Abstract
Viruses, including the novel coronavirus SARS-CoV-2, redirect infected cell metabolism to their own purposes. After binding to its receptor angiotensin-converting enzyme 2 (ACE2) on the cell surface, the SARS-CoV-2 is taken up by receptor-mediated endocytosis ending in the acidic endolysosomal compartment. The virus hijacks the endosomal machinery leading to fusion of viral and endosomal membranes and release of the viral RNA into the cytosol. This mini-review specifically highlights the membrane lipid organization of the endosomal system focusing on the unconventional and late endosome/lysosome-specific phospholipid, bis(monoacylglycero)phosphate (BMP). BMP is enriched in alveolar macrophages of lung, one of the target tissue of SARS-CoV-2. This review details the BMP structure, its unsaturated fatty acid composition and fusogenic properties that are essential for the highly dynamic formation of the intraluminal vesicles inside the endosomes. Interestingly, BMP is necessary for infection and replication of enveloped RNA virus such as SARS-CoV-1 and Dengue virus. We also emphasize the role of BMP in lipid sorting and degradation, especially cholesterol transport in cooperation with Niemann Pick type C proteins (NPC 1 and 2) and with some oxysterol-binding protein (OSBP)-related proteins (ORPs) as well as in sphingolipid degradation. Interestingly, numerous virus infection required NPC1 as well as ORPs along the endocytic pathway. Furthermore, BMP content is increased during pathological endosomal lipid accumulation in various lysosomal storage disorders. This is particularly important knowing the high percentage of patients with metabolic disorders among the SARS-CoV-2 infected patients presenting severe forms of COVID-19.
Keywords: Bis(monoacylglycero)phosphate, Cholesterol, Extracellular vesicles, Oxysterol-binding protein (OSBP)-Related proteins ORP, Niemann pick type C disease NPC, SARS-CoV-2
Abbreviations: ACE2, angiotensin-converting enzyme 2; BMP, bis(monoacylglycero)phosphate [also known as lysobisphosphatidic acid (LBPA)]; diC22:6-BMP, di-docosahexaenoyl BMP; CAD, cationic amphiphilic drug; CME, clathrin-mediated endocytosis; CoV, coronavirus; COVID-19, coronavirus disease-19; ESCRT, endosomal sorting complexes required for transport; ILV, intraluminal vesicle; LE/Lys, late endosome/lysosome; LSD, lysosomal storage disorder/disease; MCS, membrane contact sites; MERS, Middle East Respiratory Syndrome; MVE, multivesicular endosome; NPC, Niemann Pick type C; OSBP, oxysterol binding protein; ORP, OSBP-related protein; SARS, severe acute respiratory syndrome; TMPRSS2, transmembrane serine protease 2; VSV, vesicular stomatitis virus
Highlights
-
•
Host cell cholesterol is required for efficient viral infection.
-
•
NPC and ORPs regulate cholesterol exit from endolysosomes and viral infection.
-
•
Endosomal phospholipid BMP interacts with sterol transport proteins NPC and ORPs.
-
•
BMP by controlling cholesterol trafficking could be involved in virus infection.
-
•
BMP could be a potential therapeutic target for COVID-19.
1. Introduction
Coronaviruses are a group of enveloped, single-stranded positive genomic RNA viruses known to cause severe respiratory diseases in human, such as Severe Acute Respiratory Syndrome (SARS), Middle East Respiratory Syndrome (MERS) and the ongoing coronavirus disease-19 (COVID-19) due to the SARS-CoV-2 [1]. In order to treat and/or prevent the actual SARS-CoV-2 infection, a better understanding of the molecular mechanism used by this new virus is required. After binding to its receptor angiotensin-converting enzyme 2 (ACE2) found on the surface of cells lining the respiratory tract and lung [2], the virus is taken up by clathrin-mediated endocytosis (CME) and transported to the acidic endolysosomal compartment. The virus hijacks the endosomal machinery leading to fusion of viral and endosomal membranes and release of viral factors into the cytosol to infect the targeted cells. Noteworthy, viral proteins mediating this fusion usually require a specific lipid composition of the host cell membrane [3,4]. In fact, the virus is exploiting the different lipid composition of the various host cell organelles it travels through to control the intracellular location of its fusion process and thus, where its RNA will be released. These interactions between the virus and the host cell lipids can become useful therapeutic targets.
Using various viruses like the vesicular stomatitis virus (VSV) as tool has been fruitful and informative to better understand the successive steps of the endocytic pathway and the dynamic membrane traffic occurring from the early endosome to the late endosome/lysosome (LE/Lys) [[5], [6], [7], [8], [9]]. In addition, the host cell lipids are not distributed equally among the organelles. Sphingolipids and cholesterol display a gradual distribution with the highest concentration in the plasma membrane and the lowest concentration in the endoplasmic reticulum (ER) [10]. Furthermore, lipids form specific membrane domains in the plasma membrane (PM) as well as in organelle membranes. Sphingolipid- and cholesterol-enriched domains known as lipid rafts, are found on the outer leaflet of the PM and on the luminal side of the endosome [[11], [12], [13], [14]]. At the PM, these lipid domains in association with proteins play an essential role to form signaling platforms and to drive their subsequent internalization through the endosomal system [15]. In parallel to the sphingolipid- and cholesterol-enriched domains, different polyphosphoinositides enriched in the inner leaflet of the PM and in endosomes play important roles in various stages of endocytosis [9,16]. Another key anionic glycerophospholipid bis(monoacylglycero)phosphate (BMP), also named as lysobisphosphatidic acid (LBPA) is found specifically enriched in the internal membranes of the acidic LE/Lys compartment [16,17]. Interestingly, BMP and other anionic phospholipids are necessary for infection and replication of enveloped RNA virus such as SARS-CoV-1 and Dengue virus [4,18,19].
While the involvement of BMP as a key player of the anti-viral effect of chloroquine and derivatives is discussed by Carrière et al. in this special issue [20], this review will focus on the role of BMP in lipid sorting and degradation along the endocytic pathway potentially affecting virus infection. A specific focus is put on the effect of BMP on cholesterol transport regulated by Niemann Pick type C proteins (NPC 1 and 2) and some oxysterol-binding protein (OSBP)-related proteins (ORPs). This unusual endosomal phospholipid is crucial to maintain endosomal lipid storage capacity and trafficking as well as the fine-tuning of cholesterol domains at the PM known to control receptor signaling and cholesterol efflux [[21], [22], [23]]. Interestingly, the importance of the host cholesterol pools in viral fusion and replication was recently pointed out [24,25]. Since genetic NPC1 defect or drug (U18666A)-induced endosomal cholesterol accumulation were shown to inhibit Ebola virus and VSV infection [5,26], this review will provide some useful clues for the development of drugs against COVID-19, especially in the context of the fast drug repurposing occurring recently [25,27,28].
2. Bis(monoacylglycero)phosphate, an unusual endolysosomal phospholipid to control the fate of endocytosed compounds
BMP is specifically enriched in the LE/Lys compartment where it controls in particular the fate of sphingolipids and cholesterol. In fact, the late endosome is a strategic sorting station for various materials (including viruses) arriving from the endocytic, biosynthetic and autophagic pathways as well as outgoing to the lysosome for degradation, the Golgi complex, the ER or the PM. By controlling the reutilization or the degradation of endocytosed components, the LE/Lys compartment is important to inform the cell of its metabolic status.
