Abstract
The coronavirus disease (COVID-19), which is also known as acute respiratory syndrome coronavirus-2 (SARS-CoV2) is a transmissible disease, has phenotypes varying from asymptomatic to Acute Respiratory Distress Syndrome (ARDS) or multiple organ dysfunction syndrome (MODS) and ultimately death in certain cases. Coagulation disorders are being frequently reported amongst these patients and the pathogenesis is still not completely understood. Proposed mechanisms for these coagulopathies comprise a hypercoagulable state with micro- and/or macro-thrombosis in the vessels. A number of changes have been reported or proposed in circulating prothrombotic factors in COVID-19 patients and includes elevation in both factor VIII and fibrinogen, circulating prothrombotic microparticles and hyperviscosity. The COVID-19 patients are showing varied coagulopathies and are at high risk for venous thromboembolism (VTE) which demands an early intervention. This paper reviews the evolving data regarding the evaluation and managing of coagulopathies in patients with COVID-19.
Keywords: COVID-19, SARS-CoV-2, Thrombosis, Anticoagulant therapy, Disseminated intravascular coagulation (DIC)
Abbreviations: VTE, venous thromboembolism; DOACs, direct oral anticoagulants; LMWH, low molecular weight heparin; INR, international normalized ratio; DIC, disseminated intravascular coagulation
1. Introduction
Coronaviruses belonging to the Coronaviridae family are large, enveloped RNA viruses that cause respiratory, hepatic, neurologic and enteric diseases if it is spread among humans, mammals and birds (Zhu et al., 2020). There are 7 identified corona viruses currently, 3 of them are extremely pathogenic which includes, Middle East Respiratory Syndrome (MERS)-CoV, Severe Acute Respiratory Syndrome (SARS-CoV), and SARS-CoV-2 (COVID-19) (Zhu et al., 2020). The other 4 are less infectious and only causing cold symptoms in immunocompetent subjects (Su et al., 2016). SARS-CoV was first ever coronavirus originated from China’s Foshan city and caused a pandemic during 2002–2003 (Zhong et al., 2003). The second was the coronavirus- MERS, which was reported to originate from the Arabian Peninsula in 2012 (Zaki et al., 2012). The third, and most recent new coronavirus is SARS-CoV-2 that has globally spread causing a severe disease in humans (Zhu et al., 2020). It was identified in November 2019 in Wuhan, China, as the cause of a group of pneumonia patients. World Health Organization (WHO) named novel coronavirus as COVID-19, which stands for coronavirus disease 2019 and characterized it as a pandemic in March 2020 due to its widespread (Rothan and Byrareddy, 2020).
SARS-CoV and COVID-19 both share the feature of human-to-human transmissions via binding the virus spike protein (S) which is receptor binding domain to its reciprocal cellular receptor described as the angiotensin-converting enzyme 2 (ACE2) receptor (Rothan and Byrareddy, 2020). Structural and biophysical evidence indicated that the COVID-19 spike protein bound ACE2 with an affinity magnitude 10 to 20-fold higher than SARS-CoV, thus facilitating its human to human spread (Liu et al., 2020).
In early infection, COVID-19 targets ACE2 expressing cells, such as nasal and bronchial epithelial cells and pneumocytes (Wiersinga et al., 2020). ACE2 receptor is also expressed on pulmonary capillary endothelial cells, enabling the virus to infect them in the infection later stages when its replication accelerates (Wiersinga et al., 2020). Eventually the epithelial-endothelial barrier integrity becomes compromised, augmenting the inflammatory response and initiating an influx of monocytes and neutrophils. Interstitial mononuclear inflammatory infiltrates and edema develops (Wiersinga et al., 2020). Development of pulmonary edema covering the alveolar spaces with hyaline membrane follows, consistent with early-phase acute respiratory distress syndrome (ARDS) (Xu et al., 2020). Bradykinin-dependent lung angioedema might carve up the disease (van de Veerdonk et al., 2020). Collectively, the hallmark characteristics of COVID-19 are endothelial barrier disruption, defective alveolar-capillary oxygen delivery, and compromised oxygen diffusion ability (Wiersinga et al., 2020).
