Abstract
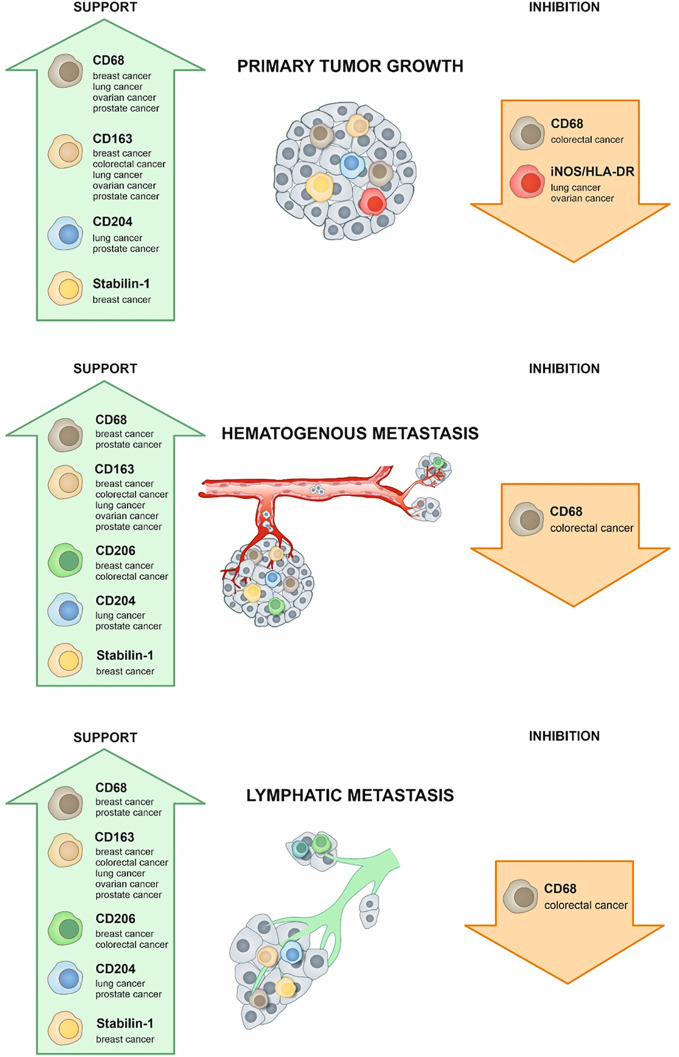
Tumor-associated macrophages (TAMs) are major innate immune cells that constitute up to 50% of the cell mass of human tumors. TAMs are highly heterogeneous cells that originate from resident tissue-specific macrophages and from newly recruited monocytes. TAMs’ variability strongly depends on cancer type, stage, and intratumor heterogeneity. Majority of TAMs are programmed by tumor microenvironment to support primary tumor growth and metastatic spread. However, TAMs can also restrict tumor growth and metastasis. In this review, we summarized the knowledge about the role of TAMs in tumor growth, metastasis and in the response to cancer therapy in patients with five aggressive types of cancer: breast, colorectal, lung, ovarian, and prostate cancers that are frequently metastasize into distant organs resulting in high mortality of the patients. Two major TAM parameters are applied for the evaluation of TAM correlation with the cancer progression: total amount of TAMs and specific phenotype of TAMs identified by functional biomarkers. We summarized the data generated in the wide range of international patient cohorts on the correlation of TAMs with clinical and pathological parameters of tumor progression including lymphatic and hematogenous metastasis, recurrence, survival, therapy efficiency. We described currently available biomarkers for TAMs that can be measured in patients’ samples (tumor tissue and blood). CD68 is the major biomarker for the quantification of total TAM amounts, while transmembrane receptors (stabilin-1, CD163, CD206, CD204, MARCO) and secreted chitinase-like proteins (YKL-39, YKL-40) are used as biomarkers for the functional TAM polarization. We also considered that specific role of TAMs in tumor progression can depend on the localization in the intratumoral compartments. We have made the conclusion for the role of TAMs in primary tumor growth, metastasis, and therapy sensitivity for breast, colorectal, lung, ovarian, and prostate cancers. In contrast to other cancer types, majority of clinical studies indicate that TAMs in colorectal cancer have protective role for the patient and interfere with primary tumor growth and metastasis. The accumulated data are essential for using TAMs as biomarkers and therapeutic targets to develop cancer-specific immunotherapy and to design efficient combinations of traditional therapy and new immunomodulatory approaches.
Keywords: tumor-associated macrophage; monocyte, CD68; lymphatic metastasis; hematogenous metastasis; chemotherapy; immunotherapy; biomarker
Introduction
Tumor-associated macrophages (TAMs) are key innate immune cells in tumor microenvironment (TME) that regulate growth of primary tumors, antitumor adaptive immune response, tumor angiogenesis, extracellular matrix remodeling, intravasation in the vasculature, extravasation in metastatic sites; they establish beneficial conditions for metastatic cells in the secondary organs, and interact with various types of therapies (1, 2). Signaling, epigenetic and metabolic mechanisms cooperate to form functional TAM phenotypes (3).
TAMs represent the major component of the innate immune system in TME and can constitute up to 50% of the tumor mass (4). Two main directions of polarization of TAMs can be defined—classically activated M1 (main markers—HLA-DR, CD80/86) and alternatively activated M2 (main markers—CD206, CD163, CD204, stabilin-1) phenotypes (1, 2, 5) ( Table 1 ). These typical M2 markers are surface receptors that are responsible for the non-inflammatory clearance of microenvironment from apoptotic bodies, ECM components, soluble mediators of activation of cancer cells and angiogenesis (6–12). In addition to scavenging function (10, 11, 13), stabilin-1 acts as an intracellular sorting receptor that targets chitinase-like proteins SI-CLP and YKL-39 to the secretory pathway (14–19). SI-CLP and YKL-39, in turn, regulate monocyte recruitment and angiogenesis (15, 17, 18, 20).
Table 1.
Variety of TAM markers in cancer.
| Macrophage marker | Function | TAM subpopulation | Type of cancer | Method of detection |
|---|---|---|---|---|
| CD68 | Transmembrane glycoprotein | General macrophage marker | Breast, colorectal, lung, ovarian, prostate | IHC, flow cytometry |
| CD80 | Immunoglobulin superfamily | M1 | Colorectal, lung | IHC |
| CD163 | Scavenger receptor for the hemoglobin–haptoglobin complex | M2 | Breast, colorectal, lung | IHC, IF |
| CD204 (MSR1) | Macrophage scavenger receptor | M2 | Breast, colorectal, lung, prostate | IHC |
| CD206 | Mannose receptor and C-type lectin | M2 | Breast, colorectal, ovarian, prostate | IHC, RNA-seq, flow cytometry |
| B7-H4 | Costimulatory protein of antigen-presenting cells | Not specified | Ovarian, lung | IF, flow cytometry |
| CCL8 (MCP2) | Monocyte chemoattractant protein | M2 | Breast | RNA-seq, qPCR |
| COX-2 | Enzyme responsible for formation of prostanoids | M2 | Breast, ovarian | IHC, multiplex IF |
| HLA-DR | MHC class II cell surface receptor | M1 | Lung, ovarian | Multiplexed IHC, IHC |
| IGF1 | Anabolic hormone | M2 | Ovarian | Gene chip analysis |
| iNOS | Enzymes catalyzing the production of NO from L-arginine | M1 | Lung, ovarian | IHC and IF analysis |
| MARCO | Class A scavenger receptor | M2 | Lung | Multiplex IF, RNA-seq |
| MMP-9 | Matrix metalloproteinase | M2 | Breast, lung | IF |
| mTORC2 | Rapamycin-insensitive protein complex | Not specified | Colorectal | IF |
| PD-L1 (CD274) | Immunosuppressive protein | Not specified | Ovarian | IF |
| SIGLEC1 (CD169) | Sialic binding receptor | M2 | Breast | RNA-seq, qPCR |
| SPP1 (Osteopontin) | Protein involved on angiogenesis and metastasis | Not specified | Lung | IHC |
| Stabilin-1 (RS1) | Scavenger receptor | M2 | Breast, colorectal | IHC, IF |
| TIE2 | Angiopoietin receptor | Not specified | Breast | IF |
| TREM-1 | Receptor, regulate inflammatory response | Not specified | Lung | IF, ELISA, Western blot |
| VEGF | Growth factor | Not specified | Colorectal, ovarian | IHC, qPCR |
| VSIG4 | Costimulatory protein of antigen-presenting cells | Not specified | Lung | IF |
| YKL-39 (CHI3L2) | Chitinase-like protein, pro-angiogenic and monocyte chemoattractant | M2 | Breast | IHC, qPCR |
| YKL-40 (CHI3L1) | Chitinase-like protein, pro-angiogenic | M1 | Breast, lung, prostate | IHC, qPCR, ELISA |
| ZEB1 | Transcription factor – driver of epithelial-mesenchymal transition | M2 | Ovarian | IHC |
All details and references are presented throughout the text. IHC, immunohistochemistry; IF, immunofluorescence; qPCR, quantitative polymerase chain reaction; TAM, tumor-associated macrophage.
It is commonly accepted that M1-like macrophages exhibit antitumor activity contributing to the activation of adaptive immune response and inflammation, while M2-like macrophages, in contrast, suppress immune function in tumor microenvironment, induce angiogenesis, and support tumor growth and metastasis (21). However, this nomenclature is based on the in vitro phenomenon and only schematically reflects vectors of the macrophage polarization in vivo, including their polarization in the complex TME. In each cancer type, TAMs can have cancer-specific phenotypes, and can be represented by the heterogeneous populations. Moreover, TAM subtypes can be distinct in various intratumoral compartments, for example in tumor nest and in tumor stroma. Individual TAM phenotypes can be defined by set of markers that not necessarily give clear classification into the M1 and M2 subtypes. The most common histological markers of macrophages belong to the class of transmembrane receptors (mostly of scavenger type); however, new biomarkers that belong to classes of cytokines, growth factors, enzymes, transcription factors, and chitinase-like proteins allow more precise phenotypic and functional characterization of TAMs ( Table 1 ).
TAMs originate from two major sources—tissue-resident macrophages and circulating monocytes recruited in tumor cite by growth factors and chemokines, such as M-CSF, CCL2, and CCL5 (21). Local resident macrophages can recognize cancer cells, and it is believed that they have intrinsic ability to eliminate sporadically transformed cells. Different origin can define TAM diversity in the TME. Transformed cells can escape local innate immune control and give origin to cancer cell clones that efficiently recruit monocytes from blood circulation and reprogram resident TAMs. The number of experimental model systems demonstrated that growing tumor can program resident and recruit macrophages to support tumor progression (22, 23). Both monocyte-derived macrophages and resident macrophages (of adult hematopoietic or embryonic origin) accumulate within an expanding tumor (24, 25). Recent study demonstrated that tissue-resident interstitial macrophages in mouse lungs contribute to the pool of TAMs and support tumor growth in vivo, while monocyte-derived TAMs contribute to tumor progression in the form of metastasis (26). Interestingly, chemotherapeutic treatment resulted in depletion of both resident and monocyte-derived macrophages, but monocyte-derived macrophages were able to recover and provided phagocytosis-mediated tumor clearance (26). However, not all tumors can do it efficiently, and monocytes and macrophages can also retain their ability to recognize tumor as an unwanted-self structure and inhibit its growth and spread (27, 28). In mouse model of ovarian cancer, CD163+ Tim4+ macrophages from omentum, which have embryonic origin and are uniquely independent of bone marrow-derived monocytes, contributed significantly to the metastatic spread (29). Depletion of CD163+ resident macrophages in tumor-bearing mice with lipid nanoparticles reduced tumor growth and progression (29). We can hypothesize that TAM heterogeneity is defined both by their high plasticity and by their origin from independent specific lineages. The contribution of each of these factors in the final tumor-specific TAM heterogeneity is a highly relevant topic for the investigation.
TAM diversity reflects and defines their specific role in different cancers. A number of studies demonstrated that high infiltration of TAMs in human tumors is associated with poor clinical outcome (1, 2). However, the role of TAMs in tumor growth, lymphatic and hematogenous metastasis and treatment outcomes is specific for each type of cancer. By studying patients, the role of TAMs cannot be defined by loss-of-functions and gain-of-function experimentation, and correlation of TAM amounts, their intratumoral localization and functional phenotypes with clinical parameters is a primary source to draw the conclusion. Therefore, precise definition and accurate selection of clinical parameter are essential. Lymphatic and vascular invasions, characterized by cancer cells’ presence within a definite, endothelial-lined space, are parameters that are potential indicators of the ability of cancer cells to metastasize to the lymph nodes and blood vessels, respectively (30, 31) Vascular invasion may reflect the risk of recurrent disease and prognosis (30). There are survival rates that define the probability of the appearance of one or more of tumor progression parameters. For example, progression-free survival (PFS) is calculated as a period of time between the dates of diagnosis and earliest progression (local recurrence or distant metastasis or death) or last follow-up for patients without progression (32). Similarly, disease-free survival (DFS) is a period of time between the dates of treatment of definite cancer and any signs or symptoms of that cancer; overall survival (OS)—the period where patients still alive for a certain period of time after they were diagnosed with or started treatment for a cancer (33).
In this review we summarize the data about TAM correlation with clinical parameters of widely distributed, dangerous and frequently metastasizing types of cancer: breast, colorectal, lung, ovarian, and prostate. We analyzed the role of TAMs in primary tumor growth and metastasis, and the role of TAMs in the tumor response to therapy with particular focus on tumor relapse and metastatic outbreak. We focus not only on the total amount of TAMs in tumor mass, but we made an accent on the functional TAM biomarkers that can be also distinct in different tumor types.
TAMs and Breast Cancer
Breast cancer (BC) is the leading cause of cancer-related female deaths in the world. More than 2 million female breast cancer cases have been diagnosed in 2018 worldwide that led to 630,000 deaths (34). Breast cancer is the most studied malignant disease; many diagnostic and therapeutic approaches have been developed for BC patients, and there are a number of ongoing clinical trials. Due to improved treatment and earlier detection, the mortality rate has decreased in most Western countries in recent years, especially in younger age groups (35). The diagnosis of breast cancer is based on the staging system, which, apart from purely anatomical information (tumor, node, metastasis), includes also prognostic information related to tumor biology such as tumor grade, estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), and gene expression data if available (36). Metastatic BC remains virtually an incurable disease with a median overall survival (OS) of around 3 years and 5-year survival of only 25% (37). The most common first metastatic site is the bone, followed by lung, brain, and liver (38, 39). Breast cancer metastasizes also through the lymphatic system to the regional lymph nodes defined as locoregional metastasis (40).
Breast cancer comprises five molecular subtypes that have distinct prognosis and treatment strategies. These five subtypes include: luminal A (ER+, PR+, Ki67 < 20%), luminal B (ER+, PR+ or PR-, Her2+ or Her2-, Ki67 > 20%), triple-negative (ER-, PR-, HER2-), and HER2-enriched breast cancer (ER+, PR+), HER2+) (41). The absence of receptors on the surface of tumor cells of breast cancer is one of the signs of aggressive status and poor prognosis (42). The most aggressive subtypes include HER2 neu-positive and triple-negative breast cancer (TNBC) (42).
BC is characterized by intratumor heterogeneity which is important for disease prognosis and therapy efficacy (43, 44). This is one of the essential difference between human tumors and mouse models, where tumor is mostly homogenous and does not reflect intratumor structures in patients. There are various approaches to describe intratumor morphological and functional heterogeneity. One of these approaches is based on the distinguishing between tumor nest (TN) and tumor stroma (TS) (45). Macrophage infiltration in TN is defined as the tumor-infiltrating macrophages within epithelial cancer cells; TAMs in TS were located in fibrous tissue surrounding the tumor nest (45).
Another approach identifies five intratumor morphological structures based on morphology of cancer cells: tubular, alveolar, solid, and trabecular structures, and discrete groups of tumor cells (44, 46). The level of morphological heterogeneity is distinct in five different molecular subtypes of breast cancer. Tumors with the presence of all five morphological structures were most frequently identified in luminal subtype in comparison with TNBC (47). TNBC was characterized by minimal out of all BC tumor intratumor heterogeneity and frequent presence of only one morphological structure (47). It was demonstrated that breast tumors with alveolar and trabecular structures often demonstrate increased risk of lymph node and distant metastasis, poor response to neoadjuvant chemotherapy (NAC), and decreased metastasis-free survival (48, 49). The distribution of macrophages varied within these morphological structures. CD68 expression was found in TME only of alveolar and trabecular structures and was absent in solid, tubular, and discrete groups (50). Gene expression of SI-CLP, CD206, and Stabilin-1 was also differentially distributed within distinct morphological structures (50).
One more classification of heterogeneity in BC is based on the level of the stromal–parenchymal interactions (51–53). In human breast cancer five distinct morphological compartments characterized by the interaction of tumor cells and immune component can be defined: areas with soft fibrous stroma; areas with coarse fibrous stroma; areas of maximum stromal and-parenchymal relationship; parenchymal elements, and gaps of ductal tumor structures (52, 53). Accordingly, TAM infiltrate localized in specific intratumor compartment or in certain molecular subtype of BC has a different clinical value in patient prognosis. The correlations of TAMs in distinct compartments with parameters of breast cancer progression are discussed below.
TAMs in Breast Tumors and Metastasis
Two main parameters are used to analyze the clinical significance of TAMs in human cancers—the amount of TAMs defined most frequently by CD68 expression and phenotype of macrophages, defined by different specific M1 and M2 markers ( Figure 1 , Table 1 ).
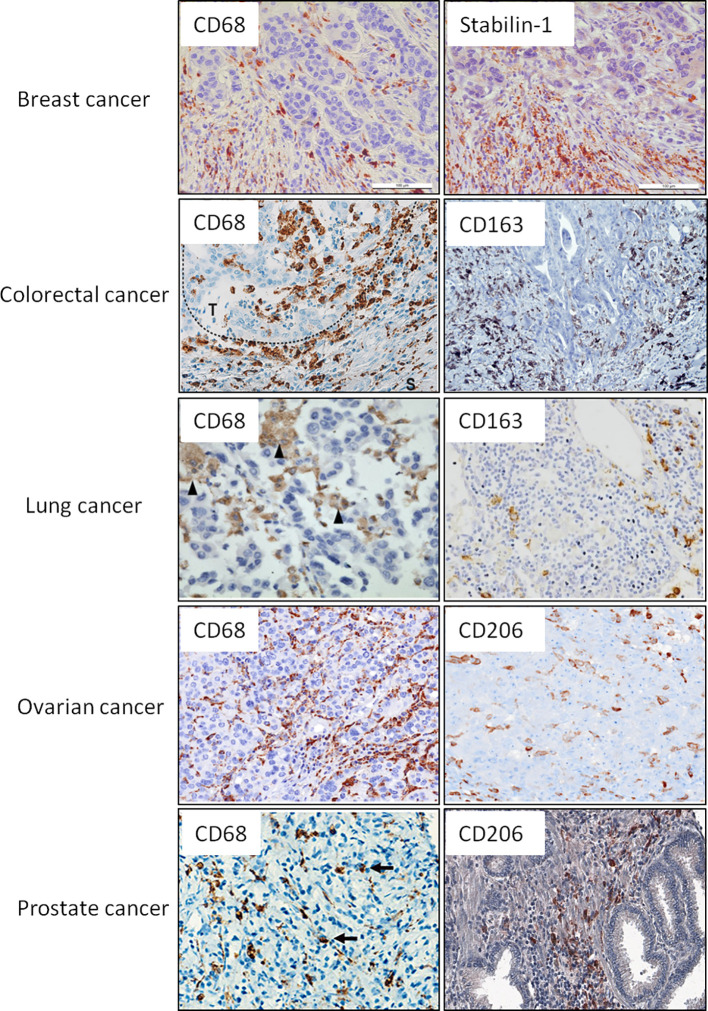
Figure 1.
Representative IHC images for the intratumoral macrophages that express CD68 as general macrophage marker and selected M2 markers. Examples of CD68 and M2 markers (CD163, CD206, stabilin-1) are presented for breast, colorectal, lung, ovarian, and prostate cancers. These examples are reproduced from the following publications: for breast cancer (9); colorectal cancer (54, 55); lung cancer (56, 57); ovarian cancer (58); prostate cancer (59). Image for CD206 expression in prostate cancer was kindly provided by Dr. K. Danilko, Bashkir State Medical University. For all published images copyright licenses have been obtained from the publisher.
Breast cancer was the first cancer type in which the tumor-supporting role of TAMs was demonstrated in various animal models (60). One of the first studies demonstrating the negative role of TAM infiltration in the pathogenesis of BC was the immunohistochemical analysis (IHC) of 101 invasive breast carcinoma samples (England, 1996) (61). In this study in a univariate Cox proportional hazard model, increased CD68+ macrophage count was a significant indicator of reduced relapse free survival (RFS) and reduced overall survival (OS) (61). Extensive experimental and clinical data, performed in European, American and Asian cohorts of patients, confirmed the importance of TAM infiltration in the pathogenesis of breast cancer and will be discussed below.
Most of the studies of the amount and phenotype of TAMs in human tumor tissues were performed by using IHC analysis. A number of studies showed that the increase in TAM number, defined by the expression of pan-macrophage marker CD68 correlated with a greater degree of severity of the tumor process ( Table 2 ). Thus, the results of meta-analysis of 16 studies (Chinese, Finnish, Swedish, Korean, UK, and USA cohorts) with a total 4,541 BC patients indicated that breast cancer with high TAM infiltration was significantly correlated with characteristics of aggressive biological behavior, such as tumor size, histological grade, ER and PR status, basal phenotype, vascular invasion (68). This meta-analysis showed that high TAM infiltration was not found to be associated with lymph node status (N0 vs. N1-3) and HER-2 status (68). Several clinical studies performed on Chinese cohorts of patients with breast cancer demonstrated the association of increased stromal infiltration of CD68+ macrophages with larger tumor size, higher histological grade, hormone receptor negativity in BC patients (45, 65). High numbers of CD11c+ macrophages in tumor stroma were associated with a larger tumor size in 367 primary BC patients from the Korean cohort (66) (66). Recent study of 60 primary BC specimens obtained from the Egyptian cohort of patients showed that high CD68+ stromal TAMs significantly correlated with nodal metastasis and vascular invasion (62). In a retrospective study of 1,579 breast cancer specimens (Chinese cohort), high density of both CD68+ TAMs significantly correlated with lymph node metastasis (65).
Table 2.
Representative studies demonstrating the association of TAMs with tumor progression parameters in breast cancer.
| Cohort of patients | Method of detection | TAM correlation with tumor growth and stage | TAM correlation with lymphatic and hematogenous metastasis | TAM correlation with survival | Reference |
|---|---|---|---|---|---|
| 101 patients with invasive breast carcinoma (UK) | IHC (Chalkley point array) | Not studied | Not studied | Increase of CD68+ TAM amount above the median (12 per HPF ×250) correlates with relapse up to 3 times and with reduced OS rate by 25% | (61) |
| 60 primary BC (Egypt) | IHC (manually) | Increased stromal CD68+ TAM amount above the median (35.3 per hot spot ×400) is indicative for larger tumor size (>5 cm) | Increased stromal CD68+ TAM amount above the median (35.3 per hot spot ×400) correlates with LN metastasis and vascular invasion | Not studied | (62) |
| 371 patients with invasive BC (USA) | Multiplex-IF in TMA (digital imaging scanning) | Presence of CD68+ TAMs positively is associated with tumor size, tumor grade and stage | Not studied | High amount of CD68+ (defined as score 3) and CD163+ (score 3 and 4) TAMs in tumor stroma correlates with reduced OS rate by 20% | (63) |
| 278 BC patients (Finland) | IHC (manually) | Increase of CD68+ TAM amount above the median (34 cells per hot spot ×400) is indicative for histological grade 3. Increase of CD163+ TAM amount above the median (26 cells per hot spot ×400) is indicative for large tumor size and grade 3 |
High amount of CD163+ TAMs (>26 per hot spot ×400) correlates with LN positivity | High amount of TAMs (CD68+ >34 and CD163+ >26 cells per hot spot ×400) correlates with reduced OS rate by 25% | (64) |
| 1,579 non-metastatic BC (China) | IHC (manually) | Increase of CD68+ and CD163+ TAM amount above the medians (33 and 21 cells, respectively, per HPF ×400) is indicative for histological grade 3 | High amount of CD68+CD163+ TAMs (>21 per hot spot ×400) correlates with positive LN status | High amount of CD163+ TAMs (>21 cells per HPF ×400) correlates with reduced OS rate by 10% | (65) |
| 367 non-metastatic primary invasive BC (South Korea) | IHC in TMA (manually) | 1.5-fold increased amount of CD68+ and twofold increased amount of CD163+ TAMs are indicative for tumors of grade 3 vs. grades 1–2 | Not significant | High amount of CD68+ TAMs (>33 cells per HPF ×400) in tumor nest correlates with reduced OS and DFS rates by 20% | (66) |
| 149 patients with invasive ductal carcinoma (Japan) | IHC (not specified) | High TAM density (>190 CD68+ cells/mm2, >145 CD163+ cells/mm2 and >200 CD204+ cells/mm2 per HPF ×200) is indicative for histological grades 2 and 3 | Not significant | Increase of CD204+ TAM density over 200 cells/mm2 correlates with reduced RFS, distant RFS and DSS rates by 25, 40 and 20%, respectively | (67) |
BC, breast cancer; DFS, disease-free survival; DSS, disease-specific survival; HPF, high-power field; IF, immunofluorescence; IHC, immunohistochemistry; LN, lymph node; TAM, tumor-associated macrophages; OS, overall survival; RFS, recurrence-free survival; TMA, tissue microarray.
The amount of CD68+ macrophages in tumor stroma in different cohorts of patients (Chinese, Finnish, Swedish, Korean, UK, and USA cohorts) was an independent prognostic factor for reduced OS, DFS, and RFS of patients with breast cancer (45, 63, 68–71) ( Table 2 ). In the two independent cohorts (totaling 677 patients) the presence of CD68high/CD4high/CD8low signature in tumors was found to be an independent predictor of decreased OS and RFS (72).
Subpopulations of TAMs in Breast Cancer Progression
The role of TAMs in the pathogenesis of cancer depends on their phenotype and functional polarization (23). A number of experimental studies in vitro and in mouse models demonstrated that M2-polarized macrophages in breast cancer stimulate proliferation of cancer cells, mediate immunosuppression, and induce angiogenesis (73). Major pro-tumor activity of TAMs was demonstrated in PyMT mouse mammary cancer model where TAMs promoted angiogenesis and vascular remodeling in tumors, while macrophage depletion inhibited the angiogenic switch and tumor growth (74). Experimental data correlate very well with the clinical studies demonstrating a supportive role of M2-like TAMs in tumor progression in patients.
Most commonly used M2 markers for the analysis of TAM phenotype in BC include CD163, CD206, CD204, stabilin-1 ( Tables 1 , 2 ). Additional markers, expressed also on other cell types, were used to characterize functional TAM phenotype—CD47, COX-2, MMP9, TIE2, YKL-39, YKL-40, PD-L1 ( Table 1 ).
