Abstract
Background
Chinese herbal medicine (CHM) has been used for severe illness caused by coronavirus disease 2019 (COVID-19), but its treatment effects and safety are unclear.
Purpose
This study reviews the effect and safety of CHM granules in the treatment of patients with severe COVID-19.
Methods
We conducteda single-center, retrospective study on patients with severe COVID-19 in a designated hospital in Wuhan from January 15, 2020 to March 30, 2020. The propensity score matching (PSM) was used to assess the effect and safety of the treatment using CHM granules. The ratio of patients who received treatment with CHM granules combined with usual care and those who received usual care alone was 1:1. The primary outcome was the time to clinical improvement within 28 days, defined as the time taken for the patients’ health to show improvement by decline of two categories (from the baseline) on a modified six-category ordinal scale, or to be dischargedfrom the hospital before Day 28.
Results
Using PSM, 43 patients (45% male) aged 65.6 (57–70) yearsfrom each group were exactly matched. No significant difference was observed in clinical improvement of patients treated with CHM granules compared with those who received usual (p = 0.851). However, the use of CHM granules reduced the 28-day mortality (p = 0.049) and shortened the duration of fever (4 days vs. 7 days, p = 0.002). The differences in the duration of cough and dyspnea and the difference in lung lesion ratio on computerized tomography scans were not significant.Commonly,patients in the CHM group had an increased D-dimer level (p = 0.036).
Conclusion
Forpatients with severe COVID-19, CHM granules, combined with usual care, showed no improvement beyond usual care alone. However, the use of CHM granules reduced the 28-day mortality rate and the time to fever alleviation. Nevertheless, CHM granules may be associated with high risk of fibrinolysis.
Keywords: COVID-19, Chinese herbal medicine, propensity score matching
Abbreviations: Aes, Adverse events; ARR, absolute risk reduction; CHM, Chinese herbal medicine; COVID-19, coronavirus disease 2019; CT, computerized tomography; ECMO, extracorporeal membrane oxygenation; IQR, interquartile range; NNT, number needed to treat; NTproBNP, N-terminal prohormone of brain natriuretic peptide; PSM, propensity score matching; RR, respiratory rate; RRR, relative risk reduction; SARS, severe acute respiratory syndrome; TCM, traditional Chinese medicine; WHO, World Health Organization
Introduction
Coronavirus disease 2019 (COVID-19) has been rapidly spreading for the past 6 months since the first case was confirmed in Wuhan, China (Li et al., 2020; WHO Coronavirus Disease (COVID-19) Pandemic). Although the COVID-19 pandemic seem to have come under control within China, the number of new infections continues to spiral upward rapidly in other regions around the world. Over 200 countries have reported more than 10,000,000 confirmed cases and more than 500,000 deaths so far (Coronavirus Disease (COVID-19) Outbreak Situation COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) “COVID-19 Global Pandemic Real-time Report DXY•DX Doctor National Health Commission of the People's Republic of China). The World Health Organization (WHO) has declared the pandemic a Public Health Emergency of International Concern (WHO Coronavirus Disease (COVID-19) Pandemic).
The clinical classification of COVID-19 ranges from mild to critical cases according to the definition by the Chinese management guideline for COVID-19 (National Health Commission of the People's Republic of China, 2020a, National Health Commission of the People's Republic of China 2020b). Most of the mild cases are self-limited, but the severe cases are associated with mortality (Guan et al., 2020; Yang et al., 2020; Zhou et al., 2020). According to the nationwide data reported to the National Health Commission of the People's Republic of China between December 2019 and January 2020, 15.7% confirmed cases were with severe conditions. Among them, 8.1% of severe cases died within 28 days compared with 0.1% fatality rate in non-severe cases. In the early stage of COVID-19 in Wuhan (i.e., before January 31), there were 35% severe and 28% critically ill patients. Of these, 22% of severe patients and up to 78% of critically ill patients had death as the outcome.
To date, however, no specific therapeutic agent has shown its efficacy in the treatment of severe COVID-19 patients, despite having hundreds of clinical trials registered for potential drugs and therapies (Chinese Clinical Trial Registry “ClinicalTrials.gov). The China's guidelines recommended the traditional Chinese medicine (TCM), which has a long history in treating influenza-like diseases and proven to be effective in treating severe acute respiratory syndrome (SARS) epidemic in 2003 (Chen and Nakamura, 2004; Lau et al., 2005; Leung, 2007), to be used jointly with modern treatment for COVID-19 patients. Therefore, we reviewed the records of severe COVID-19 patientsadmitted to one of the COVID-19 designated hospital in Wuhan and assessed the therapeutic effect and safety of Chinese herbal medicine (CHM) granules.
Materials and methods
We conducted a single-center, retrospective, observational study in Hankou Hospital, a designated hospital for COVID-19 patients in Wuhan, China. According to the classification by the China's guidelines, patients with severe disease conditions received CHM granules together with usual care and were designated as the CHM group. They were compared with patients who received usual care alone, after matching in a 1:1 ratio using propensity score matching (PSM). The protocol of this study was approved by the Ethical Committee of Guangdong Provincial Hospital of Chinese Medicine (No. ZE2020-049-01). As this study has a retrospective, observational nature, the committee waived the need for written informed consent.
Participants
In this study, patients aged 18 years or older who had received a diagnosis of severe COVID-19 were included. According to the China's guidelines, the diagnosis of COVID-19 was confirmed with a positive reverse transcription–polymerase chain reaction assay for SARS-Cov-2 in a respiratory tract sample tested by the local center for disease control or by a designated diagnostic laboratory. Severe cases were defined as patients who satisfied at least one of the following criteria: 1) shortness of breath with a respiratory rate (RR) ≥ 30/min; 2) peripheral blood oxygen saturation ≤ 93% in resting state; or 3) partial arterial oxygen pressure/fraction of inspired oxygen ≤300 mmHg. Patients with non-invasive mechanical ventilation were also included. The CHM group included patients who received CHM granules for more than 5 days in a row during their hospitalization. CHM granules were prescribed in conformity with the TCM theory (Appendix A). The control group included patients who received usual care without any TCM interventions during the observation period. Patients with abnormal renal or liver function at hospital admission were excluded from this study.
Interventions
CHM granules
Two CHM granules, Chai-hu-jie-du and Fu-zheng-jiu-fei, which were in conformity with the TCM principle of strengthening the body resistance and eliminating the virus, were used in this study. TCM doctors assessed patients and decided the granule to be given. Chai-hu-jie-du was prescribed when the patients were diagnosed with heat-toxicity in their lungs and intestines using TCM syndrome differentiation. Fu-zheng-jiu-fei was given when the patients were diagnosed with a deficiency of vital energy. Appendix A lists the composition, dosage, principle of TCM diagnosis, and prescription. All the granules were administered orally twice daily, mixed with 200 mL warm boiled water.Appendix B lists the Chinese, English, and Latin names of related Chinese herbal medicines.
Usual care
According to the guidelines of the China's guidelines, usual care comprised general treatment, supplemental oxygen, mechanical ventilation, antibiotic agents, and antiviral therapy. As there was no intensive care unit and organ-replacement equipment, including invasive ventilator and extracorporeal membrane oxygenation (ECMO), in Hankou Hospital, patients with critically ill conditions were transferred to higher-level hospitals.