The key pathogenesis of COVID-19 as a respiratory infection has been described as severe pneumonia, RNAaemia, together with incidence of ground-glass opacities, and acute cardiac damage (Rothan and Byrareddy, 2020). Aligned with inflammatory response proposed, significantly high blood levels of cytokines and chemokines were documented in patients with COVID-19 infection that included IL-1β, IL-1RA, IL-7, IL-8, IL-9, IL-10, basic FGF2, GCSF, GMCSF, IFNγ, IP10, MCP1, MIP1α, MIP1β, PDGFB, TNFα, and VEGFA (Huang et al., 2020). Severely ill patients administrated to intensive care unit (ICU) exhibited elevated levels of pro-inflammatory cytokines IL-2, IL-7, IL-10, GCSF, IP10, MCP1, MIP1α, and TNFα which are rationalized to enhance the disease severity (Huang et al., 2020, Rothan and Byrareddy, 2020).
Varying phenotypes have been reported in COVID-19 patients ranging from asymptomatic to severe, rapid MODS and sometimes death. Although COVID-19 mainly manifests as a respiratory tract infection, recent studies report that it involves several organ systems also including cardiovascular, hematopoietic, neurological, gastrointestinal, and immune system.
Coagulation disorders are also being frequently reported amongst these patients (Terpos et al., 2020). Blood hypercoagulability is not uncommon among hospitalized COVID-19 patients but the pathogenesis of hypercoagulability in these patients is not yet fully explained. A number of changes have been reported or proposed in circulating prothrombotic factors in patients with severe COVID-19 and includes an elevated factor VIII, elevated fibrinogen, circulating prothrombotic micro particles and hyperviscosity (Maier et al., 2020, Panigada et al., 2020, Ranucci et al., 2020).
The COVID-19 patients are showing varied coagulopathies and are at high risk for venous thromboembolisms (VTE) which demands an early intervention patients (Terpos et al., 2020).
This paper reviews the evolving data for the proposed pathogenesis models and management of coagulopathies in COVID-19 patients.
2. Coagulopathies in COVID-19 patients
The pathogenesis of hypercoagulability in COVID-19 is incompletely understood. Teuwen and his team in their study postulated that endothelial injury caused by direct invasion of endothelial cells by the SARS-CoV-2 virus, plays a central role in the pathogenesis of ARDS and organ catastrophe in COVID-19 severe patients (Teuwen et al., 2020). The hypercoaguable state has been confirmed by recent studies (Iba et al., 2020, Maier et al., 2020, Panigada et al., 2020, Ranucci et al., 2020, Teuwen et al., 2020) and has been termed as thromboinflammation or COVID-19-associated coagulopathy (CAC) (Yin et al., 2020). Another study (Maier et al., 2020), assessed hyperviscosity using capillary viscometry and reported an elevated hyperviscosity in all the 15 critically ill patients admitted in the ICU. CAC resembles well-characterized coagulopathies types such as sepsis-induced coagulopathy (SIC), disseminated intravascular coagulation (DIC), and thrombotic microangiopathy, in any case, has novel highlights that empower it to be characterized as another class of coagulopathy (Iba et al., 2020). The involvement of multiple factors in CAC development necessitates additional understanding of the fundamental pathophysiology for suitable management (Iba et al., 2020).
A consistent hemostatic abnormality in COVID-19 patients is increased D-dimer levels (Panigada et al., 2020), which are associated with a higher risk and requires immediate intervention. D-dimer is a degradation by-product of cross-linked fibrin which indicates augmented thrombin generation and fibrin dissolution by plasmin. Elevated D-dimer concentrations correlate with illness severity and recently significantly elevated D-dimer levels are being constantly reported in the COVID-19 studies (Panigada et al., 2020, Ranucci et al., 2020). Panigada et al., 2020 also reported high fibrinogen and high factor VIII activity in COVID-19 patients, signifying absence of consumption of these coagulation factors and this differentiates COVID-19 disease from DIC state.
