Abstract
Background
Since the declaration of COVID-19 as a global pandemic by the World Health Organization, countries are struggling with a shortage of medical capacities. It would be essential if the risk for preventable comorbidities, such as the common cold, can be reduced or prevented, so that the scarce medical resources and facilities can be focused on COVID-19.
Purpose
To evaluate the effects of two herbal medicines (Jinhaoartemisia antipyretic granules and Huoxiangzhengqi oral liquids) in reducing the risk of the common cold in community-dwelling residents in China during the COVID-19 outbreak.
Study Design
A prospective open-label, parallel-group, cluster-randomized controlled trial (RCT), was conducted in Chengdu, China.
Methods
A total of 22,065 participants from 11 communities were recruited during a period of one month. The trial started on 30 January and participants were followed up till 29 February 2020. Participants were randomly assigned to receive either a five-day herbal medicine therapy plus a reference manual or a reference manual only if they were allocated to the control group. The primary endpoint was the occurrence of patient-reported common cold symptoms. The secondary endpoint was the time in days from the receipt of herbal drugs/reference manual and the occurrence of the common cold symptoms.
Results
Use of herbal medicine reduced the risk of the common cold by 89.6% (95% CI, 52.9% to 97.7%) in all community-dwelling residents, and by 94.0% (95% CI, 52.1% to 99.2%) in residents aged between 16 and 59 years old. Sensitivity analyses showed similar results.
Conclusion
This community-based RCT found that the use of a herbal medicine therapy (Jinhaoartemisia antipyretic granules and Huoxiangzhengqi oral liquids) could significantly reduce the risks of the common cold among community-dwelling residents, suggesting that herbal medicine may be a useful approach for public health intervention to minimize preventable morbidity during COVID-19 outbreak.
Keywords: Coronavirus disease 2019, Herbal medicine, Common cold, Respiratory tract infection
Abbreviations: CONSORT, Consolidated Standards of Reporting Trials; Covid-19, coronavirus disease 2019; GEE, Generalized Estimation Equation; HAIs, hospital-acquired infections; ICD-11, International Statistical Classification of Diseases and Related Health Problems; ICTRP, International Clinical Trials Registry Platform; LMICs, low- and middle-income countries; OR, odds ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WHO, World Health Organization; 95% CI, 95% confidence interval
Introduction
Since the declaration of coronavirus disease 2019 (COVID-19) as a global pandemic by the World Health Organization (WHO), different multifaceted strategies have been implemented to stop the spread of the infection by different countries (World Health Organization, 2020a; Gates, 2020; Anderson et al., 2020; Chen et al., 2020). Only Remdesivir was authorized for emergency use for the treatment of hospitalized COVID-19 patients, and there is no treatment or vaccines available for community dwelling or quarantined patients (Anderson et al., 2020; Cao et al., 2020; U.S. Food and Drug Administration, 2020). Most recently, China began to report no new locally transmitted coronavirus cases for the first time since the pandemic, suggesting a major turning point for the world and proof of the ongoing success of China's efforts to control the virus (Chen et al., 2020).
Among many of the strategies by China is the wide use of traditional Chinese medicine, which includes herbal medicines, acupuncture, physical exercise, and other therapies (Xiao and Torok, 2020; Ren et al., 2020). Traditional herbal medicine is fully institutionalized in the China Health Care System. Reports from the National Health Commission state that the use of traditional herbal medicine for patients with COVID-19 accounted for 91.05% of the confirmed cases in Hubei province and accounted for 96.37% of the confirmed cases in non-Hubei provinces. In addition, among 564 patients who had mild or non-severe symptoms and used traditional herbal medicine in Wuhan JiangxiaFangcang hospital, none of the cases advanced to severe cases. Herbal medicine use has been the standard treatment in the National Treatment Guidelines for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in China.
As COVID-19, caused by SARS-CoV-2, spreads rapidly throughout the world, it is possible that large shortfall in inpatient hospital and intensive care unit bed capacity, along with a shortage of workforce, could pose significant challenges for current efforts to address COVID-19 (Anderson et al., 2020; Chen et al., 2020). As countries are struggling with a shortage of medical capacities, it would be essential if the risk for other preventable comorbidities, such as the common cold, can be reduced or prevented, so that the scarce medical resources and facilities can be focused on COVID-19. In addition, hospitals and healthcare facilities in low- and middle-income countries (LMICs) of Asia and West Africa do not have sufficient strategies to prevent and control hospital-acquired infections (HAIs), which was reported to have increased the mortality and financial loss during the outbreaks of SARS, Ebola, MERS, and COVID-19.