2.1. Structure, biosynthesis and metabolism of BMP
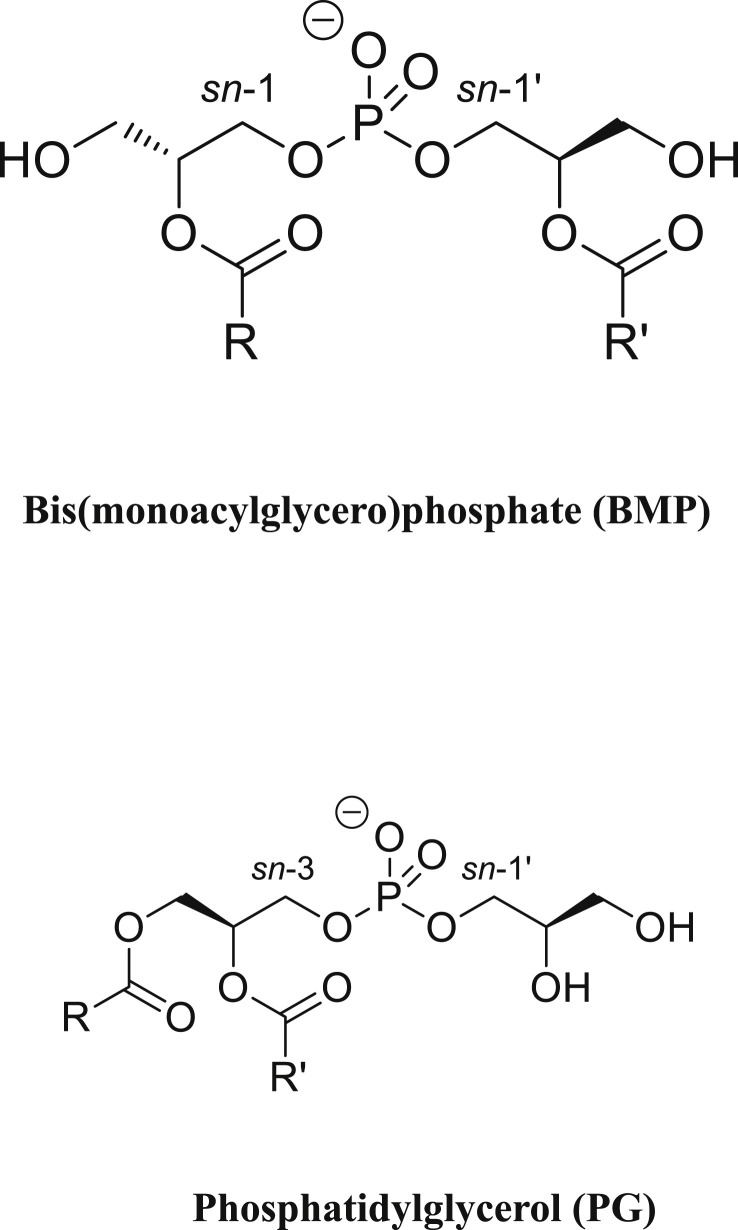
BMP is a structural isomer of phosphatidylglycerol (PG) where in contrast to PG, the two acyl chains (often identical) are not linked to the same glycerol (Fig. 1 ). It is considered as an unusual polyglycerophospholipid due to the sn-1 position of the phosphate moiety on the glycerol backbone found exclusively in the Archaea domain whereas it is linked to the sn-3 position in all the glycerophospholipids of Eukarya and Bacteria domains [22,29]. The exact position of the acyl chains on the glycerol moieties has been a matter of debate. However it seems that the native BMP in vivo is sn-2, sn-2′ acylated compared to the thermodynamically more stable sn-3, sn-3′ acylated BMP that could be formed in vitro during the purification procedure [[30], [31], [32]]. Furthermore, sn-2, sn-2′ acylated BMP and not the sn-3, sn-3’ acylated BMP is able to form multivesicular liposomes in vitro in a pH-dependent manner [33]. The other polyglycerophospholipids, PG and cardiolipin (CL), were shown as exogenous sources in vivo [22]. In addition, PG and not CL, was identified as the primary precursor of the de novo biosynthesis of BMP in CHO cells mutated for the PG phosphate synthase [34]. It was proposed that the stereoconversion of PG to BMP requires complex successive reactions involving transacylases and phospholipases [35,36]. BMP appears progressively during the maturation of the endosome visualized by a multivesicular appearance due to inward budding of the organelle limiting membrane towards its lumen leading to the formation of intraluminal vesicles (ILVs) (Fig. 2 ). The location of its biosynthesis was postulated to occur in the LE/Lys compartment [37,38]. However, it was also suggested that crosstalk through membrane contact sites (MCS) might be required for its de novo synthesis between LE/Lys and mitochondria - ER where PG and lysoPG are synthetized [39]. Due to its stereoconfiguration, BMP is resistant to the phospholipase activity present in the lysosome explaining its extended lifetime [40]. However, acyl chain turnover was demonstrated comparable to other phospholipids [41]. Phospholipase activities able to metabolize BMP have been described depending on the acidic environment and substrate presentation [42,43], including a pancreatic-lipase related protein 2 (PLRP2) also found in lysosome [44,45] and recently the monoacylglycerol hydrolase α/β hydrolase domain-containing 6 ABHD6 [46,47].
Fig. 1.
Structure of bis(monoacylglycero)phosphate BMP and its structural isomer phosphatidylglycerol PG. BMP exhibits an unusual sn-1, sn-1′ stereoconfiguration based on the position of the phosphate on each glycerol moiety, different from the sn-3, sn-1′ configuration of its precursor PG. In native BMP, the acyl chains R and R′ are esterified on the sn-2, sn-2′ positions whereas in PG, they are in sn-1 and sn-2 of the same glycerol molecule.
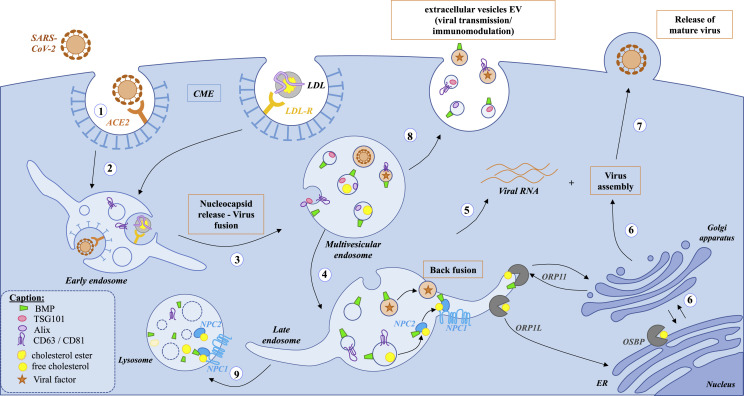
Fig. 2.
Schema of the endocytic pathway involving the endolysosomal lipid BMP and putative relation to SARS-CoV-2 infection steps. (1) binding step: SARS-CoV-2 binds through its spike S protein to its receptor angiotensin-converting enzyme 2 (ACE2) located in lipid rafts on the host cell plasma membrane. (2) internalization step: then SARS-CoV-2 is internalized by clathrin-mediated endocytosis (CME) and undergoes intracellular trafficking through the endocytic pathway: (3) from early endosome and multivesicular endosome to (4) late endosome/lysosome. The same endocytic route is used by low density lipoproteins (LDL) for cholesterol entry into mammalian cells after binding to the LDL receptor. The virus uncoating and fusion step are strongly dependent of the acidic pH of the endolysosomal compartment enriched in BMP (green conical cylinder): enzymatic cleavage by cathepsin L is crucial for subsequent release of the viral RNA genome (5) into the host cytosol. (6) Newly made viral S protein and other membrane proteins enter the secretory pathway via the endoplasmic reticulum (ER) whereas viral RNA genome and nucleocapsid protein are assembled to form the previral particles into the lumen of the ERGIC (ER-Golgi intermediate compartment). (7) Then, matured viruses are released via transport from the trans-Golgi (TGN) network to the cell surface. (8) Virus factors can also hijack the extracellular vesicle EV secretory pathway to exit infected cells [48]. Recently, BMP (green conical cylinder) was considered as a new lipid signature of endosome-derived EVs characterized by the EV protein markers ESCRT protein TSG101, associated protein Alix, and the tetraspanins CD63 and CD81 [49,50]. The cholesterol transfer between NPC2 and membrane vesicles including between inner membranes of the LE/Lys compartment is favored by BMP [51]. This occurs through the direct interaction of BMP with a domain of NPC2 [52]. This region is also involved in the interaction between the soluble intraendolysosomal NPC2 and the transmembrane protein NPC1 in the limiting membrane of the LE/Lys. This highlights the importance of BMP in NPC2-dependent cholesterol binding at inner membranes of LE/Lys and in NPC2-dependent cholesterol transfer to the N-terminal domain of NPC1. NPC1 has been proposed to be involved in infectivity of SARS-CoV-2 [25]. ORP1L as well as OSBP are required at the replication organelle for viral infection by regulating cholesterol homeostasis [53,54]. We can speculate that ORP11, by interacting with both BMP and cholesterol into late endosome, would be implicated in virus infection.
2.2. Localization and tissue distribution of BMP
BMP is found in most mammalian cells and tissues. It was also found in some strains of alkalophilic bacteria and in the amoeba Dictyostelium discoideum [55,56]. It is not detected in lower Eukaryotes such as yeast, but the improvement of recent analytical methods could be very useful to detect subminimal levels. Most human and animal cells or tissues contain relatively low amounts of BMP, not exceeding 1–2% of their total phospholipids. In the LE/Lys compartment, BMP amounts to 15% of the phospholipids and constitutes up to 70% of their internal membrane phospholipids, including ILVs [17]. In both humans and animal models, BMP was shown to dramatically increase in cationic amphiphilic drugs (CAD)-induced phospholipidosis and in inherited lysosomal storage disorders (LSD) characterized by accumulated materials in LE/Lys due to alteration of degradative pathways [57,58]. During these disorders, increasing BMP levels were detected also in extracellular biofluids (plasma, urines..) particularly docosahexaenoic acid (C22:6n-3) containing species such as diC22:6-BMP. Thus, BMP is considered as a possible biomarker to follow disease progression [59,60]. We recently showed that BMP was increased in the urines of amiodarone-treated patients, a CAD antiarrhythmic drug, and was associated with extracellular vesicles (EVs) characterized as exosomes [49]. EVs form a heterogeneous group of membrane vesicles of variable sizes: “exosomes” of about 50–120 nm in diameter, originated from the ILVs after fusion of the multivesicular endosomes (MVEs) with the PM or “microvesicles” of about 50 nm to 1 μm in diameter, obtained from shedding of the PM [50]. Presence of BMP in EVs seems to vary according to the cell type or the endolysosomal dysfunction. Due to its ubiquitous distribution in most mammalian cells and its selective localization in the endolysosome, we speculated that BMP could be a specific lipid signature of endosome-derived EVs in human urines [49]. However, this will require more analysis of EVs isolated from other extracellular biofluids. Furthermore, the precise role of BMP to control exosome biogenesis and secretion is still unclear but could be linked to its biophysical properties as detailed below.
2.3. Dynamics of ILVs and BMP membrane domains
In most cell types, BMP is characterized by a high proportion of unsaturated fatty acids, such as oleic acid. Also polyunsaturated fatty acids, such as docosahexaenoic acid, are enriched in BMP of rat uterine stromal cells, macrophages, liver and brain [31,41,47,[61], [62], [63], [64]]. The BMP polyunsaturation coupled to its cone-shape structure and its negative charge are crucial for the dynamic of endolysosomal membranes, favoring membrane invagination and the formation of ILVs. This fusion process depends also on the interaction of BMP with the endosomal sorting complexes required for transport (ESCRT)-associated protein ALIX via the exposed loop at its N-terminus exhibiting a BRO1 domain. Moreover, ALIX via its C-terminal proline-rich region binds the ESCRT-1 subunit protein, TSG101 [65]. Both proteins ALIX and TSG101 are also found in exosomes and play a role in EV biogenesis [50]. The role of BMP in EV biogenesis and secretion is thus suggested not only due to its biophysical properties [33] but also to its interaction with ALIX and its specific localization in ILVs. In addition to its role in cholesterol transport (developed below), negatively-charged BMP is considered as a stimulator of sphingolipid degradative enzymes directly for the acid sphingomyelinase or acid ceramidase, or indirectly by increasing the efficiency of their cofactors saposins [40,66]. Change in the fatty acid composition or content of BMP will alter the metabolic activity in the endolysosome compartment. Recent studies have highlighted the role of BMP for the maintenance of metabolic homeostasis and the importance of its physiological regulation. Fasting stimulates lysosomal biogenesis and BMP content [62] whereas high fat diet increases hepatic and circulating BMP concentrations [47]. All these active membrane exchanges and trafficking can be hijacked by viruses.