Yin et al., 2020 in their study, also reported higher platelet counts in COVID-19 patients than with other coronavirus infections and concluded that patients with significantly elevated D-dimer concentrations may benefit from anticoagulant treatment. Recently, similar findings have been reported (Fogarty et al., 2020), including high levels of D-dimer, fibrinogen, normal platelet counts and clotting times, between the regular medical ward and ICU patients. Another study (Zhou et al., 2020), even reported that an elevated D-dimer (≥1 μg/mL) was a strong independent risk factor for mortality. Disease severity is reported to be associated with a prolonged prothrombin time (PT), international normalized ratio (INR) (Huang et al., 2020, Zhou et al., 2020), and thrombin time (TT), and a shortened activated partial thromboplastin time (aPTT) (Gao et al., 2020). Tang et al., 2020a, Tang et al., 2020b observed with 71.4% of non-survivors meeting DIC requirements and a substantial improvement in D-dimer and fibrin degradation products (FDPs) and a PT prolongation, DIC is a good indicator of mortality (Tang et al., 2020b), with a reduction in fibrinogen in non-survivors at 10–14 days. Recently the incidence of VTE in COVID-19 patients who were not under thromboprophylaxis has been reported to be ≥25% and eventually 40% of them died (Cui et al., 2020). The authors also observed elevated levels of D-dimer (>1.5 μg/mL) in the same patients group (Cui et al., 2020).
3. Inflammation and coagulopathies in COVID-19 patients
In COVID-19 infection, the breakdown of lung cell activates a local multifactorial immune response, engaging macrophages and monocytes in response to infection, releasing cytokines, and priming adaptive T and B cell immune responses (Tay et al., 2020). The inflammatory response, particularly in COVID-19 severe cases, may also be pernicious and cause immune-mediated tissue injury and triggering a cytokine storm (Song et al., 2020). In severe COVID-19 cases, such inflammation related to the cytokine storm may spread from the local site where it started throughout the body via to the systemic circulation (Zhou et al., 2020). The sternness of COVID-19 also associates with inflammatory cytokines i.e. IL-2, IL-6, IL-7, IL-10, G-CSF, IP-10, MCP-1, MIP-1A and TNF-α, but there is still no clarity on the most likely cause of a cytokine storm (Sarzi-Puttini et al., 2020).
The inflammatory reaction will cause the coagulation cascade, which may partially explain the excessive coagulation in patients with COVD-19 (Zhang et al., 2020). Tissue factor is induced by IL-6 and other inflammatory cytokines such as IL-8, TNF-α, IFN-γ and IL-1β which in turn facilitate coagulation by activating the extrinsic coagulation pathway (Kaser et al., 2001). A significant correlation among pro-inflammatory cytokines IL-1β, IL-6, IL-8, TNF-α and coagulation-related indicators PT, aPTT, INR, TT, prothrombin activity (PTA), antithrombin (AT), and platelet count with an absence of association between anti-inflammatory features and those coagulation indicators were recently described in severe COVID-19 patients, supporting the notion that the disease pathogenesis might be mainly attributed to the cytokines (Zhang et al., 2020). Zhang et al were the first to investigate such a correlation and have additionally explained IL-6 and platelet count have a strong negative correlation. Their finding might indicate that rise in cytokine expression has caused an increase in the consumption of platelets or a decrease in their production (Zhang et al., 2020).
Collectively, recent studies highlight the significance of regular observation of these markers (both coagulation and inflammatory) and researchers are currently exploring the possible use of antithrombotics in managing COVID-19 patients.
4. Antithrombotics in COVID-19
Heparin is being explored as the potential anticoagulant because of its ability to reduce thrombi formation in microcirculation and its an anti-inflammatory action (Li and Ma, 2017). Tang et al., 2020a in their study on 449 severe COVID-19 patients, observed that anticoagulant therapy with low molecular weight heparin (LMWH) seemed to be linked with lower mortality (Tang et al., 2020a). With the established relationship between inflammation and thrombin formation, it was proposed that by blocking thrombin formation, heparin can decrease the inflammatory response. Li et al., 2018 in their meta-analysis reported a decrease in mortality with the early use of LMWH in an ARDS population with non-COVID-19 (Li et al., 2018).
Recently, a case series has projected that the usage of fibrinolytic therapy coupled with tissue plasminogen activator (tPA) in refractory hypoxia cases and has concluded that it results in improved levels during prolonged infusions (Wang et al., 2020). Despite this relation between inflammation and coagulation abnormalities, credible evidence is still lacking regarding the safety and efficacy of managing septic patients with heparin and/or antiplatelet therapies (Bikdeli et al., 2020). Conclusions to care for these patients with severe COVID-19 are challenged by the developing statistics and narratives and therefore clinicians are requested to make a thoughtful and informed clinical decision for every COVID-19 patient.