The chemical ingredients of herbs that are usually used in herbal medicine might be useful for the management of respiratory tract infection (Arora et al., 2011; Luo et al., 2020). Wang and Liu (2014) found that herbs could directly inhibit pathogens infecting the respiratory tract or coordinate the activity of the immune system to avoid or relieve infections. The purpose of this study was to evaluate the effects of two herbal medicines (Jinhaoartemisia antipyretic granules and Huoxiangzhengqi oral liquids) in reducing the risk of the common cold in community-dwelling residents during the COVID-19 outbreak.
Methods
Study design
This prospective open-label, parallel-group, cluster-randomized controlled trial (RCT) was conducted in Chengdu, China. A cluster is defined as the neighborhoods managed by the same Community Service Center. Study participants were randomly sampled from three districts in Chengdu. We sampled 11 clusters, including two communities in Jinniu District, four communities in Qingyang District, and five communities in Wuhou District. These selected clusters had a sizeable physical distance of at least four kilometers to minimize the risk for spillover effects from contamination in cluster-RCT. In addition, community quarantines and isolation enforced since 7 February 2020 in Chengdu made it possible to prevent any further contamination among residents in these communities. The trial started on 30 January and participants were followed up till 29 February 2020.
All participants provided written informed consent for participation in the study. Approval for the study protocol was obtained from the Sichuan Regional Ethical Review Committee of traditional Chinese medicine. This study was carefully designed, and registration met all the 20-item criteria suggested by the International Clinical Trials Registry Platform (ICTRP) (World Health Organization 2020b). The protocol of the study strictly followed the Consolidated Standards of Reporting Trials (CONSORT) and Standard Protocol Items for Randomized Trials guidelines (Bian et al., 2011).
Participants
Residents were eligible to participate if they were willing to volunteer to take the medications recommended by this study. Residents were excluded if they had any of the following conditions: suspected COVID-19 or common cold symptoms (fever, cough with little sputum or yellow sputum, fatigue, stuffy nose, runny nose, dry throat, thirst, poor stool or diarrhea, chest tightness, abdominal distension, constipation, and other discomfort symptoms), comorbidity (cardiovascular, cerebrovascular, lung, kidney, and hematopoietic diseases), a history of allergy to either Jinhaoartemisia antipyretic granules or Huoxiangzhengqi oral liquids used in the study, or pregnancy and lactation. Residents’ comorbidities were based on their self-reports at enrollment via phone calls.
Randomization and blinding
The selected clusters (communities) were randomly assigned in an equal ratio to either the herbal medicine group or control groups using random sequences generated by SAS (version 9.4). Randomization was carried out by an independent individual and was not involved in outcome assessments. All researchers who conducted outcome assessment, data collection, analysis, and interpretation were blinded to group allocations.
Procedures
All participants fully understood the risks and benefits of participation prior to signing the informed consent. A total of 465 participants were not fully compliant with the protocol during the follow-ups and thus intention-to-treat analysis was performed for the study. Participants received a five-day herbal medicine therapy plus a reference manual if they were allocated to the herbal medicine group, or a reference manual if they were allocated to the control group.
The interventional herbal medicines were Jinhao Artemisia Antipyretic Granules (8 g b.i.d.) and the Huoxiangzhengqi oral liquid (10 ml b.i.d.) and were prescribed by physicians to the patients according to their pathogenic and dampness factors. Two herbal medicines were administered at one-hour interval. The use of other herbal medicines indicated to prevent colds and flu-like symptoms, antiviral drugs, and antibacterial drugs were not allowed. Based on traditional Chinese medicine theory, the common cold potential is considered as pathogenic cold, and the therapy should focus on expelling “cold and dampness”. Jinhao Artemisia Antipyretic Granules and Huoxiangzhengqi Oral Liquid are indicated for the prevention of common cold due to wind and dampness and were recommended by Standard Treatment Guidelines for COVID-19.
All participants were instructed to report their outcomes via telephone calls. Follow-up for outcomes had been performed for 14 days. The first follow-up was on the day of the administration of medications; subsequent follow-ups were on the third day, the fifth day, the seventh day, and the fourteenth day after the first follow-up. During the follow-up calls, patients were also reminded to take herbal drugs or read reference manual on time to ensure medication adherence, and the use of medications was collected.