2.4. Hijacking of ILVs and EVs by viruses and role of BMP
During VSV infection, the release of viral RNA in the cytosol depends of BMP [6], Alix [8], and other ESCRT proteins through a mechanism of back-fusion, i.e upon fusion of the ILV membrane with the limiting membrane of the LE [9]. First, the viral envelope undergoes fusion with the ILV membrane or directly with the LE membrane [5]. It is not known yet if SARS-CoV-2 is using a similar pathway for cell infection and the role of BMP in this context (Fig. 2). In addition, some viruses and viral particles can be released in exosomes. Interestingly in parallel to the classical exosome formation, ceramide produced after hydrolysis of sphingomyelin by neutral sphingomelinase was shown to favor formation of exosomes containing cholesterol [67]. By hijacking the EV secretory pathway, the viruses can, not only exit infected cells, but EVs can also play a role in immune response by spreading viral and host cell components [48] (Fig. 2). The importance of this pathway for SARS-CoV-2 infection is not known yet as well as the role of BMP. Targeting the endolysosomal function that can be hijacked by the SARS-CoV-2 and particularly using drugs modifying transiently this compartment enriched in BMP has started to be evaluated [25]. But we have to keep in mind that targeting such important intracellular trafficking could have deleterious effects on lipid metabolism. This is particularly important knowing the high percentage of patients with metabolic disorders among the SARS-CoV-2 infected patients presenting severe forms of COVID-19 [68].
3. Bis(monoacylglycero)phosphate and cholesterol homeostasis
Cholesterol is a dynamic lipid that moves quickly between membranes of cell organelles. Cells have developed complex pathways to maintain cholesterol homeostasis, including vesicular and non-vesicular processes. Cholesterol traffic and distribution inside cells are mainly maintained by non-vesicular mechanisms [69]. Several families of proteins function as sterol transporters to maintain cholesterol homeostasis [70].
3.1. Cholesterol transporter proteins: focus on NPC and ORP
Among all cholesterol transport proteins, Niemann-Pick C1 and C2 proteins (NPC1, NPC2) cooperate to regulate the egress of low-density lipoprotein (LDL)-derived cholesterol out of late endosomes [52]. Other proteins like STARD3 (StAR related lipid transfer domain containing 3) [71], ORPs (OSPB (Oxysterol Binding proteins)-Related Proteins) [72] and Aster protein [73] transport cholesterol between LE, PM, ER, Golgi and mitochondria.
Exit of LDL-derived cholesterol from LE/Lys requires a cooperation between NPC1 and NPC2. NPC1 is a multi-spanning transmembrane (TM) protein located in the limiting membrane of LE/Lys and NPC2 is a globular protein present in the lumen of LE/Lys. NPC1 presents 13 TM segments among which TMs 3–7 constitute the sterol-sensing domain (SSD), and 3 luminal domains: A also called the N-terminal domain (NTD), C and I. Both NTD and SSD domains bind cholesterol [51,74]. Based on mutagenesis, model membranes and atomistic simulations, a prevailing model of cholesterol transfer is currently proposed: cholesterol-carrying NPC2 would be recruited to the domain C of NPC1 and would deliver the bound cholesterol to the NTD through a “hydrophobic hand-off” mechanism [74,75] (Fig. 2). Another functional domain of NPC1 required for cholesterol export has been identified in the SSD as a high affinity binding site for U18666A [76], a cationic amphiphile compound that strongly inhibits LDL-derived cholesterol exit from lysosomes thereby inducing NPC phenotype [77] (Fig. 3 A). Mutations in either NPC1 or NPC2 result in abnormal lipid storage, essentially cholesterol in LE/Lys, a cellular hallmark of Niemann-Pick C disease.
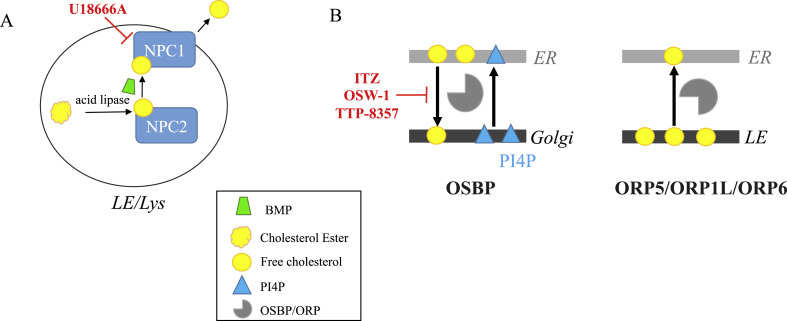
Fig. 3.
Schematic intracellular lipid flows regulated by sterol transfer proteins and their inhibitors (indicated in red): A) cholesterol efflux from LE/Lys: the cationic amphiphilic drug U18666A binds to NPC1 and inhibits the LDL-derived cholesterol exit from lysosomes thereby inducing NPC phenotype [76,77]. BMP (green conical cylinder) favors the acid hydrolysis of LDL-derived cholesterol ester [22] and stimulates the rate of cholesterol transfer by NPC2 [52,51]. B) Cholesterol transfer at MCS between ER and Golgi/LE. OSBP exchanges cholesterol with PI4P between ER and trans Golgi (TGN) membranes [78,79]. Itraconazole (ITZ) and OSW-1 interact with OSBP and ORP4 disrupting OSBP lipid-shuttling function [80]. TTP-8307 inhibits directly the OSBP-dependent activities [81]. ORP1L, ORP5 and ORP 6, localized to LE membrane, mediate cholesterol transfer from LE to ER [[82], [83], [84]] and could be implicated in virus replication [54].
Oxysterol-Binding Protein (OSBP) and their associated proteins (ORP) constitute a family of lipid binding/transfer proteins in eukaryotes [[85], [86], [87]]. In mammals, 12 Osbpl genes encode proteins of this family and variants of different sizes increase the final number of proteins to 16.
ORP proteins are mainly cytosolic proteins. However, most of them are associated with the membrane of an organelle. They are located at MCS implicating ER with other organelle limiting membrane, thanks to FFAT domain that binds the ER-resident vesicle-associated membrane protein (VAMP)-associated protein (VAP) [88,89]. ORPs are characterized by a unique binding domain to sterols (cholesterol as well as oxysterols), but also glycerophospholipids, particularly phosphatidylserine [90,91] and phosphatidylinositol-4-phosphate (PI4P) [[78], [92], [93]].
OSBP, the first oxysterol binding protein described, exchanges cholesterol with PI4P between ER and Golgi apparatus, by forming MCS and tethering ER to trans Golgi (TGN) membranes [78,79]. This phenomenon described as the OSBP cycle contributes to the maintenance of a cholesterol gradient among secretory organelle membranes.
In the context of this review, endosomes have abundant contacts with ER, and some members of the ORP families are involved in these membrane contact sites, favoring cholesterol transport between those organelles [94,95]. Among them, ORP1L, ORP5 and ORP6, that are localized to LE membrane, mediate cholesterol transfer from LE to ER at MCS [[82], [83], [84]] (Fig. 3B).
3.2. BMP modulation of NPC and ORP activities
Several lines of evidence have established that BMP is required for an efficient cholesterol transport function of NPC proteins. The potential relationships between BMP and NPC proteins initially emerged from studies showing that BMP accumulates in liver and spleen of NPC patients and in NPC mice models [[16], [22]]. A functional role of BMP in NPC-associated cholesterol defect has been first proposed by Kobayashi et al. [96], showing that internalization of anti-BMP antibody induced NPC phenotype in cultured BHK cells. Studies from Gruenberg team have further shown that increase of intracellular BMP, either by addition of exogenous BMP or drug-induced, reduced cholesterol storage disorder in fibroblasts of NPC patients and in liver of npc1−/− mice [97,98]. In addition, treatment with U18666A that induced NPC phenotype was associated with an intracellular increase of BMP [97]. The authors therefore raised the hypothesis that BMP becomes limiting in the NPC disease upon excessive cholesterol overload. Whereas Gruenberg’s studies have highlighted a functional crosstalk between BMP and NPC 1/2 proteins, it has been demonstrated that BMP regulates cholesterol transport through direct interaction with NPC2, independent of NPC1 [52]. Biochemical, structural and atomic scale studies demonstrated that BMP stimulated the extent and rate of cholesterol transfer by NPC2 through direct interaction at the hydrophobic knob domain on the surface of NPC2 [52,99]. Interestingly, this region is also involved in the direct interaction between the soluble protein NPC2 in the lumen and the transmembrane protein NPC1 in the limiting membrane of the LE/Lys and thus, suggests its role in the subsequent cholesterol “hand-off” mechanism [74]. This also highlights the importance of BMP not only in cholesterol binding by NPC2 at the inner membranes of LE/Lys, but also in NPC2-dependent cholesterol transfer to the N-terminal domain (NTD) of NPC1 (Fig. 2, Fig. 3A). The ability of NPC2 to bind to BMP may also represent a way to bring the cholesterol-rich and BMP-rich inner membranes of LE/Lys in proximity to the LE/Lys limiting membrane, but how the cholesterol will exit the compartment is still unknown.
Considering the putative involvement of NPC proteins in SARS-CoV infection and the interaction of BMP with these proteins, we raise the hypothesis that BMP could turn out to be a valuable target for therapeutic strategy against SARS-CoV including SARS-CoV-2.
