDPR1 was observed in breast cancer tissues from the GEPIA data. These data differed from the results exhibited in patients diagnosed with colorectal cancer (CRC) [13], which may be supportive of the notion that expression of EPDR1 behaves in a contrasting way in various tissues.
A series of in vitro assays was performed based on the above experimental outcomes to reveal the biological function of EPDR1 in breast cancer cells. By overexpression EPDR1 in MCF-7 and MDA-MB-45 cells, it was observed that EPDR1 inhibited cell viability and invasion, and stimulated cell apoptosis, suggesting that EPDR1 may inhibit tumorigenesis as well as prevent progression of breast cancer. However, recent research indicated that in SiEPDR1 colorectal cancer cells also showed a reduction of malignant behaviors of cancer when compared with the parental cells, indicating a pro-tumorigenic role of EPDR1, which is contradictory to the results from the present study [12,13]. Therefore, from the literature, it can be speculated that EPDR1 may function both ways in a context-dependent manner. This dual regulatory mode is common in tumor progression [16].
p53, a multifunctional anti-tumor factor, mediates a potent tumor-suppressive effect by hyperactivation or crosstalking with various signaling circuits [17-19]. Functional defects of P53 were examined in diverse types of tumors which can disturb normal cell and tissue hemostasis, progressively resulting in tumor transformation [20]. Due to its clinical applicability, multiple lines of study demonstrated that P53 can be a target of therapy for cancer patients [14,21,22]. In our study, we found that ectopic expression of EPDR1 could significantly increase the p53 signaling pathway to modulate the breast cancer cell phenotype. Hence, EPDR1 might exert its anti-tumor activity by inactivation of the P53 signaling pathway. As for the underlying mechanism of EPDR1 in cancer progression, two simultaneous studies, done by Chu et al. and Gregorio-King et al. demonstrated that differential methylation of EPDR1 may be involved in the progression of CRC patients [12,15]. Similar to ependymins, EPDR1 may be associated with cell-cell extracellular matrix interactions that facilitate EMT [23,24]. The detailed molecular mechanistic pathway of EPDR1 and its role in in vivo models should be studied.
In conclusion, EPDR1 is downregulated in breast cancer tissues and functions a tumor suppressor in breast cancer cells by deactivating the p53 signaling pathway. EPDR1/p53 might be a new avenue for breast cancer diagnosis and therapy.
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Ito H, Matsuo K. Molecular epidemiology, and possible real-world applications in breast cancer. Breast Cancer. 2016;23:33–38. doi: 10.1007/s12282-015-0609-8. [DOI] [PubMed] [Google Scholar]
- 3.Zavala VA, Serrano-Gomez SJ, Dutil J, Fejerman L. Genetic epidemiology of breast cancer in Latin America. Genes (Basel) 2019;10:153. doi: 10.3390/genes10020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woolston C. Breast cancer. Nature. 2015;527:S101. doi: 10.1038/527S101a. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez-Ericsson PI, Stovgaard ES, Sua LF, Reisenbichler E, Kos Z, Carter JM, Michiels S, Le Quesne J, Nielsen TO, Laenkholm AV, Fox SB, Adam J, Bartlett JM, Rimm DL, Quinn C, Peeters D, Dieci MV, Vincent-Salomon A, Cree I, Hida AI, Balko JM, Haynes HR, Frahm I, Acosta-Haab G, Balancin M, Bellolio E, Yang W, Kirtani P, Sugie T, Ehinger A, Castaneda CA, Kok M, McArthur H, Siziopikou K, Badve S, Fineberg S, Gown A, Viale G, Schnitt SJ, Pruneri G, Penault-Llorca F, Hewitt S, Thompson EA, Allison KH, Symmans WF, Bellizzi AM, Brogi E, Moore DA, Larsimont D, Dillon DA, Lazar A, Lien H, Goetz MP, Broeckx G, El Bairi K, Harbeck N, Cimino-Mathews A, Sotiriou C, Adams S, Liu SW, Loibl S, Chen IC, Lakhani SR, Juco JW, Denkert C, Blackley EF, Demaria S, Leon-Ferre R, Gluz O, Zardavas D, Emancipator K, Ely S, Loi S, Salgado R, Sanders M International Immuno-Oncology Biomarker Working Group. The path to a better biomarker: application of a risk management framework for the implementation of PD-L1 and TILs as immuno-oncology biomarkers into breast cancer clinical trials and daily practice. J Pathol. 2020;250:667–684. doi: 10.1002/path.5406. [DOI] [PubMed] [Google Scholar]
- 6.Monteiro AN, Bouwman P, Kousholt AN, Eccles DM, Millot GA, Masson JY, Schmidt MK, Sharan SK, Scully R, Wiesmuller L, Couch F, Vreeswijk MPG. Variants of uncertain clinical significance in hereditary breast and ovarian cancer genes: best practices in functional analysis for clinical annotation. J Med Genet. 2020;57:509–518. doi: 10.1136/jmedgenet-2019-106368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apostolopoulos J, Sparrow RL, McLeod JL, Collier FM, Darcy PK, Slater HR, Ngu C, Gregorio-King CC, Kirkland MA. Identification and characterization of a novel family of mammalian ependymin-related proteins (MERPs) in hematopoietic, nonhematopoietic, and malignant tissues. DNA Cell Biol. 2001;20:625–635. doi: 10.1089/104454901753340613. [DOI] [PubMed] [Google Scholar]
- 8.Strausberg RL, Feingold EA, Grouse LH, Derge JG, Klausner RD, Collins FS, Wagner L, Shenmen CM, Schuler GD, Altschul SF, Zeeberg B, Buetow KH, Schaefer CF, Bhat NK, Hopkins RF, Jordan H, Moore T, Max SI, Wang J, Hsieh F, Diatchenko L, Marusina K, Farmer AA, Rubin GM, Hong L, Stapleton M, Soares MB, Bonaldo MF, Casavant TL, Scheetz TE, Brownstein MJ, Usdin TB, Toshiyuki S, Carninci P, Prange C, Raha SS, Loquellano NA, Peters GJ, Abramson RD, Mullahy SJ, Bosak SA, McEwan PJ, McKernan KJ, Malek JA, Gunaratne PH, Richards S, Worley KC, Hale S, Garcia AM, Gay LJ, Hulyk SW, Villalon DK, Muzny DM, Sodergren EJ, Lu X, Gibbs RA, Fahey J, Helton E, Ketteman M, Madan A, Rodrigues S, Sanchez A, Whiting M, Madan A, Young AC, Shevchenko Y, Bouffard GG, Blakesley RW, Touchman JW, Green ED, Dickson MC, Rodriguez AC, Grimwood J, Schmutz J, Myers RM, Butterfield YS, Krzywinski MI, Skalska U, Smailus DE, Schnerch A, Schein JE, Jones SJ, Marra MA Mammalian Gene Collection Program Team. Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences. Proc Natl Acad Sci U S A. 2002;99:16899–16903. doi: 10.1073/pnas.242603899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Della Valle MC, Sleat DE, Sohar I, Wen T, Pintar JE, Jadot M, Lobel P. Demonstration of lysosomal localization for the mammalian ependymin-related protein using classical approaches combined with a novel density shift method. J Biol Chem. 2006;281:35436–35445. doi: 10.1074/jbc.M606208200. [DOI] [PubMed] [Google Scholar]
- 10.Riffo-Campos AL, Castillo J, Vallet-Sanchez A, Ayala G, Cervantes A, Lopez-Rodas G, Franco L. In silico RNA-seq and experimental analyses reveal the differential expression and splicing of EPDR1 and ZNF518B genes in relation to KRAS mutations in colorectal cancer cells. Oncol Rep. 2016;36:3627–3634. doi: 10.3892/or.2016.5210. [DOI] [PubMed] [Google Scholar]
- 11.Nimmrich I, Erdmann S, Melchers U, Chtarbova S, Finke U, Hentsch S, Hoffmann I, Oertel M, Hoffmann W, Muller O. The novel ependymin related gene UCC1 is highly expressed in colorectal tumor cells. Cancer Lett. 2001;165:71–79. doi: 10.1016/s0304-3835(01)00390-1. [DOI] [PubMed] [Google Scholar]