Clinical studies showed that CD163+ macrophages in tumor stroma positively correlated with poor histological grade, larger tumor size, Ki67 positivity and LN metastasis in patients with BC (64, 65, 69). A lot of studies from different cohorts of BC patients indicated CD163+ macrophages are predictors of poor survival. Exome-capture RNA sequencing data from 50 primary breast tumors (USA cohort) and their patient-matched metastatic tumors in brain, ovary, bone and gastrointestinal tract revealed that CD163+ macrophages were significantly more abundant in metastatic sites compared to primary tumors primary tumors (75). High amount of intratumor CD163-expressing TAMs, identified by flow cytometry in BC patients from a French cohort, was predictive for reduced survival (76). In a Finnish cohort of 278 BC patients high numbers of both CD163+ and CD68+ cells were associated with short OS of the patients (64). CD163 can be an independent macrophage biomarker indicating poor prognosis for breast cancer patients. Thus, in a study of 371 invasive breast carcinoma specimens from a USA cohort of patients, multivariate analysis revealed that high expression of stromal CD163 is an independent predictor of poor patient OS (63). In this study, the absence of quantitative parameters such as threshold numbers that were used to characterize the expression pattern of CD68 and CD163 in each quartile can potentially be a source of misunderstanding and finally contribute to reproducibility issues (63). In a Chinese study, which enrolled 1,579 non-metastatic BC specimens, CD163+ TAMs but not CD68+ TAMs were associated with poor OS (65), that might be related to the origin of TAMs. IHC analysis of 367 primary invasive BC specimens obtained from patients of a Korean cohort without hematogenous metastasis showed that CD163+ macrophages in tumor nest were an independent prognostic marker of reduced OS and DFS (66).
CD206 is the first identified marker of alternatively activated macrophages, that is induced by IL-4 and used as most specific M2 marker (77). In tumors, CD206 is frequently used to identify switch of TAM phenotype in response to new therapeutic agents and antitumor approaches in experimental models; however, CD163 is predominantly used as M2 markers in clinical studies. Thus, CD206 (M2) macrophages were significant predictor of lower PFS in patients from different racial groups (Latinas and Caucasians) (32).
Specific role of CD204 was found in the Japanese cohort, where high number of CD204+ but not CD68+ or CD163+TAMs was associated with worse relapse-free survival and breast cancer-specific survival (67). However, data about the specific prognostic value of CD206 and CD204 for BC patients is still limited.
Combinations of markers can be also used to identify correlations of TAM amount/phenotype with clinical parameters and metastatic potential BC. For example, the high number of CD68+/COX-2 TAMs in the tumor stroma (TS) and high number of COX-2/CD163 in both tumor nest (TN) and TS were observed in tumors of patients with poor survival that was demonstrated by using multiplex immunofluorescence (63). High expression of MMP-9 in the CD68+/CD163+ TAMs was associated with worse OS in ER+ tumors (78). High expression of CCL18+ and SIGLEC1+ TAMs (markers identified by RNA-seq) in 456 breast cancer (USA) was significantly associated with shorter disease-specific survival (DSS) (79). It was noted that TIE2+/CD31+ subpopulation of macrophages abundantly infiltrated metastatic LNs from human breast cancer biopsies but not reactive hyperplastic LNs (80). On the other hand, the amount of stabilin-1+ (M2 marker) TAMs in human breast cancer was mostly abundant on stage I disease (9).
TAMs in Different Tumor Compartments Are Differentially Associated With Breast Cancer Progression
The importance of TAM localization within different compartments of the tumor for BC pathogenesis was demonstrated in several studies. The localization of TAMs in tumor stroma (TS) and tumor nest (TN) showed controversial clinical value of TAMs in tumor progression and prognosis (62). Thus, high CD68+ TAMs infiltrating TS were significantly associated with larger tumor. High CD68+, and CD163+ TAM density in TS was significantly associated with LN metastasis (62). Positive correlation with OS was identified for CD68+ macrophages infiltrating TS, but not TN and for CD163+ macrophages in TN and TS structures (63, 69). Interestingly, high expression levels of CD68+ TAMs in the tumor core were significantly associated with shorter OS at the 10-year follow-up while CD68+ TAMs in the tumor periphery were not significantly associated with OS (70). Infiltration of higher number of CD11c+ macrophages in TS was higher in cases with favorable OS, but infiltration in TN did not correlate with OS (66). In the same study the infiltration of higher numbers of CD68+ or CD163+ macrophages in tumor stroma in BC patients didn’t depend on the OS, while infiltration in tumor nest was higher in patients with unfavorable OS (66). For metastatic BC patients, the numbers of CD163+ macrophages in tumor nest were an independent prognostic marker of reduced OS and DFS (66).
The importance of TAM localization in different compartments of tumor was confirmed in several studies of Russian cohort of patients. Our studies demonstrated that in patients with lymph node metastasis the amount of CD68+ macrophages in ductal gaps was lower compared to metastasis-free patients (53). Based on the intratumor morphological heterogeneity the high number of CD68+stabilin-1+ macrophages in solid structures estimated by immunofluorescent analysis was associated with an increased frequency of LN metastasis in luminal B HER2- BC (50). Solid structures demonstrated an elevated expression of factors involved in the mesenchymal type of collective cell invasion (81). So, CD68+stabilin-1+ TAMs localized in solid tumors potentially may contribute to the invasion and the induction of epithelial–mesenchymal transition (EMT) (50).
As was mentioned above, TAMs can be strongly associated with the features of BC molecular subtypes. However, presented results are somehow controversial. Thus, high CD68+TAM infiltration in triple-negative breast cancer (TNBC) had a significantly higher risk for developing distant metastasis and lower rates of DFS and OS (82). In TNBC patients, high CD163+ TAM infiltration and low level of E-cadherin were independent prognostic factors of OS and DFS (83, 84). Oppositely, the analysis of TAMs in 200 cases of basal-like BC (which is similar to TNBC) showed that increased stromal infiltration of CD68+ or CD163+ macrophages correlated with higher 5-year recurrence and 5-year breast cancer mortality (45).
A high level of infiltration of intratumor CD68+ TAMs was an independent prognostic factor for poor DFS in the hormone receptor-positive subgroup, but not in the hormone-receptor negative subgroup (85). At the same time, tissue microarray (TMA) of samples with BC revealed that CD68+ macrophage infiltrates were independently associated with improved RFS for patients with ER-negative tumors (86). In contrast, poor OS correlated with high expression of CD68 in ER− cases, while high expression of CD163 was associated with improved OS in ER− cases but not in ER+ cancers (78).
In Swedish, Norway, Chinese, and Egyptian cohorts of patients, CD163+ macrophages positively correlated with estrogen and progesterone receptor negativity, triple-negative/basal-like breast cancer and inversely correlated with luminal A breast cancer (62, 66, 69, 87). Association between high density of CD163+ TAMs and hormonal receptor negativity was also revealed in a meta-analysis of 1,672 specimens of non-metastatic invasive BC (65).
In common, higher infiltration of TAMs, expressed both pan-macrophage marker CD68 and specific M2 markers, is associated with more aggressive molecular subtypes of breast cancer. Taken together, TAM abundance correlated with unfavorable clinicopathological features and survival in patients with breast cancer. Their polarization and localization in different tumor compartments should be taken into account for determining the prognostic and/or predictive role of TAMs.
TAMs and Breast Cancer Treatment
Treatment of breast cancer is multimodal and includes surgery, radiation therapy, chemotherapy, and molecular treatments (88). Choice of therapy depends on individual course of the disease, including lymph node involvement, hormone receptor status, HER2 overexpression, and patient age and menopausal status. For HER2-positive patients, trastuzumab, an anti-HER2 monoclonal antibody, demonstrates improvement of the survival and administered in combination with chemotherapy. Patients with ER- or PR-positive breast cancer receive endocrine therapy, such as an aromatase inhibitor and selective modulator of estrogene receptors (tamoxifen) (89). For patients with high-risk disease, chemotherapeutic treatment includes an anthracyclines and taxanes, while for low-risk disease, anthracyclines are more commonly used (90). TNBC, the most aggressive type, including BRCA ½ positive patients, should be treated with platinum-based chemotherapy (carboplatin or cisplatin) in neoadjuvant regime which showed more advantages in comparison with standard schemes (91). The most important parameter for the assessment of successful chemotherapeutic treatment and improved survival is the achievement of a pathologic complete response (pCR) (92). After therapy, tumor relapse can happen in up to 40% of patients with breast cancer (93). In case of TNBC, only 30–45% of patients can achieve pCR compared to patients with ER-positive tumors (94). Below we describe how TAMs correlate with different types of therapy and show the perspectives of TAM targeting ( Table 7 ).
Table 7.
The association of TAMs with the effect of chemotherapy in patients.
| Cohort of patient | Method of detection | The type and scheme of chemotherapy (adjuvant, neoadjuvant) | The amount of TAMs in chemotherapy-treated tumors | Correlation of TAM with the effect of chemotherapy | Reference |
|---|---|---|---|---|---|
| 311 breast cancer patients (Sweden) | Flow cytometry, IHC | Neoadjuvant (PTX and FU-doxorubicin-cyclophosphamide) | fivefold increase of CD45+CD11b+CD14+ macrophage percentage of total cells is found in NAC-treated patients compared to non-treated patients | CD68 high/CD8low ratio is associated with almost fourfold decreased pCR rate compared to cases with CD68low/CD8high ratio (7 vs. 27%) | (72) |
| 7 breast cancer patients (USA) | IHC | Neoadjuvant (paclitaxel-based) | Increased amount of CD68+ TAMs in tumor post NAC treatment compared to pre-treatment biopsy | Not studied | (95) |
| 33 breast cancer patients (UK) | IHC | Neoadjuvant (capecitabine plus docetaxel preceded by adriamycin and cyclophosphamide) | Not studied | High CD163+ infiltration (defined as grades 3 and 4) in primary tumor and ALNs are associated with pCR following NAC | (98) |
| 40 breast cancer patients (Russia) | Real-time qPCR | Neoadjuvant (PTX- or taxotere-based) |
Not studied | sixfold increase of YKL-39 expression levels after NAC correlates with distant metastasis and poor response to NAC | (17) |
| 123 metastatic CRC patients (Turkey) | IHC | Adjuvant (bevacizumab plus OXP-based or irinotecan-based chemotherapy) | Not studied | Low CD68+ TAM infiltration (scored as <50% staining of stromal cells) is associated with almost twofold longer OS (26.7 ± 8.8 vs. 14.1 ± 1.7 months) and 1.5-fold longer RFS (9.3 ± 1.8 vs. 6.5 ± 1.2 months) after chemotherapy compared to patients with high CD68+ TAM infiltration | (140) |
| 208 stage III CRC patients (Italy) | IHC | Adjuvant (5-FU) | Not studied | Increase of CD68+ TAM immune-reactive area above 8% in primary tumor is associated with increased DFS rate by 30% in 5-FU treated patients with stage III | (141) |
| 521 stage II colon cancer patients (China) | TMA | Adjuvant (FU-based) | Not studied | High CD206+ TAM amount (≥74 cells per ×200 HPF) and increase of CD206/CD68 ratio (above 0.77) correlate with decreased DFS and OS rates after postoperative FU-based therapy by 20% and 30-40%, respectively. | (124) |
| 163 stage II/III NSCLC patients (USA) | Multiplex IF | Neoadjuvant (platinum-based) | twofold increase of CD68+ TAM median density in NAC-treated compared to untreated patients (609.36 vs. 298.8 cells/mm2) | Increase of epithelial and stromal CD68+ TAM densities above the medians (17 and 25 cells/mm2, respectively, under ×200) correlate with increased OS rate by almost 20% in patients who received NCT | (186) |
| 27 stage IIIA NSCLC patients (China) | IHC (manually) | Neoadjuvant (cisplatin/docetaxel) | Not studied | Decrease of CD68+ TAM amount below the median (<222 cells per HPF ×200) is associated with threefold longer DFS (median=16.3 vs. 5.3 months in high CD68+ TAMs). High islet/stromal CD68+ TAM ratio (>1.33) correlates with almost fourfold longer DFS (median = 20.7 vs. 5.5 months) and longer OS (unreached vs. 34.8 months) compared to low ratio | (187) |
| 140 ovarian cancer patients (Italy) | Flow cytometry | Adjuvant (cisplatin/carboplatin + Taxol + bevacizumab) | twofold increase of M1/M2 ratio is found in platinum-sensitive tumors compared to platinum-resistant tumors (2.6 ± 1.1 vs. 0.7 ± 0.2). | High M1/M2 ratio (≥1.4) is associated with almost twofold longer OS (34 vs. 18 months) and almost threefold longer PFS (24 vs. 9 months) compared to those with low M1/M2 ratio | (210) |
ALN, axillary lymph node; CRC, colorectal cancer; DFS, disease-free survival; DSS, disease-specific survival; FU, fluorouracile; HPF, high-power field; IF, immunofluorescence; IHC, immunohistochemistry; LN, lymph node; NAC, neoadjuvant chemotherapy; NSCLC, non-small cell lung cancer; OS, overall survival; PFS, progression-free survival; pCR, pathological complete response; RFS, recurrence-free survival; TAMs, tumor-associated macrophages; TMA, tissue microarray.
The accumulation of TAMs in breast tumors after neoadjuvant chemotherapy (NAC) was identified both in animal models and in analysis of different patient cohorts (72, 95). In a study of 311 BC patients of Swedish cohort flow cytometry analysis revealed higher percentage of tumor-infiltrating CD45+CD11b+CD14+ macrophages from women who received NAC (paclitaxel and fluorouracil–doxorubicin–cyclophosphamide) compared to the tumors from women treated with surgery alone (72). In a small cohort of patients (seven patients, USA) who received paclitaxel-based NAC the amount of CD68+ TAMs in the tumor after NAC was higher than in biopsy specimens obtained before NAC (95). Increased accumulation of TAMs after paclitaxel (PTX) treatment was detected also in tumors of PyMT-mice (95).
Predictive value of macrophages for the response to chemotherapy is still controversial. Using CIBERSORT algorithm to summarize the results of 56 studies, totaling 10,988 cases of breast cancer, it was found that M2 macrophages are strongly associated with a lack of pathological complete response (pCR) and resistance to chemotherapy (96). Positive correlation of low CD68 expression with pCR was shown in patients with BC who received trastuzumab in NAC regime (97). Gene chip analysis revealed that high CD68/CD8 ratio is also a predictive biomarker for reduced rate of pCR in 311 breast cancer patients from a Swedish cohort that underwent neoadjuvant chemotherapy (PTX and fluorouracil-doxorubicin-cyclophosphamide) (72). In contrast, in 108 patients with BC (UK cohort) who received NAC (capecitabine plus docetaxel preceded by adriamycin and cyclophosphamide), high levels of CD163+ TAMs significantly correlated with a pCR both in tumor and metastatic axillary LNs (98). However, no correlation was found between CD68 expression and pCR (98) ( Table 7 ). The semiquantitative method applied in this study for immunohistochemical analysis is useful for description of intergroup differences in CD68+ and CD163+ expression; however, it cannot guarantee the reproducibility of tissue scoring in further studies (98). It can be also hypothesized, that CD163+ TAMs differ in their origin from other CD68+TAMs.
We recently analyzed the predictive role of new TAM-released pro-angiogenic and monocyte chemotactic factor YKL-39 in patients who received PTX- or taxotere-based NAC (17). We found that high gene expression of YKL-39, in biopsies obtained before NAC, positively correlated with increased risk of distant metastasis and poor response (stabilization or progressive disease) to therapy (17) ( Table 7 ). In our other study that included 68 female patients with BC (Russian cohort) who received anthracycline-containing NAC, the absence of clinical response is associated with the presence of M2+ macrophage phenotype (YKL-39-CCL18+ or YKL-39+CCL18−) (20). In our study of patients who underwent neoadjuvant chemotherapy (multiple schemes) CD68+ TAMs in areas with parenchymal elements negatively correlated with lymphatic metastasis (52).
In contrast to YKL-39, high epithelial and stromal PD-L1 expression in biopsies obtained before NAC (PTX-based or platinum-based) correlated with increased rate of pCR after NAC, especially in hormone-positive and Her2-postive breast cancer (99).
Several studies in mouse models confirmed the effectiveness of treatment based on the combinations of chemotherapeutic agents and inhibitors of macrophage activity in tumor. Thus, in vivo in MMTV-PyMT (PyMT) tumor-bearing mice, treatment with paclitaxel (PTX) in combination with CSF1 and cKIT receptor tyrosine kinases inhibitor (PLX3397) but not with PTX alone resulted in a significant reduction in CD31+ vessel density, reduced pulmonary metastases, and activation of cytotoxic T cell response (72). Using the same mouse model, it was found that TAMs are the source of the cathepsins during PTX treatment. Combining PTX with cathepsin deletion [by JPM-OEt (JPM), a pan-cathepsin inhibitor] significantly improved therapeutic efficacy of PTX, inhibited tumor growth and metastatic burden, and improved late-stage survival (95). In this study the addition of low-dose cyclophosphamide enhanced antitumor efficacy of treatment (95). In another study using MMTV-PyMT transgenic mice, PTX showed more pronounced antitumor effect in combination with the selective class IIa histone deacetylase (HDACIIa) inhibitor TMP195 which modulates macrophage phenotypes promoting phagocytosis of cancer cells (100).
In mice bearing chemoresistant MCF-7 breast cancer xenograft treatment with combined chemotherapy (CMF—cyclophosphamide, methotrexate, 5-fluorouracil) and anti-CSF-1 Fab [murinized, polyethylene glycol-linked antigen-binding fragment (Fab) against mouse (host) CSF-1] reversed chemoresistance of MCF-7 xenografts, reduced angiogenesis, macrophage recruitment, suppressed tumor growth, down-regulated expression of the chemoresistance genes, and improved survival rates (101). In cyclophosphamide-treated mouse mammary tumors and in human breast cancer that underwent NAC (cyclophosphamide), the M2 subpopulation of TAMs (CD206+TIE2hiCXCR4hi) was found around the blood vessels, where they promoted tumor revascularization and relapse (102).
It was found that TAMs mediate the resistance of breast cancer during endocrine therapy by tamoxifen. MCF-7/THP-1 co-injected mice showing more extensive growth were characterized by tamoxifen resistance in contrast to MCF-7-injected animals (103). In vitro generated TAMs from THP-1 cells showed M2 phenotype (CD163+) when cultured with conditioned medium from tamoxifen-resistant MCF-7 cell lines (104). The possible mechanism of the resistance is a feedback loop between TAM-released CCL2 and PI3K/Akt/mTOR signaling activated in cancer cells (104). Clinically, in ER-positive and Her2-negative breast cancer, CD163+ TAMs more abundantly infiltrated tamoxifen resistant tissues in comparison with tamoxifen sensitive tissues (105).
Currently, there is no consensus about the effect of TAMs on the efficiency of chemotherapy in patients with breast cancer. However, most of mouse models demonstrated the negative role of TAMs in the tumor response to chemotherapeutic treatment.
TAMs and Colorectal Cancer
Colorectal cancer (CRC) is the fourth most commonly diagnosed malignancy and the fifth leading cause of cancer-related deaths in the world. In 2018 more than one million new cases of colorectal cancer were diagnosed and almost 550 thousands deaths were registered worldwide (34). Five-year survival of patients with CRC is still below 60% in most European countries (106).
Major pathological parameters used for the prognosis of CRC include TNM stage, microsatellite status tumor grade, and lymphovascular invasion. The mutation status of KRAS, BRAF, and NRAS has a predictive value for the response to anti-EGFR therapy in metastatic context (107). The most common site of metastasis with the worst prognosis is the liver. Other sites of metastasis include the lung, bone, multiple sites, and brain (108).
Similar to breast cancer, colorectal tumors display intratumor heterogeneity that is based on the abnormalities in three different main molecular pathways: (1) chromosomal instability (CIN) (more than 50% of cases), (2) microsatellite instability (MSI) (6–15% of cases), and (3) CpG island methylating phenotype (CIMP), or epigenetic instability (up to 20% of cases) (107, 109).
Although the colon cancer and rectal cancer are usually epidemiologically registered as CRC, they should be considered as two separate diseases due to their topography, surgical challenge, therapy, complications, and relapse pattern (108, 110). Rectal cancer is characterized by more frequent local relapses than colon cancer. Additionally, colon cancer is divided to the left and right cancer types (108). The Consensus Molecular Subtypes (CMS) classification of colon cancer was proposed in 2015 by Justin Guinney and colleagues, who analyzed the data of gene expression of 4,151 colon cancer patients (111). Four types of CMS are proposed: 1) CMS1 (MSI, immune type, 14% of total CRC) is characterized by hypermutation, high microsatellite instability, pronounced immunogenicity, mutations of the BRAF gene; 2) CMS2 (canonical, 37% of total CRC) is an epithelial type characterized by activation of Wnt and MYC signaling pathways and high frequency of copy number changes in somatic cells; 3) CMS3 (metabolic type, 13% of total CRC) is an epithelial type characterized by metabolic dysregulation and mutations of the KRAS gene and by heterogeneous microsatellite and chromosomal instability; 4) CMS4 (mesenchymal type, 23% of total CRC) is characterized by activation of the TGF-β signaling pathway, epithelial–mesenchymal transition, severe stromal infiltration, active neoangiogenesis, and poor prognosis. One subtype with mixed characteristics (13% of total CRC) is also distinguished, that can be also a transition phenotype or special case of intratumor heterogeneity (111). Both CMS1 and CMS4, which are immunogenic, showed high levels of infiltrating CD8+ cytotoxic lymphocytes and CD68+ TAMs (112). Stromal cell infiltration was significantly higher in tumors with CMS4 compared to other CMS. In contrast, the canonical (CMS2) and metabolic (CMS3) subtypes with intermediate prognosis exhibit less pronounced immune and inflammatory responses (112). Despite high heterogeneity of CRC, the prognostic role of TAM infiltrate in the context of different molecular subtypes or histological localizations remains to be investigated.
TAMs in Colorectal Tumors and Metastasis
In colorectal cancer (CRC), a number of in vitro studies showed pro-tumor activity of macrophages that induce growth and invasive behavior in colon cancer cells (113–115). For example, human colon cancer cell lines (HCT116, WiDr, SW480, and RKO) co-cultured with monocyte cell lines (THP-1 and U937) showed enhanced invasiveness compared to control tumor cells alone (113). Co-cultured HT-29 or HCT116 colorectal cell lines with TAMs (THP-1 cells stimulated by conditioned media from CRC cell lines) demonstrated enhanced EMT supporting migration, invasion, and circulating tumor cells (CTC)-mediated metastasis. Invasive phenotype of CRC tumor cells was regulated by TAM-derived IL-6 which activated the JAK2/STAT3 pathway and resulted in increased FoxQ1 expression. In turn, the production of CCL2 by tumor cells was enhanced that promoted macrophage recruitment (114). The limitation of these studies was the use of proliferative THP-1 cells which differ significantly from human primary blood monocytes. In vitro condition medium (CM) from LPS-treated macrophages containing IL-1b, IL-6, and TNF-α induced proliferation of HCT116 colon cancer cell line, increased NF-kB activity and VEGF secretion in cancer cells (116). In another study, HCT116 and HT29 colorectal cancer cells cultivated with M2 macrophages expressed reduced levels of E-cadherin but increased levels of vimentin and showed enhanced invasive ability (115). It was also found that TAMs can produce ECM proteins (the abundance of collagen types I, VI, and XIV) in CRC, that induce ECM remodeling (117).
In contrast, there is a series of convincing evidence obtained by Beelen R. and Bögels M. that indicates that macrophages in CRC have M1 phenotype with antitumor activity (27, 28, 118). Thus, they found that human monocytes incubated with the conditioned media of colon carcinoma cells (HT29, HCT116, RKO, SW620 and SW948) show high production of pro-inflammatory cytokines (IL-6, IL-12, and TNF-α) and increased gene expression of the chemokine ligand CXCL13 but decreased expression of anti-inflammatory cytokine IL-10 and the pro-angiogenic cytokine IL-8 (27). Human monocytes stimulated with conditioned media of breast carcinoma cell lines (SKBR3, MCF7 and ZR-75–1) stimulated in macrophages enhanced production of IL-10 and expression of mannose receptor 1 (MR1), CCL17, and CCL22, that are M2-associated chemokines (27). In rat model of CRC tumors, administration of flavonoids rutin and luteolin, that reduce monocyte migration, resulted in reduced number of intratumoral ED1+ immature macrophages without affecting ED2+ resident macrophages (28). Rutin and luteolin administration enhanced tumor size and increased peritoneal metastases (28). Incubation of co-culture of BMDMs and CRC cancer cells (CC531s) with MG4-c1, MG4-c2a, or MG4-c2b mAb led to increased tumor cytotoxicity and decreased tumor cell growth (118). In CRC rat model, resident liver macrophages (Kupffer cells) were involved in cytotoxic effect eliminating tumor cells under monoclonal antibody treatment (118).
Favorable Role of Total Amount of TAMs in Prognosis of CRC
CD68+ TAMs serve as a good prognostic factor for patients with CRC in different cohorts of patients ( Table 3 , Figure 1 ). Thus, IHC analysis of Japanese cohort of 30 patients with CRC showed that low levels of CD68+ TAMs in invasive front and tumor stroma were associated with more advanced colorectal cancer, while high amount of TAMs was found in patients with good prognosis (126). In European cohorts of patients, similar correlations have been identified. Tissue microarray of 100 patients with colon cancer (Germany) demonstrated that amounts of CD68+ macrophages were decreased at higher stage tumors (127). Analysis of 210 samples with primary colorectal cancer (Bulgaria) showed that lower number of CD68+ TAMs in tumor invasive front significantly correlated with the presence of metastases in local lymph nodes, with distant metastases and with more advanced tumor stage (III and IV stages) (119). Lower number of CD68+ cells in tumor border was also found in patients where tumor cell invaded the blood circulation, lymph vessels or were characterized by perineural invasion and lower grade of inflammatory infiltration. High level of TAM infiltration in tumor invasive front was an independent favorable prognostic factor for overall survival (119). High intraepithelial and stromal expression of CD68 predicted long-term OS and correlated with significantly less tumor budding at the invasive front and absence of lymph node metastasis in the Greek cohort of 201 patients with primary CRC (120). In a Swedish cohort of 488 patients with colon and rectal cancer, high infiltration of CD68+ macrophages was associated with high survival of patients (121). Significant positive association between DFS and CD68+ cells was demonstrated in the USA cohort of 188 patients with colorectal cancer liver metastasis (128).
Table 3.