5. Recommendations on antithrombotic therapy in COVID-19 patients.
International Society of Thrombosis and Haemostasis (ISTH) has published the interim guidelines on acknowledgement and management of coagulopathy and venous thromboembolism in COVID-19 patients (Thachil et al., 2020). ISTH and other scientific academies like The American Society of Hematology (ASH), The North American Thrombosis Forum (NATF), the European Society of Vascular Medicine (ESVM), and the International Union of Angiology (IUA), have endorsed this interim guide (Bikdeli et al., 2020).
5.1. Patients (outpatient) with mild COVID-19
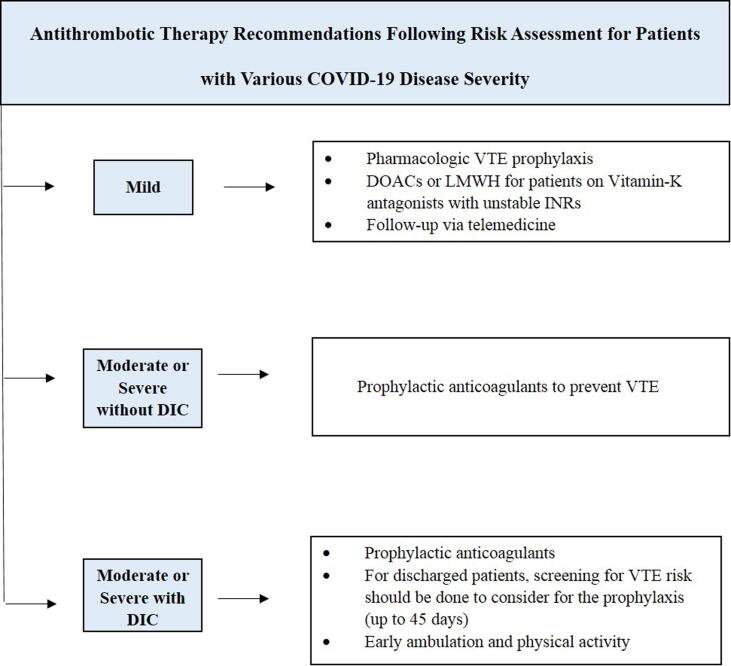
For mild category COVID-19 patients, risk assessment for VTE and outpatient administration or early discharge should be started to achieve the goal of providing antithrombotic protection while minimizing the social contact (Barco et al., 2020, Bikdeli et al., 2020). Patients must be followed-up using telemedicine and there should be reservation for in-person visits only for cases requiring hospitalization or could not be addressed by telemedicine (Bikdeli et al., 2020). Pharmacologic VTE prophylaxis should be careful after risk valuation on specific case basis.
Outpatients on vitamin-K antagonists having unstable INRs, can be directed to take direct oral anticoagulants (DOACs), if indicated and depending on drug availability and affordability (Bikdeli et al., 2020, Thachil et al., 2020). If DOACs are not indicated or available, a considerable alternative can be LMWH (Bikdeli et al., 2020).
5.2. Patients (hospitalized) with COVID-19, moderate or severe without DIC
After risk assessment in hospitalized COVID-19 without DIC, prophylactic anticoagulants can be prescribed to prevent VTE (Bikdeli et al., 2020, Thachil et al., 2020). At this stage, regular screening for VTE in hospitalized patients with elevated D-Dimer (>1,500 ng/mL), for instance via bilateral lower extremity ultrasound, is not indicated (Bikdeli et al., 2020).
5.3. Patients (hospitalized) with moderate or severe COVID-19 and suspected or confirmed DIC
Prophylactic anticoagulants should be administered immediately after risk assessment for patients with moderate or severe COVID-19 complicated with DIC but without explicit bleeding (Bikdeli et al., 2020, Thachil et al., 2020).
However, in these patients the clinician must study the suggestion for anticoagulant therapy and consider with bleeding risk for dose adjustments or discontinuation of the drug (Barco et al., 2020, Bikdeli et al., 2020). For COVID-19 patients who were admitted and have been discharged, screening for VTE risk should be done to consider for the prophylaxis (up to 45 days) (Spyropoulos et al., 2020). Clinicians must emphasize on the importance of early ambulation and should encourage the physical activity. Fig. 1 summarizes the antithrombotic therapy recommendations following risk assessment for patients with various COVID-19 disease severity.
Fig. 1.
Antithrombotic therapy recommendations for patients with various severity of COVID-19.