Recently, we demonstrated that ORP11 localizes to LE and Golgi and modulates cholesterol egress from LE in macrophages [100]. Moreover, BMP favors the egress of cholesterol from LE via an ORP11-dependent mechanism, resulting in a reduced production of cytotoxic 7-oxysterols [100].
A member of the START protein family, STARD3, localized at the endosomal compartment, participates in the formation of MCS between ER to LE membranes. Cholesterol transfer from ER to LE occurred via STARD3 interaction with VAPs [71].
3.3. Role of NPC and ORP in virus infection
NPC1 has recently been identified as a key player for infection by Ebola virus (EBOV), an enveloped RNA virus from the filovirus family. First evidence came from mutagenesis studies revealing that cells mutated for NPC1, as well as fibroblasts isolated from NPC1 patients, were resistant to EBOV infection in vitro [101]. Using NPC1−/− mouse models, it was further shown that NPC1 was required for replication and pathogenesis of EBOV in vivo [102]. By contrast, NPC2 mutants that exhibit NPC phenotype with cholesterol accumulation were not protected, suggesting that NPC1 requirement in EBOV infection was independent of cholesterol transport activity [101,102]. NPC1 binds to the cleaved form of EBOV glycoprotein GP1, thereby regulating membrane fusion and subsequent viral release into the cytoplasm of infected cells [75,[103], [104], [105]]. Of interest, U18666A and other cationic drugs inducing NPC phenotype were able to inhibit EBOV entry in a NPC1 dependent manner but independent of GP1 binding to NPC1 [26]. NPC1 and NPC2 proteins are also involved in HIV infection. HIV replication and release were significantly decreased in both NPC1 deficient cells and fibroblasts from NPC1 patients in association with cholesterol and HIV Gag protein accumulation in LE/Lys [106]. However, Coleman and colleagues reported that HIV virions released by cells lacking both NPC1 and NPC2 exhibit enhanced infectivity, probably due to their higher cholesterol content [107]. These observations suggest a rather complex mechanism in which NPC1 would favor HIV multiplication, although by controlling cholesterol transport, NPC1/NPC2 would render the viral particles less virulent. In contrast to Ebola virus, NPC requirement in HIV infection was shown to be related to the regulation of cholesterol transport out of LE/Lys [106,107].
To date, it is not known whether NPC1 is required for the entry of SARS-CoV. However, NPC1 positive compartments were shown to be an obligatory step for SARS-CoV intracellular development, probably to access to the high cathepsin activity necessary for the release of the viral nucleocapsid [108]. In a recent hypothesis paper, Ballout et al. proposed that NPC1 positive LE/Lys would also be mandatory for the successful infectivity of SARS-CoV-2 since it has an infectious life cycle similar to SARS-CoV. In addition, the authors highlight the potential role of NPC proteins for impairing SARS-CoV-2 entry and subsequent internalization and trafficking, since lipid rafts in which the ACE2 protein resides, are disrupted in NPC disorder [25]. The use of NPC1 inhibitors or NPC-disease mimetic drugs has been proposed as a potential relevant therapeutic strategy against SARS-CoV-2 [26]. Of interest, U18666A was previously reported to inhibit type I feline coronavirus in relation with NPC1 dysfunction [109].
Virus replication occurs in the cytosol of host cells, using the ER, the Golgi apparatus and endosomal membranes as replication platforms at MCS. As sterol and phospholipids of infected cells are highly implicated in the formation of the replication organelle, recently, ORPs have been described to be implicated in viral replication process.
In 2009, the role of OSBP in proliferation of RNA viruses, Hepatitis C Virus (HCV), was pointed out [110]. OSBP interacts at the Golgi membrane with NS5A, a non-structural protein of HCV, involved in virus assembly, which is anchored to ER membrane via VAP-A. OSBP is required for both replication and egress of HCV particles [110]. OSBP lipid transfer property is essential for cholesterol delivery to the HCV replication organelle in exchange for PI4P [53], strongly implicated in viral replication [111]. Other RNA viruses including poliovirus, dengue, picornavirus, as well as Aichi virus require cholesterol supply by OSBP for replication organelle efficiency [[112], [113], [114]]. ORP4, the closest OSBP homolog, was implicated in HCV replication as a negative regulator via inhibition of NS5B activity, due to alteration of lipid homeostasis and formation of lipid droplet [115]. This mechanism requires a close collaboration between ORP4 and OSBP.
The formation of replication organelles requires the hijacking of cellular lipid homeostasis and is presently explored as a target for the development of antiviral compounds. Strating and coworkers identified Itraconazole (ITZ), an antifungal drug, as inhibitor for enterovirus replication, due to its interaction with OSBP and ORP4. ITZ targets OSBP and ORP4 at the replication organelle, disrupting OSBP lipid-shuttling function. Likewise, OSW-1, an antiproliferative natural compound, interacts specifically with OSBP and ORP4 [80]. The antiviral activity is due to inhibition of 25-hydroxycholesterol binding to OSBP and ORP4 [116]. TTP-8307, an enterovirus replication inhibitor, inhibits directly OSBP activity, through the PI4P-OSBP pathway. Other viruses, as picornavirus encephalomyocarditis virus and HCV, which activities are OSBP-dependent, are sensitive to TTP-8307. Very recently, OSBP was hypothesized as a potential target to SARS-CoV-2 infection [81] (Fig. 3B).
A study of flavivirus West Nile virus (WNV) showed that the silencing of ORP1L decreased the replication of WNV as well as other types of RNA viruses. ORP1L knockdown disturbed the movement of LE and contacts between LE and ER, inhibiting the transfer of cholesterol between these organelles [117]. This activity is implicated in adenovirus infection [54], ORP1L controlling the fusion and infection by the Ebola virus [118].
ORPs, due to their intracellular localization and lipid traffic properties, are implicated in the replication of many viruses, so we can postulate that they could be involved in SARS-CoV-2 infection. Especially, ORP11, via its interaction with BMP and cholesterol, could be hypothesized as a potential target of numerous viruses including SARS-CoV-2.
3.4. BMP regulation of viral infection via other cholesterol pools
Besides regulating LDL-derived cholesterol exit out of LE/Lys, BMP is involved in the distribution of LDL-derived cholesterol from LE/Lys to other cellular compartments. Using anti-BMP antibody that accumulated in LE in cultured macrophages, or a model of BMP accumulation, we showed that BMP was involved in cholesterol transport to PM and ER [23,119]. Of specific interest, we found that cholesterol distribution in PM was altered in anti-BMP treated macrophages, especially impairing HDL accessible pools [119]. Unpublished observations suggest that BMP regulates cholesterol distribution in lipid rafts. We also reported that BMP accumulation led to reduced expression of ABCG1 transporters and related cholesterol efflux to HDL [23]. Noteworthy, it was reported that cholesterol content in lipid rafts would be determinant for SARS-CoV-2 interaction with the cellular receptor ACE2 [120]. One may therefore speculates that by regulating cholesterol-rich membrane domains, BMP could impact on SARS-CoV-2 entry in host cells.
Also of interest is our finding that BMP regulates oxysterol production, especially 7- and 25-hydroxycholesterol [100,121]. As a matter of fact, these oxysterols have been shown to exert antiviral activity against coronavirus and related virus like Ebola, Zika by decreasing virus entry or replication [[122], [123], [124]]. This raises the hypothesis of a role of BMP to favor this antiviral activity.
4. Conclusion
The unusual endosomal phospholipid BMP is crucial to maintain the endosomal lipid storage capacity and trafficking as well as the fine-tuning of cholesterol domains at the plasma membrane known to control receptor signaling and cholesterol efflux [[21], [22], [23]]. Thus, we speculated that BMP could influence SARS-CoV-2 infection in different ways: 1) by regulating cholesterol-rich membrane domains at the PM and thus, interfering with virus entry and subsequent internalization; 2) by controlling the lipid flows through the endocytic pathway and thus, interfering with viral trafficking and fusion events that are necessary for virus replication and maturation. By controlling lipid homeostasis in host cell, BMP appears as a “double face” partner of SARS-CoV-2 depending on its endosomal content: 1) in physiological conditions and within a normal concentration range of BMP in the LE/Lys, BMP will favor virus entry and infection and can be considered as a therapeutic target against COVID-19; 2) in pathological conditions and accumulation of BMP in the LE/Lys, the lipid will create deleterious conditions in the host endocytic pathway and thus, could impaired virus infection. However, the enzymes involved in the de novo biosynthetic pathway of BMP are still unknown and little is known about its physiological regulation. Therefore, it is not possible yet to favor its putative antiviral activity against SARS-CoV-2 by genetic tools.
Since genetic NPC1 defect or drug-induced endosomal cholesterol accumulation were shown to inhibit Ebola virus and VSV infection [5,26], this can provide some useful clues for drug development against COVID-19 as recently checked: 1) reduced cholesterol content in plasma membrane to modify the lipid raft-dependent ACE2 and TMPRSS2 activities [2,125] and thus, perturb host cell docking and internalization of the virus [25,126]; 2) modification of the acidic pH in endolysosome to modify trafficking and viral particle fusion [20]; 3) increase of cholesterol and oxysterol content in endolysosome to impede viral fusion and subsequent replication [27]. However targeting the endolysosomal function that can be hijacked by the SARS-CoV-2 should be only transient and reversible to avoid side effects on lipid metabolism. This is particularly important knowing the high percentage of patients with metabolic disorders among the SARS-CoV-2 infected patients presenting severe forms of COVID-19 [68].
Funding
MR is supported by a PhD fellowship from the French Ministery of education. Financial supports were from INSERM; SFD (Société Francophone du Diabète_AE 2016) and VML (Vaincre les Maladies Lysosomales_convention AO2018-6) to FHM.
Author contributions
CLC, FHM, and ID designed and wrote the manuscript; MR created the figures. All authors corrected and approved the final manuscript.
Declaration of competing interest
The authors declared no competing financial interest.