Representative studies demonstrating the association of TAMs with tumor progression parameters in colorectal cancer.
| Cohort of patients | Method of detection | TAM correlation with tumor growth and stage | TAM correlation with lymphatic and hematogenous metastasis | TAM correlation with survival | Reference |
|---|---|---|---|---|---|
| 210 patients with primary CRC (Bulgaria) | IHC (digital imaging scanning) | Amount of CD68+TAMs (per hot spot ×320) in invasive front is decreased by almost 25% in advanced III + IV stages (114.9 ± 91.9 vs. 150.2 ± 102.3 in I + II stages) | Amount of CD68+TAMs (per hot spot ×320) in invasive front is decreased by 17% in tumors with regional LN metastases (119.4 ± 96.5 vs. 143.3 ± 100.0 in cases with negative LN), and by 42% in tumors with distant metastases (150.4 ± 105.8 vs. 87.8 ± 54.3 in negative cases) | Increased amount of CD68+ TAMs above 48.6 cell/mm2 in tumor stroma and above 105.2 cell/mm2 in invasive front is associated with increased OS rates by 10 and 40%, respectively | (119) |
| 201 patients with primary CRC (Greece) | IHC in next-generation TMA (manually) | High amount of intraepithelial CD68+ TAMs (counted per hot spot ×400) predicts less tumor budding High amount of CD163+ TAMs (counted per hot spot ×400) is indicative for G1-2 grades |
High amount of CD68+ and CD163+ TAMs is associated with absence of LN metastasis | High amount of CD68+ TAMs correlates with better OS (increase by 40%) | (120) |
| 488 patients with colon and rectal cancer (Sweden) | IHC (manually) | High CD68+ infiltration (defined as grades 3 and 4 in hot spot ×200) at the invasive front is indicative for I+II stages and well-moderate grade | Not studied | High CD68+ infiltration (defined as grades 3 and 4 in hot spot ×200) at the invasive front correlates with increased DSS rate by 30% | (121) |
| 160 patients with stage IIIB and IV colon carcinoma (China) | IHC (manually) | Not significant | High CD68+ infiltration (defined as grades 3 and 4 in hot spot ×200) at the invasive front is associated with absence of hepatic metastasis | High CD68+ infiltration (defined as grades 3 and 4 in hot spot ×200) at the invasive front correlates with increased OS rate by 30% and liver-metastasis free survival rate - by 20% | (122) |
| 163 patients with rectal cancer (Sweden) | IHC in TMA (manually) | Not significant | Not studied | Presence of CD163+ TAMs in tumor tissue is associated with reduced OS and RFS rates by 40% | (123) |
| 81 patients with CRC (China) | IHC (manually using immunoreactive score) | Increase of CD163+ TAM expression above the median (measured semiquantitatively at ×400) is indicative of III TNM stage, poor tumor grade | High CD163+ expression positively correlates with lymphovascular invasion and N2-3 LN status | High CD163+ expression is associated with reduced OS rate by 30% and RFS by 20% | (114) |
| 521 and 314 patients with stage II CRC (China) | IHC in TMA (digital imaging scanning) | Increase of CD206/CD68 ratio ≥ 0.77 is indicative of poor differentiation and undifferentiation status and pathological T4 stage |
Increase of CD206/CD68 ratio ≥ 0.77 is associated with lymphatic/vascular invasion and perineural invasion |
Increase of CD206/CD68 ratio ≥ 0.77 correlates with reduced DFS rate by 40% and OS by 30% |
(124) |
| 159 patients with advanced colorectal cancer (stage IV) (Finland) | IHC (manually) | Not studied | Low amount of intratumoral stabilin-1+ TAMs (<10 cells per ×400 hotspot) correlates with low number of distant recurrences | High amount of peritumoral stabilin-1+ TAMs (≥10 cells per ×400 hotspot) correlates with longer DFS time (103 vs. 63 months in cases with low amount) at stages II and III, but correlates with reduced DSS rate by almost 2 times in stage IV patients | (125) |
CRC, colorectal cancer; DFS, disease-free survival; DSS, disease-specific survival; IF, immunofluorescence; IHC, immunohistochemistry; LN, lymph node; TAMs, tumor-associated macrophages; OS, overall survival; RFS, recurrence-free survival; TMA, tissue microarray.
IHC analysis of CD68 expression in CRC tissue in Chinese cohorts of patients revealed similar correlations. Thus, a study of 160 patients with stage IIIB and IV colon carcinoma demonstrated that high density of CD68+ macrophages in invasive front of tumor was associated with higher 5-year survival rate and lower hepatic metastasis (122). However, in this study, the exact quantitative parameters have to be interpreted carefully, since the semiquantitative method applied relies on a subjective visual assessment that could affect reproducibility (122). In 521 patients with stage II colon cancer after radical resection, low CD68+ TAM density was significantly associated with perineural invasion (124). This finding was confirmed by using validation cohorts (314 eligible patients) (124). IHC staining of 118 CRC tissues demonstrated positive association of intratumoral CD68+ TAM count with depth of invasion, lymph node metastasis, and tumor staging. Besides, a significant association between CD68 expression and MMP-2 and MMP-9 expression in CRC was found (113). The difference of this study was the fact that CD68+ TAM infiltration was estimated only in intratumor compartment where they have very low density. For M1 macrophages expressing NOS2, their high infiltration was demonstrated to be significantly associated with improved cancer-specific survival in patients with colon cancer of the Swedish cohort (54).
Negative Role of M2-Like TAMs in Prognosis of CRC
In contrast to the total amount of macrophages defined mostly by CD68 marker, M2-like phenotype of macrophages is rather indicative for the negative prognosis of patients with CRC ( Table 3 ). IHC analysis of Chinese cohort of 81 patients with CRC showed that high expression of stromal CD163 at tumor invasive front was significantly associated with tumor grade, lymphovascular invasion, tumor invasion, lymph node metastasis, and TNM stage and correlated with poor RFS. High level of CD163 was also associated with reduction of E-cadherin and high expression of vimentin in cancer cells, an indication of EMT (114). In the same cohort high CD163+/CD68+ ratio in the tumor front, but not in tumor stroma, was closely correlated with enhanced lymphovascular invasion, tumor invasion, and TNM stage as well as recurrence-free survival (RFS) and OS of patients with CRC. Moreover, CD163+/CD68+ ratio in tumor front was also significantly associated with EMT program and CTC amount (115). A study of 150 patients of Spanish cohort demonstrated that CD163+ macrophages were found in tumor invasive front while CD80+ cells located in adjacent normal mucosa in less invasive T1 colorectal cancer that was detected by immunohistochemistry. At stage III CRC, higher CD68 and lower CD80/CD163 ratio was associated with decreased OS (129). Tissue microarray of samples obtained from 163 patients with rectal cancer from the South Eastern region in Sweden demonstrated that CD163+ biopsies have earlier local recurrence and poor survival (123, 130). One contradictory study was found. In 201 patients with primary CRC (Greece), improved survival was identified in tumors with strong stromal infiltration of CD163+ M2 macrophages, which presented 40% of the total macrophage population (120). CD163+ macrophages were also predictive of the lower tumor grade and less lymph node metastasis that was demonstrated by next-generation tissue microarray construction (120). In this study, expression scores were dichotomized according to the mean into low and high groups; however, the authors did not provide the information about mean number used as threshold, thereby limiting our ability to compare the obtained results with data from other studies (120).
Using two independent cohorts of Chinese patients with stage II CRC (521 patients and 314 patients) it was found that high CD206+ TAM density was significantly associated with stage II of CRC characterized by poor differentiation (124). A high CD206/CD68 ratio was significantly associated with poor differentiation, pathological T4 stage, lymphatic/vascular invasion, and perineural invasion. Besides, patients with CD206+ TAM density and high CD206/CD68 ratio had significantly worse DFS and OS (124). CD204+ TAMs were abundantly detected in high-grade colorectal adenomas in comparison with low-grade adenomas that was shown immunohistochemically in 88 tubular or tubulovillous adenomas (131). In advanced colorectal cancer (stage IV), patients with a high number of peritumoral or intratumoral stabilin-1+ macrophages had a shorter DSS that was found in the Finland cohort of 159 patients. Moreover, a low number of suppressive intratumoral stabilin-1+ macrophages in this cohort correlated with a low number of distant recurrences (125).
TAMs were also found to be involved in tumor progression by expressing several markers expressed also by other cell types. Interestingly, VEGF+ TAMs/stroma in colon cancer is indicative for the increased survival in comparison with patients with the absence of VEGF expression in stroma (132). Patients with CRC (Swiss cohort) tumors with VEGFA gene amplification have reduced CD68+ and CD163+ TAM infiltration, while high-grade tumors are associated with increased CD163+ and reduced CD68+ macrophage infiltration (55). In another study, high percentage of VEGFR1+ macrophages in lymph node metastasis was associated with worse outcome in patient with CRC (133). VEGFR1+ circulating monocytes in blood of patients with LM predicted reduced PFS and site of recurrence (liver) in CRC (133). In contrast, mTORC2 activity (pPKCα staining) in macrophages was negatively associated with tumor stage and LN metastasis in the Austrian cohort of CRC patients. Low mTORC2 activity in macrophages in tumors was significantly associated with lower survival rate (134).
TAMs and Colorectal Cancer Treatment
The main strategies in the treatment of colorectal cancer are surgery, radiation therapy (or chemoradiation), chemotherapeutic treatment (135). Chemoradiation and short-course radiotherapy have more advantages than chemotherapy alone and result in improved survival. Conventional chemoradiation regimens include fluorouracil or capecitabine. Addition of oxaliplatin to fluorouracil improved DFS (135). FOLFOX (oxaliplatin-containing regimens) and FOLFIRI (irinotecan-containing regimens) showed more efficacy than 5-FU alone (135). Neoadjuvant FOLFOX chemotherapy combined with radiotherapy followed by radical resection is the standard combined therapy in patients with locally advanced colon cancer (136). However, the treatment response to neoadjuvant CRT is variable from a pathological complete response (pCR) to total resistance. pCR was associated with the favorable survival, however, has ranged from 10 to 30% (137).
The presence of activating mutations in the KRAS, NRAS, and BRAF genes is the criterion to refuse the therapy with the anti-EGFR agents. Mutations in these genes occur in about 55–60% of colorectal cancers. Patients with KRAS, NRAS, or BRAF mutations do not benefit from anti-EGFR therapies (138). Targeted drugs, such as bevacizumab (human anti-VEGF antibody), cetuximab, and panitumumab (human EGFR monoclonal antibodies) have been proven to be effective against metastatic CRC in patients (139). Survival of patients with metastatic CRC increased with the addition of irinotecan or oxaliplatin to 5-FU. However, the recurrence rate remains high, especially in rectal cancer.
The role of TAMs in the efficiency of treatment is strictly limited in the studies of patients with CRC ( Table 7 ). High CD68+ TAM infiltration in tumor tissue of 123 patients with metastatic CRC decreased the efficacy of bevacizumab plus FOLFIRI scheme (folinic acid, 5-fluorouracil, irinotecan) of chemotherapy (140). In stage II colon cancer with high CD206/CD68 ratio, adjuvant chemotherapy significantly improved the DFS rate from 38.9 to 68.0% at 3 years and from 33.1 to 66.0% at 5 years (124). Oppositely, for 208 patients resected for stage III colorectal cancer, high CD68+ TAMs in invasive front of tumor and in metastatic lymph node were associated with better DFS only in 5-fluorouracil-treated patients compared to untreated ones (141).
Clinical trial of bevacizumab plus FOLFIRI treatment in patients with metastatic colorectal cancer demonstrated that single-nucleotide polymorphisms in genes regulating TAM-related functions significantly associated with clinical outcome in metastatic CRC patients (142). CCL2 rs4586, CCL18 rs14304, and IRF3 rs2304205 correlated with PFS in KRAS mutant patients of the TRIBE cohort; TBK1 rs7486100 correlated with OS in KRAS wild-type patients of TRIBE cohort (142).
Most pieces of evidence are found in vitro or in animal models. In several studies TAMs were found to be involved in the resistance of tumor to 5-fluorouracile (5-FU). Thus, 5-FU treatment significantly increased the infiltration of CD68+TAMs in the mouse subcutaneous CT-26 tumors (143). In vitro putrescine (polyamine) secreted by TAMs significantly attenuated 5-FU-induced growth inhibition of SW-480 and HCT-116 cell lines (143). 5-FU treatment induces CCL22 secretion by M2 macrophages in vitro (144). Co-culture of colon cancer cells and M2 macrophages treated with 5-FU indicated that macrophages mediate cell migration and invasion in CRC cells inducing EMT and activating PI3K/AKT pathway (144). CCL22 neutralizing antibody increased the apoptosis in cancer cells. Clinically, CCL22 expression was elevated in patients with colorectal adenocarcinoma and was positively associated with CD163+ TAMs. Patients with higher CD163+ M2 macrophages and high expression of CCL22 in CRC tissue had worse overall survival (OS) (144).
Administration of oxaliplatin (OXP) with other potential antitumor drugs demonstrated antitumor effect in several mouse models of CRC. The expression of F4/80 and iNOS significantly decreased under oxaliplatin (OXP) treatment in tumor-bearing mice (145). OXP inhibited the M1-like macrophages polarization while had little effect on differentiation into M2-like macrophages in vitro (145). Administration of oxaliplatin combined with Toll-like receptor agonists R848 reversed the functional orientation of MDSCs towards M1-like macrophages and strengthened antitumor effect of oxaliplatin in vivo (145). In an abdominal implantation model of colon cancer intraperitoneal administration of OXP inhibits tumor cell growth by a decrease in CD11b+F4/80high macrophages in tumors (146). Treatment of CT26 tumor-bearing mice with combination of oxaliplatin with trifluridine/tipiracil (FTD/TPI), a new antimetabolite agent, induced TAM depletion and promoted CD8+ T-cell infiltration in tumors (147).
Contradictory results were found for cetuximab interaction with macrophages. In AOM/DSS-induced colon cancer mouse model, cetuximab (anti-EGFR antibody) treatment inhibited total F4/80+/CD11b+ TAMs and M2 (F4/80+/CD206+) TAM accumulation (148). Down-regulation of gene expression of M2 polarization markers, ARG1, IL-10, and IL-4, was observed in tumor. In vitro THP-1 cells stimulated with conditioned medium from HCT116 cell with EGFR knockdown acquired M1 phenotype (by upregulation of IL-12, CCR7, and TNF-α) and down-regulation of M2-related markers (IL-10, ARG1, CCL17, CCL22, and IL-4) (148). In contrast, cetuximab induced production of anti-inflammatory and tumor-promoting mediators, including IL-10 and VEGF activating M2-macrophages in co-culture of CRC cell line and human monocyte-derived macrophages (149).
In summary, there is still no agreement about the role of TAMs in the treatment of CRC. Such contradictory results clearly depend on the animal model, type of in vitro study, patient cohort, and type of anti-cancer drug. Most of presented studies indicate that TAMs enhance tumor resistance to chemotherapy in colorectal adenocarcinoma. Therefore, to achieve the maximum efficiency of chemotherapy in CRC, the combined approaches that include targeting of TAMs should be developed.
TAMs and Lung Cancer
Lung cancer is the leading cause of cancer-related death and the second most diagnosed cancer worldwide. More than two million new cases and more than 1.7 million deaths were registered in 2018 worldwide (34).
Lung cancer is highly heterogenic and can be localized in different anatomic compartments of the lung and manifests in variable symptoms (34, 150). There are two main histological types of lung cancer: non-small cell lung carcinomas (NSCLC) (85% of patients) and small-cell lung carcinomas (SCLC) (15%). These two types differed by growth, metastatic spread, and treatment strategy. NSCLC is classified into three subtypes: adenocarcinoma, squamous cell carcinoma, and large cell carcinoma (34, 150). Unfortunately, around 70% of patients are diagnosed at the advanced stages of the disease (stage III or IV) (34). Around 40% of the newly diagnosed patients have stage IV of NSCLC (151). The 60-month OS rate for NSCLC remains poor, from 50 to 70% in patients with early-stage operable disease, dropped to 2–5% in patients with stage IVA–IVB (150). The brain is the most frequent site of distant metastasis in lung cancer patients, and metastatic process is a major cause of morbidity and mortality. Brain metastases are found in 80% of SCLC and 30% NSCLC (152, 153).
The lung is one of the major barrier organs for the defense of the organism against foreign particles and pathogens. The lung anatomy and cellular composition are ideal to fulfil this defense function without induction of unnecessary inflammation (77). Numerous components of the immune system, including abundant alveolar macrophages (AMs), are involved in the maintenance of the immunological homeostasis. The role of AMs in lung cancer remains contradictory. Lung tumors activate tumor-supporting role of resident AMs by decreasing their antibody-mediated cytotoxicity and antigen processing and presentation ability and by enhancing their pro-angiogenic activity (154, 155). However, in numerous studies antitumor activity of AMs has also been demonstrated (155). The mechanism of AM programming by TME remains to be investigated.
We focused on TAMs located in lung tumor tissue and discussed the prognostic relevance of TAMs below.
TAMs in Lung Tumors and Metastasis
In lung cancer TAMs represent the most abundant immune cell component of TME (154) ( Figure 1 ). TAMs in lung cancer promote cancer proliferation, epithelial–mesenchymal transition (EMT), invasion and metastasis, resulting in poor patient outcome (156, 157). Lung cancer cells activate macrophages and other non-malignant stromal cells, such as fibroblasts and vascular endothelial cells, in the TME to form a positive feedback between tumor cells and TAMs promoting tumor progression (158–160). However, the detailed mechanisms by which TAMs promote malignancy in lung cancer remain largely unclear.
Numerous studies confirmed that in lung cancer TAMs contribute to tumor progression and metastasis through the production of variety of chemokines and growth factors (156, 161–163). In vitro lung carcinoma cells (human NSCLC A549 cells) induce polarization of THP-1 cells to CD206+ M2 phenotype (156). In turn, M2 macrophages promoted EMT and invasion in lung cancer cells upregulating CRYAB expression on tumor cells and activating the ERK1/2/Fra-1/SLUG signaling pathway. Clinically, high expression of CRYAB on tumor cells was associated with lymph node metastasis and tumor stage (III–IV) (156). In human and mouse tumors TAM accumulation correlated with the expression of integrin αvβ3 on cancer cells, a known driver of epithelial cancer progression and drug resistance (164). In mouse model of Lewis lung carcinoma (LLC), macrophage depletion with clodronate in combination with genetic ablation of CCR2 and CX3CR1 (receptors responsible for monocyte recruitment) inhibited cancer cell growth and metastasis enhancing survival in mouse (160). In human lung cancer samples from 72 NSCLC patients, intratumor CD68+ TAM infiltration and CCR2 expression correlated with tumor stage and metastasis (160).
Total Amount of TAMs in Lung Cancer Progression
Lung macrophages are major component of lung tissue due to their essential role in the clearance of the infectious and non-infectious contaminants of the air (77). Due to their high abundance, their increased amount is not the critical factor for the progression of lung cancer. However, there are still some reports in Chinese cohorts where the correlation of CD68+ cells with clinical parameters of lung cancer was examined ( Table 4 ).
Table 4.
Representative studies demonstrating the association of TAMs with tumor progression parameters in lung cancer.
| Cohort of patients | Method of detection | TAM correlation with tumor growth and stage | TAM correlation with lymphatic and hematogenous metastasis | TAM correlation with survival | Reference |
|---|---|---|---|---|---|
| 68 NSCLC patients (China) | IHC (not specified) | Positive CD68+ expression correlates with higher TNM stage (III and IV) | Positive CD68+ expression correlates with the presence of LN metastases | Not studied | (165) |
| 160 NSCLC patients (Japan) | IHC (manually) | High stromal (>380/mm2 in ×400 HPF) and alveolar CD163+ TAM densities (>400/mm2) are indicative for increase of CRP level up to 2 times, increase of invasive size by 20–45%, poor differentiation and advanced stages (II and III) | 1,4-fold increase of stromal and alveolar CD163+ TAM densities is indicative for tumors with N1–N3 nodal status vs. cases without LN metastases | In early stages (0 and I), high stromal CD163+ TAM density correlates with reduced DFS rate by 20% and OS by 12%. In advanced stages (II and III), high alveolar CD163+ TAM density correlates with reduced DFS rate by 22% and OS by 17% |
(166) |
| 335 NSCLC patients (Danmark) | IHC (digital imaging scanning) | Not significant | twofold increase of median area fraction of CD163+ TAMs in tumor nest and 1.5-fold increase in tumor stroma are found in cases with N1/N2 nodal status vs. those without LN metastases | Not significant | (167) |
| 297 NSCLC patients (Japan) | IHC (digital imaging scanning) | Increase of stromal CD68+ and CD204+ TAM amounts above the medians (48 and 15, respectively, under ×200) positively correlates with Ib-IV stages and G2-G4 histological grade | High amount of CD68+ (>48) and CD204+ (>15) TAMs correlates with pleural invasion and LN metastasis | High amount of CD68+ (>48) and CD204+ (>15) TAMs in tumor stroma correlates with decreased DFS rates by 10% | (168) |
| 553 primary NSCLC patients (Norway). | Multiplexed-IHC in TMA (digital imaging scanning) | Increase of stromal HLA-DR+/CD68+ TAM amount >1.0 under ×200 is indicative for lower T stages (T1 and T2) | Not studied | High amount of intratumoral and stromal HLA-DR+/CD68+, CD204+ and CD68+ TAMs correlates with increased DSS rates (appr. by 10-20%) | (169) |
| 80 NSCLC patients (Lithuania) | IHC (manually) | High amount of CD163+TAMs is found in tumors with poor differentiation (median 118 per 10 HPFs under ×400) versus moderate and well differentiated (median 108) | High amount of stromal CD68+ TAMs is found in tumors with N1-N3 nodal status (median 77 per 10 HPFs under ×400) vs. cases without LN metastases (median 64) | High CD68+iNOS+ and low CD68+ CD163+ amount correlates with increased OS rates by almost 50% | (170) |
DFS, disease-free survival; DSS, disease-specific survival; CRP, C-reactive protein; HPF, high-power field; IF, immunofluorescence; IHC, immunohistochemistry; LN, lymph node; NSCLC, non-small lung cancer; TAMs, tumor-associated macrophages; OS, overall survival; RFS, recurrence-free survival; TMA, tissue microarray.
Thus, in patients with NSCLC, the expression of CD68 in tumor tissue was significantly higher in comparison with normal tissue, and high amount of CD68+ macrophages positively correlated with a higher TNM stage, peritumoral LVD, and LN metastasis (56, 165). Association between infiltration of CD68+ macrophages and EGFR-status was demonstrated in study of 105 surgically resected tumor samples (50 EGFR mutated and 55 EGFR wild-type) (171). CD68+ cells within the tumor niche exhibited more intensive infiltration in wild-type EGFR than in mutated tumors, and were related to lymph node invasion (171).
Similar to breast cancer the intratumoral localization of TAMs can have distinct role on the prognosis. IHC analysis of 99 patients with NSCLC demonstrated that the number of CD68+ macrophages in the tumor islets was positively associated with OS, whereas the number of macrophages in the tumor stroma was negatively associated with OS (172). However, specific phenotypes in tumor islets and stroma were not identified in this study, and the role of CD68+ TAM amounts in lung cancer metastasis was not clarified.
Subpopulations of TAMs in Lung Cancer Progression
TAM phenotype in lung cancer is characterized mostly by M2-like markers, such as CD163, CD204, and MARCO. A number of studies demonstrated that M2 macrophage phenotype positively correlates with poor survival and efficient development of metastasis in lung cancer. In order to elucidate the biological and clinical significance of M2 TAMs, a comprehensive clinical study that assessed tissue distribution of CD163+ TAMs in tumor stroma, tumor islets, and alveolar space in 160 NSCLC patients from the Japanese cohort was performed (166). Thus, high stromal and alveolar density of CD163+ TAMs significantly correlated with the C-reactive protein (CRP) level in circulation, the Ki-67 proliferation index and invasive size, tumor differentiation, lymph node metastasis and pathological stage (166). The DFS and OS were significantly lower in patients with high infiltration of stromal and alveolar CD163+ TAMs. The islet CD163+ TAMs were not associated with these parameters (166). Availability of all quantitative parameters in this study used as thresholds for TAM density in stromal and alveolar compartments merits our attention as an example of scientific transparency and clarity (166).
A study of 335 patients with stage I–IIIA NSCLC from the Danish cohort revealed the association of the density of CD163+ macrophages in tumor nests and stroma with elevated CRP level and LN metastases, but no correlation with RFS or OS was found (167). The significant accumulation of CD163+ TAMs in malignant pleural effusion of lung cancer patients closely correlated with reduced PFS (173). CD163+ macrophages were the predominant macrophage subpopulation detected in bronchoalveolar lavage fluid (BALF) from lung cancer patients (174, 175). However, no significant correlation of CD163+ macrophages in BALF with clinical and pathological parameters was found, indicating prognostic role of CD163+ TAMs in tumor tissue, but not in BALF.
In contrast to other tumor types that are considered in the present review, most pronounced prognostic significance of CD204+ macrophages in lung cancer was shown in a number of studies of Japanese cohort of patients ( Table 4 ). Thus, in 297 samples obtained from patients with NSCLC, high density of CD68+ or CD204+ TAMs (assessed independently by IHC) in tumor stroma, but not in tumor islets or alveolar space, positively correlate with an advanced disease stage and histological grade, pleural invasion, node status, and wild-type EGFR gene status, and poor DFS of NSCLC patients (168). Similarly, CD204+ macrophages in the tumor stroma of 201 patients with lung adenocarcinoma positively correlated with tumor differentiation, pathologic stage, T status, nodal involvement, lymphatic permeation, vessel invasion, and pleural invasion (176). Besides, the numbers of CD204+ macrophages significantly correlated with microvessel density and the numbers of Foxp3+ lymphocytes and the expression levels of IL-10 and MCP-1 (176, 177). High levels of CD14+CD204+ cells in the pulmonary vein (PV) of patients with NSCLC were identified in cases of early recurrence and were positively related to the expression of CD204 in the tumor stroma of 207 stage I lung adenocarcinoma patients from Japanese cohort (178).
Controversial data have been obtained in a Norway study of 553 primary NSCLCs. It was found that high levels of CD204+ M2 as well as CD68+/HLA-DR+ M1 and CD68+ infiltration in stromal and intratumor compartments were independently associated with improved NSCLC-specific survival (169). HLA-DR+/CD68+ M1 TAM level significantly decreased from pathological stage I to stage III. In lymph nodes, the intratumoral level of HLA-DR+/CD68+M1 was an independent positive prognostic indicator (169). Technologically, this study differed from the previous ones by using multiplex chromogenic immunohistochemistry in tissue microarrays.
MARCO was defined as one more M2 marker of TAMs in lung cancer. Multiplex immunofluorescent staining of tumor samples from NSCLC Swedish patients demonstrated the co-localization of CD68, CD163, and MARCO (179). Co-staining of PD-L1, MARCO, and CD68 revealed MARCO+ TAMs are in direct contact with PD-L1+ tumor cells and demonstrated co-localization of MARCO and PD-L1 in TAMs (179). RNA-seq analysis of 199 tumor tissues from the same Swedish cohort showed the positive correlation of MARCO gene expression with the expression of genes associated with immunosuppressive TAMs (CD163, CD204, IL4R, CHIA, TGFB1, and IL10), genes of regulatory T-cells (FOXP3, TGFB1, IL10, EBI3, PDCD1, and CTLA4), genes of exhausted T-cells (PDCD1, CTLA4, TIGIT, BTLA, HAVCR2, and LAG3), genes of cytotoxic T-cells (CD8A, PRF1, GZMA, and GZMB) and genes of immune checkpoint molecules PD-L1, VISTA, PD-1, and CTLA4 (179). MARCO-expressing TAMs which may be considered as a specific macrophage subpopulation contributed to an immunosuppressive mechanism protecting cancer cells.
The distribution of M1 and M2 macrophages in tumor islets and tumor stroma may differ and can be associated with survival rates in NSCLC patients (170). Thus high infiltration of M1 macrophages (CD68+iNOS+) in tumor islets was associated with increased overall survival (OS) in NSCLC, while high infiltration of total M2 macrophages (CD68+CD163+) in tumor islets and stroma was associated with reduced OS in NSCLC (170).