5.4. Patients with COVID-19 presenting with acute coronary syndrome (ACS)
For patients presenting with ST-segment elevation myocardial infarction (STEMI) and COVID-19, the risks and severity of the condition in the patient should be considered by the clinician and accordingly decide for a primary percutaneous coronary interference or fibrinolytic therapy (Bikdeli et al., 2020, Roffi et al., 2016).
5.5. Patients with a history of thrombotic disease but without COVID-19
There is no documented risk of developing a severe form of COVID-19 attributed to antithrombotic agents. Patients should continue administrating their antithrombotic agents as recommended by their clinicians (Barco et al., 2020, Bikdeli et al., 2020). Nevertheless, to decrease the risk of transmission of COVID-19 to healthcare worker and other community interactions, affected patients should be followed-up with using telemedicine as the preferred mode (Bikdeli et al., 2020, WHO, 2020).
5.6. Patients who develop new thrombotic disease but without COVID-19
For this category of patients, outpatient management or early discharge should be instituted to achieve the goal of providing antithrombotic protection while minimizing the social contact (Barco et al., 2020, Bikdeli et al., 2020) and affected patients should be followed-up using telemedicine.
5.7. Patients with co-morbid conditions but without COVID-19
Patients with co-morbid condition such as past VTE, active cancer, severe cardiopulmonary disease are included in this group and the guidelines for this group include enhanced mobility, and risk assessment for VTE risk and risk of bleeding are appropriate. Clinicians should consider the administration of VTE prophylaxis following risk assessment on an individual case basis (Bikdeli et al., 2020). Fig. 2 summarizes the antithrombotic therapy recommendations following risk assessment for patients with coexisting diseases with or without COVID-19.
Fig. 2.
Antithrombotic Therapy Recommendations for Patients with Coexisting Diseases with or without COVID-19 Following Risk Assessment.
6. Conclusion
The clinical understanding of COVID-19 patients management is inadequate and as more evidence is evolving to fill the gaps in the data for its management. Real incidence of VTE in COVID-19 is possibly underestimated because of many undiagnosed or asymptomatic cases and or the lack of systematic diagnosis. The noticeable manifestation from the hematopoietic system is often associated with a major blood hypercoagulability. Cautious assessment of laboratory baseline data during the disease course assists clinicians in planning a custom-made treatment for the affected patient and if required, and provide timely intensive care. Preventive thromboprophylaxis and early diagnosis of potentially complications will improve patient treatment outcomes. Continuous care is necessary and future studies must research whether optimal anticoagulation regimen with or without adjunctive antithrombotic therapies can be supportive in COVID-19 patients.
Financial support and sponsorship
Nil.
Declaration of Competing Interest
The author declares that she has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
None.
Footnotes
Peer review under responsibility of King Saud University.
References
- Barco S., Schmidtmann I., Ageno W., Bauersachs R.M., Becattini C., Bernardi E., Beyer-Westendorf J., Bonacchini L., Brachmann J., Christ M., Czihal M., Duerschmied D., Empen K., Espinola-Klein C., Ficker J.H., Fonseca C., Genth-Zotz S., Jiménez D., Harjola V.P., Held M., Prat L.I., Lange T.J., Manolis A., Meyer A., Mustonen P., Rauch-Kroehnert U., Ruiz-Artacho P., Schellong S., Schwaiblmair M., Stahrenberg R., Westerweel P.E., Wild P.S., Konstantinides S.V., Lankeit M. Early discharge and home treatment of patients with low-risk pulmonary embolism with the oral factor Xa inhibitor rivaroxaban: An international multicentre single-arm clinical trial. Eur. Heart J. 2020;41:509–518. doi: 10.1093/eurheartj/ehz367. [DOI] [PubMed] [Google Scholar]