Outcomes
The primary endpoint was the occurrence of a patient-reported and clinically diagnosed common cold, including fever, cough with little sputum or yellow sputum, fatigue, stuffy nose, runny nose, dry throat, thirst, poor stool or diarrhea, chest tightness, abdominal distension, constipation, and other discomfort symptoms. The secondary endpoint was the time in days from the receipt of herbal drugs and the occurrence of the common cold symptoms. The common cold diagnosis was made by two community doctors. A consensus would be reached following discussions among the doctors and investigators should there be disagreement. Community doctors were available for verification if there was any confusion about the outcomes. Participants were instructed to stop taking their herbal medicines when having common cold symptoms. They would be contacted by a community physician for further assessments.
Statistical analyses
The incidence of the common cold in China was 2‰ in 2018. Assuming an incidence between 1.5‰ and 2.5‰, effect size of 80% to 90% to be effective in prevention over a two-week period after receiving treatment between the herbal medicine and control groups, significance level at 0.05, and a power at 80%, the estimated number of participants in each group ranged from 4,004 to 9,799, suggesting the study needed to recruit 19,598 participants.
The incidence rates of the common cold were expressed with the 95% Clopper-Pearson exact confidence interval. The Generalized Estimation Equation (GEE) model was employed to compare the incidence rates of the two groups. The model with logit link function included the disease status as the dependent variable, group as a fixed effect and family as a random effect. The odds ratio (OR) between intervention and non-intervention groups and corresponding 95% CI were estimated based on the model. The Kaplan-Meier curve of the incident common cold was plotted. Sensitivity analyses were performed after excluding the whole families in which any resident had a common cold during the period of screening. Subgroup analyses were performed in different groups of age (< 16, 16–59, ≥ 60).
All reported p values were two-sided, with a p-value of less than 0.05 deemed to be significant. All analyses were done with SAS (version 9.4).
Results
Participants and baseline characteristics
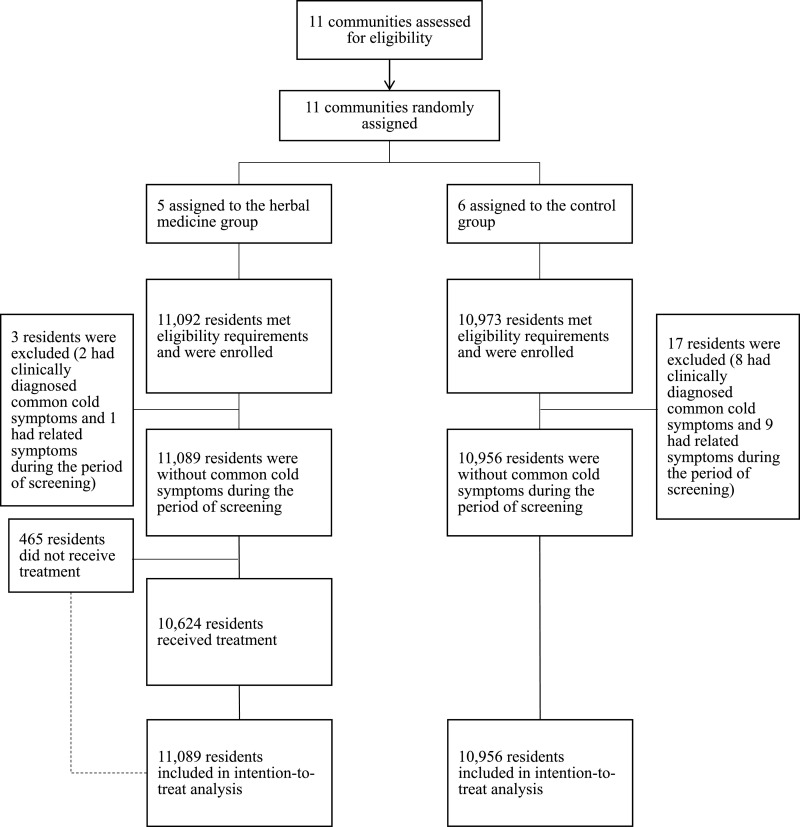
Between January 30, 2020, and February 29, 2020, five communities were randomly allocated to the herbal medicine group and six communities were assigned to the control group. After screening, a total of 4,344 families or 11,092 residents in the herbal medicine group met the selection criteria, and a total of 4,394 families or 10,973 residents in the control group met the selection criteria. Twenty residents from the control group had COVID-19 or common cold symptoms before taking herbal medicines and thus were excluded from the study. Specifically, a total of 3 participants were excluded in the herbal medicine group, including 2 individuals with clinically diagnosed common cold symptoms and 1 with diarrhea before the start of the intervention; 17 residents were excluded in the control group, including 8 with clinically diagnosed common cold symptoms, 3 with pharyngitis, 2 with chronic cough, 1 with emphysema, 1 with diarrhea, 1 with cardiac arrhythmia, and 1 with mouth sores (Fig. 1 ).