In lung cancer TAMs have a great heterogeneity, and a number of studies demonstrated the prognostic value of TAMs expressed specific markers. For example, TAMs isolated from 96 primary lung cancer tissues displayed the elevated level of cathepsin K, COX-2, MMP-9, PDGF-B, uPA, VEGFA, and HGF (180). MMP9 and VEGF expression was significantly higher in patients with LN metastasis and lymphovascular invasion (180). Recently, using LLC-induced tumors of MafB-GFP knock-in heterozygous mice, transcription factor MafB was detected to be specifically expressed in CD204+ TAMs (181). Immunostaining analysis of human lung cancer tissue revealed that MafB is expressed in the same region and mostly in severe samples together with CD204+ and CD68+ TAMs (181). In peripheral blood collected from patients with lung carcinoma, B7-H4-expressing CD68+ macrophages were found. The level of B7-H4-expressing macrophages was significantly higher in lung cancer patients in comparison with healthy donors and was related to tumor size, lymph node metastasis, and TNM stage (182). CD68+ macrophages also expressed the protein V-set and Ig domain-containing 4 (VSIG4), a novel B7 family-related macrophage protein which has the capacity to inhibit T-cell activation; however, no correlations of VSIG4+ TAMs with patient’s outcome was found up to this date (183). Triggering receptor expressed on myeloid cells (TREM)-1, a molecule crucial for the triggering and amplification of inflammatory response was found to be expressed on TAMs in NSCLC. TREM-1+ TAMs in tumor tissue of patients with NSCLC were associated with reduced DFS and OS (184). SPP1 expressed by TAMs was indicated as an independent predictor for OS and DFS, especially for stage I NSCLC (185). TMA analysis of 159 lung cancer tissue samples demonstrated that MVD was increased in patients with positive expression of SPP1 in TAMs compared with that in the SPP1-negative group (185). IHC analysis of 213 cases of human lung adenocarcinoma specimens revealed that PD-1 is preferentially expressed by CD163+ TAMs in the tumor stroma, and these stromal PD-1+ TAMs were an independent predictor of reduced survival in lung cancer patients (57). Furthermore, PD-1+ TAMs possess a unique transcriptional profile as compared to PD-1− TAMs as was shown in mouse allografts of lung adenocarcinoma (57).
TAMs and Lung Cancer Treatment
The primary treatment for early stage lung cancer (Stages I and II) is surgery which provides long-term survival in patients. Five-year OS after surgical resection is 60–80% for patients with stage I NSCLC and 30–50% for patients with stage II NSCLC (151). In patients with unrespectable tumors, primary radiotherapy is used. The platinum-based chemotherapy used in adjuvant regimen is beneficial for stage II NSCLC patients (151).
For advanced lung cancer (Stage IV) the treatment with platinum (cisplatin or carboplatin)-based chemotherapy in combination with taxanes (paclitaxel, docetaxel, or vinorelbine), antimetabolites (gemcitabine or pemetrexed), or vinca alkaloids (vinblastine) is recommended as a first-line therapy (151, 153).
Lung cancer cells can carry mutations in a number of proto-oncogenes including KRAS, EGFR, BRAF, PI3K, MEK, and HER2, making targeted drug to be attractive treatment strategy (152, 153). The first of the approved targeted drugs for NSCLC patients are anti-EGFR agents, tyrosine kinase inhibitor (TKI) Erlotinib (Tarceva) and gefitinib (Iressa). Gefitinib might be recommended as a first-line therapy for patients with EGFR mutations, while chemotherapy is preferred if EGFR mutation status is negative or unknown. Anti-VEGF inhibitor (Bevacizumab) is also used for the treatment of lung cancer (151). Bevacizumab in combination with first-line platinum-based chemotherapy showed significantly improved response rates, PFS, and OS compared to chemotherapy alone (153). Several clinical trials investigated therapeutic approaches that combine Immune Checkpoint Inhibitors (anti-CTLA4, anti-PD1, anti-PD-L1) and chemotherapy in NSCLC (152). However, resistance to these treatments frequently occurs that makes the development of new antitumor strategies based on immunomodulation highly relevant.
Contradicting results are available for the association between macrophage polarization and the antitumor effect of distinct drugs (e.g. chemotherapy, tyrosine kinase inhibitors) ( Table 7 ). In patients with stage II/III NSCLC (USA cohort), treated by platinum-based NAC, density of CD68+ TAMs was higher than in untreated patients (186). In NAC treated patients higher levels of TAMs both in tumor nest and stroma were associated with better OS (186). In contrast, low total macrophage number defined by CD68 expression is an independent factor for better DFS in pN2 stage IIIA NSCLC patients receiving neoadjuvant chemotherapy (NAC) (cisplatin/docetaxel) from the Chinese cohort (187). However, high tumor islet/stromal macrophage ratio was significantly associated with longer DFS and OS (187). In a French study of 122 stage III-N2 NSCLC patients treated with cisplatin-based chemotherapy, no correlation of CD68+ TAMs with survival rates was found (188). These data indicated TAMs located in tumor nest (islets) as a favorable prognostic factor after platinum-containing chemotherapeutic treatment.
Several studies indicated the influence of chemotherapy on circulating monocytes in lung cancer. Thus, the absolute number of total CD14+ monocytes (taken before treatment) in peripheral blood of patients received adjuvant cisplatin-based chemotherapy was significantly increased in patients with progressive disease (PD) after chemotherapy in comparison to patients with partial response (PR) or stable disease (SD) (189). Percentage and absolute number of CD14+HLA-DR−/low MDSCs were significantly increased in patients with PD compared with patients with PR and SD after chemotherapy (189). Besides, the low amount of CD14+HLA-DR−/low cells was associated with longer PFS (189). Significant increase of IL-1beta (M1 cytokine) and significant decrease of IL-1ra (M2 cytokine) production by alveolar macrophages isolated from BALF after platinum-based chemotherapy were demonstrated in patients with small cell lung cancer from the Japanese cohort (190). It was also found that platinum-containing drug oxaliplatin induced immunogenic cell death (ICD) in LLC cells, activating dendritic cells with CD80+CD86+ phenotype and enhancing cytotoxic CD8+ T cells in LLC tumor tissues, which resulted in tumor regression in a mouse model of lung cancer (191). However, no effect of oxaliplatin on macrophages was investigated in this study (191).
Tyrosine kinase inhibitors (TKIs) were found to have an impact on the polarization of TAMs. In the study of 206 stage IIIb or IV NSCLC patients treated with EGFR-tyrosine kinase inhibitors (gefitinib or erlotinib), stromal TAMs were the predominant CD163+ TAMs (192). Among all patients as well as patients with EGFR mutation, TAM density was significantly related to poor PFS and OS (192). In contrast, in LLC-derived mouse model, Gefitinib (EGFR inhibitor) and Imatinib (tyrosine kinase inhibitor) inhibited the M2-like polarization of macrophages by reducing expression of CD206 and CD163 and M2-like genes (Arg1, Mgl2, Ym1, Fizz1, IL-10, CDH1, CCL2). This promotes anti-metastatic effect of Gefitinib and imatinib (193, 194). The combination of Gefitinib/simvastatin with anti-PD-L1-modified liposomes or with Vorinostat (histone deacetylase inhibitor) demonstrated better antitumor effect by repolarization of macrophages (inhibition of CD206, ARG-1 expression and activation of CD86, iNOS expression, and ROS production) and inhibition of revascularization (downregulation of VEGF, HIF-1a and CD31 expression) in lung cancer cell lines (195, 196). Vorinostat had an impact on TAM re-polarization. In mouse lung tumor tissues, the percentages of F4/80+ CD206+ cells and CD68+CD206+ cells were decreased at the 7th day after the administration of Imatinib (194).
Recent case report is available that suggested that TAMs in lung cancer can be a predictor of a positive response to anti-PD-1 antibodies (nivolumab) in patents with EGFR-mutated lung cancer (197). In this case report a 72-year old male patient with lung adenocarcinoma (cT1bN2M0, cStage IIIA) was harboring anEGFRexon19 deletion. The patient was subjected to right upper lobectomy after NAC. Twelve months after the surgery, recurrence of multiple brain metastases was identified, and the brain lesions were treated with γ-knife therapy. Thirteen months after radiosurgery, multiple lung metastases have been identified by CT. Chemotherapies, including EGFR tyrosine kinase inhibitors (TKIs), erlotinib, carboplatin plus paclitaxel, and docetaxel, were then administered consecutively. Erlotinib as second-line therapy was continued for seven months with a partial response. However, multiple lung metastatic lesions regrew. Although, the PD-L1 expression was negative, nivolumab was administered as sixth-line therapy. After seven cycles of nivolumab administration, the patient has continued treatment with nivolumab for more than two years with no evidence of tumor regrowth or serious immune-related adverse events (197). TAMs were analyzed in lung tumor by IHC, and CD68, CD206 and PD-L1 expression was detected (197). However, this study does not provide any evidence for the dynamic changes of TAM amounts or phenotypes in primary tumor and metastatic sites and also during different chemotherapy approaches. The presence of TAMs does not explain their role in the tumor spread and response to various chemotherapy approaches. In lung cancer patients of Italian cohort, CD163+CD33+PD-L1+ macrophages with epithelioid morphology (alveolar macrophage-like) defined by the authors as “complete immunophenotype,” were detected in all patients with hyperprogression. The authors suggested that CD163+CD33+PD-L1+ TAMs are statistically significantly associated with hyperprogression compared to patients without hyperprogression (198). However, it is hard to understand whether CD163+CD33+PD-L1+ TAMs can be also found in small amounts in patients without hyperprogression. These reports show that our knowledge about the role of TAMs in response to various types of chemotherapy as well as to immunotherapy in patients is strictly limited. They highlight the urgent need to intensify investigations in this field.
In summary, several lines of evidence show that TAMs can improve the response of lung cancer patients to chemotherapy, in particular their higher amount in tumor nest in case of platinum-based chemotherapy. Increased amount of circulating monocyte that can be recruited to tumor mass and differentiate into TAMs is rather a negative factor for the patient response to cisplatin-based chemotherapy. TAMs correlated with poor response to EGFR-tyrosine kinase inhibitor Gefitinib, while in mouse models Gefitinib induced re-polarization of TAMs to antitumor phenotype. The role of TAM in immunotherapy of lung cancer needs careful analysis. The mechanism of TAM interaction with of anti-lung cancer treatments has to be identified in order to develop new immunomodulating approaches.
TAMs and Ovarian Cancer
Ovarian cancer (OC) is the most lethal gynecological cancer (199). Around 300 thousand new cases of ovarian carcinoma are diagnosed worldwide in 2018, with around 184 thousand deaths (34). The origin of more than 90% of malignant ovarian tumors is epithelial. Epithelial OC is a heterogeneous disease with histological subtypes that differ by cellular origin, pathogenesis, and prognosis (199, 200). Epithelial OC consists of five main histotypes: high-grade serous (HGSOC; 62%), endometrioid (ENOC; 20%), clear cell (CCOC; 8%), mucinous (MOC; 5%), and low-grade serous (LGSOC; 5%) (199, 200). High-grade serous ovarian carcinoma (HGSOC) is often diagnosed at the late stages and exhibits the highest aggressiveness and mortality (201).
The biological behavior of ovarian carcinoma differs from other tumors by the pattern of hematogenous metastasis through transcoelomic dissemination of tumor cells via the peritoneal fluid (202, 203). In ascite, cancer cells detached from the primary tumor obtain EMT phenotype, form multicellular spheroids and attach preferentially on the abdominal peritoneum or omentum through a passive mechanism, carried by the physiological movement of peritoneal fluid (203). Floating spheroids form a continuously repopulated chemoresistant niche, that leads to the high mortality of patients with cure rate of only 30% (203).
There are no effective criteria to diagnose OC at early stages, and screening tests for ovarian cancer are limited in sensitivity. Therefore, up to 70% of cases are detected at the advanced stages (204). The five-year survival of patients with disseminated tumors is only about 25% at the stage III and not more than 5% at the stage IV stage (according to International Federation of Gynaecologists and Obstetricians (FIGO) (205). Despite a good response of disease to the first line of standard platinum/taxane-based chemotherapy (cisplatin or carboplatin and paclitaxel or docetaxel), development of recurrence associated with multidrug resistance is detected within a short period in 70% patients (206). Moreover, it was shown that these chemotherapeutic agents, as well as anthracyclines and cyclophosphamide, can contribute to metastasis (206). It is not excluded that such pro-metastatic effect can be due to the detrimental effects of the therapeutic agents on the components of TME, including TAMs. However, the effects of chemotherapeutic agents on TAMs in ovarian cancer remain to be investigated. So it is necessary to develop more effective approach to cure the patients who have acquired drug resistance during standard chemotherapy, and this approach has to include programming of intratumoral immunity.
TAMs in Ovarian Tumors and Metastasis
By analysis of the role of macrophages in OC progression both TAMs infiltrating tumor mass and TAMs intimately interacting with cancer cells in ascitic fluid should be taken into account.
The total number of TAMs as well as specific subpopulations in the tumor mass was examined in the patient cohorts from a broad spectrum of countries, including UK, Italy, Canada, China, Korea. The correlation of TAMs with clinical-pathological parameters (TNM stage, histotypes, lymph node metastasis, hematogenious metastasis) and survival rates was analyzed. Similar to breast cancer, a number of studies demonstrated positive correlation of TAMs with poor prognosis in OC. However, in contrast to breast cancer, CD68 was not frequently used as TAM marker to evaluate TAM levels ( Table 5 , Figure 1 ). Thus, in the study of 332 patients with high-grade serous ovarian carcinoma (HGSOC) from the UK cohort, stromal CD68 expression was found to be positively associated with survival rates (207). In 112 ovarian cancer patients from the Chinese cohort, intratumoral CD68+ TAM density significantly increased with increasing cancer stage and grade, however, displayed no prognostic significance in both the Kaplan–Meier survival and multivariate Cox regression analyses (208).
Table 5.
Representative studies demonstrating the association of TAMs with tumor progression parameters in ovarian cancer.
| Cohort of patients | Method of detection | TAM correlation with tumor growth and stage | TAM correlation with lymphatic and hematogenous metastasis | TAM correlation with survival | Reference |
|---|---|---|---|---|---|
| 332 HGSOC patients (UK) | IHC in TMA (digital imaging scanning) | Not studied | Not studied | High amount of stromal CD68+ TAMs is associated with increased OS rate by 15% | (207) |
| 112 ovarian cancer patients (China) | IHC (manually) | 1.6–2.0-fold increase of CD68+ and CD163+ TAM densities is found in tumors with grade G3 vs. grade G1. Decrease in M1/M2 TAM ratio is observed from stage I (1.4 ± 0.5 cells/mm2) to stage IV (1.0 ± 0.5) | Not studied | Increase of overall M1/M2 ratio above the mean 1.731 is associated with increased 5-year OS by 19.7% | (208) |
| 110 EOC patients (China) | IHC (manually) | 1.7-fold increase of CD163+ TAM amount is found in tumors with grade 2–3 (median = 79 cells) compared to grade 1 (median = 47 cells) | Not studied | Increase of CD163+ TAM amount above the median (76 cells per ×400 HPF) correlates with decreased PFS rate by 25.7% and OS rate by 26.9% | (209) |
| 140 ovarian cancer patients (Italy) | Flow cytometry | Not studied | Not studied | High M1/M2 ratio (defined as 1.4) is associated with prolonged OS by 16 months, and PFS – by 15 months compared to low M1/M2 ratio (< 1.4). | (210) |
| 199 HGSOC patients (Canada) | IHC of TMA (digital imaging scanning) | Not studied | Not studied | Increased CD206+/CD68+ ratio correlates with decreased OS and PFS rates by 40% | (58) |
DFS, disease-specific survival; DSS, disease-free survival; HPF, high-power field; IF, immunofluorescence; HGSOC, high-grade serous ovarian cancer; IHC, immunohistochemistry; LN, lymph node; TAMs, tumor-associated macrophages; OS, overall survival; RFS, recurrence-free survival; TMA, tissue microarray.
Subpopulations of TAMs in Ovarian Cancer Progression
The association of macrophage polarization with survival of ovarian cancer patients was demonstrated in numerous studies that used M1 and M2 markers for the phenotyping of TAMs or M1/M2 ratio ( Table 5 ). Meta-analysis of nine studies (eight from Chinese cohorts and one from USA cohort), including 794 patients, revealed that higher M1(iNOS+ or HLA-DR+)/M2(CD163+) ratio, but not just CD68 or CD163 expression in tumor tissues, was associated with a favorable OS (211). Besides, elevated M1/M2 ratio predicted better PFS of ovarian cancer (211). In contrast, worse PFS was associated with high density of CD163+ TAMs and higher ratio of CD163/CD68. High density of CD163+ and CD68+ TAMs was observed in OC with advanced TNM stage (211). IHC analysis of 110 Chinese patients with stages III–IV epithelial ovarian cancer revealed that PFS and OS rates were higher in the low-CD163 expression group than in the high-CD163 expression group (209). CD68 expression did not show significant differences, while the high CD163/CD68 ratio was a negative predictor for PFS and OS (209). In the study of the Chinese cohort that enrolled 112 OC patients, the M1 (HLA-DR+)/M2(CD163+) ratio also positively correlated with 5-year survival rates (208). Decrease in M1/M2 ratio was observed in cancer specimens from Stage I through Stage IV. At the same time, high number of CD163+ TAMs was associated with increasing cancer stage and the size of the residual site (208). In patients from the Italian cohort a positive relationship between the M1(CD14+CD80+)/M2(CD14+CD163+) ratio and OS and PFS was found in patients with HGSOC and patients with other histotypes or ovarian metastases (210). High serum levels of CD163 in Korean patients with EOC were associated with advanced stage and with short DFS and OS (212). The density of CD206+ macrophages was not prognostic, but a higher ratio of CD206+/CD68+ cells was strongly associated with worse PFS and poorer OS that was found by IHC analysis in a cohort of 199 HGSOC patients from the Canadian cohort (58).
There is evidence about the differences in TAM clinical value between different histological types of ovarian cancer. Thus, the numbers of CD68+ macrophages, as well as the numbers of macrophages positive for M2 markers (CD163 and CD204) in borderline and malignant tumors were significantly higher in both serous and mucinous ovarian tumors than in benign tumors (213). As for serous carcinoma, total CD68+ macrophage infiltration together with CD163 expression was significantly increased in high-grade serous ovarian cancer (HGSOC) compared to low-grade serous ovarian cancer (LGSOC) (214). At the same time LGSOC had significantly lower microvessel density assessed by CD31 and lower MMP9 expression (214).
Other studies found the associations of macrophages expressed different specific factors with clinical and pathological parameters in ovarian cancer ( Table 1 ). In peripheral blood of 51 patients with pathologically diagnosed ovarian cancer the proportion of PD-L1+ CD68+ cell among CD68+ cells and the intensity of PD-L1 staining on CD68+ cell were significantly higher in the ovarian cancer group in comparison with the healthy group (215). Besides, these parameters were increased at the late stage cancer (stages III–IV) compared to early stage cancer (stage I–II) (215). IHC and immunofluorescent analysis of tumor samples from 102 OC patients of Chinese cohort showed that reduced ratio of M1(HLA-DR+ or iNOS+)/M2(CD163+ or VEGF+) TAMs and the increased densities of COX-2+ TAMs were the predictors of poor prognosis (216).
B7-H4 (the member of the B7 family of T cell costimulatory molecules, is a negative regulator of T cell responses) was found to be expressed by TAMs in ovarian cancer. Primary ovarian tumor cells express intracellular B7-H4, whereas TAMs have surface B7-H4 expression (217). B7-H4+ tumor macrophages expressed higher levels of CD86 than B7-H4-tumor macrophages, but the expression of other molecules responsible for T cell activation (HLA-DR, HLA-ABC, CD40, and CD80) did not differ. In vitro and in vivo, B7-H4+ TAMs, but not cancer cells, suppressed T cell immunity. Blocking B7-H4, but not arginase, inducible nitric oxide synthase or B7-H1 restored the T cell stimulating capacity of the macrophages and contributed to tumor regression in vivo (217).
Gene chip analysis showed that human TAMs express significantly higher levels of insulin-like growth factor 1 (IGF1) than undifferentiated M0 myeloid cells (218). In vitro TAMs may increase the proliferation and migration of ID8 mouse EOC cells by upregulation of IGF1. Blockade of the IGF1 pathway in ID8 cells with an IGF1 neutralizing antibody effectively inhibited the proliferation and migration of ID8 cancer cells (218). Using histological data obtained from 395 EOC patients, it was found that CD163+ TAM infiltration correlates with higher expression of ZEB1 that drives EMT in ovarian cancer cells (219). ZEB1 expression was identified in TAMs, and ZEB1+TAMs correlated with poorer survival and higher expression of CCR2 and MMP9 in patients with EOC. Mouse TAMs that expressed Zeb1 were prone to the polarization toward an F4/80low pro-tumor phenotype and accelerated tumor growth (219). IHC study of 108 samples from patients with EOC demonstrated that CD68+ TAM infiltration and high-mobility group box protein 1 (HMGB1) expression closely correlated with lymph node metastasis and with poor OS (220). In vitro, TAMs isolated from ascites of EOC patients and HMGB1 facilitated lymphangiogenesis by inducing LEC proliferation, migration, and capillary-like tube formation (220).
Ascitic TAMs in Metastasis of Ovarian Cancer
In ovarian cancer TAMs have a clinical significance not only by infiltrating tumor mass but also by the interacting closely with cancer cells in ascites. Ascite, which is a hallmark of OC, contains a large number of components of unique peritoneal TME, including tumor spheroids and immune cells, such as TAMs and T cells (201, 202). Experimental mouse models have demonstrated that TAMs constitute a major cell fraction in ascites that support the survival of cancer cells and promote cancer progression, chemoresistance, and immunosuppression (202, 204, 221–223).
Interestingly, TAMs were found to maintain transcoelomic metastasis by tumor spheroids (221). As was shown, in tumor spheroids isolated from 128 patients (USA cohort) with advanced stage OC, higher amounts of CD68+ macrophages were found in poorly differentiated OC compared with more-differentiated OCs, and their amount correlated with lymphovascular invasion (LVI) and ascite volume. High number of CD68+ macrophages in these spheroids was significantly associated with lower 5-year OS of patients (221). In a mouse model of ovarian cancer, EGF, secreted by TAMs, promoted early transcoelomic metastasis. Immunostaining of mouse tumor spheroids isolated from ascite, confirmed that EGF was specifically detected in TAMs that were surrounded by EGFR+ tumor cells. Pharmacological blockade of EGFR or neutralizing antibody for ICAM-1 in TAMs blunted spheroid formation and ovarian cancer progression in mouse models. These findings suggest that TAMs play an essential role in spheroid formation during the process of transcoelomic metastasis of OC (221).
The possibility to isolate high amount of pure macrophages from the ascitic fluid enables high throughput analysis of their transcriptome and proteome. The transcriptomic and proteomic analysis of TAMs in ascites of OC patients was performed in detail by the group of R. Muller (224–226). Transcriptomic analysis (RNA-seq) of TAMs isolated from 18 ascites of ovarian cancer patients (Germany cohort, serouse, and clear cell carcinoma) revealed two signatures of expressing genes: signature A, characterized by the hyperexpression of pro-tumor markers (CD163, PCOLCE2, IL6) related to ECM remodeling and signature B with low expression of pro-tumorigenic and immunosuppressive markers and an upregulation of genes linked to interferon signaling (225). It was shown that subgroup A of TAMs correlated with a short OS, while subgroup B linked to a favorable clinical outcome in OC patients (225).
RNA-seq analysis also revealed that CD163+ or CD206+ TAMs isolated from the ascites of HGSOC patients (Germany cohort) have elevated expression of protumorigenic growth factors and cytokines, e.g. CCL18, KITLG, SEMA6B, S100B, and VEGFB and downregulated tumor suppressive mediators, e.g. CXCL10, CXCL11, IL15, TNFSF10, and TNFSF14 (226). The increased expression of proteins involved in ECM remodeling (ADAMTS2, CTSB, FBLN5) and complement factors (C1QC and CR1L) was also found in CD163 or CD206-expressing TAMs. TAMs from ascites also produce CCL5, CXCL8, IL1RN, CCL18, CXCL2, CXCL3, acting as a chemokines for the monocyte/macrophage recruitment (226). The gene expression of IL10, TGFbeta1, S100A8, S100A9, and IL10RA was upregulated in TAMs compared to tumor cells isolated from the ascites of OC patients (227).
Surprisingly, flow cytometry analysis identified that neither CD163 nor CD206 distinguishes TAMs (from ascite of 79 OC patients) from resident peritoneal macrophages (pMPHs) (from 11 patients undergoing hysterectomy for non-malignant diseases (ovarian cyst, uterine myomatosis, endometriosis) (224). RNA-seq data confirmed that TAMs closely resemble pMPHs (224). Both TAMs and pMPHs expressed a number of macrophage markers, including phagocytosis-associated receptor genes (CD36, MSR1, SCAR family genes,TIMD4, CD163), FCGR genes, complement receptor genes (CD93/C1Q-R1, C3AR, CR1, C5AR1), and polarization marker genes (IL10). However, upregulation of ECM remodeling genes (COL family genes, LUM, PCOLCE2) was selectively observed only in ovarian cancer TAMs (224). The limitation of this study may be due to the comparison of TAMs from OC patients and pMPHs from the patients with non-malignant diseases, but not pMPHs from healthy donors.
TAMs and Ovarian Cancer Treatment
Patients with stage I ovarian cancer undergo surgery. Treatment of stages II–IV of epithelial OC includes complete surgical resection, followed by platinum-based chemotherapy. Another option is NACT, interval cytoreductive surgery, followed by adjuvant platinum/taxane chemotherapy (228, 229). Platinum and taxane combination as chemotherapeutic treatment showed improved survival in early stage OC of high-grade lesions (216). In the past 2 years the interest to the problem of the interaction of chemotherapy and TAMs in OC has been increased and some novel data were accumulated.
Cisplatin is a most frequently used conventional drug in ovarian cancer patient (228). In vitro, cisplatin stimulated human macrophage-like THP-1 to become classically activated (CAMs) and to produce CCL20, chemokine ligand 20 (macrophage inflammatory protein-3 (MIP3A), that activates CCR6 on ovarian cancer cells, promoting EMT and migration (230). Cisplatin has only limited effect on the polarization of CAMs, by increasing IL-1β expression, but not affecting other typical M1 (TNFα, iNOS) and M2 (IL-10, ARG-1, CCL18) polarization markers. The specific blockade of CCL20 on CAMs as well as inactivation CCR6 on tumor cells by siRNA diminished cisplatin-induced cancer cell migration. Thus, a novel pro-migration mechanism driven by the crosstalk between cisplatin and CAMs, allow to consider the CCL20-CCR6 axis for therapeutic targeting to reduce chemotherapy-induced metastasis in advanced stage ovarian cancer (230). In vitro in co-culture of THP-1 macrophages and A2780 cancer cells, cisplatin downregulated expression of CD274, IL-6 and HLA-DRA without inducing M2-type markers in M1-type macrophages, while doxorubicin caused the decrease in HLA-DRA and increase in CD206 (231). In M2 macrophages, downregulation of CD163 and IL10 under doxorubicin treatment was observed (231).