Acknowledgements
The authors apologize to all the researchers who cannot be cited due not only to space constraints, but also to the so fast release of new findings and literature on COVID-19.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. China novel coronavirus Investigating and research team, A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roth S.L., Whittaker G.R. Promotion of vesicular stomatitis virus fusion by the endosome-specific phospholipid bis(monoacylglycero)phosphate (BMP) FEBS (Fed. Eur. Biochem. Soc.) Lett. 2011;585:865–869. doi: 10.1016/j.febslet.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 4.Carpio L.E., Villalain J. Identification of the phospholipid binding regions of the envelope E protein of flaviviruses by molecular dynamics. J. Biomol. Struct. Dyn. 2019:1–12. doi: 10.1080/07391102.2019.1697368. [DOI] [PubMed] [Google Scholar]
- 5.Le Blanc I., Luyet P.P., Pons V., Ferguson C., Emans N., Petiot A., Mayran N., Demaurex N., Faure J., Sadoul R., Parton R.G., Gruenberg J. Endosome-to-cytosol transport of viral nucleocapsids. Nat. Cell Biol. 2005;7:653–664. doi: 10.1038/ncb1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luyet P.P., Falguieres T., Pons V., Pattnaik A.K., Gruenberg J. The ESCRT-I subunit TSG101 controls endosome-to-cytosol release of viral RNA. Traffic. 2008;9:2279–2290. doi: 10.1111/j.1600-0854.2008.00820.x. [DOI] [PubMed] [Google Scholar]
- 7.Falguieres T., Luyet P.P., Gruenberg J. Molecular assemblies and membrane domains in multivesicular endosome dynamics. Exp. Cell Res. 2009;315:1567–1573. doi: 10.1016/j.yexcr.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Bissig C., Lenoir M., Velluz M.C., Kufareva I., Abagyan R., Overduin M., Gruenberg J. Viral infection controlled by a calcium-dependent lipid-binding module in ALIX. Dev. Cell. 2013;25:364–373. doi: 10.1016/j.devcel.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gruenberg J. Life in the lumen: the multivesicular endosome. Traffic. 2020;21:76–93. doi: 10.1111/tra.12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Meer G., Voelker D.R., Feigenson G.W. Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lingwood D., Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 12.Sezgin E., Levental I., Mayor S., Eggeling C. The mystery of membrane organization: composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Biol. 2017;18:361–374. doi: 10.1038/nrm.2017.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carquin M., D’Auria L., Pollet H., Bongarzone E.R., Tyteca D. Recent progress on lipid lateral heterogeneity in plasma membranes: from rafts to submicrometric domains. Prog. Lipid Res. 2016;62:1–24. doi: 10.1016/j.plipres.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sobo K., Chevallier J., Parton R.G., Gruenberg J., van der Goot F.G. Diversity of raft-like domains in late endosomes. PloS One. 2007;2:e391. doi: 10.1371/journal.pone.0000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scott C.C., Vacca F., Gruenberg J. Endosome maturation, transport and functions. Semin. Cell Dev. Biol. 2014;31:2–10. doi: 10.1016/j.semcdb.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 16.Hullin-Matsuda F., Taguchi T., Greimel P., Kobayashi T. Lipid compartmentalization in the endosome system. Semin. Cell Dev. Biol. 2014;31:48–56. doi: 10.1016/j.semcdb.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi T., Beuchat M.H., Chevallier J., Makino A., Mayran N., Escola J.M., Lebrand C., Cosson P., Gruenberg J. Separation and characterization of late endosomal membrane domains. J. Biol. Chem. 2002;277:32157–32164. doi: 10.1074/jbc.M202838200. [DOI] [PubMed] [Google Scholar]
- 18.Stadler K., Ha H.R., Ciminale V., Spirli C., Saletti G., Schiavon M., Bruttomesso D., Bigler L., Follath F., Pettenazzo A., Baritussio A. Amiodarone alters late endosomes and inhibits SARS coronavirus infection at a post-endosomal level. Am. J. Respir. Cell Mol. Biol. 2008;39:142–149. doi: 10.1165/rcmb.2007-0217OC. [DOI] [PubMed] [Google Scholar]
- 19.Zaitseva E., Yang S.-T., Melikov K., Pourmal S., Chernomordik L.V. Dengue virus ensures its fusion in late endosomes using compartment-specific lipids. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carriere F., Longhi S., Record M. The endosomal lipid bis(monoacylglycero) phosphate as a potential key player in the mechanism of action of chloroquine against SARS-COV-2 and other enveloped viruses hijacking the endocytic pathway. Biochimie. 2020 doi: 10.1016/j.biochi.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi T., Beuchat M.H., Lindsay M., Frias S., Palmiter R.D., Sakuraba H., Parton R.G., Gruenberg J. Late endosomal membranes rich in lysobisphosphatidic acid regulate cholesterol transport. Nat. Cell Biol. 1999;1:113–118. doi: 10.1038/10084. [DOI] [PubMed] [Google Scholar]
- 22.Hullin-Matsuda F., Luquain-Costaz C., Bouvier J., Delton-Vandenbroucke I. Bis(monoacylglycero)phosphate, a peculiar phospholipid to control the fate of cholesterol: implications in pathology. Prostaglandins Leukot. Essent. Fatty Acids. 2009;81:313–324. doi: 10.1016/j.plefa.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Luquain-Costaz C., Lefai E., Arnal-Levron M., Markina D., Sakaï S., Euthine V., Makino A., Guichardant M., Yamashita S., Kobayashi T., Lagarde M., Moulin P., Delton-Vandenbroucke I. Bis(Monoacylglycero)Phosphate accumulation in macrophages Induces intracellular cholesterol redistribution, attenuates liver-X receptor/ATP-binding cassette transporter A1/ATP-binding cassette transporter G1 pathway, and impairs cholesterol efflux. Arterioscler. Thromb. Vasc. Biol. 2013;33:1803–1811. doi: 10.1161/atvbaha.113.301857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ilnytska O., Santiana M., Hsu N.-Y., Du W.-L., Chen Y.-H., Viktorova E.G., Belov G., Brinker A., Storch J., Moore C., Dixon J.L., Altan-Bonnet N. Enteroviruses harness the cellular endocytic machinery to remodel the host cell cholesterol landscape for effective viral replication. Cell Host Microbe. 2013;14:281–293. doi: 10.1016/j.chom.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ballout R.A., Sviridov D., Bukrinsky M.I., Remaley A.T. The lysosome: a potential juncture between SARS-CoV-2 infectivity and Niemann-Pick disease type C, with therapeutic implications. Faseb. J. 2020;34:7253–7264. doi: 10.1096/fj.202000654R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shoemaker C.J., Schornberg K.L., Delos S.E., Scully C., Pajouhesh H., Olinger G.G., Johansen L.M., White J.M. Multiple cationic amphiphiles induce a Niemann-Pick C phenotype and inhibit Ebola virus entry and infection. PLoS One. 2013;8 doi: 10.1371/journal.pone.0056265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sturley S.L., Rajakumar T., Hammond N., Higaki K., Márka Z., Márka S., Munkacsi A.B. Potential COVID-19 therapeutics from a rare disease: weaponizing lipid dysregulation to combat viral infectivity. J. Lipid Res. 2020;61:972–982. doi: 10.1194/jlr.R120000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sauvat A., Ciccosanti F., Colavita F., Di Rienzo M., Castilletti C., Capobianchi M.R., Kepp O., Zitvogel L., Fimia G.M., Piacentini M., Kroemer G. On-target versus off-target effects of drugs inhibiting the replication of SARS-CoV-2. Cell Death Dis. 2020;11:656. doi: 10.1038/s41419-020-02842-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan H.H., Makino A., Sudesh K., Greimel P., Kobayashi T. Spectroscopic evidence for the unusual stereochemical configuration of an endosome-specific lipid. Angew Chem. Int. Ed. Engl. 2012;51:533–535. doi: 10.1002/anie.201106470. [DOI] [PubMed] [Google Scholar]
- 30.Luquain C., Laugier C., Lagarde M., Pageaux J.F. High-performance liquid chromatography determination of bis(monoacylglycerol) phosphate and other lysophospholipids. Anal. Biochem. 2001;296:41–48. doi: 10.1006/abio.2001.5158. [DOI] [PubMed] [Google Scholar]
- 31.Luquain C., Dolmazon R., Enderlin J.M., Laugier C., Lagarde M., Pageaux J.F. Bis(monoacylglycerol) phosphate in rat uterine stromal cells: structural characterization and specific esterification of docosahexaenoic acid. Biochem. J. 2000;351:795–804. [PMC free article] [PubMed] [Google Scholar]
- 32.Chevallier J., Sakai N., Robert F., Kobayashi T., Gruenberg J., Matile S. Rapid access to synthetic lysobisphosphatidic acids using P(III) chemistry. Org. Lett. 2000;2:1859–1861. doi: 10.1021/ol0059246. [DOI] [PubMed] [Google Scholar]
- 33.Matsuo H., Chevallier J., Mayran N., Le Blanc I., Ferguson C., Faure J., Blanc N.S., Matile S., Dubochet J., Sadoul R., Parton R.G., Vilbois F., Gruenberg J. Role of LBPA and Alix in multivesicular liposome formation and endosome organization. Science. 2004;303:531–534. doi: 10.1126/science.1092425. [DOI] [PubMed] [Google Scholar]