- Bikdeli B., Madhavan M.V., Gupta A., Jimenez D., Burton J.R., Der Nigoghossian C., Chuich T., Nouri S.N., Dreyfus I., Driggin E., Sethi S., Sehgal K., Chatterjee S., Ageno W., Madjid M., Guo Y., Tang L.V., Hu Y., Bertoletti L., Giri J., Cushman M., Quéré I., Dimakakos E.P., Gibson C.M., Lippi G., Favaloro E.J., Fareed J., Tafur A.J., Francese D.P., Batra J., Falanga A., Clerkin K.J., Uriel N., Kirtane A., McLintock C., Hunt B.J., Spyropoulos A.C., Barnes G.D., Eikelboom J.W., Weinberg I., Schulman S., Carrier M., Piazza G., Beckman J.A., Leon M.B., Stone G.W., Rosenkranz S., Goldhaber S.Z., Parikh S.A., Monreal M., Krumholz H.M., Konstantinides S.V., Weitz J.I., Lip G.Y.H. Pharmacological Agents Targeting Thromboinflammation in COVID-19: Review and Implications for Future Research. Thromb. Haemost. 2020;120:1004–1024. doi: 10.1055/s-0040-1713152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18:1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty H., Townsend L., Ni Cheallaigh C., Bergin C., Martin-Loeches I., Browne P., Bacon C.L., Gaule R., Gillett A., Byrne M., Ryan K., O’Connell N., O’Sullivan J.M., Conlon N., O’Donnell J.S. COVID19 coagulopathy in Caucasian patients. Br. J. Haematol. 2020;189:1044–1049. doi: 10.1111/bjh.16749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Li T., Han M., Li X., Wu D., Xu Y., Zhu Y., Liu Y., Wang X., Wang L. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J. Med. Virol. 2020;92:791–796. doi: 10.1002/jmv.25770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba T., Levy J.H., Connors J.M., Warkentin T.E., Thachil J., Levi M., Su S., Wong G., Shi W., Liu J., Lai A.C.K., Zhou J., Liu W., Bi Y., Gao G.F., Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P., Xu Z., Shi L., Wang Y., Zhang J.Y., Huang L., Zhang C., Liu S.S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J.W., Xia P., Dong J., Zhao J., Wang F.F.S., van de Veerdonk F.L., Netea M.G., van Deuren M., van der Meer J.W.M., de Mast Q., Brüggemann R.J., van der Hoeven H., Vieira-de-Abreu A., Campbell R.A., Weyrich A.S., Zimmerman G.A., Zhong N.S., Zheng B.J., Li Y.M., Poon L.L.M., Xie Z.H., Chan K.H., Li P.H., Tan S.Y., Chang Q., Xie J.P., Liu X.Q., Xu J., Li D.X., Yuen K.Y., Peiris J.S.M., Guan Y., Barco S., Schmidtmann I., Ageno W., Bauersachs R.M., Becattini C., Bernardi E., Beyer-Westendorf J., Bonacchini L., Brachmann J., Christ M., Czihal M., Duerschmied D., Empen K., Espinola-Klein C., Ficker J.H., Fonseca C., Genth-Zotz S., Jiménez D., Harjola V.P., Held M., Prat L.I., Lange T.J., Manolis A., Meyer A., Mustonen P., Rauch-Kroehnert U., Ruiz-Artacho P., Schellong S., Schwaiblmair M., Stahrenberg R., Westerweel P.E., Wild P.S., Konstantinides S.V., Lankeit M., Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E., Nigoghossian C.D., Ageno W., Madjid M., Guo Y., Tang L.V., Hu Y., Giri J., Cushman M., Quéré I., Dimakakos E.P., Gibson C.M., Lippi G., Favaloro E.J., Fareed J., Caprini J.A., Tafur A.J., Burton J.R., Francese D.P., Wang E.Y., Falanga A., McLintock C., Hunt B.J., Spyropoulos A.C., Barnes G.D., Eikelboom J.W., Weinberg I., Schulman S., Carrier M., Piazza G., Beckman J.A., Steg P.G., Stone G.W., Rosenkranz S., Goldhaber S.Z., Parikh S.A., Monreal M., Krumholz H.M., Konstantinides S.V., Weitz J.I., Lip G.Y.H., Cui S., Chen S., Li X., Liu S.S., Wang F.F.S., Fogarty H., Townsend L., Ni Cheallaigh C., Bergin C., Martin-Loeches I., Browne P., Bacon C.L., Gaule R., Gillett A., Byrne M., Ryan K., O’Connell N., O’Sullivan J.M., Conlon N., O’Donnell J.S., Terpos E., Ntanasis-Stathopoulos I., Elalamy I., Kastritis E., Sergentanis T.N., Politou M., Psaltopoulou T., Gerotziafas G., Dimopoulos M.A., Maier C.L., Truong A.D., Auld S.C., Polly D.M., Tanksley C.L., Duncan A., Zhang D.W.D., Zhou X., Yan S., Tian R., Su L., Ding