Recently molecular profiling of more than 500 genes was performed, and 22 immune subsets were estimated with computational analysis CIBERSORT in 13 studies that enrolled 2,218 patients with HGSOC, who underwent platinum-based chemotherapy. As was found, a high fraction of M1 and M0 macrophages was associated with favorable OS, whereas the M2 macrophages conferred worse OS that was found by CIBERSORT approach (232). In the study from Netherlands, which enrolled 69 peritoneal samples from patients with HGSOC who underwent NAC, an increase in CD3+ cells in peritoneal metastases of HGSOC was observed and an increase of CD3+ and CD8+ cells was associated with improved PFS and OS; however, no correlation between TAM number and outcome was found after NAC (233). Patients with HGSOC from the Italian cohort treated with adjuvant cisplatin-based chemotherapy (cisplatin/carboplatin + Taxol + bevacizumab) had a significantly higher M1/M2 ratio in platinum-sensitive tumors compared to platinum-resistant tumors (210) ( Table 7 ).
Paclitaxel is the antitumor agent which enables the rearrangement of microtubules resulting in cell cycle arrest in tumor cells (2). Paclitaxel can also program the immune system for tumor inhibition. The microarray analysis of tumors derived from OC patients undergoing paclitaxel chemotherapy revealed that paclitaxel exposure results in the increase in genes linked to the M1 macrophage activation profile (IFNg-stimulated macrophages) in comparison with gene profile before treatment (234). In vitro TAM phenotype skewed to M1-like one mediated by TLR4 innate immunity receptor. This study endows new evidence that the antitumor effect of paclitaxel occurs in part via reactivation of the immune response against cancer, with repolarization of TAMs toward the M1-like antitumor phenotype (234).
In vitro and in vivo treatment with paclitaxel and carboplatin increased MCP-1 expression in ovarian cancer cells that is known to be responsible for inducing macrophage migration (235). Chemotherapy with paclitaxel or carboplatin may generate debris in ID8 ovarian cancer cells which triggers macrophage production of the proinflammatory cytokines TNF-α, MIP-2/CXCL2, MIP-1β/CCL4, CCL2/MCP-1, as well as sICAM-1/CD54 and G-CSF (236). Cytokine storm induced by debris-stimulated macrophages was prevented by the dual cyclooxygenase-2 (COX-2) and soluble epoxide hydrolase (sEH) inhibitor PTUPB. Indeed it may be an approach to suppress debris-stimulated ovarian tumor growth by preventing the therapy-induced surge of cytokines and lipid mediators (236). Hyaluronic acid-based nanoparticles encapsulating miR-125b (HA-PEI-miR-125b) specifically target TAMs in the peritoneal cavity of a syngeneic ID8-VEGF ovarian cancer mouse model and repolarize macrophages to an immune-activating phenotype (increased CD80 and iNOS and reduced CD206 and ARG1 expression) (237). Intraperitoneal administration of paclitaxel in combination with HA-PEI-miR-125b nanoparticles enhanced the antitumor efficacy of paclitaxel mediating by the significant reduction in the ascite fluid and peritoneal VEGF levels (237). Docetaxel treatment increased the infiltration of macrophages in ID8 tumor-bearing mice. Docetaxel in combination with BLZ945 (CSF-1R inhibitor) treatment significantly inhibited tumor growth, reduced the abundance of TAMs, increased CD8+ T cell infiltration and prevented lung metastasis in a mouse epithelial ovarian cancer (238). Imminofluorescence/confocal analysis of 24 patients with OC (Belgium cohort) who underwent platinum-based neoadjuvant chemotherapy (carboplatin and paclitaxel) revealed an increase in vessel width, TAMs, and M2-like macrophages after NAC (239). Blood vessel width was correlated with M2 presence. The additional use of bevacizumab (anti-VEGF therapy) resulted in more pronounced increase in the number of TAMs and M2 macrophages compared to paclitaxel–carboplatin alone (239).
A phase 1/2 study of 18 patients who had platinum-resistant ovarian cancer (the Netherlands) showed that gemcitabine reduced myeloid-derived suppressor cells and increased immune-supportive M1 macrophages (240). Combination of gemcitabine and Pegintron (IFN-alpha) stimulated higher portions of circulating CD4+ and CD8+ T-cells but not regulatory T-cells. All patients vaccinated with p53 synthetic long peptide (SLP) vaccine showed strong specific T-cell responses. Combination of gemcitabine, the immune modulator Pegintron and therapeutic peptide vaccination is a new approach of combined chemo-immunotherapeutic regimens to treat ovarian cancer that has anti-cancer programming effect on innate and adaptive immune systems (240).
In summary, published data about the interaction of TAMs with anti-ovarian cancer treatment are highly diverse. Most of the results were generated in animal models or in vitro, while data from clinical studies is strictly limited. In vitro and animal studies demonstrated opposite effects of treatment on TAMs that depend on both experimental models and chemotherapeutic agent with different mechanisms of action. For example, cisplatin, which is a DNA intercalating agent, supported tumor-promoting functions of TAMs, while paclitaxel, affecting microtubules, induced pro-inflammatory program in TAMs. Mouse pre-clinical models and clinical trials provided promising data for the combination of chemotherapy and TAM-blocking agents that opens the perspectives for using integrated approachs in the treatment of ovarian cancer.
TAMs and Prostate Cancer
Prostate cancer (PC) represents the second most frequent malignancy in men with an estimated over 1.5 million new cases diagnosed annually worldwide and ranks as the fifth leading cause of cancer-associated mortality globally (241). The incidence and mortality rates of PC are trending upwards due to population aging and urbanization, thereby having a significant social and financial burden on global healthcare system (242).
PC belongs to hormonally driven malignancy, whose primary progression relies on functional activity of androgen receptors (243). Accordingly, three stages in prostate carcinogenesis are distinguished: precancerous intraepithelial neoplasia, androgen-dependent, and followed by aggressive androgen-independent PC (244). Adenocarcinoma is the most common prostatic tumor, whereas other histological subtypes such as urothelial, small cell, squamous cell, and basal cell carcinomas are diagnosed quite rarely (245). The major routes for PC progression include extracapsular extension and spread to pelvic lymph nodes, as well as metastasis to lungs and bones (246). Furthermore, given the abundant innervation of prostate peripheral zone, primary tumors arising in this area tend to escape the organ through perineural invasion (247).
Routine screening of PC involves an evaluation of serum levels of prostate specific antigen (PSA), a serine protease produced by prostate epithelium, while the gold standard for diagnosis confirmation is prostate biopsy analysis (248). Apart from the TNM staging system, Gleason score is used to characterize the PC metastatic potential on the basis of differentiation patterns. Thus, high-grade PC (Gleason score over 7) has higher risk of metastasis as compared to less aggressive primary tumors with Gleason score below 6 (249).
Given the hormone dependent nature of PC, androgen deprivation therapy (ADT) has been regarded as a standard treatment approach for patients with PC (250). Despite the initial efficacy and improvement in OS rates, prolonged hormonal treatment is eventually associated with the emergence of aggressive castration-resistant prostate cancer (CRPC) associated with high mortality and poor patient outcomes (251). Current evidence suggests that inflammatory microenvironment, especially TAMs, is involved in the onset of prostate carcinogenesis and acts as an essential modulator of further malignant progression, metastasis, and overall therapeutic response (252).
TAMs in Prostate Tumors and Metastasis
In human prostate cancer, the inflammatory component of local TME is considered as an essential modulator of malignant progression and determinant of the overall therapeutic response (253). To date, a number of investigations have focused on the patterns of macrophage infiltration in prostate cancer specimens in attempts to validate its clinical and pathological significance (254) ( Table 6 ). The primary analysis of TAMs in 85 prostate carcinomas (Sweden, 2000) demonstrated significant increase of the cell profile area and volume density of CD68+ macrophages in cases with higher Gleason score (260). A positive correlation was also found between the size of individual macrophage and angiogenesis measured as the number of von Willebrand factor-positive microvessels in the most vascularized area (260). In the same cohort, increased density and cell profile area of CD68+ TAMs were recognized as predictors of shorter cancer-specific survival (CSS) (260). Next study of a cohort of 81 prostate cancer patients from USA cohort revealed an increase of macrophage density in tumor versus adjacent benign tissue (255). Interestingly, a negative association between the amount of CD68+ TAM infiltrate in total tumor tissue and TNM clinical stage was found, while TAM density within cancer cell area positively correlated with Gleason score (255). Such contradicting results may reflect the heterogeneous distribution of TAMs in the tissue samples and highlights the importance of the compartment-specific macrophages in prostate tumorigenesis. High levels of CD68 in biopsy specimens of 859 patients from the USA cohort with benign prostatic hyperplasia were associated with increased risk for overall clinical progression (261). Several independent investigations confirmed high expression of CD68 in advanced prostate cancer. Thus, IHC study of 131 Japanese prostate cancer patients detected abundant CD68+ macrophage infiltration in tumor mass in patients with higher serum prostate-specific antigen (PSA) and Gleason score (59). The relapse-free survival rates in the same cohort were significantly lower in patients with greater TAM counts (59). Appropriate reporting of methodology, quantitative assessment and statistical analysis in this study could be necessary to ensure the quality of data interpretation in accordance with scientific rigor (59). Tissue microarray (TMA) containing 332 radical prostatectomy specimens (USA cohort) revealed greater abundance of CD68+ cells in malignant areas in comparison to benign tissues, as well as increase in mean TAM numbers in Gleason grade 4 versus grade 3 (262). IHC analysis of 100 specimens of prostate adenocarcinoma of the Turkish cohort demonstrated positive correlation between the density of CD68+ TAM infiltration and such clinical–pathological parameters as tumor stage, Gleason score, extracapsular extension, perineural invasion, and positive surgical margins (256). Furthermore, a study involving 93 prostate cancer patients from the Italian cohort identified that high expression of CD68 in primary tumor identified by IHC was an independent predictor of biochemical recurrence (defined as elevation of PSA level) after radical prostatectomy (263). Increased CD68+ macrophage count was observed in metastases from the lymph nodes, liver, bladder, rectum, and seminal vesicles in comparison to the corresponding primary tumors collected from 59 prostate cancer patients from the Norway cohort (264). Recent study of representative TMA collected from over 400 patient cohort from Germany confirmed the increase of CD68+ cell numbers in prostate cancers with Gleason score over 8 (265). Microarray analysis of 9,393 prostate cancer samples demonstrated that elevated expression signature of TAMs is strongly associated with worse distant metastasis-free survival (266). Thus, a number of studies indicated that higher CD68+ macrophage abundance in tumor tissue reflects aggressive tumor behavior and unfavorable patient outcomes in prostate cancer.
Table 6.
Representative studies demonstrating the association of TAMs with tumor progression parameters in prostate cancer.
| Cohort of patients | Method of detection | TAM correlation with tumor growth and stage | TAM correlation with lymphatic and hematogenous metastasis | TAM correlation with survival | Reference |
|---|---|---|---|---|---|
| 81 prostate cancer patients (USA) | IHC (manually) | 1.94-fold increase of stromal CD68+ TAM amount is found in tumors with T1a–T2a stages (mean = 228.5) vs. T3a stage (mean = 118.0). fivefold increase of CD68+ TAM amount is found in cancer area with Gleason score 8–10 (mean = 138.0) vs. Gleason score 4–6 (mean = 27.6) |
Decrease of mean CD68+ TAM amount in primary cancer by 48% (from 59.3 to 30.7 cells at ×400) is associated with LN metastases |
Increase of CD68+ TAM amount above the mean (185.8) is associated with increased RFS rate by 44% | (255) |
| 131 prostate cancer patients (Japan) | IHC (not specified) | 1.6-fold increase of CD68+ TAM amount is found in tumors of stage T ≥ 3 (mean = 40.54) vs. T ≤ 2 stage (mean = 25.26). 1.87-fold increase of CD68+ TAM amount is found in cases with Gleason score≥8 (mean = 44.94) vs. Gleason ≤ 6 (mean = 24.03). | Not studied | High amount of CD68+ TAMs (≥22 per ×400 HPF) correlates with decreased RFS rate by 75% | (59) |
| 100 prostate adenocarcinoma patients (Turkey) | IHC (manually) | Increase of CD68+ TAM amount (≥15 cells under ×400, defined as score 3) is indicative for Gleason score ≥8 and stage III | High amount of CD68+ TAMs (≥15 cells under ×400) correlates with extracapsular extension and perineural invasion | Not significant | (256) |
| 93 prostate cancer patients (Italy) | IHC (manually) | fourfold increase of mean amount of CD163+ TAMs vs. CD68+ TAMs is associated with Gleason score ≥ 7 | Not studied | Patients with tumors of high CD163+ TAM amount show reduced biochemical RFS rates by 16% compared to those with high CD68+ TAM amount | (257) |
| 234 prostate cancer patients (Sweden) | IHC (digital imaging scanning) | 1.7-fold increase of CD163+ TAM amount is found in tumors with Gleason score ≥ 8 (mean = 100.0) vs. Gleason < 6 (mean = 60.1) | 1.3-fold increase of mean CD163+ TAM amount (from 74.8 to 99.9) in primary cancer is associated with presence of bones metastases | Increase of CD163+ TAM amount (above 99) correlates with reduced DSS | (258) |
| 135 prostate cancer patients (Japan) | IHC in TMA | Low amount of CD204+ TAMs (<24 cells per 0.06175 mm2) is associated with high PSA level (>20 ng/ml) 1.6-fold decrease of CD204+ TAM amount in tumors with Gleason score ≥ 8 (mean = 19.17) vs. Gleason ≤ 6 (mean = 30.2) |
Not studied | Low amount of CD204+ TAMs (<24 cells per 0.06175 mm2) is associated with decreased RFS rate by 25% | (259) |
DFS, disease-free survival; DSS, disease-specific survival; HPF, high-power field; IF, immunofluorescence; IHC, immunohistochemistry; LN, lymph node; TAM, tumor-associated macrophages; OS, overall survival; RFS, recurrence-free survival; TMA, tissue microarray.
Subpopulations of TAMs in Prostate Cancer Progression
Not only total macrophage amount but also specific macrophage subtypes were found to be correlated with clinical and pathological characteristics of prostate cancer patients ( Table 6 ). IHC analysis of tissue specimens derived from 93 Italian prostate cancer patients has identified that high amount of CD163+ TAMs was associated with extracapsular extension (Gleason score > 7) and worse biochemical recurrence-free survival rates (257). Increased infiltration of CD163+ cells correlated with higher Gleason score and incidence of metastasis, as well as lower rates of CSS in a cohort of 234 Swedish prostate cancer patients (258). These findings were further confirmed in a study involving 592 patients with diagnosed prostate cancer from the Swedish cohort demonstrating greater CD163+ macrophage infiltration in aggressive tumors with Gleason scores ranging from 8 to 10 (267). The risk of death from prostate cancer in the same cohort was almost twofold higher in patients with high amount of CD163+ TAMs versus those with lower numbers (267). Positive correlation between the number of CD206+ macrophages and Gleason scores was found in Chinese cohort of 42 prostate adenocarcinoma patients (268). TMA of 192 prostate cancer samples from the USA cohort revealed greater amount of CD206+ TAMs in primary adenocarcinoma and lymphatic metastases in comparison to benign prostate tissues (269). IHC analysis of 373 prostate biopsy samples (Japanese cohort) demonstrated significantly lower numbers CD204+ TAMs in cases with prostate cancer in comparison to benign specimens (270). Negative correlation between the density of CD204+ TAMs and the clinical T stage was confirmed in the retrospective study of 135 PC patients from the Japanese cohort (259). Inverse association was demonstrated between the expression of MSR-A in primary tumors and the presence of lymph node metastases in the USA cohort of 90 prostate cancer patients (271). YKL-40 is an emerging TAM biomarker that is produced by both macrophages and cancer cells and enhances inflammation in TME (272). YKL-40 is also a strong inducer of tumor angiogenesis (273). In macrophages, YKL-40 is induced by IFNγ and can be considered as M1 biomarker (14, 16). Significantly higher concentrations of YKL-40 were detected in the serum of 153 patients (from Denmark) with metastatic prostate cancer compared to healthy donors (274). Accordingly, elevated plasma YKL-40 levels at the time of diagnosis were predictive of shorter OS rates in the same cohort of patients (274).
TAMs and Prostate Cancer Treatment
To date, androgen deprivation therapy (ADT) is accepted as a standard treatment approach for patients with advanced prostate cancer (250). Despite initial efficacy and improvement in the OS, prolonged hormonal treatment is eventually associated with aggressive castration-resistant prostate cancer (CRPC) (251). Multiple lines of evidence indicate crucial role of TAMs in therapeutic response and in post-treatment recurrence of prostate cancer (275). In comparison with tumor tissues from hormone-naïve prostate cancer patients, CRPC samples displayed higher number of CD68+ macrophages expressing cathepsin S enzyme known to be involved in angiogenesis and remodeling of extracellular matrix (276). IHC analysis of 75 prostate cancer specimens (Canadian cohort) was performed in two groups of patients—patients pre-treated with Cyproterone (antiandrogen agent) or Leuprolide (gonadotropin-releasing hormone analogue) in combination with Flutamide (nonsteroidal antiandrogen) before radical prostatectomy and patients who underwent surgery only. Increase in the amount of CD68+ TAMs within tumor tissues of pre-treated patients compared to the untreated group was demonstrated (277). Increased CD68+ and CD163+ macrophage infiltration was found in a cohort of 60 Chinese prostate cancer patients receiving preoperative Bicalutamide-based ADT (278). TMA analysis was performed for retrospective cohort of 366 prostatectomized patients (Canada) divided into two groups—hormone ablation-treated patients (luteinizing hormone-releasing hormone-agonists and/or antiandrogen prior to surgery) and hormone-naïve patients. This analysis confirmed significantly higher amount of CD163+ TAMs in treated group of patients in comparison with hormone-naïve patients (279). Mouse model of prostate cancer further confirmed dramatic recruitment of TAMs in response to ADT. Substantial overexpression of VEGF-A, MMP-9, and ARG1 was found in tumors of castrated animals treated by ADT (279). Also, concentrations of CSF1, major macrophage differentiation, and chemotactic factor, were enhanced in the serum of animals in response to ADT treatment (279). In parallel, co-culture of Myc-CaP prostate cancer cells and RAW264.7 macrophages treated with antiandrogen Enzalutamide resulted in significant increase in the expression of M2 markers—VEGF-A, MMP- 9, ARG1, IL-10, and CSF1 (279). Importantly, higher levels of CD163+ macrophages were detected in the prostate cancer sections (Chinese cohort) resected after preoperative ADT in comparison to the corresponding tissues collected before therapy (280). IHC study of 126 prostate cancer patients (Italian cohort) using pelvic lymph node metastases samples obtained from those patients who received neoadjuvant hormonal treatment flutamide combined with Leuprolide acetate before radical prostatectomy was performed (281). Double IHC revealed the co-localization of CD68+ TAMs and TARC/CCL17 (thymus- and activation-regulated chemokine), chemokine regulated Treg function, in treated patients in contrast to the untreated group (281).
Clinical trial on 17 patients (USA) with Gleason score 7–10 prostate cancer, treated with anti-PD-1 therapy, revealed significant upregulation of inhibitory molecules PD-L1 and VISTA on CD68+ TAMs in tumor after treatment in comparison with baseline tumor (10-fold and fourfold increase in expression, respectively) (282). The authors suggested that VISTA expression is a compensatory pathway limiting efficiency of ipilimumab therapy of prostate cancer (282), and targeting of VISTA on TAMs can be suggested as next therapeutic approach to develop.
Monitoring of serum YKL-40 concentrations can also be considered as promising prognostic approach for the management of CRPC. Thus, post-treatment increase of serum YKL-40 was an independent prognostic factor of earlier death in 106 metastatic prostate cancer patients (Denmark cohort) treated with total androgen ablation or parenteral estrogen (283). Retrospective analysis of 109 patients with CRPC receiving first-line chemotherapy with docetaxel revealed significance of high pre-treatment YKL-40 serum levels as predictive parameter of shorter OS and DSS (284).
These data demonstrate the essential role of TAMs in prostate cancer progression and emphasize on the promise of targeting TAMs to prevent the recurrence of disease and achieve sustained improvements in patient outcomes. Further in-depth investigations must be done to characterize macrophage phenotypes within certain intratumor compartments of prostate cancer and determine their potential diagnostic and therapeutic value.
Conclusions
In our review we compile existing lines of evidence about the clinical role of TAMs in the context of metastasis (including survival rate) and antitumor treatment in different cohorts of patients that come out of a number of courtiers worldwide. We compared the role of TAMs in worldwide leading types of malignant diseases: breast, colorectal, lung, ovarian, and prostate cancers that very frequently give life-threatening distant metastasis. Systematic analysis of TAM biomarkers identified that CD68, and in some cases CD163, are the best markers for the quantification of TAMs in tumor tissue, while several other surface receptors (scavenger receptor stabilin-1, mannose receptor CD206, CD204, MARCO) and chitinase-like proteins (YKL-39, YKL-40) are very informative biomarkers of functional TAM polarization.
In patients with breast, ovarian, and prostate cancer, increased amount of TAMs is a clear indicator for rapid tumor growth, aggressive metastatic process, and limited efficiency of therapy ( Tables 2 – 6 ) ( Figure 2 ). In lung and ovarian cancer, the major parameter associated with prognosis was not the total amount of CD68+ macrophages, but M1/M2 index. The prevalence of M1 macrophages was favorable for the patients, indicating that in lung tumor M1 TAMs have the ability to limit tumor progression. Moreover, in lung cancer, high amount of TAMs in tumor nest correlated with the chemotherapy efficiency. The most distinct from other types of cancer was colorectal cancer, where high amounts of TAMs were indicative of the favorable prognosis and restricted ability of primary tumors to grow and to metastasize ( Figure 2 ). In contrast to the total amount of macrophages, M2-like phenotype of TAMs is rather indicative for the negative prognosis for patients with CRC.
Figure 2.
TAMs in primary tumor growth and metastasis. Role of TAMs in primary tumor growth, hematogenous metastasis, and lymphatic metastasis is illustrated. Green arrow indicates supportive role of TAMs for each process, and orange arrow indicates the suppressive role of TAMs. The role of each specific macrophage marker in the individual type of cancer is indicated within the arrows.
TAMs may contribute to resistance to therapy facilitating tumor progression by suppression of T cell immunity, the maintenance of tumor cell survival, and the stimulation of tumor revascularization. Chemotherapy can stimulate antitumor immunity, thereby increasing the pathological complete response (pCR) to the treatment. There is no agreement about the role of TAMs in chemotherapy response. The results are contradictory and depend on the animal model, type of in vitro study, patient cohort, and type of anti-cancer drug ( Table 7 ). Therefore, to achieve the maximum efficiency of chemotherapy, the molecular mechanisms of the interaction of chemotherapeutic agents with TAMs have to be investigated. Understanding of these interactions will also allow developing targeting strategies for TAMs. The investigation of TAM-mediated tumor resistance to therapy is of particular relevance in the era of the development of immunomodulatory approaches aimed to enhance T-cell immunity, to inhibit macrophage recruitment into a tumor, to modify polarization of TAMs, and to enhance phagocytosis of cancer cells by TAMs.
Author Contributions
Conceptualization: IL and JK. Writing—original draft preparation: IL, JK, GT, AP, MS, VP, EC, and NC. Writing—review and editing: IL, GT, and JK. Figure preparation: IL. Supervision: JK. Funding acquisition: JK. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the Russian Science Foundation, grant number #19-15-00151. The publication costs were covered by the Tomsk State University competitiveness improvement program.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to Dr. Ksenia Danilko, Bashkir State Medical University for providing the image for CD206 expression in prostate cancer. We would like to thank Mrs Ekaterina Chitrinskaya for the graphical support in the preparation of illustrations.
Abbreviations
ADT, androgen deprivation therapy; BC, breast cancer; CRC, colorectal cancer; CRPC, castration-resistant prostate cancer; DFS, disease-free survival; DSS, disease-specific survival; EMT, epithelial–mesenchymal transition; EOC, epithelial ovarian cancer; IF, immunofluorescence; IHC, immunohistochemical analysis; LN, lymph node; LVI, lymphovascular invasion; NAC, neoadjuvant chemotherapy; NSCLC, non-small cell lung carcinoma; OC, ovarian cancer; OS, overall survival; OXP, oxaliplatin; PC, prostate cancer; pCR, pathologic complete response; PFS, progression-free survival; RFS, relapse-free survival; PTX, paclitaxel:TAMs, tumor-associated macrophages; TMA, tissue microarray; TME, tumor microenvironment; TNBC, triple-negative breast cancer; TN, tumor nest; TS, tumor stroma; PSA, prostate-specific antigen.