Data collection
Electronic medical records of patients included in this study were retrospectively screened from January 15, 2020 to March 30, 2020. Data in the form of photographs were collected and double entered into a pre-established database. Missing data, disallowed values, and outliers were manually checked and corrected as appropriate. Thefollowing datawere extracted: sex; age; ethnicity; height; weight; exposure history; chronic medical histories (chronic pulmonary disease, chronic cardiovascular disease, chronic nephritic disease, diabetes, malignancy, and so on); duration from onset to hospital admission; presence and severity of symptoms (fever, cough, dyspnea, chest tightness, fatigue, and so on); vital signs (RR, pulse/heart rate, blood pressure, and peripheral blood oxygen saturation); laboratory examinations (complete blood count, blood chemical analysis, measures of electrolytes, high-sensitivity C-reactive protein, procalcitonin, liver and renal function test, lactate dehydrogenase, and so on); chest computed tomography findings; complications (coexisted infection and chronic disease progression); and supporting treatment (oxygen therapy, ventilation, antiviral and antibacterial agents, corticosteroids, and so on).
Outcome measures
The primary outcome was the time to clinical improvement up to Day 28, which was defined as the time from admission of the patient to an improvement in disease status by decline of two categories on a modified six-category ordinal scale, or the time to be discharged alive from the hospital before Day 28. The scale was recommended by the WHO R&D Blueprint expert group (WHO, 2020) and has been used as the primary outcome in clinical trials for patients hospitalized with COVID-19 (Cao et al., 2020). The six-category ordinal scale consisted of the following categories: 1, not hospitalized; 2, hospitalized, not requiring supplemental oxygen; 3, hospitalized, requiring supplemental oxygen; 4, hospitalized, requiring nasal high-flow oxygen therapy, non-invasive mechanical ventilation, or both; 5, hospitalized, requiring ECMO, invasive mechanical ventilation, or both; and 6, death.
Other outcomes included the mortality rate on Day 28; duration of main symptoms (fever, cough, and dyspnea); and difference in the lung lesion ratio on computerized tomography (CT) calculated before and after treatment during the 28 days of hospitalization. The lung lesion ratio, defined as the ratio of total area of pneumonia to the entire lung field measured from CT images (Zhang et al., 2020), was calculated by qualified radiologists based on the manually labeled pneumonia and lung areas. The images were exported as Joint Photographic Experts Group, and a computer program (Aitrox, Shanghai, China) was developed to calculate the percentage of area of pneumonia (Li et al., 2019).
Safety assessment
Safety assessment included analysis of adverse events (AEs) and serious AEs that occurred in both groups. Physicians checked and confirmed the AEs experienced by patients from their medical records. The details of the AEs included the time of occurrence, severity, causality to intervention, categorization as serious or non-serious, interventions, and AE outcome. The WHO–Uppsala Monitoring Centre System for Standardized Case Causality Assessment (WHO, 2009) was used to assess the causality between AEs and intervention.
Statistical analysis
PSM was used to balance baseline characteristics between the CHM and control groups and reduce confounding bias. A multivariable logistic regression model that included sex, age, disease severity (severe and critical), time from symptom onset to admission, and appearance of comorbidities (hypertension, diabetes and coronary heart disease, fever during admission, cough, and dyspnea) were used to obtain propensity scores for the matched probabilities. Next, we used the caliper matching method with a caliper width equal to 0.2 to obtain pair-matched cases.
Descriptive statistics were summarized as mean (standard deviation) or median (interquartile range [IQR]) for continuous variables and frequency (proportion) for categorical variables. Student's t-test or Wilcoxon rank sum test was used to evaluate continuous variables. The chi-squared test or Fisher's exact test was used to assess categorical variables. The linear association or the rank sum test was used to analyze the ordinal data. Furthermore, we classified the treatment duration of CHM granules into three levels (< 7, 7–14, and > 14 days) for the calculation of the effect of TCM treatment. The significance level was set at α = 0.05 (two-sided test). All analyses were done using software PASW Statistics 18.0 (SPSS Inc., Chicago, IL, USA) and STATA 14.0 (StataCorp., College Station, TX, USA).
Results
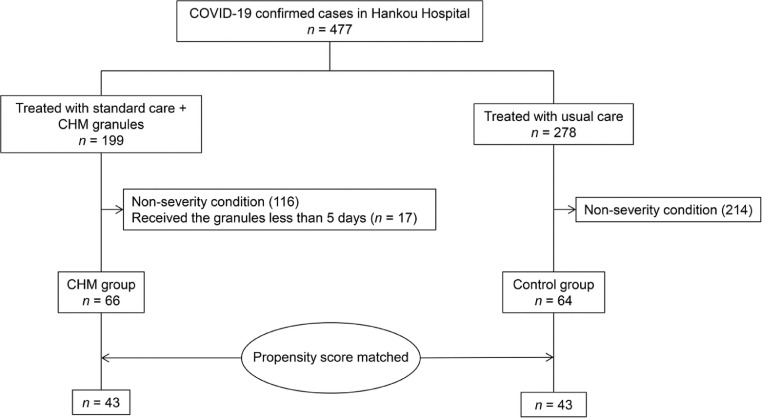
During the 2-month period of this study, 477 laboratory-confirmed COVID-19 patients were admitted to Hankou Hospital in Wuhan, China. All hospitalized patients received usual care. Among them, 199 adult patients were given a combination of usual care and TCM therapy. Of these, 116 patients who did not have severe illness and 17 patients who received granules for less than 5 days were excluded from the study. Therefore, the final CHM group consisted of 66 patients. The treatment with usual care alone was provided to 278 patients; of which, 64 patients were in severe condition and constituted the control group (Fig. 1 ).
Fig. 1.
Study flow chart.
After PSM, 43 patients from each group were exactly matched. The median age of patients was 65.6 years (IQR, 57–70), and 45% patients were men (Table 1 ). The demographic and clinical characteristics of patients, including medical history, symptoms, vital signs, and laboratory tests at admission were well-balanced at baseline (Table 1). In total, 14% (12/86) patients received non-invasive mechanical ventilation and the remaining patients were provided with oxygen support through nasal catheters or masks. During hospitalization, 71% (59/83) patients received antiviral drugs, 96% (80/83) received antibiotics, and 83% (69/83) received systemic methylprednisolone. No significant differences were observed between the two groups in medications and respiratory support (Table 2 ).
Table 1.