Fig. 1.
Flowchart of randomization, enrollment, screening, and follow-up.
Table 1 showed the baseline characteristics of participants before receiving the interventions. The majority of the participants were aged between 16 and 59; 75.58% in the herbal medicine group and 59.92% in the control group.
Table 1.
Baseline information of participants.
| Characteristics | Herbal medicine group N = 11,092 | Control group N = 10,973 |
|---|---|---|
| Age, years | ||
| < 16 (No., %) | 35 (0.32) | 70 (0.64) |
| 16–59 (No., %) | 8,383 (75.58) | 6,574 (59.92) |
| ≥ 60 (No., %) | 2,659 (23.97) | 4,269 (38.91) |
| Gender | ||
| Male (No., %) | 5,398 (48.67) | 5,186 (47.27) |
| Female (No., %) | 5,694 (51.33) | 5,786 (52.73) |
| Had common cold or related symptoms during the screening period | ||
| Common cold symptoms (No., %) | 2 (0.02) | 8 (0.07) |
| Other related symptoms (No., %) | 1 (0.01) | 9 (0.08) |
| No symptoms (No., %) | 11,089 (99.97) | 10,956 (99.85) |
Herbal medicines and incidence of the common cold
Table 2 compares the incidence of common cold between herbal medicine and control groups. Two participants or 0.02% (95% CI, 0.00 to 0.07%) receiving herbal medicines developed incident common cold over a 14-day period; while 18 participants or 0.16% (95% CI, 0.10% to 0.26%) in the control group developed symptoms. The odds ratio (OR) of having an incident common cold in the herbal medicine group was 0.104 (95% CI, 0.023 to 0.471) compared to the control group.
Table 2.
Effects of herbal medicine on the risk of the common cold and by age group.
| Herbal medicine group, n (%; 95% CI) | Control group, n (%; 95% CI) | OR (95% CI) | Protective effect (95% CI) | p-value | |
|---|---|---|---|---|---|
| Alla | 2 (0.02; 0.00–0.07) | 18 (0.16; 0.10–0.26) | 0.104 (0.023–0.471) | 89.6% (52.9%–97.7%) | 0.0033 |
| Age group, years | |||||
| < 16b | 0 (0.00; 0.00–10.00) | 0 (0.00; 0.00–5.13) | – | – | – |
| 16–59c | 1 (0.01; 0.00–0.07) | 13 (0.20; 0.11–0.34) | 0.060 (0.008–0.479) | 94.0% (52.1%–99.2%) | 0.0079 |
| ≥ 60d | 1 (0.04; 0.00–0.21) | 5 (0.12; 0.04–0.27) | 0.315 (0.037–2.673) | 68.5% (-167.3%–93.3%) | 0.2899 |
OR: Odds Ratio.
11,089 participants in the herbal medicine group and 10,956 in the control group.
35 patients in the herbal medicine group and 70 in the control group.
8,383 patients in the herbal medicine group and 6,574 in the control group.
2,659 patients in the herbal medicine group and 4,269 in the control group.
According to the results of the GEE model (Table 2), using herbal medicine reduced the risk of incident common cold by 89.6% (95% CI, 52.9% to 97.7%) in all community-dwelling residents. We also found that using herbal medicines could reduce the risk of having common cold by 94.0% (95% CI, 52.1% to 99.2%) in residents between 16 and 59 years old. No significant protective effect of herbal medicine was observed in participants younger than 16 years old, or in those aged 60 years or older.
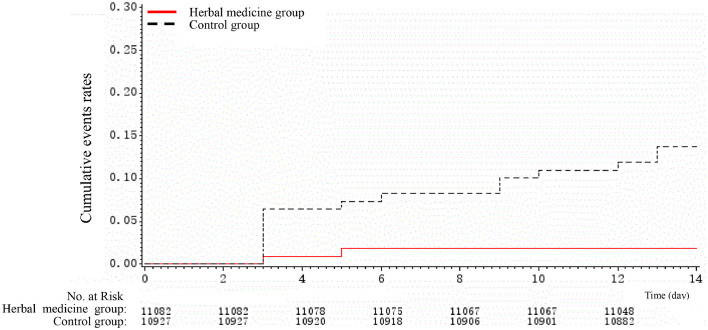
In the sensitivity analyses, we excluded families in which any family member had common cold symptoms during the period of screening and yielded similar results (Table 3 ).