References
- 1. Cassetta L, Pollard JW. Tumor-associated macrophages. Curr Biol CB (2020) 30(6):R246–R8. 10.1016/j.cub.2020.01.031 [DOI] [PubMed] [Google Scholar]
- 2. Larionova I, Cherdyntseva N, Liu T, Patysheva M, Rakina M, Kzhyshkowska J. Interaction of tumor-associated macrophages and cancer chemotherapy. Oncoimmunology (2019) 8(7):1596004. 10.1080/2162402X.2019.1596004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Larionova I, Kazakova E, Patysheva M, Kzhyshkowska J. Transcriptional, Epigenetic and Metabolic Programming of Tumor-Associated Macrophages. Cancers (2020) 12(6):1411. 10.3390/cancers12061411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukocyte Biol (2009) 86(5):1065–73. 10.1189/jlb.0609385 [DOI] [PubMed] [Google Scholar]
- 5. Kzhyshkowska J, Grigoryeva E, Larionova I. Targeting the Tumor-Associated Macrophages for ‘Normalizing’ Cancer. In: Bizzarri M, editor. Approaching Complex Diseases, Human Perspectives in Health Sciences and Technologies 2. Cham: Springer Nature Switzerland AG; (2020). p. 245–74. [Google Scholar]
- 6. Martinez-Pomares L. The mannose receptor. J leukocyte Biol (2012) 92(6):1177–86. 10.1189/jlb.0512231 [DOI] [PubMed] [Google Scholar]
- 7. Van Gorp H, Delputte PL, Nauwynck HJ. Scavenger receptor CD163, a Jack-of-all-trades and potential target for cell-directed therapy. Mol Immunol (2010) 47(7-8):1650–60. 10.1016/j.molimm.2010.02.008 [DOI] [PubMed] [Google Scholar]
- 8. Kzhyshkowska J. Multifunctional receptor stabilin-1 in homeostasis and disease. TheScientificWorldJournal (2010) 10:2039–53. 10.1100/tsw.2010.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Riabov V, Yin S, Song B, Avdic A, Schledzewski K, Ovsiy I, et al. Stabilin-1 is expressed in human breast cancer and supports tumor growth in mammary adenocarcinoma mouse model. Oncotarget (2016) 7(21):31097–110. 10.18632/oncotarget.8857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kzhyshkowska J, Workman G, Cardo-Vila M, Arap W, Pasqualini R, Gratchev A, et al. Novel function of alternatively activated macrophages: stabilin-1-mediated clearance of SPARC. J Immunol (2006) 176(10):5825–32. 10.4049/jimmunol.176.10.5825 [DOI] [PubMed] [Google Scholar]
- 11. Kzhyshkowska J, Gratchev A, Brundiers H, Mamidi S, Krusell L, Goerdt S. Phosphatidylinositide 3-kinase activity is required for stabilin-1-mediated endosomal transport of acLDL. Immunobiology (2005) 210(2-4):161–73. 10.1016/j.imbio.2005.05.022 [DOI] [PubMed] [Google Scholar]
- 12. Kzhyshkowska J, Neyen C, Gordon S. Role of macrophage scavenger receptors in atherosclerosis. Immunobiology (2012) 217(5):492–502. 10.1016/j.imbio.2012.02.015 [DOI] [PubMed] [Google Scholar]
- 13. Park SY, Jung MY, Lee SJ, Kang KB, Gratchev A, Riabov V, et al. Stabilin-1 mediates phosphatidylserine-dependent clearance of cell corpses in alternatively activated macrophages. J Cell Sci (2009) 122(Pt 18):3365–73. 10.1242/jcs.049569 [DOI] [PubMed] [Google Scholar]
- 14. Kzhyshkowska J, Mamidi S, Gratchev A, Kremmer E, Schmuttermaier C, Krusell L, et al. Novel stabilin-1 interacting chitinase-like protein (SI-CLP) is up-regulated in alternatively activated macrophages and secreted via lysosomal pathway. Blood (2006) 107(8):3221–8. 10.1182/blood-2005-07-2843 [DOI] [PubMed] [Google Scholar]
- 15. Yin S, Wang N, Riabov V, Mossel DM, Larionova I, Schledzewski K, et al. SI-CLP inhibits the growth of mouse mammary adenocarcinoma by preventing recruitment of tumor-associated macrophages. Int J Cancer (2020) 146(5):1396–408. 10.1002/ijc.32685 [DOI] [PubMed] [Google Scholar]
- 16. Kzhyshkowska J, Yin S, Liu T, Riabov V, Mitrofanova I. Role of chitinase-like proteins in cancer. Biol Chem (2016) 397(3):231–47. 10.1515/hsz-2015-0269 [DOI] [PubMed] [Google Scholar]
- 17. Liu T, Larionova I, Litviakov N, Riabov V, Zavyalova M, Tsyganov M, et al. Tumor-associated macrophages in human breast cancer produce new monocyte attracting and pro-angiogenic factor YKL-39 indicative for increased metastasis after neoadjuvant chemotherapy. Oncoimmunology (2018) 7(6):e1436922. 10.1080/2162402X.2018.1436922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kzhyshkowska J, Larionova I, Liu T. YKL-39 as a Potential New Target for Anti-Angiogenic Therapy in Cancer. Front Immunol (2019) 10:2930. 10.3389/fimmu.2019.02930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kzhyshkowska J, Gratchev A, Martens JH, Pervushina O, Mamidi S, Johansson S, et al. Stabilin-1 localizes to endosomes and the trans-Golgi network in human macrophages and interacts with GGA adaptors. J Leukocyte Biol (2004) 76(6):1151–61. 10.1189/jlb.0504300 [DOI] [PubMed] [Google Scholar]
- 20. Litviakov N, Tsyganov M, Larionova I, Ibragimova M, Deryusheva I, Kazantseva P, et al. Expression of M2 macrophage markers YKL-39 and CCL18 in breast cancer is associated with the effect of neoadjuvant chemotherapy. Cancer Chemother Pharmacol (2018) 82(1):99–109. 10.1007/s00280-018-3594-8 [DOI] [PubMed] [Google Scholar]
- 21. Chavez-Galan L, Olleros ML, Vesin D, Garcia I. Much More than M1 and M2 Macrophages, There are also CD169(+) and TCR(+) Macrophages. Front Immunol (2015) 6:263. 10.3389/fimmu.2015.00263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell (2010) 141(1):39–51. 10.1016/j.cell.2010.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Riabov V, Gudima A, Wang N, Mickley A, Orekhov A, Kzhyshkowska J. Role of tumor associated macrophages in tumor angiogenesis and lymphangiogenesis. Front Physiol (2014) 5:75. 10.3389/fphys.2014.00075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Laviron M, Boissonnas A. Ontogeny of Tumor-Associated Macrophages. Front Immunol (2019) 10:1799. 10.3389/fimmu.2019.01799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Franklin RA, Li MO. Ontogeny of Tumor-associated Macrophages and Its Implication in Cancer Regulation. Trends Cancer (2016) 2(1):20–34. 10.1016/j.trecan.2015.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Loyher PL, Hamon P, Laviron M, Meghraoui-Kheddar A, Goncalves E, Deng Z, et al. Macrophages of distinct origins contribute to tumor development in the lung. J Exp Med (2018) 215(10):2536–53. 10.1084/jem.20180534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bogels M, Braster R, Nijland PG, Gul N, van de Luijtgaarden W, Fijneman RJ, et al. Carcinoma origin dictates differential skewing of monocyte function. Oncoimmunology (2012) 1(6):798–809. 10.4161/onci.20427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van der Bij GJ, Bogels M, Oosterling SJ, Kroon J, Schuckmann DT, de Vries HE, et al. Tumor infiltrating macrophages reduce development of peritoneal colorectal carcinoma metastases. Cancer Lett (2008) 262(1):77–86. 10.1016/j.canlet.2007.11.040 [DOI] [PubMed] [Google Scholar]
- 29. Etzerodt A, Moulin M, Doktor TK, Delfini M, Mossadegh-Keller N, Bajenoff M, et al. Tissue-resident macrophages in omentum promote metastatic spread of ovarian cancer. J Exp Med (2020) 217(4):e20191869. 10.1084/jem.20191869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fujii T, Yajima R, Hirakata T, Miyamoto T, Fujisawa T, Tsutsumi S, et al. Impact of the prognostic value of vascular invasion, but not lymphatic invasion, of the primary tumor in patients with breast cancer. Anticancer Res (2014) 34(3):1255–9. [PubMed] [Google Scholar]
- 31. Rakha EA, Martin S, Lee AH, Morgan D, Pharoah PD, Hodi Z, et al. The prognostic significance of lymphovascular invasion in invasive breast carcinoma. Cancer (2012) 118(15):3670–80. 10.1002/cncr.26711 [DOI] [PubMed] [Google Scholar]
- 32. Koru-Sengul T, Santander AM, Miao F, Sanchez LG, Jorda M, Gluck S, et al. Breast cancers from black women exhibit higher numbers of immunosuppressive macrophages with proliferative activity and of crown-like structures associated with lower survival compared to non-black Latinas and Caucasians. Breast Cancer Res Treat (2016) 158(1):113–26. 10.1007/s10549-016-3847-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wilson MK, Karakasis K, Oza AM. Outcomes and endpoints in trials of cancer treatment: the past, present, and future. Lancet Oncol (2015) 16(1):e32–42. 10.1016/S1470-2045(14)70375-4 [DOI] [PubMed] [Google Scholar]
- 34. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2018) 68(6):394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 35. Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang XS, et al. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet (2015) 385(9972):977–1010. 10.1016/S0140-6736(14)62038-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rubio IT, et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-updagger. Ann Oncol (2019) 30(8):1194–220. 10.1093/annonc/mdz173 [DOI] [PubMed] [Google Scholar]
- 37. Cardoso F, Spence D, Mertz S, Corneliussen-James D, Sabelko K, Gralow J, et al. Global analysis of advanced/metastatic breast cancer: Decade report (2005-2015). Breast (2018) 39:131–8. 10.1016/j.breast.2018.03.002 [DOI] [PubMed] [Google Scholar]
- 38. Yousefi M, Nosrati R, Salmaninejad A, Dehghani S, Shahryari A, Saberi A. Organ-specific metastasis of breast cancer: molecular and cellular mechanisms underlying lung metastasis. Cell Oncol (2018) 41(2):123–40. 10.1007/s13402-018-0376-6 [DOI] [PubMed] [Google Scholar]
- 39. Wu Q, Li J, Zhu S, Wu J, Chen C, Liu Q, et al. Breast cancer subtypes predict the preferential site of distant metastases: a SEER based study. Oncotarget (2017) 8(17):27990–6. 10.18632/oncotarget.15856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nathanson SD, Krag D, Kuerer HM, Newman LA, Brown M, Kerjaschki D, et al. Breast cancer metastasis through the lympho-vascular system. Clin Exp Metastasis (2018) 35(5-6):443–54. 10.1007/s10585-018-9902-1 [DOI] [PubMed] [Google Scholar]
- 41. Tsang JYS, Tse GM. Molecular Classification of Breast Cancer. Adv Anat Pathol (2020) 27(1):27–35. 10.1097/PAP.0000000000000232 [DOI] [PubMed] [Google Scholar]
- 42. Saraiva DP, Guadalupe Cabral M, Jacinto A, Braga S. How many diseases is triple negative breast cancer: the protagonism of the immune microenvironment. ESMO Open (2017) 2(4):e000208. 10.1136/esmoopen-2017-000208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Denisov EV, Litviakov NV, Zavyalova MV, Perelmuter VM, Vtorushin SV, Tsyganov MM, et al. Intratumoral morphological heterogeneity of breast cancer: neoadjuvant chemotherapy efficiency and multidrug resistance gene expression. Sci Rep (2014) 4:4709. 10.1038/srep04709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gerashchenko TS, Novikov NM, Krakhmal NV, Zolotaryova SY, Zavyalova MV, Cherdyntseva NV, et al. Markers of Cancer Cell Invasion: Are They Good Enough? J Clin Med (2019) 8(8):1092. 10.3390/jcm8081092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yang M, Li Z, Ren M, Li S, Zhang L, Zhang X, et al. Stromal Infiltration of Tumor-Associated Macrophages Conferring Poor Prognosis of Patients with Basal-Like Breast Carcinoma. J Cancer (2018) 9(13):2308–16. 10.7150/jca.25155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Denisov EV, Skryabin NA, Gerashchenko TS, Tashireva LA, Wilhelm J, Buldakov MA, et al. Clinically relevant morphological structures in breast cancer represent transcriptionally distinct tumor cell populations with varied degrees of epithelial-mesenchymal transition and CD44(+)CD24(-) stemness. Oncotarget (2017) 8(37):61163–80. 10.18632/oncotarget.18022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zavyalova MV, Denisov EV, Tashireva LA, Gerashchenko TS, Litviakov NV, Skryabin NA, et al. Phenotypic drift as a cause for intratumoral morphological heterogeneity of invasive ductal breast carcinoma not otherwise specified. Biores Open Access (2013) 2(2):148–54. 10.1089/biores.2012.0278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zavyalova MV, Perelmuter VM, Vtorushin SV, Denisov EV, Litvyakov NV, Slonimskaya EM, et al. The presence of alveolar structures in invasive ductal NOS breast carcinoma is associated with lymph node metastasis. Diagn Cytopathol (2013) 41(3):279–82. 10.1002/dc.21852 [DOI] [PubMed] [Google Scholar]
- 49. Gerashchenko TS, Zavyalova MV, Denisov EV, Krakhmal NV, Pautova DN, Litviakov NV, et al. Intratumoral Morphological Heterogeneity of Breast Cancer As an Indicator of the Metastatic Potential and Tumor Chemosensitivity. Acta Naturae (2017) 9(1):56–67. 10.32607/20758251-2017-9-1-56-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tashireva LA, Denisov EV, Gerashchenko TS, Pautova DN, Buldakov MA, Zavyalova MV, et al. Intratumoral heterogeneity of macrophages and fibroblasts in breast cancer is associated with the morphological diversity of tumor cells and contributes to lymph node metastasis. Immunobiology (2017) 222(4):631–40. 10.1016/j.imbio.2016.11.012 [DOI] [PubMed] [Google Scholar]
- 51. Mitrofanova I, Zavyalova M, Riabov V, Cherdyntseva N, Kzhyshkowska J. The effect of neoadjuvant chemotherapy on the correlation of tumor-associated macrophages with CD31 and LYVE-1. Immunobiology (2018) 223(6-7):449–59. 10.1016/j.imbio.2017.10.050 [DOI] [PubMed] [Google Scholar]
- 52. Mitrofanova I, Zavyalova M, Telegina N, Buldakov M, Riabov V, Cherdyntseva N, et al. Tumor-associated macrophages in human breast cancer parenchyma negatively correlate with lymphatic metastasis after neoadjuvant chemotherapy. Immunobiology (2017) 222(1):101–9. 10.1016/j.imbio.2016.08.001 [DOI] [PubMed] [Google Scholar]
- 53. Buldakov M, Zavyalova M, Krakhmal N, Telegina N, Vtorushin S, Mitrofanova I, et al. CD68+, but not stabilin-1+ tumor associated macrophages in gaps of ductal tumor structures negatively correlate with the lymphatic metastasis in human breast cancer. Immunobiology (2017) 222(1):31–8. 10.1016/j.imbio.2015.09.011 [DOI] [PubMed] [Google Scholar]
- 54. Edin S, Wikberg ML, Dahlin AM, Rutegard J, Oberg A, Oldenborg PA, et al. The distribution of macrophages with a M1 or M2 phenotype in relation to prognosis and the molecular characteristics of colorectal cancer. PLoS One (2012) 7(10):e47045. 10.1371/journal.pone.0047045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Burmeister K, Quagliata L, Andreozzi M, Eppenberger-Castori S, Matter MS, Perrina V, et al. Vascular endothelial growth factor A amplification in colorectal cancer is associated with reduced M1 and M2 macrophages and diminished PD-1-expressing lymphocytes. PLoS One (2017) 12(4):e0175563. 10.1371/journal.pone.0175563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang BC, Gao J, Wang J, Rao ZG, Wang BC, Gao JF. Tumor-associated macrophages infiltration is associated with peritumoral lymphangiogenesis and poor prognosis in lung adenocarcinoma. Med Oncol (2011) 28(4):1447–52. 10.1007/s12032-010-9638-5 [DOI] [PubMed] [Google Scholar]
- 57. Chen L, Cao MF, Xiao JF, Ma QH, Zhang H, Cai RL, et al. Stromal PD-1(+) tumor-associated macrophages predict poor prognosis in lung adenocarcinoma. Hum Pathol (2020) 97:68–79. 10.1016/j.humpath.2019.12.007 [DOI] [PubMed] [Google Scholar]
- 58. Le Page C, Marineau A, Bonza PK, Rahimi K, Cyr L, Labouba I, et al. BTN3A2 expression in epithelial ovarian cancer is associated with higher tumor infiltrating T cells and a better prognosis. PLoS One (2012) 7(6):e38541. 10.1371/journal.pone.0038541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nonomura N, Takayama H, Nakayama M, Nakai Y, Kawashima A, Mukai M, et al. Infiltration of tumour-associated macrophages in prostate biopsy specimens is predictive of disease progression after hormonal therapy for prostate cancer. BJU Int (2011) 107(12):1918–22. 10.1111/j.1464-410X.2010.09804.x [DOI] [PubMed] [Google Scholar]
- 60. Cassetta L, Pollard JW. Repolarizing macrophages improves breast cancer therapy. Cell Res (2017) 27(8):963–4. 10.1038/cr.2017.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res (1996) 56(20):4625–9. [PubMed] [Google Scholar]
- 62. Mwafy SE, El-Guindy DM. Pathologic assessment of tumor-associated macrophages and their histologic localization in invasive breast carcinoma. J Egyptian Natl Cancer Inst (2020) 32(1):6. 10.1186/s43046-020-0018-8 [DOI] [PubMed] [Google Scholar]
- 63. Esbona K, Yi Y, Saha S, Yu M, Van Doorn RR, Conklin MW, et al. The Presence of Cyclooxygenase 2, Tumor-Associated Macrophages, and Collagen Alignment as Prognostic Markers for Invasive Breast Carcinoma Patients. Am J Pathol (2018) 188(3):559–73. 10.1016/j.ajpath.2017.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tiainen S, Tumelius R, Rilla K, Hamalainen K, Tammi M, Tammi R, et al. High numbers of macrophages, especially M2-like (CD163-positive), correlate with hyaluronan accumulation and poor outcome in breast cancer. Histopathology (2015) 66(6):873–83. 10.1111/his.12607 [DOI] [PubMed] [Google Scholar]
- 65. Ni C, Yang L, Xu Q, Yuan H, Wang W, Xia W, et al. CD68- and CD163-positive tumor infiltrating macrophages in non-metastatic breast cancer: a retrospective study and meta-analysis. J Cancer (2019) 10(19):4463–72. 10.7150/jca.33914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jeong H, Hwang I, Kang SH, Shin HC, Kwon SY. Tumor-Associated Macrophages as Potential Prognostic Biomarkers of Invasive Breast Cancer. J Breast Cancer (2019) 22(1):38–51. 10.4048/jbc.2019.22.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Miyasato Y, Shiota T, Ohnishi K, Pan C, Yano H, Horlad H, et al. High density of CD204-positive macrophages predicts worse clinical prognosis in patients with breast cancer. Cancer Sci (2017) 108(8):1693–700. 10.1111/cas.13287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhao X, Qu J, Sun Y, Wang J, Liu X, Wang F, et al. Prognostic significance of tumor-associated macrophages in breast cancer: a meta-analysis of the literature. Oncotarget (2017) 8(18):30576–86. 10.18632/oncotarget.15736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Medrek C, Ponten F, Jirstrom K, Leandersson K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer (2012) 12:306. 10.1186/1471-2407-12-306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Morita Y, Zhang R, Leslie M, Adhikari S, Hasan N, Chervoneva I, et al. Pathologic evaluation of tumor-associated macrophage density and vessel inflammation in invasive breast carcinomas. Oncol Lett (2017) 14(2):2111–8. 10.3892/ol.2017.6466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhang Y, Cheng S, Zhang M, Zhen L, Pang D, Zhang Q, et al. High-infiltration of tumor-associated macrophages predicts unfavorable clinical outcome for node-negative breast cancer. PLoS One (2013) 8(9):e76147. 10.1371/journal.pone.0076147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov (2011) 1(1):54–67. 10.1158/2159-8274.CD-10-0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity (2014) 41(1):49–61. 10.1016/j.immuni.2014.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, et al. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res (2006) 66(23):11238–46. 10.1158/0008-5472.CAN-06-1278 [DOI] [PubMed] [Google Scholar]
- 75. Zhu L, Narloch JL, Onkar S, Joy M, Broadwater G, Luedke C, et al. Metastatic breast cancers have reduced immune cell recruitment but harbor increased macrophages relative to their matched primary tumors. J Immunother Cancer (2019) 7(1):265. 10.1186/s40425-019-0755-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ramos RN, Rodriguez C, Hubert M, Ardin M, Treilleux I, Ries CH, et al. CD163(+) tumor-associated macrophage accumulation in breast cancer patients reflects both local differentiation signals and systemic skewing of monocytes. Clin Trans Immunol (2020) 9(2):e1108. 10.1002/cti2.1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gordon S, Pluddemann A. Tissue macrophages: heterogeneity and functions. BMC Biol (2017) 15(1):53. 10.1186/s12915-017-0392-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pelekanou V, Villarroel-Espindola F, Schalper KA, Pusztai L, Rimm DL. CD68, CD163, and matrix metalloproteinase 9 (MMP-9) co-localization in breast tumor microenvironment predicts survival differently in ER-positive and -negative cancers. Breast Cancer Res BCR (2018) 20(1):154. 10.1186/s13058-018-1076-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cassetta L, Fragkogianni S, Sims AH, Swierczak A, Forrester LM, Zhang H, et al. Human Tumor-Associated Macrophage and Monocyte Transcriptional Landscapes Reveal Cancer-Specific Reprogramming, Biomarkers, and Therapeutic Targets. Cancer Cell (2019) 35(4):588–602 e10. 10.1016/j.ccell.2019.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kim OH, Kang GH, Noh H, Cha JY, Lee HJ, Yoon JH, et al. Proangiogenic TIE2(+)/CD31 (+) macrophages are the predominant population of tumor-associated macrophages infiltrating metastatic lymph nodes. Molecules Cells (2013) 36(5):432–8. 10.1007/s10059-013-0194-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Krakhmal NV, Zavyalova MV, Denisov EV, Vtorushin SV, Perelmuter VM. Cancer Invasion: Patterns and Mechanisms. Acta Naturae (2015) 7(2):17–28. 10.32607/20758251-2015-7-2-17-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yuan ZY, Luo RZ, Peng RJ, Wang SS, Xue C. High infiltration of tumor-associated macrophages in triple-negative breast cancer is associated with a higher risk of distant metastasis. OncoTargets Ther (2014) 7:1475–80. 10.2147/OTT.S61838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zhang WJ, Wang XH, Gao ST, Chen C, Xu XY, Sun Q, et al. Tumor-associated macrophages correlate with phenomenon of epithelial-mesenchymal transition and contribute to poor prognosis in triple-negative breast cancer patients. J Surg Res (2018) 222:93–101. 10.1016/j.jss.2017.09.035 [DOI] [PubMed] [Google Scholar]
- 84. Zhou J, Wang XH, Zhao YX, Chen C, Xu XY, Sun Q, et al. Cancer-Associated Fibroblasts Correlate with Tumor-Associated Macrophages Infiltration and Lymphatic Metastasis in Triple Negative Breast Cancer Patients. J Cancer (2018) 9(24):4635–41. 10.7150/jca.28583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gwak JM, Jang MH, Kim DI, Seo AN, Park SY. Prognostic value of tumor-associated macrophages according to histologic locations and hormone receptor status in breast cancer. PLoS One (2015) 10(4):e0125728. 10.1371/journal.pone.0125728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mohammed ZM, Going JJ, Edwards J, Elsberger B, Doughty JC, McMillan DC. The relationship between components of tumour inflammatory cell infiltrate and clinicopathological factors and survival in patients with primary operable invasive ductal breast cancer. Br J Cancer (2012) 107(5):864–73. 10.1038/bjc.2012.347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Klingen TA, Chen Y, Aas H, Wik E, Akslen LA. Tumor-associated macrophages are strongly related to vascular invasion, non-luminal subtypes, and interval breast cancer. Hum Pathol (2017) 69:72–80. 10.1016/j.humpath.2017.09.001 [DOI] [PubMed] [Google Scholar]
- 88. Wormann B. Breast cancer: basics, screening, diagnostics and treatment. Medizinische Monatsschrift fur Pharmazeuten (2017) 40(2):55–64. [PubMed] [Google Scholar]
- 89. Maughan KL, Lutterbie MA, Ham PS. Treatment of breast cancer. Am Fam Physician (2010) 81(11):1339–46. [PubMed] [Google Scholar]
- 90. McDonald ES, Clark AS, Tchou J, Zhang P, Freedman GM. Clinical Diagnosis and Management of Breast Cancer. J Nucl Med (2016) 57(Suppl 1):9S–16S. 10.2967/jnumed.115.157834 [DOI] [PubMed] [Google Scholar]
- 91. Khosravi-Shahi P, Cabezon-Gutierrez L, Custodio-Cabello S. Metastatic triple negative breast cancer: Optimizing treatment options, new and emerging targeted therapies. Asia-Pacific J Clin Oncol (2018) 14(1):32–9. 10.1111/ajco.12748 [DOI] [PubMed] [Google Scholar]
- 92. Spring LM, Fell G, Arfe A, Sharma C, Greenup RA, Reynolds KL, et al. Pathological complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: a comprehensive meta-analysis. Clin Cancer Res (2020) 26(12):2838–48. 10.1158/1078-0432.CCR-19-3492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Gerber B, Freund M, Reimer T. Recurrent breast cancer: treatment strategies for maintaining and prolonging good quality of life. Deutsches Arzteblatt Int (2010) 107(6):85–91. 10.3238/arztebl.2010.0085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Chun KH, Park JH, Fan S. Predicting and Overcoming Chemotherapeutic Resistance in Breast Cancer. Adv Exp Med Biol (2017) 1026:59–104. 10.1007/978-981-10-6020-5_4 [DOI] [PubMed] [Google Scholar]
- 95. Shree T, Olson OC, Elie BT, Kester JC, Garfall AL, Simpson K, et al. Macrophages and cathepsin proteases blunt chemotherapeutic response in breast cancer. Genes Dev (2011) 25(23):2465–79. 10.1101/gad.180331.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ali HR, Chlon L, Pharoah PD, Markowetz F, Caldas C. Patterns of Immune Infiltration in Breast Cancer and Their Clinical Implications: A Gene-Expression-Based Retrospective Study. PLoS Med (2016) 13(12):e1002194. 10.1371/journal.pmed.1002194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Raphael J, Gong IY, Nofech-Mozes S, Bartlett J, Nafisi H, Verma S. Tumour infiltrating lymphocytes and stromal CD68 in early stage HER2 positive breast cancer. J Clin Pathol (2016) 69(6):552–5. 10.1136/jclinpath-2015-203493 [DOI] [PubMed] [Google Scholar]