Demographic and clinical characteristics of patients (after PSM).
| Total |
Control group |
CHM group | p | ||||
|---|---|---|---|---|---|---|---|
| N | N | N | |||||
| Male sex (%) | 39 | 45% | 22 | 51% | 17 | 40% | 0.386 |
| Age, median (IQR) (years) | 86 | 65.5(57–70) | 43 | 66(56–71) | 43 | 65(61–68) | 0.938 |
| Coexisting disorder, No. (%) | 86 | 63 (67%) | 43 | 29(67%) | 43 | 30(70%) | 0.816 |
| COPD | 86 | 1(1%) | 43 | 0(0%) | 43 | 1(2%) | 1.000 |
| Hypertension | 86 | 35(41%) | 43 | 18(42%) | 43 | 17(40%) | 0.826 |
| Coronary heart disease | 86 | 16(19%) | 43 | 9(21%) | 43 | 7(16%) | 0.782 |
| Diabetes | 86 | 14(16%) | 43 | 8(19%) | 43 | 6(14%) | 0.559 |
| Cerebrovascular disease | 86 | 2(2%) | 43 | 2(5%) | 43 | 0(0%) | 0.494 |
| Cancer | 86 | 2(2%) | 43 | 1(2%) | 43 | 1(2%) | 1.000 |
| Immunodeficiency | 86 | 1(1%) | 43 | 0(0%) | 43 | 1(2%) | 1.000 |
| Vital Signs, median (IQR) | |||||||
| Body temperature (°C) | 85 | 36.9 (36.5–37.6) | 42 | 36.6 (36.4–37.8) | 43 | 37 (36.5–37.5) | 0.567 |
| Systolic pressure (mmHg) | 86 | 125.5 (120–134) | 43 | 123 (120–130) | 43 | 130 (120–139) | 0.407 |
| Diastolic pressure (mmHg) | 86 | 72 (70–80) | 43 | 75 (70–80) | 43 | 72 (70–80) | 0.943 |
| Peripheral oxygen saturation, No. (%) | 86 | 43 | 43 | 1.000 | |||
| ≥ 95% | 84 (98%) | 42 (98%) | 42 (98%) | ||||
| < 95% | 2 (2%) | 1 (2%) | 1 (2%) | ||||
| RR, No. (%) | 78 | 39 | 39 | 1.000 | |||
| > 20/min | 29 (37%) | 14 (36%) | 15 (39%) | ||||
| 12–20/min | 49 (63%) | 25 (64%) | 24 (62%) | ||||
| Heart rate, No. (%) | 86 | 43 | 43 | 0.81 | |||
| > 100/min | 24 (28%) | 11 (26%) | 13 (30%) | ||||
| 60–100/min | 62 (72%) | 32 (74%) | 30 (70%) | ||||
| Symptoms and signs, No. (%) | |||||||
| Fever* | 86 | 30 (35%) | 43 | 15 (35%) | 43 | 15 (35%) | 1.000 |
| Cough | 86 | 75 (87%) | 43 | 38 (88%) | 43 | 37 (86%) | 1.000 |
| Dyspnea | 86 | 71 (83%) | 43 | 36 (84%) | 43 | 35 (81%) | 1.000 |
| Headache | 86 | 4 (4.70%) | 43 | 1 (2%) | 43 | 3 (7%) | 0.616 |
| Sputum production | 86 | 27 (31%) | 43 | 15 (35%) | 43 | 12 (28%) | 0.643 |
| Chest tightness | 86 | 22 (26%) | 43 | 13 (30%) | 43 | 9 (21%) | 0.459 |
| Chest pain | 86 | 1 (1%) | 43 | 1 (2%) | 43 | 0 (0%) | 1.000 |
| Sore throat | 86 | 3 (4%) | 43 | 0 (0%) | 43 | 3 (7%) | 0.241 |
| Fatigue | 86 | 41 (48%) | 43 | 21 (49%) | 43 | 20 (47%) | 1.000 |
| Laboratory indicators, No. (%) | |||||||
| Whitecell count (× 109/l) | 80 | 40 | 40 | ||||
| < 3.5 | 13 (16%) | 4 (10%) | 9 (23%) | 0.206 | |||
| 3.5–9.5 | 53 (66%) | 30 (75%) | 23 (58%) | ||||
| > 9.5 | 14 (18%) | 6 (15%) | 8 (20%) | ||||
| Neutrophil count (× 109/l) | 80 | 40 | 40 | ||||
| < 1.8 | 8 (10%) | 3 (8%) | 5 (13%) | 0.760 | |||
| 1.8–6.3 | 46 (58%) | 23 (58%) | 23 (58%) | ||||
| >6.3 | 26 (33%) | 14 (35%) | 12 (30%) | ||||
| Lymphocyte count (× 109/l) | 80 | 40 | 40 | ||||
| < 1.1 | 68 (85%) | 33 (83%) | 35 (88%) | 0.755 | |||
| 1.1–3.2 | 12 (15%) | 7 (18%) | 5 (13%) | ||||
| Platelet count (× 109/l) | 80 | 40 | 40 | ||||
| < 125 | 17 (21%) | 11 (28%) | 6 (15%) | 0.339 | |||
| 125–350 | 56 (70%) | 25 (63%) | 31 (78%) | ||||
| > 350 | 7 (9%) | 4 (10%) | 3 (8%) | ||||
| C-reactive protein (mg/l) | 55 | 25 | 30 | 1.000 | |||
| > 5.000 | 51 (93%) | 23 (92%) | 28 (93%) | ||||
| 0.000–5.000 | 4 (7%) | 2 (8%) | 2 (7%) | ||||
| Procalcitonin (ng/ml) | 46 | 18 | 28 | 1.000 | |||
| > 0.050 | 38 (83%) | 15 (83%) | 23 (82%) | ||||
| 0.000–0.050 | 8 (17%) | 3 (17%) | 5 (18%) | ||||
| Alanine aminotransferase (U/l) | 58 | 24 | 34 | 0.165 | |||
| > 40 | 20 (35%) | 11 (46%) | 9 (27%) | ||||
| 0–40 | 38 (66%) | 13 (54%) | 25 (74%) | ||||
| Aspartate aminotransferase (U/l) | 59 | 25 | 34 | 0.598 | |||
| > 40 | 24 (41%) | 9 (36%) | 15 (44%) | ||||
| 0–40 | 35 (59%) | 16 (64%) | 19 (56%) | ||||
| Urea nitrogen (mmol/l) | 55 | 24 | 31 | 0.843 | |||
| 3.1–8.8 | 38 (69%) | 14 (58%) | 24 (77%) | ||||
| > 8.8 | 9 (16%) | 5 (21%) | 4 (13%) | ||||
| Serum creatinine (μmol/l) | 56 | 25 | 31 | 0.519 | |||
| < 50 | 7 (13%) | 4 (16%) | 3 (10%) | ||||
| 50–120 | 44 (79%) | 19 (76%) | 25 (81%) | ||||
| > 120 | 5 (9%) | 2 (8%) | 3 (10%) | ||||
| Lactate dehydrogenase (U/l) | 50 | 21 | 29 | 0.184 | |||
| 120–250 | 13 (26%) | 3 (14%) | 10 (35%) | ||||
| > 250 | 35 (70%) | 17 (81%) | 18 (62%) | ||||
| D-dimer (mg/l) | 34 | 15 | 19 | 0.165 | |||
| > 0.5 | 19 (56%) | 6 (40%) | 13 (68%) | ||||
| 0.0–0.5 | 15 (44%) | 9 (60%) | 6 (32%) | ||||
PSM: propensity score matching; IQR: interquartile range; COPD: chronic obstructive pulmonary diseases; RR, Respiratory rate.
