Dear Editor,
Giardia spp. is a protozoan parasite that may inhabit the upper small intestine of mammals and is the aetiological agent of giardiasis, a self-limited illness in over 85% of affected individuals [1]. Giardiasis may be reactivated in immunocompromised hosts, thus indicating the effectiveness of normal host defence mechanisms against the parasite [1,2].
In December 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was associated with a cluster of respiratory tract infections in Wuhan, Hubei province, China. It rapidly spread worldwide, currently with more than 38.5 million confirmed cases and over 1,000,000 deaths [3].
During the peak of contagion on April the 10th in Italy, a 66-year-old man was admitted to the hospital with an acute respiratory distress syndrome due to SARS-CoV-2 infection. He was rapidly transported in intensive care unit (ICU) with endotracheal intubation and mechanical ventilation. During the third week of hospitalization in ICU, eosinophils rose up to 24.9%, with an absolute count of 2970/mm3, without constitutional symptoms, except for unformed feces, and increased progressively. However, respiratory symptoms and lymphopenia were improving at that time of the ICU admission: pressure support decreased, mechanical ventilation was stopped, total lymphocytes raised from 630 at admission in ICU to 2050 cells/mm3) (Table 1 ). The diagnostic dilemma of hypereosinophilia was solved with ova, cysts and parasite tests, over three consecutive days, which revealed a positive antigen test for Giardia spp. and a large amount of trophozoites and cysts of Giardia lamblia in the stool test. Moreover, other bacteriological, virological and parasitological stool tests were negative. In the complex cascade of the immune system function, after careful epidemiological considerations, we therefore hypothized that some mechanisms may intertwine between the anti-giardiasis and the anti-SARS-CoV-2 response. Upon administration of metronidazole, his hypereosinophilia improved returning to normal without recurrences (Table 1). Innate and adaptive immune responses were elicited in humans with Giardia spp. Moreover, this protozoan parasite does not invade epithelia, but it can induce local and systemic antibody and T-cell responses [1]. Early in giardiasis, operating defence mechanisms are B-cell-independent and include nitric oxide, reactive oxygen species, defensins, lactoferrins, and immune cells, such as phagocytes, mast cells, dendritic cells, and chemokines [2]. Subsequently, there is production of large amounts of parasite-specific IgA following infection, with the contribution of CD4+ T cell responses [1,2]. Among cytokines, interleukin (IL)-6 and IL-17 are important during Giardia infections in both animals and humans [1,2]. It has been suggested that the CD 4+ T cells play a double role during the anti-Giardia immune response. First, they activate and stimulate the differentiation of B cells to generate Giardia-specific antibodies. Second, they act through a B-cell-independent mechanism that is likely mediated by T-helper type 17 (Th17) cells [1,2].
Table 1.
Blood cells count and main laboratory values between admission (Day 0) to discharge from Intensive Care Unit (Day 42).
| Day 0 | Day 3 | Day 7 | Day 10 | Day 14 | Day 21 | Day 28 | Day 35 | Day 42 | |
|---|---|---|---|---|---|---|---|---|---|
| Total WBC count (× 10 × 9/L) | 13,7 | 7,9 | 13 | 9,4 | 7,4 | 12,1 | 8 | 14 | 12 |
| Lymphocytes (absolute count) | 940 | 630 | 580 | 540 | 620 | 3.230 | 1.870 | 2.040 | 2.050 |
| Eosinophils (absolute count) | 4 | 34 | 2 | 30 | 790 | 2970 | 590 | 520 | 460 |
| LDH (U/L) | 949 | 944 | 741 | 422 | 414 | NA | 599 | 489 | 422 |
| CRP (mg/L) | 417 | 111 | 11 | 37 | 95 | 64 | 177 | 81 | 26 |
| PCT (ng/mL) | 1,7 | 0,72 | 0,13 | 0,18 | 0,34 | 0,17 | 0,63 | 0,15 | 0,25 |
| IL-6 (pg/mL) | 121 | NA | 360 | NA | NA | 303 | 70,6 | 30,3 | NA |
| Ferritin (ng/mL) | 2203 | NA | 1242 | 1653 | NA | NA | NA | 2066 | NA |
Abbreviations: WBC, White Blood Cell; LDH, lactate dehydrogenases; CRP, C-reactive protein; PCT, procalcitonin; IL-6, interleuchin 6.
Concerning SARS-CoV-2, infected patients may present with lymphopenia, but the disease has also been associated with immune hyper-responsiveness referred to as a cytokine storm [4,5]. The acute lung injury observed in patients with severe COVID-19 is characterized by inflammation and tissue damage in the respiratory tract that has is highly correlated to Th17 cell responses, as IL-17 can prompt the apoptosis of alveolar epithelial cells and the progression to pulmonary fibrosis [4,5].