- 98. Kaewkangsadan V, Verma C, Eremin JM, Cowley G, Ilyas M, Satthaporn S, et al. The Differential Contribution of the Innate Immune System to a Good Pathological Response in the Breast and Axillary Lymph Nodes Induced by Neoadjuvant Chemotherapy in Women with Large and Locally Advanced Breast Cancers. J Immunol Res (2017) 2017:1049023. 10.1155/2017/1049023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Wimberly H, Brown JR, Schalper K, Haack H, Silver MR, Nixon C, et al. PD-L1 Expression Correlates with Tumor-Infiltrating Lymphocytes and Response to Neoadjuvant Chemotherapy in Breast Cancer. Cancer Immunol Res (2015) 3(4):326–32. 10.1158/2326-6066.CIR-14-0133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Guerriero JL, Sotayo A, Ponichtera HE, Castrillon JA, Pourzia AL, Schad S, et al. Class IIa HDAC inhibition reduces breast tumours and metastases through anti-tumour macrophages. Nature (2017) 543(7645):428–32. 10.1038/nature21409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Paulus P, Stanley ER, Schafer R, Abraham D, Aharinejad S. Colony-stimulating factor-1 antibody reverses chemoresistance in human MCF-7 breast cancer xenografts. Cancer Res (2006) 66(8):4349–56. 10.1158/0008-5472.CAN-05-3523 [DOI] [PubMed] [Google Scholar]
- 102. Hughes R, Qian BZ, Rowan C, Muthana M, Keklikoglou I, Olson OC, et al. Perivascular M2 Macrophages Stimulate Tumor Relapse after Chemotherapy. Cancer Res (2015) 75(17):3479–91. 10.1158/0008-5472.CAN-14-3587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Castellaro AM, Rodriguez-Baili MC, Di Tada CE, Gil GA. Tumor-Associated Macrophages Induce Endocrine Therapy Resistance in ER+ Breast Cancer Cells. Cancers (2019) 11(2):189. 10.3390/cancers11020189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Li D, Ji H, Niu X, Yin L, Wang Y, Gu Y, et al. Tumor-associated macrophages secrete CC-chemokine ligand 2 and induce tamoxifen resistance by activating PI3K/Akt/mTOR in breast cancer. Cancer Sci (2020) 111(1):47–58. 10.1111/cas.14230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Xuan QJ, Wang JX, Nanding A, Wang ZP, Liu H, Lian X, et al. Tumor-associated macrophages are correlated with tamoxifen resistance in the postmenopausal breast cancer patients. Pathol Oncol Res POR (2014) 20(3):619–24. 10.1007/s12253-013-9740-z [DOI] [PubMed] [Google Scholar]
- 106. Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut (2017) 66(4):683–91. 10.1136/gutjnl-2015-310912 [DOI] [PubMed] [Google Scholar]
- 107. Sagaert X, Vanstapel A, Verbeek S. Tumor Heterogeneity in Colorectal Cancer: What Do We Know So Far? Pathobiol J Immunopathol Mol Cell Biol (2018) 85(1-2):72–84. 10.1159/000486721 [DOI] [PubMed] [Google Scholar]
- 108. Paschke S, Jafarov S, Staib L, Kreuser ED, Maulbecker-Armstrong C, Roitman M, et al. Are Colon and Rectal Cancer Two Different Tumor Entities? A Proposal to Abandon the Term Colorectal Cancer. Int J Mol Sci (2018) 19(9):2577. 10.3390/ijms19092577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kang GH. Four molecular subtypes of colorectal cancer and their precursor lesions. Arch Pathol Lab Med (2011) 135(6):698–703. 10.1043/2010-0523-RA.1 [DOI] [PubMed] [Google Scholar]
- 110. Jafarov S, Link KH. Colon and rectal cancer are different tumor entities according to epidemiology, carcinogenesis, molecular- and tumor biology, primary and secondary prevention: preclinical evidence. Siberian J Oncol (2018) 17(4):88–98. 10.21294/1814-4861-2018-17-4-88-98 [DOI] [Google Scholar]
- 111. Guinney J, Dienstmann R, Wang X, de Reynies A, Schlicker A, Soneson C, et al. The consensus molecular subtypes of colorectal cancer. Nat Med (2015) 21(11):1350–6. 10.1038/nm.3967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Roelands J, Kuppen PJK, Vermeulen L, Maccalli C, Decock J, Wang E, et al. Immunogenomic Classification of Colorectal Cancer and Therapeutic Implications. Int J Mol Sci (2017) 18(10):2229. 10.3390/ijms18102229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kang JC, Chen JS, Lee CH, Chang JJ, Shieh YS. Intratumoral macrophage counts correlate with tumor progression in colorectal cancer. J Surg Oncol (2010) 102(3):242–8. 10.1002/jso.21617 [DOI] [PubMed] [Google Scholar]
- 114. Wei C, Yang C, Wang S, Shi D, Zhang C, Lin X, et al. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Mol Cancer (2019) 18(1):64. 10.1186/s12943-019-0976-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Yang C, Wei C, Wang S, Shi D, Zhang C, Lin X, et al. Elevated CD163(+)/CD68(+) Ratio at Tumor Invasive Front is Closely Associated with Aggressive Phenotype and Poor Prognosis in Colorectal Cancer. Int J Biol Sci (2019) 15(5):984–98. 10.7150/ijbs.29836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Jedinak A, Dudhgaonkar S, Sliva D. Activated macrophages induce metastatic behavior of colon cancer cells. Immunobiology (2010) 215(3):242–9. 10.1016/j.imbio.2009.03.004 [DOI] [PubMed] [Google Scholar]
- 117. Zhong X, Chen B, Yang Z. The Role of Tumor-Associated Macrophages in Colorectal Carcinoma Progression. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol (2018) 45(1):356–65. 10.1159/000486816 [DOI] [PubMed] [Google Scholar]
- 118. van der Bij GJ, Bogels M, Otten MA, Oosterling SJ, Kuppen PJ, Meijer S, et al. Experimentally induced liver metastases from colorectal cancer can be prevented by mononuclear phagocyte-mediated monoclonal antibody therapy. J Hepatol (2010) 53(4):677–85. 10.1016/j.jhep.2010.04.023 [DOI] [PubMed] [Google Scholar]
- 119. Gulubova M, Ananiev J, Yovchev Y, Julianov A, Karashmalakov A, Vlaykova T. The density of macrophages in colorectal cancer is inversely correlated to TGF-beta1 expression and patients’ survival. J Mol Histol (2013) 44(6):679–92. 10.1007/s10735-013-9520-9 [DOI] [PubMed] [Google Scholar]
- 120. Koelzer VH, Canonica K, Dawson H, Sokol L, Karamitopoulou-Diamantis E, Lugli A, et al. Phenotyping of tumor-associated macrophages in colorectal cancer: Impact on single cell invasion (tumor budding) and clinicopathological outcome. Oncoimmunology (2016) 5(4):e1106677. 10.1080/2162402X.2015.1106677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Forssell J, Oberg A, Henriksson ML, Stenling R, Jung A, Palmqvist R. High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin Cancer Res (2007) 13(5):1472–9. 10.1158/1078-0432.CCR-06-2073 [DOI] [PubMed] [Google Scholar]
- 122. Zhou Q, Peng RQ, Wu XJ, Xia Q, Hou JH, Ding Y, et al. The density of macrophages in the invasive front is inversely correlated to liver metastasis in colon cancer. J Trans Med (2010) 8:13. 10.1186/1479-5876-8-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Shabo I, Olsson H, Sun XF, Svanvik J. Expression of the macrophage antigen CD163 in rectal cancer cells is associated with early local recurrence and reduced survival time. Int J Cancer (2009) 125(8):1826–31. 10.1002/ijc.24506 [DOI] [Google Scholar]
- 124. Feng Q, Chang W, Mao Y, He G, Zheng P, Tang W, et al. Tumor-associated Macrophages as Prognostic and Predictive Biomarkers for Postoperative Adjuvant Chemotherapy in Patients with Stage II Colon Cancer. Clin Cancer Res (2019) 25(13):3896–907. 10.1158/1078-0432.CCR-18-2076 [DOI] [PubMed] [Google Scholar]
- 125. Algars A, Irjala H, Vaittinen S, Huhtinen H, Sundstrom J, Salmi M, et al. Type and location of tumor-infiltrating macrophages and lymphatic vessels predict survival of colorectal cancer patients. Int J Cancer (2012) 131(4):864–73. 10.1002/ijc.26457 [DOI] [PubMed] [Google Scholar]
- 126. Nakayama Y, Nagashima N, Minagawa N, Inoue Y, Katsuki T, Onitsuka K, et al. Relationships between tumor-associated macrophages and clinicopathological factors in patients with colorectal cancer. Anticancer Res (2002) 22(6C):4291–6. [PubMed] [Google Scholar]
- 127. Sickert D, Aust DE, Langer S, Haupt I, Baretton GB, Dieter P. Characterization of macrophage subpopulations in colon cancer using tissue microarrays. Histopathology (2005) 46(5):515–21. 10.1111/j.1365-2559.2005.02129.x [DOI] [PubMed] [Google Scholar]
- 128. Cavnar MJ, Turcotte S, Katz SC, Kuk D, Gonen M, Shia J, et al. Tumor-Associated Macrophage Infiltration in Colorectal Cancer Liver Metastases is Associated With Better Outcome. Ann Surg Oncol (2017) 24(7):1835–42. 10.1245/s10434-017-5812-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Pinto ML, Rios E, Duraes C, Ribeiro R, Machado JC, Mantovani A, et al. The Two Faces of Tumor-Associated Macrophages and Their Clinical Significance in Colorectal Cancer. Front Immunol (2019) 10:1875. 10.3389/fimmu.2019.01875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Shabo I, Olsson H, Elkarim R, Sun XF, Svanvik J. Macrophage Infiltration in Tumor Stroma is Related to Tumor Cell Expression of CD163 in Colorectal Cancer. Cancer Microenviron (2014) 7(1-2):61–9. 10.1007/s12307-014-0145-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Taniyama D, Taniyama K, Kuraoka K, Yamamoto H, Zaitsu J, Saito A, et al. CD204-Positive Tumor-associated Macrophages Relate to Malignant Transformation of Colorectal Adenoma. Anticancer Res (2019) 39(6):2767–75. 10.21873/anticanres.13403 [DOI] [PubMed] [Google Scholar]
- 132. Khorana AA, Ryan CK, Cox C, Eberly S, Sahasrabudhe DM. Vascular endothelial growth factor, CD68, and epidermal growth factor receptor expression and survival in patients with Stage II and Stage III colon carcinoma: a role for the host response in prognosis. Cancer (2003) 97(4):960–8. 10.1002/cncr.11152 [DOI] [PubMed] [Google Scholar]
- 133. Freire Valls A, Knipper K, Giannakouri E, Sarachaga V, Hinterkopf S, Wuehrl M, et al. VEGFR1(+) Metastasis-Associated Macrophages Contribute to Metastatic Angiogenesis and Influence Colorectal Cancer Patient Outcome. Clin Cancer Res (2019) 25(18):5674–85. 10.1158/1078-0432.CCR-18-2123 [DOI] [PubMed] [Google Scholar]
- 134. Katholnig K, Schutz B, Fritsch SD, Schorghofer D, Linke M, Sukhbaatar N, et al. Inactivation of mTORC2 in macrophages is a signature of colorectal cancer that promotes tumorigenesis. JCI insight (2019) 4(20):e124164. 10.1172/jci.insight.124164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Ikoma N, Raghav K, Chang G. An Update on Randomized Clinical Trials in Metastatic Colorectal Carcinoma. Surg Oncol Clin North Am (2017) 26(4):667–87. 10.1016/j.soc.2017.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Huang CM, Huang MY, Ma CJ, Yeh Y, Tsai HL, Huang CW, et al. Neoadjuvant FOLFOX chemotherapy combined with radiotherapy followed by radical resection in patients with locally advanced colon cancer. Radiat Oncol (2017) 12(1):48. 10.1186/s13014-017-0790-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Casadaban L, Rauscher G, Aklilu M, Villenes D, Freels S, Maker AV. Adjuvant chemotherapy is associated with improved survival in patients with stage II colon cancer. Cancer (2016) 122(21):3277–87. 10.1002/cncr.30181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Testa U, Pelosi E, Castelli G. Colorectal cancer: genetic abnormalities, tumor progression, tumor heterogeneity, clonal evolution and tumor-initiating cells. Med Sci (2018) 6(2):31. 10.3390/medsci6020031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Piawah S, Venook AP. Targeted therapy for colorectal cancer metastases: A review of current methods of molecularly targeted therapy and the use of tumor biomarkers in the treatment of metastatic colorectal cancer. Cancer (2019) 125(23):4139–47. 10.1002/cncr.32163 [DOI] [PubMed] [Google Scholar]
- 140. Dost Gunay FS, Kirmizi BA, Ensari A, Icli F, Akbulut H. Tumor-associated Macrophages and Neuroendocrine Differentiation Decrease the Efficacy of Bevacizumab Plus Chemotherapy in Patients With Advanced Colorectal Cancer. Clin colorectal Cancer (2019) 18(2):e244–e50. 10.1016/j.clcc.2018.12.004 [DOI] [PubMed] [Google Scholar]
- 141. Malesci A, Bianchi P, Celesti G, Basso G, Marchesi F, Grizzi F, et al. Tumor-associated macrophages and response to 5-fluorouracil adjuvant therapy in stage III colorectal cancer. Oncoimmunology (2017) 6(12):e1342918. 10.1080/2162402X.2017.1342918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Sunakawa Y, Stintzing S, Cao S, Heinemann V, Cremolini C, Falcone A, et al. Variations in genes regulating tumor-associated macrophages (TAMs) to predict outcomes of bevacizumab-based treatment in patients with metastatic colorectal cancer: results from TRIBE and FIRE3 trials. Ann Oncol (2015) 26(12):2450–6. 10.1093/annonc/mdv474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Zhang X, Chen Y, Hao L, Hou A, Chen X, Li Y, et al. Macrophages induce resistance to 5-fluorouracil chemotherapy in colorectal cancer through the release of putrescine. Cancer Lett (2016) 381(2):305–13. 10.1016/j.canlet.2016.08.004 [DOI] [PubMed] [Google Scholar]
- 144. Wei C, Yang C, Wang S, Shi D, Zhang C, Lin X, et al. M2 macrophages confer resistance to 5-fluorouracil in colorectal cancer through the activation of CCL22/PI3K/AKT signaling. OncoTargets Ther (2019) 12:3051–63. 10.2147/OTT.S198126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Liu Z, Xie Y, Xiong Y, Liu S, Qiu C, Zhu Z, et al. TLR 7/8 agonist reverses oxaliplatin resistance in colorectal cancer via directing the myeloid-derived suppressor cells to tumoricidal M1-macrophages. Cancer Lett (2020) 469:173–85. 10.1016/j.canlet.2019.10.020 [DOI] [PubMed] [Google Scholar]
- 146. Gou HF, Zhou L, Huang J, Chen XC. Intraperitoneal oxaliplatin administration inhibits the tumor immunosuppressive microenvironment in an abdominal implantation model of colon cancer. Mol Med Rep (2018) 18(2):2335–41. 10.3892/mmr.2018.9219 [DOI] [PubMed] [Google Scholar]
- 147. Limagne E, Thibaudin M, Nuttin L, Spill A, Derangere V, Fumet JD, et al. Trifluridine/Tipiracil plus Oxaliplatin Improves PD-1 Blockade in Colorectal Cancer by Inducing Immunogenic Cell Death and Depleting Macrophages. Cancer Immunol Res (2019) 7(12):1958–69. 10.1158/2326-6066.CIR-19-0228 [DOI] [PubMed] [Google Scholar]
- 148. Zhang W, Chen L, Ma K, Zhao Y, Liu X, Wang Y, et al. Polarization of macrophages in the tumor microenvironment is influenced by EGFR signaling within colon cancer cells. Oncotarget (2016) 7(46):75366–78. 10.18632/oncotarget.12207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Pander J, Heusinkveld M, van der Straaten T, Jordanova ES, Baak-Pablo R, Gelderblom H, et al. Activation of tumor-promoting type 2 macrophages by EGFR-targeting antibody cetuximab. Clin Cancer Res (2011) 17(17):5668–73. 10.1158/1078-0432.CCR-11-0239 [DOI] [PubMed] [Google Scholar]
- 150. Duma N, Santana-Davila R, Molina JR. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin Proc (2019) 94(8):1623–40. 10.1016/j.mayocp.2019.01.013 [DOI] [PubMed] [Google Scholar]
- 151. Oberndorfer F, Mullauer L. Molecular pathology of lung cancer: current status and perspectives. Curr Opin Oncol (2018) 30(2):69–76. 10.1097/CCO.0000000000000429 [DOI] [PubMed] [Google Scholar]
- 152. Lemjabbar-Alaoui H, Hassan OU, Yang YW, Buchanan P. Lung cancer: Biology and treatment options. Biochim Biophys Acta (2015) 1856(2):189–210. 10.1016/j.bbcan.2015.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ, Jr., Wu YL, et al. Lung cancer: current therapies and new targeted treatments. Lancet (2017) 389(10066):299–311. 10.1016/S0140-6736(16)30958-8 [DOI] [PubMed] [Google Scholar]
- 154. Mukaida N, Nosaka T, Nakamoto Y, Baba T. Lung Macrophages: Multifunctional Regulator Cells for Metastatic Cells. Int J Mol Sci (2018) 20(1):116. 10.3390/ijms20010116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Almatroodi SA, McDonald CF, Pouniotis DS. Alveolar Macrophage Polarisation in Lung Cancer. Lung Cancer Int (2014) 2014:721087. 10.1155/2014/721087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Guo Z, Song J, Hao J, Zhao H, Du X, Li E, et al. M2 macrophages promote NSCLC metastasis by upregulating CRYAB. Cell Death Dis (2019) 10(6):377. 10.1038/s41419-019-1618-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Argyle D, Kitamura T. Targeting Macrophage-Recruiting Chemokines as a Novel Therapeutic Strategy to Prevent the Progression of Solid Tumors. Front Immunol (2018) 9:2629. 10.3389/fimmu.2018.02629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Sarode P, Schaefer MB, Grimminger F, Seeger W, Savai R. Macrophage and Tumor Cell Cross-Talk Is Fundamental for Lung Tumor Progression: We Need to Talk. Front Oncol (2020) 10:324. 10.3389/fonc.2020.00324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Rolny C, Mazzone M, Tugues S, Laoui D, Johansson I, Coulon C, et al. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell (2011) 19(1):31–44. 10.1016/j.ccr.2010.11.009 [DOI] [PubMed] [Google Scholar]
- 160. Schmall A, Al-Tamari HM, Herold S, Kampschulte M, Weigert A, Wietelmann A, et al. Macrophage and cancer cell cross-talk via CCR2 and CX3CR1 is a fundamental mechanism driving lung cancer. Am J respiratory Crit Care Med (2015) 191(4):437–47. 10.1164/rccm.201406-1137OC [DOI] [PubMed] [Google Scholar]
- 161. Yuan S, Dong Y, Peng L, Yang M, Niu L, Liu Z, et al. Tumor-associated macrophages affect the biological behavior of lung adenocarcinoma A549 cells through the PI3K/AKT signaling pathway. Oncol Lett (2019) 18(2):1840–6. 10.3892/ol.2019.10483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Chen Y, Tan W, Wang C. Tumor-associated macrophage-derived cytokines enhance cancer stem-like characteristics through epithelial-mesenchymal transition. OncoTargets Ther (2018) 11:3817–26. 10.2147/OTT.S168317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Takeo S, Yasumoto K, Nagashima A, Nakahashi H, Sugimachi K, Nomoto K. Role of tumor-associated macrophages in lung cancer. Cancer Res (1986) 46(6):3179–82. [PubMed] [Google Scholar]
- 164. Wettersten HI, Weis SM, Pathria P, Von Schalscha T, Minami T, Varner JA, et al. Arming Tumor-Associated Macrophages to Reverse Epithelial Cancer Progression. Cancer Res (2019) 79(19):5048–59. 10.1158/0008-5472.CAN-19-1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Yusen W, Xia W, Shengjun Y, Shaohui Z, Hongzhen Z. The expression and significance of tumor associated macrophages and CXCR4 in non-small cell lung cancer. J BUON (2018) 23(2):398–402. [PubMed] [Google Scholar]
- 166. Sumitomo R, Hirai T, Fujita M, Murakami H, Otake Y, Huang CL. M2 tumor-associated macrophages promote tumor progression in non-small-cell lung cancer. Exp Ther Med (2019) 18(6):4490–8. 10.3892/etm.2019.8068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Carus A, Ladekarl M, Hager H, Pilegaard H, Nielsen PS, Donskov F. Tumor-associated neutrophils and macrophages in non-small cell lung cancer: no immediate impact on patient outcome. Lung Cancer (2013) 81(1):130–7. 10.1016/j.lungcan.2013.03.003 [DOI] [PubMed] [Google Scholar]
- 168. Li Z, Maeda D, Yoshida M, Umakoshi M, Nanjo H, Shiraishi K, et al. The intratumoral distribution influences the prognostic impact of CD68- and CD204-positive macrophages in non-small cell lung cancer. Lung Cancer (2018) 123:127–35. 10.1016/j.lungcan.2018.07.015 [DOI] [PubMed] [Google Scholar]
- 169. Rakaee M, Busund LR, Jamaly S, Paulsen EE, Richardsen E, Andersen S, et al. Prognostic Value of Macrophage Phenotypes in Resectable Non-Small Cell Lung Cancer Assessed by Multiplex Immunohistochemistry. Neoplasia (2019) 21(3):282–93. 10.1016/j.neo.2019.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Jackute J, Zemaitis M, Pranys D, Sitkauskiene B, Miliauskas S, Vaitkiene S, et al. Distribution of M1 and M2 macrophages in tumor islets and stroma in relation to prognosis of non-small cell lung cancer. BMC Immunol (2018) 19(1):3. 10.1186/s12865-018-0241-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Gong Z, Chen J, Cheng JN, Sun C, Jia Q, Diao X, et al. Tumor Microenvironment Properties are Associated With Low CD68-positive Cell Infiltration and Favorable Disease-free Survival in EGFR-mutant Lung Adenocarcinoma. Clin Lung Cancer (2018) 19(5):e551–e8. 10.1016/j.cllc.2018.03.011 [DOI] [PubMed] [Google Scholar]
- 172. Dai F, Liu L, Che G, Yu N, Pu Q, Zhang S, et al. The number and microlocalization of tumor-associated immune cells are associated with patient’s survival time in non-small cell lung cancer. BMC Cancer (2010) 10:220. 10.1186/1471-2407-10-220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Yang L, Wang F, Wang L, Huang L, Wang J, Zhang B, et al. CD163+ tumor-associated macrophage is a prognostic biomarker and is associated with therapeutic effect on malignant pleural effusion of lung cancer patients. Oncotarget (2015) 6(12):10592–603. 10.18632/oncotarget.3547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Chen L, Li Q, Zhou XD, Shi Y, Yang L, Xu SL, et al. Increased pro-angiogenic factors, infiltrating neutrophils and CD163(+) macrophages in bronchoalveolar lavage fluid from lung cancer patients. Int Immunopharmacol (2014) 20(1):74–80. 10.1016/j.intimp.2014.02.025 [DOI] [PubMed] [Google Scholar]
- 175. Kwiecien I, Polubiec-Kownacka M, Dziedzic D, Wolosz D, Rzepecki P, Domagala-Kulawik J. CD163 and CCR7 as markers for macrophage polarization in lung cancer microenvironment. Central-European J Immunol (2019) 44(4):395–402. 10.5114/ceji.2019.92795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Ohtaki Y, Ishii G, Nagai K, Ashimine S, Kuwata T, Hishida T, et al. Stromal macrophage expressing CD204 is associated with tumor aggressiveness in lung adenocarcinoma. J Thorac Oncol (2010) 5(10):1507–15. 10.1097/JTO.0b013e3181eba692 [DOI] [PubMed] [Google Scholar]
- 177. Hirayama S, Ishii G, Nagai K, Ono S, Kojima M, Yamauchi C, et al. Prognostic impact of CD204-positive macrophages in lung squamous cell carcinoma: possible contribution of Cd204-positive macrophages to the tumor-promoting microenvironment. J Thorac Oncol (2012) 7(12):1790–7. 10.1097/JTO.0b013e3182745968 [DOI] [PubMed] [Google Scholar]
- 178. Maeda R, Ishii G, Neri S, Aoyagi K, Haga H, Sasaki H, et al. Circulating CD14+CD204+ cells predict postoperative recurrence in non-small-cell lung cancer patients. J Thorac Oncol (2014) 9(2):179–88. 10.1097/JTO.0000000000000044 [DOI] [PubMed] [Google Scholar]
- 179. La Fleur L, Boura VF, Alexeyenko A, Berglund A, Ponten V, Mattsson JSM, et al. Expression of scavenger receptor MARCO defines a targetable tumor-associated macrophage subset in non-small cell lung cancer. Int J Cancer (2018) 143(7):1741–52. 10.1002/ijc.31545 [DOI] [PubMed] [Google Scholar]
- 180. Wang R, Zhang J, Chen S, Lu M, Luo X, Yao S, et al. Tumor-associated macrophages provide a suitable microenvironment for non-small lung cancer invasion and progression. Lung Cancer (2011) 74(2):188–96. 10.1016/j.lungcan.2011.04.009 [DOI] [PubMed] [Google Scholar]
- 181. Yadav MK, Inoue Y, Nakane-Otani A, Tsunakawa Y, Jeon H, Samir O, et al. Transcription factor MafB is a marker of tumor-associated macrophages in both mouse and humans. Biochem Biophys Res Commun (2020) 521(3):590–5. 10.1016/j.bbrc.2019.10.125 [DOI] [PubMed] [Google Scholar]
- 182. Chen C, Zhu YB, Shen Y, Zhu YH, Zhang XG, Huang JA. Increase of circulating B7-H4-expressing CD68+ macrophage correlated with clinical stage of lung carcinomas. J Immunother (2012) 35(4):354–8. 10.1097/CJI.0b013e31824212c4 [DOI] [PubMed] [Google Scholar]
- 183. Liao Y, Guo S, Chen Y, Cao D, Xu H, Yang C, et al. VSIG4 expression on macrophages facilitates lung cancer development. Lab Invest (2014) 94(7):706–15. 10.1038/labinvest.2014.73 [DOI] [PubMed] [Google Scholar]
- 184. Ho CC, Liao WY, Wang CY, Lu YH, Huang HY, Chen HY, et al. TREM-1 expression in tumor-associated macrophages and clinical outcome in lung cancer. Am J Respir Crit Care Med (2008) 177(7):763–70. 10.1164/rccm.200704-641OC [DOI] [PubMed] [Google Scholar]
- 185. Li Y, Sun BS, Pei B, Li CG, Zhang ZF, Yin YS, et al. Osteopontin-expressing macrophages in non-small cell lung cancer predict survival. Ann Thorac Surg (2015) 99(4):1140–8. 10.1016/j.athoracsur.2014.11.054 [DOI] [PubMed] [Google Scholar]
- 186. Parra ER, Villalobos P, Behrens C, Jiang M, Pataer A, Swisher SG, et al. Effect of neoadjuvant chemotherapy on the immune microenvironment in non-small cell lung carcinomas as determined by multiplex immunofluorescence and image analysis approaches. J Immunother Cancer (2018) 6(1):48. 10.1186/s40425-018-0368-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Feng PH, Yu CT, Wu CY, Lee MJ, Lee WH, Wang LS, et al. Tumor-associated macrophages in stage IIIA pN2 non-small cell lung cancer after neoadjuvant chemotherapy and surgery. Am J Trans Res (2014) 6(5):593–603. [PMC free article] [PubMed] [Google Scholar]
- 188. Remark R, Lupo A, Alifano M, Biton J, Ouakrim H, Stefani A, et al. Immune contexture and histological response after neoadjuvant chemotherapy predict clinical outcome of lung cancer patients. Oncoimmunology (2016) 5(12):e1255394. 10.1080/2162402X.2016.1255394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Huang A, Zhang B, Wang B, Zhang F, Fan KX, Guo YJ. Increased CD14(+)HLA-DR (-/low) myeloid-derived suppressor cells correlate with extrathoracic metastasis and poor response to chemotherapy in non-small cell lung cancer patients. Cancer Immunol Immunother CII (2013) 62(9):1439–51. 10.1007/s00262-013-1450-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Haku T, Yanagawa H, Ohmoto Y, Takeuchi E, Yano S, Hanibuchi M, et al. Systemic chemotherapy alters interleukin-1 beta and its receptor antagonist production by human alveolar macrophages in lung cancer patients. Oncol Res (1996) 8(12):519–26. [PubMed] [Google Scholar]