- 34.Hullin-Matsuda F., Kawasaki K., Delton-Vandenbroucke I., Xu Y., Nishijima M., Lagarde M., Schlame M., Kobayashi T. De novo biosynthesis of the late endosome lipid, bis(monoacylglycero)phosphate. J. Lipid Res. 2007;48:1997–2008. doi: 10.1194/jlr.M700154-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Thornburg T., Miller C., Thuren T., King L., Waite M. Glycerol reorientation during the conversion of phosphatidylglycerol to bis(monoacylglycerol)phosphate in macrophage-like RAW 264.7 cells. J. Biol. Chem. 1991;266:6834–6840. [PubMed] [Google Scholar]
- 36.Amidon B., Schmitt J.D., Thuren T., King L., Waite M. Biosynthetic conversion of phosphatidylglycerol to sn-1:sn-1’ bis(monoacylglycerol) phosphate in a macrophage-like cell line. Biochemistry. 1995;34:5554–5560. doi: 10.1021/bi00016a029. [DOI] [PubMed] [Google Scholar]
- 37.Joutti A., Brotherus J., Renkonen O., Laine R., Fischer W. The stereochemical configuration of lysobisphosphatidic acid from rat liver, rabbit lung and pig lung. Biochim. Biophys. Acta. 1976;450:206–209. doi: 10.1016/0005-2760(76)90092-8. [DOI] [PubMed] [Google Scholar]
- 38.Poorthuis B.J., Hostetler K.Y. Studies on the subcellular localization and properties of bis(monoacylglyceryl)phosphate biosynthesis in rat liver. J. Biol. Chem. 1976;251:4596–4602. [PubMed] [Google Scholar]
- 39.Poorthuis B.J., Hostetler K.Y. Conversion of diphosphatidylglycerol to bis(monoacylglyceryl)phosphate by lysosomes. J. Lipid Res. 1978;19:309–315. [PubMed] [Google Scholar]
- 40.Schulze H., Kolter T., Sandhoff K. Principles of lysosomal membrane degradation Cellular topology and biochemistry of lysosomal lipid degradation. Biochim. Biophys. Acta. 2009;1793:674–683. doi: 10.1016/j.bbamcr.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 41.Besson N., Hullin-Matsuda F., Makino A., Murate M., Lagarde M., Pageaux J.F., Kobayashi T., Delton-Vandenbroucke I. Selective incorporation of docosahexaenoic acid into lysobisphosphatidic acid in cultured THP-1 macrophages. Lipids. 2006;41:189–196. doi: 10.1007/s11745-006-5087-5. [DOI] [PubMed] [Google Scholar]
- 42.Abe A., Shayman J.A. The role of negatively charged lipids in lysosomal phospholipase A2 function. JLR (J. Lipid Res.) 2009;50:2027–2035. doi: 10.1194/jlr.M900008-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ito M., Tchoua U., Okamoto M., Tojo H. Purification and properties of a phospholipase A2/lipase preferring phosphatidic acid, bis(monoacylglycerol) phosphate, and monoacylglycerol from rat testis. J. Biol. Chem. 2002;277:43674–43681. doi: 10.1074/jbc.M202817200. [DOI] [PubMed] [Google Scholar]
- 44.Record M., Amara S., Subra C., Jiang G., Prestwich G.D., Ferrato F., Carrière F. Bis (monoacylglycero) phosphate interfacial properties and lipolysis by pancreatic lipase-related protein 2, an enzyme present in THP-1 human monocytes. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2011;1811:419–430. doi: 10.1016/j.bbalip.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 45.Gilleron M., Lepore M., Layre E., Cala-De Paepe D., Mebarek N., Shayman J.A., Canaan S., Mori L., Carrière F., Puzo G., De Libero G. Lysosomal lipases PLRP2 and LPLA2 process mycobacterial multi-acylated lipids and generate T cell stimulatory antigens. Cell Chem Biol. 2016;23:1147–1156. doi: 10.1016/j.chembiol.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 46.Pribasnig M.A., Mrak I., Grabner G.F., Taschler U., Knittelfelder O., Scherz B., Eichmann T.O., Heier C., Grumet L., Kowaliuk J., Romauch M., Holler S., Anderl F., Wolinski H., Lass A., Breinbauer R., Marsche G., Mark Brown J., Zimmermann R. Alpha/beta hydrolase domain-containing 6 (ABHD6) degrades the late endosomal/lysosomal lipid bis(monoacylglycero)phosphate. J. Biol. Chem. 2015 doi: 10.1074/jbc.M115.669168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grabner G.F., Fawzy N., Pribasnig M.A., Trieb M., Taschler U., Holzer M., Schweiger M., Wolinski H., Kolb D., Horvath A., Breinbauer R., Rülicke T., Rabl R., Lass A., Stadlbauer V., Hutter-Paier B., Stauber R.E., Fickert P., Zechner R., Marsche G., Eichmann T.O., Zimmermann R. Metabolic disease and ABHD6 alter the circulating bis(monoacylglycerol)phosphate profile in mice and humans. JLR (J. Lipid Res.) 2019;60:1020–1031. doi: 10.1194/jlr.M093351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Urbanelli L., Buratta S., Tancini B., Sagini K., Delo F., Porcellati S., Emiliani C. The role of extracellular vesicles in viral infection and transmission. Vaccines. 2019:7. doi: 10.3390/vaccines7030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rabia M., Leuzy V., Soulage C., Durand A., Fourmaux B., Errazuriz-Cerda E., Köffel R., Draeger A., Colosetti P., Jalabert A., Di Filippo M., Villard-Garon A., Bergerot C., Luquain-Costaz C., Moulin P., Rome S., Delton I., Hullin-Matsuda F. Bis(monoacylglycero)phosphate, a new lipid signature of endosome-derived extracellular vesicles. Biochimie. 2020;178:26–38. doi: 10.1016/j.biochi.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 50.van Niel G., D’Angelo G., Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 51.Storch J., Xu Z. Niemann-Pick C2 (NPC2) and intracellular cholesterol trafficking. Biochim. Biophys. Acta. 2009;1791:671–678. doi: 10.1016/j.bbalip.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCauliff L.A., Langan A., Li R., Ilnytska O., Bose D., Waghalter M., Lai K., Kahn P.C., Storch J. Intracellular cholesterol trafficking is dependent upon NPC2 interaction with lysobisphosphatidic acid. Elife. 2019;8 doi: 10.7554/eLife.50832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang H., Perry J.W., Lauring A.S., Neddermann P., De Francesco R., Tai A.W. Oxysterol-binding protein is a phosphatidylinositol 4-kinase effector required for HCV replication membrane integrity and cholesterol trafficking. Gastroenterology. 2014;146:1373–1385. doi: 10.1053/j.gastro.2014.02.002. e1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cianciola N.L., Chung S., Manor D., Carlin C.R. Adenovirus modulates toll-like receptor 4 signaling by reprogramming ORP1L-VAP protein contacts for cholesterol transport from endosomes to the endoplasmic reticulum. J. Virol. 2017;91 doi: 10.1128/JVI.01904-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nishihara M., Morii H., Koga Y. Bis(monoacylglycero)phosphate in alkalophilic bacteria. J. Biochem. (Tokyo) 1982;92:1469–1479. doi: 10.1093/oxfordjournals.jbchem.a134071. [DOI] [PubMed] [Google Scholar]
- 56.Rodriguez-Paris J.M., Nolta K.V., Steck T.L. Characterization of lysosomes isolated from Dictyostelium discoideum by magnetic fractionation. J. Biol. Chem. 1993;268:9110–9116. [PubMed] [Google Scholar]
- 57.Meikle P.J., Duplock S., Blacklock D., Whitfield P.D., Macintosh G., Hopwood J.J., Fuller M. Effect of lysosomal storage on bis(monoacylglycero)phosphate. Biochem. J. 2008;411:71–78. doi: 10.1042/BJ20071043. [DOI] [PubMed] [Google Scholar]
- 58.Sawada H., Takami K., Asahi S. A toxicogenomic approach to drug-induced phospholipidosis: analysis of its induction mechanism and establishment of a novel in vitro screening system. Toxicol. Sci. 2005;83:282–292. doi: 10.1093/toxsci/kfh264. [DOI] [PubMed] [Google Scholar]
- 59.Tengstrand E.A., Miwa G.T., Hsieh F.Y. Bis(monoacylglycerol)phosphate as a non-invasive biomarker to monitor the onset and time-course of phospholipidosis with drug-induced toxicities. Expet Opin. Drug Metabol. Toxicol. 2010;6:555–570. doi: 10.1517/17425251003601961. [DOI] [PubMed] [Google Scholar]
- 60.Liu N., Tengstrand E.A., Chourb L., Hsieh F.Y. Di-22:6-bis(monoacylglycerol)phosphate: a clinical biomarker of drug-induced phospholipidosis for drug development and safety assessment. Toxicol. Appl. Pharmacol. 2014;279:467–476. doi: 10.1016/j.taap.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 61.Bouvier J., Zemski Berry K.A., Hullin-Matsuda F., Makino A., Michaud S., Geloen A., Murphy R.C., Kobayashi T., Lagarde M., Delton-Vandenbroucke I. Selective decrease of bis(monoacylglycero)phosphate content in macrophages by high supplementation with docosahexaenoic acid. J. Lipid Res. 2009;50:243–255. doi: 10.1194/jlr.M800300-JLR200. [DOI] [PubMed] [Google Scholar]
- 62.Grabner G.F., Fawzy N., Schreiber R., Pusch L.M., Bulfon D., Koefeler H., Eichmann T.O., Lass A., Schweiger M., Marsche G., Schoiswohl G., Taschler U., Zimmermann R. Metabolic regulation of the lysosomal cofactor bis(monoacylglycero)phosphate in mice. J. Lipid Res. 2020 doi: 10.1194/jlr.RA119000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Akgoc Z., Iosim S., Seyfried T.N. Bis(monoacylglycero)phosphate as a macrophage enriched phospholipid. Lipids. 2015;50:907–912. doi: 10.1007/s11745-015-4045-5. [DOI] [PubMed] [Google Scholar]