In total, 86 cases were matched with a matching tolerance of 0.2 by PSM. The matching criteria was based on of gender; age; disease classification; duration from onset to hospitalization; history of hypertension, diabetes, or coronary heart disease; and symptoms of fever, cough, and dyspnea. The normality test showed that the age, vital signs, and laboratory indicators did not meet the normal distribution. Then they were analyzed using the rank sum test with a result of no statistical significance. The remaining variables were analyzed using chi-squared test and showed no statistical significance as well. Baseline balance between the two groups was better after matching.
*Fever was defined as the body temperature ≥ 37.3 °C on admission.
Table 2.
Patients’ status during admission and medication received in the observation period.
| Total | Control group |
CHM group |
p | ||||
|---|---|---|---|---|---|---|---|
| N | No. (%) | N | No. (%) | N | No. (%) | ||
| Score on six-category scale at admission | 1.000 | ||||||
| 6. Death | 0 | 0 | 0 | ||||
| 5. Hospitalization, requiring ECMO, invasive mechanical ventilation, or both | 0 | 0 | 0 | ||||
| 4. Hospitalization, requiring HFNC or non-invasive mechanical ventilation | 86 | 12 (14%) | 43 | 6 (14%) | 43 | 6 (14%) | |
| 3. Hospitalization, requiring supplemental oxygen | 86 | 74 (86%) | 43 | 37 (86%) | 43 | 37 (86%) | |
| 2. Hospitalization, not requiring supplemental oxygen | 0 | 0 | 0 | ||||
| 1. Discharge from hospital | 0 | 0 | 0 | ||||
| Medications | |||||||
| Antiviral drugs | 83 | 59 (71%) | 43 | 29 (67%) | 40 | 30 (75%) | 0.478 |
| Oseltamivir | 83 | 51 (61%) | 43 | 24 (56%) | 40 | 27 (68%) | 0.367 |
| Lopinavir and ritonavir tablets | 83 | 8 (10%) | 43 | 5 (12%) | 40 | 3 (8%) | 0.714 |
| Arbidol | 86 | 6 (7%) | 43 | 4 (9%) | 40 | 2 (5%) | 0.677 |
| Chloroquine | 83 | 3 (4%) | 43 | 1 (2%) | 40 | 2 (5%) | 0.607 |
| Antibiotics | 83 | 80 (96%) | 43 | 41 (95%) | 40 | 39 (98%) | 1.000 |
| Cefoperazone–sulbactam | 83 | 41 (49%) | 43 | 22 (51%) | 40 | 19 (48%) | 0.827 |
| Moxifloxacin | 83 | 75 (90%) | 43 | 40 (93%) | 40 | 35 (88%) | 0.473 |
| Glucocorticoids | 83 | 69 (83%) | 43 | 32 (74%) | 40 | 37 (93%) | 0.039 |
| Days of methylprednisolone, median (IQR) | 52 | 5.5 (4–10.8) | 22 | 9 (4–12.3) | 30 | 5 (3.8–8.3) | 0.053 |
| Total dose ofmethylprednisolone (mg), median (IQR) | 52 | 360 (165–520) | 22 | 420 (195–560) | 30 | 300 (160–480) | 0.286 |
| Nutritional support | 84 | 65 (77%) | 43 | 36 (84%) | 41 | 29 (71%) | 0.196 |
Primary outcome
On Day 28 of hospitalization, patients who received treatment with CHM granules did not show clinical improvement compared with those who received usual care alone (p = 0.518; Table 3 ). Furthermore, no significant difference was observed (p = 0.437) in disease improvement or deterioration (defined as at least one-category increase or decrease from baseline in the six-category ordinal scale) when compared between the two groups. Moreover, on Day 28, no significant difference was observed in the six-category scale when compared between the two groups (p = 0.775).
Table 3.
The primary outcome of the groups.
| Total | Control group | CHM group | p | |
|---|---|---|---|---|
| Clinical improvement* | N = 86 | N = 43 | N = 43 | 0.518 |
| No improvement, No. (%) | 44 (51%) | 20 (47%) | 24 (56%) | |
| Improvement, No. (%) | 42 (49%) | 23 (54%) | 19 (44%) | |
| Disease improvement or deterioration† | N = 86 | N = 43 | N = 43 | 0.437 |
| Improvement, No. (%) | 53 (62%) | 25 (58%) | 28 (65%) | |
| Non-improvement, No. (%) | 18 (21%) | 9 (21%) | 9 (21%) | |
| Worse, No. (%) | 15 (17%) | 9 (21%) | 6 (14%) | |
| Score on six-category scale on Day 28 | N = 86 | N = 43 | N = 43 | 0.775 |
| 6. Death, No. (%) | 11 (13%) | 9 (21%) | 2 (5%) | |
| 5. Hospitalization, requiring ECMO, invasive mechanical ventilation, or both, No. (%) | 1 (1%) | 0 (0%) | 1 (2%) | |
| 4. Hospitalization, requiring HFNC or non-invasive mechanical ventilation, No. (%) | 2 (2%) | 1 (2%) | 1 (2%) | |
| 3. Hospitalization, requiring supplemental oxygen, No. (%) | 23 (27%) | 9 (21%) | 14 (33%) | |
| 2. Hospitalization, not requiring supplemental oxygen, No. (%) | 4 (5%) | 0 (0%) | 4 (9%) | |
| 1. Discharge from hospital, No. (%) | 45 (52%) | 24 (56%) | 21 (49%) |
* Clinical improvement was defined as a decline of two categories on the modified six-category ordinal scale of clinical status or hospital discharge.
The disease improvement or deterioration was defined as at least one-category increase or decrease on the six-category scale.
The chi-squared test was used to analyze the six-category scale on Day 28, and the results showed no statistical significance.
The clinical improvement of the two groups on Day 28 were classified as one-way ordered data (grouping variable disorder, response variable ordering). The nonparametric test, Wilcoxon rank sum test showed no significant difference.
The disease improvement or deterioration on Day 28 and mortality rates were two-way disordered data. The chi-squared test showed no significant difference.
Secondary outcome
In this study, the 28-day mortality rate was lower in the CHM group than in the usual care group (2/43 vs. 9/43, p = 0.049) (Table 4 ). Additionally, on Day 28, compared with the control group, relative risk in the CHM group was 0.222 (95% CI: 0.051–0.969), relative risk reduction (RRR) was 0.775, absolute risk reduction (ARR) was 16%, and number needed to treat (NNT) was 6.2. Furthermore, the independent variables were group (CHM vs. control) anduse of glucocorticoid use (vs. non-use). The stepwise forward regression method was used in the logistic regression model, and the results showed that glucocorticoid use did not affect the mortality rate within 28 days (Table 5 ).
Table 4.