Table 3.
Sensitivity analysis of protective effect of herbal medicine and by age group.
| Herbal medicine group, n (%; 95% CI) | Control group, n (%; 95% CI) | OR (95% CI) | Protective effect (95% CI) | p-value | |
|---|---|---|---|---|---|
| Alla | 2 (0.02; 0.00–0.07) | 18 (0.16; 0.10–0.26) | 0.104 (0.023–0.470) | 89.6% (52.9%–97.7%) | 0.0033 |
| Age group, years | |||||
| < 16b | 0 (0.00; 0.00–10.00) | 0 (0.00; 0.00–5.13) | – | – | – |
| 16–59c | 1 (0.01; 0.00–0.07) | 13 (0.20; 0.11–0.34) | 0.060 (0.008–0.478) | 94.0% (52.2%–99.2%) | 0.0079 |
| ≥ 60d | 1 (0.04; 0.00–0.21) | 5 (0.12; 0.04–0.27) | 0.315 (0.037–2.665) | 68.5% (-166.5%–93.3%) | 0.2888 |
OR: Odds Ratio.
11,086 participants in the herbal medicine group and 10,931 in the control group.
35 patients in the herbal medicine group and 70 in the control group.
8,381 patients in the herbal medicine group and 6,553 in the control group.
2,655 patients in the herbal medicine group and 4,248 in the control group.
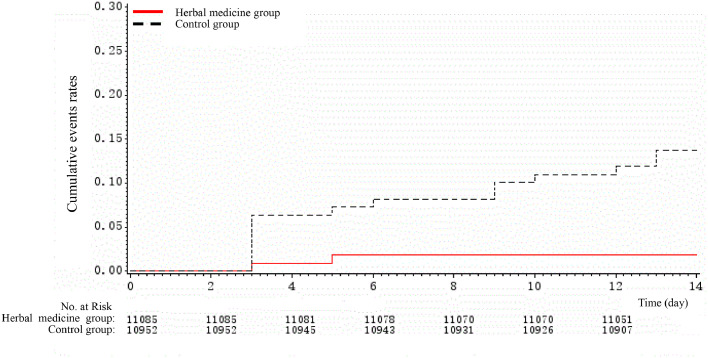
To assess differences in rates of developing common cold symptoms over time, we compared Kaplan-Meier curves of the event rates between herbal medicine and control groups, which are presented in Fig. 2., Fig. 3. . Kaplan-Meier curves revealed substantial differences in the rates of events over time; herbal medicine was associated with significantly lower risks of having common cold symptoms, compared to the control group.
Fig. 2.
Kaplan-Meier cumulative event rates for the primary outcome.
Fig. 3.
Sensitivity analysis of Kaplan-Meier cumulative event rates for the primary outcome (excluding families with patients who had symptoms at baseline).
Discussion
To the best of our knowledge, this is the first large, open-label, randomized clinical trial to examine the effect of herbal medicine on lowing the risks of the common cold. This study provided the highest level of evidence, derived from the RCT, to support the use of herbal medicine to lower risks of the common cold. Traditional herbal medicine is fully institutionalized in China Health Care system and is widely used for the prevention and treatment of the common cold. However, very few studies were conducted in accordance with internationally recognized standards. For instance, clinical studies that examined herbal medicines often fail to follow internationally recognized research guidelines, resulting in concerns about the validity and reliability of these studies (Wu et al., 2008). This randomized clinical trial, however, met all the 20-item criteria suggested by the ICTRP (World Health Organization 2020b). In addition, the protocol of the study strictly followed the CONSORT and Standard Protocol Items for Randomized Trials guidelines (Bian et al., 2011).
The findings from this study show the beneficial effect of using herbal medicine as a preventive approach to reduce the risks of the common cold during the COVID-19 outbreak. We found that the Jinhao Artemisia Antipyretic Granules and the Huoxiangzhengqi oral liquid were clinically and statistically beneficial in reducing risks of the common cold compared to the control group among large community-dwelling residents in the overall participants. Similar results were illustrated by using Kaplan-Meier cumulative event rates for the primary outcome, or in the sensitivity analyses in which families were excluded if any of its residents had a common cold during the period of screening. Subgroup analyses were performed in different groups of age (< 16, 16–59, ≥60). In subgroup analyses, the clinical benefit of herbal medicine was established in individuals between 16 and 59 years old. We did not, however, find the same effect among individuals younger than 16 years or people aged 60 years or older probably because of the very low incidence of the common cold and small sample size. For individuals younger than 16 years old, no incident common cold was observed during the follow-up; for individuals aged 60 years or older, only one incident case in the treatment group and five cases in the control group were observed. Although it shows that the herbal medicine was associated with a reduced risk of common cold, the results did not reach statistical significance level because of the small number of cases in the subgroup analyses. This very low incidence was due to the quarantines during the COVID-19 outbreak. Future studies with a larger sample size focusing on younger or older individuals are warranted.