X., Xiao M., Chen Y., Zhao H., Chen H., Zhang H., Li Z., Li Q., Xu Y., Yan X., Li Y.M., Zhang S., Rothan H.A., Byrareddy S.N., Matheson B.N.J., Lehner P.J., Song J.W., Zhang C., Fan X., Meng F.P., Xu Z., Xia P., Cao W.J., Yang T., Dai X.P., Wang S.Y., Xu R.N., Jiang T.J., Li W.G., Zhang D.W.D., Zhao P., Shi M., Agrati C., Ippolito G., Maeurer M., Zumla A., Wang F.F.S., Zhang J.Y., Zhou Y., Fu B., Zheng X., Wang D., Zhao C., Qi Y., Sun R., Tian Z., Xu X., Wei H., Thachil J., Tang N., Gando S., Falanga A., Cattaneo M., Levi M., Clark C., Iba T., Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C., Iba T., Levy J.H., Connors J.M., Warkentin T.E., Thachil J., Levi M., Yin S., Huang M., Li D.X., Tang N. The unique characteristics of COVID-19 coagulopathy. Crit. Care. 2020;24:834–847. doi: 10.1186/s13054-020-03077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaser A., Brandacher G., Steurer W., Kaser S., Offner F.A., Zoller H., Theurl I., Widder W., Molnar C., Ludwiczek O., Atkins M.B., Mier J.W., Tilg H. Interleukin-6 stimulates thrombopoiesis through thrombopoietin: Role in inflammatory thrombocytosis. Blood. 2001;98:2720–2725. doi: 10.1182/blood.V98.9.2720. [DOI] [PubMed] [Google Scholar]
- Li J., Li Y., Yang B., Wang H., Li L. Low-molecular-weight heparin treatment for acute lung injury/acute respiratory distress syndrome: a meta-analysis of randomized controlled trials. Int. J. Clin. Exp. Med. 2018;11:414–422. [Google Scholar]
- Li X., Ma X. The role of heparin in sepsis: much more than just an anticoagulant. Br. J. Haematol. 2017;179:389–398. doi: 10.1111/bjh.14885. [DOI] [PubMed] [Google Scholar]
- Liu M., Wang T., Zhou Y., Zhao Y., Zhang Y., Li J. Potential role of ACE2 in coronavirus disease 2019 (COVID-19) prevention and management. J. Transl. Intern. Med. 2020;8:9–19. doi: 10.2478/jtim-2020-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier C.L., Truong A.D., Auld S.C., Polly D.M., Tanksley C.L., Duncan A. COVID-19-associated hyperviscosity: a link between inflammation and thrombophilia? Lancet. 2020;395:1758–1759. doi: 10.1016/S0140-6736(20)31209-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panigada M., Bottino N., Tagliabue P., Grasselli G., Novembrino C., Chantarangkul V., Pesenti A., Peyvandi F., Tripodi A. Hypercoagulability of COVID-19 patients in intensive care unit: A report of thromboelastography findings and other parameters of hemostasis. J. Thromb. Haemost. 2020;18:1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranucci M., Ballotta A., Di Dedda U., Bayshnikova E., Dei Poli M., Resta M., Falco M., Albano G., Menicanti L. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J. Thromb. Haemost. 2020;18:1747–1751. doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roffi M., Patrono C., Collet J.P., Mueller C., Valgimigli M., Andreotti F., Bax J.J., Borger M.A., Brotons C., Chew D.P., Gencer B., Hasenfuss G., Kjeldsen K., Lancellotti P., Landmesser U., Mehilli J., Mukherjee D., Storey R.F., Windecker S., Baumgartner H., Gaemperli O., Achenbach S., Agewall S., Badimon L., Baigent C., Bueno H., Bugiardini R., Carerj S., Casselman F., Cuisset T., Erol Ç., Fitzsimons D., Halle M., Hamm C., Hildick-Smith D., Huber K., Iliodromitis E., James S., Lewis B.S., Lip G.Y.H., Piepoli M.F., Richter D., Rosemann T., Sechtem U., Steg P.G., Vrints C., Zamorano J.L. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent st-segment elevation: Task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of. Eur. Heart J. 2016;37:267–315. doi: 10.1093/eurheartj/ehv320. [DOI] [PubMed] [Google Scholar]
- Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarzi-Puttini P., Giorgi V., Sirotti S., Marotto D., Ardizzone S., Rizzardini G., Antinori S., Galli M. COVID-19, cytokines and immunosuppression: what can we learn from severe acute respiratory syndrome? Clin. Exp. Rheumatol. 2020;38:337–342. [PubMed] [Google Scholar]