Secondary outcomes of the groups.
| Variables | Total |
Control group |
CHM group |
p | |||
|---|---|---|---|---|---|---|---|
| Mortality rate on Day 28 | N | No. (%) | N | No. (%) | N | No. (%) | |
| Survival | 86 | 75 (87%) | 43 | 34 (79%) | 43 | 41(95%) | 0.049 |
| Death | 86 | 11 (13%) | 43 | 9 (21%) | 43 | 2 (5%) | |
| Duration of main symptoms (days) | N | Median (IQR) | N | Median (IQR) | N | Median (IQR) | |
| Duration of fever | 29 | 5 (3.5–7.5) | 14 | 7 (5–9.3) | 15 | 4 (2–5) | 0.002 |
| Duration of cough | 76 | 18 (13.9–22.1) | 38 | 21 (13.0–29.0) | 38 | 16 (11.6–20.4) | 0.441 |
| Duration of dyspnea | 76 | 20 (15.4–24.5) | 36 | 20 (13.7–26.3) | 40 | 19 (12.6–25.4) | 0.793 |
| Lung lesion ratio from CT images | N | Percentage | N | Percentage | N | Percentage | |
| Before treatment | 55 | 30 ± 18 | 29 | 26 ± 16 | 26 | 35 ± 19 | 0.081 |
| After treatment | 55 | 29 ± 19 | 29 | 28 ± 18 | 26 | 29 ± 21 | 0.832 |
| Difference | 55 | 1 ± 18 | 29 | 2 ± 13 | 26 | 5 ± 22 | 0.267 |
CHM: Chinese herbal medicine.
Table 5.
Glucocorticoid use vs. non-use with respect to mortality rate.
| Factors | p | OR | 95% CI |
|
|---|---|---|---|---|
| Lower | Upper | |||
| CHM group | 0.02 | 0.146 | 0.029 | 0.738 |
| Use of glucocorticoids | 0.998 | 4.90E + 08 | 0 | – |
CHM: Chinese herbal medicine.
We evaluated the duration of three main symptoms: fever, cough, and dyspnea in patients of the two groups. In the CHM group, the duration of fever was found to be shorter than that in the control group (4 days vs. 7 days, p = 0.002). However, no significant difference was observed in durations of cough or dyspnea between the groups.
The complete CT scan images of 55 patients (26/43 vs. 29/43) were considered for the comparison of lung lesion ratio before and after the treatment. The difference in their lung lesion ratio was calculated. In the control group, this ratio increased by 2 ± 13%, while in the CHM group, it decreased by 5 ± 22%, indicating no significant difference (p = 0.267).
Safety
A total of 28 patients (65%) in the CHM group and 22 patients (51%) in the control group reported AEs between the day of admission and Day 28 (Table 6 ). A significant difference (p = 0.036) was observed in D-dimer levels between the two groups. In the CHM group, 33% (14/43) patients hadincreased D-dimer levels, while in the control group, only 12% (5/43) patients had increased D-dimer levels. Abnormal liver function (evaluated by increased alanine aminotransferase and aspartate aminotransferase) and gastrointestinal AEs, including diarrhea and constipation, were more common in the CHM group, but no differences were observed when compared with the control group. Serious AEs occurred in five patients: three in the CHM group and two in the control group. All three serious events and all deaths in the CHM group were judged by clinical physicians and found unrelated to the Chinese herbs.
Table 6.
AEs in the groups.
| Total, N = 86 | Control group, N = 43 | CHM group, N = 43 | ||
|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | p | |
| Any Aes | 50 (58%) | 22 (51%) | 28(65%) | 0.274 |
| Increased D-dimer | 19(22%) | 5 (12%) | 14(33%) | 0.036 |
| Dyspnea | 14 (16%) | 5 (12%) | 9 (21%) | 0.382 |
| Fever | 7 (8%) | 3 (7%) | 4 (9%) | 1.000 |
| Increased alanine aminotransferase | 6 (7%) | 1 (2%) | 5 (12%) | 0.202 |
| Diarrhea | 6 (7%) | 2 (5%) | 4 (9%) | 0.676 |
| Increased aspartate aminotransferase | 5 (6%) | 1 (2%) | 4 (9%) | 0.360 |
| Decreased peripheral oxygen saturation | 4 (5%) | 4 (9%) | 0 (0%) | 0.116 |
| Constipation | 4 (5%) | 1 (2%) | 3 (7%) | 0.616 |
| Chest tightness | 3 (4%) | 0 (0%) | 3 (7%) | 0.241 |
| Cough | 3 (4%) | 0 (0%) | 3 (7%) | 0.241 |
| Skin rash | 3 (4%) | 0 (0%) | 3 (7%) | 0.241 |
| Increased neutrophil percentage | 3 (4%) | 3 (7%) | 0 (0%) | 0.241 |
| Increased whitecell count | 2 (2%) | 1 (2%) | 1 (2%) | 1.000 |
| Thrombocytopenia | 2 (2%) | 1 (2%) | 1 (2%) | 1.000 |
| Melena | 2 (2%) | 1 (2%) | 1 (2%) | 1.000 |
| Fatigue | 2 (2%) | 1 (2%) | 1 (2%) | 1.000 |
| Sleep disorders | 2 (2%) | 1 (2%) | 1 (2%) | 1.000 |
| Urination disorder | 2 (2%) | 1 (2%) | 1 (2%) | 1.000 |
| Palpitation | 2 (2%) | 0 (0%) | 2 (5%) | 0.494 |
| Chest pain | 2 (2%) | 0 (0%) | 2 (5%) | 0.494 |
| Pruritus | 2 (2%) | 0 (0%) | 2 (5%) | 0.494 |
| Dysphoria | 2 (2%) | 2 (5%) | 0 (0%) | 0.494 |
| Hemoptysis | 1 (1%) | 0 (0%) | 1 (2%) | 1.000 |
| Nausea and vomiting | 1 (1%) | 0 (0%) | 1 (2%) | 1.000 |
| Abdominal discomfort | 1 (1%) | 0 (0%) | 1 (2%) | 1.000 |
| Anorexia | 1 (1%) | 0 (0%) | 1 (2%) | 1.000 |
| Headache and ophthalmodynia | 1 (1%) | 0 (0%) | 1 (2%) | 1.000 |
| Oral ulcer | 1 (1%) | 0 (0%) | 1 (2%) | 1.000 |
| Lymphopenia | 1 (1%) | 1 (2%) | 0 (0%) | 1.000 |
| Increased C-reactive protein | 1 (1%) | 1 (2%) | 0 (0%) | 1.000 |
| Increased NTproBNP | 1 (1%) | 1 (2%) | 0 (0%) | 1.000 |
| Atrial fibrillation | 1 (1%) | 1 (2%) | 0 (0%) | 1.000 |
| Hypoalbuminemia | 1 (1%) | 1 (2%) | 0 (0%) | 1.000 |
| Hyponatremia | 1 (1%) | 1 (2%) | 0 (0%) | 1.000 |
| Increased glutamyl transpeptidase | 1 (1%) | 1 (2%) | 0 (0%) | 1.000 |
| Increased creatinine | 1 (1%) | 1 (2%) | 0 (0%) | 1.000 |
| Increased creatine kinase | 1 (1%) | 1 (2%) | 0 (0%) | 1.000 |
| Increased lactate dehydrogenase | 1 (1%) | 1 (2%) | 0 (0%) | 1.000 |
| Increased urea nitrogen | 1 (1%) | 1 (2%) | 0 (0%) | 1.000 |
| Costalgia | 1 (1%) | 1 (2%) | 0 (0%) | 1.000 |
| Serious Aes | 5 (6%) | 2 (5%) | 3 (7%) | 1.000 |
| Respiratory failure | 2 (2%) | 0 (0%) | 2 (5%) | 0.494 |
| Shock | 1 (1%) | 0 (0%) | 1 (2%) | 1.000 |
| Severe metabolic acidosis | 1 (1%) | 0 (0%) | 1 (2%) | 1.000 |
| Unconsciousness | 2 (2%) | 2 (5%) | 0 (0%) | 0.494 |
AE: Adverse event; NTproBNP: N-terminal prohormone of brain natriuretic peptide.