During the outbreak of COVID-19, reducing preventable comorbidities is of great importance to maintain a functioning health care system. First, the common cold is common in the Spring season. It is too much burden for the health care system if patients with other conditions compete for hospital beds and other scarce medical resources. Second, hospital-acquired COVID-19 is possible and has been reported. More clinics and hospital visits due to the common cold or flu are associated with an increased likelihood of acquiring COVID-19. Third, the supply of testing kits for COVID-19 was extremely limited at the beginning of the outbreak in China. If the risks of the common cold are reduced, testing kits can be saved for those who most need them. Fourth, having symptoms that may be confused with COVID-19 can increase the frequency and severity of pandemic worry. Pandemic worry may result in a negative impact on mental health. Given the extensive media coverage of COVID-19 in China, when patients have a common cold, there is a concern of unnecessary pandemic worry among communities. Taking herbal medicine as a preventive strategy can reduce the pandemic worry of COVID-19 among the general public.
Although herbal medicine is considered as a safe way to boost the immune system and has very few side effects, safety should always be the top priority in the use of herbal medicines, as it might cause herb-induced liver injury (HILI) to patients (Xiao et al., 2019). According to Teschke et al., from 2006 to 2019, there were 21 published studies of HILI cases assessed for causality using the Roussel Uclaf Causality Assessment Method (RUCAM) in China (Teschke et al., 2020). However, these studies did not report any HILI cases caused by the herb medicines used in this specific trial. Therefore, HILI was not a concern for this study and Jinhaoartemisia antipyretic granules and Huoxiangzhengqi oral liquids were safe therapies to prevent the common cold during the COVID-19 pandemic.
Aside from the fact that some herbal medicines might induce liver injury, evidence has shown that some of the herbal medicines have a protective effect against liver injury (Zhao et al., 2014). According to Zhang et al, several trials on COVID-19 characteristicshave shown that COVID-19 was associated with abnormal liver tests, with rates ranging from 16.1% to 53.1% (Zhang et al., 2020). However, in this trial, we did not perform liver function tests for the residents, because there was no literature showing the herbal medicines used in this trial would induce liver injury or have a protective effect against liver injury, and during the period of the trial, it was difficult to perform relevant tests due to self-quarantine and constraint medical resources. Future studies are needed to evaluate whether herbal medicines have protective effects against COVID-19-induced liver test abnormalities.
Herbal medicine is one of the oldest healing systems of medical practice from ancient China and has a history of more than 2000 years. While herbal medicine originated in China, it is widely practiced in Asia such as Japan, South Korea, and Singapore. Non-Asian countries have also recognized the potentials of therapeutic benefit and herbal medicine is practiced in over 100 countries worldwide. In 2019, the WHO adopted the eleventh revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-11), in which the herbal medicine was included for the first time, suggesting that WHO recognizes the potential of traditional herbal medicine in the practice of healthcare systems among its members.