- 191. Sun F, Cui L, Li T, Chen S, Song J, Li D. Oxaliplatin induces immunogenic cells death and enhances therapeutic efficacy of checkpoint inhibitor in a model of murine lung carcinoma. J Recept Signal Transduct Res (2019) 39(3):208–14. 10.1080/10799893.2019.1655050 [DOI] [PubMed] [Google Scholar]
- 192. Chung FT, Lee KY, Wang CW, Heh CC, Chan YF, Chen HW, et al. Tumor-associated macrophages correlate with response to epidermal growth factor receptor-tyrosine kinase inhibitors in advanced non-small cell lung cancer. Int J Cancer (2012) 131(3):E227–35. 10.1002/ijc.27403 [DOI] [PubMed] [Google Scholar]
- 193. Tariq M, Zhang JQ, Liang GK, He QJ, Ding L, Yang B. Gefitinib inhibits M2-like polarization of tumor-associated macrophages in Lewis lung cancer by targeting the STAT6 signaling pathway. Acta Pharmacol Sin (2017) 38(11):1501–11. 10.1038/aps.2017.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Yao Z, Zhang J, Zhang B, Liang G, Chen X, Yao F, et al. Imatinib prevents lung cancer metastasis by inhibiting M2-like polarization of macrophages. Pharmacol Res (2018) 133:121–31. 10.1016/j.phrs.2018.05.002 [DOI] [PubMed] [Google Scholar]
- 195. Peng H, Chen B, Huang W, Tang Y, Jiang Y, Zhang W, et al. Reprogramming Tumor-Associated Macrophages To Reverse EGFR(T790M) Resistance by Dual-Targeting Codelivery of Gefitinib/Vorinostat. Nano Lett (2017) 17(12):7684–90. 10.1021/acs.nanolett.7b03756 [DOI] [PubMed] [Google Scholar]
- 196. Yin W, Yu X, Kang X, Zhao Y, Zhao P, Jin H, et al. Remodeling Tumor-Associated Macrophages and Neovascularization Overcomes EGFR(T790M) -Associated Drug Resistance by PD-L1 Nanobody-Mediated Codelivery. Small (2018) 14(47):e1802372. 10.1002/smll.201802372 [DOI] [PubMed] [Google Scholar]
- 197. Watanabe H, Ohashi K, Nishii K, Seike K, Makimoto G, Hotta K, et al. A Long-term Response to Nivolumab in a Case of PD-L1-negative Lung Adenocarcinoma with an EGFR Mutation and Surrounding PD-L1-positive Tumor-associated Macrophages. Internal Med (2019) 58(20):3033–7. 10.2169/internalmedicine.2875-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Lo Russo G, Moro M, Sommariva M, Cancila V, Boeri M, Centonze G, et al. Antibody-Fc/FcR Interaction on Macrophages as a Mechanism for Hyperprogressive Disease in Non-small Cell Lung Cancer Subsequent to PD-1/PD-L1 Blockade. Clin Cancer Res (2019) 25(3):989–99. 10.1158/1078-0432.CCR-18-1390 [DOI] [PubMed] [Google Scholar]
- 199. Narod S. Can advanced-stage ovarian cancer be cured? Nat Rev Clin Oncol (2016) 13(4):255–61. 10.1038/nrclinonc.2015.224 [DOI] [PubMed] [Google Scholar]
- 200. Reid BM, Permuth JB, Sellers TA. Epidemiology of ovarian cancer: a review. Cancer Biol Med (2017) 14(1):9–32. 10.20892/j.issn.2095-3941.2016.0084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Baci D, Bosi A, Gallazzi M, Rizzi M, Noonan DM, Poggi A, et al. The Ovarian Cancer Tumor Immune Microenvironment (TIME) as Target for Therapy: A Focus on Innate Immunity Cells as Therapeutic Effectors. Int J Mol Sci (2020) 21(9)3125. 10.3390/ijms21093125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Steitz AM, Steffes A, Finkernagel F, Unger A, Sommerfeld L, Jansen JM, et al. Tumor-associated macrophages promote ovarian cancer cell migration by secreting transforming growth factor beta induced (TGFBI) and tenascin C. Cell Death Dis (2020) 11(4):249. 10.1038/s41419-020-2438-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Lengyel E. Ovarian cancer development and metastasis. Am J Pathol (2010) 177(3):1053–64. 10.2353/ajpath.2010.100105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Yin M, Shen J, Yu S, Fei J, Zhu X, Zhao J, et al. Tumor-Associated Macrophages (TAMs): A Critical Activator In Ovarian Cancer Metastasis. OncoTargets Ther (2019) 12:8687–99. 10.2147/OTT.S216355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. DiSaia PJ. Clin Gynecol Oncol. Amsterdam: Elsevier Science; (2012). [Google Scholar]
- 206. Henderson JT, Webber EM, Sawaya GF. Screening for Ovarian Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA (2018) 319(6):595–606. 10.1001/jama.2017.21421 [DOI] [PubMed] [Google Scholar]
- 207. Montfort A, Owen S, Piskorz AM, Supernat A, Moore L, Al-Khalidi S, et al. Combining measures of immune infiltration shows additive effect on survival prediction in high-grade serous ovarian carcinoma. Br J Cancer (2020) 122(12):1803–10. 10.1038/s41416-020-0822-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Zhang M, He Y, Sun X, Li Q, Wang W, Zhao A, et al. A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J Ovarian Res (2014) 7:19. 10.1186/1757-2215-7-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Lan C, Huang X, Lin S, Huang H, Cai Q, Wan T, et al. Expression of M2-polarized macrophages is associated with poor prognosis for advanced epithelial ovarian cancer. Technol Cancer Res Treat (2013) 12(3):259–67. 10.7785/tcrt.2012.500312 [DOI] [PubMed] [Google Scholar]
- 210. Maccio A, Gramignano G, Cherchi MC, Tanca L, Melis L, Madeddu C. Role of M1-polarized tumor-associated macrophages in the prognosis of advanced ovarian cancer patients. Sci Rep (2020) 10(1):6096. 10.1038/s41598-020-63276-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Yuan X, Zhang J, Li D, Mao Y, Mo F, Du W, et al. Prognostic significance of tumor-associated macrophages in ovarian cancer: A meta-analysis. Gynecol Oncol (2017) 147(1):181–7. 10.1016/j.ygyno.2017.07.007 [DOI] [PubMed] [Google Scholar]
- 212. No JH, Moon JM, Kim K, Kim YB. Prognostic significance of serum soluble CD163 level in patients with epithelial ovarian cancer. Gynecol Obstet Invest (2013) 75(4):263–7. 10.1159/000349892 [DOI] [PubMed] [Google Scholar]
- 213. Kawamura K, Komohara Y, Takaishi K, Katabuchi H, Takeya M. Detection of M2 macrophages and colony-stimulating factor 1 expression in serous and mucinous ovarian epithelial tumors. Pathol Int (2009) 59(5):300–5. 10.1111/j.1440-1827.2009.02369.x [DOI] [PubMed] [Google Scholar]
- 214. Ciucci A, Zannoni GF, Buttarelli M, Martinelli E, Mascilini F, Petrillo M, et al. Ovarian low and high grade serous carcinomas: hidden divergent features in the tumor microenvironment. Oncotarget (2016) 7(42):68033–43. 10.18632/oncotarget.10797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Qu QX, Huang Q, Shen Y, Zhu YB, Zhang XG. The increase of circulating PD-L1-expressing CD68(+) macrophage in ovarian cancer. Tumour Biol J Int Soc Oncodev Biol Med (2016) 37(4):5031–7. 10.1007/s13277-015-4066-y [DOI] [PubMed] [Google Scholar]
- 216. He YF, Zhang MY, Wu X, Sun XJ, Xu T, He QZ, et al. High MUC2 expression in ovarian cancer is inversely associated with the M1/M2 ratio of tumor-associated macrophages and patient survival time. PLoS One (2013) 8(12):e79769. 10.1371/journal.pone.0079769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med (2006) 203(4):871–81. 10.1084/jem.20050930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Liu L, Wang X, Li X, Wu X, Tang M, Wang X. Upregulation of IGF1 by tumor-associated macrophages promotes the proliferation and migration of epithelial ovarian cancer cells. Oncol Rep (2018) 39(2):818–26. 10.3892/or.2017.6148 [DOI] [PubMed] [Google Scholar]
- 219. Cortes M, Sanchez-Moral L, de Barrios O, Fernandez-Acenero MJ, Martinez-Campanario MC, Esteve-Codina A, et al. Tumor-associated macrophages (TAMs) depend on ZEB1 for their cancer-promoting roles. EMBO J (2017) 36(22):3336–55. 10.15252/embj.201797345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Zhang W, Tian J, Hao Q. HMGB1 combining with tumor-associated macrophages enhanced lymphangiogenesis in human epithelial ovarian cancer. Tumour Biol J Int Soc Oncodev Biol Med (2014) 35(3):2175–86. 10.1007/s13277-013-1288-8 [DOI] [PubMed] [Google Scholar]
- 221. Yin M, Li X, Tan S, Zhou HJ, Ji W, Bellone S, et al. Tumor-associated macrophages drive spheroid formation during early transcoelomic metastasis of ovarian cancer. J Clin Invest (2016) 126(11):4157–73. 10.1172/JCI87252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Gupta V, Yull F, Khabele D. Bipolar Tumor-Associated Macrophages in Ovarian Cancer as Targets for Therapy. Cancers (2018) 10(10):366. 10.3390/cancers10100366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Robinson-Smith TM, Isaacsohn I, Mercer CA, Zhou M, Van Rooijen N, Husseinzadeh N, et al. Macrophages mediate inflammation-enhanced metastasis of ovarian tumors in mice. Cancer Res (2007) 67(12):5708–16. 10.1158/0008-5472.CAN-06-4375 [DOI] [PubMed] [Google Scholar]
- 224. Finkernagel F, Reinartz S, Lieber S, Adhikary T, Wortmann A, Hoffmann N, et al. The transcriptional signature of human ovarian carcinoma macrophages is associated with extracellular matrix reorganization. Oncotarget (2016) 7(46):75339–52. 10.18632/oncotarget.12180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Adhikary T, Wortmann A, Finkernagel F, Lieber S, Nist A, Stiewe T, et al. Interferon signaling in ascites-associated macrophages is linked to a favorable clinical outcome in a subgroup of ovarian carcinoma patients. BMC Genomics (2017) 18(1):243. 10.1186/s12864-017-3630-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Worzfeld T, Finkernagel F, Reinartz S, Konzer A, Adhikary T, Nist A, et al. Proteotranscriptomics Reveal Signaling Networks in the Ovarian Cancer Microenvironment. Mol Cell Proteomics MCP (2018) 17(2):270–89. 10.1074/mcp.RA117.000400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Reinartz S, Finkernagel F, Adhikary T, Rohnalter V, Schumann T, Schober Y, et al. A transcriptome-based global map of signaling pathways in the ovarian cancer microenvironment associated with clinical outcome. Genome Biol (2016) 17(1):108. 10.1186/s13059-016-0956-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Orr B, Edwards RP. Diagnosis and Treatment of Ovarian Cancer. Hematology/oncology Clin North Am (2018) 32(6):943–64. 10.1016/j.hoc.2018.07.010 [DOI] [PubMed] [Google Scholar]
- 229. Eisenhauer EA. Real-world evidence in the treatment of ovarian cancer. Ann Oncol (2017) 28(suppl_8):viii61–viii5. 10.1093/annonc/mdx443 [DOI] [PubMed] [Google Scholar]
- 230. Liu W, Wang W, Wang X, Xu C, Zhang N, Di W. Cisplatin-stimulated macrophages promote ovarian cancer migration via the CCL20-CCR6 axis. Cancer Lett (2020) 472:59–69. 10.1016/j.canlet.2019.12.024 [DOI] [PubMed] [Google Scholar]
- 231. Mlynska A, Povilaityte E, Zemleckaite I, Zilionyte K, Strioga M, Krasko J, et al. Platinum sensitivity of ovarian cancer cells does not influence their ability to induce M2-type macrophage polarization. Am J Reprod Immunol (2018) 80(3):e12996. 10.1111/aji.12996 [DOI] [PubMed] [Google Scholar]
- 232. Liu R, Hu R, Zeng Y, Zhang W, Zhou HH. Tumour immune cell infiltration and survival after platinum-based chemotherapy in high-grade serous ovarian cancer subtypes: A gene expression-based computational study. EBioMedicine (2020) 51:102602. 10.1016/j.ebiom.2019.102602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. van Baal J, Lok CAR, Jordanova ES, Horlings H, van Driel WJ, Amant FC, et al. The effect of the peritoneal tumor microenvironment on invasion of peritoneal metastases of high-grade serous ovarian cancer and the impact of NEOADJUVANT chemotherapy. Virchows Archiv (2020) 477(4):535–44. 10.1007/s00428-020-02795-8 [DOI] [PubMed] [Google Scholar]
- 234. Wanderley CW, Colon DF, Luiz JPM, Oliveira FF, Viacava PR, Leite CA, et al. Paclitaxel Reduces Tumor Growth by Reprogramming Tumor-Associated Macrophages to an M1 Profile in a TLR4-Dependent Manner. Cancer Res (2018) 78(20):5891–900. 10.1158/0008-5472.CAN-17-3480 [DOI] [PubMed] [Google Scholar]
- 235. Geller MA, Bui-Nguyen TM, Rogers LM, Ramakrishnan S. Chemotherapy induces macrophage chemoattractant protein-1 production in ovarian cancer. Int J Gynecol Cancer (2010) 20(6):918–25. 10.1111/IGC.0b013e3181e5c442 [DOI] [PubMed] [Google Scholar]
- 236. Gartung A, Yang J, Sukhatme VP, Bielenberg DR, Fernandes D, Chang J, et al. Suppression of chemotherapy-induced cytokine/lipid mediator surge and ovarian cancer by a dual COX-2/sEH inhibitor. Proc Natl Acad Sci U S A (2019) 116(5):1698–703. 10.1073/pnas.1803999116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Parayath NN, Gandham SK, Leslie F, Amiji MM. Improved anti-tumor efficacy of paclitaxel in combination with MicroRNA-125b-based tumor-associated macrophage repolarization in epithelial ovarian cancer. Cancer Lett (2019) 461:1–9. 10.1016/j.canlet.2019.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Lu X, Meng T. Depletion of tumor-associated macrophages enhances the anti-tumor effect of docetaxel in a murine epithelial ovarian cancer. Immunobiology (2019) 224(3):355–61. 10.1016/j.imbio.2019.03.002 [DOI] [PubMed] [Google Scholar]
- 239. Vankerckhoven A, Wouters R, Mathivet T, Ceusters J, Baert T, Van Hoylandt A, et al. Opposite Macrophage Polarization in Different Subsets of Ovarian Cancer: Observation from a Pilot Study. Cells (2020) 9(2):305. 10.3390/cells9020305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Dijkgraaf EM, Santegoets SJ, Reyners AK, Goedemans R, Nijman HW, van Poelgeest MI, et al. A phase 1/2 study combining gemcitabine, Pegintron and p53 SLP vaccine in patients with platinum-resistant ovarian cancer. Oncotarget (2015) 6(31):32228–43. 10.18632/oncotarget.4772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Pernar CH, Ebot EM, Wilson KM, Mucci LA. The Epidemiology of Prostate Cancer. Cold Spring Harb Perspect Med (2018) 8(12):a030361. 10.1101/cshperspect.a030361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Global Burden of Disease Cancer C. Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol (2019) 5(12):1749–68. 10.1001/jamaoncol.2019.2996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Watson PA, Arora VK, Sawyers CL. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat Rev Cancer (2015) 15(12):701–11. 10.1038/nrc4016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Wang G, Zhao D, Spring DJ, DePinho RA. Genetics and biology of prostate cancer. Genes Dev (2018) 32(17-18):1105–40. 10.1101/gad.315739.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Testa U, Castelli G, Pelosi E. Cellular and Molecular Mechanisms Underlying Prostate Cancer Development: Therapeutic Implications. Medicines (2019) 6(3):82. 10.3390/medicines6030082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Budnik J, Suri J, Bates JE, Bylund KC, Milano MT. Prognostic Significance of Sites of Visceral Metastatic Disease in Prostate Cancer: A Population-based Study of 12,180 Patients. Clin Genitourin Cancer (2019) 17(4):260–7. 10.1016/j.clgc.2019.03.020 [DOI] [PubMed] [Google Scholar]
- 247. Harryman WL, Hinton JP, Rubenstein CP, Singh P, Nagle RB, Parker SJ, et al. The Cohesive Metastasis Phenotype in Human Prostate Cancer. Biochim Biophys Acta (2016) 1866(2):221–31. 10.1016/j.bbcan.2016.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Welch HG, Albertsen PC. Reconsidering Prostate Cancer Mortality - The Future of PSA Screening. N Engl J Med (2020) 382(16):1557–63. 10.1056/NEJMms1914228 [DOI] [PubMed] [Google Scholar]
- 249. Nagpal K, Foote D, Liu Y, Chen PC, Wulczyn E, Tan F, et al. Development and validation of a deep learning algorithm for improving Gleason scoring of prostate cancer. NPJ Digit Med (2019) 2:48. 10.1038/s41746-019-0112-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Strasner A, Karin M. Immune Infiltration and Prostate Cancer. Front Oncol (2015) 5:128. 10.3389/fonc.2015.00128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Lo CH, Lynch CC. Multifaceted Roles for Macrophages in Prostate Cancer Skeletal Metastasis. Front Endocrinol (2018) 9:247. 10.3389/fendo.2018.00247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Sfanos KS, Yegnasubramanian S, Nelson WG, De Marzo AM. The inflammatory microenvironment and microbiome in prostate cancer development. Nat Rev Urol (2018) 15(1):11–24. 10.1038/nrurol.2017.167 [DOI] [PubMed] [Google Scholar]
- 253. Tyekucheva S, Bowden M, Bango C, Giunchi F, Huang Y, Zhou C, et al. Stromal and epithelial transcriptional map of initiation progression and metastatic potential of human prostate cancer. Nat Commun (2017) 8(1):420. 10.1038/s41467-017-00460-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Cao J, Liu J, Xu R, Zhu X, Zhao X, Qian BZ. Prognostic role of tumour-associated macrophages and macrophage scavenger receptor 1 in prostate cancer: a systematic review and meta-analysis. Oncotarget (2017) 8(47):83261–9. 10.18632/oncotarget.18743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Shimura S, Yang G, Ebara S, Wheeler TM, Frolov A, Thompson TC. Reduced infiltration of tumor-associated macrophages in human prostate cancer: association with cancer progression. Cancer Res (2000) 60(20):5857–61. [PubMed] [Google Scholar]
- 256. Ok Atilgan A, Ozdemir BH, Akcay EY, Ataol Demirkan O, Tekindal MA, Ozkardes H. Role of tumor-associated macrophages in the Hexim1 and TGFbeta/SMAD pathway, and their influence on progression of prostatic adenocarcinoma. Pathol Res Pract (2016) 212(2):83–92. 10.1016/j.prp.2015.10.011 [DOI] [PubMed] [Google Scholar]
- 257. Comito G, Giannoni E, Segura CP, Barcellos-de-Souza P, Raspollini MR, Baroni G, et al. Cancer-associated fibroblasts and M2-polarized macrophages synergize during prostate carcinoma progression. Oncogene (2014) 33(19):2423–31. 10.1038/onc.2013.191 [DOI] [PubMed] [Google Scholar]
- 258. Lundholm M, Hagglof C, Wikberg ML, Stattin P, Egevad L, Bergh A, et al. Secreted Factors from Colorectal and Prostate Cancer Cells Skew the Immune Response in Opposite Directions. Sci Rep (2015) 5:15651. 10.1038/srep15651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Takayama H, Nonomura N, Nishimura K, Oka D, Shiba M, Nakai Y, et al. Decreased immunostaining for macrophage scavenger receptor is associated with poor prognosis of prostate cancer. BJU Int (2009) 103(4):470–4. 10.1111/j.1464-410X.2008.08013.x [DOI] [PubMed] [Google Scholar]
- 260. Lissbrant IF, Stattin P, Wikstrom P, Damber JE, Egevad L, Bergh A. Tumor associated macrophages in human prostate cancer: relation to clinicopathological variables and survival. Int J Oncol (2000) 17(3):445–51. 10.3892/ijo.17.3.445 [DOI] [PubMed] [Google Scholar]
- 261. Torkko KC, Wilson RS, Smith EE, Kusek JW, van Bokhoven A, Lucia MS. Prostate Biopsy Markers of Inflammation are Associated with Risk of Clinical Progression of Benign Prostatic Hyperplasia: Findings from the MTOPS Study. J Urol (2015) 194(2):454–61. 10.1016/j.juro.2015.03.103 [DOI] [PubMed] [Google Scholar]
- 262. Gollapudi K, Galet C, Grogan T, Zhang H, Said JW, Huang J, et al. Association between tumor-associated macrophage infiltration, high grade prostate cancer, and biochemical recurrence after radical prostatectomy. Am J Cancer Res (2013) 3(5):523–9. [PMC free article] [PubMed] [Google Scholar]
- 263. Lanciotti M, Masieri L, Raspollini MR, Minervini A, Mari A, Comito G, et al. The role of M1 and M2 macrophages in prostate cancer in relation to extracapsular tumor extension and biochemical recurrence after radical prostatectomy. BioMed Res Int (2014) 2014:486798. 10.1155/2014/486798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Richardsen E, Uglehus RD, Due J, Busch C, Busund LT. The prognostic impact of M-CSF, CSF-1 receptor, CD68 and CD3 in prostatic carcinoma. Histopathology (2008) 53(1):30–8. 10.1111/j.1365-2559.2008.03058.x [DOI] [PubMed] [Google Scholar]
- 265. Jones JD, Sinder BP, Paige D, Soki FN, Koh AJ, Thiele S, et al. Trabectedin Reduces Skeletal Prostate Cancer Tumor Size in Association with Effects on M2 Macrophages and Efferocytosis. Neoplasia (2019) 21(2):172–84. 10.1016/j.neo.2018.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Zhao SG, Lehrer J, Chang SL, Das R, Erho N, Liu Y, et al. The Immune Landscape of Prostate Cancer and Nomination of PD-L2 as a Potential Therapeutic Target. J Natl Cancer Institute (2019) 111(3):301–10. 10.1093/jnci/djy141 [DOI] [PubMed] [Google Scholar]
- 267. Erlandsson A, Carlsson J, Lundholm M, Falt A, Andersson SO, Andren O, et al. M2 macrophages and regulatory T cells in lethal prostate cancer. Prostate (2019) 79(4):363–9. 10.1002/pros.23742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Hu W, Qian Y, Yu F, Liu W, Wu Y, Fang X, et al. Alternatively activated macrophages are associated with metastasis and poor prognosis in prostate adenocarcinoma. Oncol Lett (2015) 10(3):1390–6. 10.3892/ol.2015.3400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Zarif JC, Baena-Del Valle JA, Hicks JL, Heaphy CM, Vidal I, Luo J, et al. Mannose Receptor-positive Macrophage Infiltration Correlates with Prostate Cancer Onset and Metastatic Castration-resistant Disease. Eur Urol Oncol (2019) 2(4):429–36. 10.1016/j.euo.2018.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Nonomura N, Takayama H, Kawashima A, Mukai M, Nagahara A, Nakai Y, et al. Decreased infiltration of macrophage scavenger receptor-positive cells in initial negative biopsy specimens is correlated with positive repeat biopsies of the prostate. Cancer Sci (2010) 101(6):1570–3. 10.1111/j.1349-7006.2010.01563.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Yang G, Addai J, Tian WH, Frolov A, Wheeler TM, Thompson TC. Reduced infiltration of class A scavenger receptor positive antigen-presenting cells is associated with prostate cancer progression. Cancer Res (2004) 64(6):2076–82. 10.1158/0008-5472.can-03-4072 [DOI] [PubMed] [Google Scholar]
- 272. Libreros S, Iragavarapu-Charyulu V. YKL-40/CHI3L1 drives inflammation on the road of tumor progression. J Leukocyte Biol (2015) 98(6):931–6. 10.1189/jlb.3VMR0415-142R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Pouyafar A, Heydarabad MZ, Mahboob S, Mokhtarzadeh A, Rahbarghazi R. Angiogenic potential of YKL-40 in the dynamics of tumor niche. Biomed Pharmacother (2018) 100:478–85. 10.1016/j.biopha.2018.02.050 [DOI] [PubMed] [Google Scholar]
- 274. Brasso K, Christensen IJ, Johansen JS, Teisner B, Garnero P, Price PA, et al. Prognostic value of PINP, bone alkaline phosphatase, CTX-I, and YKL-40 in patients with metastatic prostate carcinoma. Prostate (2006) 66(5):503–13. 10.1002/pros.20311 [DOI] [PubMed] [Google Scholar]
- 275. Kalina JL, Neilson DS, Comber AP, Rauw JM, Alexander AS, Vergidis J, et al. Immune Modulation by Androgen Deprivation and Radiation Therapy: Implications for Prostate Cancer Immunotherapy. Cancers (2017) 9(2):13. 10.3390/cancers9020013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Lindahl C, Simonsson M, Bergh A, Thysell E, Antti H, Sund M, et al. Increased levels of macrophage-secreted cathepsin S during prostate cancer progression in TRAMP mice and patients. Cancer Genomics Proteomics (2009) 6(3):149–59. [PubMed] [Google Scholar]
- 277. Gannon PO, Poisson AO, Delvoye N, Lapointe R, Mes-Masson AM, Saad F. Characterization of the intra-prostatic immune cell infiltration in androgen-deprived prostate cancer patients. J Immunol Methods (2009) 348(1-2):9–17. 10.1016/j.jim.2009.06.004 [DOI] [PubMed] [Google Scholar]
- 278. Liu Q, Tong D, Liu G, Gao J, Wang LA, Xu J, et al. Metformin Inhibits Prostate Cancer Progression by Targeting Tumor-Associated Inflammatory Infiltration. Clin Cancer Res (2018) 24(22):5622–34. 10.1158/1078-0432.CCR-18-0420 [DOI] [PubMed] [Google Scholar]
- 279. Escamilla J, Schokrpur S, Liu C, Priceman SJ, Moughon D, Jiang Z, et al. CSF1 receptor targeting in prostate cancer reverses macrophage-mediated resistance to androgen blockade therapy. Cancer Res (2015) 75(6):950–62. 10.1158/0008-5472.CAN-14-0992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Wang C, Peng G, Huang H, Liu F, Kong DP, Dong KQ, et al. Blocking the Feedback Loop between Neuroendocrine Differentiation and Macrophages Improves the Therapeutic Effects of Enzalutamide (MDV3100) on Prostate Cancer. Clin Cancer Res (2018) 24(3):708–23. 10.1158/1078-0432.CCR-17-2446 [DOI] [PubMed] [Google Scholar]
- 281. Sorrentino C, Musiani P, Pompa P, Cipollone G, Di Carlo E. Androgen deprivation boosts prostatic infiltration of cytotoxic and regulatory T lymphocytes and has no effect on disease-free survival in prostate cancer patients. Clin Cancer Res (2011) 17(6):1571–81. 10.1158/1078-0432.CCR-10-2804 [DOI] [PubMed] [Google Scholar]
- 282. Gao J, Ward JF, Pettaway CA, Shi LZ, Subudhi SK, Vence LM, et al. VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat Med (2017) 23(5):551–5. 10.1038/nm.4308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Johansen JS, Brasso K, Iversen P, Teisner B, Garnero P, Price PA, et al. Changes of biochemical markers of bone turnover and YKL-40 following hormonal treatment for metastatic prostate cancer are related to survival. Clin Cancer Res (2007) 13(11):3244–9. 10.1158/1078-0432.CCR-06-2616 [DOI] [PubMed] [Google Scholar]
- 284. Darr C, Krafft U, Hadaschik B, Tschirdewahn S, Sevcenco S, Csizmarik A, et al. The Role of YKL-40 in Predicting Resistance to Docetaxel Chemotherapy in Prostate Cancer. Urol Int (2018) 101(1):65–73. 10.1159/000489891 [DOI] [PubMed] [Google Scholar]