Discussion
In this study using PSM, we found that the use of CHM granules in addition to usual care was not significantly associated with clinical improvement on Day 28 in patients with severe COVID-19, when compared with patients who received usual care alone. However, assessment of secondary outcomes indicated reduced mortality rate and fever alleviation in the patients who received CHM granules.
The 28-day mortality rate was significantly lower in the CHM group than that in the control group. The RRR of 0.775 also showed a potential clinical significance. It is the expression of the number of patients who must be treated to prevent one AE, which was first reported by Laupacis et al. in 1988 (Nuovo et al., 2002). In our study, the mortality rates indicated that about 20.9% patients with severe COVID-19 who received usual care alone would be expected to have died on Day 28. This risk was reduced to 4.7% when the use of CHM granules was added to usual care. The ARR was estimated to be 16.2% and NNT was about 6.2, implying that if every 6.2 severe patients received CHM granules, it was expected that one death could be prevented on Day 28. Therefore, even though the use of CHM granules is not statistically effective in clinical improvement, physicians would still prescribe these granules for patients with severe COVID-19 to reduce mortality rate by 77.5% until a more effective drug is available.
Fever, cough, and dyspnea have been reported as the most common symptoms in COVID-19 patients (Guan et al., 2020; Huang et al., 2020). In this study, we investigated the duration for relief from these symptoms. The resolution of fever was found to be faster in patients who were given CHM granules along with usual care than in those with usual care alone. However, the improvement in symptoms of cough and dyspnea was not significantly rapid. The mechanism of CHM is complex. Studies have shown beneficial antivirus and immunomodulatory effects of CHM when administered for viral infections. Lianhuaqingwen,a Chinese patent medicine, has showed significant in vitro inhibition of SARS-COV-2 replication, effect on virus morphology, and anti-inflammatory activity (Runfeng et al., 2020). These results may partly explain the effectiveness of CHM granules in reducing fever.
The changes in lung lesion ratio calculated from CT images have been considered an effective prognosis indicator in patients with COVID-19 due to the quantitative measurement of lung involvement (Zhang et al., 2020). Although there was no significance, the reduced lung lesion ratio in the CHM group showed a trend of pulmonary inflammatory resorption. The increased lesion ratio in the control group further demonstrated that CHM granules might prevent deterioration of COVID-19 pneumonia.
As an indicator in helping diagnose thrombosis, D-dimer has been widely used in clinical practice to exclude thromboembolic disease where the probability is low (Adam et al., 2009). Recent studies pointed out that the increase in D-dimer levels is a strong indicator of mortality in those suffering from COVID-19 (Tang et al., 2020a, 2020b; Velavan and Meyer, 2020; Zhou et al., 2020). Interestingly, 14 patients in the CHM group had increased D-dimer levels but survived on Day 28 (Appendix C). Most of these patients were in a relatively stable condition or discharged alive; only two of them needed mechanical ventilation. Few studies have investigated the relationship between Chinese herbs and D-dimer. On one hand,we could consider it an AE in the CHM group, but on the other hand, particularly when taking into account the lower mortality of the CHM group, we could speculate that the use of CHM granules might have prevented death in those patients with abnormal D-dimerlevels. Besides, as this study was of retrospective nature, not every patient was tested for D-dimer, which may also cause bias. Further studies are required to investigate the intrinsic interaction and mechanism of Chinese herbs and fibrinolysis.
Our study has some limitations. First, the small sample size may cause bias and limit its representativeness of the real population. Second, it was a single-center study, which might limit the possibility of generalizing its findings. Third, we did not collect data on virology. Fourth, although we used PSM to minimize the bias due to confounding factors, the results of PSM could be generalized only among those in the range of propensity scores included in the analysis, and they may not be applicable to those who were out of this range. Moreover, there might have been unmeasured confounders that were not addressed in the PSM model.
Conclusion
In this study, the use of CHM granules in combination with usual care did not show significant clinical improvement in patients with severe COVID-19. Nevertheless, the patients who used CHM granules showed an inclination toward reduction in mortality rates and time to fever resolution, but these patients may acquire hypercoagulable states. Further well-designed trials are needed to explore the potential treatment benefits of CHM.
Ethics approval and consent to participate
Consent for publication
Not applicable.
Availability of data and materials
After publication, the data will be made available to others on reasonable requests to the corresponding author. A proposal with detailed description of study objectives and statistical analysis plan will be needed for evaluation of the reasonability of requests. Additional materials might also be required during the process of evaluation. De-identified participant data will be provided after approval from the corresponding author and Hankou Hospital, Wuhan (Tables B.1 and C.1).
Declaration of Competing Interest
This study was approved by the ethical committees of Guangdong Provincial Hospital of Chinese Medicine, Guangzhou, China (GPHCM; No. ZE2020-049-01). As the study was of retrospective, observational nature, the committee waived the need for a written informed consent.
Acknowledgments
Funding
The study was supported by National Administration of Traditional Chinese Medicine (Grant No.2020ZYLCYJ05-11);National Key Research and Development Project of China (Grant Nos. 2020YFA0708001 and 2020YFC0845300); Guangdong Provincial Key Laboratory of TCM Research on Emergency (Grant No. 2017B030314176); the Zhen's Miscellaneous Diseases School in Lingnan; and Zhang Zhongde's workshop of TCM celebrity doctors in Guangdong province. The funding body was not involved in the design, conduct, data collection, analysis, interpretation, or writing of this study.
Author Contributions
All data were generated in-house, and no paper mill was used. All authors agree to be accountable for the aspects of their work ensuring integrity and accuracy.YW, YL, QL, and DZ were responsible for the design, data collection, and writing of the manuscript. LZ, WO, and ZW contributed to data management and statistical analysis. BL, JC, TL, CF, QF, and YW were responsible for the analysis of CT scan images. BD, XZ, FY, FL, and AC contributed to supervising data collection and improved the manuscript. CSL was responsible for supervision or mentorship. JG, ZW, and ZZ were responsible for the research idea and result interpretation. All authors reviewed and approved the final version of the manuscript.
Footnotes
Yuanyuan Wang, Yuntao Liu, Qingquan LV, Danwen Zheng are co-first authors.
Appendix A. Composition, dosage, principle of TCM diagnosis, and prescription
-
1)
Epidemic toxin closing lung syndrome
Main symptoms: Greasy or dull-red complexion, stuffy chest, constipation, yellow greasy or yellow-white greasy coating on the tongue, and replete pulse (wiry and thready pulse, wiry and slippery pulse, or wiry and forceful pulse).
Aided symptoms: Dysphoria, bright red eyelids, aversion to heat, feverish palms and feet, and poor sleep at night.
Prescription: Chai-hu-jie-du granule (Bupleurum detoxification granule)
Composition: Chaihu (Radix Bupleuri), 30 g; Huangqin (Radix Scutellaria), 15 g; Fabanxia (Rhizoma Pinelliae Praeparatum), 15 g; Shengjiang (Rhizoma Zingiberis Recens), 15 g; Dazao (Ziziphus jujuba Mill), 5 g; Zhishi (Fructus Aurantii Immaturus), 20 g; Dahuang (Radix et Rhizoma Rhei), 10 g; Taoren (Semen Persicae), and 10 g; Baishao (Radix Paeoniae Alba), 15 g.