This study has several strengths. First, this study is a community-based RCT. RCT is the gold standard to establish a causal relationship between the herbal medicine and the common cold. All observed and unobserved confounding variables may be adjusted in the RCT design. Second, this study identified the herbal drug as a potentially useful and inexpensive public health prevention strategy to reduce preventable comorbidities during COVID-19. Given the fact that currently there is no medication to treat and vaccine to prevent COVID-19, identifying effective and affordable intervention strategies is very important to the government and the general public. Third, this study provided scientific-based evidence on the effect of the herbal medicine in the prevention of diseases. Herbal medicine has been used to prevent the common cold for thousands of years in China. However, the practice is mainly based on experience rather than evidence. This study provided the highest level of evidence to support herbal medicine practice in disease prevention. Several limitations of the study should be noted. First, because of the use of community-based randomization, we could not generate a perfect balance of covariates between the two groups, and the covariates between the two groups might be imbalanced by chance (Carter, 2010; Ivers et al., 2012). At baseline, two residents in the herbal medicine group had clinically diagnosed common cold symptoms, while there were eight people in the control group. However, the Chi-square test for such prevalence showed that the p-value was 0.056 and greater than 0.05 between herbal medicine and control group, which was not statistically significant, suggesting that this imbalance might be generated by chance (Robert and Torgerson, 1999; Carter, 2010). Moreover, the reported OR of the incidence of common colds during the follow-up between the two groups was controlled for covariates using GEE model with group as a fixed effect and family as a random effect, which could further rule out the chance bias caused by potential imbalanced characteristics of patients (Robert and Torgerson, 1999). Second, the primary outcome was the self-reported incidence of the common cold and is subject to bias. However, it is unlikely that participants would over- or under-estimate these symptoms if they have developed, and community doctors helped to clarify if there were any confusion. In addition, patient-reported outcomes are well in line with the four traditional diagnosis methods of looking, listening and smelling, asking, and touching, which are based on specific symptoms rather than laboratory works. Third, this study was conducted during the quarantine period. Due to social distancing and behavior change, we observed a lower risk for the common cold in this study. Fourth, only two herbal medicines were investigated in the study. Other herbal medicines might have different effects on possible preventive approaches.
Conclusion
The findings of this randomized clinical trial suggest that Jinhao Artemisia Antipyretic Granules and Huoxiangzhengqi oral liquid may be associated with significant reductions in the risk of common cold among community-dwelling residents. This study provides important insights into the merits of herbal medicine approach to public health intervention to minimize preventable morbidity during the COVID-19 outbreak, at a time when patients are competing for limited medical resources, and a time when two of the biggest global exporters of herbal medicines, China and India, become key players (The Lancet Oncology, 2015). Herbal medications are an essential component of both the goal of Healthy China 2030 and China's ongoing long-term medical reform. It is also an integral part of the Diagnosis and Treatment Protocols of COVID-19 by the National Health Commission of China during the COVID-19 pandemic. Although the traditional Chinese medicine theory may be complicated or obscure for Western health care practitioners in that prescriptions of herbal medications are based on patients’ symptoms and signs, it may be the right time to embrace this millennium-old practice for the world in face of the challenges of COVID-19 outbreak.
Funding
This work was supported by the Department of Science and Technology of Sichuan Province New Coronavirus Science and Technology Emergency Response Project [2020YFS0012 and 2020YFS0013); Chengdu Science and Technology Bureau's key R & D support plan [002090591030];and Chengdu University of Traditional Chinese Medicine Fund [XGZX2001 and XGZX2002).
Author Contributions
Bohua Yan and Jing Yuan conceived of the study, Jianyuan Tang and Kevin Lu initiated the study design, Zhiwei Jiang formulated a statistical analysis plan, Jielai Xia carried out statistical analysis, Minghui Li, Jieping Zeng, Hong Ding, Qiaoling Wang, Jundong Wang, HongyanXie, and Yan Li helped with implementation, Wenyuan Li, Na Zhang, Haiyan Li and Xiaoya Sang put forward supplementary opinions on the protocol, Lina Wu, XiaomoXiong, Shiyun Tang, and Mengyao Tao is responsible for trial registration and ethical issues. Qiaoling Wang, Yan Li, and Mengyao Tao provided statistical expertise in clinical trial design,ChunguangXie and Shuguang Yu are in charge of the sponsor. All data were generated in-house, and no paper mill was used. All authors agree to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Competing Interest
All authors declare no competing interests.
Acknowledgments
The authors thank the families and residents who participated in the study for their time and contributions.