- Song J.W., Zhang C., Fan X., Meng F.P., Xu Z., Xia P., Cao W.J., Yang T., Dai X.P., Wang S.Y., Xu R.N., Jiang T.J., Li W.G., Zhang D.W., Zhao P., Shi M., Agrati C., Ippolito G., Maeurer M., Zumla A., Wang F.S., Zhang J.Y. Immunological and inflammatory profiles in mild and severe cases of COVID-19. Nat. Commun. 2020;11 doi: 10.1038/s41467-020-17240-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyropoulos A.C., Lipardi C., Xu J., Peluso C., Spiro T.E., De Sanctis Y., Barnathan E.S., Raskob G.E. Modified IMPROVE VTE Risk Score and Elevated D-Dimer Identify a High Venous Thromboembolism Risk in Acutely Ill Medical Population for Extended Thromboprophylaxis. TH Open. 2020;04:e59–e65. doi: 10.1055/s-0040-1705137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S., Wong G., Shi W., Liu J., Lai A.C.K., Zhou J., Liu W., Bi Y., Gao G.F. Epidemiology, Genetic Recombination, and Pathogenesis of Coronaviruses. Trends Microbiol. 2016;24:490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpos E., Ntanasis-Stathopoulos I., Elalamy I., Kastritis E., Sergentanis T.N., Politou M., Psaltopoulou T., Gerotziafas G., Dimopoulos M.A. Hematological findings and complications of COVID-19. Am. J. Hematol. 2020;95:834–847. doi: 10.1002/ajh.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuwen L.A., Geldhof V., Pasut A., Carmeliet P. COVID-19: the vasculature unleashed. Nat. Rev. Immunol. 2020;20:389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thachil J., Tang N., Gando S., Falanga A., Cattaneo M., Levi M., Clark C., Iba T. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J. Thromb. Haemost. 2020;18:1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Veerdonk F.L., Netea M.G., van Deuren M., van der Meer J.W.M., de Mast Q., Brüggemann R.J., van der Hoeven H. Kallikrein-kinin blockade in patients with covid-19 to prevent acute respiratory distress syndrome. Elife. 2020;9:1–10. doi: 10.7554/ELIFE.57555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Hajizadeh N., Moore E.E., McIntyre R.C., Moore P.K., Veress L.A., Yaffe M.B., Moore H.B., Barrett C.D. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): A case series. J. Thromb. Haemost. 2020;18:1752–1755. doi: 10.1111/jth.14828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO WHO siterep 73. World Heal. Organ. 2020;2020:2633. doi: 10.1056/NEJMoa2001316.4. [DOI] [Google Scholar]
- Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA - J. Am. Med. Assoc. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin S., Huang M., Li D., Tang N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J. Thromb. Thrombolysis. 2020;3–6 doi: 10.1007/s11239-020-02105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D.M.E., Fouchier R.A.M. Isolation of a Novel Coronavirus from a Man with Pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367:1814–1820. doi: 10.1056/nejmoa1211721. [DOI] [PubMed] [Google Scholar]
- Zhang D., Zhou X., Yan S., Tian R., Su L., Ding X., Xiao M., Chen Y., Zhao H., Chen H., Zhang H., Li Z., Li Q., Xu Y., Yan X., Li Y., Zhang S. Correlation between cytokines and coagulation-related parameters in patients with coronavirus disease 2019 admitted to ICU. Clin. Chim. Acta. 2020;510:47–53. doi: 10.1016/j.cca.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong N.S., Zheng B.J., Li Y.M., Poon L.L.M., Xie Z.H., Chan K.H., Li P.H., Tan S.Y., Chang Q., Xie J.P., Liu X.Q., Xu J., Li D.X., Yuen K.Y., Peiris J.S.M., Guan Y. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People’s Republic of China, in February, 2003. Lancet. 2003;362:1353–1358. doi: 10.1016/S0140-6736(03)14630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Fu B., Zheng X., Wang D., Zhao C., Qi Y., Sun R., Tian Z., Xu X., Wei H. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl. Sci. Rev. 2020;7:998–1002. doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/nejmoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]