- 12.Chu CH, Chang SC, Wang HH, Yang SH, Lai KC, Lee TC. Prognostic values of EPDR1 hypermethylation and its inhibitory function on tumor invasion in colorectal cancer. Cancers (Basel) 2018;10:393. doi: 10.3390/cancers10100393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gimeno-Valiente F, Riffo-Campos AL, Ayala G, Tarazona N, Gambardella V, Rodriguez FM, Huerta M, Martinez-Ciarpaglini C, Monton-Bueno J, Rosello S, Roda D, Cervantes A, Franco L, Lopez-Rodas G, Castillo J. EPDR1 up-regulation in human colorectal cancer is related to staging and favours cell proliferation and invasiveness. Sci Rep. 2020;10:3723. doi: 10.1038/s41598-020-60476-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duffy MJ, Synnott NC, Crown J. Mutant p53 in breast cancer: potential as a therapeutic target and biomarker. Breast Cancer Res Treat. 2018;170:213–219. doi: 10.1007/s10549-018-4753-7. [DOI] [PubMed] [Google Scholar]
- 15.Gregorio-King CC, McLeod JL, Collier FM, Collier GR, Bolton KA, Van Der Meer GJ, Apostolopoulos J, Kirkland MA. MERP1: a mammalian ependymin-related protein gene differentially expressed in hematopoietic cells. Gene. 2002;286:249–257. doi: 10.1016/s0378-1119(02)00434-1. [DOI] [PubMed] [Google Scholar]
- 16.Falcomata C, Barthel S, Schneider G, Saur D, Veltkamp C. Deciphering the universe of genetic context-dependencies using mouse models of cancer. Curr Opin Genet Dev. 2019;54:97–104. doi: 10.1016/j.gde.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Chao CC. Mechanisms of p53 degradation. Clin Chim Acta. 2015;438:139–147. doi: 10.1016/j.cca.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 18.Parrales A, Iwakuma T. Targeting oncogenic mutant p53 for cancer therapy. Front Oncol. 2015;5:288. doi: 10.3389/fonc.2015.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong B, van den Heuvel AP, Prabhu VV, Zhang S, El-Deiry WS. Targeting tumor suppressor p53 for cancer therapy: strategies, challenges and opportunities. Curr Drug Targets. 2014;15:80–89. doi: 10.2174/1389450114666140106101412. [DOI] [PubMed] [Google Scholar]
- 20.Mello SS, Attardi LD. Deciphering p53 signaling in tumor suppression. Curr Opin Cell Biol. 2018;51:65–72. doi: 10.1016/j.ceb.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bertheau P, Lehmann-Che J, Varna M, Dumay A, Poirot B, Porcher R, Turpin E, Plassa LF, de Roquancourt A, Bourstyn E, de Cremoux P, Janin A, Giacchetti S, Espie M, de The H. p53 in breast cancer subtypes and new insights into response to chemotherapy. Breast. 2013;22(Suppl 2):S27–29. doi: 10.1016/j.breast.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Kaur RP, Vasudeva K, Kumar R, Munshi A. Role of p53 gene in breast cancer: focus on mutation spectrum and therapeutic strategies. Curr Pharm Des. 2018;24:3566–3575. doi: 10.2174/1381612824666180926095709. [DOI] [PubMed] [Google Scholar]
- 23.Dolmans GH, Werker PM, Hennies HC, Furniss D, Festen EA, Franke L, Becker K, van der Vlies P, Wolffenbuttel BH, Tinschert S, Toliat MR, Nothnagel M, Franke A, Klopp N, Wichmann HE, Nürnberg P, Giele H, Ophoff RA, Wijmenga C Dutch Dupuytren Study Group; German Dupuytren Study Group; LifeLines Cohort Study; BSSH-GODD Consortium. Wnt signaling and Dupuytren’s disease. N Engl J Med. 2011;365:307–317. doi: 10.1056/NEJMoa1101029. [DOI] [PubMed] [Google Scholar]
- 24.Staats KA, Wu T, Gan BS, O’Gorman DB, Ophoff RA. Dupuytren’s disease susceptibility gene, EPDR1, is involved in myofibroblast contractility. J Dermatol Sci. 2016;83:131–137. doi: 10.1016/j.jdermsci.2016.04.015. [DOI] [PubMed] [Google Scholar]