References
- Anderson R.M., Heesterbeek H., Klinkenberg D., Hollingsworth TD. How will country-based mitigation measures influence the course of the COVID-19 epidemic? Lancet. 2020;395(10228):931–934. doi: 10.1016/S0140-6736(20)30567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora R., Chawla R., Marwah R., Arora P., Sharma R.K., Kaushik V., Goel R., Kaur A., Silambarasan M., Tripathi R.P., Bhardwaj J.R. Potential of complementary and alternative medicine in preventive management of novel H1N1 flu (swine flu) pandemic: thwarting potential disasters in the bud. Evid. Based Complement. Alternat. Med. 2011;2011 doi: 10.1155/2011/586506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian Z., Liu B., Moher D., Wu T., Li Y., Shang H., Cheng C. Consolidated standards of reporting trials (CONSORT) for traditional Chinese medicine: current situation and future development. Front. Med. 2011;5(2):171–177. doi: 10.1007/s11684-011-0132-z. [DOI] [PubMed] [Google Scholar]
- Carter B. Cluster size variability and imbalance in cluster randomized controlled trials. Stat. Med. 2010;29(29):2984–2993. doi: 10.1002/sim.4050. [DOI] [PubMed] [Google Scholar]
- Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M., Li X., Xia J., Chen N., Xiang J., Yu T., Bai T., Xie X., Zhang L., Li C., Yuan Y., Chen H., Li H., Huang H., Tu S., Gong F., Liu Y., Wei Y., Dong C., Zhou F., Gu X., Xu J., Liu Z., Zhang Y., Li H., Shang L., Wang K., Li K., Zhou X., Dong X., Qu Z., Lu S., Hu X., Ruan S., Luo S., Wu J., Peng L., Cheng F., Pan L., Zou J., Jia C., Wang J., Liu X., Wang S., Wu X., Ge Q., He J., Zhan H., Qiu F., Guo L., Huang C., Jaki T., Hayden F.G., Horby P.W., Zhang D., Wang C. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. N. Engl. J. Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Yang J., Yang W, Wang C., Bärnighausen T. COVID-19 control in China during mass population movements at New Year. Lancet. 2020;395(10226):764–766. doi: 10.1016/S0140-6736(20)30421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates B. Responding to covid-19 - a once-in-a-century pandemic? N. Engl. J. Med. 2020;382(18):1677–1679. doi: 10.1056/NEJMp2003762. [DOI] [PubMed] [Google Scholar]
- Ivers N.M., Halperin I.J., Barnsley J., Grimshaw J.M., Shah B.R., Tu K., Upshur R., Zwarenstein M. Allocation techniques for balance at baseline in cluster randomized trials: a methodological review. Trials 1. 2012;13:120. doi: 10.1186/1745-6215-13-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H., Tang Q.L., Shang Y.X., Liang S.B., Yang M., Robinson N., Liu J.P. Can Chinese medicine be used for prevention of corona virus disease 2019 (COVID-19)? A review of historical classics, research evidence and current prevention programs. Chin. J. Integr. Med. 2020;26(4):243–250. doi: 10.1007/s11655-020-3192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J., Zhang A., Wang X. Traditional Chinese medicine for COVID-19 treatment. Pharmacol. Res. 2020;155 doi: 10.1016/j.phrs.2020.104743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts C., Torgerson D.J. Understanding controlled trials: baseline imbalance in randomised controlled trials. BMJ 17. 1999;319(7203):185. doi: 10.1136/bmj.319.7203.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teschke R., Zhu Y., Jing J. Herb-induced liver injury in asia and current role of RUCAM for causality assessment in 11,160 published cases. J. Clin. Transl. Hepatol. 2020;28(2):200–214. doi: 10.14218/JCTH.2020.00009. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Lancet Oncology Rethinking traditional Chinese medicines for cancer. Lancet Oncol. 2015;16(15):1439. doi: 10.1016/S1470-2045(15)00406-4. [DOI] [PubMed] [Google Scholar]
- U.S. Food & Drug Administration, 2020. https://www.fda.gov/media/137564/download.
- World Health Organization, 2020a. WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020.
- World Health Organization, 2020b. International Clinical Trials Registry Platform (ICTRP).https://www.who.int/ictrp/network/trds_1.2.1/en.
- Wang X., Liu Z. Prevention and treatment of viral respiratory infections by traditional Chinese herbs. Chin. Med. J. (Engl.) 2014;127(7):1344–1350. [PubMed] [Google Scholar]
- Wu T., Yang X., Zeng X., Poole P. Traditional Chinese medicine in the treatment of acute respiratory tract infections. Respir. Med. 2008;102(8):1093–1098. doi: 10.1016/j.rmed.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y., Torok M.E. Taking the right measures to control COVID-19. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30152-3. 10.1016%2FS1473-3099(20)30152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Tang J, Mao Y, et al. Guidance for the clinical evaluation of traditional Chinese medicine-induced liver injury issued by China Food and Drug Administration. Acta Pharm. Sin. B. 2019;9(3):648–658. doi: 10.1016/j.apsb.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Shi L., Wanga FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol. Hepatol. 2020;5(5):428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao CQ., Zhou Y., Ping J., Xu LM. Traditional Chinese medicine for treatment of liver diseases: progress, challenges and opportunities. J. Integr. Med. 2014;12(5):401–408. doi: 10.1016/S2095-4964(14)60039-X. [DOI] [PMC free article] [PubMed] [Google Scholar]