- 64.Akgoc Z., Sena-Esteves M., Martin D.R., Han X., d’Azzo A., Seyfried T.N. Bis(monoacylglycero)phosphate: a secondary storage lipid in the gangliosidoses. J. Lipid Res. 2015;56:1006–1013. doi: 10.1194/jlr.M057851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bissig C., Gruenberg J. ALIX and the multivesicular endosome: ALIX in Wonderland. Trends Cell Biol. 2014;24:19–25. doi: 10.1016/j.tcb.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 66.Schulze H., Sandhoff K. Sphingolipids and lysosomal pathologies. Biochim. Biophys. Acta. 2014;1841:799–810. doi: 10.1016/j.bbalip.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 67.Trajkovic K., Hsu C., Chiantia S., Rajendran L., Wenzel D., Wieland F., Schwille P., Brügger B., Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 68.Dugail I., Amri E.-Z., Vitale N. High prevalence for obesity in severe COVID-19: possible links and perspectives towards patient stratification. Biochimie. 2020 doi: 10.1016/j.biochi.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Iaea D.B., Maxfield F.R. Cholesterol trafficking and distribution. Essays Biochem. 2015;57:43–55. doi: 10.1042/bse0570043. [DOI] [PubMed] [Google Scholar]
- 70.Luo J., Jiang L.-Y., Yang H., Song B.-L. Intracellular cholesterol transport by sterol transfer proteins at membrane contact sites. Trends Biochem. Sci. 2019;44:273–292. doi: 10.1016/j.tibs.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 71.Wilhelm L.P., Wendling C., Védie B., Kobayashi T., Chenard M.-P., Tomasetto C., Drin G., Alpy F. STARD3 mediates endoplasmic reticulum-to-endosome cholesterol transport at membrane contact sites. EMBO J. 2017;36:1412–1433. doi: 10.15252/embj.201695917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Olkkonen V.M., Li S. Oxysterol-binding proteins: sterol and phosphoinositide sensors coordinating transport, signaling and metabolism. Prog. Lipid Res. 2013;52:529–538. doi: 10.1016/j.plipres.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 73.Sandhu J., Li S., Fairall L., Pfisterer S.G., Gurnett J.E., Xiao X., Weston T.A., Vashi D., Ferrari A., Orozco J.L., Hartman C.L., Strugatsky D., Lee S.D., He C., Hong C., Jiang H., Bentolila L.A., Gatta A.T., Levine T.P., Ferng A., Lee R., Ford D.A., Young S.G., Ikonen E., Schwabe J.W.R., Tontonoz P. Aster proteins facilitate nonvesicular plasma membrane to ER cholesterol transport in mammalian cells. Cell. 2018;175:514–529. doi: 10.1016/j.cell.2018.08.033. e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pfeffer S.R. NPC intracellular cholesterol transporter 1 (NPC1)-mediated cholesterol export from lysosomes. J. Biol. Chem. 2019;294:1706–1709. doi: 10.1074/jbc.TM118.004165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gong X., Qian H., Zhou X., Wu J., Wan T., Cao P., Huang W., Zhao X., Wang X., Wang P., Shi Y., Gao G.F., Zhou Q., Yan N. Structural Insights into the niemann-pick C1 (NPC1)-mediated cholesterol transfer and Ebola infection. Cell. 2016;165:1467–1478. doi: 10.1016/j.cell.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lu F., Liang Q., Abi-Mosleh L., Das A., De Brabander J.K., Goldstein J.L., Brown M.S. Identification of NPC1 as the target of U18666A, an inhibitor of lysosomal cholesterol export and Ebola infection. Elife. 2015;4 doi: 10.7554/eLife.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liscum L., Faust J.R. The intracellular transport of low density lipoprotein-derived cholesterol is inhibited in Chinese hamster ovary cells cultured with 3-beta-[2-(diethylamino)ethoxy]androst-5-en-17-one. J. Biol. Chem. 1989;264:11796–11806. [PubMed] [Google Scholar]
- 78.Antonny B., Bigay J., Mesmin B. The oxysterol-binding protein cycle: burning off PI(4)P to transport cholesterol. Annu. Rev. Biochem. 2018;87:809–837. doi: 10.1146/annurev-biochem-061516-044924. [DOI] [PubMed] [Google Scholar]
- 79.Mesmin B., Bigay J., Moser von Filseck J., Lacas-Gervais S., Drin G., Antonny B. A four-step cycle driven by PI(4)P hydrolysis directs sterol/PI(4)P exchange by the ER-Golgi tether OSBP. Cell. 2013;155:830–843. doi: 10.1016/j.cell.2013.09.056. [DOI] [PubMed] [Google Scholar]
- 80.Strating J.R.P.M., van der Linden L., Albulescu L., Bigay J., Arita M., Delang L., Leyssen P., van der Schaar H.M., Lanke K.H.W., Thibaut H.J., Ulferts R., Drin G., Schlinck N., Wubbolts R.W., Sever N., Head S.A., Liu J.O., Beachy P.A., De Matteis M.A., Shair M.D., Olkkonen V.M., Neyts J., van Kuppeveld F.J.M. Itraconazole inhibits enterovirus replication by targeting the oxysterol-binding protein. Cell Rep. 2015;10:600–615. doi: 10.1016/j.celrep.2014.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shahmohamadnejad S., Nabavi S.F., Habtemariam S., Sarkar K., Sil P.C., Dowran R., Nabavi S.M. May we target double-membrane vesicles and oxysterol-binding protein to combat SARS-CoV-2 infection? Cell Biol. Int. 2020 doi: 10.1002/cbin.11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhao K., Ridgway N.D. Oxysterol-binding protein-related protein 1L regulates cholesterol egress from the endo-lysosomal system. Cell Rep. 2017;19:1807–1818. doi: 10.1016/j.celrep.2017.05.028. [DOI] [PubMed] [Google Scholar]
- 83.Du X., Kumar J., Ferguson C., Schulz T.A., Ong Y.S., Hong W., Prinz W.A., Parton R.G., Brown A.J., Yang H. A role for oxysterol-binding protein-related protein 5 in endosomal cholesterol trafficking. J. Cell Biol. 2011;192:121–135. doi: 10.1083/jcb.201004142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ouimet M., Hennessy E.J., van Solingen C., Koelwyn G.J., Hussein M.A., Ramkhelawon B., Rayner K.J., Temel R.E., Perisic L., Hedin U., Maegdefessel L., Garabedian M.J., Holdt L.M., Teupser D., Moore K.J. miRNA targeting of oxysterol-binding protein-like 6 regulates cholesterol trafficking and efflux. Arterioscler. Thromb. Vasc. Biol. 2016;36:942–951. doi: 10.1161/ATVBAHA.116.307282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Olkkonen V.M. Macrophage oxysterols and their binding proteins: roles in atherosclerosis. Curr. Opin. Lipidol. 2012;23:462–470. doi: 10.1097/MOL.0b013e328356dba0. [DOI] [PubMed] [Google Scholar]
- 86.Olkkonen V.M., Béaslas O., Nissilä E. Oxysterols and their cellular effectors. Biomolecules. 2012;2:76–103. doi: 10.3390/biom2010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pietrangelo A., Ridgway N.D. Bridging the molecular and biological functions of the oxysterol-binding protein family. Cell. Mol. Life Sci. 2018;75:3079–3098. doi: 10.1007/s00018-018-2795-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kentala H., Weber-Boyvat M., Olkkonen V.M. OSBP-related protein family: mediators of lipid transport and signaling at membrane contact sites. Int Rev Cell Mol Biol. 2016;321:299–340. doi: 10.1016/bs.ircmb.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 89.Olkkonen V.M. OSBP-related protein family in lipid transport over membrane contact sites. Lipid Insights. 2015;8:1–9. doi: 10.4137/LPI.S31726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maeda K., Anand K., Chiapparino A., Kumar A., Poletto M., Kaksonen M., Gavin A.C. Interactome map uncovers phosphatidylserine transport by oxysterol-binding proteins. Nature. 2013;501:257–261. doi: 10.1038/nature12430. [DOI] [PubMed] [Google Scholar]
- 91.Chung J., Torta F., Masai K., Lucast L., Czapla H., Tanner L.B., Narayanaswamy P., Wenk M.R., Nakatsu F., De Camilli P. INTRACELLULAR TRANSPORT. PI4P/phosphatidylserine countertransport at ORP5- and ORP8-mediated ER-plasma membrane contacts. Science. 2015;349:428–432. doi: 10.1126/science.aab1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tong J., Yang H., Yang H., Eom S.H., Im Y.J. Structure of Osh 3 reveals a conserved mode of phosphoinositide binding in oxysterol-binding proteins. Structure. 2013;21:1203–1213. doi: 10.1016/j.str.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 93.Moser von Filseck J., Čopič A., Delfosse V., Vanni S., Jackson C.L., Bourguet W., Drin G. INTRACELLULAR TRANSPORT. Phosphatidylserine transport by ORP/Osh proteins is driven by phosphatidylinositol 4-phosphate. Science. 2015;349:432–436. doi: 10.1126/science.aab1346. [DOI] [PubMed] [Google Scholar]
- 94.Friedman J.R., Dibenedetto J.R., West M., Rowland A.A., Voeltz G.K. Endoplasmic reticulum-endosome contact increases as endosomes traffic and mature. Mol. Biol. Cell. 2013;24:1030–1040. doi: 10.1091/mbc.E12-10-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Raiborg C., Wenzel E.M., Stenmark H. ER–endosome contact sites: molecular compositions and functions. EMBO J. 2015;34:1848–1858. doi: 10.15252/embj.201591481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kobayashi T., Beuchat M.H., Lindsay M., Frias S., Palmiter R.D., Sakuraba H., Parton R.G., Gruenberg J. Late endosomal membranes rich in lysobisphosphatidic acid regulate cholesterol transport. Nat. Cell Biol. 1999;1:113–118. doi: 10.1038/10084. [DOI] [PubMed] [Google Scholar]
- 97.Chevallier J., Chamoun Z., Jiang G., Prestwich G., Sakai N., Matile S., Parton R.G., Gruenberg J. Lysobisphosphatidic acid controls endosomal cholesterol levels. J. Biol. Chem. 2008;283:27871–27880. doi: 10.1074/jbc.M801463200. [DOI] [PubMed] [Google Scholar]