Administration and dosage: Oral or nasal feeding of 1–2 doses mixed with 100–200 mL waterfor 2 times a day.
-
1)
Syndromes of impaired primordial Qi and uncleared epidemic toxin closing lung
Main symptoms: Fatigue and weakness, light and dark tongue, and feeble pulse (thready pulse, sunken and thready pulse, deep and weak pulse, or deep and faint pulse).
Aided symptoms: Pale face, whitish eyelids, low faint voice, exertional dyspnea, intolerance to cold, deficient sweating, anorexia, cold limbs, and loose stool.
Prescription: Fu-zheng-jiu-fei granule (granule for strengthening body resistance and rescuing the lung)
Composition: Zhifuzi (Radix Aconiti Lateralis Preparata), 10 g; Ganjiang (Rhizoma Zingiberis), 15 g; Zhigancao (Radix et Rhizoma Glycyrrhizae Preparata), 20 g; Jinyinhua (Flos Lonicera), 10 g; Zaojiaoci (Spina Gleditsiae), 10 g; Wuzhaolong (Radix Ficus Hirta), 20 g; Guanghuoxiang (Herba Pogostemonis), 10 g; Chenpi (Pericarpium Citri Reticulatae), 5 g.
Administration and dosage: Oral or nasal feeding of 1–2 doses mixed with 100–200 mL waterfor 2 times a day.
-
1)
Adjustment of prescription
a. Continuing with the same prescription
If the patient's symptoms subsided progressively and had no new discomfort with the tongue and pulse improving gradually, the original prescription should be continued until all the main symptoms are relieved. b. Transformation from deficient syndrome to excessive syndrome
If main symptoms of patients with the syndrome of primordial Qi impaired by the epidemic toxin have no obvious relief after taking Fu-zheng-jiu-fei granules for 2 days or if excessive symptoms, such as constipation, restlessness, bright red eyelids, aversion to heat, feverish palms and feet, poor sleep at night, yellow greasy or yellow-white greasy tongue coating, replete pulse, and so on, have appeared, the prescription should be changed to Chai-hu-jie-du granules. c. Transformation from excessive syndrome to deficient syndrome
If the symptoms of patients with the syndrome of epidemic toxin closing lung did not alleviate obviously after taking Chai-hu-jie-du granules for 2 days or new deficient syndromes, such as fatigue and weakness, intolerance to cold, deficient sweating, anorexia, cold limbs, loose stool, light and dark tongue, feeble pulse, and so on, have appeared, the prescription should be adjusted to Fu-zheng-jiu-fei granules. d. Concurrence of the two syndromes
If the patient has symptoms of epidemic toxin closing lung syndrome and impairment of primordial Qi simultaneously, Chai-hu-jie-du granules and Fu-zheng-jiu-fei granules were prescribed in combination. Patients could be prescribed one Chai-hu-jie-du granule and one Fu-zheng-jiu-fei granule mixed together, to be taken 2 times a day; or oral or nasal feeding of each granule with 100–200 ml water, 1 time a day.
Appendix B
Granule preparation: Every herb, except Guanghuoxiang, was boiled with water and then filtered. The filtrate was concentrated into an ointment with maltodextrin for dry spraying. The primary extracts of the herb varieties were obtained after mixing with maltodextrin and magnesium stearate. According to the converted equivalent weight from herbs to granules, the granules were mixed and dried again with appropriate maltodextrin and silicon dioxide, and finally packed. For Guanghuoxiang, the active ingredient was volatile oil. In this case, the first step of granule preparation was to extract the volatile oil from the herb. The oil was then included with beta-cyclodextrin. After mixed with the filtrate, which was filtered from boiled herb and water, the primary extracts of Guanghuoxiang were obtained. The remaining steps were similar to that of preparation of granules from other herbs.
Table B.1.
The Chinese, English, and Latin names of related Chinese herbal medicines.
| Chinese names | English names | Latin names |
|---|---|---|
| 柴胡 | Chaihu | Radix Bupleuri |
| 黄芩 | Huangqin | Radix Scutellaria |
| 法半夏 | Fabanxia | Rhizoma Pinelliae Praeparatum |
| 生姜 | Shengjiang | Rhizoma Zingiberis Recens |
| 大枣 | Dazao | Ziziphus jujuba Mill |
| 枳实 | Zhishi | Fructus Aurantii Immaturus |
| 大黄 | Dahuang | Radix et Rhizoma Rhei |
| 桃仁 | Taoren | Semen Persicae |
| 白芍 | Baishao | Radix Paeoniae Alba |
| 制附子 | Zhifuzi | Radix Aconiti Lateralis Preparata |
| 干姜 | Ganjiang | Rhizoma Zingiberis |
| 炙甘草 | Zhigancao | Radix et Rhizoma Glycyrrhizae Preparata |
| 金银花 | Jinyinhua | Flos Lonicera |
| 皂角刺 | Zaojiaoci | Spina Gleditsiae |
| 五爪龙 | Wuzhaolong | Radix Ficus Hirta |
| 广藿香 | Guanghuoxiang | Herba Pogostemonis |
| 陈皮 | Chenpi | Pericarpium Citri Reticulatae |
All herbs were manufactured and industrially extracted into granules by Sichuan Neo-Green Pharmaceutical Technology Development Co., Ltd. (Chengdu, Sichuan, China).
Appendix C
Table C.1.
The incidence of the increased D-dimer in the groups.
| Patient IDs | Testing day of hospitalization | D-dimer (mg/l)* | Groups | Scores on six-category scale onDay 28† |
|---|---|---|---|---|
| 1042 | 20 | 3.55 | Control | 4 |
| 1048 | 21 | 3.88 | Control | 3 |
| 1040 | 10 | 1.9 | Control | 1 |
| 1004 | 20 | 0.66 | Control | 1 |
| 1035 | 27 | 1.15 | Control | 1 |
| 136 | 8 | >8.00 | CHM | 5 |
| 128 | 7 | 4.93 | CHM | 4 |
| 85 | 4 | 0.68 | CHM | 3 |
| 52 | 7 | > 8 | CHM | 3 |
| 37 | 16 | 2.34 | CHM | 3 |
| 49 | 16 | > 8 | CHM | 3 |
| 23 | 26 | 0.88 | CHM | 3 |
| 61 | 28 | 2.92 | CHM | 3 |
| 54 | 5 | 2.74 | CHM | 2 |
| 104 | 7 | 0.68 | CHM | 2 |
| 3 | 7 | 3.27 | CHM | 1 |
| 105 | 11 | 5.08 | CHM | 1 |
| 19 | 18 | 0.61 | CHM | 1 |
| 46 | 24 | 1.49 | CHM | 1 |
*The normal value of D-dimer is < 0.5 mg/l. Patients survived on Day 28 were included.