It is well established that CD4+ T cells are essential for the clearance of Giardia, since T cell-deficient mice cannot control infections, whereas B cell-deficient mice can eliminate parasites. CD8+ T cells are not required for parasite clearance [1,2]. It is becoming increasingly evident that IL-17, within the context of a Giardia infection, is essential for protective immunity [1,2]. This pro-inflammatory cytokine is produced by Th17 cells that require both IL-6 and TGF-β for their development. IL-6 deficent mice fail to clear infections and also lack IL-17 expression, which suggests Th17 cells are required for parasite clearance in an IL-6 dependent manner [1,2].
Cases in literature stated that giardiasis and hypereosinophilia association are extremely rare but with a relatively good prognosis [6].
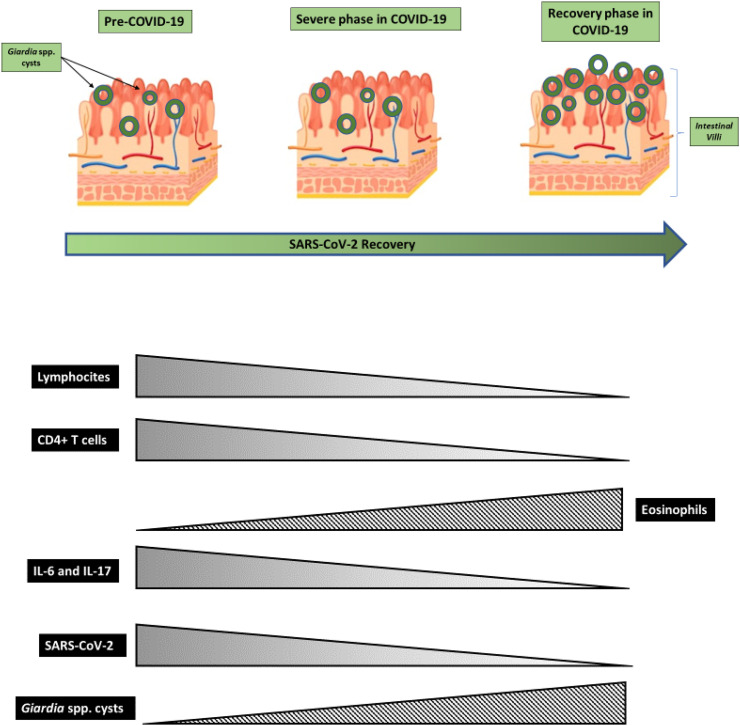
In our hypothesis, CD4+ T cells lymphopenia occurring in severe SARS-CoV-2 infected subjects may enable the avoidance of the clearance of Giardia lamblia infection or the development of invasive infection due to, albeit transitory, impaired immune response. High amounts of IL-17 and IL-6 in the acute phase of SARS-CoV-2 infection, however, kept the infection at bay until the cytokine storm passed away in the recovery phase (Fig. 1 ).
Fig. 1.
Hyphotetic trends in Lymphocites count, interleukins levels, viral and parasitic load during the different phases of SARS-CoV-2 infections.
Based on these observations, the risk of reactivation of latent parasite infection should be considered during SARS-CoV-2 infection, especially during the acute or recovery phase.
Funding
None.
Availability of data and material
Not requested.
Code availability
Not requested.
Authors' contributions
Each author contributed equally to this manuscript.
Declaration of Competing Interest
None.
References
- 1.Singer S.M., Fink M.Y., Angelova V.V. Recent insights into innate and adaptive immune responses to Giardia. Adv. Parasitol. 2019;106:171–208. doi: 10.1016/bs.apar.2019.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopez-Romero G., Quintero J., Astiazarán-García H., Velazquez C. Host defences against Giardia lamblia. Parasite Immunol. 2015;37(8):394–406. doi: 10.1111/pim.12210. [DOI] [PubMed] [Google Scholar]
- 3.https://coronavirus.jhu.edu/map.html
- 4.Azkur A.K., Akdis M., Azkur D. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy. 2020;75(7):1564–1581. doi: 10.1111/all.14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang L.X., Miao S.Y., Qin Z.H. Preliminary analysis of B- and T-cell responses to SARS-CoV-2 [published online ahead of print, 2020 Jul 24] Mol. Diagn. Ther. 2020 doi: 10.1007/s40291-020-00486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki Y., Nakamura T., Tokoro M. A case of giardiasis expressing severe systemic symptoms and marked hypereosinophilia. Parasitol. Int. 2010 Sep;59(3):487–489. doi: 10.1016/j.parint.2010.06.006. (Epub 2010 Jun 23. PMID: 20601107) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not requested.