- 98.Moreau D., Vacca F., Vossio S., Scott C., Colaco A., Paz Montoya J., Ferguson C., Damme M., Moniatte M., Parton R.G., Platt F.M., Gruenberg J. Drug-induced increase in lysobisphosphatidic acid reduces the cholesterol overload in Niemann-Pick type C cells and mice. EMBO Rep. 2019;20 doi: 10.15252/embr.201847055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Enkavi G., Mikkolainen H., Güngör B., Ikonen E., Vattulainen I. Concerted regulation of npc2 binding to endosomal/lysosomal membranes by bis(monoacylglycero)phosphate and sphingomyelin. PLoS Comput. Biol. 2017;13 doi: 10.1371/journal.pcbi.1005831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Arnal-Levron M., Chen Y., Greimel P., Calevro F., Gaget K., Riols F., Batut A., Bertrand-Michel J., Hullin-Matsuda F., Olkkonen V.M., Delton I., Luquain-Costaz C. Bis(monoacylglycero)phosphate regulates oxysterol binding protein-related protein 11 dependent sterol trafficking. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2019;1864:1247–1257. doi: 10.1016/j.bbalip.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 101.Carette J.E., Raaben M., Wong A.C., Herbert A.S., Obernosterer G., Mulherkar N., Kuehne A.I., Kranzusch P.J., Griffin A.M., Ruthel G., Dal Cin P., Dye J.M., Whelan S.P., Chandran K., Brummelkamp T.R. Ebola virus entry requires the cholesterol transporter Niemann-Pick C1. Nature. 2011;477:340–343. doi: 10.1038/nature10348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Herbert A.S., Davidson C., Kuehne A.I., Bakken R., Braigen S.Z., Gunn K.E., Whelan S.P., Brummelkamp T.R., Twenhafel N.A., Chandran K., Walkley S.U., Dye J.M. Niemann-pick C1 is essential for ebolavirus replication and pathogenesis in vivo. mBio. 2015;6 doi: 10.1128/mBio.00565-15. e00565-00515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Côté M., Misasi J., Ren T., Bruchez A., Lee K., Filone C.M., Hensley L., Li Q., Ory D., Chandran K., Cunningham J. Small molecule inhibitors reveal Niemann-Pick C1 is essential for Ebola virus infection. Nature. 2011;477:344–348. doi: 10.1038/nature10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Miller E.H., Obernosterer G., Raaben M., Herbert A.S., Deffieu M.S., Krishnan A., Ndungo E., Sandesara R.G., Carette J.E., Kuehne A.I., Ruthel G., Pfeffer S.R., Dye J.M., Whelan S.P., Brummelkamp T.R., Chandran K. Ebola virus entry requires the host-programmed recognition of an intracellular receptor. EMBO J. 2012;31:1947–1960. doi: 10.1038/emboj.2012.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang H., Shi Y., Song J., Qi J., Lu G., Yan J., Gao G.F. Ebola viral glycoprotein bound to its endosomal receptor niemann-pick C1. Cell. 2016;164:258–268. doi: 10.1016/j.cell.2015.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tang Y., Leao I.C., Coleman E.M., Broughton R.S., Hildreth J.E.K. Deficiency of niemann-pick type C-1 protein impairs release of human immunodeficiency virus type 1 and results in Gag accumulation in late endosomal/lysosomal compartments. J. Virol. 2009;83:7982–7995. doi: 10.1128/JVI.00259-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Coleman E.M., Walker T.N., Hildreth J.E.K. Loss of Niemann Pick type C proteins 1 and 2 greatly enhances HIV infectivity and is associated with accumulation of HIV Gag and cholesterol in late endosomes/lysosomes. Virol. J. 2012;9:31. doi: 10.1186/1743-422X-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mingo R.M., Simmons J.A., Shoemaker C.J., Nelson E.A., Schornberg K.L., D’Souza R.S., Casanova J.E., White J.M. Ebola virus and severe acute respiratory syndrome coronavirus display late cell entry kinetics: evidence that transport to NPC1+ endolysosomes is a rate-defining step. J. Virol. 2015;89:2931–2943. doi: 10.1128/JVI.03398-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Takano T., Endoh M., Fukatsu H., Sakurada H., Doki T., Hohdatsu T. The cholesterol transport inhibitor U18666A inhibits type I feline coronavirus infection. Antivir. Res. 2017;145:96–102. doi: 10.1016/j.antiviral.2017.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Amako Y., Sarkeshik A., Hotta H., Yates J., Siddiqui A. Role of oxysterol binding protein in hepatitis C virus infection. J. Virol. 2009;83:9237–9246. doi: 10.1128/JVI.00958-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang H., Tai A.W. Nir 2 is an effector of VAPs necessary for efficient hepatitis C virus replication and phosphatidylinositol 4-phosphate enrichment at the viral replication organelle. J. Virol. 2019;93 doi: 10.1128/JVI.00742-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Arita M. Phosphatidylinositol-4 kinase III beta and oxysterol-binding protein accumulate unesterified cholesterol on poliovirus-induced membrane structure. Microbiol. Immunol. 2014;58:239–256. doi: 10.1111/1348-0421.12144. [DOI] [PubMed] [Google Scholar]
- 113.Meutiawati F., Bezemer B., Strating J.R.P.M., Overheul G.J., Žusinaite E., van Kuppeveld F.J.M., van Cleef K.W.R., van Rij R.P. Posaconazole inhibits dengue virus replication by targeting oxysterol-binding protein. Antivir. Res. 2018;157:68–79. doi: 10.1016/j.antiviral.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 114.Ishikawa-Sasaki K., Nagashima S., Taniguchi K., Sasaki J. Model of OSBP-mediated cholesterol supply to Aichi virus RNA replication sites involving protein-protein interactions among viral proteins, ACBD3, OSBP, VAP-A/B, and SAC1. J. Virol. 2018;92 doi: 10.1128/JVI.01952-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Park I.-W., Ndjomou J., Wen Y., Liu Z., Ridgway N.D., Kao C.C., He J.J. Inhibition of HCV replication by oxysterol-binding protein-related protein 4 (ORP4) through interaction with HCV NS5B and alteration of lipid droplet formation. PloS One. 2013;8 doi: 10.1371/journal.pone.0075648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Roberts B.L., Severance Z.C., Bensen R.C., Le A.T., Kothapalli N.R., Nuñez J.I., Ma H., Wu S., Standke S.J., Yang Z., Reddig W.J., Blewett E.L., Burgett A.W.G. Transient compound treatment Induces a multigenerational reduction of oxysterol-binding protein (OSBP) levels and prophylactic antiviral activity. ACS Chem. Biol. 2019;14:276–287. doi: 10.1021/acschembio.8b00984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Courtney S.C., Di H., Stockman B.M., Liu H., Scherbik S.V., Brinton M.A. Identification of novel host cell binding partners of Oas1b, the protein conferring resistance to flavivirus-induced disease in mice. J. Virol. 2012;86:7953–7963. doi: 10.1128/JVI.00333-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.van der Kant R., Fish A., Janssen L., Janssen H., Krom S., Ho N., Brummelkamp T., Carette J., Rocha N., Neefjes J. Late endosomal transport and tethering are coupled processes controlled by RILP and the cholesterol sensor ORP1L. J. Cell Sci. 2013;126:3462–3474. doi: 10.1242/jcs.129270. [DOI] [PubMed] [Google Scholar]
- 119.Delton-Vandenbroucke I., Bouvier J., Makino A., Besson N., Pageaux J.F., Lagarde M., Kobayashi T. Anti-bis(monoacylglycero)phosphate antibody accumulates acetylated LDL-derived cholesterol in cultured macrophages. J. Lipid Res. 2007;48:543–552. doi: 10.1194/jlr.M600266-JLR200. [DOI] [PubMed] [Google Scholar]
- 120.Glende J., Schwegmann-Wessels C., Al-Falah M., Pfefferle S., Qu X., Deng H., Drosten C., Naim H.Y., Herrler G. Importance of cholesterol-rich membrane microdomains in the interaction of the S protein of SARS-coronavirus with the cellular receptor angiotensin-converting enzyme 2. Virology. 2008;381:215–221. doi: 10.1016/j.virol.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Arnal-Levron M., Chen Y., Delton-Vandenbroucke I., Luquain-Costaz C. Bis(monoacylglycero)phosphate reduces oxysterol formation and apoptosis in macrophages exposed to oxidized LDL. Biochem. Pharmacol. 2013;86:115–121. doi: 10.1016/j.bcp.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 122.Liu S.-Y., Aliyari R., Chikere K., Li G., Marsden M.D., Smith J.K., Pernet O., Guo H., Nusbaum R., Zack J.A., Freiberg A.N., Su L., Lee B., Cheng G. Interferon-inducible cholesterol-25-hydroxylase broadly inhibits viral entry by production of 25-hydroxycholesterol. Immunity. 2013;38:92–105. doi: 10.1016/j.immuni.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Willard K.A., Elling C.L., Stice S.L., Brindley M.A. The oxysterol 7-ketocholesterol reduces Zika virus titers in vero cells and human neurons. Viruses. 2018;11 doi: 10.3390/v11010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang Y., Song Z., Wang M., Lan M., Zhang K., Jiang P., Li Y., Bai J., Wang X. Cholesterol 25-hydroxylase negatively regulates porcine intestinal coronavirus replication by the production of 25-hydroxycholesterol. Vet. Microbiol. 2019;231:129–138. doi: 10.1016/j.vetmic.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Radenkovic D., Chawla S., Pirro M., Sahebkar A., Banach M. Cholesterol in relation to COVID-19: should we care about it? J. Clin. Med. 2020;9 doi: 10.3390/jcm9061909. [DOI] [PMC free article] [PubMed] [Google Scholar]