†Score on six-category scale on Day 28:
6. Death
5.Hospitalization, requiring ECMO, invasive mechanical ventilation, or both
4.Hospitalization, requiring HFNC or noninvasive mechanical ventilation
3.Hospitalization, requiring supplemental oxygen
2.Hospitalization, not requiring supplemental oxygen
1.Discharge from hospital
References
- Adam S.S., Key N.S., Greenberg C.S. D-dimer antigen: Current concepts and future prospects. Blood. 2009;113:2878–2887. doi: 10.1182/blood-2008-06-165845. [DOI] [PubMed] [Google Scholar]
- Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M., Li X., Xia J., Chen N., Xiang J., Yu T., Bai T., Xie X., Zhang L., Li C., Yuan Y., Chen H., Li H., Huang H., Tu S., Gong F., Liu Y., Wei Y., Dong C., Zhou F., Gu X., Xu J., Liu Z., Zhang Y., Li Hui, Shang L., Wang K., Li K., Zhou X., Dong X., Qu Z., Lu S., Hu X., Ruan S., Luo S., Wu J., Peng L., Cheng F., Pan L., Zou J., Jia C., Wang J., Liu X., Wang S., Wu X., Ge Q., He J., Zhan H., Qiu F., Guo L., Huang C., Jaki T., Hayden F.G., Horby P.W., Zhang D., Wang C. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 2020:1–13. doi: 10.1056/nejmoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Nakamura T. Statistical evidence for the usefulness of Chinese medicine in the treatment of SARS. Phyther. Res. 2004;18:592–594. doi: 10.1002/ptr.1485. [DOI] [PubMed] [Google Scholar]
- Chinese Clinical Trial Registry, n.d. URL http://www.chictr.org.cn/index.aspx.
- ClinicalTrials.gov, n.d. URL https://clinicaltrials.gov/.
- Coronavirus Disease (COVID-19) Outbreak Situation, n.d. URL https://who.sprinklr.com/.
- COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU), n.d. URL https://coronavirus.jhu.edu/map.html.
- COVID-19 Global Pandemic Real-time Report DXY•DX Doctor.COVID-19 Global Pandemic Real-time Report DXY•DX Doctor, n.d. URL https://ncov.dxy.cn/ncovh5/view/en_pneumonia?from=dxy&source=&link=&share=.
- Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X., Liu L., Shan H., Lei C.-L., Hui D.S.C., Du B., Li L.-J., Zeng G., Yuen K.-Y., Chen R.-C., Tang C.-L., Wang T., Chen P.-Y., Xiang J., Li S.-Y., Wang J.-L., Liang Z.-J., Peng Y.-X., Wei L., Liu Y., Hu Y.-H., Peng P., Wang J.-M., Liu J.-Y., Chen Z., Li G., Zheng Z.-J., Qiu S.-Q., Luo J., Ye C.-J., Zhu S.-Y., Zhong N.-S. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020:1–13. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;6736:497–506. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau T.F., Leung P.C., Wong E.L.Y., Fong C., Cheng K.F., Zhang S.C., Lam C.W.K., Wong V., Choy K.M., Ko W.M. Using herbal medicine as a means of prevention experience during the SARS crisis. Am. J. Chin. Med. 2005;33:345–356. doi: 10.1142/S0192415X05002965. [DOI] [PubMed] [Google Scholar]
- Leung P.-C. The efficacy of Chinese medicine for SARS: A review of Chinese publications after the crisis. Am. J. Chin. Med. 2007;35:575–581. doi: 10.1142/S0192415X07005077. [DOI] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K.S.M., Lau E.H.Y., Wong J.Y., Xing X., Xiang N., Wu Y., Li C., Chen Q., Li D., Liu T., Zhao J., Liu M., Tu W., Chen C., Jin L., Yang R., Wang Q., Zhou S., Wang R., Liu H., Luo Y., Liu Y., Shao G., Li H., Tao Z., Yang Y., Deng Z., Liu B., Ma Z., Zhang Y., Shi G., Lam T.T.Y., Wu J.T., Gao G.F., Cowling B.J., Yang B., Leung G.M., Feng Z. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Li Q, Cao R., Wang X., Chen R., Liu K., Fan L., Xiao Y., Zhang Z., Fu C.-C., Song Q., Liu W., Fang Q., Liu S. Detectability of pulmonary nodules by deep learning: results from a phantom study. Chinese J. Acad. Radiol. 2019;2:1–12. doi: 10.1007/s42058-019-00015-0. [DOI] [Google Scholar]
- National Health Commission of the People's Republic of China, n.d. [DOI] [PMC free article] [PubMed]
- National Health Commission of the People's Republic of China, N.A. of T.C.M. of the P., 2020a. Guidance for Corona Virus Disease 2019: Prevention, Control, Diagnosis and Management.
- National Health Commission of the People's Republic of China, N.A. of T.C.M. of the P., 2020b. Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7), pp. 1–17.
- Nuovo J., Melnikow J., Chang D. Reporting number needed to treat and absolute risk reduction in randomized controlled trials. J. Am. Med. Assoc. 2002;287:2813–2814. doi: 10.1001/jama.287.21.2813. [DOI] [PubMed] [Google Scholar]
- Runfeng L., Yunlong H., Jicheng H., Weiqi P., Qinhai M., Yongxia S., Chufang L., Jin Z., Zhenhua J., Haiming J., Kui Z., Shuxiang H., Jun D., Xiaobo L., Xiaotao H., Lin W., Nanshan Z., Zifeng Y. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacol. Res. 2020;156 doi: 10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velavan T.P., Meyer C.G. Mild versus severe COVID-19: Laboratory markers. Int. J. Infect. Dis. 2020;95:304–307. doi: 10.1016/j.ijid.2020.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2020. WHO R&D Blueprint: novel coronavirus: outline of trial designs for experimental therapeutics.
- WHO, 2009. The use of the WHO-UMC system for standardised case causality assessment. Good Pharmacovigil. Pract. Guid. URL https://www.who.int/medicines/areas/quality_safety/safety_efficacy/pharmvigi/en/.
- WHO Coronavirus Disease (COVID-19) Pandemic, n.d. URL https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
- Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y., Pan S., Zou X., Yuan S., Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020;2600:1–7. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Liu X., Shen J., Li Z., Sang Y., Wu X., Zha Y., Liang W., Wang C., Wang K., Ye L., Gao M., Zhou Z., Li L., Wang J., Yang Z., Cai H., Xu J., Yang L., Cai W., Xu W., Wu S., Zhang W., Jiang S., Zheng L., Zhang X., Wang L., Lu L., Li J., Yin H., Wang W., Li O., Zhang C., Liang L., Wu T., Deng R., Wei K., Zhou Y., Chen T., Lau J.Y.-N., Fok M., He J., Lin T., Li W., Wang G. Clinically applicable AI system for accurate diagnosis, quantitative measurements, and prognosis of COVID-19 pneumonia using computed tomography. Cell. 2020;181 doi: 10.1016/j.cell.2020.04.045. 1423.E11–1433.E11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
After publication, the data will be made available to others on reasonable requests to the corresponding author. A proposal with detailed description of study objectives and statistical analysis plan will be needed for evaluation of the reasonability of requests. Additional materials might also be required during the process of evaluation. De-identified participant data will be provided after approval from the corresponding author and Hankou Hospital, Wuhan (Tables B.1 and C.1).